Bioengineered Skin Microbiome: The Next Frontier in Personalized Cosmetics
Abstract
1. Introduction
2. The Current Understanding of Skin Microbiome in Cosmetics
2.1. Role of Natural Skin Bacteria in Beauty and Skin Health
2.2. Commercial Probiotics and Prebiotics in Skincare
3. Bioengineering and Synthetic Biology for Skincare
3.1. Advances in Genetically Modified Bacteria for Dermatology
3.2. Engineering Skin-Friendly Microbes for Cosmetic Applications
3.3. Potential of CRISPR and Synthetic Biology in Beauty Industry
4. Next-Generation Personalized Microbiome-Based Cosmetics
4.1. Customizing Skincare Through Microbiome Sequencing
4.2. AI-Driven Microbiome Analysis for Tailor-Made Cosmetics
5. Regulatory and Ethical Considerations
5.1. Safety of Engineered Microbes in Cosmetics
5.2. Challenges in Global Regulations and Public Acceptance
6. Sustainability Aspects
6.1. Environmental Impact of Traditional vs. Bioengineered Cosmetics
6.2. Sustainable Production of Engineered Microbes
6.3. Packaging and Product Lifecycle
6.4. Social and Economic Sustainability
6.5. Metrics and Certification Pathways
7. Prospective Advances in Cosmetic Bioengineering
Strengths and Limitations
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Swaney, M.H.; Kalan, L.R. Living in Your Skin: Microbes, Molecules, and Mechanisms. Infect. Immun. 2021, 89, e00695-20. [Google Scholar] [CrossRef] [PubMed]
- Kannenberg, S.M.H. The Microbiome’s Impact on Skin Health and Disease. Curr. Allergy Clin. Immunol. 2024, 37, 63–69. [Google Scholar]
- Haykal, D.; Cartier, H.; Dréno, B. Dermatological Health in the Light of Skin Microbiome Evolution. J. Cosmet. Dermatol. 2024, 23, 3836–3846. [Google Scholar] [CrossRef]
- Barnard, E. Shaping of Cutaneous Function by Encounters with Commensals. J. Physiol. 2017, 595, 437–450. [Google Scholar] [CrossRef]
- Gościńska, A.; Będzichowska, A.; Lipińska-Opałka, A. Atopic dermatitis and the human skin microbiota. Pediatr. Med. Rodz. 2023, 19, 78–82. [Google Scholar] [CrossRef]
- Biskanaki, F.; Beloukas, A.; Letsiou, S.; Chaniotis, D.; Kefala, V. The Use of Probiotics and Prebiotics in the Restoration of Aesthetic Problems. What is a proteome? Ep. Klin. Farmakol. Kai Farmakokinet. 2024, 42, 5–8. [Google Scholar] [CrossRef]
- Arora, R.; Kaur, R.; Babbar, R.; Dhingra, S.; Dhingra, A.K.; Grewal, A.S. Evolving Advances in the Cosmetic Use of Probiotics and Postbiotics: Health, Regulatory and Marketing Aspects. Curr. Pharm. Biotechnol. 2024, 25, 1349–1361. [Google Scholar] [CrossRef] [PubMed]
- Broadhead, R.; Craeye, L.; Callewaert, C. The Future of Functional Clothing for an Improved Skin and Textile Microbiome Relationship. Microorganisms 2021, 9, 1192. [Google Scholar] [CrossRef]
- Herrmann, A.; Bänziger, S.; Walzel, B. Strategies for the Skin Microbiome. COSSMA 2021, 22, 10–13. [Google Scholar]
- Benner, S.A.; Sismour, A.M. Synthetic Biology. Nat. Rev. Genet. 2005, 6, 533–543. [Google Scholar] [CrossRef]
- Chávez, M.N.; Schenck, T.L.; Hopfner, U.; Centeno-Cerdas, C.; Somlai-Schweiger, I.; Schwarz, C.; Machens, H.-G.; Heikenwalder, M.; Bono, M.R.; Allende, M.L.; et al. Towards Autotrophic Tissue Engineering: Photosynthetic Gene Therapy for Regeneration. Biomaterials 2016, 75, 25–36. [Google Scholar] [CrossRef]
- Colliec-Jouault, S. Skin Tissue Engineering Using Functional Marine Biomaterials. In Functional Marine Biomaterials: Properties and Applications; Woodhead Publishing: Cambridge, UK, 2015; pp. 69–90. [Google Scholar]
- Supp, D.M.; Neely, A.N. Cutaneous Antimicrobial Gene Therapy: Engineering Human Skin Replacements to Combat Wound Infection. Expert Rev. Dermatol. 2008, 3, 73–84. [Google Scholar] [CrossRef]
- Bui, N.-L.; Nguyen, M.A.; Nguyen, M.-L.; Bui, Q.-C.; Chu, D.-T. Phage for Regenerative Medicine and Cosmetics. Progress Mol. Biol. Transl. Sci. 2023, 201, 241–259. [Google Scholar] [CrossRef]
- Guerrero-Aspizua, S.; Conti, C.J.; Zapatero-Solana, E.; Larcher, F.; Del Río, M. Current Applications for Bioengineered Skin. In Translating Regenerative Medicine to the Clinic; Academic Press: Cambridge, MO, USA, 2016; pp. 107–120. [Google Scholar]
- Urciuolo, F.; Casale, C.; Imparato, G.; Netti, P.A. Bioengineered Skin Substitutes: The Role of Extracellular Matrix and Vascularization in the Healing of Deep Wounds. J. Clin. Med. 2019, 8, 2083. [Google Scholar] [CrossRef]
- Gautam, P.; Chaurasia, A.; Bhattacharya, A.; Grover, R.; Consortium, I.G.V.; Mukerji, M.; Natarajan, V.T. Population Diversity and Adaptive Evolution in Keratinization Genes: Impact of Environment in Shaping Skin Phenotypes. Mol. Biol. Evol. 2015, 32, 555–573. [Google Scholar] [CrossRef]
- Amano, S.; Yoshikawa, T.; Ito, C.; Mabuchi, I.; Kikuchi, K.; Ooguri, M.; Yasuda, C. Prediction and Association Analyses of Skin Phenotypes in Japanese Females Using Genetic, Environmental, and Physical Features. Skin Res. Technol. 2023, 29, e13231. [Google Scholar] [CrossRef]
- Tokarz-Deptuła, B.; Śliwa-Dominiak, J.; Adamiak, M.; Bak, K.; Deptuła, W. Commensal Bacteria and Immunity of the Gastrointestinal, Respiratory and Genitourinary Tracts. Postep. Hig. Med. Dosw. 2016, 70, 599–609. [Google Scholar] [CrossRef]
- Tlaskalová-Hogenová, H.; Štěpánková, R.; Hudcovic, T.; Tučková, L.; Cukrowska, B.; Lodinová-Žádníková, R.; Kozáková, H.; Rossmann, P.; Bártová, J.; Sokol, D.; et al. Commensal Bacteria (Normal Microflora), Mucosal Immunity and Chronic Inflammatory and Autoimmune Diseases. Immunol. Lett. 2004, 93, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Nyugen, U.T.; Kalan, L.R. Forgotten Fungi: The Importance of the Skin Mycobiome. Curr. Opin. Microbiol. 2022, 70, 102235. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Yao, Y.-F.; Li, J.-Y.; Li, Y. Human mycobiome and diseases. Hua Xi Kou Qiang Yi Xue Za Zhi 2019, 37, 314–319. [Google Scholar] [CrossRef]
- Ruchti, F.; LeibundGut-Landmann, S. New Insights into Immunity to Skin Fungi Shape Our Understanding of Health and Disease. Parasite Immunol. 2023, 45, e12948. [Google Scholar] [CrossRef] [PubMed]
- Chueachavalit, C.; Meephansan, J.; Payungporn, S.; Sawaswong, V.; Chanchaem, P.; Wongpiyabovorn, J.; Thio, H.B. Comparison of Malassezia Spp. Colonization between Human Skin Exposed to High- and Low-Ambient Air Pollution. Exp. Dermatol. 2022, 31, 1454–1461. [Google Scholar] [CrossRef]
- Graham, E.H.; Tom, W.A.; Neujahr, A.C.; Adamowicz, M.S.; Clarke, J.L.; Herr, J.R.; Fernando, S.C. The Persistence and Stabilization of Auxiliary Genes in the Human Skin Virome. Virol. J. 2023, 20, 49. [Google Scholar] [CrossRef] [PubMed]
- Hannigan, G.D.; Meisel, J.S.; Tyldsley, A.S.; Zheng, Q.; Hodkinson, B.P.; SanMiguel, A.J.; Minot, S.; Bushman, F.D.; Grice, E.A. The Human Skin Double-Stranded DNA Virome: Topographical and Temporal Diversity, Genetic Enrichment, and Dynamic Associations with the Host Microbiome. mBio 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.T.; Götz, F. How Bacterial Molecules Manipulate Our Immune System. BioSpektrum 2017, 23, 759–761. [Google Scholar] [CrossRef]
- Nemati, M.; Larussa, T.; Khorramdelazad, H.; Mahmoodi, M.; Jafarzadeh, A. Toll-like Receptor 2: An Important Immunomodulatory Molecule during Helicobacter Pylori Infection. Life Sci. 2017, 178, 17–29. [Google Scholar] [CrossRef]
- van Bergenhenegouwen, J.; Plantinga, T.S.; Joosten, L.A.B.; Netea, M.G.; Folkerts, G.; Kraneveld, A.D.; Garssen, J.; Vos, A.P. TLR2 & Co: A Critical Analysis of the Complex Interactions between TLR2 and Coreceptors. J. Leukoc. Biol. 2013, 94, 885–902. [Google Scholar] [CrossRef]
- Mitchell, J.A.; Sorrentino, R.; Cartwright, N.; Paul-Clark, M. Toll-like Receptor 2 as Therapeutic Target in Lung Diseaseate. In New Drugs and Targets for Asthma and COPD; Karger Medical and Scientific Publishers: Basel, Switzerland, 2010; ISBN 978-3-8055-9566-7. [Google Scholar]
- Farhat, K.; Riekenberg, S.; Heine, H.; Debarry, J.; Lang, R.; Mages, J.; Buwitt-Beckmann, U.; Röschmann, K.; Jung, G.; Wiesmüller, K.-H.; et al. Heterodimerization of TLR2 with TLR1 or TLR6 Expands the Ligand Spectrum but Does Not Lead to Differential Signaling. J. Leukoc. Biol. 2008, 83, 692–701. [Google Scholar] [CrossRef]
- Lopez, C.A.; Skaar, E.P. Crossed Wires: Interspecies Interference Blocks Pathogen Colonization. Cell Host Microbe 2017, 22, 721–723. [Google Scholar] [CrossRef][Green Version]
- Wanke, I.; Steffen, H.; Christ, C.; Krismer, B.; Götz, F.; Peschel, A.; Schaller, M.; Schittek, B. Skin Commensals Amplify the Innate Immune Response to Pathogens by Activation of Distinct Signaling Pathways. J. Investig. Dermatol. 2011, 131, 382–390. [Google Scholar] [CrossRef]
- Paller, A.S.; Kong, H.H.; Seed, P.; Naik, S.; Scharschmidt, T.C.; Gallo, R.L.; Luger, T.; Irvine, A.D. The Microbiome in Patients with Atopic Dermatitis. J. Allergy Clin. Immunol. 2019, 143, 26–35. [Google Scholar] [CrossRef]
- Zheng, Y.; Hunt, R.L.; Villaruz, A.E.; Fisher, E.L.; Liu, R.; Liu, Q.; Cheung, G.Y.C.; Li, M.; Otto, M. Commensal Staphylococcus epidermidis Contributes to Skin Barrier Homeostasis by Generating Protective Ceramides. Cell Host Microbe 2022, 30, 301–313.e9. [Google Scholar] [CrossRef] [PubMed]
- Scott, E.; Burkhart, C. Cutibacterium Acnes as a Cause of Post-Surgical Prosthesis Infection. Open Dermatol. J. 2023, 17, e187437222309130. [Google Scholar] [CrossRef]
- Coenye, T.; Spittaels, K.-J.; Achermann, Y. The Role of Biofilm Formation in the Pathogenesis and Antimicrobial Susceptibility of Cutibacterium acnes. Biofilm 2022, 4, 100063. [Google Scholar] [CrossRef]
- Lam, M.; Hu, A.; Fleming, P.; Lynde, C.W. The Impact of Acne Treatment on Skin Bacterial Microbiota: A Systematic Review. J. Cutan. Med. Surg. 2022, 26, 93–97. [Google Scholar] [CrossRef]
- Niedźwiedzka, A.; Micallef, M.P.; Biazzo, M.; Podrini, C. The Role of the Skin Microbiome in Acne: Challenges and Future Therapeutic Opportunities. Int. J. Mol. Sci. 2024, 25, 11422. [Google Scholar] [CrossRef]
- Guerra-Tapia, A.; Martínez, H.; Nieto, C.; Ruiz Alonso, C.; Bermejo, R.; Carrón, N.; Garcia-Segura, S.; Gonzalez-Torres, P.; Palacios-Martínez, D.; Bou, L.; et al. A New Topical Biotechnological Phytocomplex for Truncal Mild-Moderate Acne Restores Skin Microbiota Balance. Skin Res. Technol. 2024, 30, e13806. [Google Scholar] [CrossRef]
- Reztsova, P.A.; Raznatovskiy, K.I.; Vashkevich, A.A.; Kotrekhova, L.P.; Elishakova, L.I.; Bukiya, M.T. Phototherapy Efficacy Evaluation for the Patients’ Treatment with Microbial Eczema. Klin. Dermatol. I Venerol. 2020, 19, 655–660. [Google Scholar] [CrossRef]
- Lee, E.; Min, K.; Ahn, H.; Jeon, B.-N.; Park, S.; Yun, C.; Jeon, H.; Yeon, J.-S.; Kim, H.; Park, H. Potential Therapeutic Skin Microbiomes Suppressing Staphylococcus aureus-Derived Immune Responses and Upregulating Skin Barrier Function-Related Genes via the AhR Signaling Pathway. Int. J. Mol. Sci. 2022, 23, 9551. [Google Scholar] [CrossRef]
- Tlish, M.M.; Popandopulo, E.K. Evaluation of the Effectiveness of Ultratonotherapy in Patients with Microbial Eczema. Probl. Balneol. Physiother. Ther. Phys. Cult. 2022, 99, 48–53. [Google Scholar] [CrossRef]
- Zhang, H.; Li, L.; Wang, M.; Dong, Y.; Meng, H.; Yi, F. Role of Probiotics in the Treatment of Acne. Chin. J. Dermatol. 2023, 56, 283–285. [Google Scholar] [CrossRef]
- Hülpüsch, C.; Rohayem, R.; Reiger, M.; Traidl-Hoffmann, C. Exploring the Skin Microbiome in Atopic Dermatitis Pathogenesis and Disease Modification. J. Allergy Clin. Immunol. 2024, 154, 31–41. [Google Scholar] [CrossRef]
- Permatasari, F.; Zhou, B.; Luo, D. Epidermal Barrier: Adverse and Beneficial Changes Induced by Ultraviolet B Irradiation Depending on the Exposure Dose and Time (Review). Exp. Ther. Med. 2013, 6, 287–292. [Google Scholar] [CrossRef]
- Kim, H.-S.; Kim, H.-J.; Hong, Y.-D.; Son, E.D.; Cho, S.-Y. β-Endorphin Suppresses Ultraviolet B Irradiation-Induced Epidermal Barrier Damage by Regulating Inflammation-Dependent mTORC1 Signaling. Sci. Rep. 2023, 13, 22357. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, T. Ultraviolet radiation--immune response. J. Dtsch. Dermatol. Ges. 2005, 3 (Suppl. S2), S11–S18. [Google Scholar] [CrossRef]
- Khatoon, U.T.; Rao, G.V.S.N.; Mohan, M.K.; Romanaviciene, A.; Romanavicius, A. Comparative Study of Antifungal Activity of Silver and Gold Nanoparticles Synthesized by Facile Chemical Approach. J. Environ. Chem. Eng. 2018, 6, 5837–5844. [Google Scholar] [CrossRef]
- Das, R.; Nath, S.S.; Chakdar, D.; Gope, G.; Bhattacharjee, R. Synthesis of Silver Nanoparticles and Their Optical Properties. J. Exp. Nanosci. 2010, 5, 357–362. [Google Scholar] [CrossRef]
