From Lasers to Longevity: Exploring Energy-Based Devices as Senotherapeutic Tools in Dermatology
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Selection Criteria
2.2. Data Extraction and Thematic Analysis
3. Results
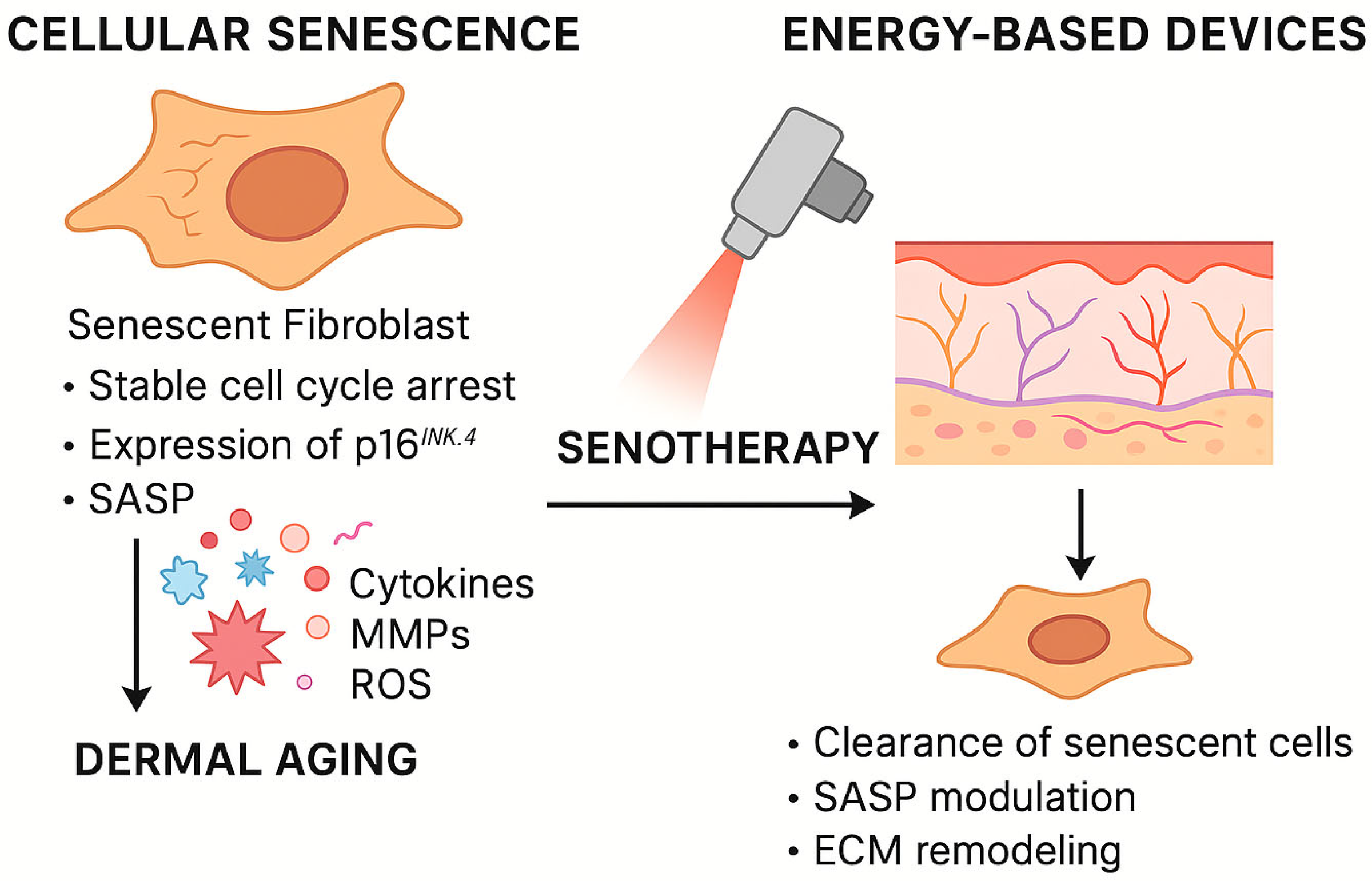
3.1. Skin Senescence: Molecular and Clinical Landscape
3.1.1. Mechanisms of Cellular Senescence in the Skin
3.1.2. Clinical Consequences of Dermal Senescence
3.2. Energy-Based Devices in Dermatology
3.2.1. Classification and Mechanisms of Action
- Non-ablative fractional lasers (e.g., 1550 nm Er: Glass and 1927 nm Thulium): create dermal microthermal zones (MTZs) while sparing the epidermis, enabling controlled wound healing with minimal downtime [51];
- Pulsed dye lasers (PDL) and Nd:YAG lasers: primarily for vascular lesions but also capable of dermal remodeling [52];
- High-intensity focused ultrasound (HIFU): delivers ultrasound energy to the SMAS and deep dermis, inducing collagen denaturation and neocollagenesis [56].
3.2.2. Biological Effects of EBD Treatment Relevant to Senescence
3.2.3. Safety, Precision, and Adaptability
3.2.4. Senotherapeutic Effects of EBD Treatments
In Vitro and Ex Vivo Studies
Animal and Clinical Studies
Limitations of Current Evidence
- Lack of standardized biomarkers: Many studies rely on collagen quantification or wrinkle scoring rather than direct senescence markers [86].
- Short follow-up periods: Persistence of anti-senescent effects remains uncertain [87].
- Limited human biopsy data: More clinical trials with serial biopsies are needed, particularly in older populations [88].
