The Potential of Coffee and Olive by Products as Ingredient in Cosmetics Formulations and Their Extraction Techniques
Abstract
1. Introduction
2. Methodology of Search
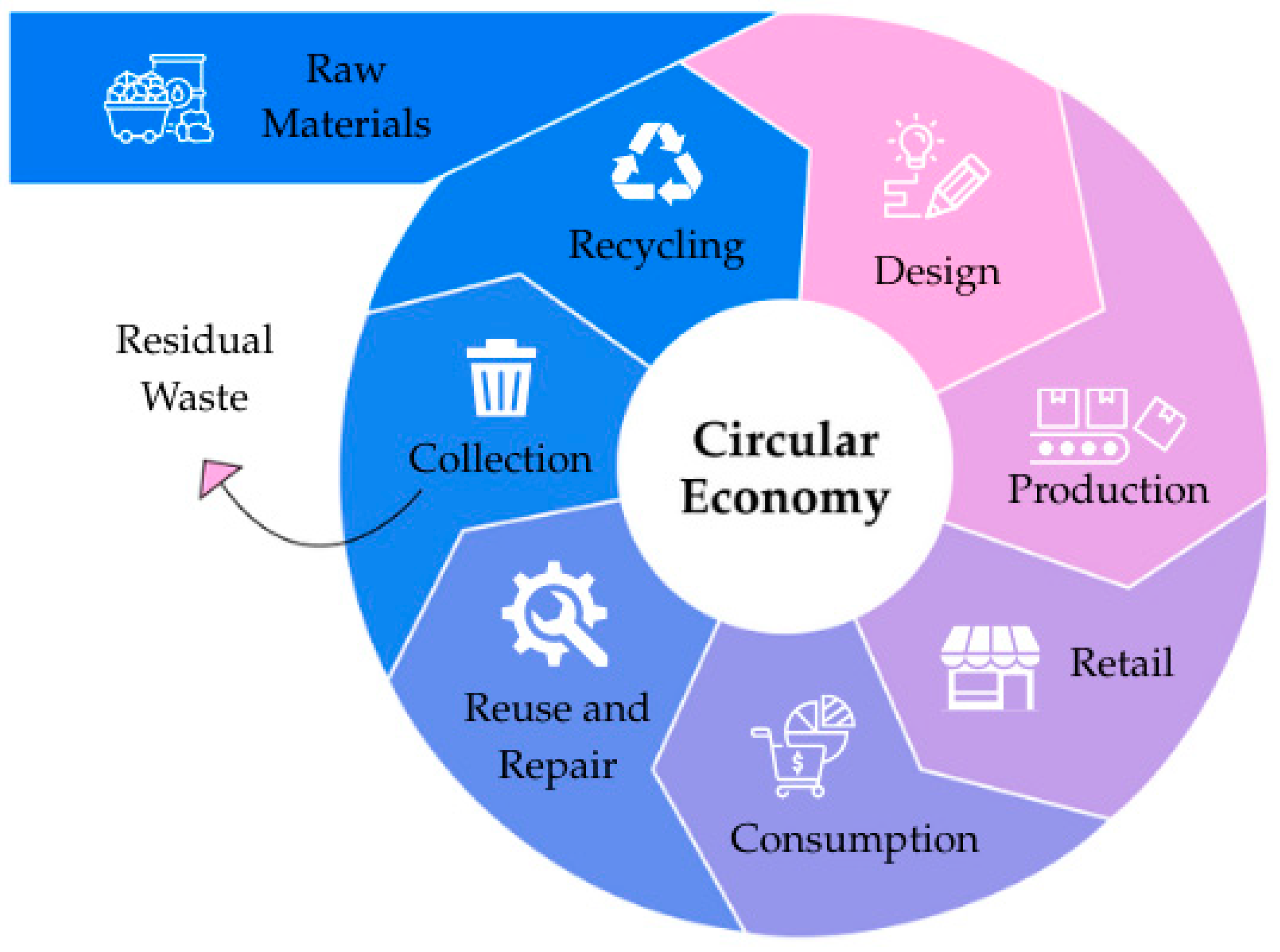
3. Circular Economy
4. Natural Products in Cosmetics
5. Coffee By-Products
5.1. Bioactive Compounds of Coffee By-Products
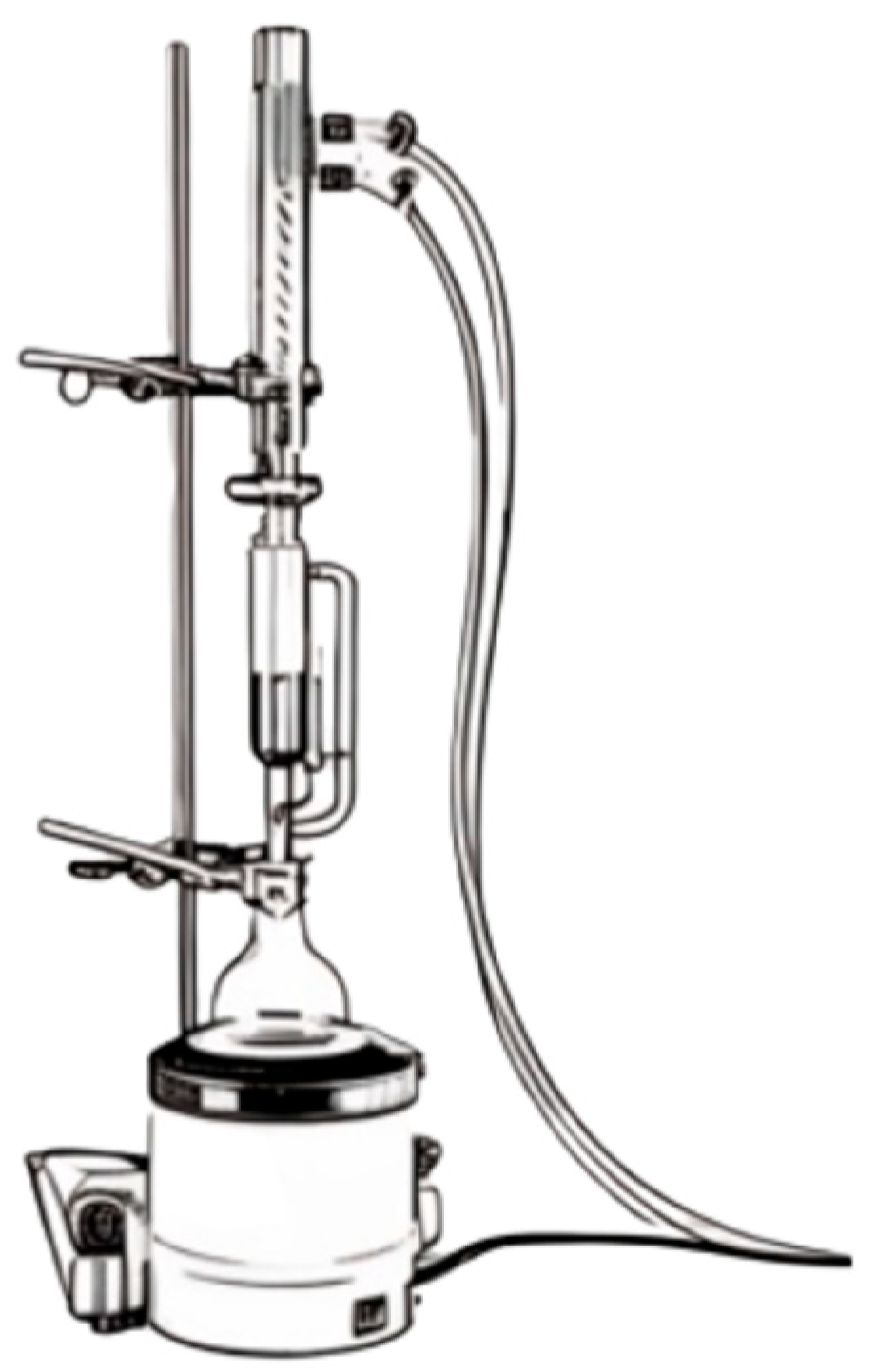
5.2. Extraction Methods for Coffe By-Products
| Category | Method | Pros | Cons | Yield |
|---|---|---|---|---|
| Conventional | Solid–Liquid Extraction (e.g., Soxhlet) | Simple; Economic; Accuracy/reproducibility [49] | Not environmentally friendly; Time consuming; Poor processing adaptability [49] | (ethanol-water solvent) Chlorogenic acid (9.69 mg/g) p-coumaric acid (0.155 to 0.348 mg/g) rutin (0.086 mg/g) [53] |
| Green | Microwave-assisted (MAE) | Less time consuming; Efficient and environmentally friendly; [29,57] | Expensive equipment; Limitations concerning extraction uniformity [29,57] | Chlorogenic acid (84 ± 2.8 mg/g) Caffeine (approx. 72.5 mg/g) [57] |
| Supercritical fluid Extraction (SFE) | Chlorogenic acid (0.55 mg/g) Caffeine (19.49 mg/g) [57] | |||
| Water Ultrasound-Assisted Extraction (UAE) | Chlorogenic acid (1.15 mg/g) Caffeine (0.972 mg/g) [58] | |||
| Water and Vortex Extraction | Chlorogenic acid (0.827 mg/g) Caffeine (0.766 mg/g) [58] | |||
| Supramolecular Solvent Extraction | Chlorogenic acid (0.116 mg/g) Caffeine (0.885 mg/g) [58] |
| Category | Method | Temperature or Power | Time | Solvent Ratio | Ref. |
| Conventional | Solid–Liquid Extraction (e.g., Soxhlet) | 60 °C | 30 min | EtOH:H2O (50:50) | [59] |
| Green | Microwave-assisted (MAE) | 500 W | 2 min | H2O 15 mL/g | [60] |
| Supercritical fluid Extraction (SFE) | Room temperature | 5 min | EtOH: H2O 4:1 v/w | [61] | |
| Water Ultrasound-Assisted Extraction (UAE) | 30–50 °C 100–300 W | 5–45 min | EtOH 5:1–30:1 | [52] | |
| Water and Vortex Extraction | 50/60 Hz, 195 W | 1 min | 0.7 g of SCG to 4 mL of ultrapure water. | [58] | |
| Supramolecular Solvent Extraction | - | 1 min | 0.7 g of SCG to ethanol (1.2 mL), 1-hexanol (0.96 mL), and water until a total volume of 4 mL | [58] |
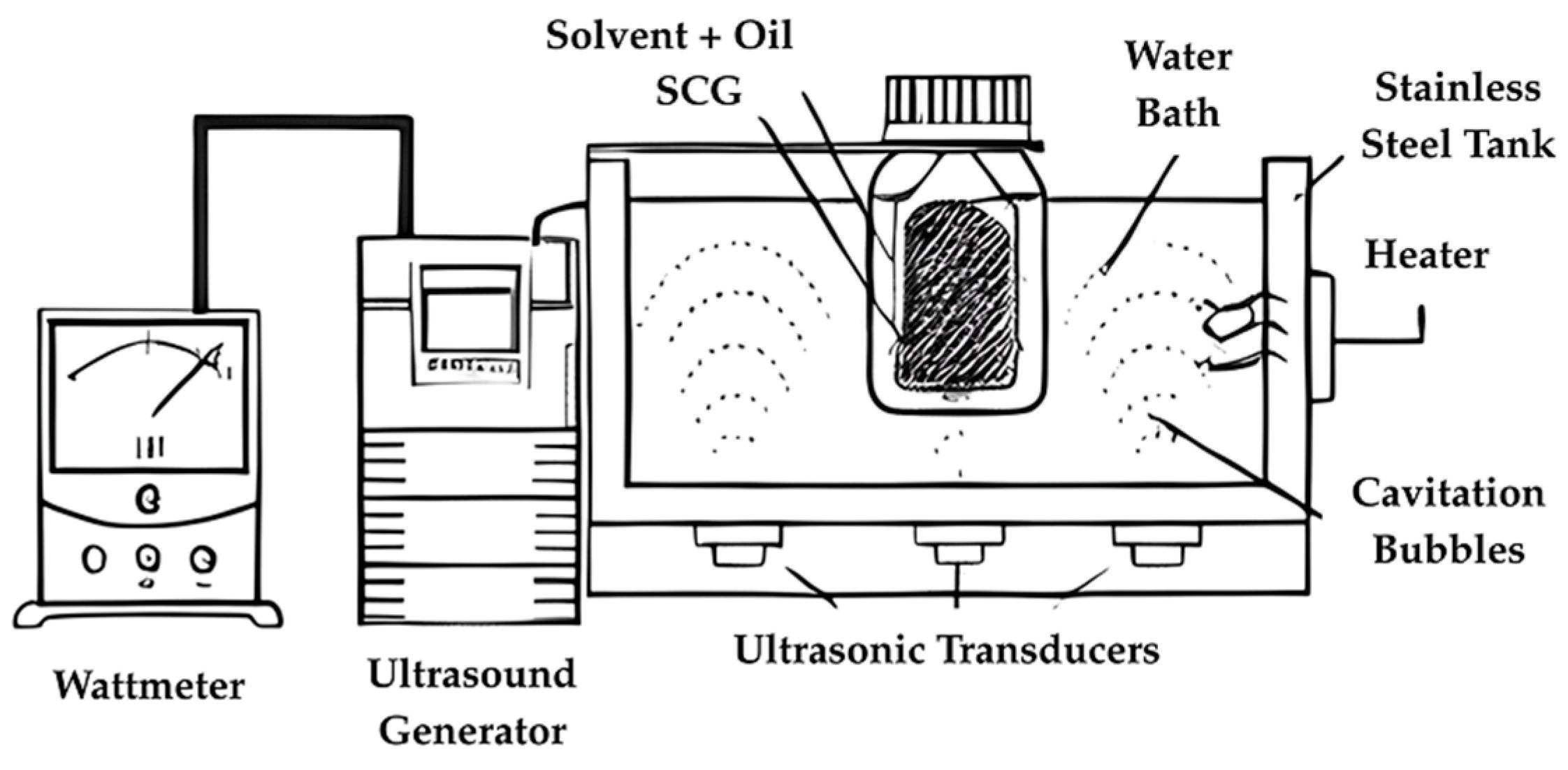
5.3. Applications in Cosmetic Formulations
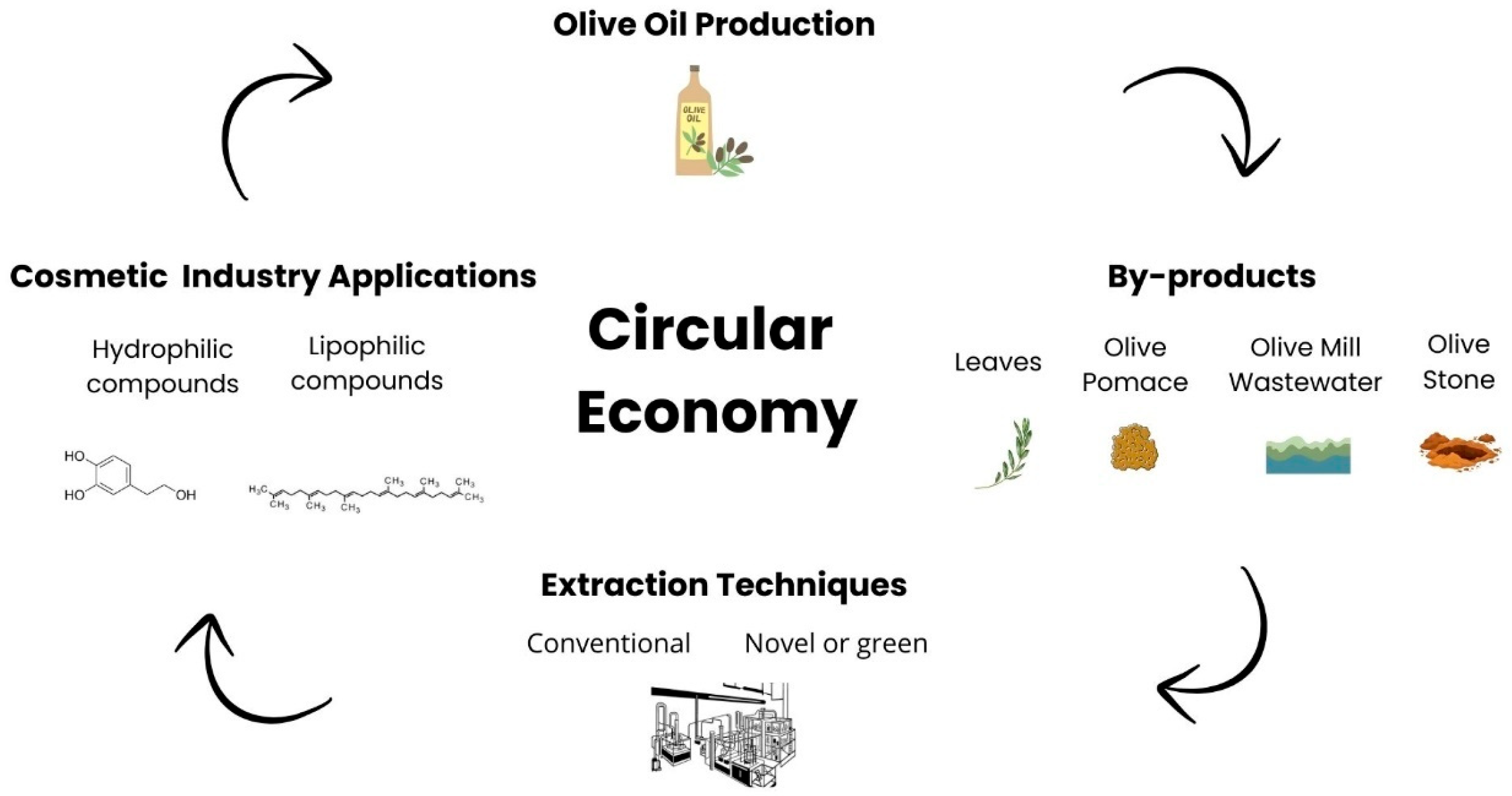
6. Olive By-Products
6.1. Bioactive Compounds of Olive By-Products
6.2. Extraction Methods for Olive By-Products
| Category | Method | Pros | Cons | Yield |
|---|---|---|---|---|
| Conventional | Solid–Liquid Extraction (e.g., Soxhlet) | Simple; Economic; Accuracy/reproducibility [104] | Not environmentally friendly; Time consuming; Poor processing adaptability [104] | (ethanol-water solvent) Hydroxytyrosol (10–15 mg/g) [103] |