- Ifuku, S.; Tsukiyama, Y.; Yukawa, T.; Egusa, M.; Kaminaka, H.; Izawa, H.; Morimoto, M.; Saimoto, H. Facile Preparation of Silver Nanoparticles Immobilized on Chitin Nanofiber Surfaces to Endow Antifungal Activities. Carbohydr. Polym. 2015, 117, 813–817. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.-C.; Chen, Y.-J. Skin Microbiome in Acne Vulgaris, Skin Aging, and Rosacea: An Evidence-Based Review. Dermatol. Sin. 2022, 40, 129. [Google Scholar] [CrossRef]
- Greugny, E.T.; Stamatas, G.N.; Fages, F. Stability Versus Meta-Stability in a Skin Microbiome Model. In Proceedings of the Computational Methods in Systems Biology, Bucharest, Romania, 14–16 September 2022; Petre, I., Păun, A., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 179–197. [Google Scholar]
- Hülpüsch, C.; Reiger, M. Hautmikrobiom—Wertvoll für Diagnostik und Therapie? Hautarzt 2021, 72, 579–585. [Google Scholar] [CrossRef]
- Woo, T.E.; Sibley, C.D. The Emerging Utility of the Cutaneous Microbiome in the Treatment of Acne and Atopic Dermatitis. J. Am. Acad. Dermatol. 2020, 82, 222–228. [Google Scholar] [CrossRef]
- Boyajian, J.L.; Ghebretatios, M.; Schaly, S.; Islam, P.; Prakash, S. Microbiome and Human Aging: Probiotic and Prebiotic Potentials in Longevity, Skin Health and Cellular Senescence. Nutrients 2021, 13, 4550. [Google Scholar] [CrossRef]
- Woo, Y.R.; Kim, H.S. Interaction between the Microbiota and the Skin Barrier in Aging Skin: A Comprehensive Review. Front. Physiol. 2024, 15, 1322205. [Google Scholar] [CrossRef]
- Rawal, S.; Ali, S.A. Probiotics and Postbiotics Play a Role in Maintaining Dermal Health. Food Funct. 2023, 14, 3966–3981. [Google Scholar] [CrossRef] [PubMed]
- Chaiyasut, C.; Sivamaruthi, B.S.; Tansrisook, C.; Peerajan, S.; Chaiyasut, K.; Bharathi, M. Influence of Paraprobiotics-Containing Moisturizer on Skin Hydration and Microbiome: A Preliminary Study. Appl. Sci. 2022, 12, 12483. [Google Scholar] [CrossRef]
- Kianmehr, S.; Jahani, M.; Moazzen, N.; Ahanchian, H.; Khameneh, B. The Potential of Probiotics for Treating Skin Disorders: A Concise Review. Curr. Pharm. Biotechnol. 2022, 23, 1851–1863. [Google Scholar] [CrossRef] [PubMed]
- McLoughlin, I.J.; Voss, A.L.; Hale, J.D.F.; Jain, R. Cosmetic Efficacy of the Topical Probiotic Micrococcus luteus Q24 in Healthy Human Adults. Cosmetics 2024, 11, 122. [Google Scholar] [CrossRef]
- Alves, A.C.; Martins, S.M.D.S.B., Jr.; Belo, J.V.T.; Lemos, M.V.C.; Lima, C.E.d.M.C.; Silva, C.D.d.; Zagmignan, A.; Nascimento da Silva, L.C. Global Trends and Scientific Impact of Topical Probiotics in Dermatological Treatment and Skincare. Microorganisms 2024, 12, 2010. [Google Scholar] [CrossRef] [PubMed]
- Catic, T.; Pehlivanovic, B.; Pljakic, N.; Balicevac, A. The Moisturizing Efficacy of a Proprietary Dermo-Cosmetic Product (CLS02021) Versus Placebo in a 4-Week Application Period. Med. Arch. 2022, 76, 108–114. [Google Scholar] [CrossRef]
- Theodorou, I.M.; Kapoukranidou, D.; Theodorou, M.; Tsetis, J.K.; Menni, A.E.; Tzikos, G.; Bareka, S.; Shrewsbury, A.; Stavrou, G.; Kotzampassi, K. Cosmeceuticals: A Review of Clinical Studies Claiming to Contain Specific, Well-Characterized Strains of Probiotics or Postbiotics. Nutrients 2024, 16, 2526. [Google Scholar] [CrossRef]
- Majeed, M.; Nagabhushanam, K.; Paulose, S.; Rajalakshmi, H.; Mundkur, L. A Randomized Double-Blind, Placebo-Controlled Study to Evaluate the Anti-Skin-Aging Effect of LactoSporin—The Extracellular Metabolite from Bacillus coagulans (Weizmannia coagulans) MTCC 5856 in Healthy Female Volunteers. Clin. Cosmet. Investig. Dermatol. 2023, 16, 769–782. [Google Scholar] [CrossRef] [PubMed]
- Paramita, S.; Kusuma, I.W.; Sulistioadi, Y.B.; Kiswanto. Environmental and Safety Aspects of Bio-Based Cosmetics in Indonesia. In Biomass-Based Cosmetics: Research Trends and Future Outlook; Springer Nature: Berlin/Heidelberg, Germany, 2024; pp. 545–568. [Google Scholar]
- Handore, A.V.; Khandelwal, S.R.; Gupte, A.M.; Morankar, P.G.; Ghangale, S.S.; More, A.N.; Kale, S.V.; Handore, D.V. Revolutionizing Sustainable Cosmetic Formulations with Endophytic Bacterial Biosurfactants. In Microbial Surfactants in Pharmaceuticals and Cosmetics; CRC Press: Boca Raton, FL, USA, 2025; pp. 435–461. [Google Scholar]
- Baria, D.M.; Patel, N.Y.; Raval, S.Y.; Panchal, R.R.; Rajput, K.N.; Raval, V.H. Microbial Biosurfactants and Bioemulsifiers: Properties, Types and Applications in Cosmetic Industry. In Microbial Surfactants in Pharmaceuticals and Cosmetics; CRC Press: Boca Raton, FL, USA, 2025; pp. 144–172. [Google Scholar]
- Tanabe, K.; Moriguchi, C.; Fujiyama, N.; Shigematsu, Y.; Haraguchi, N.; Hirano, Y.; Dai, H.; Inaba, S.; Tokudome, Y.; Kitagaki, H. A Trial for the Construction of a Cosmetic Pattern Map Considering Their Effects on Skin Microbiota—Principal Component Analysis of the Effects on Short-Chain Fatty Acid Production by Skin Microbiota Staphylococcus epidermidis. Fermentation 2023, 9, 647. [Google Scholar] [CrossRef]
- Fiume, M.M.; Heldreth, B.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; et al. Safety Assessment of Microbial Polysaccharide Gums as Used in Cosmetics. Int. J. Toxicol. 2016, 35, 5S–49S. [Google Scholar] [CrossRef]
- Janssens-Böcker, C.; Doberenz, C.; Monteiro, M.; de Oliveira Ferreira, M. Influence of Cosmetic Skincare Products with pH < 5 on the Skin Microbiome: A Randomized Clinical Evaluation. Dermatol. Ther. 2025, 15, 141–159. [Google Scholar] [CrossRef]
- Field, D.; Hill, C.; Cotter, P.D.; Ross, R.P. The Dawning of a “Golden Era” in Lantibiotic Bioengineering. Mol. Microbiol. 2010, 78, 1077–1087. [Google Scholar] [CrossRef]
- Zhu, H.; Cheng, X.; Wu, H. Research Progress on Antimicrobial Peptides in Terms of the Prevention and Treatment of Dental Caries. J. Prev. Treat. Stomatol. Dis. 2023, 31, 434–439. [Google Scholar] [CrossRef]
- Zorko, M.; Jerala, R. Production of Recombinant Antimicrobial Peptides in Bacteria. Methods Mol. Biol. 2010, 618, 61–76. [Google Scholar] [CrossRef] [PubMed]
- McInturff, J.E.; Wang, S.-J.; Machleidt, T.; Lin, T.R.; Oren, A.; Hertz, C.J.; Krutzik, S.R.; Hart, S.; Zeh, K.; Anderson, D.H.; et al. Granulysin-Derived Peptides Demonstrate Antimicrobial and Anti-Inflammatory Effects against Propionibacterium acnes. J. Investig. Dermatol. 2005, 125, 256–263. [Google Scholar] [CrossRef]
- Wen, X.; Feng, J.; Liu, J.; Xu, J.; Chen, X.; Xie, X. Research Progress of Staphylococcus epidermidis Applied in Cosmetics. China Surfactant Deterg. Cosmet. 2025, 55, 89–97. [Google Scholar] [CrossRef]
- Lai, Y.; Cogen, A.L.; Radek, K.A.; Park, H.J.; MacLeod, D.T.; Leichtle, A.; Ryan, A.F.; Di Nardo, A.; Gallo, R.L. Activation of TLR2 by a Small Molecule Produced by Staphylococcus epidermidis Increases Antimicrobial Defense against Bacterial Skin Infections. J. Investig. Dermatol. 2010, 130, 2211–2221. [Google Scholar] [CrossRef]
- An Antitumor T-Cell Response Was Induced by Engineered Commensal Bacteria. Cancer Discov. 2023, 13, OF15. [CrossRef]
- Martínez, B.; Rodríguez, A.; Suárez, E. Antimicrobial Peptides Produced by Bacteria: The Bacteriocins. In New Weapons to Control Bacterial Growth; Springer International Publishing: Cham, Switzerland, 2016; pp. 15–38. [Google Scholar]
- Vogel, V.; Olari, L.-R.; Jachmann, M.; Reich, S.J.; Häring, M.; Kissmann, A.-K.; Rosenau, F.; Riedel, C.U.; Münch, J.; Spellerberg, B. The Bacteriocin Angicin Interferes with Bacterial Membrane Integrity through Interaction with the Mannose Phosphotransferase System. Front. Microbiol. 2022, 13, 991145. [Google Scholar] [CrossRef]
- Bartholomae, M.; Buivydas, A.; Viel, J.H.; Montalbán-López, M.; Kuipers, O.P. Major Gene-Regulatory Mechanisms Operating in Ribosomally Synthesized and Post-Translationally Modified Peptide (RiPP) Biosynthesis. Mol. Microbiol. 2017, 106, 186–206. [Google Scholar] [CrossRef]
- Quadri, L.E.N. Regulation of Class II Bacteriocin Production by Cell-Cell Signaling. J. Microbiol. 2003, 41, 175–182. [Google Scholar]
- Aswathanarayan, J.B.; Ravishankar Rai, V. Quorum-Sensing Systems in Pseudomonas. In Quorum Sensing vs Quorum Quenching: A Battle with no end in Sight; Springer: Berlin/Heidelberg, Germany, 2015; pp. 73–84. [Google Scholar]
- Smith, J.L.; Fratamico, P.M.; Novak, J.S. Quorum Sensing: A Primer for Food Microbiologists. J. Food Prot. 2004, 67, 1053–1070. [Google Scholar] [CrossRef]
- Zhang, X.; Li, P. Quorum Sensing in Class II Bacteriocin-Producing Lactic Acid Bacteria and Its Application—A Review. Wei Sheng Wu Xue Bao = Acta Microbiol. Sin. 2011, 51, 1152–1157. [Google Scholar]
- Fang, T.; Zheng, T.; Jiang, Q.; Li, X.; Tang, L. Synergistic Effect of Silk Peptides Thermophilus Fermentation on the Skin Anti-Inflammatory and Anti-Aging Activities. China Surfactant Deterg. Cosmet. 2023, 53, 1057–1064. [Google Scholar] [CrossRef]
- Kim, N.Y.; Yim, T.B.; Lee, H.Y. Skin Anti-Aging Activities of Bacteriochlorophyll a from Photosynthetic Bacteria, Rhodobacter Sphaeroides. J. Microbiol. Biotechnol. 2015, 25, 1589–1598. [Google Scholar] [CrossRef] [PubMed]
- Suryaningsih, B.E. Melanogenesis and Its Associated Signalings. Bali Med. J. 2020, 9, 327–331. [Google Scholar] [CrossRef]
- Lu, Y.; Tonissen, K.F.; Di Trapani, G. Modulating Skin Colour: Role of the Thioredoxin and Glutathione Systems in Regulating Melanogenesis. Biosci. Rep. 2021, 41, BSR20210427. [Google Scholar] [CrossRef]
- Passeron, T.; Ballotti, R.; Ortonne, J.-P. Melanogenesis. EMC Dermatol. Cosmetol. 2005, 2, 204–216. [Google Scholar] [CrossRef]
- Orhan, I.E.; Deniz, F.S.S. Inhibition of Melanogenesis by Some Well-Known Polyphenolics: A Review. Curr. Pharm. Biotechnol. 2021, 22, 1412–1423. [Google Scholar] [CrossRef]
- Hida, T.; Kamiya, T.; Kawakami, A.; Ogino, J.; Sohma, H.; Uhara, H.; Jimbow, K. Elucidation of Melanogenesis Cascade for Identifying Pathophysiology and Therapeutic Approach of Pigmentary Disorders and Melanoma. Int. J. Mol. Sci. 2020, 21, 6129. [Google Scholar] [CrossRef] [PubMed]
- Serre, C.; Busuttil, V.; Botto, J.-M. Intrinsic and Extrinsic Regulation of Human Skin Melanogenesis and Pigmentation. Int. J. Cosmet. Sci. 2018, 40, 328–347. [Google Scholar] [CrossRef]
- Kim, Y.-M.; Lim, H.-M.; Lee, E.-C.; Seo, Y.-K. Pigmentation Effect of Rice Bran Extract in Hair Follicle-Like Tissue and Organ Culture Models. Tissue Eng. Regen. Med. 2020, 17, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Jin, C.L.; Oh, J.H.; Oh, I.G.; Park, C.H.; Chung, J.H. Ardisia Crenata Extract Stimulates Melanogenesis in B16F10 Melanoma Cells through Inhibiting ERK1/2 and Akt Activation. Mol. Med. Rep. 2015, 11, 653–657. [Google Scholar] [CrossRef]
- Chandra, R.; Pons-Faudoa, F.P.; Parra Saldívar, R.; Rittmann, B.E. Effect of Ultra-Violet Exposure on Production of Mycosporine-like Amino Acids and Lipids by Lyngbya Purpurem. Biomass Bioenergy 2020, 134, 105475. [Google Scholar] [CrossRef]
- Tanner, K.; Martorell, P.; Genovés, S.; Ramón, D.; Zacarías, L.; Rodrigo, M.J.; Peretó, J.; Porcar, M. Bioprospecting the Solar Panel Microbiome: High-Throughput Screening for Antioxidant Bacteria in a Caenorhabditis elegans Model. Front. Microbiol. 2019, 10, 986. [Google Scholar] [CrossRef]
- Fuentes-Tristan, S.; Parra-Saldivar, R.; Iqbal, H.M.N.; Carrillo-Nieves, D. Bioinspired Biomolecules: Mycosporine-like Amino Acids and Scytonemin from Lyngbya Sp. with UV-Protection Potentialities. J. Photochem. Photobiol. B Biol. 2019, 201, 111684. [Google Scholar] [CrossRef]
- Jain, S.; Prajapat, G.; Ledwani, L.; Agrawat, A. Cyanobacterial Sunscreen: Past, Present and Future. In Sunscreens: Source, Formulations, Efficacy and Recommendations; Nova Science Publishers: New York, NY, USA, 2018; pp. 201–221. [Google Scholar]
- Fawcett, C.A.; Laamanen, C.A.; Senhorinho, G.N.; Scott, J.A. The Effect of Ultraviolet Radiation on Production of Antioxidant Compounds from Bioprospected Acid Tolerant Microalgae Used to Mitigate Industrial CO2. Ind. Biotechnol. 2025, 21, 111–118. [Google Scholar] [CrossRef]
- Budzianowska, A.; Banaś, K.; Budzianowski, J.; Kikowska, M. Antioxidants to Defend Healthy and Youthful Skin—Current Trends and Future Directions in Cosmetology. Appl. Sci. 2025, 15, 2571. [Google Scholar] [CrossRef]
- Wollert, D. Wet & Dry Lab Activities to Introduce Students to CRISPR-Based Gene Editing. Am. Biol. Teach. 2020, 82, 315–322. [Google Scholar] [CrossRef]