3.3. Comparison with Pharmacologic Senotherapeutics
3.3.1. Pharmacologic Senotherapeutics: Mechanisms and Limitations
3.3.2. EBDs as Localized Senotherapeutic Agents
3.3.3. Synergistic Potential
4. Discussion
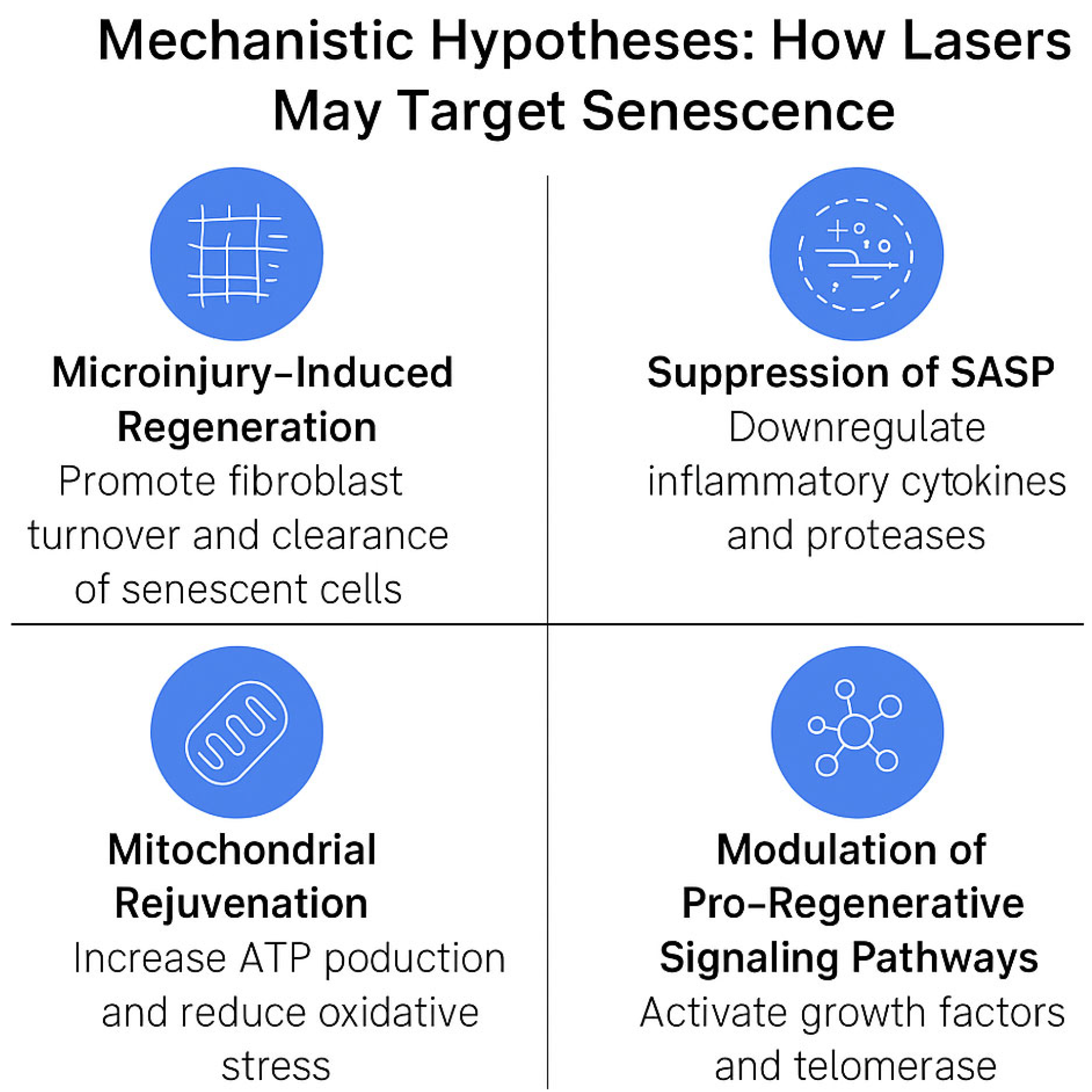
4.1. Mechanistic Hypotheses: How Lasers May Target Senescence
4.2. Future Directions and Clinical Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EBDs | Energy-based devices |
| SASP | Senescence-associated secretory phenotype |
| MMPs | Matrix metalloproteinases |
| ECM | Extracellular matrix |
| IPL | Intense pulsed light |
| RF | Radiofrequency |
| HIFU | High-intensity focused ultrasound |
| UV | Ultraviolet |
| MTZs | Dermal microthermal zones |
| LLLT | Low-level light therapy |
| ROS | Reactive oxygen species |
| EM | Electromagnetic radiation |
References
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Wyles, S.P.; Carruthers, J.D.; Dashti, P.; Yu, G.; Yap, J.Q.; Gingery, A.; Tchkonia, T.; Kirkland, J. Cellular senescence in human skin aging: Leveraging senotherapeutics. Gerontology 2024, 70, 7–14. [Google Scholar] [CrossRef]
- Campisi, J. Aging, cellular senescence, and cancer. Annu. Rev. Physiol. 2013, 75, 685–705. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.W.; Kwon, S.H.; Choi, J.Y.; Na, J.I.; Huh, C.H.; Choi, H.R.; Park, K.-C. Molecular mechanisms of dermal aging and antiaging approaches. Int. J. Mol. Sci. 2019, 20, 2126. [Google Scholar] [CrossRef]
- Park, J.; Shin, D.W. Senotherapeutics and Their Molecular Mechanism for Improving Aging. Biomol. Ther. 2022, 30, 490–500. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chin, T.; Lee, X.E.; Ng, P.Y.; Lee, Y.; Dreesen, O. The role of cellular senescence in skin aging and age-related skin pathologies. Front. Physiol. 2023, 14, 1297637. [Google Scholar] [CrossRef]
- Ajoolabady, A.; Pratico, D.; Bahijri, S.; Eldakhakhny, B.; Tuomilehto, J.; Wu, F.; Ren, J. Hallmarks and mechanisms of cellular senescence in aging and disease. Cell Death Discov. 2025, 11, 364. [Google Scholar] [CrossRef]
- Kirkland, J.L.; Tchkonia, T. Senolytic drugs: From discovery to translation. J. Intern. Med. 2020, 288, 518–536. [Google Scholar] [CrossRef]
- Kim, J.; Lee, S.G.; Boo, J.; Kim, H.; Hwang, S.; Liu, C.; Yan, X.; Brieva, P.; Kim, J. Fractional microneedle radiofrequency with the application of vitamin C, E, and ferulic acid serum for neck skin rejuvenation: A prospective, double-blinded, split-neck, placebo-controlled trial. J. Dermatol. Treat. 2025, 36, 2504655. [Google Scholar] [CrossRef]
- Hwang, J.M.; Lee, S.H.; Baek, E.J.; Kim, H.C.; Oh, J.H.; Lee, J.S.; Lee, S.H. Comparison of the effects of fractional microneedle radiofrequency and microneedling on modulating the senescent fibroblast milieu in aged skin. Sci. Rep. 2025, 15, 18296. [Google Scholar] [CrossRef]
- Waibel, J.S.; Schallen, K.P. Non-ablative fractional 1940-nm diode laser for skin resurfacing and treatment of benign pigmented lesions: A randomized controlled trial. Lasers Surg. Med. 2025, 57, 63–70. [Google Scholar] [CrossRef]
- Wang, Y.J.; Chang, C.C.; Wu, Y.H.; Huang, L.; Chen, I.L.; Shih, Y.C.; Cheng, H.; Shen, J.W.; Lu, M.E.; Chiang, H.M.; et al. Activated melanocytes and senescent collagen fibers predict laser-treated melasma outcomes: An optical biopsy–based prospective cohort study. Photodiagn. Photodyn. Ther. 2025, 54, 104648. [Google Scholar] [CrossRef]
- Hong, J.Y.; Park, K.Y. Pilot study on the efficacy of a dual-length microneedle radiofrequency device with microblade design for neck rejuvenation. Dermatol. Ther. 2025, 2025, 8855222. [Google Scholar] [CrossRef]
- Chen, C.; Ke, Y. Picosecond alexandrite laser with diffractive lens array combined with long-pulse alexandrite laser for the treatment of facial photoaging in Chinese women: A retrospective study. Skin Res. Technol. 2024, 30, e70091. [Google Scholar] [CrossRef] [PubMed]
- Piccolo, D.; Cannarozzo, G.; Segreto, F.; Fusco, I.; Sannino, M.; Nisticò, S.P. A faster CO2 fractional scanner system mode for skin rejuvenation: A clinical study. Skin Res. Technol. 2024, 30, e13383. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.H.; Wang, Y.M.; Wang, C.Y.; Chiou, C.S.; Tsai, C.Y.; Tsai, K.J. Organic light-emitting diode therapy promotes longevity through the upregulation of SIRT1 in senescence-accelerated mouse prone 8 mice. J. Photochem. Photobiol. 2024, 257, 112957. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; He, Y.; Shu, X.; Ma, X.; Wu, J.; Du, Z.; Xu, J.; Chen, N.; You, J.; Liu, Y.; et al. Photomodulation alleviates cellular senescence of aging adipose-derived stem cells. Cell Commun. Signal. 2023, 21, 95. [Google Scholar] [CrossRef]
- Jia, C.; Gong, C.; Lu, Y.; Xu, N. Low-energy green light alleviates senescence-like phenotypes in a cell model of photoaging. J. Cosmet. Dermatol. 2023, 22, 410–418. [Google Scholar] [CrossRef]
- Couturaud, V.; Fouéré, S.; Boucaud, A.; Bernerd, F. Reverse skin aging signs by red-light photobiomodulation. Skin Res. Technol. 2023, 29, e13391. [Google Scholar] [CrossRef]