| Green | Microwave-assisted (MAE) | Less time consuming; Efficient and environmentally friendly; [107] | Expensive equipment; Limitations concerning extraction uniformity; [107] | Hydroxytyrosol (5.87 mg/g) Manitol (46.70 mg/g) [107] |
| Deep eutectic solvents (DES) | Solvents easy to prepare; Efficient; Inexpensive, and environmentally friendly; [27,111] | Challenges in compound and solvent recovery; [119] | Hydroxytyrosol (4.98 mg/g) [112] | |
| Water Ultrasound-Assisted Extraction (UAE) | Less time consuming; Efficient and environmentally friendly; [108] | Expensive equipment; Limitations concerning extraction uniformity [108] | Hydroxytyrosol (36 ± 2 mg/g) Tyrosol (14 ± 1 mg/g) [106] | |
| Natural Deep Eutectic Solvents (NaDES) | Biocompatible and environmentally friendly; Efficient; [113] | High viscosity; [114] | Hydroxytyrosol (1.24 mg/g) [115] | |
| Enzyme-Assisted Extraction (EAE) | Environmentally friendly and cost-effective; [116] | Enzymes can be expensive; Extraction efficiency may vary with substrate and enzyme; [57] | (Using cellulase and viscoenzyme) Hydroxytyrosol (0.512 mg/g) [117] |
| Category | Method | Temperature or Power | Time | Solvent Ratio | Ref. |
|---|---|---|---|---|---|
| Conventional | Solid–Liquid Extraction (e.g., Soxhlet) | 25 °C | 180 min | EtOH: H2O 5:1 v/w | [120] |
| Green | Microwave-assisted (MAE) | 250–350 W | 2–3 min | Solvent free Sample Amount 5–10 g. | [121] |
| Deep eutectic solvents (DES) | 55 °C | 5 min | ChCl:AA (1:2) ethanol (80:20 w/w) | [122] | |
| Water Ultrasound-Assisted Extraction (UAE) | 500 W 30 °C | 10 min | EtOH: H2O 50% v/v | [123] | |
| Natural Deep Eutectic Solvents (NaDES) | 60 °C | 10 min | Choline chloride and caffeic acid (1:2) | [124] | |
| Enzyme-Assisted Extraction (EAE) | 50 °C | 120 min | Cellulase or viscoenzyme 1.0% | [125] |
6.3. Applications in Cosmetic Formulations
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| C. arabica | Coffea arabica |
| C. robusta | Coffea canephora |
| CH | Coffee Husk |
| CP | Coffee Pulp |
| CS | Coffee Silver skin |
| CPSR | Cosmetic Product Safety Report |
| DES | Deep Eutectic Solvents |
| IGF-1 | Insulin-like growth factor 1 |
| EAE | Enzyme-Assisted Extraction |
| EC | European Commission |
| EVOO | Extra Virgin Olive Oil |
| GMP | Good Manufacturing Practices |
| MAE | Microwave-Assisted Extraction |
| MF | Microfiltration |
| NF | Nanofiltration |
| OMWW | Olive Mill Wastewater |
| O/W | Oil-in-Water |
| RO | Reverse Osmosis |