- Wollert, D. The Fascinating & Controversial New Science of CRISPR. Am. Biol. Teach. 2020, 82, 279–288. [Google Scholar] [CrossRef]
- Li, Y.; Li, J. CRISPR Bioanalytical Chemistry Technology. Progress Chem. 2020, 32, 5–13. [Google Scholar] [CrossRef]
- Biagioni, A.; Chillà, A.; Andreucci, E.; Laurenzana, A.; Margheri, F.; Peppicelli, S.; Del Rosso, M.; Fibbi, G. Type II CRISPR/Cas9 Approach in the Oncological Therapy. J. Exp. Clin. Cancer Res. 2017, 36, 80. [Google Scholar] [CrossRef] [PubMed]
- Contiliani, D.F.; Moraes, V.N.; Passos, G.A.; Pereira, T.C. What Is the CRISPR System and How It Is Used? Adv. Exp. Med. Biol. 2023, 1429, 1–11. [Google Scholar] [CrossRef]
- Ansori, A.N.M.; Antonius, Y.; Susilo, R.J.K.; Hayaza, S.; Kharisma, V.D.; Parikesit, A.A.; Zainul, R.; Jakhmola, V.; Saklani, T.; Rebezov, M.; et al. Application of CRISPR-Cas9 Genome Editing Technology in Various Fields: A Review. Narra J. 2023, 3, e184. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.; Ikram, A.R.; Azeem, F.; Tahir ul Qamar, M.; Shaheen, T. Mehboob-ur-Rahman Precision Genome Editing with CRISPR-Cas9. Methods Mol. Biol. 2024, 2788, 355–372. [Google Scholar] [CrossRef] [PubMed]
- Son, J.; Lee, H.J.; Woo, H.M. CRISPRi-Assisted Metabolic Engineering of Cyanobacteria for Photosynthetic Hyaluronic Acid from CO2. J. Biol. Eng. 2025, 19, 26. [Google Scholar] [CrossRef]
- Ma, Y.; Qiu, Y.; Yu, C.; Li, S.; Xu, H. Design and Construction of a Bacillus Amyloliquefaciens Cell Factory for Hyaluronic Acid Synthesis from Jerusalem Artichoke Inulin. Int. J. Biol. Macromol. 2022, 205, 410–418. [Google Scholar] [CrossRef]
- Chu, L.L. CRISPR-Cas System in Microbial Hosts for Terpenoid Production. Crit. Rev. Biotechnol. 2022, 42, 1116–1133. [Google Scholar] [CrossRef]
- Zhang, C.-D.; Qi, Y.-H.; Mi, Y.-X.; Zhang, Y.-Z.; Qin, H.-J.; Liu, D.; Li, X.-B.; Ren, L.-M. Application of CRISPR/Cas9 Technology in Industrial Microorganisms. Progress Biochem. Biophys. 2023, 50, 1629–1637. [Google Scholar] [CrossRef]
- Oulhen, N.; Chi-Yi, L.; Yi-Hsien, S.; Wessel, G. CRISPR/Cas9-Mediated Genome Editing in Sea Urchins. In Methods in Cell Biology; Academic Press: Cambridge, MO, USA, 2019; Volume 151, pp. 305–321. [Google Scholar]
- Chornyi, S.; Koster, J.; Waterham, H.R. Applying CRISPR-Cas9 Genome Editing to Study Genes Involved in Peroxisome Biogenesis or Peroxisomal Functions. In Peroxisomes: Methods and Protocols; Schrader, M., Ed.; Springer US: New York, NY, USA, 2023; pp. 233–245. ISBN 978-1-0716-3048-8. [Google Scholar]
- Chaudhari, P.; Ranjan, M. Advances in CRISPR-Based Technologies for Genome Editing in Microorganisms. IP Int. J. Med. Microbiol. Trop. Dis. 2024, 10, 11–16. [Google Scholar] [CrossRef]
- Kursheed, F.; Naz, E.; Mateen, S.; Kulsoom, U. CRISPR Applications in Microbial World: Assessing the Opportunities and Challenges. Gene 2025, 935, 149075. [Google Scholar] [CrossRef]
- Moon, T.S. Probiotic and Microbiota Engineering for Practical Applications. Curr. Opin. Food Sci. 2024, 56, 101130. [Google Scholar] [CrossRef]
- Johns, N.I.; Blazejewski, T.; Gomes, A.L.C.; Wang, H.H. Principles for Designing Synthetic Microbial Communities. Curr. Opin. Microbiol. 2016, 31, 146–153. [Google Scholar] [CrossRef]
- McCarty, N.S.; Ledesma-Amaro, R. Synthetic Biology Tools to Engineer Microbial Communities for Biotechnology. Trends Biotechnol. 2019, 37, 181–197. [Google Scholar] [CrossRef]
- Wan, X.; Saltepe, B.; Yu, L.; Wang, B. Programming Living Sensors for Environment, Health and Biomanufacturing. Microb. Biotechnol. 2021, 14, 2334–2342. [Google Scholar] [CrossRef] [PubMed]
- Brooks, S.M.; Alper, H.S. Applications, Challenges, and Needs for Employing Synthetic Biology beyond the Lab. Nat. Commun. 2021, 12, 1390. [Google Scholar] [CrossRef]
- Woo, H.M.; Park, J.-B. Recent Progress in Development of Synthetic Biology Platforms and Metabolic Engineering of Corynebacterium Glutamicum. J. Biotechnol. 2014, 180, 43–51. [Google Scholar] [CrossRef]
- Igielska-Kalwat, J.; Gościańska, J.; Witkowska, B.; Nowak, I. In Vivo Studies of Substances Used in the Cosmetic Industry. Postep. Dermatol. Alergol. 2016, 33, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Sajna, K.V.; Gottumukkala, L.D.; Sukumaran, R.K.; Pandey, A. White Biotechnology in Cosmetics. In Industrial Biorefineries and White Biotechnology; Elsevier: Amsterdam, The Netherlands, 2015; pp. 607–652. [Google Scholar]
- Reisch, M.S. Super Natural Cosmetic Potions: Advances in Biotechnology Enable a New Generation of Plant-Derived Ingredients. Chem. Eng. News 2011, 89, 21–22. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, L.; Hao, H.; Yan, M.; Zhu, Z. Applications of Engineered Skin Tissue for Cosmetic Component and Toxicology Detection. Cell Transplant. 2024, 33, 24. [Google Scholar] [CrossRef] [PubMed]
- Michalak, I.; Dmytryk, A.; Chojnacka, K. Algae Cosmetics. In Encyclopedia of Marine Biotechnology; John Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 65–85. [Google Scholar]
- Tang, P.; Si, Y.; Song, X.; Sun, G. Hierarchically Porous Bacterial Cellulose Nanofibrous Membranes for Selective Adsorption and Real-Time Colorimetric Monitoring of Volatile Carboxylic Acids. Cellulose 2024, 31, 381–393. [Google Scholar] [CrossRef]
- Dal’Belo, S.E.; Rigo Gaspar, L.; Maia Campos, P.M.B.G. Moisturizing Effect of Cosmetic Formulations Containing Aloe Vera Extract in Different Concentrations Assessed by Skin Bioengineering Techniques. Skin Res. Technol. 2006, 12, 241–246. [Google Scholar] [CrossRef]
- Satam, H.; Joshi, K.; Mangrolia, U.; Waghoo, S.; Zaidi, G.; Rawool, S.; Thakare, R.P.; Banday, S.; Mishra, A.K.; Das, G.; et al. Next-Generation Sequencing Technology: Current Trends and Advancements. Biology 2023, 12, 997. [Google Scholar] [CrossRef]
- Malla, M.A.; Dubey, A.; Kumar, A.; Yadav, S.; Hashem, A.; Allah, E.F.A. Exploring the Human Microbiome: The Potential Future Role of next-Generation Sequencing in Disease Diagnosis and Treatment. Front. Immunol. 2019, 10, 2868. [Google Scholar] [CrossRef]
- Oulas, A.; Pavloudi, C.; Polymenakou, P.; Pavlopoulos, G.A.; Papanikolaou, N.; Kotoulas, G.; Arvanitidis, C.; Iliopoulos, I. Metagenomics: Tools and Insights for Analyzing next-Generation Sequencing Data Derived from Biodiversity Studies. Bioinform. Biol. Insights 2015, 9, 75–88. [Google Scholar] [CrossRef]
- Carlos, N.; Tang, Y.-W.; Pei, Z. Pearls and Pitfalls of Genomics-Based Microbiome Analysis. Emerg. Microbes Infect. 2012, 1, 1–3. [Google Scholar] [CrossRef]
- Jovel, J.; Patterson, J.; Wang, W.; Hotte, N.; O’Keefe, S.; Mitchel, T.; Perry, T.; Kao, D.; Mason, A.L.; Madsen, K.L.; et al. Characterization of the Gut Microbiome Using 16S or Shotgun Metagenomics. Front. Microbiol. 2016, 7, 459. [Google Scholar] [CrossRef]
- Wensel, C.R.; Pluznick, J.L.; Salzberg, S.L.; Sears, C.L. Next-Generation Sequencing: Insights to Advance Clinical Investigations of the Microbiome. J. Clin. Investig. 2022, 132, e154944. [Google Scholar] [CrossRef]
- Jung, S. Advances in Functional Analysis of the Microbiome: Integrating Metabolic Modeling, Metabolite Prediction, and Pathway Inference with Next-Generation Sequencing Data. J. Microbiol. 2025, 63, e.2411006. [Google Scholar] [CrossRef]
- Gelbach, P.E.; Cetin, H.; Finley, S.D. Flux Sampling in Genome-Scale Metabolic Modeling of Microbial Communities. BMC Bioinform. 2024, 25, 45. [Google Scholar] [CrossRef]
- Giannari, D.; Ho, C.H.; Mahadevan, R. A Gap-Filling Algorithm for Prediction of Metabolic Interactions in Microbial Communities. PLoS Comput. Biol. 2021, 17, e1009060. [Google Scholar] [CrossRef]
- Pylro, V.S.; Morais, D.K.; de Oliveira, F.S.; dos Santos, F.G.; Lemos, L.N.; Oliveira, G.; Roesch, L.F.W. BMPOS: A Flexible and User-Friendly Tool Sets for Microbiome Studies. Microb. Ecol. 2016, 72, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Koh, Y.-S. The Role of Skin Microbiome in Human Health and Diseases. J. Bacteriol. Virol. 2024, 54, 191–202. [Google Scholar] [CrossRef]
- Rahimi Ardali, D.; Rüether, L.; Popov, V.; Schlippe, G.; Vuksanovic, B. Human Skin Profiling by Physical Skin Biomarkers: A Machine Learning Approach. Adv. Intell. Syst. Comput. 2019, 797, 151–160. [Google Scholar] [CrossRef]
- Locker, J.; Serrage, H.J.; Ledder, R.G.; Deshmukh, S.; O’Neill, C.A.; McBain, A.J. Microbiological Insights and Dermatological Applications of Live Biotherapeutic Products. J. Appl. Microbiol. 2024, 135, lxae181. [Google Scholar] [CrossRef]
- Khmaladze, I.; Butler, É.; Fabre, S.; Gillbro, J.M. Lactobacillus reuteri DSM 17938—A Comparative Study on the Effect of Probiotics and Lysates on Human Skin. Exp. Dermatol. 2019, 28, 822–828. [Google Scholar] [CrossRef] [PubMed]
- Hwang, B.K.; Lee, S.; Myoung, J.; Hwang, S.J.; Lim, J.M.; Jeong, E.T.; Park, S.G.; Youn, S.H. Effect of the Skincare Product on Facial Skin Microbial Structure and Biophysical Parameters: A Pilot Study. MicrobiologyOpen 2021, 10, e1236. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, M.; Sekar, P.; Pasupathi, M.; Mukhopadhyay, T. Self-Preserving Personal Care Products. Int. J. Cosmet. Sci. 2017, 39, 301–309. [Google Scholar] [CrossRef]
- Morris, C.; Leech, R. Natural and Physical Preservative Systems. In Microbial Quality Assurance in Pharmaceuticals, Cosmetics, and Toiletries; CRC Press: Boca Raton, FL, USA, 1996; ISBN 978-0-203-97845-0. [Google Scholar]
- Baikuni, A.; Hawari, F.L.; Sutriyo; Ramadon, D.; Malik, A. Untargeted LC-QTOF-MS/MS Based Metabolomic Profile Approach of Bacterial Ferment Lysates and Skin Commensal Bacterial Cocktail Ferment Lysates. HAYATI J. Biosci. 2023, 30, 576–587. [Google Scholar] [CrossRef]
- Shishkov, V.V.; Bikmulina, P.Y.; Kardosh, A.V.; Tsibulnikov, S.V.; Grekova, E.V.; Kolesova, Y.V.; Zakharova, P.A.; Nesterova, A.M.; Alencar de Sena Pereira, F.D.; Kotova, S.L.; et al. Advancing the in Vitro Drug Screening Models: Microbiome as a Component of Tissue-Engineered Skin. Bioprinting 2025, 45, e00379. [Google Scholar] [CrossRef]
- Fábrega, M.-J.; Knödlseder, N.; Nevot, G.; Sanvicente, M.; Toloza, L.; Santos-Moreno, J.; Güell, M. Establishing a Cell-Free Transcription-Translation Platform for Cutibacterium acnes to Prototype Engineered Metabolic and Synthetic Biology. ACS Biomater. Sci. Eng. 2023, 9, 5101–5110. [Google Scholar] [CrossRef]
- Perumal, I.; Kalaivani, P.; Naveenkumar, A.; Saran, V.; Sriram, K.; Suruthi, N. AI-Driven Personalized Skincare Recommendations. In Proceedings of the International Conference on Electronics and Renewable Systems (ICEARS), Tuticorin, India, 11–13 February 2025; pp. 1086–1091. [Google Scholar]
- Baldisserotto, A.; Baldini, E.; Ravarotto, S.; Cesa, E.; De Lucia, D.; Durini, E.; Vertuani, S.; Manfredini, S.; Michniak-Kohn, B.B. Expert Systems for Predicting the Bioavailability of Sun Filters in Cosmetic Products, Software vs. Expert Formulator: The Benzophenone-3 Case. Pharmaceutics 2022, 14, 1815. [Google Scholar] [CrossRef]
- Hamaguchi, M.; Miwake, H.; Nakatake, R.; Arai, N. Predicting the Performance of Functional Materials Composed of Polymeric Multicomponent Systems Using Artificial Intelligence—Formulations of Cleansing Foams as an Example. Polymers 2023, 15, 4216. [Google Scholar] [CrossRef]
- Cheng, M.; Zhou, H.; Zhang, H.; Zhang, X.; Zhang, S.; Bai, H.; Zha, Y.; Luo, D.; Chen, D.; Chen, S.; et al. Hidden Links Between Skin Microbiome and Skin Imaging Phenome. Genom. Proteom. Bioinform. 2024, 22, qzae040. [Google Scholar] [CrossRef] [PubMed]
- Johnson, H.R.; Trinidad, D.D.; Guzman, S.; Khan, Z.; Parziale, J.V.; DeBruyn, J.M.; Lents, N.H. A Machine Learning Approach for Using the Postmortem Skin Microbiome to Estimate the Postmortem Interval. PLoS ONE 2016, 11, e0167370. [Google Scholar] [CrossRef]
- Davis, T.; Decker, K.T.; Hosseini, D.; Jameson, G.; Borazanci, E. Skin Microbiome Differences in Pancreatic Adenocarcinoma, Other Cancers, and Healthy Controls: A Pilot Study. Front. Oncol. 2025, 15, 1495500. [Google Scholar] [CrossRef]
- Sherier, A.J.; Woerner, A.E.; Budowle, B. Determining Informative Microbial Single Nucleotide Polymorphisms for Human Identification. Appl. Environ. Microbiol. 2022, 88, e0005222. [Google Scholar] [CrossRef]
- Li, G.; Huang, W.; Sun, H.; Li, Y. Applications of Machine Learning in Predicting Host Phenotype Based on Microbiome. Acta Microbiol. Sin. 2021, 61, 2581–2593. [Google Scholar] [CrossRef]
- Guru Prasad, M.S.; Devadas, R.M.; Hiremani, V.; Jahan, A.; Praveen Gujjar, J.; Dahiya, A. Metagenomic Face: Applying Machine Learning to Facial Microbiome Data for Pattern Recognition and Disease Classification. In Proceedings of the 4th International Conference on Intelligent Technologies (CONIT), Bangalore, India, 21–23 June 2024. [Google Scholar]
