Abstract
Free radicals can cause damage to the structure of the dermis layer, which makes skin lose its elasticity and leads to the formation of wrinkles. A strategy to prevent this problem is by using antioxidants. A plant that has been reported to contain good antioxidant activity is sacha inchi seed (Plukenetia volubilis L.); apart from that, its oil has quite a high omega-3 content and potentially can act as an anti-aging agent stimulating the skin-cell-regeneration process, maintaining skin moisture and elasticity and stimulating collagen production. This research aims to analyze the physicochemical characteristics and determine the fatty acid profile, the levels of vitamins A, D, and E, and the antioxidant activity of sacha inchi seed oil. This research was conducted through eight main stages: sacha inchi seed extraction, quality parameters checking, phytochemical screening, determining fatty acid profiles, vitamin analysis, antioxidant activity tests, microbiological contamination tests, and heavy-metal contamination tests. In this study, quality inspection results were obtained: organoleptic form (liquid), color (yellow), odor (typical), relative density value (0.91 g/cm3), acid number (0.38 ± 0.02 mg/g), peroxide value (11.01 mEq/Kg), iodine value (179.32 g/100 g), and refractive index (1.479). The phytochemical screening results of sacha inchi seed oil were positive for containing flavonoids, triterpenoids, and steroids. The results of the fatty acid profile were omega-3 (48.5%), omega-6 (34.8%), and omega-9 (7.7%). The results of the vitamin contents analysis were vitamin A (123.42 mg/100 g), vitamin D (899.46 mg/100 g), and vitamin E (145.06 mg/100 g). The antioxidant activity test showed an IC50 value of 8.859 ppm (very strong), and the microbial and heavy-metal contamination tests were negative.
1. Introduction
Skin, as the outermost part of our body, is the defense system against internal and external factors that can damage the skin. Skin damage is usually caused by free radicals in the skin, which damage the structure or layers of the dermis and lead to the loss of skin elasticity and the formation of wrinkles [1]. With the increasing incidence of skin damage, it is necessary to carry out chemoprevention strategies and develop therapies. A potential approach is through the application of natural extracts.
There are several mechanisms for natural ingredients to protect the skin. It includes reducing the reactivity of reactive oxygen species (ROS), inhibiting the oxidation process, absorbing UV rays, or suppressing enzyme activity. Their collective effect aims to minimize the formation of wrinkles on the skin and protect the skin from aging. The effectiveness of these mechanisms is due to the presence of chemical components such as phenolics, flavonoids, and triterpenoids in natural ingredient extracts, which appear as antioxidants [2].
Antioxidants are compounds that can provide one or two electrons against free radicals, inhibiting oxidation reactions in cells and minimizing cell damage, which causes skin aging. Antioxidants are available in various forms, including vitamins, minerals, and secondary metabolites found in plants [3].
The sacha inchi seed oil (Plukenetia volubilis L.) can act as a practical antioxidant component. Sacha inchi, a native plant of Peru, Ecuador, and Colombia, has gained global recognition due to its anti-aging properties. As a result, it is now being cultivated in countries beyond South America and has emerged as a promising cosmetic ingredient in the future. Sacha inchi is commercially cultivated, particularly in Southeast Asia [4], with Indonesia being one of the countries where it is grown. One specific location in Indonesia that cultivates sacha inchi is the sacha inchi plantation in Cibokor Village, Cibeber District, Cianjur Regency, West Java. The plant of sacha inchi is represented in Figure 1.

Figure 1.
Sacha inchi (Plukenetia volubilis L.).
In Peru, sacha inchi seed oil is conventionally applied as a regular skin care oil to promote skin health [5]. According to the research of Puangpronpitag et al [6], sacha inchi seed oil has significant antioxidant activity, with an IC50 value of 0.007 ± 0.001 (mg/mL). Hadzich et al. [7] and Chirinos et al. [8] also reported that the bioactive compounds contained in sacha inchi seed oil have the potential to act as antioxidants, including vitamin E, vitamin A, tannins, phytosterols, phenolic compounds, and terpenoids. Aside from that, sacha inchi seed oil has many unsaturated fatty acids, such as omega-3 (48%), omega-6 (37%), and omega-9 (8%). These acids maintain healthy skin by promoting the growth of new skin cells, keeping skin moist and elastic, and increasing collagen production [8].
The natural cosmetics market has developed in recent years due to the market transition of cosmetics from synthetic ingredients to natural ingredients [9]. Based on a review of the Centre for the Promotion of Imports from Developing Countries (CBI), one of the natural ingredients that is predicted to become a trend in the next few years is sacha inchi seed oil due to its abundance of omega-3 content, which represents 48% of the total fatty acids found in sacha inchi seed oil. This value is higher than the omega-3 content found in olive oil (1%) or in argan oil (0.5%). Omega-3 is effective in skin regeneration by controlling inflammation and collagen synthesis. Therefore, it is widely used in skin care products—especially as an anti-aging agent [10].
Prevention of aging can be achieved through cosmetic treatments, cell regulators, and topical agents containing antioxidants. Topical formulations with antioxidants and cell regulators have shown the potential to reduce the effects of aging, such as hyperpigmentation and wrinkles [2]. Vitamin B3, C, D, and E are the most important antioxidants because they penetrate the skin through their small molecular weight [11]. Vitamin B3 is capable of reducing matrix degradation enzymes (ECMs) and enhancing collagen synthesis in dermal fibroblasts. In addition, it also can escalate the structural and functional integrity of the skin barrier by improving lipid synthesis in the skin [12]. Vitamin C is an important antioxidant for the synthesis of collagen, and it indirectly eliminates superoxide by the formation of intermediate radicals. It also serves as a cofactor for prolyl and lysyl hydroxylase, the key enzymes that cross-bind and stabilize collagen fibers [13,14]. Vitamin D (cholecalciferol) can stimulate the production of metallothionein (MT)-mRNA. MT-mRNA functions as an antioxidant by effectively scavenging free radicals generated by UV radiation. In addition, vitamin D also contributes to increasing collagen synthesis and clinically improves skin elasticity [15]. Vitamin E (α-tocopherol) shows properties that can reduce inflammation and inhibit cell proliferation. The mechanism of action of vitamin E involves improving skin smoothness and increasing the capability of the stratum corneum to maintain moisture. It also facilitates epithelization processes and acts as a photoprotective agent in the skin. In contrast, cell regulators such as vitamin A (retinol) provide their effects by directly affecting collagen metabolism and stimulating the synthesis of collagen and elastic fibers [11].
Sacha inchi seed oil contains bioactive compounds such as omega-3, 6, and 9; vitamins A, D, and E; as well as secondary metabolite compounds such as flavonoids, steroids, and triterpenoids [6,7,8]. The synergistic combination of these factors enhances the efficacy of sacha inchi seed oil in preventing aging, surpassing the effectiveness of cosmetic therapies that solely rely on a single bioactive ingredient. Therefore, this research aimed to analyze the physicochemical characteristics, determine the fatty acid profile, determine the levels of vitamins A, D, and E, and test the antioxidant activity of sacha inchi seed oil. Furthermore, examinations were conducted to detect the presence of microorganisms and heavy metals to guarantee safety. These findings could form the basis for sacha inchi oil for cosmetic formulation that has anti-aging properties due to its antioxidant activity and can be produced according to applicable regulatory standards. In addition, the sacha inchi seed oil used in this research originates from Indonesia. However, there is a scarcity of research on this particular oil, as no scientific papers have been published on the issue.