- Oh, S.; Rhee, D.Y.; Batsukh, S.; Son, K.H.; Byun, K. High-Intensity Focused Ultrasound Increases Collagen and Elastin Fiber Synthesis by Modulating Caveolin-1 in Aging Skin. Cells 2023, 12, 2275. [Google Scholar] [CrossRef]
- Ventura, N.; Weinstein Velez, M.C. Non-ablative lasers offer a gentle approach to healthy skin. Dermatol. Times 2023, 44, 11. [Google Scholar]
- Zhang, M.; Huang, Y.; Wu, Q.; Lin, T.; Gong, X.; Chen, H.; Wang, Y. Comparison of 1064-nm and Dual-Wavelength (532/1064-nm) Picosecond-Domain Nd:YAG Lasers in the Treatment of Facial Photoaging: A Randomized Controlled Split-Face Study. Lasers Surg. Med. 2021, 53, 1158–1165. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.I.; Kim, E.; Lee, D.W.; Kim, J.; Kim, J.; Lee, W.J.; Lee, J.H. Synergistic Effect of 300 μm Needle-Depth Fractional Microneedling Radiofrequency on the Treatment of Senescence-Induced Aging Hyperpigmentation of the Skin. Int. J. Mol. Sci. 2021, 22, 7480. [Google Scholar] [CrossRef] [PubMed]
- Robati, R.M.; Abdollahimajd, F.; Shahidi-Dadras, M.; Yeganeh, O.; Naraghi, Z.S. Efficacy of microneedling versus fractional Er:YAG laser in facial rejuvenation. J. Cosmet. Dermatol. 2020, 19, 2956–2963. [Google Scholar] [CrossRef]
- Borges, J.; Araújo, L.; Cuzzi, T.; Martinez, L.; Gonzales, Y.; Manela-Azulay, M. Fractional laser resurfacing treats photoaging by promoting neocollagenesis and cutaneous edema. J. Clin. Aesthet. Dermatol. 2020, 13, 22–27. [Google Scholar]
- Wang, H.; Guo, B.; Hui, Q.; Lin, F.; Tao, K. CO2 lattice laser reverses skin aging caused by UVB. Aging 2020, 12, 7056–7065. [Google Scholar] [CrossRef]
- Jipu, R.; Serban, I.L.; Goriuc, A.; Jipu, A.G.; Luchian, I.; Amititeloaie, C.; Tarniceriu, C.C.; Hurjui, I.; Butnaru, O.M.; Hurjui, L.L. Targeting dermal fibroblast senescence: From cellular plasticity to anti-aging therapies. Biomedicines 2025, 13, 1927. [Google Scholar] [CrossRef]
- Victorelli, S.; Passos, J.F. Telomeres and cell senescence: Size matters not. eBioMedicine 2017, 21, 14–20. [Google Scholar] [CrossRef]
- Saretzki, G. Telomeres, telomerase and ageing. In Biochemistry and Cell Biology of Ageing: Part I Biomedical Science; Springer: Singapore, 2019; pp. 221–308. [Google Scholar]
- Zhu, T.Y.; Hu, P.; Mi, Y.H.; Zhang, J.L.; Xu, A.N.; Gao, M.T.; Zhang, Y.Y.; Shen, S.B.; Yang, G.M.; Pan, Y. Telomerase reverse transcriptase gene knock-in unleashes enhanced longevity and accelerated damage repair in mice. Aging Cell 2025, 24, e14445. [Google Scholar] [CrossRef]
- Wang, A.S.; Dreesen, O. Biomarkers of cellular senescence and skin aging. Front. Genet. 2018, 9, 247. [Google Scholar] [CrossRef]
- Birch, J.; Gil, J. Senescence and the SASP: Many therapeutic avenues. Genes Dev. 2020, 34, 1565–1576. [Google Scholar] [CrossRef]
- Wang, B.; Kohli, J.; Demaria, M. Senescent cells in cancer therapy: Friends or foes? Trends Cancer 2020, 6, 838–857. [Google Scholar] [CrossRef]
- Gorgoulis, V.; Adams, P.D.; Alimonti, A.; Bennett, D.C.; Bischof, O.; Bishop, C.; Campisi, J.; Collado, M.; Evangelou, K.; Ferbeyre, G.; et al. Cellular senescence: Defining a path forward. Cell 2019, 179, 813–827. [Google Scholar] [CrossRef]
- Kirkland, J.L.; Tchkonia, T. Cellular senescence: A translational perspective. eBioMedicine 2017, 21, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Freund, A.; Orjalo, A.V.; Desprez, P.Y.; Campisi, J. Inflammatory networks during cellular senescence: Causes and consequences. Trends Mol. Med. 2010, 16, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Victorelli, S.; Passos, J.F. Mechanisms of telomere shortening. Biochem. Soc. Trans. 2017, 45, 197–205. [Google Scholar]
- Bernardes de Jesus, B.; Blasco, M.A. Telomerase at the intersection of cancer and aging. Trends Genet. 2013, 29, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Quan, T.; Fisher, G.J. Role of age-associated alterations of the dermal extracellular matrix microenvironment in human skin aging: A mini-review. Gerontology 2015, 61, 427–434. [Google Scholar] [CrossRef]
- Acosta, J.C.; Banito, A.; Wuestefeld, T.; Georgilis, A.; Janich, P.; Morton, J.P.; Athineos, D.; Kang, T.-W.; Lasitschka, F.; Andrulis, M.; et al. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat. Cell Biol. 2013, 15, 978–990. [Google Scholar] [CrossRef]
- Nelson, G.; Wordsworth, J.; Wang, C.; Jurk, D.; Lawless, C.; Martin-Ruiz, C.; von Zglinicki, T. A senescent cell bystander effect: Senescence-induced senescence. Aging Cell 2012, 11, 345–349. [Google Scholar] [CrossRef]
- Waaijer, M.E.; Parish, W.E.; Strongitharm, B.H.; van Heemst, D.; Slagboom, P.E.; de Craen, A.J.; Sedivy, J.M.; Westendorp, R.G.J.; Gunn, D.A.; Maier, A.B. The number of p16INK4a positive cells in human skin reflects biological age. Aging Cell 2012, 11, 722–725. [Google Scholar] [CrossRef]