| SCGs | Spent Coffee Grounds |
| SFE | Supercritical Fluid Extraction |
| UAE | Ultrasound-Assisted Extraction |
| UF | Ultrafiltration |
| W/O | Water-in-Oil |
References
- Baki, G.; Alexander, K.S. Introduction to Cosmetic Formulation and Technology, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2023. [Google Scholar]
- Mondello, A.; Salomone, R.; Mondello, G. Exploring circular economy in the cosmetic industry: Insights from a literature review. Environ. Impact Assess. Rev. 2024, 105, 107443. [Google Scholar] [CrossRef]
- Ferreira, S.M.; Falé, Z.; Santos, L. Sustainability in Skin Care: Incorporation of Avocado Peel Extracts in Topical Formulations. Molecules 2022, 27, 1782. [Google Scholar] [CrossRef]
- Nhani, G.B.B.; Di Filippo, L.D.; de Paula, G.A.; Mantovanelli, V.R.; da Fonseca, P.P.; Tashiro, F.M.; Monteiro, D.C.; Fonseca-Santos, B.; Duarte, J.L.; Chorilli, M. High-Tech Sustainable Beauty: Exploring Nanotechnology for the Development of Cosmetics Using Plant and Animal By-Products. Cosmetics 2024, 11, 112. [Google Scholar] [CrossRef]
- Fortunati, S.; Martiniello, L.; Morea, D. The strategic role of the corporate social responsibility and circular economy in the cosmetic industry. Sustainability 2020, 12, 5120. [Google Scholar] [CrossRef]
- Martins, A.M.; Marto, J.M. A sustainable life cycle for cosmetics: From design and development to post-use phase. Sustain. Chem. Pharm. 2023, 35, 101178. [Google Scholar] [CrossRef]
- Khajuria, A.; Atienza, V.A.; Chavanich, S.; Henning, W.; Islam, I.; Kral, U.; Liu, M.; Liu, X.; Murthy, I.K.; Oyedotun, T.D.T.; et al. Accelerating circular economy solutions to achieve the 2030 agenda for sustainable development goals. Circ. Econ. 2022, 1, 100001. [Google Scholar] [CrossRef]
- Circular Economy Action Plan. Available online: https://environment.ec.europa.eu/strategy/circular-economy-action-plan_en (accessed on 28 April 2025).
- Khajuria, A. Integrated Approach between DPSIR, Planetary Boundaries and Sustainable Development Goals towards 3Rs and Resource Efficiency. World Environ. 2020, 10, 52–56. [Google Scholar] [CrossRef]
- Kirchherr, J.; Reike, D.; Hekkert, M. Conceptualizing the circular economy: An analysis of 114 definitions. Resour. Conserv. Recycl. 2017, 127, 221–232. [Google Scholar] [CrossRef]
- Circular Economy: Definition, Importance and Benefits. Available online: https://www.europarl.europa.eu/topics/en/article/20151201STO05603/circular-economy-definition-importance-and-benefits#:~:text=What%20is%20the%20circular%20economy,products%20as%20long%20as%20possible (accessed on 8 February 2025).