2. Materials and Methods
2.1. Plant Materials
The sacha inchi seeds (Plukenetia volubilis L.) were collected from Cianjur Regency, West Java, Indonesia. They were taxonomically identified by botanists at the Plant Taxonomy Laboratory, Biology Department, Faculty of Mathematics and Natural Sciences, Padjadjaran University.
2.2. Chemicals
The chemicals used in this study are toluene, distilled water, ethanol 95% (Merck, Germany), phenolphthalein indicator (Merck, Germany), potassium hydroxide (KOH) (Merck, Germany), oxalic acid (C2H2O4) (Merck, Germany), potassium iodide (KI) (Merck, Germany), chloroform pa (Merck, Germany), sodium thiosulfate (Na2S3O3), starch indicator, carbon tetrachloride (CCL4) (Merck, Germany), Wijs solution, ammonia, hydrochloric acid (HCl) (Merck, Germany), Mayer’s reagent, Dragendorff’s reagent, magnesium (Merck), amyl alcohol (Merck, Germany), iron (III) solution chloride (FeCl3), ether (Merck, Germany), Liebermann Burchard’s reagent, vanillin solution, sulfuric acid (H2SO4) (Merck, Germany), boron trifluoride-methanol (BF3-methanol) reagent, n-hexane, Sabouraud Dextrose Agar (SDA) media (Merck, Germany), Plate Count Agar (PCA) media (Merck, Germany), standard retinol, standard cholecalciferol, and standard α-tocopherol (Sigma Aldrich), 2,2-diphenyl-1-picrylhydrazyl (DPPH) (Sigma-Aldrich Co., St. Louis, MO, USA), ascorbic acid (Merck, Germany), and ethanol pa.
2.3. Methods
This research consisted of eight stages, starting with the extraction of sacha inchi seeds, followed by examining the quality parameters of sacha inchi seed oil, phytochemical screening, determining the fatty acid profile, determining microbiological contamination, heavy-metal contamination, vitamin analysis, and antioxidant activity testing.
2.3.1. Production of Sacha Inchi Seed Oil (Plukenetia volubilis L.)
Preparation of Sacha Inchi Seed
Sacha inchi seeds are cleaned and dried in a chamber at 40 °C for 7 days until the water content reaches 13%. Then, the sacha inchi seeds are separated from the brown skin.
Seeds Extraction
Sacha inchi seeds (2 kg) were weighed, peeled, and dried before being put into the press machine. The dried sacha inchi seeds were continuously compressed until the oil came out. The resulting oil was left for 2–3 days and then separated from the sediment [16]. The percentage yield of the sacha inchi seed oil was determined using the following Formula (1), with the weight of oil (a) and the sample material (seeds) (b):
2.3.2. Quality Parameters of Sacha Inchi Seed Oil (Plukenetia volubilis L.)
Organoleptic
Organoleptic parameters were observed, including the five senses to describe consistency, color, and odor [17].
Ash Content
The oil (2–3 g) was carefully weighed inside a porcelain cup of known weight. The oil was then burned in an electric furnace at a maximum temperature of 550 °C until complete ash was obtained. The ash was then cooled in a desiccator and weighed [18]. The ash content was calculated in percent of the initial sample weight, which was determined using the following formula (Equation (2)) in which W is the weight of the sample before ashing in grams, W1 is the weight of the sample and crucible after ashing in grams, and W2 is the weight of the empty crucible in grams (2):
Water Content
A certain amount of oil was put into a round bottom flask, then 200 mL of toluene was added, and the apparatus was installed. The oil was distilled at a speed of ±2 drops per second until some of the water was distilled, and the receiving tube was left to cool at room temperature. The water and toluene were separated, and the percent of water content was determined [19].
Relative Density
The relative density was calculated using a pycnometer. A clean, dry, and calibrated pycnometer was used to determine the weight of the pycnometer and the weight of freshly boiled water at 25 °C. The temperature of the liquid sample was adjusted to approximately 20 °C, and the sample was then inserted into the pycnometer. The temperature of the filled pycnometer was set to 25 °C; the excess oil was removed and weighed. Next, the weight of the empty pycnometer was subtracted from the weight of the pycnometer that was filled with water. The specific gravity of a liquid sample was obtained by dividing the sample weight by the weight of water in the pycnometer at a temperature of 25 °C. The specific gravity of the sample was determined using the following Formula (3) [18]:
Acid Value
The acid value was determined by weighing 1 g of the sample, then placing it in a 100 mL beaker and mixing it slowly with 50 mL of 95% ethanol pa using a stir bar on a magnetic stirrer for 5 min. After that, the solution was added with 1% phenolphthalein indicator and titrated with a standard solution of 0.1 N KOH, which had been standardized with 0.1 N C2H2O4 until a pink color appeared. The acid value was calculated using the equation below [20], in which V is the numerical volume of 0.1 N KOH required in the titer (mL), T is the normality of KOH 0.1 solution (N), m is the sample weight (grams), and 56.1 is the KOH equivalent weight (4):
Peroxide Value
The peroxide value was calculated with 1 g of sample and 1 g of potassium iodide dissolved in 20 mL of chloroform. The mixture was then boiled in boiling water for 30 s and immediately transferred into a flask containing 20 mL of 5% KI solution. After that, the solution was titrated with 0.01 M Na2S2O3 solution using 0.5 mL of starch as an indicator. The peroxide number was calculated using the following equation [17], in which V0 is the numerical value of the volume of 0.1 N Na2S2O3 solution required in the blank meter (mL), V1 is the numerical value of the volume of 0.1 N Na2S2O3 solution required for sample titration (mL), T is the normality of Na2S2O3 0.1 (N), and m is the sample weight (grams) (5):
Iodin Value
The iodine value was calculated with 1 g of oil and 15 g of CCl4 dissolved in 25 mL of Wijs solution. The solution mixture was incubated at room temperature for 1–2 h and protected from light. Then, 10 mL of 20% KI solution and 100 mL of distilled water were added. After that, the solution was closed immediately, shaken, and titrated with 0.1 N Na2S2O3 solution using starch solution as an indicator. The iodine number was calculated using the equation below [17], in which V0 is the numerical value of the volume of 0.1 N Na2S2O3 solution required in the blank meter (mL), V1 is the numerical value of the volume of 0.1 N Na2S2O3 solution required for sample titration (mL), T is the normality of 0.1 Na2S2O3 solution (N), m is the sample weight (grams), and 12.69 is the equivalent weight of iodine (6):
Refractive Index
The refractive index was determined using a refractometer. To begin, rotate the locking mechanism to unlock the illumination prism. Some of the liquid was added to the measuring prism. Then, the illumination prism was lowered back down, and the locking device was used to keep it in place. After that, the protective plate was opened, and the reflected mirror was closed. The adjustment wheel was turned right or left while looking through the eyepiece to focus the image. Then, the measurement range adjustment wheel was turned to the left or right to change the measurement range. The value was read from the bottom window when the line between the light and dark in the upper window lined up with the crosshairs [21].
2.3.3. Phytochemical Screening
The qualitative phytochemistry test was conducted according to Indonesian Materia Medica and Harborne [22,23]:
Alkaloid
The oil was alkalized with 5 mL of 25% ammonia and ground with a mortar and stamper. Then, 20 mL of chloroform was added and ground vigorously. The chloroform layer was pipetted, 2 N HCl was added, and the mixture was shaken vigorously until the two layers formed. Next, the formed acid layer was pipetted and divided into three parts [22,23]:
- Filtrate I: The filtrate was added with 3 drops of Mayer’s reagent (mercury (II) chloride and potassium iodide). Positive results were indicated by the formation of turbidity or white precipitate, which indicated the presence of alkaloids.