- Namkung, J. Machine Learning Methods for Microbiome Studies. J. Microbiol. 2020, 58, 206–216. [Google Scholar] [CrossRef]
- Chen, H.; Tao, Y.; Mao, Z.; Xing, P. A Review of Machine Learning Algorithms for Environmental Microbiology. Acta Microbiol. Sin. 2022, 62, 4646–4662. [Google Scholar] [CrossRef]
- Wu, S.; Chen, Y.; Li, Z.; Li, J.; Zhao, F.; Su, X. Towards Multi-Label Classification: Next Step of Machine Learning for Microbiome Research. Comput. Struct. Biotechnol. J. 2021, 19, 2742–2749. [Google Scholar] [CrossRef]
- Waldor, M.K.; Tyson, G.; Borenstein, E.; Ochman, H.; Moeller, A.; Finlay, B.B.; Kong, H.H.; Gordon, J.I.; Nelson, K.E.; Dabbagh, K.; et al. Where Next for Microbiome Research? PLoS Biol. 2015, 13, e1002050. [Google Scholar] [CrossRef]
- Slezak, T.; Hart, B.; Jaing, C. Design of Genomic Signatures for Pathogen Identification and Characterization. In Microbial Forensics; Academic Press: Cambidge, MO, USA, 2020; pp. 299–312. [Google Scholar]
- Mallott, E.K.; Malhi, R.S.; Amato, K.R. Assessing the Comparability of Different DNA Extraction and Amplification Methods in Gut Microbial Community Profiling. Access Microbiol. 2019, 1, e000060. [Google Scholar] [CrossRef]
- Bartolomaeus, T.U.P.; Forslund, S.K. Hitchhiker’s Guide to Microbiome Studies. Cardiovasc. Res. 2020, 116, E44–E47. [Google Scholar] [CrossRef] [PubMed]
- Juelg, P.; Kipf, E.; Specht, M.; Fillies, M.; Eckert, C.; Paust, N.; Zengerle, R.; Lehnert, M.; Hutzenlaub, T. The MRD Disk: Automated Minimal Residual Disease Monitoring by Highly Sensitive Centrifugal Microfluidic Multiplex qPCR. Lab Chip 2021, 21, 558–570. [Google Scholar] [CrossRef] [PubMed]
- Pak, N.; Saunders, D.C.; Phaneuf, C.R.; Forest, C.R. Plug-and-Play, Infrared, Laser-Mediated PCR in a Microfluidic Chip. Biomed. Microdevices 2012, 14, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.A.J.; Karakochuk, C.D. Feasibility of an At-Home Adult Stool Specimen Collection Method in Rural Cambodia. Int. J. Environ. Res. Public Health 2021, 18, 12430. [Google Scholar] [CrossRef]
- Suleman, H. Reflections on Design Principles for a Digital Repository in a Low Resource Environment; CEUR: Aachen, Germany, 2019. [Google Scholar]
- Agbeyangi, A.; Suleman, H. Advances and Challenges in Low-Resource-Environment Software Systems: A Survey. Informatics 2024, 11, 90. [Google Scholar] [CrossRef]
- Zhang, M.T.; Niu, S.; Deng, S.; Zhang, Z.; Li, Q.; Zheng, L. Hierarchical Capacity Planning with Reconfigurable Kits in Global Semiconductor Assembly and Test Manufacturing. IEEE Trans. Autom. Sci. Eng. 2007, 4, 543–552. [Google Scholar] [CrossRef]
- Ballard, Z.S.; Brown, C.; Ozcan, A. Mobile Technologies for the Discovery, Analysis, and Engineering of the Global Microbiome. ACS Nano 2018, 12, 3065–3082. [Google Scholar] [CrossRef]
- Abdelbasset, W.K.; Savina, S.V.; Mavaluru, D.; Shichiyakh, R.A.; Bokov, D.O.; Mustafa, Y.F. Smartphone Based Aptasensors as Intelligent Biodevice for Food Contamination Detection in Food and Soil Samples: Recent Advances. Talanta 2023, 252, 123769. [Google Scholar] [CrossRef]
- Kholafazad-Kordasht, H.; Hasanzadeh, M.; Seidi, F. Smartphone Based Immunosensors as next Generation of Healthcare Tools: Technical and Analytical Overview towards Improvement of Personalized Medicine. TrAC Trends Anal. Chem. 2021, 145, 116455. [Google Scholar] [CrossRef]
- Liang, B.; Fan, W.; Han, J.G.; Chen, N.; Zhao, N. An Implementation of Infants’ Gut Microbiome Maturation Analyses by 16s rRNA from Stool Samples in Extraction Solution of Room Temperature. In Proceedings of the 2017 IEEE International Conference on Bioinformatics and Biomedicine (BIBM), Kansas City, MO, USA, 13–16 November 2017; pp. 910–914. [Google Scholar]
- Di Bella, J.M.; Bao, Y.; Gloor, G.B.; Burton, J.P.; Reid, G. High Throughput Sequencing Methods and Analysis for Microbiome Research. J. Microbiol. Methods 2013, 95, 401–414. [Google Scholar] [CrossRef]
- Fanning, S.; Proos, S.; Jordan, K.; Srikumar, S. A Review on the Applications of next Generation Sequencing Technologies as Applied to Food-Related Microbiome Studies. Front. Microbiol. 2017, 8, 1829. [Google Scholar] [CrossRef]
- Laurence, M. Metagenomics in Microbiomic Studies. In Microbiomics: Dimensions, Applications, and Translational Implications of Human and Environmental Microbiome Research; Academic Press: Cambridge, MO, USA, 2020; pp. 121–154. [Google Scholar]
- Kumar, A.; Bisht, A.; SammraMaqsood; SaiqaAmjad; Baghel, S.; Jaiswal, S.G.; Wei, S. The Role of Micro-Biome Engineering in Enhancing Food Safety and Quality. Biotechnol. Notes 2025, 6, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Rouskas, K.; Guela, M.; Pantoura, M.; Pagkalos, I.; Hassapidou, M.; Lalama, E.; Pfeiffer, A.F.H.; Decorte, E.; Cornelissen, V.; Wilson-Barnes, S.; et al. The Influence of an AI-Driven Personalized Nutrition Program on the Human Gut Microbiome and Its Health Implications. Nutrients 2025, 17, 1260. [Google Scholar] [CrossRef] [PubMed]
- Turroni, S.; Rampelli, S. The Potential of Microbiome Big Data in Precision Medicine: Predicting Outcomes Through Machine Learning. In Big Data Analysis and Artificial Intelligence for Medical Sciences; John Wiley & Sons: Hoboken, NJ, USA, 2024; pp. 149–160. [Google Scholar]
- Dabban, I.A.; Ahmad, M.; Enejiyon, S.O.; Hauwau, A.N.; Gani, M.; Oyewole, O.A.; Adetunji, C.O. Isolation Techniques Used for Molecular Characterization of Beneficial Microorganisms: Cultural, Biochemical and Molecular Characterization. In Handbook of Agricultural Biotechnology: Volume V Nanobiofertilizers; John Wiley & Sons: Hoboken, NJ, USA, 2024; pp. 491–545. [Google Scholar]
- Harrison, X.A.; Cameron, S.J.S. Analytical Approaches for Microbiome Research. In Microbiomes of Soils, Plants and Animals: An Integrated Approach; Cambridge University Press & Assessment: Cambridge, UK, 2020; pp. 8–28. [Google Scholar]
- Dakal, T.C.; Xu, C.; Kumar, A. Advanced Computational Tools, Artificial Intelligence and Machine-Learning Approaches in Gut Microbiota and Biomarker Identification. Front. Med. Technol. 2024, 6, 1434799. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, D.C.; da Rocha Fernandes, G.; Waitzberg, D.L. Artificial Intelligence and Human Microbiome: A Brief Narrative Review. Clin. Nutr. Open Sci. 2025, 59, 134–142. [Google Scholar] [CrossRef]
- Smitha, M.S.; Siddiqui, M.S. Enhancing Microbiology with Artificial Intelligence: Future of Disease Detection and Treatment. Methods Microbiol. 2025, 56, 297–312. [Google Scholar] [CrossRef]
- Ferreira, D.D.; Ferreira, L.G.; Amorim, K.A.; Delfino, D.C.T.; Ferreira, A.C.B.H.; Souza, L.P.C.E. Assessing the Links Between Artificial Intelligence and Precision Nutrition. Curr. Nutr. Rep. 2025, 14, 47. [Google Scholar] [CrossRef]
- Gupta, S.; Kumar, D. Constructing and Analysing Microbial Interaction Networks Using AI. In Genomic Intelligence: Metagenomics and Artificial Intelligence; CRC Press: Boca Raton, FL, USA, 2024; pp. 207–219. [Google Scholar]
- Prakash, D.; Verma, S.; Bhatia, R.; Tiwary, B.N. Risks and Precautions of Genetically Modified Organisms. Int. Sch. Res. Not. 2011, 2011, 369573. [Google Scholar] [CrossRef]
- Ekici, K.; Sancak, Y.C. A Perspective on Genetically Modified Food Crops. Afr. J. Agric. Res. 2011, 6, 1639–1642. [Google Scholar]
- Demir, A. Impacts of Genetically Modified Organisms (GMOs) on the Environment and Human Health. J. Food Agric. Environ. 2014, 12, 808–810. [Google Scholar]
- Fox, S.; Morrison-Saunders, A.; Katscherian, D. Biotechnology Risk Assessment in Australia: A Molecular Perspective. Environ. Plan. Law J. 2006, 23, 236–248. [Google Scholar]
- Craig, W.; Tepfer, M.; Degrassi, G.; Ripandelli, D. An Overview of General Features of Risk Assessments of Genetically Modified Crops. Euphytica 2008, 164, 853–880. [Google Scholar] [CrossRef]
- Whomsley, R.; Palmi Reig, V.; Hidalgo-Simon, A. Environmental Risk Assessment of Advanced Therapies Containing Genetically Modified Organisms in the EU. Br. J. Clin. Pharmacol. 2021, 87, 2450–2458. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, J.; Gomes, A.R.; Olaru, I. Principles for the Risk Assessment of Genetically Modified Microorganisms and Their Food Products in the European Union. Int. J. Food Microbiol. 2013, 167, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Bergmans, H.; Logie, C.; Maanen, K.V.; Hermsen, H.; Meredyth, M.; Vlugt, C.V.D. Identification of Potentially Hazardous Human Gene Products in GMO Risk Assessment. Environ. Biosaf. Res. 2008, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Boy, C.; Lesage, J.; Alfenore, S.; Guillouet, S.E.; Gorret, N. Investigation of the Robustness of Cupriavidus Necator Engineered Strains during Fed-Batch Cultures. AMB Express 2021, 11, 151. [Google Scholar] [CrossRef]
- Allen, J.R.; Torres-Acosta, M.A.; Mohan, N.; Lye, G.J.; Ward, J.M. Segregationally Stabilised Plasmids Improve Production of Commodity Chemicals in Glucose-Limited Continuous Fermentation. Microb. Cell Factories 2022, 21, 229. [Google Scholar] [CrossRef]
- Wang, G.; Li, Q.; Zhang, Z.; Yin, X.; Wang, B.; Yang, X. Recent Progress in Adaptive Laboratory Evolution of Industrial Microorganisms. J. Ind. Microbiol. Biotechnol. 2023, 50, kuac023. [Google Scholar] [CrossRef]
- Hospet, R.; Thangadurai, D.; Cruz-Martins, N.; Sangeetha, J.; Anu Appaiah, K.A.; Chowdhury, Z.Z.; Bedi, N.; Soytong, K.; Al Tawaha, A.R.M.; Jabeen, S.; et al. Genome Shuffling for Phenotypic Improvement of Industrial Strains through Recursive Protoplast Fusion Technology. Crit. Rev. Food Sci. Nutr. 2023, 63, 2960–2969. [Google Scholar] [CrossRef]
- Muir, W.M.; Howard, R.D. Characterization of Environmental Risk of Genetically Engineered (GE) Organisms and Their Potential to Control Exotic Invasive Species. Aquat. Sci. 2004, 66, 414–420. [Google Scholar] [CrossRef]
- Schiemann, J. The OECD Blue Book on Recombinant DNA Safety Considerations: It’s Influence on ISBR and EFSA Activities. Environ. Biosaf. Res. 2006, 5, 233–235. [Google Scholar] [CrossRef][Green Version]
- Korolik, V.V.; Sheina, N.I.; Mjalina, L.P.; Sazonova, L.P.; Drugova, E.D. Primary Sanitary and Hygienic Assessment of Microorganisms Used in Biotechnology. Gig. Sanit. 2023, 102, 135–140. [Google Scholar] [CrossRef]
- Schmidt, M.; De Lorenzo, V. Synthetic Constructs in/for the Environment: Managing the Interplay between Natural and Engineered Biology. FEBS Lett. 2012, 586, 2199–2206. [Google Scholar] [CrossRef] [PubMed]
- Kochhar, H.P.S.; Evans, B.R. Current Status of Regulating Biotechnology-Derived Animals in Canada-Animal Health and Food Safety Considerations. Theriogenology 2007, 67, 188–197. [Google Scholar] [CrossRef]
- Manful, M.E.; Ahmed, L.; Barry-Ryan, C. Cosmetic Formulations from Natural Sources: Safety Considerations and Legislative Frameworks in the European Union. Cosmetics 2024, 11, 72. [Google Scholar] [CrossRef]
- Vinardell, M.P. The Use of Non-Animal Alternatives in the Safety Evaluations of Cosmetics Ingredients by the Scientific Committee on Consumer Safety (SCCS). Regul. Toxicol. Pharmacol. 2015, 71, 198–204. [Google Scholar] [CrossRef]
- Ross, G. A Perspective on the Safety of Cosmetic Products: A Position Paper of The American Council on Science and Health. Int. J. Toxicol. 2006, 25, 269–277. [Google Scholar] [CrossRef]
- Kim, K.-B.; Kwack, S.J.; Lee, J.Y.; Kacew, S.; Lee, B.-M. Current Opinion on Risk Assessment of Cosmetics. J. Toxicol. Environ. Health Part B Crit. Rev. 2021, 24, 137–161. [Google Scholar] [CrossRef]
- Juncan, A.M.; Rus, L.-L.; Morgovan, C.; Loghin, F. Evaluation of the Safety of Cosmetic Ingredients and Their Skin Compatibility through In Silico and In Vivo Assessments of a Newly Developed Eye Serum. Toxics 2024, 12, 451. [Google Scholar] [CrossRef]
- Dent, M.; Amaral, R.T.; Da Silva, P.A.; Ansell, J.; Boisleve, F.; Hatao, M.; Hirose, A.; Kasai, Y.; Kern, P.; Kreiling, R.; et al. Principles Underpinning the Use of New Methodologies in the Risk Assessment of Cosmetic Ingredients. Comput. Toxicol. 2018, 7, 20–26. [Google Scholar] [CrossRef]
- Antezana, L.; Hwang, J. Cosmetics. In Translational Orthopedics; Elsevier: Amsterdam, The Netherlands, 2024; pp. 421–425. [Google Scholar]
- Garcia, A.; DiBartolo, R. Cosmetics and FDA Regulation; Food and Drug Administration (FDA): Maryland, MD, USA, 2013; p. 102.