- Baker, D.J.; Wijshake, T.; Tchkonia, T.; LeBrasseur, N.K.; Childs, B.G.; van de Sluis, B.; Kirkland, J.L.; Van Deursen, J.M. Clearance of p16INK4a-positive senescent cells delays ageing-associated disorders. Nature 2011, 479, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Waibel, J.S.; Wulkan, A.J.; Shumaker, P.R. Treatment of hypertrophic scars using laser and laser-assisted corticosteroid delivery. Lasers Surg. Med. 2013, 45, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Majid, I.; Imran, S. Efficacy and safety of fractional CO2 laser resurfacing in non-hypertrophic traumatic and burn scars. J. Cutan. Aesthetic Surg. 2015, 8, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Wat, H.; Wu, D.C.; Chan, H.H. Fractional resurfacing in the Asian patient: Current state of the art. Lasers Surg. Med. 2017, 49, 45–59. [Google Scholar] [CrossRef]
- Avci, P.; Gupta, A.; Sadasivam, M.; Vecchio, D.; Pam, Z.; Pam, N.; Hamblin, M.R. Low-level laser (light) therapy in skin: Stimulating, healing, restoring. Semin. Cutan. Med. Surg. 2013, 32, 41–52. [Google Scholar]
- Barolet, D.; Boucher, A. Radiant near-infrared light emitting diode exposure as skin preparation to enhance the effect of photodynamic therapy in actinic keratosis. J. Cosmet. Laser Ther. 2010, 12, 236–240. [Google Scholar]
- Araújo, A.R.; Soares, V.P.C.; Silva, F.S.; Moreira, T.S. Radiofrequency for the treatment of skin laxity: Myth or truth. An. Bras. Dermatol. 2015, 90, 707–721. [Google Scholar] [CrossRef]
- Suh, D.H.; Shin, M.K.; Lee, S.J.; Rho, J.H.; Lee, M.H.; Kim, N.I.; Song, K.Y. Intense focused ultrasound tightening in Asian skin: Clinical and pathologic results. Dermatol. Surg. 2011, 37, 1595–1602. [Google Scholar] [CrossRef]
- Gold, M.H. Update on fractional laser technology. J. Clin. Aesthet. Dermatol. 2010, 3, 42–50. [Google Scholar]
- Tierney, E.P.; Hanke, C.W. Fractionated carbon dioxide laser treatment of photoaging: Prospective study in 45 patients and review of the literature. Dermatol. Surg. 2011, 37, 1279–1290. [Google Scholar] [CrossRef]
- Alexiades-Armenakas, M.R.; Dover, J.S.; Arndt, K.A. Fractional laser skin resurfacing. J. Drugs Dermatol. 2012, 11, 1274–1287. [Google Scholar] [PubMed]
- Ma, H.; Yang, J.P. Effect of low-level laser therapy on proliferation and collagen synthesis of human fibroblasts in vitro. J. Wound Manag. Res. 2018, 14, 1–6. [Google Scholar] [CrossRef]
- Miller-Kobisher, B.; Suárez-Vega, D.V.; Velazco de Maldonado, G.J. Epidermal growth factor in aesthetics and regenerative medicine: Systematic review. J. Cutan. Aesthet. Surg. 2021, 14, 137–146. [Google Scholar]
- Park, G.H.; Chang, S.E.; Bang, S.; Won, K.H.; Won, C.H.; Lee, M.W.; Choi, J.H.; Moon, K.C. Usefulness of skin explants for histologic analysis after fractional photothermolysis. Ann. Dermatol. 2015, 27, 283–290. [Google Scholar] [CrossRef]
- Hantash, B.M.; Bedi, V.P.; Kapadia, B.; Rahman, Z.; Jiang, K.; Tanner, H.; Chan, K.F.; Zachary, C.B. In vivo histological evaluation of a novel ablative fractional resurfacing device. Lasers Surg. Med. 2007, 39, 96–107. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, Y.J.; Cho, K.H. Fractional CO2 laser-induced collagen remodeling and reduction of senescence markers in photoaged skin. J. Cosmet. Laser Ther. 2015, 17, 159–165. [Google Scholar]
- Jung, K.E.; Jung, K.H.; Park, Y.M.; Lee, J.Y.; Kim, T.Y.; Kim, H.O.; Kim, H.S. A split-face comparison of ablative fractional lasers (CO2 and Er:YAG) in Asian patients: Postprocedure erythema, pain and patient satisfaction. J. Cosmet. Laser Ther. 2013, 15, 70–73. [Google Scholar] [CrossRef]
- Abijo, A.; Lee, C.Y.; Huang, C.Y.; Ho, P.C.; Tsai, K.J. The beneficial role of photobiomodulation in neurodegenerative diseases. Biomedicines 2023, 11, 1828. [Google Scholar] [CrossRef]
- Ortiz, A.E.; Goldman, M.P.; Fitzpatrick, R.E. Ablative CO2 lasers for skin tightening: Traditional versus fractional. Dermatol. Surg. 2014, 40 (Suppl. 12), S147–S151. [Google Scholar] [CrossRef]
- Hsiao, F.C.; Bock, G.N.; Eisen, D.B. Recent advances in fractional laser resurfacing: New paradigm in optimal parameters and post-treatment wound care. Adv. Wound Care 2012, 1, 207–212. [Google Scholar] [CrossRef]
- Borges, J.; Cuzzi, T.; Mandarim-de-Lacerda, C.A.; Manela-Azulay, M. Fractional erbium laser in the treatment of photoaging: Randomized comparative, clinical and histopathological study of ablative (2940 nm) vs. non-ablative (1540 nm) methods after 3 months. An. Bras. Dermatol. 2014, 89, 764–771. [Google Scholar] [CrossRef] [PubMed]
- Weiss, R.A.; McDaniel, D.H.; Geronemus, R.G.; Weiss, M.A.; Beasley, K.L.; Munavalli, G. Clinical trial of a 675 nm laser for skin rejuvenation: Mitochondrial and dermal effects. Lasers Surg. Med. 2019, 51, 686–693. [Google Scholar]
- Chung, H.; Dai, T.; Sharma, S.K.; Huang, Y.Y.; Carroll, J.D.; Hamblin, M.R. The nuts and bolts of low-level laser therapy. Ann. Biomed. Eng. 2012, 40, 516–533. [Google Scholar] [CrossRef]
- Hamblin, M.R. Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS Biophys. 2017, 4, 337–361. [Google Scholar] [CrossRef]