- Filtrate II: The filtrate was added with 3 drops of Dragendorff’s reagent (bismuth subnitrate and potassium iodide). Positive results were indicated by the formation of an orange-brown precipitate, which indicated the presence of alkaloids.
- Filtrate III: Used as the blank.
Flavonoid
The oil (10 g) was added to 100 mL of distilled water, boiled for 5 min, and then filtered. The filtrate is referred to as solution A. Solution A (5 mL) was added with magnesium powder, 1 mL of hydrochloric acid, and 2 mL of amyl alcohol, then shaken vigorously and allowed to separate. Positive results were indicated by the formation of a yellow-to-red layer of amyl alcohol, indicating the presence of flavonoids [22,23].
Tannins
Solution A (5 mL) was added with 1% gelatin solution. A positive result was indicated by the formation of a white precipitate, indicating the presence of tannin [22,23].
Polyphenols
Solution A (5 mL) was added with 1% iron (III) chloride (FeCl3 1%). A positive result was marked by the formation of a blue-to-black color, indicating the presence of polyphenolic compounds [22,23].
Steroids and Triterpenoids
The oil (5 g) was added with 20 mL of ether, crushed with a mortar and stamper, then pipetted and filtered to obtain the filtrate. The filtrate was placed in an evaporating dish and then allowed to evaporate, and a residue remained. Liebermann Burchard’s reagent (20 parts anhydrous acetic acid and 1 part concentrated sulfuric acid) was added in 2 to 3 drops to the residue resulting from evaporation. Positive results were indicated by the formation of a purple color, which indicated the presence of triterpenoids, and a blue-green color, which indicated the presence of steroids [22,23].
Monoterpenoids and Sesquiterpenoids
The oil (5 g) was added with 20 mL of ether, crushed with a mortar and stamper, then the sample was pipetted and filtered to obtain the filtrate. The filtrate was placed in an evaporating dish and then allowed to evaporate, and a residue remained. Vanillin solution (10%) in concentrated H2SO4 was added and dripped with a dropper through the edge of the cup. Positive results were indicated by the formation of colors indicating the presence of monoterpenoids and sesquiterpenoids [22,23].
Saponins
Solution A (10 mL) was taken and then shaken vertically in a test tube for 10 min, and then one drop of 2 N HCl was added. A positive result was indicated by the formation of persistent foam that lasts for 10 min, indicating the presence of saponin [22,23].
Quinones
Solution A (5 mL) was added with 5% KOH solution. A positive result was indicated by the formation of colored salt (red) from the reaction of hydroquinone with strong alkali (KOH 5%) [22,23].
2.3.4. Determination of Fatty Acid Profile of Sacha Inchi Seed Oil
The fatty acid composition of the sacha inchi seed oil was determined by gas chromatography. The sample was converted into methyl ester by adding 2 mL of sodium methylate in methanol (5% w/v) to 100 mg of sacha inchi seed oil sample, then incubated at 100 °C for 15 min. After that, the mixture was cooled down and 1 mL of boron trifluoride-methanol (20% BF3) reagent was added. Then, it was heated at 100 °C for 15 min. Next, the cooled-down solution was mixed with 1 mL of n-hexane and 1 mL of distilled water before centrifuging for 2 min at 1300 rpm. The top layer containing fatty acid methyl ester (FAME) was transferred into a 2 mL vial and stored at 4 °C before analysis. Analysis was conducted on an Agilent-6820 gas chromatograph with a manual injector and a flame ionization detector (FID) detector. FAME separation was carried out on an Agilent J&W GC column (DBFFAP; length 30 m, diameter 0.25 mm, film thickness 0.25 µm), and gas (He) was used as a carrier at a constant flow of 1.2 mL·min−1. The oven temperature was set at 180 °C and 220 °C for the start and end points, respectively, and increased at a rate of 10 °C·min−1 and held for 5 min. The injector and detector temperatures were set at 260 °C. The sample size was 1 mL with a separation ratio of 30:1. Data analysis was carried out using Agilent Cerity NDS (A.04.05). Based on the chromatogram obtained, the retention time was matched to the same or close to the standard retention time of fatty acids [24]. Fatty acid levels were calculated using the following Formula (7):
2.3.5. Antioxidant Activity
The DPPH radical scavenging experiment was modified slightly from Liua et al. [24] to assess the antioxidant ability of sacha inchi seed oil. A total of 4 mg of DPPH was weighed and dissolved in 25 mL of ethanol pa so that the concentration of the DPPH reagent was 160 µg/mL, and it was diluted to reach the concentration of 40 µg/mL. The standard used was ascorbic acid with varying concentrations of 1 µg/mL, 2 µg/mL, 3 µg/mL, 4 µg/mL, 5 µg/mL, and 6 µg/mL in ethanol pa. Then, various concentrations of sacha inchi seed oil were made: 15.625 µg/mL, 31.25 µg/mL, 62.5 µg/mL, 125 µg/mL, 250 µg/mL, and 500 µg/mL in ethanol pa. In each, a total of 1 mL of sample and standard solution was put into a different vial and 1 mL of DPPH solution was added to each solution, then homogenized and incubated for 30 min in dark conditions and not exposed to light directly. After that, the absorbance value at the maximum wavelength (λ), 515 nm, was measured. All experiments were carried out in triplicate, and the IC50 value was calculated by % inhibition using the following Formula (8):
If the value is 0%, the solution has no free radical scavenging power. However, if the value reaches 100%, it has a total immersion value. The % inhibition value at various concentration variations is created by a linear regression equation with sample concentration as the X axis and % inhibition as the Y axis. The IC50 value is obtained when the % inhibition is 50% with the equation Y = aX + b.
2.3.6. Analysis of Vitamins A (Retinol), D (Cholecalciferol), and E (α-Tocopherol)
The retinol, cholecalciferol, and tocopherol contents of the oils were elucidated by high-performance liquid chromatography (HPLC) [24]. Chromatographic analysis of retinol, cholecalciferol, and tocopherol in sacha inchi seed oil was carried out using the isocratic elution method. A total of 50 mg of sacha inchi seed oil was placed in a vial, dissolved with 1 mL of hexane (HPLC grade), and analyzed using HPLC-PDA (Shimadzu SIL-20AD HPLC system; Kyoto, Japan) with a binary pump coupled to a SPD-M30A PDA detector. Separation was performed by reversed-phase high-performance liquid chromatography (RP-HPLC) using a Supelco Kromasil Eternity C18, 4.6 × 150 mm column (Millipore Sigma, St. Louis, MO, USA) maintained at room temperature. The mobile phase used was a mixture of methanol in acetonitrile (13:87 v/v) with a flow rate of 0.5 mL/minute and an injection volume of 20 μL. The optimal responses of retinol, cholecalciferol, and α-tocopherol were identified at wavelengths of 325 nm, 264 nm, and 294 nm, respectively. The measurement results are expressed in mg/kg oil.
2.3.7. Determination of Microbiological Contamination
Total Plate Count (TPC)
Three test tubes were prepared, each filled with 9 mL of sterile distilled water, and then 1 mL of the 10−1 dilution was pipetted into the tube containing the first distilled water. Further dilutions were made until the dilution was 10−3. Next, 1 mL of each dilution was pipetted into a Petri dish and made in triplicate. Then, 15–20 mL of PCA media was poured into each cup. A control test was also carried out to determine the sterility of the media. After the medium solidified, all Petri dishes were incubated at a temperature of 32.5 ± 2.5 °C for 24–48 h with the dish upside down. After that, the dishes were observed, and the number of colonies that grew were counted [25].