- Tlais, A.Z.A.; Fiorino, G.M.; Polo, A.; Filannino, P.; Di Cagno, R. High-value compounds in fruit, vegetable and cereal byproducts: An overview of potential sustainable reuse and exploitation. Molecules 2020, 25, 2987. [Google Scholar] [CrossRef]
- Faria-Silva, C.; Ascenso, A.; Costa, A.M.; Marto, J.; Carvalheiro, M.; Ribeiro, H.M.; Simões, S. Feeding the skin: A new trend in food and cosmetics convergence. Trends Food Sci. Technol. 2020, 95, 21–32. [Google Scholar] [CrossRef]
- Hoang, H.T.; Moon, J.Y.; Lee, Y.C. Natural antioxidants from plant extracts in skincare cosmetics: Recent applications, challenges and perspectives. Cosmetics 2021, 8, 108. [Google Scholar] [CrossRef]
- Scott, D.A. A review of ancient Egyptian pigments and cosmetics. Stud. Conserv. 2016, 61, 185–202. [Google Scholar] [CrossRef]
- González-Minero, F.J.; Bravo-Díaz, L. The use of plants in skin-care products, cosmetics and fragrances: Past and present. Cosmetics 2019, 5, 50. [Google Scholar] [CrossRef]
- McMullen, R.L.; Dell’Acqua, G. History of Natural Ingredients in Cosmetics. Cosmetics 2023, 10, 71. [Google Scholar] [CrossRef]
- Mansoor, K.; Aburjai, T.; Al-Mamoori, F.; Schmidt, M. Plants with cosmetic uses. Phytother. Res. 2023, 37, 5755–5768. [Google Scholar] [CrossRef]
- Menaa, F. Skin Anti-Aging Benefits of Phytotherapeutics-based Emulsions. Pharm. Anal. Acta 2014, 5, e168. [Google Scholar] [CrossRef]
- Jadoon, S.; Karim, S.; Asad, M.H.H.; Bin Akram, M.R.; Kalsoom Khan, A.; Malik, A.; Chen, C.; Murtaza, G. Anti-aging potential of phytoextract loaded-pharmaceutical creams for human skin cell longetivity. Oxid. Med. Cell. Longev. 2015, 2015, 709628. [Google Scholar] [CrossRef] [PubMed]
- Michalak, M. Plant Extracts as Skin Care and Therapeutic Agents. Int. J. Mol. Sci. 2023, 24, 15444. [Google Scholar] [CrossRef] [PubMed]
- Ugolini, T.; Cecchi, L.; Sani, G.; Digiglio, I.; Adinolfi, B.; Ciaccheri, L.; Zanoni, B.; Melani, F.; Mulinacci, N. Seasonal and Cultivar-Dependent Phenolic Dynamics in Tuscan Olive Leaves: A Two-Year Study by HPLC-DAD-MS for Food By-Product Valorization. Separations 2025, 12, 192. [Google Scholar] [CrossRef]
- Dias, E.A.; Miho, H.; Escobar, L.; Moral, J.; Diez, C.M.; Capote, P.F. Influence of genetic and interannual factors on bioactive compounds of olive pomace determined through a germplasm survey. Food Chem. 2022, 378, 1322107. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, Y.; Xiang, L.; Picardo, M. Deciphering the role of skin aging in pigmentary disorders. Free Radic. Biol. Med. 2025, 227, 638–655. [Google Scholar] [CrossRef]
- Saewan, N.; Jimtaisong, A.; Panyachariwat, N.; Chaiwut, P. In Vitro and In Vivo Anti-Aging Effect of Coffee Berry Nanoliposomes. Molecules 2023, 28, 6830. [Google Scholar] [CrossRef]
- Rodrigues, R.; Oliveira, M.B.P.P.; Alves, R.C. Chlorogenic Acids and Caffeine from Coffee By-Products: A Review on Skincare Applications. Cosmetics 2023, 10, 12. [Google Scholar] [CrossRef]
- Dauber, C.; Parente, E.; Zucca, M.P.; Gámbaro, A.; Vieitez, I. Olea europea and By-Products: Extraction Methods and Cosmetic Applications. Cosmetics 2023, 10, 112. [Google Scholar] [CrossRef]
- Hasballah, K.; Lestari, W.; Listiawan, M.Y.; Sofia, S. Coffee by-products as the source of antioxidants: A systematic review. F1000Research 2022, 11, 220. [Google Scholar] [CrossRef]
- Ribeiro, R.C.; Mota, M.F.S.; Silva, R.M.V.; Silva, D.C.; Novaes, F.J.M.; da Veiga, V.F.; Bizzo, H.R.; Teixeira, R.S.S.; Rezende, C.M. Coffee Oil Extraction Methods: A Review. Foods 2024, 13, 2601. [Google Scholar] [CrossRef]
- Lee, Y.G.; Cho, E.J.; Maskey, S.; Nguyen, D.T.; Bae, H.J. Value-Added Products from Coffee Waste: A Review. Molecules 2023, 28, 3562. [Google Scholar] [CrossRef]
- Oussou, K.F.; Guclu, G.G.; Kelebek, H.; Selli, S. Valorization of cocoa, tea and coffee processing by-products-wastes. In Advances in Food and Nutrition Research; Çapanoğlu Güven, E., Navarro-Hortal, M.D., Forbes-Hernández, T.Y., Battino, M., Eds.; Academic Press: Cambridge, MA, USA, 2023; Volume 107, pp. 91–130. [Google Scholar]
- De Bruyn, F.; Zhang, S.J.; Pothakos, V.; Torres, J.; Lambot, C.; Moroni, A.V.; Callanan, M.; Sybesma, W.; Weckx, S.; De Vuyst, L. Exploring the impacts of postharvest processing on the microbiota and metabolite profiles during green coffee bean production. Appl. Environ. Microbiol. 2017, 83, e02398-16. [Google Scholar] [CrossRef]
- Figueroa Campos, G.A.; Sagu, S.T.; Celis, P.S.; Rawel, H.M. Comparison of batch and continuous wet-processing of coffee: Changes in the main compounds in beans, by-products and wastewater. Foods 2020, 9, 1135. [Google Scholar] [CrossRef]
- Oliveira, G.; Passos, C.P.; Ferreira, P.; Coimbra, M.A.; Gonçalves, I. Coffee by-products and their suitability for developing active food packaging materials. Foods 2021, 10, 683. [Google Scholar] [CrossRef]
- Galali, Y.; Omar, Z.A.; Sajadi, S.M. Biologically active components in by-products of food processing. Food Sci. Nutr. 2020, 8, 3004–3022. [Google Scholar] [CrossRef] [PubMed]
- de Melo Pereira, G.V.; de Carvalho Neto, D.P.; Magalhães Júnior, A.I.; do Prado, F.G. Chemical composition and health properties of coffee and coffee by-products. In Advances in Food and Nutrition Research; Toldrá, F., Ed.; Academic Press: Cambridge, MA, USA, 2020; Volume 91, pp. 65–96. [Google Scholar]
- Machado, M.; Espírito Santo, L.; Machado, S.; Lobo, J.C.; Costa, A.S.G.; Oliveira, M.B.P.P.; Ferreira, H.; Alves, R.C. Bioactive Potential and Chemical Composition of Coffee By-Products: From Pulp to Silverskin. Foods 2023, 12, 2354. [Google Scholar] [CrossRef]
- Martínez-Inda, B.; Jiménez-Moreno, N.; Esparza, I.; Ancín-Azpilicueta, C. Coffee and Cocoa By-Products as Valuable Sources of Bioactive Compounds: The Influence of Ethanol on Extraction. Antioxidants 2025, 14, 42. [Google Scholar] [CrossRef] [PubMed]
- Bessada, S.M.F.; Alves, R.C.; Oliveira, M.B.P.P. Coffee silverskin: A review on potential cosmetic applications. Cosmetics 2018, 5, 5. [Google Scholar] [CrossRef]
- Zengin, G.; Sinan, K.I.; Mahomoodally, M.F.; Angeloni, S.; Mustafa, A.M.; Vittori, S.; Maggi, F.; Caprioli, G. Chemical composition, antioxidant and enzyme inhibitory properties of different extracts obtained from spent coffee ground and coffee silverskin. Foods 2020, 9, 713. [Google Scholar] [CrossRef] [PubMed]
- Janissen, B.; Huynh, T. Chemical composition and value-adding applications of coffee industry by-products: A review. Resour. Conserv. Recycl. 2018, 128, 110–117. [Google Scholar] [CrossRef]
- Costa, A.S.G.; Peixoto, J.A.B.; Machado, S.; Espírito Santo, L.; Soares, T.F.; Andrade, N.; Azevedo, R.; Almeida, A.; Costa, H.S.; Oliveira, M.B.P.P.; et al. Coffee Pulp from Azores: A Novel Phytochemical-Rich Food with Potential Anti-Diabetic Properties. Foods 2025, 14, 306. [Google Scholar] [CrossRef]
- Maiyah, N.; Kerdpiboon, S.; Supapvanich, S.; Kerr, W.L.; Sriprom, P.; Chotigavin, N.; Klaypradit, W.; Puttongsiri, T. Recovering bioactive compounds and antioxidant capacity of medium roasted spent coffee grounds through varied hydrothermal brewing cycles. J. Agric. Food Res. 2025, 20, 101789. [Google Scholar] [CrossRef]
- Lu, L.; Tibpromma, S.; Karunarathna, S.C.; Jayawardena, R.S.; Lumyong, S.; Xu, J.; Hyde, K.D. Comprehensive Review of Fungi on Coffee. Pathogens 2022, 11, 411. [Google Scholar] [CrossRef]
- Hermansen, C.; Chong, Q.K.; Ho, S.; Natali, F.; Weingarten, M.; Peterson, E.C. Microbiome Evolution of Brewer’s Spent Grain and Spent Coffee Ground Solid Sidestreams Under Industrial Storage Conditions. Appl. Sci. 2024, 14, 9759. [Google Scholar] [CrossRef]
- Nolasco, A.; Squillante, J.; Esposito, F.; Velotto, S.; Romano, R.; Aponte, M.; Giarra, A.; Toscanesi, M.; Montella, E.; Cirillo, T. Coffee Silverskin: Chemical and Biological Risk Assessment and Health Profile for Its Potential Use in Functional Foods. Foods 2022, 11, 2834. [Google Scholar] [CrossRef]
- Bessaire, T.; Perrin, I.; Tarres, A.; Bebius, A.; Reding, F.; Theurillat, V. Mycotoxins in green coffee: Occurrence and risk assessment. Food Control 2019, 96, 59–67. [Google Scholar] [CrossRef]
- López-Rodríguez, C.; Verheecke-Vaessen, C.; Strub, C.; Fontana, A.; Schorr-Galindo, S.; Medina, A. Reduction in Ochratoxin A Occurrence in Coffee: From Good Practices to Biocontrol Agents. J. Fungi 2024, 10, 590. [Google Scholar] [CrossRef]
- Felten Bondam, A.; da Silveira, D.D.; Pozzada dos Santos, J.; Hoffmann, J.F. Phenolic compounds from coffee by-products: Extraction and application in the food and pharmaceutical industries. Trends Food Sci. Technol. 2022, 123, 172–186. [Google Scholar] [CrossRef]
- Pyrzynska, K. Useful Extracts from Coffee By-Products: A Brief Review. Separations 2024, 11, 334. [Google Scholar] [CrossRef]
- Mwaurah, P.W.; Kumar, S.; Kumar, N.; Attkan, A.K.; Panghal, A.; Singh, V.K.; Garg, M.K. Novel oil extraction technologies: Process conditions, quality parameters, and optimization. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3–20. [Google Scholar] [CrossRef]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochem. 2020, 70, 105325. [Google Scholar] [CrossRef]
- Ramón-Gonçalves, M.; Gómez-Mejía, E.; Rosales-Conrado, N.; León-González, M.E.; Madrid, Y. Extraction, identification and quantification of polyphenols from spent coffee grounds by chromatographic methods and chemometric analyses. Waste Manag. 2019, 96, 15–24. [Google Scholar] [CrossRef]
- Pham, Q.D.; Topgaard, D.; Sparr, E. Tracking solvents in the skin through atomically resolved measurements of molecular mobility in intact stratum corneum. Proc. Natl. Acad. Sci. USA 2017, 114, E112–E121. [Google Scholar] [CrossRef]
- ISO 16128-2:2017; Cosmetics—Guidelines on Technical Definitions and Criteria for Natural and Organic Cosmetic Ingredients Part 2: Criteria for Ingredients and Products. ISO Copyright Office: Geneva, Switzerland, 2017.
- Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32009R1223 (accessed on 19 June 2025).
- Ramadan, M.; Fadel, A.; Bahi, A.; Alajlani, S.; Al Neyadi, M.S.S.; Alshamsi, E.S.H.; Naser, S.; Shahbaz, H.M.; Samuel, R.; Ranneh, Y. Optimizing Chlorogenic Acid Extraction From Spent Coffee Grounds: A Comparative Review of Conventional and Non-Conventional Techniques. Food Sci. Nutr. 2025, 13, e70315. [Google Scholar] [CrossRef]
- Bouhzam, I.; Cantero, R.; Balcells, M.; Margallo, M.; Aldaco, R.; Bala, A.; Fullana-i-Palmer, P.; Puig, R. Environmental and Yield Comparison of Quick Extraction Methods for Caffeine and Chlorogenic Acid from Spent Coffee Grounds. Foods 2023, 12, 779. [Google Scholar] [CrossRef]
- Brzezińska, R.; Wirkowska-Wojdyła, M.; Piasecka, I.; Górska, A. Application of Response Surface Methodology to Optimize the Extraction Process of Bioactive Compounds Obtained from Coffee Silverskin. Appl. Sci. 2023, 13, 5388. [Google Scholar] [CrossRef]
- Savić, I.M.; Savić, G.I.M.; Gajić, D.G. Optimization of the Microwave-Assisted Extraction of Caffeine from Roasted Coffee Beans. Foods 2024, 13, 2333. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, L.S.; Gómez, A.B.; Rubio, S. Supramolecular solvent extraction of bioactives from coffee cherry pulp. J. Food Eng. 2020, 278, 109933. [Google Scholar] [CrossRef]
- Kanlayavattanakul, M.; Lourith, N.; Chaikul, P. Valorization of spent coffee grounds as the specialty material for dullness and aging of skin treatments. Chem. Biol. Technol. Agric. 2021, 8, 55. [Google Scholar] [CrossRef]
- Miladi, M.; Martins, A.A.; Mata, T.M.; Vegara, M.; Pérez, I.M.; Remmani, R.; Ruiz-Canales, A.; Núñez-Gómez, D. Optimization of Ultrasound-Assisted Extraction of Spent Coffee Grounds Oil Using Response Surface Methodology. Processes 2021, 9, 2085. [Google Scholar] [CrossRef]
- dos Santos, É.M.; de Macedo, L.M.; Tundisi, L.L.; Ataide, J.A.; Camargo, G.A.; Alves, R.C.; Oliveira, M.B.P.P.; Mazzola, G. Coffee by-products in topical formulations: A review. Trends Food Sci. Technol. 2021, 111, 280–291. [Google Scholar] [CrossRef]
- Dias, R.; Oliveira, H.; Fernandes, I.; Simal-Gandara, J.; Perez-Gregorio, R. Recent advances in extracting phenolic compounds from food and their use in disease prevention and as cosmetics. Crit. Rev. Food Sci. Nutr. 2020, 61, 1130–1151. [Google Scholar] [CrossRef]
- Cho, Y.-H.; Bahuguna, A.; Kim, H.-H. Potential effect of compounds isolated from Coffea arabica against UV-B induced skin damage by protecting fibroblast cells. J. Photochem. Photobiol. B 2017, 174, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.F.; Magalhães, W.V.; Di Stasi, L.C. Recent Advances in Herbal-Derived Products with Skin Anti-Aging Properties and Cosmetic Applications. Molecules 2022, 27, 7518. [Google Scholar] [CrossRef] [PubMed]
- Hahn, H.J.; Kim, K.B.; Bae, S.; Choi, B.G.; An, S.; Ahn, K.J.; Kim, S.Y. Pretreatment of ferulic acid protects human dermal fibroblasts against ultraviolet a irradiation. Ann. Dermatol. 2016, 28, 740–748. [Google Scholar] [CrossRef]
- Stevanato, R.; Bertelle, M.; Fabris, S. Photoprotective characteristics of natural antioxidant polyphenols. Regul. Toxicol. Pharmacol. 2014, 69, 71–77. [Google Scholar] [CrossRef]
- Ng, S.Y.; Eh Suk, V.R.; Gew, L.T. Plant polyphenols as green sunscreen ingredients: A systematic review. J. Cosmet. Dermatol. 2022, 21, 5409–5444. [Google Scholar] [CrossRef] [PubMed]
- Toschi, T.G.; Cardenia, V.; Bonaga, G.; Mandrioli, M.; Rodriguez, E.M.T. Coffee silverskin: Characterization, possible uses, and safety aspects. J. Agric. Food Chem. 2014, 62, 10836–10844. [Google Scholar] [CrossRef]
- Szendzielorz, E.; Spiewak, R. Caffeine as an Active Molecule in Cosmetic Products for Hair Loss: Its Mechanisms of Action in the Context of Hair Physiology and Pathology. Molecules 2025, 30, 167. [Google Scholar] [CrossRef] [PubMed]
- Fischer, T.W.; Herczeg, L.E.; Funk, W.; Zillikens, D.; Bíró, T.; Paus, R. Differential effects of caffeine on hair shaft elongation, matrix and outer root sheath keratinocyte proliferation, and transforming growth factor-β2/insulin-like growth factor-1-mediated regulation of the hair cycle in male and female human hair follicles in vitro. Br. J. Dermatol. 2014, 171, 1031–1043. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, F.; Alves, A.C.; Nunes, C.; Sarmento, B.; Amaral, M.; Reis, S.; Oliveira, M.B.P.P. Permeation of topically applied caffeine from a food by—Product in cosmetic formulations: Is nanoscale in vitro approach an option? Int. J. Pharm. 2016, 513, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Cherian, P.A.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C. Safety Assessment of Methylxanthines as Used in Cosmetics. Int. J. Toxicol. 2024, 43, 42–77. [Google Scholar] [CrossRef]
- Donejko, M.; Przylipiak, A.; Rysiak, E.; Głuszuk, K.; Surażyński, A. Influence of caffeine and hyaluronic acid on collagen biosynthesis in human skin fibroblasts. Drug Des. Dev. Ther. 2014, 8, 1923–1928. [Google Scholar] [CrossRef]
- Rivelli, D.P.; Filho, C.A.H.; Almeida, R.L.; Ropke, C.D.; Sawada, T.C.H.; Barros, S.B.M. Chlorogenic acid UVA-UVB photostability. Photochem. Photobiol. 2010, 86, 1005–1007. [Google Scholar] [CrossRef]
- Grigolon, G.; Nowak, K.; Poigny, S.; Hubert, J.; Kotland, A.; Waldschütz, L.; Wandrey, F. From Coffee Waste to Active Ingredient for Cosmetic Applications. Int. J. Mol. Sci. 2023, 24, 8516. [Google Scholar] [CrossRef]
- Rodrigues, F.; Matias, R.; Ferreira, M.; Amaral, M.H.; Oliveira, M.B.P.P. In vitro and in vivo comparative study of cosmetic ingredients Coffee silverskin and hyaluronic acid. Exp. Dermatol. 2016, 25, 572–574. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, F.; Sarmento, B.; Amaral, M.H.; Oliveira, M.B. Cytotoxicity evaluation of a new ingredient with cosmetic proposals incorporated into a hand cream. Toxicol. Lett. 2015, 238, S354. [Google Scholar] [CrossRef]
- Al-Yousef, H.M.; Amina, M. Essential oil of Coffee arabica L. husks: A brilliant source of antimicrobial and antioxidant agents. Biomed. Res. 2018, 29, 174–180. [Google Scholar] [CrossRef]
- Andrade, C.; Perestrelo, R.; Câmara, J.S. Bioactive Compounds and Antioxidant Activity from Spent Coffee Grounds as a Powerful Approach for Its Valorization. Molecules 2022, 27, 7504. [Google Scholar] [CrossRef]
- Silva, V.; Igrejas, G.; Falco, V.; Santos, T.P.; Torres, C.; Oliveira, A.M.P.; Pereira, J.E.; Amaral, J.S.; Poeta, P. Chemical composition, antioxidant and antimicrobial activity of phenolic compounds extracted from wine industry by-products. Food Control 2018, 92, 516–522. [Google Scholar] [CrossRef]
- VITCHY Laboratoires. Available online: https://www.vichy.pt (accessed on 26 August 2025).