- Rossi, F.; Magni, G.; Tatini, F.; Banchelli, M.; Cherchi, F.; Rossi, M.; Coppi, E.; Pugliese, A.M.; Degl’innocenti, D.R.; Alfieri, D.; et al. Photobiomodulation of human fibroblasts and keratinocytes with blue light: Implications in wound healing. Biomedicines 2021, 9, 41. [Google Scholar] [CrossRef]
- Hsiao, C.Y.; Huang, C.H.; Hu, S.; Ko, Y.S.; Sung, H.C.; Chen, C.C.; Huang, S.Y. Fractional carbon dioxide laser treatment to enhance skin permeation of ascorbic acid 2-glucoside with minimal skin disruption. Dermatol. Surg. 2012, 38, 1284–1293. [Google Scholar] [CrossRef]
- El-Domyati, M.; Moawad, O.; Abdel-Wahab, H.; Behairy, E.F.; Rezk, A.F. A new approach with combined microneedle and sublative fractional radiofrequency for photoaging management: A clinical, histometric, and immunohistochemical study. Aesthetic Plast. Surg. 2025, 49, 1435–1443. [Google Scholar] [CrossRef]
- Manuskiatti, W.; Iamphonrat, T.; Wanitphakdeedecha, R.; Eimpunth, S. Comparison of fractional erbium-doped yttrium aluminum garnet and carbon dioxide lasers in resurfacing of atrophic acne scars in Asians: A randomized controlled trial. Dermatol. Surg. 2013, 39, 111–120. [Google Scholar] [CrossRef]
- Rodier, F.; Campisi, J. Four faces of cellular senescence. J. Cell Biol. 2011, 192, 547–556. [Google Scholar] [CrossRef]
- Koch, C.M.; Suschek, C.V.; Lin, Q.; Bork, S.; Goergens, M.; Joussen, S.; Pallua, N.; Ho, A.D.; Zenke, M.; Wagner, W.; et al. Specific age-associated DNA methylation changes in human dermal fibroblasts. PLoS ONE 2011, 6, e16679. [Google Scholar] [CrossRef] [PubMed]
- Orioli, D.; Dellambra, E. Epigenetic regulation of skin cells in natural aging and premature aging diseases. Cells 2018, 7, 268. [Google Scholar] [CrossRef] [PubMed]
- McHugh, D.; Gil, J. Senescence and aging: Causes, consequences, and therapeutic avenues. J. Cell Biol. 2018, 217, 65–77. [Google Scholar] [CrossRef]
- Wlaschek, M.; Maity, P.; Makrantonaki, E.; Scharffetter-Kochanek, K. Connective tissue and fibroblast senescence in skin aging. J. Investig. Dermatol. 2021, 141, 985–992. [Google Scholar] [CrossRef]
- Konstantinou, E.; Longange, E.; Kaya, G. Mechanisms of senescence in cutaneous biology and their implications for skin aging. Biology 2024, 13, 647. [Google Scholar]
- Alqahtani, S.; Alqahtani, T.; Venkatesan, K.; Sivadasan, D.; Ahmed, R.; Sirag, N.; Elfadil, H.; Mohamed, H.A.; Taha, H.; Ahmed, R.E.; et al. SASP modulation for cellular rejuvenation and tissue homeostasis: Therapeutic strategies and molecular insights. Cells. 2025, 14, 608. [Google Scholar] [CrossRef]
- Zorina, A.; Zorin, V.; Kudlay, D.; Kopnin, P. Age-related changes in the fibroblastic differon of the dermis: Role in skin aging. Int. J. Mol. Sci. 2022, 23, 6135. [Google Scholar] [CrossRef]
- Talbott, H.E.; Mascharak, S.; Griffin, M.; Wan, D.C.; Longaker, M.T. Wound healing, fibroblast heterogeneity, and cellular senescence in skin repair. Cell Stem Cell 2022, 29, 1161–1180. [Google Scholar] [CrossRef]
- Deng, C.C.; Hu, Y.F.; Zhu, D.H.; Cheng, Q.; Gu, J.J.; Feng, Q.L.; Zhang, L.-X.; Xu, Y.-P.; Wang, D.; Rong, Z.; et al. Single-cell RNA-seq reveals fibroblast heterogeneity in mouse dermis and its role in wound healing. Nat. Commun. 2021, 12, 3709. [Google Scholar] [CrossRef]
- Mamalis, A.; Koo, E.; Tepper, C.; Jagdeo, J. MicroRNA expression analysis of human skin fibroblasts treated with senotherapeutic agents. J. Biophotonics 2019, 12, e201800207. [Google Scholar] [CrossRef]
- Lee, S.S.; Al Halawani, A.; Teo, J.D.; Weiss, A.S.; Yeo, G.C. The matrix protein tropoelastin in skin biology and aging. Adv. Sci. 2024, 11, 2402168. [Google Scholar] [CrossRef] [PubMed]
- Heinz, A. Elastic fibers during aging and disease. Ageing Res. Rev. 2021, 66, 101255. [Google Scholar] [CrossRef] [PubMed]
- McCabe, M.C.; Hill, R.C.; Calderone, K.; Cui, Y.; Yan, Y.; Quan, T.; Fisher, G.J.; Hansen, K.C. Alterations in extracellular matrix composition during skin aging. Matrix Biol. Plus 2020, 8, 100041. [Google Scholar] [CrossRef]
- Charoenchon, N.; Rhodes, L.E.; Nicolaou, A.; Al-Saleh, S.; Suriyaphol, G. Ultraviolet radiation-induced skin damage: Molecular pathways and protection. Clin. Exp. Dermatol. 2022, 47, 1314–1323. [Google Scholar] [CrossRef]
- Feng, C.; Chen, X.; Yin, X.; Jiang, Y.; Zhao, C. Matrix metalloproteinases on skin photoaging: Insights into pathogenesis and intervention. J. Cosmet. Dermatol. 2024, 23, 3847–3862. [Google Scholar] [CrossRef]
- Wang, S.T.; Neo, B.H.; Betts, R.J. Glycosaminoglycans: Sweet as sugar targets in skin aging and regeneration. Clin. Cosmet. Investig. Dermatol. 2021, 14, 1227–1246. [Google Scholar] [CrossRef]
- Xu, M.; Pirtskhalava, T.; Farr, J.N.; Weigand, B.M.; Palmer, A.K.; Weivoda, M.M.; Inman, C.L.; Ogrodnik, M.B.; Hachfeld, C.M.; Fraser, D.G.; et al. Senolytics improve physical function and increase lifespan in old age. Nat. Med. 2018, 24, 1246–1256. [Google Scholar] [CrossRef]