Yeast Mold Count (YMC)
Three test tubes were prepared, each filled with 9 mL of sterile distilled water, and then 1 mL of the 10−1 dilution was pipetted into the tube containing the first distilled water. Further dilutions were made until the dilution was 10−3. Next, 1 mL of each dilution was pipetted into a Petri dish and made in triplicate. A total of 15–20 mL of SDA media was poured into each cup. A control test was also carried out to determine the sterility of the media. After the medium solidified, all Petri dishes were incubated at a temperature of 25 ± 2.5 °C for 3–5 days with the dish upside down. After that, the dishes were observed, and the number of colonies that grew were counted [25].
2.3.8. Determination of Heavy-Metal Contamination
Sample preparation was carried out by weighing 0.1 g of sacha inchi seed oil and placing it in a 25 mL Erlenmeyer flask. Next, 5 mL of 0.07 N HCl was measured and added to the sample, then stirred for approximately 1 min. The pH of the solution was measured using universal pH. Then, the sample was put into a water bath shaker at a speed of 150 rpm at 37 °C for 60 ± 2 min. The samples were then left for 1 h at 37 °C. Then, the sample that had been sedimented was filtered with the help of a sampling manifold fitted with a filter membrane (0.45 µm pore size). If necessary, centrifugation was carried out on the solution containing sediment. The sample was then analyzed using an inductively coupled plasma-optical emission spectrophotometry (ICP-OES) instrument. Standard solutions were prepared with varying concentrations. The metal content in the sample was calculated based on a standard curve [26].
3. Result
3.1. Quality Parameter of Sacha Inchi Seed Oil Result
To formulate an active ingredient into a dosage form, it must undergo a quality parameter inspection stage so that the ingredient used meets the specified safety requirements. The results of the quality inspection of sacha inchi seed oil are represented in Table 1.

Table 1.
Quality parameter of sacha inchi seed oil.
Table 1.
Quality parameter of sacha inchi seed oil.
| Parameters | Specifications | Observations | Reference |
|---|---|---|---|
| Appearance | Liquid | Liquid | [17] |
| Color | Yellow | Yellow | [17] |
| Odor | Characteristic | Characteristic | [17] |
| Moisture Content | - | 0.06% | - |
| Ash Content | - | <0.02% | - |
| Relative Density | 0.91–0.94 g/cm3 | 0.91 g/cm3 | [27,28,29] |
| Acid Value | 0.37–2.40 mg/g | 0.38 ± 0.02 mg/g | [23,27,29,30,31,32] |
| Peroxide Value | 1.50–19.10 mEq/kg | 11.01 mEq/kg | [23,27,29,30,32,33,34] |
| Iodine Value | 183.69–195.05 g/100 g | 179.32 g/100 g | [23,29,31,32,33] |
| Refractive Index | 1.475–1.482 | 1.479 | [27,28,31,33] |
3.2. Phytochemical Screening of Sacha Inchi Seed Oil Results
The phytochemical content of sacha inchi seed oil has been analyzed using various reagents to detect the presence of various secondary metabolite compounds. Visuals of sacha inchi seed oil with reagents after treatment are illustrated in Figure 2. Table 2 presents the interpretation of the test results for secondary metabolite content. The sacha inchi seed oil used has been confirmed to contain positive flavonoids, triterpenoids, and steroids. However, the extract did not show the presence of alkaloids, tannins, and saponins.

Figure 2.
Phytochemical screening results of sacha inchi seed oil (A) using reagents, including FeCl3 5% (B), FeCl3 1% (C), concentrated HCl + Mg (D), H2SO4 (E), NaOH 10% (F), agitation followed by heating (G), concentrated H2SO4 + acetic anhydric, and Dragendorff (H).

Table 2.
Phytochemical screening of sacha inchi seed oil.
3.3. Determination of the Fatty Acid Profile of Sacha Inchi Seed Oil
The content of omega-3, 6, and 9 fatty acids obtained from sacha inchi seed oil is presented in Table 3. These results show that sacha inchi seed oil has a higher omega-3 content than olive oil (1.5%). Furthermore, the omega-6 content of sacha inchi seed oil is also equivalent to argan oil (35%). According to the 2018 NTP regulations, the fatty acid content of sacha inchi seed oil must contain at least omega-3 (greater than 32%), omega-6 (greater than 42%), and omega-9 (greater than 8.5%) [35].

Table 3.
Results of determination of the fatty acid profile of sacha inchi seed oil.
3.4. Determination of the Vitamin A, D, and E Content of Sacha Inchi Seed Oil
The results of the analysis of vitamin A (retinol), vitamin D (cholecalciferol), and vitamin E (tocopherol) content using HPLC are shown in Table 4. The results of vitamin A (retinol) content in this study have a higher value when compared with the results of research by Wang et al. [36] of 0.07–0.09 mg/100 g. However, the results of vitamin E (tocopherol) content tend to have lower values, with a difference in value of 133.94 mg/100 g.

Table 4.
Results of determination of the vitamin A, D, and E content of sacha inchi seed oil.
3.5. Antioxidant Activity
The results of the antioxidant activity in sacha inchi seed oil using a UV–Vis spectrophotometer with the DPPH method are presented in Table 5 and Figure 3. The IC50 value produced by this research is classified into the very strong category at 8.859 ppm. However, the IC50 value is still lower than the results of research conducted by Puangpronpitag et al. [6] at 7 ppm.

Table 5.
Antioxidant activity in sacha inchi seed oil.
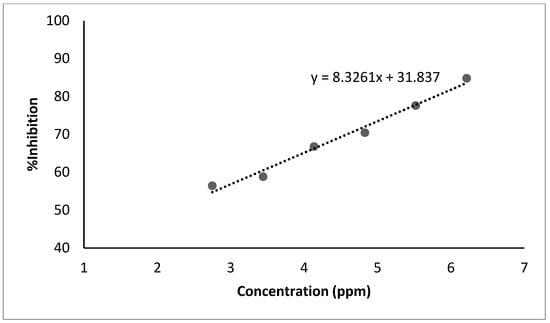
Figure 3.
Antioxidant activity diagram (DPPH) of sacha inchi seed oil.
3.6. Determination of Quantitation Limits for Microbial Contamination
The complete results of the microbial contamination analysis are presented in Table 6. The results of the total plate number and yeast mold analysis were zero; this indicates that sacha inchi seed oil is safe to use as an active raw material.

Table 6.
Determination of quantitation limits for microbial contamination result.
3.7. Determination of Quantitation Limits for Heavy-Metal Contamination
The complete results of the analysis of heavy-metal contamination, including arsenic, cadmium, lead, and mercury, are presented in Table 7. The analysis results of heavy-metal contamination were <0.0001 mg/kg; this indicates that sacha inchi seed oil is safe to use as an active raw material.

Table 7.
Determination of quantitation limits for heavy-metal contamination result.
4. Discussion
The sacha inchi plant has the scientific name Plukenetia volubilis L. This plant belongs to the Tracheophyta division, class Magnoliopsida, order Malpighiales, family Euphorbiaceae, and genus Plukenetia L. The sacha inchi plant comes from Peru and is commonly known as the Inca bean, wild bean, or bean sacha [8,38]. Initially, the sacha inchi plant originated from Peru, Ecuador, and Colombia. However, because of its popularity as an anti-aging agent and its inclusion in the projected list of promising cosmetic ingredients in the future, countries outside South America began to cultivate the sacha inchi plant commercially, especially in the Southeast Asia region, one of which is Indonesia. Raw materials for sacha inchi seed oil were developed to maximize the potential of sacha inchi seed oil as a raw material for anti-aging cosmetics. This started with the sacha inchi seed extraction process, followed by examining the quality parameters of sacha inchi seed oil, phytochemical screening, and determining its content (fatty acid profile, determination of microbiological contamination, heavy-metal contamination, vitamin analysis, and antioxidant activity testing) [38].