- Erskine, E.; Gültekin, S.B.; Vahapoglu, B.; Capanoglu, E. Coffee Phenolics and Their Interaction with Other Food Phenolics: Antagonistic and Synergistic Effects. ACS Omega 2022, 7, 1595–1601. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Yang, L.; Zhu, Y.; Liao, M.; Chu, L.; Li, X.; Lin, L.; Zheng, G. Collaborative effects of chlorogenic acid and caffeine on lipid metabolism via the AMPKα-LXRα/SREBP-1c pathway in high-fat diet-induced obese mice. Food Funct. 2019, 10, 7489–7497. [Google Scholar] [CrossRef]
- Nemzer, B.; Edwards, J.; Kalita, D. Matrix-Specific Effects on Caffeine and Chlorogenic Acid Complexation in a Novel Extract of Whole Coffea arabica Coffee Cherry by NMR Spectroscopy. Molecules 2022, 27, 7803. [Google Scholar] [CrossRef]
- Valvez, S.; Maceiras, A.; Santos, P.; Reis, P.N.B. Olive stones as filler for polymer-based composites: A review. Materials 2021, 14, 845. [Google Scholar] [CrossRef]
- Romani, A.; Ieri, F.; Urciuoli, S.; Noce, A.; Marrone, G.; Nediani, C.; Bernini, R. Health effects of phenolic compounds found in extra-virgin olive oil, by-products, and leaf of Olea europaea L. Nutrients 2019, 11, 1776. [Google Scholar] [CrossRef]
- Rodrigues, F.; Pimentel, F.B.; Oliveira, M.B.P.P. Olive by-products: Challenge application in cosmetic industry. Ind. Crops Prod. 2015, 70, 116–124. [Google Scholar] [CrossRef]
- Rodrigues, R.; Alves, R.C.; Oliveira, M.B.P.P. Exploring Olive Pomace for Skincare Applications: A Review. Cosmetics 2023, 10, 35. [Google Scholar] [CrossRef]
- Rodrigues, R.; Lobo, J.C.; Ferreira, D.M.; Senderowicz, E.; Nunes, M.A.; Amaral, M.H.; Alves, R.C.; Oliveira, M.B.P.P. Chemical and Rheological Characterization of a Facial Mask Containing an Olive Pomace Fraction. Cosmetics 2023, 10, 64. [Google Scholar] [CrossRef]
- Otero, P.; Oliveira, P.G.; Carpena, M.; Martinez, M.B. Applications of by-products from the olive oil processing: Revalorization strategies based on target molecules and green extraction technologies. Trends Food Sci. Technol. 2021, 116, 1084–1104. [Google Scholar] [CrossRef]
- Domingues, E.; Fernandes, E.; Gomes, J.; Castro-Silva, S.; Martins, R.C. Olive oil extraction industry wastewater treatment by coagulation and Fenton’s process. J. Water Process Eng. 2021, 39, 101818. [Google Scholar] [CrossRef]
- Madureira, J.M.A.; Margaça, F.; Santos-Buelga, C. Applications of bioactive compounds extracted from olive industry wastes: A review. Compr. Rev. Food Sci. Food Saf. 2021, 21, 453–476. [Google Scholar] [CrossRef]
- Cuffaro, D.; Bertolini, A.; Bertini, S.; Ricci, C.; Cascone, M.G.; Danti, S.; Saba, A.; Macchia, M.; Digiacomo, M. Olive Mill Wastewater as Source of Polyphenols with Nutraceutical Properties. Nutrients 2023, 15, 3746. [Google Scholar] [CrossRef]
- Sosa, A.; Armienta, M.A.; Aguayo, A.; Cruz, O. Evaluation of the influence of main groundwater ions on arsenic removal by limestones through column experiments. Sci. Total Environ. 2020, 727, 138459. [Google Scholar] [CrossRef]
- Madureira, J.; Dias, M.I.; Pinela, J.; Calhelha, R.C.; Barros, L.; Santos-Buelga, C.; Margaça, F.M.A.; Ferreira, I.C.F.R.; Cabo Verde, S. The use of gamma radiation for extractability improvement of bioactive compounds in olive oil wastes. Sci. Total Environ. 2020, 727, 138706. [Google Scholar] [CrossRef]
- Abbattista, R.; Ventura, G.; Calvano, C.D.; Cataldi, T.R.I.; Losito, I. Bioactive compounds in waste by-products from olive oil production: Applications and structural characterization by mass spectrometry techniques. Foods 2021, 10, 1236. [Google Scholar] [CrossRef]
- Mir-Cerdà, A.; Ramos-Calero, V.; Beltrán, J.L.; Cortina, J.L.; Saurina, J.; Sentellas, S.; Granados, M. Towards a sustainable recovery of polyphenols from agrifood waste: Performance of polymeric sorbents with natural deep eutectic solvent extracts. LWT 2024, 207, 116632. [Google Scholar] [CrossRef]
- Ben Saad, A.; Tiss, M.; Keskes, H.; Chaari, A.; Sakavitsi, M.E.; Hamden, K.; Halabalaki, M.; Allouche, N. Antihyperlipidemic, Antihyperglycemic, and Liver Function Protection of Olea europaea var. Meski Stone and Seed Extracts: LC-ESI-HRMS-Based Composition Analysis. J. Diabetes Res. 2021, 2021, 6659415. [Google Scholar] [CrossRef]
- Clodoveo, M.L.; Crupi, P.; Annunziato, A.; Corbo, F. Innovative Extraction Technologies for Development of Functional Ingredients Based on Polyphenols from Olive Leaves. Foods 2022, 11, 103. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, C.S.; Lyri, P.; Xintaropoulou, I.; Diamantopoulos, I.; Zagklis, D.P.; Paraskeva, C.A. High-Yield Production of a Rich-in-Hydroxytyrosol Extract from Olive (Olea europaea) Leaves. Antioxidants 2022, 11, 1042. [Google Scholar] [CrossRef]
- Markhali, F.S.; Teixeira, J.A.; Rocha, C.M.R. Olive tree leaves—A source of valuable active compounds. Processes 2020, 8, 1177. [Google Scholar] [CrossRef]
- Panzella, L.; Moccia, F.; Nasti, R.; Marzorati, S.; Verotta, L.; Napolitano, A. Bioactive Phenolic Compounds from Agri-Food Wastes: An Update on Green and Sustainable Extraction Methodologies. Front. Nutr. 2020, 7, 60. [Google Scholar] [CrossRef]
- Madureira, J.; Albuquerque, B.; Dias, M.I.; Pinela, J.; Calhelha, R.C.; Santos-Buelga, C.; Margaça, F.M.A.; Ferreira, I.C.F.R.; Cabo Verde, S.; Barros, L. Ultrasound-assisted extraction of hydroxytyrosol and tyrosol from olive pomace treated by gamma radiation: Process optimization and bioactivity assessment. Food Funct. 2023, 14, 3038–3050. [Google Scholar] [CrossRef]
- Gómez, C.I.; Del Mar, C.M.; Romero, I.; Castro, E. Optimization of Microwave-Assisted Water Extraction to Obtain High Value-Added Compounds from Exhausted Olive Pomace in a Biorefinery Context. Foods 2022, 11, 2002. [Google Scholar] [CrossRef]
- Gómez, C.I.; Del Mar, C.M.; Carvalheiro, F.; Duarte, L.C.; Roseiro, L.B.; Romero, I.; Castro, E. Recovery of Bioactive Compounds from Industrial Exhausted Olive Pomace through Ultrasound-Assisted Extraction. Biology 2021, 10, 514. [Google Scholar] [CrossRef] [PubMed]
- Expósito, A.X.; Munguía, U.Á.; Duque, S.C.; Borrás, L.I.; Quirante, P.R.; Lozano, S.J. Optimized Ultrasound-Assisted Extraction for Enhanced Recovery of Valuable Phenolic Compounds from Olive By-Products. Antioxidants 2025, 14, 938. [Google Scholar] [CrossRef] [PubMed]
- Contreras, A.L.A.; Laaroussi, H.; Ousaaid, D.; Bakour, M.; Lyoussi, B.; Ferreira, S.P. Sustainable Valorization of Olive Oil By-Products: Green Extraction of Phytochemicals, Encapsulation Strategies, and Food Applications. J. Food Sci. 2025, 90, e70412. [Google Scholar] [CrossRef]
- de Almeida Pontes, P.V.; Ayumi Shiwaku, I.; Maximo, G.J.; Caldas Batista, E.A. Choline chloride-based deep eutectic solvents as potential solvent for extraction of phenolic compounds from olive leaves: Extraction optimization and solvent characterization. Food Chem. 2021, 352, 129346. [Google Scholar] [CrossRef]