- Yousefzadeh, M.J.; Zhu, Y.; McGowan, S.J.; Angelini, L.; Fuhrmann-Stroissnigg, H.; Xu, M.; Ling, Y.Y.; Melos, K.I.; Pirtskhalava, T.; Inman, C.L.; et al. Fisetin is a senotherapeutic that extends health and lifespan. eBioMedicine 2018, 36, 18–28. [Google Scholar] [CrossRef]
- Hickson, L.J.; Langhi Prata, L.G.P.; Bobart, S.A.; Evans, T.K.; Giorgadze, N.; Hashmi, S.K.; Herrmannc, S.M.; Jensenf, M.D.; Jiaf, Q.; Jordan, K.L.; et al. Senolytics decrease senescent cells in humans: Pilot study. eBioMedicine 2019, 47, 446–456. [Google Scholar] [CrossRef]
- Zhang, L.; Pitcher, L.E.; Prahalad, V.; Niedernhofer, L.J.; Robbins, P.D. Recent advances in the discovery of senolytics. Mech. Ageing Dev. 2021, 200, 111587. [Google Scholar] [CrossRef]
- Zheng, L.; He, S.; Wang, H.; Li, J.; Liu, Y.; Liu, S. Targeting cellular senescence in skin aging and disease. Aging Dis. 2024, 15, 2554–2574. [Google Scholar]
- Raffaele, M.; Vinciguerra, M. The costs and benefits of senotherapeutics for human healthspan. Lancet Healthy Longev. 2022, 3, e67–e77. [Google Scholar] [CrossRef]
- Smer-Barreto, V.; Quintanilla, A.; Elliott, R.J.R.; Dawson, J.C.; Sun, J.; Campa, V.M.; Lorente-Macías, Á.; Unciti-Broceta, A.; Carragher, N.O.; Acosta, J.C.; et al. Discovery of senolytics using machine learning. Nat. Commun. 2023, 14, 3445. [Google Scholar] [CrossRef]
- Lee, H.; Hong, Y.; Kim, M. Structural and functional changes and possible molecular mechanisms in aged skin. Int. J. Mol. Sci. 2021, 22, 12489. [Google Scholar] [CrossRef]
- Fullard, N.; Wordsworth, J.; Welsh, C.; Maltman, V.; Bascom, C.; Tasseff, R.; Isfort, R.; Costello, L.; Scanlan, R.-L.; Przyborski, S.; et al. Cell senescence-independent changes of human skin fibroblasts with age. Cells 2024, 13, 659. [Google Scholar] [CrossRef]
- Jeong, S.; Kim, J.; Jeon, H.M.; Kim, K.; Sung, G.Y. Development of an aged full-thickness skin model using periodic mechanical stimulation: A flexible skin-on-a-chip. Int. J. Mol. Sci. 2021, 22, 12788. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.H.; Lee, Y.H.; Rho, N.K.; Park, K.Y. Skin aging from mechanisms to interventions: Focusing on dermal aging. Front. Physiol. 2023, 14, 1195272. [Google Scholar] [CrossRef] [PubMed]
- Cervelli, V.; Pizzicannella, J.; Marcelli, M.; Lucarini, L.; Brandi, C. Sequential fractional CO2 and 1540/1570 nm lasers: A narrative review of preclinical and clinical evidence. Lasers Med. Sci. 2024, 39, 17–29. [Google Scholar]
- Hussen, N.H.; Abdulla, S.K.; Ali, N.M.; Ahmed, V.A.; Hasan, A.H.; Qadir, E.E. Role of antioxidants in skin aging and the molecular mechanism of ROS: A comprehensive review. Aspects Mol. Med. 2025, 5, 100063. [Google Scholar] [CrossRef]
- Le Couteur, D.G.; Barzilai, N. New horizons in life extension, healthspan extension and exceptional longevity. Age Ageing 2022, 51, afac156. [Google Scholar] [CrossRef]
- Moskalev, A.; Chernyagina, E.; de Magalhães, J.P.; Barardo, D.; Thoppil, H.; Shaposhnikov, M.; Budovsky, A.; Fraifeld, V.E.; Garazha, A.; Tsvetkov, V.; et al. Geroprotectors.org: A new, structured and curated database of current therapeutic interventions in aging and age-related disease. Aging 2015, 7, 616–628. [Google Scholar] [CrossRef]
- Haykal, D.; Cartier, H.; Goldberg, D.; Gold, M. Advancements in laser technologies for skin rejuvenation: A comprehensive review of efficacy and safety. J. Cosmet. Dermatol. 2024, 23, 3078–3089. [Google Scholar] [CrossRef]
- Kang, B.Y.; Wyles, S.P.; Levin, Y.; Almukhtar, R.; Cullison, S.R.J.; Joo, J.S.; Saikaly, S.K.; Mahadeo, S.; Ong, M.; Salingaros, S.; et al. Barriers to clinical cosmetic and laser dermatology research in the academic setting by source of funding: A systematic review. Arch. Dermatol. Res. 2025, 317, 791. [Google Scholar] [CrossRef] [PubMed]
- Weshahy, R.H.; Aly, D.G.; Shalaby, S.; Mohammed, F.N.; Sayed, K.S. Clinical and histological assessment of combined fractional CO2 laser and growth factors versus fractional CO2 laser alone in the treatment of facial mature burn scars: A pilot split-face study. Lasers Surg. Med. 2020, 52, 952–958. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Yeo, H.L.; Wong, S.W.; Zhao, Y. Cellular senescence: Mechanisms and therapeutic potential. Biomedicines 2021, 9, 1769. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.H.; Kim, K.J.; Park, D.J.; Ji, Y.H.; Yoon, E.S.; Park, S.H. Early treatment effects of nonablative fractional lasers on hypertrophic scars in an animal model. Lasers Surg. Med. 2021, 53, 537–548. [Google Scholar] [CrossRef]
- Wissler Gerdes, E.O.; Zhu, Y.; Tchkonia, T.; Kirkland, J.L. Discovery, development, and future application of senolytics: Theories and predictions. FEBS J. 2020, 287, 2418–2427. [Google Scholar] [CrossRef]
- Kunishige, J.H.; Katz, T.M.; Goldberg, L.H.; Friedman, P.M. Fractional photothermolysis for the treatment of surgical scars. Dermatol. Surg. 2010, 36, 538–541. [Google Scholar] [CrossRef]
- Gupta, A.; Singh, A.P. Natural sun-screening compounds and DNA-repair enzymes: Photoprotection and photoaging. Catalysts 2023, 13, 745. [Google Scholar] [CrossRef]