Empirically, sacha inchi seed oil is milled; sacha inchi seeds are ground into flour and then extracted [5]. However, for commercial purposes, the way to obtain sacha inchi seed oil is to extract the seeds using the cold-pressing method, Soxhlet extraction, supercritical carbon dioxide (CO2) method, and enzymatic methods. In this research, sacha inchi seed oil was obtained using the cold-pressing method utilizing an oil-pressing machine. The screw-pressing method is receiving renewed interest as an alternative to solvent extraction, especially for specialty oils. Mechanical screw presses usually recover 75–95% of the oil from oil seeds [39]. Also, screw pressing is generally preferred as it retains more of those components that are beneficial to health, such as antioxidants, thereby increasing the nutritional value of the oil. The yield of sacha inchi seed oil obtained was 1000 mL with a percentage of 50%. In this study, the yield value of sacha inchi oil was higher than sacha inchi seed oil from Peru and Colombia; this was influenced by several factors, such as location and plant growth conditions and the extraction method used [40,41].
Sacha inchi seed oil is a natural ingredient and is predicted to become popular in the next few years because of its high omega-3 content, which is 48% of the total fatty acids in sacha inchi seed oil. The omega-3 content in sacha inchi seed oil is very high compared to argan and olive oil, which contain <0.5% and 0–1.5% omega-3, respectively [9,38,42]. Omega-3 is effective in skin regeneration by controlling inflammation and collagen synthesis. Therefore, it is widely used in skincare products, especially as an anti-aging agent. Another advantage is that it contains vitamins A, D, and E, which have anti-aging properties, and bioactive compounds that potentially act as antioxidants, including flavonoids and terpenoids. However, sacha inchi seed oil has a distinctive and quite strong odor, which may be unpleasant to some individuals [7,9,38].
The potential mechanism of sacha inchi in anti-aging is supported by the chemical compounds contained in its seed oil, such as omega-3, vitamins A, D, and E, flavonoids, and triterpenoids. Omega-3 has an anti-aging mechanism by suppressing damage to keratinocytes due to UV light, which is possibly due to the regulation of the COX-2 pathway, NF-κB, and mitogen-activated protein kinase (MAPK)/extracellular-signal-regulated kinase (ERK) pathway. Exposure to UV rays can induce metalloproteinases (MMPs), which can cause damage to connective tissue, resulting in aging and wrinkles on the skin [43]. Vitamin A (retinol) provides its effects by directly affecting collagen metabolism and stimulating the synthesis of collagen and elastic fibers [11]. Vitamin D increases collagen synthesis and clinically improves skin elasticity [15]. Vitamin E exhibits its antioxidant activity in biological systems by protecting unsaturated fatty acids from oxidation. Flavonoids have the potential to act as antioxidants, which play a role in preventing the formation and elimination of free radicals by inhibiting hydrogen peroxide and reducing the activity of iron ions [38].
The quality parameters of sacha inchi seed oil need to be inspected. This is because an active ingredient that will be formulated into a dosage form must first go through a quality parameter inspection stage so that the ingredient used meets the specified safety requirements. Oil quality parameter testing includes organoleptic, water content, ash content, relative density, acid value, peroxide value, iodine, and refractive index testing.
The organoleptic examination of sacha inchi seed oil was conducted as an initial introduction that was as simple and objective as possible. Organoleptic parameter tests are carried out by observing samples using the five senses to describe shape, color, odor, and consistency [17]. From organoleptic observations, sacha inchi oil is a thick yellow liquid with a characteristic odor.
Relative density is a comparison of the density of oil at a temperature of 25 °C to the density of water at the same temperature. The determination of relative density was performed using a clean, dry, and calibrated pycnometer. The specific gravity of an organic compound is influenced by molecular weight, polarity, temperature, and pressure. In general, the relative density value of oil ranges from 0.696 to 1.188 g/cm3 [44]. Determination of relative density aims to provide an overview of the mass per volume and indicate the level of purity and contaminants that may be contained in the oil. Oil with a specific gravity value different from the standard may contain contaminants. The results of determining the relative density of sacha inchi seed oil show a value of 0.91 g/cm3. This value corresponds to the reference relative density value of 0.91–0.94 g/cm3 [27,29].
The acid value measures the amount of free fatty acids from the hydrolysis of oil or fat. It can also be said to be milligrams of KOH, used to neutralize the free fatty acids in 1 g of oil or fat. A large acid number can indicate poor oil processing, where the higher the acid number, the lower the oil quality produced. The results of determining the acid number of sacha inchi seed oil show a value of 0.38 ± 0.02 mg/g. This value concurs with the reference acid number value of 0.37–2.40 mg/g. According to the Food and Agriculture Organization, the acid number for cold-pressed vegetable oils should not exceed 4 mg/g. The low acidity of this acid reflects the low refining and good quality of sacha inchi seed oil [29].
Determining the peroxide value aims to determine the degradation or degree of damage to the oil. As mentioned by Hildago et al. [29] this test directly measures the concentration of hydroperoxide from the primary oxidation in the oxidation of products, which, according to the FAO/OMS, must not exceed 10 mEq/kg in the case of vegetable oils. The results of the peroxide number in sacha inch oil obtained in this study were 11.01 mEq/kg. This value corresponds to the reference peroxide value, 1.50–19.10 mEq/kg [27,29,32,33,34,41].
The iodine number examination aims to measure the amount of unsaturated fatty acids in the oil. The higher the amount of iodine absorbed, the more saturated the oil. According to Muangrat et al. [45] this characteristic of the oil is related to its unsaturation, which, as seen in Table 1, gives a value of 179.32 g/100 g. Comparing it with the report by Paucar-Menacho et al. [28] for olive oil (56.15 g I2/100 g), the sacha inchi oil shows a very high value in the iodine index, reflecting that this oil has greater unsaturation compared to the other oil.
The refractive index of oil is the ratio between the sine of the angle of incidence and the sine of the angle of refraction if a beam of light with a particular wavelength falls from air to oil at a certain angle. The tool used to measure the refractive index is a Guenther refractometer. When determining the refractive index, the oil must be kept away from hot and humid weather. Hot and humid weather can condense on the cold surface of the prism and cause blurry separation between the dark and light prisms. Therefore, the dividing lines will appear sharper and cause the refractive index value to be low. Determination of the refractive index can be used to determine an oil’s purity. The smaller the refractive index value, the greater the water content in the oil due to the nature of water, which efficiently refracts the incoming light, so oil with an immense refractive index value is better than oil with a small refractive index value. The result of determining the refractive index of sacha inch oil is 1.479. This value concurs with the reference relative density value of 1.475–1.482 [27,28,31,33].
Screening for phytochemicals was also performed, along with quality parameter evaluation. Phytochemical screening was performed on sacha inchi seed oil to qualitatively identify the secondary metabolite content [23]. The results of the phytochemical screening of sacha inchi seed oil showed the presence of several active compounds of flavonoids, steroids, and terpenoids, as shown in Table 2. Puangpronpitag et al. [6] revealed flavonoid, steroid, and terpenoid compounds in sacha inchi seed oil. This compound is known to have therapeutic activities, including antimicrobial, anti-inflammatory, and antioxidant [46]. Antioxidant compounds can inhibit the formation of free radicals and their activity against the enzymes collagenase, elastase, hyaluronidase, and tyrosinase, where these enzymes can accelerate aging if they experience increased activity [2,6].