- Hu, M.; Han, B.; Xie, L.; Lu, B.; Bai, D.; Shi, N.; Liao, Y.; Wang, Y.; Liu, L.; Wu, S.; et al. Ultrasonic assisted natural deep eutectic solvents as a green and efficient approach for extraction of hydroxytyrosol from olive leaves. Ind. Chem. Mater. 2024, 2, 309–320. [Google Scholar] [CrossRef]
- Oyoun, F.; Toncheva, A.; Henríquez, L.C.; Grougnet, R.; Laoutid, F.; Mignet, N.; Alhareth, K.; Corvis, Y. Deep Eutectic Solvents: An Eco-friendly Design for Drug Engineering. ChemSusChem 2023, 16, 938. [Google Scholar] [CrossRef]
- Hikmawanti, N.P.E.; Ramadon, D.; Jantan, I.; Mun’im, A. Natural deep eutectic solvents (Nades): Phytochemical extraction performance enhancer for pharmaceutical and nutraceutical product development. Plants 2021, 10, 2091. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, L.; Xavier, L.; Zecchi, B. Extraction of phenolic compounds with antioxidant activity from olive pomace using natural deep eutectic solvents: Modelling and optimization by response surface methodology. Discov. Food 2024, 4, 29. [Google Scholar] [CrossRef]
- Łubek-Nguyen, A.; Ziemichód, W.; Olech, M. Application of Enzyme-Assisted Extraction for the Recovery of Natural Bioactive Compounds for Nutraceutical and Pharmaceutical Applications. Appl. Sci. 2022, 12, 3232. [Google Scholar] [CrossRef]
- Martins, V.F.R.; Ribeiro, T.B.; Lopes, A.I.; Pintado, M.E.; Morais, R.M.S.C.M.; Morais, A.M.M.B. Comparison among Different Green Extraction Methods of Polyphenolic Compounds from Exhausted Olive Oil Pomace and the Bioactivity of the Extracts. Molecules 2024, 29, 1935. [Google Scholar] [CrossRef]
- Dobrinčić, A.; Repajić, M.; Garofulić, I.E.; Tuden, L.; Dragović, U.V.; Levaj, B. Comparison of different extraction methods for the recovery of olive leaves polyphenols. Processes 2020, 8, 1008. [Google Scholar] [CrossRef]
- Ivanović, M.; Razboršek, M.I.; Kolar, M. Innovative extraction techniques using deep eutectic solvents and analytical methods for the isolation and characterization of natural bioactive compounds from plant material. Plants 2020, 9, 1428. [Google Scholar] [CrossRef]
- Lafka, T.I.; Lazou, A.E.; Sinanoglou, V.J.; Lazos, E.S. Phenolic extracts from wild olive leaves and their potential as edible oils antioxidants. Foods 2013, 2, 18–31. [Google Scholar] [CrossRef]
- Sahin, S.; Samli, R.; Birteks, Z.; Tan, A.S.; Barba, F.J.; Chemat, F.; Cravotto, G.; Lorenzo, J.M. Solvent-free microwave-assisted extraction of polyphenols from olive tree leaves: Antioxidant and antimicrobial properties. Molecules 2017, 22, 1056. [Google Scholar] [CrossRef]
- Boli, E.; Prinos, N.; Louli, V.; Pappa, G.; Stamatis, H.; Magoulas, K.; Voutsas, E. Recovery of Bioactive Extracts from Olive Leaves Using Conventional and Microwave-Assisted Extraction with Classical and Deep Eutectic Solvents. Separations 2022, 9, 255. [Google Scholar] [CrossRef]
- Niknam, S.M.; Kashaninejad, M.; Escudero, I.; Sanz, M.T.; Beltrán, S.; Benito, J.M. Valorization of olive mill solid residue through ultrasound-assisted extraction and phenolics recovery by adsorption process. J. Clean. Prod. 2021, 316, 128340. [Google Scholar] [CrossRef]
- Chanioti, S.; Katsouli, M.; Tzia, C. Novel processes for the extraction of phenolic compounds from olive pomace and their protection by encapsulation. Molecules 2021, 26, 1781. [Google Scholar] [CrossRef] [PubMed]
- Difonzo, G.; Aresta, A.; Cotugno, P.; Ragni, R.; Squeo, G.; Summo, C.; Massari, F.; Pasqualone, A.; Faccia, M.; Zambonin, C.; et al. Supercritical CO2 Extraction of Phytocompounds from Olive Pomace Subjected to Different Drying Methods. Molecules 2021, 26, 598. [Google Scholar] [CrossRef]
- Lecci, R.M.; D’antuono, I.; Cardinali, A.; Garbetta, A.; Linsalata, V.; Logrieco, A.F.; Leone, A. Antioxidant and pro-oxidant capacities as mechanisms of photoprotection of olive polyphenols on uva-damaged human keratinocytes. Molecules 2021, 26, 2153. [Google Scholar] [CrossRef]
- Pourzand, C.; Albieri-Borges, A.; Raczek, N.N. Shedding a New Light on Skin Aging, Iron-and Redox-Homeostasis and Emerging Natural Antioxidants. Antioxidants 2022, 11, 471. [Google Scholar] [CrossRef]
- Viola, P.; Viola, M. Virgin olive oil as a fundamental nutritional component and skin protector. Clin. Dermatol. 2009, 27, 159–165. [Google Scholar] [CrossRef]
- Nunes, M.A.; Costa, A.S.G.; Bessada, S.; Santos, J.; Puga, H.; Alves, R.C.; Freitas, V.; Oliveira, M.B.P.P. Olive pomace as a valuable source of bioactive compounds: A study regarding its lipid- and water-soluble components. Sci. Total Environ. 2018, 644, 229–236. [Google Scholar] [CrossRef]
- Wanitphakdeedecha, R.; Ng, J.N.C.; Junsuwan, N.; Phaitoonwattanakij, S.; Phothong, W.; Eimpunth, S.; Manuskiatti, W. Efficacy of olive leaf extract–containing cream for facial rejuvenation: A pilot study. J. Cosmet. Dermatol. 2020, 19, 1662–1666. [Google Scholar] [CrossRef]
- Nunes, A.; Gonçalves, L.; Marto, J.; Martins, A.M.; Silva, A.N.; Pinto, P.; Martins, M.; Fraga, C.; Ribeiro, H.M. Investigations of olive oil industry by-products extracts with potential skin benefits in topical formulations. Pharmaceutics 2021, 13, 465. [Google Scholar] [CrossRef]
- Al-Rimawi, F.; Sbeih, M.; Amayreh, M.; Rahhal, B.; Mudalal, S. Evaluation of the effectiveness of natural extract as a substituent for synthetic preservatives and antioxidants in pharmaceutical preparations. Saudi Pharm. J. 2024, 32, 102014. [Google Scholar] [CrossRef]
- Ramírez, E.; Brenes, M.; Garcia, P. Oleuropein Hydrolysis in Natural Green Olives: Importance of the Endogenous Enzymes. Food Chem. 2016, 206, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.F.R.; Resende, D.; Monteiro, M.; Coimbra, M.A.; Silva, A.M.S.; Cardoso, S.M. Application of hydroxytyrosol in the functional foods field: From ingredient to dietary supplements. Antioxidants 2020, 9, 1246. [Google Scholar] [CrossRef]
- Albini, A.; Corradino, P.; Morelli, D.; Albini, F.; Noonan, D. Cosmeceutical and Dermatological Potential of Olive Mill Wastewater: A Sustainable and Eco-Friendly Source of Natural Ingredients. Cosmetics 2025, 12, 142. [Google Scholar] [CrossRef]
- Jeon, S.; Choi, M. Anti-inflammatory and anti-aging effects of hydroxytyrosol on human dermal fibroblasts (HDFs). Biomed. Dermatol. 2018, 2, 21. [Google Scholar] [CrossRef]
- Smeriglio, A.; Denaro, M.; Mastracci, L.; Grillo, F.; Cornara, L.; Shirooie, S.; Nabavi, S.M.; Trombetta, D. Safety and efficacy of hydroxytyrosol-based formulation on skin inflammation: In vitro evaluation on reconstructed human epidermis model. DARU J. Pharm. Sci. 2019, 27, 283–293. [Google Scholar] [CrossRef]