- Smith, P.; Carroll, B. Senescence in the ageing skin: A new focus on mTORC1 and the lysosome. FEBS J. 2024, 292, 960–975. [Google Scholar] [CrossRef]
- Fisher, G.J.; Varani, J.; Voorhees, J.J. Looking older: Fibroblast collapse and therapeutic implications. Arch. Dermatol. 2012, 148, 169–175. [Google Scholar] [CrossRef]
- Werschler, W.P.; Calkin, J.M.; Laub, D.A.; Mauricio, T.; Narurkar, V.A.; Rich, P. Aesthetic dermatologic treatments: Consensus from the experts. J. Clin. Aesthet. Dermatol. 2015, 8 (Suppl. 10), S2–S7. [Google Scholar]
- Demidova-Rice, T.N.; Hamblin, M.R.; Herman, I.M. Acute and impaired wound healing: Pathophysiology and current methods for drug delivery. Adv. Skin. Wound Care 2012, 25, 304–314. [Google Scholar] [CrossRef]
- Wilkinson, H.N.; Hardman, M.J. Cellular senescence in acute and chronic wound repair. Cold Spring Harb. Perspect. Biol. 2022, 14, a041221. [Google Scholar] [CrossRef]
- Poon, F.; Kang, S.; Chien, A.L. Mechanisms and treatments of photoaging. Photodermatol. Photoimmunol. Photomed. 2015, 31, 65–74. [Google Scholar] [CrossRef]
- Mansouri, V.; Arjmand, B.; Rezaei Tavirani, M.; Razzaghi, M.; Rostami-Nejad, M.; Hamdieh, M. Evaluation of efficacy of low-level laser therapy. J. Lasers Med. Sci. 2020, 11, 369–380. [Google Scholar] [CrossRef]
| Year | Study | EBD Type | Device/Params | Model/Population | Design | Evidence Tag | Biomarkers | Effect on Senescence/SASP | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 2025 | Sci Rep: FMR with topical antioxidant serum for neck | Fractional microneedle RF | FMR + antioxidant serum | Human clinical (neck skin) | Clinical | clinical (human) | Clinical + histology | Enhanced rejuvenation outcomes vs. FMR alone | [9] |
| 2025 | Fractional microneedle RF vs. microneedling on senescent milieu | Fractional microneedle RF | FMRF vs. MN | Human aged skin | Clinical + molecular | clinical (human) | Senescent fibroblast milieu markers | FMRF modulated senescent fibroblast niche; superior to MN | [10] |
| 2025 | Non-ablative fractional 1940-nm diode laser for rejuvenation | NAFL (1940 nm) | 1940 nm diode | Human clinical | Clinical | clinical (human) | Clinical outcomes; superficial thermal remodeling | Improved superficial remodeling; supports senomorphic effect | [11] |
| 2025 | Activated melanocytes and senescent collagen fibers—PAL DLA protocol | Picosecond alexandrite (DLA) | 755 nm; DLA; 3 sessions | Human clinical (protocol) | Protocol/clinical | clinical (human) | Optical markers; collagen | Targets senescent collagen fibers; remodeling | [12] |
| 2025 | Pilot: microblade RF for neck rejuvenation | Microblade RF | Dual length | Human clinical (neck) | Pilot clinical | clinical (human) | Skin properties; wrinkles | Clinical improvement; remodeling | [13] |
| 2024 | Picosecond 755 nm DLA laser for wrinkles (clinical) | Picosecond alexandrite (DLA) | 755 nm; diffractive lens array | Human clinical (wrinkles) | Prospective clinical | clinical (human) | Clinical wrinkle scales; histology subset | Improved wrinkles; supports photomechanical remodeling | [14] |
| 2024 | A faster CO2 fractional scanner mode for full-face rejuvenation | CO2 fractional laser | Scanner “moveo” vs. standard | Human clinical | Clinical performance | clinical (human) | Treatment time; clinical outcomes | Improved efficiency; remodeling effects implied | [15] |
| 2024 | OLED therapy reduces aging signs; stem-cell senescence recovery | OLED PBM | OLED panel | Cell/animal | Preclinical | in vitro | Stem cell senescence | Recovery from senescence | [16] |
| 2023 | Photomodulation alleviates cellular senescence of aging ASCs | Photobiomodulation (LED) | Light activation at P3 | Human adipose-derived stem cells | In vitro | in vitro | p16, p21, p53 | ↓ p16/p21/p53 in aged cells | [17] |
| 2023 | Green-light pretreatment reduces SA-β-Gal (clinical context) | Green-light PBM | Low-energy green light | In vitro + concept to clinic | Preclinical | clinical (human) | SA-β-gal, collagen I/III, MMP-1 | ↓ SA-β-gal; ↑ collagen | [18] |
| 2023 | Reverse skin aging signs by red-light PBM (clinical) | Photobiomodulation (red LED 630 ± 10 nm) | 15.6 J/cm2; 12 min; 2×/week; 3 months | Human clinical (facial skin) | Prospective clinical | clinical (human) | Clinical: elasticity, texture; (mechanistic narrative) | Improved clinical aging signs; consistent with PBM-induced mitochondrial benefits | [19] |
| 2023 | HIFU increases collagen/elastin via Cav-1 modulation | HIFU | 0.5 J LINEAR mode | Senescent fibroblasts + aging skin model | In vitro + in vivo | in vivo + in vitro | Ac-p53, p21, cyclin D1, PCNA; collagen/elastin | ↓ p53/p21; ↑ proliferation markers; ↑ collagen/elastin | [20] |
| 2023 | Non-ablative lasers offer a gentle approach (news + study refs) | Non-ablative fractional lasers | NAFL vs. MFR | Human clinical | Clinical (summarized) | clinical (human) | Clinical laxity, wrinkles | Improved remodeling; indirect senomorphic effect | [21] |