- Vigan, M. European Regulatory Guidelines for Cosmetics. EMC Dermatol. Cosmetol. 2004, 1, 154–163. [Google Scholar] [CrossRef]
- Filaire, E.; Nachat-Kappes, R.; Laporte, C.; Harmand, M.-F.; Simon, M.; Poinsot, C. Alternative in Vitro Models Used in the Main Safety Tests of Cosmetic Products and New Challenges. Int. J. Cosmet. Sci. 2022, 44, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Finlay, T.A.; Andrew, D.J. Cosmetic Products. In Issues in Toxicology; Andersen, F.A., Basketter, D.A., Eds.; Royal Society of Chemistry: Cambridge, UK, 2018; Volume 36, pp. 505–538. [Google Scholar] [CrossRef]
- Lawrence, S. What Would You Do with a Fluorescent Green Pig: How Novel Transgenic Products Reveal Flaws in the Foundational Assumptions for the Regulation of Biotechnology. Ecol. Law Q. 2007, 34, 201. [Google Scholar]
- Von Wright, A. Regulatory Aspects of Probiotics and Other Microbial Products Intended for Skin Care: The European Approach. In Skin Microbiome Handbook: From Basic Research to Product Development; John Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 321–341. [Google Scholar]
- Franciosa, G.; Guida, S.; Miguel, M.J.G.; Hunolstein, C.V. Live Biotherapeutic Products and Their Regulatory Framework in Italy and Europe. Ann. Dell’istituto Super. Di Sanità 2023, 59, 56–67. [Google Scholar] [CrossRef]
- Chung, M.-H.; Huang, W.-S.; Chang, Y.-C.; Chen, Y.-H.; Lee, M.-S.; Huang, S.-C.; Chen, Y.-P.; Shih, D.Y.-C.; Cheng, H.-F. A Review of Quality Surveillance Projects on Cosmetics in Taiwan. J. Food Drug Anal. 2014, 22, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Halla, N.; Fernandes, I.P.; Heleno, S.A.; Costa, P.; Boucherit-Otmani, Z.; Boucherit, K.; Rodrigues, A.E.; Ferreira, I.C.F.R.; Barreiro, M.F. Cosmetics Preservation: A Review on Present Strategies. Molecules 2018, 23, 1571. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.S.B.; Tüysüz, M.; Ötük, G. Investigation of Preservative Efficacy and Microbiological Content of Some Cosmetics Found on the Market. Pak. J. Pharm. Sci. 2013, 26, 153–157. [Google Scholar]
- Klaschka, U. Trust, but Verify! Personal Care Products in the Rapid Alert System Database RAPEX. Sustain. Chem. Pharm. 2017, 5, 30–41. [Google Scholar] [CrossRef]
- Ahmed, E.; Hens, K. Microbiome in Precision Psychiatry: An Overview of the Ethical Challenges Regarding Microbiome Big Data and Microbiome-Based Interventions. AJOB Neurosci. 2022, 13, 270–286. [Google Scholar] [CrossRef]
- Ponce de Leon-Derecho, C.M.; Dable-Tupas, G. Ethical Considerations in Microbiome Research. In Human Microbiome Drug Targets: Modern Approaches in Disease Management; Elsevier: Amsterdam, The Netherlands, 2024; pp. 179–188. [Google Scholar]
- Ma, Y.; Yang, J.; Cui, B.; Xu, H.; Xiao, C.; Zhang, F. How Chinese Clinicians Face Ethical and Social Challenges in Fecal Microbiota Transplantation: A Questionnaire Study. BMC Med. Ethics 2017, 18, 39. [Google Scholar] [CrossRef]
- Singh, V.; Rastogi, M. Future and Challenges of Microbiome Engineering. In Microbiome Engineering: The New Dimension of Biotechnology; CRC Press: Boca Raton, FL, USA, 2024; pp. 263–280. [Google Scholar]
- Métris, A.; Barrett, P.; Price, L.; Klamert, S.; Fernandez-Piquer, J. A Tiered Approach to Risk Assess Microbiome Perturbations Induced by Application of Beauty and Personal Care Products. Microb. Risk Anal. 2022, 20, 100188. [Google Scholar] [CrossRef]
- Lange, L.; Berg, G.; Cernava, T.; Champomier-Vergès, M.-C.; Charles, T.; Cocolin, L.; Cotter, P.; D’Hondt, K.; Kostic, T.; Maguin, E.; et al. Microbiome Ethics, Guiding Principles for Microbiome Research, Use and Knowledge Management. Environ. Microbiomes 2022, 17, 50. [Google Scholar] [CrossRef]
- Cummings, C.; Landreville, K.D.; Kuzma, J. Taking the Temperature of the United States Public Regarding Microbiome Engineering. Front. Public Health 2024, 12, 1477377. [Google Scholar] [CrossRef]
- Slashinski, M.J.; McCurdy, S.A.; Achenbaum, L.S.; Whitney, S.N.; McGuire, A.L. Snake-Oil, Quack Medicine, and Industrially Cultured Organisms: Biovalue and the Commercialization of Human Microbiome Research. BMC Med. Ethics 2012, 13, 28. [Google Scholar] [CrossRef]
- Traill, W.B.; Jaeger, S.R.; Yee, W.M.S.; Valli, C.; House, L.O.; Lusk, J.L.; Moore, M.; Morrow, J.L., Jr. Categories of GM Risk-Benefit Perceptions and Their Antecedents. AgBioForum 2004, 7, 176–186. [Google Scholar]
- Krajišnik, D.; Đekić, L. Microbiological Safety of Cosmetic Products and Potential Risks for Consumers. Arh. Za Farm. 2018, 68, 949–970. [Google Scholar] [CrossRef]
- Stewart, S.E.; Parker, M.D.; Amézquita, A.; Pitt, T.L. Microbiological Risk Assessment for Personal Care Products. Int. J. Cosmet. Sci. 2016, 38, 634–645. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, C.F.; Desvignes, C.; Kling, F.; Voisin, E.M.; Ruthsatz, M. Microbiome Product Toxicology: Regulatory View on Translational Challenges In Regulatory Toxicology, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2021; Volume 2, pp. 1401–1430. [Google Scholar]
- Suhag, J.; Dureja, H. Cosmetic Regulations: A Comparative Study. SKINmed 2015, 13, 191–194. [Google Scholar]
- Srikanth, T.; Hussen, S.S.; Abha, A.; Vasantharaju, S.G.; Gummudavelly, S. A Comparative View on Cosmetic Regulations: USA, EU and INDIA. Der Pharm. Lett. 2011, 3, 334–341. [Google Scholar]
- Gagliardi, L.; Dorato, S. General Concepts. Current Legislation on Cosmetics in Different Countries. In Analysis of Cosmetic Products; Elsevier: Amsterdam, The Netherlands, 2007; pp. 3–28. [Google Scholar]
- Zhu, H.; Sun, S.; Zhang, J.; Liu, S.; Liu, Q.; Zou, H. Recent Progress on the Application Status of Cosmetic Preservatives and Their Detection Technology. China Surfactant Deterg. Cosmet. 2023, 53, 679–685. [Google Scholar] [CrossRef]
- Kim, H.W.; Seok, Y.S.; Lee, H.G.; Song, M.K.; Rhee, M.S. Consumers’ Lack of Understanding of Customized Cosmetics Made on the Spot and Implications for Regulations and Controls. Regul. Toxicol. Pharmacol. 2021, 124, 104979. [Google Scholar] [CrossRef]
- Lionetti, N.; Rigano, L. Labeling of Cosmetic Products. Cosmetics 2018, 5, 22. [Google Scholar] [CrossRef]
- Corby-Edwards, A.K. FDA Regulation of Cosmetics and Personal Care Products. In Cosmetics and FDA Regulation; Food and Drug Administration (FDA): Maryland, MD, USA, 2013. [Google Scholar]
- Renner, G.; Audebert, F.; Burfeindt, J.; Calvet, B.; Caratas-Perifan, M.; Leal, M.E.; Gorni, R.; Long, A.; Meredith, E.; O’Sullivan, Ú.; et al. Cosmetics Europe Guidelines on the Management of Undesirable Effects and Reporting of Serious Undesirable Effects from Cosmetics in the European Union. Cosmetics 2017, 4, 1. [Google Scholar] [CrossRef]
- Grossman, M.R. Genetically Modified Crops in the United States: The Federal Regulatory Framework, and State Tort Liability. Environ. Law Rev. 2003, 5, 86–108. [Google Scholar] [CrossRef]
- Ferreira, M.; Matos, A.; Couras, A.; Marto, J.; Ribeiro, H. Overview of Cosmetic Regulatory Frameworks around the World. Cosmetics 2022, 9, 72. [Google Scholar] [CrossRef]
- Cubas, A.L.V.; Bianchet, R.T.; Reis, I.M.A.S.D.; Gouveia, I.C. Plastics and Microplastic in the Cosmetic Industry: Aggregating Sustainable Actions Aimed at Alignment and Interaction with UN Sustainable Development Goals. Polymers 2022, 14, 4576. [Google Scholar] [CrossRef] [PubMed]
- Aziz, Z.A.A.; Setapar, S.H.M.; Khatoon, A.; Ahmad, A. The Potential Use of Biosurfactants in Cosmetics and Dermatological Products: Current Trends and Future Prospects. In Biosurfactants for a Sustainable Future: Production and Applications in the Environment and Biomedicine; John Wiley & Sons: Hoboken, NJ, USA, 2021; pp. 397–421. [Google Scholar]
- Chen, X.; Li, X. The Impact of Hazardous Substances in Cosmetics, and Treatment Measures; IOP Publishing Ltd.: Bristol, UK, 2021; Volume 1011. [Google Scholar]
- Purwanto, P.; Permana-Citra, A.D. Recycling and Processing of Solid Waste into Products of the Cosmetic Packaging Industry; IOP Publishing Ltd.: Bristol, UK, 2019; Volume 1295. [Google Scholar]
- Rathore, S.; Schuler, B.; Park, J. Life Cycle Assessment of Multiple Dispensing Systems Used for Cosmetic Product Packaging. Packag. Technol. Sci. 2023, 36, 533–547. [Google Scholar] [CrossRef]
- Carvalho, M.J.; Oliveira, A.L.S.; Santos Pedrosa, S.; Pintado, M.; Pinto-Ribeiro, I.; Madureira, A.R. Skin Microbiota and the Cosmetic Industry. Microb. Ecol. 2023, 86, 86–96. [Google Scholar] [CrossRef]
- Kadam, S.; Deore, S.; Tare, H.; Wagh, V.; Thube, U. The Use of Biological Pigments in Cosmetics for Eco-Friendly and Sustainable Coloring. Int. J. Pharm. Qual. Assur. 2024, 15, 539–545. [Google Scholar] [CrossRef]
- Vrabič-Brodnjak, U.; Jestratijević, I. The Future of Baby Cosmetics Packaging and Sustainable Development: A Look at Sustainable Materials and Packaging Innovations—A Systematic Review. Sustain. Dev. 2024, 32, 2208–2222. [Google Scholar] [CrossRef]
- Borowska, S.; Brzóska, M.M. Metals in Cosmetics: Implications for Human Health. J. Appl. Toxicol. 2015, 35, 551–572. [Google Scholar] [CrossRef]
- Orisakwe, O.E.; Otaraku, J.O. Metal Concentrations in Cosmetics Commonly Used in Nigeria. Sci. World J. 2013, 2013, 959637. [Google Scholar] [CrossRef]
- Hashim, A.M.; Abd-Alameer, A.M.; Rashed, A.H. Ctrophotometyric Determination of Heavy Metal (Lead) in Cosmetics (Lipsticks) in Commercial Markets. Indian J. Forensic Med. Toxicol. 2020, 14, 2405–2409. [Google Scholar] [CrossRef]
- Bocca, B.; Pino, A.; Alimonti, A.; Forte, G. Toxic Metals Contained in Cosmetics: A Status Report. Regul. Toxicol. Pharmacol. 2014, 68, 447–467. [Google Scholar] [CrossRef]
- Abed, M.S.; Moosa, A.A.; Alzuhairi, M.A. Heavy Metals in Cosmetics and Tattoos: A Review of Historical Background, Health Impact, and Regulatory Limits. J. Hazard. Mater. Adv. 2024, 13, 100390. [Google Scholar] [CrossRef]
- Machado, M.; Silva, S.; Costa, E.M. Byproducts as a Sustainable Source of Cosmetic Ingredients. Appl. Sci. 2024, 14, 10241. [Google Scholar] [CrossRef]
- Krzyżostan, M.; Wawrzyńczak, A.; Nowak, I. Use of Waste from the Food Industry and Applications of the Fermentation Process to Create Sustainable Cosmetic Products: A Review. Sustainability 2024, 16, 2757. [Google Scholar] [CrossRef]
- Martins, A.M.; Marto, J.M. A Sustainable Life Cycle for Cosmetics: From Design and Development to Post-Use Phase. Sustain. Chem. Pharm. 2023, 35, 101178. [Google Scholar] [CrossRef]
- Glew, D.; Lovett, P.N. Life Cycle Analysis of Shea Butter Use in Cosmetics: From Parklands to Product, Low Carbon Opportunities. J. Clean. Prod. 2014, 68, 73–80. [Google Scholar] [CrossRef]
- Vaz, B.M.C.; Contieri, L.S.; Sosa, F.H.B.; Martins, M.; Conde, A.; Dias, A.C.R.V.; Rostagno, M.A.; de Souza Mesquita, L.M.; Ventura, S.P.M. Unleashing the Potential of Castor Oil as Extraction Solvent of Carotenoids from Tomatoes. Sep. Purif. Technol. 2025, 358, 130278. [Google Scholar] [CrossRef]