- Nunes, M.A.; Palmeira, J.D.; Melo, D.; Machado, S.; Lobo, J.C.; Costa, A.S.G.; Alves, R.C.; Ferreira, H.; Oliveira, M.B.P.P. Chemical composition and antimicrobial activity of a new olive pomace functional ingredient. Pharmaceuticals 2021, 14, 913. [Google Scholar] [CrossRef]
- Guo, L.; Gong, S.; Wang, Y.; Sun, Q.; Duo, K.; Fei, P. Antibacterial Activity of Olive Oil Polyphenol Extract Against Salmonella Typhimurium and Staphylococcus aureus: Possible Mechanisms. Foodborne Pathog. Dis. 2020, 17, 396–403. [Google Scholar] [CrossRef]
- Bernini, R.; Carastro, I.; Santoni, F.; Clemente, M. Synthesis of lipophilic esters of tyrosol, homovanillyl alcohol and hydroxytyrosol. Antioxidants 2019, 8, 174. [Google Scholar] [CrossRef]
- González, A.A.; Ramos, T.J.; Illescas, M.R.; Costela, R.V.J.; Ruiz, C.; Melguizo, R.L.; García-Martínez, O. The Benefits of Olive Oil for Skin Health: Study on the Effect of Hydroxytyrosol, Tyrosol, and Oleocanthal on Human Fibroblasts. Nutrients 2023, 15, 2077. [Google Scholar] [CrossRef]
- Kováčik, A.; Kopečná, M.; Hrdinová, I.; Opálka, L.; Boncheva, B.M.; Vávrová, K. Time-Dependent Differences in the Effects of Oleic Acid and Oleyl Alcohol on the Human Skin Barrier. Mol. Pharm. 2023, 20, 6237–6245. [Google Scholar] [CrossRef]
- Michalak, M. Plant-Derived Antioxidants: Significance in Skin Health and the Ageing Process. Int. J. Mol. Sci. 2022, 23, 585. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.L.S.; Gondim, S.; Gómez-García, R.; Ribeiro, T.; Pintado, M. Olive leaf phenolic extract from two Portuguese cultivars—Bioactivities for potential food and cosmetic application. J. Environ. Chem. Eng. 2021, 9, 106175. [Google Scholar] [CrossRef]
- Aggoun, M.; Arhab, R.; Cornu, A.; Portelli, J.; Barkat, M.; Graulet, B. Olive mill wastewater microconstituents composition according to olive variety and extraction process. Food Chem. 2016, 209, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Hernández, M.L.; Muñoz-Ocaña, C.; Posada, P.; Sicardo, M.D.; Hornero-Méndez, D.; Gómez-Coca, R.B.; Belaj, A.; Moreda, W.; Martínes-Rivas, J.M. Functional Characterization of Four Olive Squalene Synthases with Respect to the Squalene Content of the Virgin Olive Oil. J. Agric. Food Chem. 2023, 71, 15701–15712. [Google Scholar] [CrossRef]
- Oliveira, A.L.S.; Valente, D.; Moreira, H.R.; Pintado, M.; Costa, P. Effect of squalane-based emulsion on polyphenols skin penetration: Ex vivo skin study. Colloids Surf. B Biointerfaces 2022, 218, 112779. [Google Scholar] [CrossRef]
- Olive Tree People. Available online: https://olivetreepeople.com/en/collections/oliveda (accessed on 26 August 2025).
- Safety Assessment of Olea europaea (Olive)-Derived Ingredients as Used in Cosmetics. 2023. Available online: https://www.cir-safety.org/sites/default/files/Olive.pdf (accessed on 26 August 2025).
- SCCS. Notes of Guidance for the Testing of Cosmetic Ingredients and Their Safety Evaluation, 12th Revision, 22 December 2023. Available online: https://health.ec.europa.eu/publications/sccs-notes-guidance-testing-cosmetic-ingredients-and-their-safety-evaluation-12th-revision_en (accessed on 29 April 2025).
- De Leo, V.; Maurelli, A.M.; Giotta, L.; Daniello, V.; Di Gioia, S.; Conese, M.; Ingrosso, C.; Ciriaco, F.; Catucci, L. Polymer Encapsulated Liposomes for Oral Co-Delivery of Curcumin and Hydroxytyrosol. Int. J. Mol. Sci. 2023, 24, 790. [Google Scholar] [CrossRef]
- Katsouli, M.; Thanou, I.V.; Raftopoulou, E.; Ntzimani, A.; Taoukis, P.; Giannakourou, M.C. Bioaccessibility and Stability Studies on Encapsulated Phenolics and Carotenoids from Olive and Tomato Pomace: Development of a Functional Fruit Beverage. Appl. Sci. 2024, 14, 10495. [Google Scholar] [CrossRef]
- Nanonourish—Hair Care Ingredient for Nourishing Scalp and Hair Structure Based on Bioactives from Food Byproducts. Available online: https://upin.up.pt/pt-pt/tecnologias/nanonourish-hair-care-ingredient-nourishing-scalp-and-hair-structure-based-bioactives (accessed on 30 August 2025).


| Bioactive Compounds | Properties | Cosmetic | References |
|---|---|---|---|
| Formulations | |||
| Chlorogenic acid | Photoprotective; Antioxidant; Anti-wrinkle; Anti-aging; Anti-inflammatory; Antimicrobial | Anti-aging products; Products with therapeutic effects; Suncreams | [64,66,77] |
| Caffeine | Antioxidant; Anti-aging; Photoprotective; Lipolytic activity; Microcirculation enhancer | Products for cellulite treatment, hair growth, oxidative damage, photoprotection and skin protection and vitality | [26,71,72,73,74,82] |
| Cafestol; Kahweol; Acylglycerols; β-sitosterol | Antioxidant; Moisturizer; Regenerator; Photoprotective | Skin hydration and firmness creams; Products for skin regeneration; | [78,79,80] |
| Bioactive Compounds | Properties | Cosmetic Formulations | References |
|---|---|---|---|
| Hydroxytyrosol | Whitening, lightning and depigmenting Antioxidant Anti-aging Photoprotective Anti-inflammatory Antimicrobial | Formulations for reducing skin pigmentation; Creams to enhance the epidermal barrier, inflammatory responses and for skin repair; | [95,126,127,131,134,136] |
| Tyrosol | Antioxidant Anti-aging Photoprotective Anti-inflammatory Surfactant | Emulsions, creams and oil-based formulations. | [126,140] |
| Oleic acid Vitamin E Squalene | Antioxidant Emolient Moisturizer Anti-inflammatory | Formulations with healing and lubricant activity; Formulations to prevent oxidative stress; Dermo-protective creams | [27,125,128,129,142,147] |
| Carotenoids | Antioxidant Photoprotective Anti-inflammatory | Formulations to prevent UVA induced dermal damage and reduce oxidative stress. | [145] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, A.M.; Alves, R.C.; Bastos, B.; Oliveira, M.B.P.P.; Casas, A.; Almeida, H. The Potential of Coffee and Olive by Products as Ingredient in Cosmetics Formulations and Their Extraction Techniques. Cosmetics 2025, 12, 206. https://doi.org/10.3390/cosmetics12050206
Ferreira AM, Alves RC, Bastos B, Oliveira MBPP, Casas A, Almeida H. The Potential of Coffee and Olive by Products as Ingredient in Cosmetics Formulations and Their Extraction Techniques. Cosmetics. 2025; 12(5):206. https://doi.org/10.3390/cosmetics12050206
Chicago/Turabian StyleFerreira, Ana Matilde, Rita C. Alves, Bernardo Bastos, Maria Beatriz P. P. Oliveira, Ana Casas, and Hugo Almeida. 2025. "The Potential of Coffee and Olive by Products as Ingredient in Cosmetics Formulations and Their Extraction Techniques" Cosmetics 12, no. 5: 206. https://doi.org/10.3390/cosmetics12050206
APA StyleFerreira, A. M., Alves, R. C., Bastos, B., Oliveira, M. B. P. P., Casas, A., & Almeida, H. (2025). The Potential of Coffee and Olive by Products as Ingredient in Cosmetics Formulations and Their Extraction Techniques. Cosmetics, 12(5), 206. https://doi.org/10.3390/cosmetics12050206