Sacha inchi seed oil has high levels of fatty acids. Determination of the fatty acid profile of sacha inchi seed oil was conducted using a gas chromatography flame ionization detector (GC-FID). According to GC-FID analysis, the fatty acid profile of the oil is shown in Table 3. The oil was found to be rich in linolenic acid (omega-3, 48.5%), linoleic acid (omega-6, 34.8%), and oleic acid (omega-9, 7.7%). Similar to recent reports by Maurer et al. [34] and Gutierrez et al. [47], the fatty acid composition of the sacha inchi oil was dominated by linolenic, linoleic, and oleic acids, which were present at higher levels than found in other vegetable oils, such as olive oil. Previous studies have found that topical use of omega-3 and omega-6 can restore the permeability barrier and improve skin appearance [17,24]. Therefore, sacha inchi oil might be an alternative source of essential fatty acids for improving skin appearance and function through topical administration.
Sacha inchi oil is an excellent antioxidant source. Antioxidant activity was tested in ascorbic acid, sacha inchi seed oil, facial serum base, and facial serum preparations containing sacha inchi seed oil. The method used was DPPH [24]. The free radical scavenging activities of the oil were determined using a DPPH scavenging assay, and the results are displayed in Table 5. From these data, the IC50 value produced by this research is classified in the very strong category with a value of 8.859 ppm based on the IC50 antioxidant category grouping (Table 8). However, the IC50 value is still lower than the results of research conducted by Puangpronpitag et al. [6] of 7 ppm.

Table 8.
Antioxidant characteristics based on IC50 values.
Sacha inchi seed oil produced an IC50 value classified as very strong [48]. These effects happen because the sacha inchi seed oil has chemicals such as antioxidants, vitamin E, and poly and monounsaturated fatty acids. This aligns with research conducted by Cardenas et al. [49]. The antioxidant potential of the flavonoids in sacha inchi seed oil plays a role in preventing the formation and elimination of free radicals by inhibiting hydrogen peroxide and reducing the activity of iron ions forming iron. Vitamin E also shows its antioxidant activity in biological systems by protecting unsaturated fatty acids from oxidation [6,36]. In addition, the antioxidant properties of flavonoids come from their ability to transfer an electron to free radical compounds and form complexes with metals. These two mechanisms cause flavonoids to have several effects, including inhibiting lipid peroxidation, suppressing tissue damage by free radicals, and inhibiting the activity of several enzymes [50]. The complete reaction between flavonoids and free radicals can be seen in Figure 4.
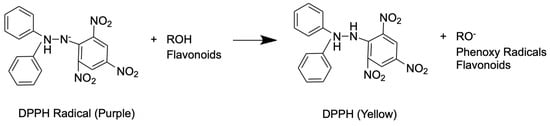
Figure 4.
Mechanism of flavonoids against free radicals.
In flavonoid compounds, the free radical scavenging activity is believed to be influenced by the number and position of phenolic hydrogens in the molecule. Thus, a higher antioxidant activity will be produced in phenolic compounds that have a more significant number of hydroxyl groups in their flavonoid core. These phenolic compounds can donate hydrogen; therefore, the antioxidant activity of phenolic compounds can be produced in the free radical neutralization reaction, which initiates the oxidation process or terminates the chain radical reaction that occurs [1,4,51].
Moreover, sacha inchi seed oil also contains other active compounds with anti-aging potential, such as vitamins A, D, and E, which have antioxidant activity. Vitamin A contributes to skin rejuvenation and smoothness, resulting in a more youthful and smoother appearance. Vitamin E is a major fat-soluble non-enzymatic antioxidant that protects the skin from the adverse effects of oxidative stress, including photoaging. Previous research found that sacha inchi seed oil contains vitamins A and E with specifications of 0.07–0.09 mg/100 g and 279 mg/100 g, respectively [32]. This study found that sacha inchi seed oil contained vitamin A (retinol) of 123.42 mg/100 g, which was higher than the specifications in the literature. Meanwhile, the vitamin E found in this study had a lower value than the specifications, 145.06 mg/100 g. This study also found a vitamin D content of 899.46 mg/100 g, which has not been found in other studies.
In this research, microbial contamination and heavy-metal content were also tested. Microbial contamination is internal contamination originating from microbes that are harmful to human health. Microbial contamination was measured by the TPC and YMC methods. The total plate number test shows the amount of bacterial contamination in a sample, while the yeast mold test shows the amount of yeast mold contamination in a sample. The purpose of this test is to ensure that sacha inchi seed oil produced from the cold-press process meets the contamination limit requirements required for standardized cosmetic raw materials. Microbial contamination can cause health risks due to the presence of pathogenic bacteria. The requirements for microbial contamination in the TPC and YMC tests for cosmetics other than for children under 3 years, areas around the eyes, and mucous membranes are no more than 103 colonies/mL. The TPC and YMC test results are presented in Table 6, indicating that the test results meet the requirements of no more than 103 colonies/mL. Furthermore, the results of the heavy-metal contamination test can be seen in Table 7. The test results showed no heavy-metal contamination, such as As, Cd, Pb, and Hg. Heavy-metal contamination is contamination in the form of metal and metalloid chemical elements, which have a high atomic weight and specific gravity and are toxic to living things. Evaluation of heavy metals is one of the tests that must be carried out to ensure the safety of cosmetic raw materials [37].
5. Conclusions
This study focuses mainly on identifying and assessing the safety of sacha inchi seed oil to provide valuable information for its application in cosmetic manufacturing and formulation. This research analyzed the results of the physicochemical characteristics, fatty acid profile, vitamins A, D, and E contents, and antioxidant activity of sacha inchi seed oil as an active raw material. Sacha inchi seed oil has the characteristics of a liquid consistency, yellow color, and a distinctive odor, with quality parameters of a relative density value (0.91 g/cm3), acid number (0.38 ± 0.02 mg/g), peroxide value (11.01 mEq/kg), iodine value (179.32 g/100 g), and a refractive index of 1.479. The standardized flavonoid, steroid, and triterpenoid contents in sacha inchi seed oil also show a good effect in terms of its antioxidant activity in vitro, as proven by the IC50 value in the DPPH antioxidant activity test amounting to 8.859 ppm, which is categorized as very strong. This chemical content shows a synergistic effect with unsaturated fatty acids, including omega-3 (48.5%), omega 6- (34.8%), and omega-9 (7.7%) as well as with vitamin A (123.42 mg/100 g), vitamin D (899.46 mg/100 g), and vitamin E (145.06 mg/100 g), playing a role in regenerating skin cells, maintaining skin moisture and elasticity, and stimulating collagen production. These discoveries are envisioned to serve as the foundation for formulating sacha inchi oil into cosmetic products with anti-aging properties and producing it by applicable regulatory standards for societal benefits.
Author Contributions
Conceptualization, I.M., S.S. and C.K.K.; methodology, E.A. and S.R.M.; investigation, I.M. and N.A.P.; resources, S.S. and C.K.K.; data curation, S.R.M.; writing—original draft preparation, I.M. and S.S.; writing—review and editing, I.M., S.S. and D.O.W.; visualization, I.M. and D.O.W.; supervision, N.A.P. and E.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported in part by the Directorate General of Higher Education, Research and Technology (DGHERT); the Ministry of Education, Culture, Research, and Technology (MOECRT) of the Republic of Indonesia, and the Education Fund Management Institute (LPDP) SK No. 290/E1/HK.02.02/2023.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Acknowledgments
The authors thank the Ministry of Education, Culture, Research, and Technology (MOECRT) of the Republic of Indonesia, and the Education Fund Management Institute (LPDP).