- de Albuquerque Vita, N.; Rodrigues de Souza, I.; Di Pietro Micali Canavez, A.; Brohem, C.A.; Cristine Marios Ferreira Pinto, D.; Schuck, D.C.; Leme, D.M.; Lorencini, M. The Development and Application of a Novel Hazard Scoring Tool for Assessing Impacts of Cosmetic Ingredients on Aquatic Ecosystems: A Case Study of Rinse-off Cosmetics. Integr. Environ. Assess. Manag. 2023, 19, 1619–1635. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Chen, X.-B.; Chen, J.-C.; Wu, Q.; Chen, G.-Q. Open and Continuous Fermentation: Products, Conditions and Bioprocess Economy. Biotechnol. J. 2014, 9, 1503–1511. [Google Scholar] [CrossRef]
- Zhao, Z.-T.; Ding, J.; Pang, J.-W.; Yang, S.-S.; Wang, B.-Y.; Bao, M.-Y.; Liu, B.-F.; Ren, N.-Q. Advances in the Biomass Valorization in Dark Fermentation Systems: A Sustainable Approach for Biohydrogen Production. Chem. Eng. J. 2024, 481, 148444. [Google Scholar] [CrossRef]
- Ramprakash, B.; Lindblad, P.; Eaton-Rye, J.J.; Incharoensakdi, A. Current Strategies and Future Perspectives in Biological Hydrogen Production: A Review. Renew. Sustain. Energy Rev. 2022, 168, 112773. [Google Scholar] [CrossRef]
- Cowan, A.E.; Klass, S.H.; Winegar, P.H.; Keasling, J.D. Microbial Production of Fuels, Commodity Chemicals, and Materials from Sustainable Sources of Carbon and Energy. Curr. Opin. Syst. Biol. 2023, 36, 100482. [Google Scholar] [CrossRef]
- Sakshi; Singh, P.; Mishra, A.P.; Mishra, P.; Nigam, M.; Singh, S. Recent Trends in the Valorization of Value-Added Biomass from Microbes for Sustainable Development. Environ. Sci. Eng. 2025, Part F1, 377–395. [Google Scholar] [CrossRef]
- de Oliveira Santos, V.T.; Siqueira, G.; Milagres, A.M.F.; Ferraz, A. Role of Hemicellulose Removal during Dilute Acid Pretreatment on the Cellulose Accessibility and Enzymatic Hydrolysis of Compositionally Diverse Sugarcane Hybrids. Ind. Crops Prod. 2018, 111, 722–730. [Google Scholar] [CrossRef]
- Sun, W.-L.; Tao, W.-Y. Simultaneous Saccharification and Fermentation of Rice Straw Pretreated by a Sequence of Dilute Acid and Dilute Alkali at High Dry Matter Content. Energy Sources Part A Recovery Util. Environ. Eff. 2013, 35, 741–752. [Google Scholar] [CrossRef]
- Foston, M.; Ragauskas, A.J. Changes in the Structure of the Cellulose Fiber Wall during Dilute Acid Pretreatment in Populus Studied by 1H and 2H NMR. Energy Fuels 2010, 24, 5677–5685. [Google Scholar] [CrossRef]
- Sheng, Y.; Tan, X.; Gu, Y.; Zhou, X.; Tu, M.; Xu, Y. Effect of Ascorbic Acid Assisted Dilute Acid Pretreatment on Lignin Removal and Enzyme Digestibility of Agricultural Residues. Renew. Energy 2021, 163, 732–739. [Google Scholar] [CrossRef]
- Luo, X.; Gong, Z.; Shi, J.; Chen, L.; Zhu, W.; Zhou, Y.; Huang, L.; Liu, J. Integrating Benzenesulfonic Acid Pretreatment and Bio-Based Lignin-Shielding Agent for Robust Enzymatic Conversion of Cellulose in Bamboo. Polymers 2020, 12, 191. [Google Scholar] [CrossRef]
- Nägele, H.J.; Steinbrenner, J.; Hermanns, G.; Holstein, V.; Haag, N.L.; Oechsner, H. Innovative Additives for Chemical Desulphurisation in Biogas Processes: A Comparative Study on Iron Compound Products. Biochem. Eng. J. 2017, 121, 181–187. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, L.; Liu, S.; Yang, S.; Shangguan, J. Role of Iron-Based Catalysts in Reducing NOx Emissions from Coal Combustion. Chin. J. Chem. Eng. 2023, 59, 1–8. [Google Scholar] [CrossRef]
- Amiri, H.; Karimi, K. Efficient Dilute-Acid Hydrolysis of Cellulose Using Solvent Pretreatment. Ind. Eng. Chem. Res. 2013, 52, 11494–11501. [Google Scholar] [CrossRef]
- Naseema Rasheed, R.; Pourbakhtiar, A.; Mehdizadeh Allaf, M.; Baharlooeian, M.; Rafiei, N.; Alishah Aratboni, H.; Morones-Ramirez, J.R.; Winck, F.V. Microalgal Co-Cultivation-Recent Methods, Trends in Omic-Studies, Applications, and Future Challenges. Front. Bioeng. Biotechnol. 2023, 11, 1193424. [Google Scholar] [CrossRef] [PubMed]
- Praveen, M.; Brogi, S. Microbial Fermentation in Food and Beverage Industries: Innovations, Challenges, and Opportunities. Foods 2025, 14, 114. [Google Scholar] [CrossRef]
- Rozina; Emmanuel, O.; Ezeji, T.C. Exploring the Synergy of Nanomaterials and Microbial Cell Factories during Biohydrogen and Biobutanol Production from Different Carbon Sources. Sustain. Chem. Environ. 2024, 6, 100098. [Google Scholar] [CrossRef]
- Villacreses-Freire, D.; Ketzer, F.; Rösch, C. Advanced Metabolic Engineering Approaches and Renewable Energy to Improve Environmental Benefits of Algal Biofuels: LCA of Large-Scale Biobutanol Production with Cyanobacteria Synechocystis PCC6803. Bioenergy Res. 2022, 15, 1515–1530. [Google Scholar] [CrossRef]
- Mitra, S.; Paliya, S.; Mandpe, A. Microbial Engineering in Biofuel Production—A Global Outlook, Advances, and Roadmap. Environ. Sci. Eng. 2024, 2491 Part F, 547–593. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, Y.; Li, Y. Production of Fuels and Chemicals from Renewable Resources Using Engineered Escherichia coli. Biotechnol. Adv. 2019, 37, 107402. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Chaudhary, J.; Singh, P.; Alsanie, W.F.; Grammatikos, S.A.; Thakur, V.K. Synthesis of Bio-Based Monomers and Polymers Using Microbes for a Sustainable Bioeconomy. Bioresour. Technol. 2022, 344, 126156. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, J.A.; Lee, S.Y. Biobased Production of Succinic Acid and Its Derivatives Using Metabolically Engineered Microorganisms. Ind. Biotechnol. 2023, 19, 125–137. [Google Scholar] [CrossRef]
- Jung, J.-H.; Ponnusamy, V.K.; Kumar, G.; Igliński, B.; Kumar, V.; Piechota, G. Industrial–Scale Production of Various Bio–Commodities by Engineered Microbial Cell Factories: Strategies of Engineering in Microbial Robustness. Chem. Eng. J. 2024, 502, 157679. [Google Scholar] [CrossRef]
- Lyu, X.; Nuhu, M.; Candry, P.; Wolfanger, J.; Betenbaugh, M.; Saldivar, A.; Zuniga, C.; Wang, Y.; Shrestha, S. Top-down and Bottom-up Microbiome Engineering Approaches to Enable Biomanufacturing from Waste Biomass. J. Ind. Microbiol. Biotechnol. 2024, 51, kuae025. [Google Scholar] [CrossRef]
- Jerold, M.; Sivasubramanian, V. Biochemical and Environmental Bioprocessing: Challenges and Developments; Biochemical and Environmental Bioprocessing: Challenges and Developments; CRC Press: Boca Raton, FL, USA, 2019; p. 256. [Google Scholar]
- Pagliano, G.; Ventorino, V.; Panico, A.; Pepe, O. Integrated Systems for Biopolymers and Bioenergy Production from Organic Waste and By-Products: A Review of Microbial Processes. Biotechnol. Biofuels 2017, 10, 113. [Google Scholar] [CrossRef] [PubMed]
- Gavrilescu, M. From Pollutants to Products: Microbial Cell Factories Driving Sustainable Biomanufacturing and Environmental Conservation. Chem. Eng. J. 2024, 500, 157152. [Google Scholar] [CrossRef]
- Kowalczyk, S.; Grymel, M.; Bilik, J.; Kula, W.; Wawoczny, A.; Grymel, P.; Gillner, D. Selected Plants as Sources of Natural and Active Ingredients for Cosmetics of the Future. Appl. Sci. 2024, 14, 3487. [Google Scholar] [CrossRef]
- Zhao, M.; Chi, Z.; Lai, D.; Liu, K.; Liu, Z.; Zheng, Y. Advances in the microbial synthesis of active ingredients in cosmetics. Sheng Wu Gong Cheng Xue Bao 2024, 40, 2489–2512. [Google Scholar] [CrossRef]
- Savary, G.; Grisel, M.; Picard, C. Cosmetics and Personal Care Products. In Natural Polymers: Industry Techniques and Applications; Olatunji, O., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 219–261. ISBN 978-3-319-26414-1. [Google Scholar]
- Ifuku, O. Botanical Ingredients. In Cosmetic Science and Technology: Theoretical Principles and Applications; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 978-0-12-802054-8. [Google Scholar]
- Yahya, E.B.; Ali, S.R.; Lalung, J.; Zain, M.S.C.; Danish, M.; John, A. Exploring the Potential of Biopolymers in Cosmetic Applications: Sustainable, Biocompatible, and High-Performance Materials for Future Innovations. Polym. Eng. Sci. 2025, 65, 2789–2802. [Google Scholar] [CrossRef]
- Dailin, D.J.; Rithwan, F.; Azelee, N.I.W.; Zainan, N.; Low, L.Z.M.I.; Zaidel, D.N.A.; El Enshasy, H. Trends in Bio-Based Cosmetic Ingredients. In Biomass-Based Cosmetics: Research Trends and Future Outlook; Arung, E.T., Fatriasari, W., Kusuma, I.W., Kuspradini, H., Shimizu, K., Kim, Y., Azelee, N.I.W., Edis, Z., Eds.; Springer Nature: Singapore, 2024; pp. 27–47. ISBN 978-981-97-1908-2. [Google Scholar]
- Amro, B.S.; Abu Hajleh, M.N.; Affifi, F. Evidence-Based Potential of Some Edible, Medicinal and Aromatic Plants as Safe Cosmetics and Cosmeceuticals. Trop. J. Nat. Product Res. 2021, 5, 16–48. [Google Scholar]
- Hasmida, M.N.; Siti Hamidah, M.S. Natural Ingredients in Cosmetics from Malaysian Plants: A Review. Sains Malays. 2018, 47, 951–959. [Google Scholar] [CrossRef]
- Xiaowei, C.; Wei, W.; Hong, G.; Hui, C.; Xiaofei, Z.; Haonan, W.; Yumeng, W.; Xuelan, Z.; Chunchao, H. Review of Polygonatum Sibiricum: A New Natural Cosmetic Ingredient. Die Pharm. An. Int. J. Pharm. Sci. 2019, 74, 513–519. [Google Scholar] [CrossRef]
- Caiola, S.; Palleschi, L.; Draisci, R.; Mancinelli, R. Cosmetics, chemical exposure and gender differences. J. Sex Gend. Specif. Med. 2018, 4, 21–26. [Google Scholar]
- Nosáľová, M.; Loučanová, E.; Parobek, J.; Olšiaková, M. Paper-Based Cosmetic Packaging. Proceedings of Current Trends and Challenges for Forest-based Sector: Carbon Neutrality and Bioeconomy, Prague, Czech Republic, 14–16 June 2023; pp. 94–98. [Google Scholar]
- Vassalo, N.; Refalo, P. Life Cycle Assessment of Various Initiatives towards Sustainable Plastic Packaging. Procedia CIRP 2024, 122, 372–377. [Google Scholar] [CrossRef]
- Vishal Srivastav; Singh, S.; Das, D. Enhancing Packaging Sustainability with Natural Fiber Reinforced Biocomposites: An Outlook into the Future; EDP Sciences: Les Ulis, France, 2023; Volume 436. [Google Scholar]
- Mifsud, S.; Refalo, P.; Rochman, A. Biodegradable Polymers for Cosmetic Packaging: A Technical and Life Cycle Perspective. In Proceedings of the Material Forming, Kraków, Poland, 25 May 2023; pp. 2015–2024. [Google Scholar]
- Klein, M.; Oleynikova, A.; Neumair, C.; Tacker, M.; Apprich, S. Assessment of Sustainability Indicators for Cosmetic Product Packaging in the DACH Region. Cosmetics 2025, 12, 56. [Google Scholar] [CrossRef]
- Aguiar, J.B.; Martins, A.M.; Almeida, C.; Ribeiro, H.M.; Marto, J. Water Sustainability: A Waterless Life Cycle for Cosmetic Products. Sustain. Prod. Consum. 2022, 32, 35–51. [Google Scholar] [CrossRef]
- Colwill, J.A.; Rahimifard, S. Impact of the Use of Renewable Materials on Ecoefficiency of Manufacturing Processes. Plast. Rubber Compos. 2013, 42, 129–133. [Google Scholar] [CrossRef]
- Del Greco, A.; Bani, M.; Rampoldi, G.; Ardenghi, S.; Galli, P.; Strepparava, M.G.; Russo, S. Re-Evaluating Beauty: Attitudes and Perceptions of Eco-Friendly Packaging in Beauty Care Products—A Systematic Review. Sustain. Prod. Consum. 2024, 52, 458–468. [Google Scholar] [CrossRef]
- Bogusz, M.; Matysik-Pejas, R.; Krasnodębski, A.; Dziekański, P. Sustainable Consumption of Households According to the Zero Waste Concept. Energies 2023, 16, 6516. [Google Scholar] [CrossRef]