| 2021 | Picosecond Nd:YAG (532/1064) improves photoaged skin | Picosecond Nd:YAG | 532/1064 nm | Human clinical/ex vivo | Clinical/ex vivo | ex vivo | Collagen III increase | Photomechanical remodeling | [22] |
| 2021 | Synergistic effect of 300 μm FMR needle depth on pigmentation | Fractional microneedle RF | 300 μm needle depth | Human clinical (pigmentation disorders) | Clinical observational | clinical (human) | Basement membrane markers; keratinocyte senescence context | Suggests removal of senescent keratinocytes; repairs BM | [23] |
| 2020 | Microneedling vs. fractional Er:YAG for rejuvenation | Fractional Er:YAG | Er:YAG fractional; microneedling comparator | Human clinical | Clinical comparative | clinical (human) | Clinical scores; histology subset | Improved wrinkles /texture; suggests remodeling | [24] |
| 2020 | Non-ablative vs. ablative Er:YAG fractional resurfacing | Er:YAG (ablative and NAFL) | Er:YAG fractional | Human clinical | Clinical comparative | clinical (human) | Collagen I/III (histology), fibroblast markers | ↑ Collagen I/III; fibroblast activation | [25] |
| 2020 | CO2 lattice laser reverses UVB-induced skin aging | CO2 fractional laser | CO2 lattice; UVB photoaging model | Human fibroblasts + UVB-mice | in vitro + in vivo | in vivo + in vitro | SA-β-gal, collagen I/III, SOD, SMAD3 | ↓ SA-β-gal; ↑ collagen; antioxidant upregulation | [26] |
| Device Type | Wavelength/Modality | Penetration Depth/Target | Chromophore/Target | Mechanism of Action | Clinical Indications | Key Features/Notes |
|---|---|---|---|---|---|---|
| Ablative CO2 Laser | 10,600 nm | Epidermis + dermis | Water | Vaporizes tissue → stimulates neocollagenesis | Wrinkles, scars, and deep resurfacing | Gold standard for deep rejuvenation |
| Ablative Er:YAG Laser | 2940 nm | Epidermis | Water | Precise ablation → collagen remodeling | Fine wrinkles and superficial scars | High precision and minimal thermal damage |
| Non-Ablative Fractional Lasers | 1550 nm/1927 nm | Dermal MTZ | Water | Microthermal injury → repair response | Rejuvenation, downtime reduction | Deep remodeling and low downtime |
| Pulsed Dye Lasers (PDLs) | ~585–595 nm | Superficial dermis | Hemoglobin | Vascular targeting → stimulates remodeling | Vascular lesions and rejuvenation | Multifaceted impact |
| Intense Pulsed Light (IPL) | Broad 400–1200 nm | Variable | Melanin and hemoglobin | Broad-spectrum light → chromophore absorption | Pigmentation and photorejuvenation | Versatile but less specific |
| Radiofrequency (RF) (monopolar/tri-/microneedling) | Non-ionizing EM | Deep dermis | Water and collagen frameworks | Thermal heating → neocollagenesis | Tightening and collagen induction | Safe for all skin types and minimal pigment risk |
| High-Intensity Focused Ultrasound (HIFU) | Ultrasound energy | SMAS + deep dermis | Acoustic focus | Thermal coagulation → collagen remodeling | Skin lifting and tightening | Deep-targeting and non-invasive |
| Light-Emitting Diodes (LEDs and LLLT) | 600–1100 nm (e.g., 660 nm) | Mitochondrial level | Cytochrome c oxidase | Photobiomodulation → improves ATP and reduces ROS | Biostimulation and mild rejuvenation | Non-thermal and low risk |
| Parameter | Pharmacologic Senotherapeutics | Energy-Based Devices (EBDs) |
|---|---|---|
| Delivery | Systemic or topical (limited skin penetration) | Localized and operator controlled |
| Mechanism | Molecular inhibition or apoptosis | Controlled injury → regeneration/remodeling |
| Selectivity | Pathway dependent | Tissue compartment specific |
| Onset of Action | Delayed (weeks to months) | Immediate to short term |
| Side Effects | Systemic toxicity and off-target effects | Minimal with appropriate parameters |
| Evidence in Skin | Mostly preclinical | Clinical studies available |
| Reversibility of ECM damage | Limited | Promotes neocollagenesis and structural recovery |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hrițcu, O.M.C.; Costan, V.-V.; Toader, Ș.V.; Brănișteanu, D.E.; Toader, M.P. From Lasers to Longevity: Exploring Energy-Based Devices as Senotherapeutic Tools in Dermatology. Cosmetics 2025, 12, 201. https://doi.org/10.3390/cosmetics12050201
Hrițcu OMC, Costan V-V, Toader ȘV, Brănișteanu DE, Toader MP. From Lasers to Longevity: Exploring Energy-Based Devices as Senotherapeutic Tools in Dermatology. Cosmetics. 2025; 12(5):201. https://doi.org/10.3390/cosmetics12050201
Chicago/Turabian StyleHrițcu, Oana Mihaela Condurache, Victor-Vlad Costan, Ștefan Vasile Toader, Daciana Elena Brănișteanu, and Mihaela Paula Toader. 2025. "From Lasers to Longevity: Exploring Energy-Based Devices as Senotherapeutic Tools in Dermatology" Cosmetics 12, no. 5: 201. https://doi.org/10.3390/cosmetics12050201
APA StyleHrițcu, O. M. C., Costan, V.-V., Toader, Ș. V., Brănișteanu, D. E., & Toader, M. P. (2025). From Lasers to Longevity: Exploring Energy-Based Devices as Senotherapeutic Tools in Dermatology. Cosmetics, 12(5), 201. https://doi.org/10.3390/cosmetics12050201