Conflicts of Interest
Cahya Khairani Kusumawulan was employed by the company. The remaining authors declare that research was conducted in the absence of any commercial of financial relationships that could be constructed as a potential conflict of interest.
References
- Barel, A.; Paye, M.; Maibach, H. Handbook of Cosmetic Science and Technology, 3rd ed.; Informa Healthcare USA Inc.: New York, NY, USA, 2009. [Google Scholar]
- Irianti, T.; Suwijoyo, P.; Sugiyanto, S. Penuaan dan Pencegahannya; Universitas Gadjah Mada Press: Yogyakarta, Indonesia, 2021. [Google Scholar]
- Hernani, R. Tanaman Berkhasiat Antioksidan; CV Trans Info Media: Jakarta, Indonesia, 2005. [Google Scholar]
- Baumann, L.; Shagari, S.; Weisberg, E. Dermatology; McGraw Hill: New York, NY, USA, 2009. [Google Scholar]
- Gonzalez-Aspajo, G.; Belkhelfa, H.; Haddioui-Hbabi, L.; Bourdy, G.; Deharo, E. Sacha Inchi Oil (Plukenetia volubilis L.), Effect on Adherence of Staphylococus aureus to Human Skin Explant and Keratinocytes in Vitro. J. Ethnopharmacol. 2015, 171, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Puangpronpitag, D.; Tankitjanon, P.; Sumalee, A.; Konsue, A. Phytochemical Screening and Antioxidant Activities of the Seedling Extracts from Inca Peanut Plukenetia Volubilis. Pharmacogn. J. 2021, 13, 52–58. [Google Scholar] [CrossRef]
- Hadzich, A.; Gross, G.; Leimbach, M.; Ispas, A.; Bund, A.; Flores, S. Characterization of Plukenetia volubilis L. Fatty Acid-Based Alkyd Resins. Polym. Test. 2020, 82, 106296. [Google Scholar] [CrossRef]
- Chirinos, R.; Necochea, O.; Pedreschi, R.; Campos, D. Sacha Inchi (Plukenetia volubilis L.) Shell: An Alternative Source of Phenolic Compounds and Antioxidants. Int. J. Food Sci. Technol. 2016, 51, 986–993. [Google Scholar] [CrossRef]
- Murargo, Y. Potensi Kosmetik Natural Indonesia dan Persyaratan Berkelanjutan Sebagai Referensi Pasar di Uni Eropa; Atase Keuangan KBRI Brussel: Woluwe Saint Pierre, Belgium, 2021. [Google Scholar]
- Brinckmann, J. Market Analysis for Three Peruvian Natural Ingredients; International Trade Center: Geneva, Switzerland, 2009. [Google Scholar]
- Ganceviciene, R.; Liakou, A.; Theodoridis, A.; Makrantonaki, E.; Zouboulis, C. Skin Anti-Aging Strategies. Derm.-Endocrinol. 2012, 4, 308–319. [Google Scholar] [CrossRef]
- Boo, Y. Mechanistic Basis and Clinical Evidence for the Applications of Nicotinamide (Niacinamide) to Control Skin Aging and Pigmentation. Antioxidants 2021, 10, 1315. [Google Scholar] [CrossRef] [PubMed]
- Al-Niaimi, F.; Chiang, N. Topical Vitamin C and the Skin: Mechanisms of Action and Clinical Applications. J. Clin. Aesthetic Dermatol. 2017, 10, 14–17. [Google Scholar]
- Boo, Y. Ascorbic Acid (Vitamin C) as a Cosmeceutical to Increase Dermal Collagen for Skin Antiaging Purposes: Emerging Combination Therapies. Antioxidants 2022, 11, 1663. [Google Scholar] [CrossRef]
- Danimayostu, A.; Martien, R.; Lukitaningsih, E.; Danarti, R. Vitamin D3 and the Molecular Pathway of Skin Aging. Indones. J. Pharm. 2023, 34, 357–371. [Google Scholar] [CrossRef]
- Sembiring, E.; Elya, B.; Sauriasari, R. Phytochemical Screening, Total Flavonoid and Total Phenolic Content and Antioxidant Activity of Different Parts of Caesalpinia bonduc (L.) Roxb. Pharmacogn. J. 2018, 10, 123–127. [Google Scholar] [CrossRef]
- Soimee, W.; Nakyai, W.; Charoensit, P.; Grandmottet, F.; Worasakwutiphong, S.; Phimnuan, P.; Viyoch, J. Evaluation of Moisturizing and Irritation Potential of Sacha Inchi Oil. J. Cosmet. Dermatol. 2019, 19, 915–924. [Google Scholar] [CrossRef] [PubMed]
- Kementrian Kesehatan Republik Indonesia. Farmakope Herbal Indonesia; Kementrian Kesehatan Republik Indonesia: Jakarta, Indonesia, 2017.
- Departmen Kesehatan Republik Indonesia. Parameter Standar Umum Ekstrak Tumbuhan Obat; Departemen Kesehatan Republik Indonesia: Jakarta, Indonesia, 2000.
- SNI 01-3555-1998; Cara Uji Minyak Dan Lemak. Badan Standar Nasional: Jakarta, Indonesia, 1998.
- AOCS. Official Methods and Recommended Practice of the American Oil Chemists’ Society; Method Cc 7-25 (Refractive Index); AOCS: Boulder Urbana, IL, USA, 2009. [Google Scholar]
- Departemen Kesehatan Republik Indonesia. Materia Medika Indonesia Jilid VI; Departemen Kesehatan Republik Indonesia: Jakarta, Indonesia, 1995.
- Harborne, J. Phytochemical Methods a Guide to Modern Techniques of Plant Analysis, 3rd ed.; Chapman and Hall: London, UK, 1987. [Google Scholar]
- Liua, Q.; Xu, Y.K.; Zhang, P.; Na, Z.; Tang, T.; Shi, Y.X. Chemical Composition and Oxidative Evolution of Sacha Inchi (Plukentia volubilis L.) Oil from Xishuangbanna (China). Grasas Aceites 2014, 65, 012. [Google Scholar] [CrossRef]
- Peraturan Kepala Badan Pengawas Obat dan Makanan Republik Indonesia Nomor HK.03.1.23.08.11.07331 Tahun 2011 Tentang Metode Analisis Kosmetika; BPOM RI: Jakarta, Indonesia, 2011.