- Ali, S.; Shirazi, F. The Paradigm of Circular Economy and an Effective Electronic Waste Management. Sustainability 2023, 15, 1998. [Google Scholar] [CrossRef]
- de Koeijer, B.; ten Klooster, R.; Hesseling, I.; Huijben, C. Designing Reusable Packaging: Tool Development, Validation, and Implications. In Proceedings of the 23rd IAPRI World Conference on Packaging, IAPRI 2022, Thailand, Bangkok, 12–16 June 2022; pp. 436–443. [Google Scholar]
- Klein, M.; Tacker, M.; Apprich, S. Product Waste Resulting from Insufficient Emptiability of Cosmetic Packaging and Its Economic and Environmental Implications. Sustainability 2025, 17, 1056. [Google Scholar] [CrossRef]
- Sung, B.; Yoon, K.-K.; Yu, S. Who Drives Market Access for Genetically Modified Organisms and Products in Korea? A Political Economy Approach to Sustainable Development. Sci. Technol. Soc. 2016, 21, 271–295. [Google Scholar] [CrossRef]
- Merritt, H.; Barragán-Ocaña, A. The Impact of Market Factors on the Development of Eco-Friendly Energy Technologies: The Case of Bioethanol. Clean Technol. Environ. Policy 2023, 25, 313–321. [Google Scholar] [CrossRef]
- Gaur, S.; Kaur, M.; Kalra, R.; Rene, E.R.; Goel, M. Application of Microbial Resources in Biorefineries: Current Trend and Future Prospects. Heliyon 2024, 10, e28615. [Google Scholar] [CrossRef]
- Maqsood, Q.; Khan, M.I.; Ashraf, M.H.; Khan, U.; Hussain, N. Harnessing Microbiome for Health and Sustainable Waste Management. Adv. Chem. Pollut. Environ. Manag. Prot. 2025, 12, 597–624. [Google Scholar] [CrossRef]
- Kostic, T.; Schloter, M.; Arruda, P.; Berg, G.; Charles, T.C.; Cotter, P.D.; Kiran, G.S.; Lange, L.; Maguin, E.; Meisner, A.; et al. Concepts and Criteria Defining Emerging Microbiome Applications. Microb. Biotechnol. 2024, 17, e14550. [Google Scholar] [CrossRef]
- Festel, G.; Knöll, J.; Götz, H.; Zinke, H. Impact of Biotechnology Production Processes in the Chemical Industry. Chem. Ing. Tech. 2004, 76, 307–312. [Google Scholar] [CrossRef]
- Soetaert, W.; Vandamme, E. The Impact of Industrial Biotechnology. Biotechnol. J. 2006, 1, 756–769. [Google Scholar] [CrossRef]
- Wydra, S.; Nusser, M. Diffusion and Economic Impacts of Biotechnology—A Case Study for Germany. Int. J. Biotechnol. 2011, 12, 87–103. [Google Scholar] [CrossRef]
- Otero, G. Neoliberal Globalism and the Biotechnology Revolution: Economic and Historical Context. In Food for the Few: Neoliberal Globalism and Biotechnology in Latin America; University of Texas Press: Austin, TX, USA, 2008; pp. 1–29. [Google Scholar]
- Bil, G. The Representation of Plants. In A Cultural History of Plants: In the Modern Era; Bloomsbury Academic: London, UK, 2023; Volume 6, pp. 171–191. [Google Scholar]
- Grobbelaar, S.; Tijssen, R.; Dijksterhuis, M. University-Driven Inclusive Innovations in the Western Cape of South Africa: Towards a Research Framework of Innovation Regimes. Afr. J. Sci. Technol. Innov. Dev. 2017, 9, 7–19. [Google Scholar] [CrossRef]
- Ruan, J.; Song, S. Design for Sustainability Drives Inclusive Innovation of Urban Agricultural Communities: Taking Projects in China and Italy as Examples. In Urban and Transit Planning; Springer: Cham, Switzerland, 2025; pp. 143–153. [Google Scholar] [CrossRef]
- Nakad, M.; Charif, H.; Gardelle, L.; Abboud, R.J. Empowering Sustainability: Can Women Engineers Play a Key Role in Achieving a Sustainable Future? Sustain. Futures 2025, 10, 100977. [Google Scholar] [CrossRef]
- Galipeau, D. A New Era of Sustainable Innovation. In Business and Management in Asia: Digital Innovation and Sustainability; Springer: Berlin/Heidelberg, Germany, 2022; pp. 49–66. [Google Scholar]
- Romano, A.L.; Ferreira, L.M.D.F.; Caeiro, S.S.F.S. Modelling Sustainability Risk in the Brazilian Cosmetics Industry. Sustainability 2021, 13, 13771. [Google Scholar] [CrossRef]
- McGrath, P.; McCarthy, L.; Marshall, D.; Rehme, J. Tools and Technologies of Transparency in Sustainable Global Supply Chains. Calif. Manag. Rev. 2021, 64, 67–89. [Google Scholar] [CrossRef]
- Patón-Romero, J.D.; Baldassarre, M.T.; Rodríguez, M.; Piattini, M. Application of ISO 14000 to Information Technology Governance and Management. Comput. Stand. Interfaces 2019, 65, 180–202. [Google Scholar] [CrossRef]
- de Vries, H.J.; Bayramoglu, D.K.; van der Wiele, T. Business and Environmental Impact of ISO 14001. Int. J. Qual. Reliab. Manag. 2012, 29, 425–435. [Google Scholar] [CrossRef]
- Mulej, M.; Hrast, A. International Organization for Standards ISO 26000. In The Palgrave Handbook of Global Sustainability; Springer: Berlin/Heidelberg, Germany, 2023; Volume 2–3, pp. 1279–1301. [Google Scholar]
- dos Santos Ferreira, C.; Poltronieri, C.F.; Gerolamo, M.C. ISO 14001:2015 and ISO 9001:2015: Analyse the Relationship between These Management Systems Standards and Corporate Sustainability. Gest. Prod. 2019, 26, e3906. [Google Scholar] [CrossRef]
- Minkov, N.; Bach, V.; Finkbeiner, M. Characterization of the Cradle to Cradle CertifiedTM Products Program in the Context of Eco-Labels and Environmental Declarations. Sustainability 2018, 10, 738. [Google Scholar] [CrossRef]
- Del Vitto, A.; Marazzina, D.; Stocco, D. ESG Ratings Explainability through Machine Learning Techniques. Ann. Oper. Res. 2023, 208, 1–30. [Google Scholar] [CrossRef]
- Bozza, A.; Campi, C.; Garelli, S.; Ugazio, E.; Battaglia, L. Current Regulatory and Market Frameworks in Green Cosmetics: The Role of Certification. Sustain. Chem. Pharm. 2022, 30, 100851. [Google Scholar] [CrossRef]
- İstanbulluoğlu, S.; Timur, S.S.; Gürsoy, R.N. Biotechnological Active Ingredients and Excipients Used in Cosmetics. Hacet. Univ. J. Fac. Pharm. 2019, 39, 98–112. [Google Scholar]
- Sarang-Sieminski, A.L.; Chachra, D. Developing a Small-Footprint Bioengineering Program. In Proceedings of the ASEE Annual Conference & Exposition, San Antonio, TX, USA, 10–13 June 2012. [Google Scholar]
- Bezerra, K.G.O.; Rufino, R.D.; Luna, J.M.; Sarubbo, L.A. Saponins and Microbial Biosurfactants: Potential Raw Materials for the Formulation of Cosmetics. Biotechnol. Progress 2018, 34, 1482–1493. [Google Scholar] [CrossRef]
- Costa, J.P.; Custódio, L.; Reis, C.P. Exploring the Potential of Using Marine-Derived Ingredients: From the Extraction to Cutting-Edge Cosmetics. Mar. Drugs 2023, 21, 620. [Google Scholar] [CrossRef]
- Morocho-Jácome, A.L.; Ruscinc, N.; Martinez, R.M.; de Carvalho, J.C.M.; Santos de Almeida, T.; Rosado, C.; Costa, J.G.; Velasco, M.V.R.; Baby, A.R. (Bio)Technological Aspects of Microalgae Pigments for Cosmetics. Appl. Microbiol. Biotechnol. 2020, 104, 9513–9522. [Google Scholar] [CrossRef]
- Morganti, P. Use and Potential of Nanotechnology in Cosmetic Dermatology. Clin. Cosmet. Investig. Dermatol. 2010, 3, 5–13. [Google Scholar] [CrossRef]
- Aziz, Z.A.A.; Peng, W.L.; Nasir, H.M.; Asmak, N.; Setapar, S.H.M.; Ahmad, A. Survey of Nanotechnology in Beauty Products Development. In Nanotechnology for the Preparation of Cosmetics Using Plant-Based Extracts; Elsevier: Amsterdam, The Netherlands, 2022; pp. 13–25. [Google Scholar]
- Kesharwani, P.; Dubey, S.K. Nanocosmetics: Delivery Approaches, Applications and Regulatory Aspects; CRC Press: Boca Raton, FL, USA, 2023; p. 408. [Google Scholar]
- Polshettiwar, S.; Chapadgaonkar, S.S.; Kannur, D.; Musale, V.; Kabra, S. Cosmeceuticals from the Sea. In Handbook of Research in Marine Pharmaceutics: Exploring Oceanic Microbial Diversity for Human Health and Wellness. Faiyazuddin, M., Hakeem, K.R., Eds.; Apple Academic Press: Palm Bay, FL, USA, 2025; pp. 435–446. ISBN 978-1-040-16456-3. [Google Scholar]
- Kousar, S.S.; Hasse, R.; Mukherjee, A.; Mukunthan, K.S. Biomaterials at the Forefront: A Comprehensive Review of Patented Biomedical Applications. J. Appl. Pharm. Sci. 2025, 15, 21–31. [Google Scholar] [CrossRef]
- Callewaert, C.; Knödlseder, N.; Karoglan, A.; Güell, M.; Paetzold, B. Skin Microbiome Transplantation and Manipulation: Current State of the Art. Comput. Struct. Biotechnol. J. 2021, 19, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Paetzold, B.; Willis, J.R.; Pereira De Lima, J.; Knödlseder, N.; Brüggemann, H.; Quist, S.R.; Gabaldón, T.; Güell, M. Skin Microbiome Modulation Induced by Probiotic Solutions. Microbiome 2019, 7, 95. [Google Scholar] [CrossRef] [PubMed]
- Perin, B.; Addetia, A.; Qin, X. Transfer of Skin Microbiota between Two Dissimilar Autologous Microenvironments: A Pilot Study. PLoS ONE 2019, 14, e0226857. [Google Scholar] [CrossRef] [PubMed]
- Taglialegna, A. Cutibacterium Improves Skin Condition. Nat. Rev. Microbiol. 2024, 22, 119. [Google Scholar] [CrossRef]
- Dodds, D.; Bose, J.L.; Deng, M.-D.; Dubé, G.R.; Grossman, T.H.; Kaiser, A.; Kulkarni, K.; Leger, R.; Mootien-Boyd, S.; Munivar, A.; et al. Controlling the Growth of the Skin Commensal Staphylococcus epidermidis Using D-Alanine Auxotrophy. mSphere 2020, 5, 13. [Google Scholar] [CrossRef]
- Taylor, M.; Liu, X.; Denniston, A.; Esteva, A.; Ko, J.; Daneshjou, R.; Chan, A.-W. Raising the Bar for Randomized Trials Involving Artificial Intelligence: The SPIRIT-Artificial Intelligence and CONSORT-Artificial Intelligence Guidelines. J. Investig. Dermatol. 2021, 141, 2109–2111. [Google Scholar] [CrossRef]

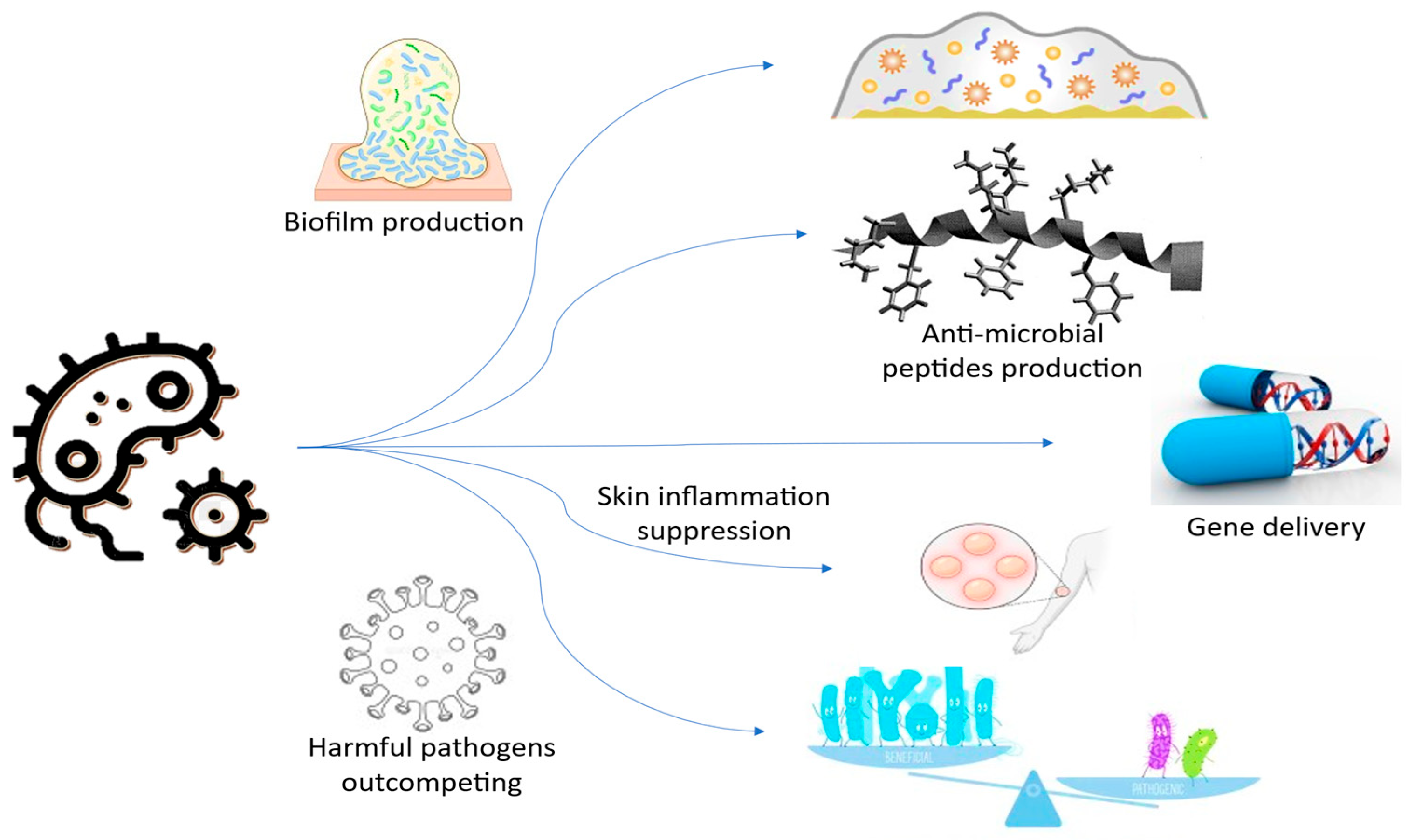
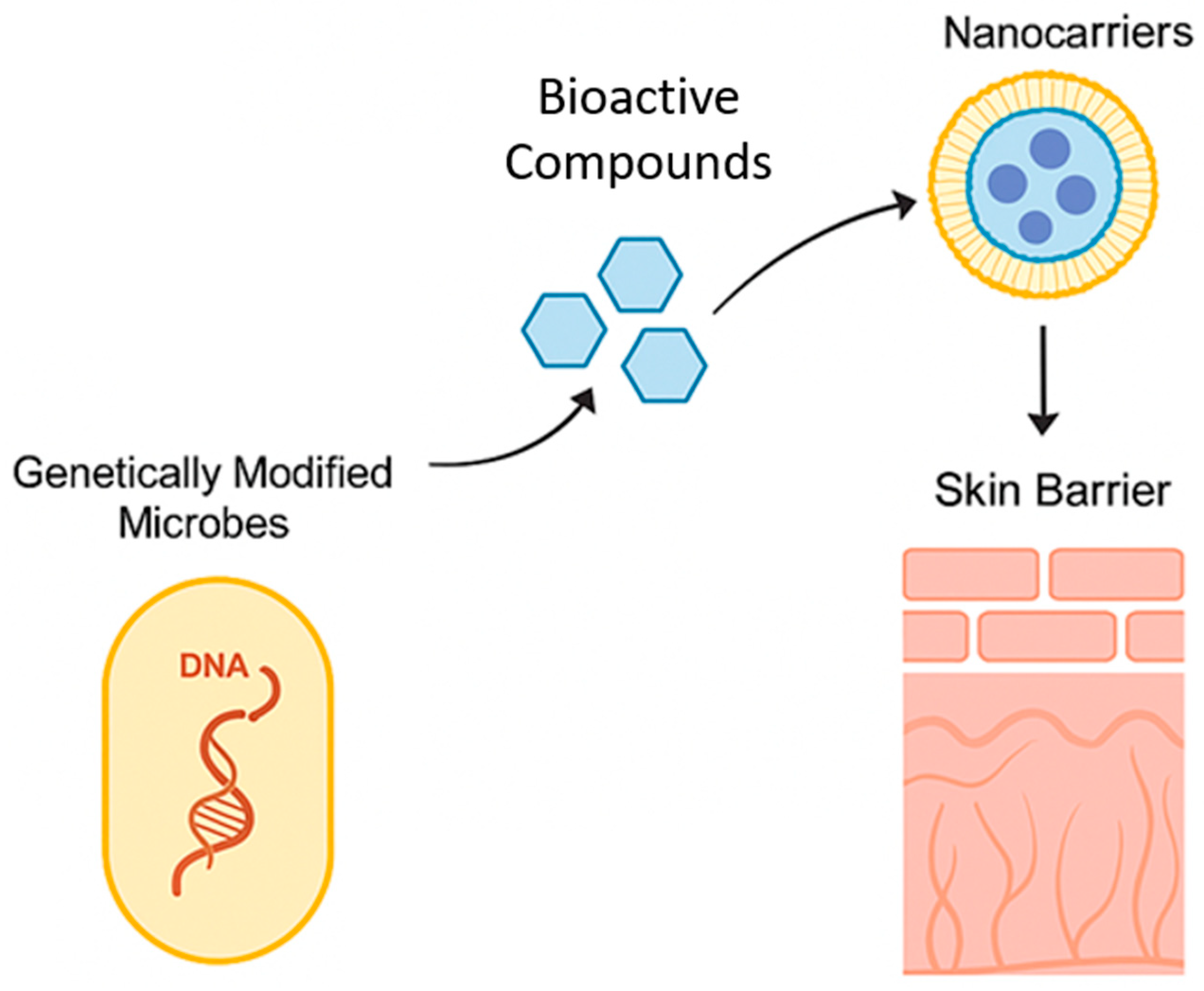
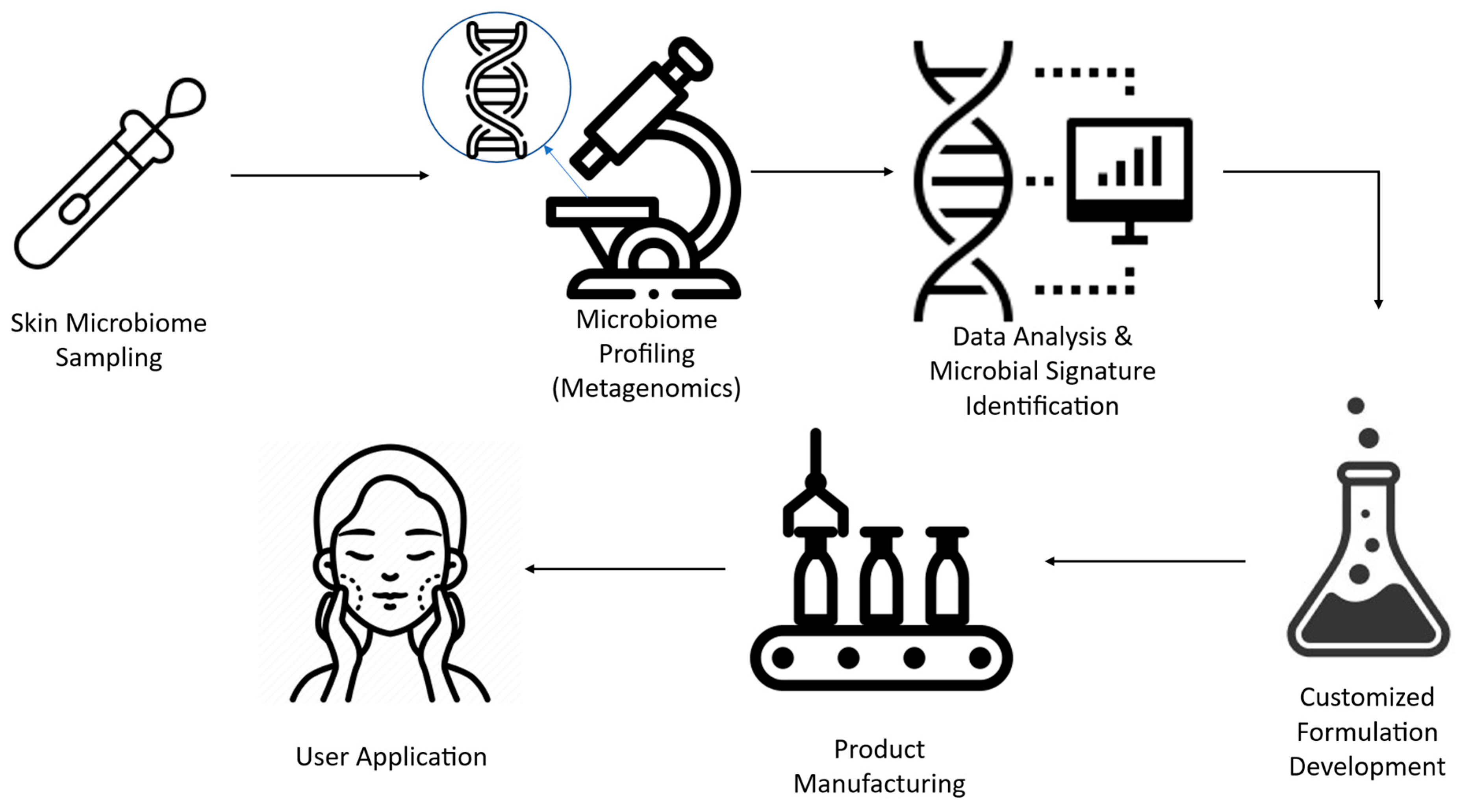
| Condition | Therapeutic Target | Key Information | Efficiency | References |
|---|---|---|---|---|
| Acne | Cutibacterium acnes (C. acnes). |
| Colonization rate: 96% of patients | [52,53] |
| Eczema | Staphylococcus aureus (S. aureus). |
| Lesional Skin: 70.2% colonized, with 47.3% being S. aureus. Non-lesional Skin: 32.7% colonized, with 27.9% being S. aureus | [54,55] |
| Aging | General microbial balance (Usage of probiotics and prebiotics). |
| Bifidobacteria increase was observed during synbiotic supplementation and Reduction in Inflammatory Markers: Enhanced IL-10 production and decreased TNF-α. | [56,57] |
| Category | Description | Examples | Composition | Effects on Microbiome | Efficiency | References |
|---|---|---|---|---|---|---|
| Probiotics | Beneficial bacteria used in cosmetics to promote healthy skin microbiome. | Micrococcus luteus Q24. | Lactobacillus and Bifidobacterium. | Hydrates the skin, reduces pores, wrinkles, spots and impurities. | 45–80% reduction in skin impurities and 83.1 ± 6.1% encapsulation efficiency. | [61] |
| Prebiotics | Non-digestible ingredients that support the growth of beneficial bacteria on the skin to enhance its natural defenses. | Prebiotic formulations such as serums and face creams. | Inulin, fructo-oligosaccharides, and other oligosaccharides. | Strengthens skin’s proteome, and stimulates beneficial microbiota. | Reduces the severity of atopic dermatitis (AD) and xerosis, improves skin barrier properties, and provides itch relief within 4 weeks. | [10] |
| Natural Antimicrobial Agents | Ingredients derived from natural sources that control harmful bacteria without altering beneficial microbiota. | Plant extracts, essential oils, phenolic compounds, alkaloids, and polyphenols. | Tea tree oil, neem oil, and other plant-derived antimicrobial compounds such as antibiotics, antifungals, antivirals and antiprotozoals. | Helps maintain microbiome balance and provides antimicrobial benefits. | 35% reduction in burn wound infection. | [10,66] |
| Bio-surfactants | Eco-friendly alternatives to synthetic surfactants, naturally produced by bacteria such as Pseudomonas aeruginosa and Bacillus species. | Bacterial bio-surfactants. | Sophorolipids, rhamnolipids and lipopeptides. | Biodegradable, non-toxic, and compatible with skin microbiome. | Not specifically mentioned. | [67,68] |
| Fermented ingredients | Ingredients obtained through fermentation processes; often contain beneficial microbial metabolites. | Ferments of rice and soy. Fermented oils such as F-Shiunko, F-Artemisia and F-Glycyrrhiza. | Fermented rice extract, fermented soy extract, and other fermented botanicals which includes plant-derived products. | Have varying effects on skin microbiota with properties like skin moisturizing, antioxidant, and skin-whitening. | Improved skin condition and gut microbiota. | [69] |
| Microbial Polysaccharide Gums | Polysaccharides produced by microbes, used for their functional properties in cosmetics. | Various microbial polysaccharide gums. | Xanthan gum, gellan gum, and other polysaccharides derived from microbial fermentation. | Have functions like emulsion stabilization and skin conditioning which provides safe use in cosmetics. | Not specifically mentioned. | [70] |
| Bioengineering Technique | Cosmetic Application | Description | References |
|---|---|---|---|
| Recombinant DNA Technology | Production of bioactive molecules such as vitamins and oils, peptides and proteins, amino acids like serine, alanine and threonine, and biotechnologically derived compounds. | Used for large-scale production of ingredients like epidermal growth factor, botulinum toxin, collagen, ceramide, and kojic acid. These ingredients are used for skin and hair care, replacing harmful synthetic compounds. | [124] |
| Cell Culture Techniques | Creation of cosmetic ingredients like plant cell culture-derived ingredients and microbial and fermentation-derived ingredients. | Ingredients are created in sterile fermentation reactors, avoiding the need for growing, harvesting, and extracting. This leads to safer and more effective products. | [125] |
| Biorefinery and Bioconversion | Purification of bioactive molecules. | Enables the addition of pure phytochemicals in cosmetic formulations, eliminating the need for crude plant extracts and reducing side effects. | [124] |
| Synthetic Biology | Development of cosmetic ingredients such as hyaluronic acid, kojic acid, collagen, resveratrol, peptides, ceramides, Epidermal Growth Factor (EGF), Botulinum Toxin and polyphenols and terpenes. | Focuses on creating effective, safe, and environmentally friendly ingredients. | |
| Phage Display | Skin infection and acne treatment. | Bacteriophages are used as nanomaterials, delivery vectors, and growth factor alternatives. | |
| Engineered Skin Tissue | Cosmetic testing and toxicological evaluation. | Used for in vitro testing of cosmetic products, providing a scientific method for safety assessment. | [126] |
| Algal Biotechnology | Production of active ingredients, such as brown macroalgae, green macroalgae and red macroalgae. | Extracts from algae are used for anti-aging, moisturizing, whitening, UV protection and anti-cellulite care. | [127] |
| Bioengineering of Volatile Carboxylic Acids (VCAs) | Ingredient production. | VCAs are used in various cosmetic formulations, with advanced techniques for their efficient separation and collection. | [128] |
| Freeze-dried Aloe Vera Extract | Skin hydration. | Used in moisturizing formulations to improve skin hydration. | [129] |
| Technique/Method | Description | Applications/ Examples | Key Points | References |
|---|---|---|---|---|
| High-Throughput Sequencing | Rapid sequencing of DNA/RNA to study microbial communities. | Analyzing prokaryotes in various niches. | Improved quality, speed, and cost. | [176] |
| 16S rRNA Sequencing | Sequencing of the 16S ribosomal RNA gene to identify bacteria. | Taxonomic identification of microbiomes related to food. | Helps in understanding taxonomic composition. | [177] |
| Metagenomics | Sequencing of DNA from environmental samples to study microbial communities. | Studying human microbiome. | Offers species-level characterization. | [178] |
| Meta-transcriptomics | Sequencing of RNA to study gene expression in microbial communities. | Functional characterization of microbial interactions. | Provides insights into microbial functions. | [177] |
| Synthetic Biology | Engineering microbial communities for specific purposes. | Enhancing food safety and quality. | Food production processes revolution. | [179] |
| Artificial Intelligence (AI) | Analysing and interpreting complex microbiome data. | Personalized nutrition programs. | Dietary recommendations and health improvements. | [180] |
| Machine Learning (ML) | Applying algorithms to predict and analyse microbiome data. | Predicting clinical outcomes in obesity and cancer. | Identifying biomarkers and developing personalized therapies. | [181] |
| PCR (Polymerase Chain Reaction) | Amplifying DNA sequences to identify specific microbes. | Identification of bacteria, fungi, algae, and actinomycetes. | Isolating and characterizing microbial species. | [182] |
| Bioinformatics Tools | Software and algorithms to analyse sequencing data. | Analysing amplicon sequences, metagenome shotgun sequences. | Essential for processing and interpreting large datasets. | [176] |
| Microbial Culture | Growing microbes in controlled environments to study their properties. | Measuring functional capacity of individual microbes. | Provides detailed study of microbial traits and interactions. | [183] |
| Metabolomics | Study of metabolites produced by microbial communities. | Identifying gut microbiota’s role in health and disease. | Offers insights into metabolic interactions and processes. | [184] |
| Proteomics | Study of proteins expressed by microbial communities. | Identifying functional proteins in microbiomes. | Understanding protein functions and interactions. | [183] |
| AI-Driven Biomarker Discovery | Identifying key microbial markers for health and disease by using AI. | Identifying biomarkers for treatments and diagnostics. | Accelerates the discovery of important microbial indicators. | [185] |
| Next Generation Sequencing (NGS) | Advanced sequencing technologies for detailed microbial analysis. | Investigating microbiomes of fermented and non-fermented foods. | Deep understanding of microbial communities. | [177] |
| Deep Learning (DL) | Advanced ML techniques for analysing complex microbiome data. | Predicting microbial interactions and health outcomes. | Uncovers complex patterns within microbiome data. | [184] |
| AI in Microbial Diagnostics | Using AI to enhance the accuracy and speed of microbial diagnostics. | Rapid diagnosis of microbial infections. | Improves workflow speed and accuracy in clinical settings. | [186] |
| Microbiome Engineering | Modifying microbial communities to improve food quality and safety. | Extending shelf life and enhancing food production. | Requires robust public engagement and standardized frameworks. | [179] |
| AI in Precision Nutrition | Tailoring dietary recommendations based on individual microbiome data. | Managing conditions like obesity and diabetes. | Potential in improving personalized dietary recommendations. | [187] |
| Microbial Interaction Networks | Discovering the relationships between different microbial species. | Understanding ecological dynamics and biotechnological applications. | Constructing and deciphering complex microbial networks. | [188] |
| Region | Regulatory Framework | Key Requirements | Specifics for Bioengineered Cosmetics | References |
|---|---|---|---|---|
| European Union (EU) | Cosmetics Products Regulation (EC) No 1223/2009. | Safety assessment, ban on animal testing, responsible person for legal accountability. | Strict safety and efficacy, emphasis on consumer adverse effect reporting. | [216,243] |
| United States (US) | Federal Food, Drug, and Cosmetic Act (FDCA). | Classification of products as food, drugs or cosmetics, and status for ingredients. | Rigorous scrutiny for drugs compared to cosmetics and duplicative regulatory efforts. | [217,244] |
| Asia | Depends on the country (like Japan, India, China). | Japan has strict regulations similar to the EU and the US while India is regulated under the Drug and Cosmetic Act, 1940. | Japan has high safety standards while China and India have an increasing regulatory alignment with global standards. | [236,238,245] |
| MENA Region | Depends on the country. | Saudi Arabia follows guidelines similar to international standards. | Regulatory frameworks are evolving. | [238] |
| Aspect | Natural/Bioengineered Ingredients | Synthetic Ingredients | References |
|---|---|---|---|
| Source | Derived from plants, animals and microorganisms. | Chemically synthesized in laboratories. | [291,292,293] |
| Environmental Impact | Biodegradable and eco-friendly. | Could generate environmental concerns. | [294,295] |
| Safety | May contain less contaminants but generally it is safe. | Can cause irritation due to parabens but is regulated for safety. | [296,297] |
| Efficacy | Sustained effects with benefits like antioxidants and anti-inflammatory properties. | Higher potency but may cause adverse effects. | [298,299] |
| Regulation | The use of preservatives makes it less restricted. | Strictly regulated to ensure consumer safety. | [206] |
| Examples of Ingredients | Essential oils, plant extracts, natural antioxidants (polyphenols, carotenoids, tannins). | Parabens (methylparaben), synthetic emulsifiers (polyglycerol fatty acid esters), artificial fragrances (linalool). | [297,300] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atallah, C.; El Abiad, A.; El Abiad, M.; Nakad, M.; Assaf, J.C. Bioengineered Skin Microbiome: The Next Frontier in Personalized Cosmetics. Cosmetics 2025, 12, 205. https://doi.org/10.3390/cosmetics12050205
Atallah C, El Abiad A, El Abiad M, Nakad M, Assaf JC. Bioengineered Skin Microbiome: The Next Frontier in Personalized Cosmetics. Cosmetics. 2025; 12(5):205. https://doi.org/10.3390/cosmetics12050205
Chicago/Turabian StyleAtallah, Cherelle, Ayline El Abiad, Marita El Abiad, Mantoura Nakad, and Jean Claude Assaf. 2025. "Bioengineered Skin Microbiome: The Next Frontier in Personalized Cosmetics" Cosmetics 12, no. 5: 205. https://doi.org/10.3390/cosmetics12050205
APA StyleAtallah, C., El Abiad, A., El Abiad, M., Nakad, M., & Assaf, J. C. (2025). Bioengineered Skin Microbiome: The Next Frontier in Personalized Cosmetics. Cosmetics, 12(5), 205. https://doi.org/10.3390/cosmetics12050205