- Indrawijaya, B.; Oktavia, H.; Cahyani, W.E.; Studi, P.; Kimia, T.; Teknik, F.; Pamulang, U.; Selatan, T. Penentuan Kadar Logam Berat (As, Ba, Cd, Cr, Hg, Pb, Sb, Se) Pada Mainan Anak Dengan Metode SNI ISO 8124-3:2010 Menggunakan ICP-OES. J. Ilm. Tek. Kim. 2019, 3, 87–94. [Google Scholar]
- Chasquibol, N.A.; Del Aguila, C.; Yacono, J.C.; Guinda, A.; Moreda, W.; Beatriz Gómez-Coca, R.; Del Carmen Pérez-Camino, M. Characterization of Glyceridic and Unsaponifiable Compounds of Sacha Inchi (Plukenetia huayllabambana L.) Oils. J. Agric. Food Chem. 2014, 17, 10162–10169. [Google Scholar] [CrossRef] [PubMed]
- Paucar-Menacho, L.M.; Salvador-Reyes, R.; Guillén-Sánchez, J.; Capa-Robles, J.; Moreno-Rojo, C. Comparative Study of Physical-Chemical Features of Sacha Inchi Oil (Plukenetia volubilis L.), Olive Oil (Olea europaea) and Fish Oil. Sci. Agropecu. 2015, 6, 279–290. [Google Scholar] [CrossRef]
- Hildago, R.; Eduardo, L.; Rogel, V.; Jefferson, C.; Bermeo, B.; Michelle, S. Characterization of Sacha Inchi Seed Oil (Plukenetia volubilis) from Canton San Vincente, Manabi, Ecuador Obtained by Non-Thermal Extrusion Processes. La Granja Rev. Cienc. La Vida 2019, 30, 70–79. [Google Scholar]
- Chirinos, R.; Zuloeta, G.; Pedreschi, R.; Mignolet, E.; Larondelle, Y.; Campos, D. Sacha Inchi (Plukenetia volubilis): A Seed Source of Polyunsaturated Fatty Acids, Tocopherols, Phytosterols, Phenolic Compounds and Antioxidant Capacity. Food Chem. 2013, 141, 1732–1739. [Google Scholar] [CrossRef] [PubMed]
- Vicente, J.; de-Carvalho, M.; Garcia-Rojas, E. Fatty Acids Profile of Sacha Inchi Oil and Blends by 1H NMR and GC–FID. Food Chem. 2015, 181, 215–221. [Google Scholar] [CrossRef]
- Kyaw, T.; New, T.; Khaing, M.; San, P.; Kyaing, K.; Thet, T.; Htun, E. Studies on Nutritional Compositions of Sacha Inchi Seed and Physicochemical Characteristics of Sacha Inchi Oil. Int. Eur. Ext. Enablement Sci. Eng. Manag. (IEEESEM) 2019, 7, 111–119. [Google Scholar]
- Penagos-Calvete, D.; Duque, V.; Marimon, C.; Parra, D.M.; Restrepo-Arango, S.K.; Scherf-Clavel, O.; Holzgrabe, U.; Montoya, G.; Salamanca, C.H. Glycerolipid Composition and Advanced Physicochemical Considerations of Sacha Inchi Oil toward Cosmetic Products Formulation. Cosmetics 2019, 6, 70. [Google Scholar] [CrossRef]
- Maurer, N.; Hatta-Sakoda, B.; Pascual-Chagman, G.; Rodriguez-Saona, L. Characterization and Authentication of a Novel Vegetable Source of Omega-3 Fatty Acids, Sacha Inchi (Plukenetia volubilis L.). Food Chem. 2012, 134, 1173–1180. [Google Scholar] [CrossRef]
- R.D. N° 047-2018-INACAL/DN; Norma Tecnica Peruana 151.400, Amendment to NTP 151.400, 2014. Sacha Inchi Oil. Requirements. Norma Tecnica Peruana: Lima, Peru, 2018.
- Wang, S.; Zhu, F.; Kakuda, Y. Sacha Inchi (Plukenetia volubilis L.): Nutritional Composition, Biological Activity, and Uses. Food Chem. 2018, 265, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Badan Pengawas Obat dan Makanan Republik Indonesia. Peraturan Badan Pengawas Obat dan Makanan Nomor 12 Tahun 2019 Tentang Cemaran Dalam Kosmetika; Badan Pengawas Obat dan Makanan Republik Indonesia: Jakarta, Indonesia, 2019. [Google Scholar]
- Maya, I.; Sriwidodo, S. Review: Potensi Minyak Biji Sacha Inchi (Plukenetia volubilis) Sebagai Anti-Aging Dalam Formula Kosmetik. Maj. Farmasetika 2022, 7, 407–423. [Google Scholar]
- Martinez, M.; Penci, M.; Marin, M.; Ribotta, P.; Maestri, D. Screw Press Extraction of Almond (Prunus Dulcis (Miller) D.A. Webb): Oil Recovery and Oxidative Stability. J. Food Eng. 2013, 119, 40–45. [Google Scholar] [CrossRef]
- Zanqui, A.; da Silva, C.; de Morais, D.; Santos, J.; Ribeiro, S.; Eberlin, M.; Cardozo-Filho, L.; Visentainer, J.; Gomes, S.; Matsushita, M. Sacha Inchi (Plukenetia volubilis L.) Oil Composition Varies with Changes in Temperature and Pressure in Subcritical Extraction with n-Propane. Ind. Crops Prod. 2016, 87, 64–70. [Google Scholar] [CrossRef]
- Chirinos, R.; Pedreschi, R.; Domínguez, G.; Campos, D. Comparison of the Physico-Chemical and Phytochemical Characteristics of the Oil of Two Plukenetia Species. Food Chem. 2015, 173, 1203–1206. [Google Scholar] [CrossRef]
- Miklavcic, M.; Taous, F.; Valencic, V.; Elghali, T.; Podgornik, M.; Strojnik, L.; Ogrinc, N. Fatty Acid Composition of Cosmetic Argan Oil: Provenience and Authenticity Criteria. Molecules 2020, 25, 4080. [Google Scholar] [CrossRef]
- Huang, T.; Wang, P.; Yang, S.; Chou, W.; Fang, J. Cosmetic and Therapeutic Applications of Fish Oil’s Fatty Acids on the Skin. Mar. Drugs 2018, 16, 256. [Google Scholar] [CrossRef]
- Guenther, E. Minyak Atsiri Jilid I; UI Press: Jakarta, Indonesia, 1987. [Google Scholar]
- Muangrat, R.; Veeraphong, P.; Chantee, N. Screw Press Extraction of Sacha Inchi Seeds: Oil Yield and Its Chemical Composition and Antioxidant Properties. J. Food Process. Preserv. 2018, 42, e13635. [Google Scholar] [CrossRef]
- Srichamnong, W.; Ting, P.; Pitchakarn, P.; Nuchuchua, O.; Temviriyanukul, P. Safety Assessment of Plukenetia volubilis (Inca Peanut) Seeds, Leaves, and Their Products. Food Sci. Nutr. 2018, 6, 962–969. [Google Scholar] [CrossRef]
- Gutiérrez, L.; Rosada, L.; Jiménez, A. Chemical Composition of Sacha Inchi (Plukenetia volubilis L.) Seeds and Characteristics of Their Lipid Fraction. Grasas Aceites 2011, 62, 76–83. [Google Scholar] [CrossRef]
- Molyneux, P. The Use of The Stable Free Radikal Diphenylpicrylhydrazyl (DPPH) For Estimating Antioxidant Activity. J. Sci. Technol. 2004, 26, 211–219. [Google Scholar]
- Cárdenas, D.M.; Rave, L.J.G.; Soto, J.A. Biological Activity of Sacha Inchi (Plukenetia volubilis Linneo) and Potential Uses in Human Health: A Review. Food Technol. Biotechnol. 2021, 59, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Porat, Y.; Abramowitz, A.; Gazit, E. Inhibition of Amyloid Fibril Formation by Polyphenols: Structural Similarity and Aromatic Interactions as a Common Inhibition Mechanism. Chem. Biol. Drug Des. 2006, 67, 27–37. [Google Scholar] [CrossRef]
- Purwaningsih, S.; Salamah, E.; Budiarti, T.A.; Perikanan, F.; Kelautan, I.; Pertanian Bogor, I. Formulasi Skin Lotion Dengan Penambahan Karagenan Dan Antioksidan Alami Dari Rhizophora Mucronata Lamk. J. Akuatika 2014, 5, 55–62. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).