Abstract
We sought to incorporate a community-based solution with a family health history (FHH) clinical support program (MeTree) integrated into well-patient appointments with the novel partnership of a public health state-level health information exchange (HIE). The Arkansas—Making History pilot project tested informatics compatibility among these systems and the patients’ electronic medical record (EPIC) in a rural clinic in the north central region of the state, having the state HIE as a means for patients to store and share their FHHs across multiple healthcare providers with updates in real time. We monitored for unexpected issues during the pilot and asked for the perspectives of patients and healthcare providers throughout the project to have a clear understanding of how to implement this project on a larger scale. The greatest barrier to project implementation was the inability of the state HIE to host or share the FHH data. We compensated for the lack of systems compatibility and documented valuable information about patient acceptability and usability of the MeTree platform, as well as gleaning important clinical outcome data from those who completed MeTree FHH accounts in an underserved area. Rural patients need additional technological support in the larger scaling of this project, both in available linkages to community clinics with patient-controlled options for how their data is stored and shared and in Internet connectivity and software options available for ease of use.
1. Introduction
Our understanding of the genetic mechanisms influencing human disease has grown exponentially since the completion of the Human Genome Project in 2003 [1]. This abundance of new knowledge has dramatically expanded our capability for assessing an individual’s personalized risk for genetically influenced disease(s) based on their family health history (FHH) [2]. As a result, clinical guidelines for a plethora of diseases and conditions now include recommendations to collect and analyze an individual’s FHH; however, healthcare providers (HCPs), particularly in remote rural areas, are unable to fully leverage the existing science due to several critical barriers, including managing diverse under-resourced populations with complex medical conditions, limited access to specialists and specialty guidance, and inadequate face time during appointments. In addition, patients are often unable to recall, or do not know, details of their FHH and are unprepared to provide full FHHs at their appointments.
Arkansas (AR) is a predominantly rural state in the southern United States (US). The state ranks among the top 10 US states for the highest death rates due to heart disease, cancer, chronic lower respiratory disease, stroke, Alzheimer’s disease, diabetes, and kidney disease [3]. Access barriers to genetic services in Arkansas include sparse services that are clustered in the central and northwestern regions of the state, making it challenging for already overwhelmed HCPs to provide individual-level genetic risk assessments to maximize preventive care and treatment for their patients. To mitigate this problem and increase access to genetic services in rural areas of AR, we sought a comprehensive solution that would address two issues—access to quality patient data for HCPs and access to quality healthcare for patients. Our objectives were as follows: (1) to aid HCPs, especially in rural areas with little existing support, to identify patients at increased risk for genetically influenced disease(s) by providing guidance on genetic screening, testing, and referral within a shared decision-making framework that would engage patients and their families in decisions around their risk and recommended actions to address that risk; and (2) to eliminate time-intensive, inefficient, and redundant updates of FHHs at each patient appointment by enabling a single comprehensive patient-updated FHH to be available across multiple HCPs in real time—for example, for ophthalmologic care, well-woman care, dental care, etc.
To achieve this goal, we proposed incorporating the patient-facing MeTree FHH risk assessment platform (MeTree) into the AR Department of Health’s SHARE (State Health Alliance for Records Exchange), and the patients’ electronic health record (EHR). SHARE integrates with providers’ EHR using a blue-button approach to receiving and sharing patient health data from clinical sites across the state. Access to the health exchange promotes greater portability and real-time access to valuable information generated in different locations around the state. MeTree guides patients in entering a comprehensive FHH for 145 medical conditions on 3+ generations of relatives and analyzes that information across 45 different genetic risk assessment guidelines [4]. By integrating MeTree with SHARE (and thereby the EHR), providers would be able to access the most up-to-date FHH information, current level of risk for genetically influenced disease(s), and evidence-based recommendations for reducing that risk. We used an adaptation of the Genomic Medicine Integrative Research (GMIR) Framework to organize the overall development and implementation of the project and to demonstrate the project’s potential broader application to rural areas beyond this state [5].
We implemented this pilot project with the following objectives:
- Establish informatics compatibility between the MeTree clinical decision support platform and the AR SHARE health information exchange (HIE) and confirm the successful transfer of information from MeTree to SHARE and from SHARE to the EHR (Epic) at a rural primary care clinic in Arkansas;
- Identify and correct any issues that may impede larger program implementation; and
- Explore the perspectives of the HCPs and patients in the pilot by understanding how the program impacted care and workflow during well-care patient appointments and measure patient-reported usability and acceptability.
The most important component of this pilot study was the incorporation of the FHH clinical support software platform, MeTree, into well-patient appointments to help HCPs identify patients at increased risk for genetically influenced disease(s) using automated computerized algorithms. MeTree is a software solution designed to help patients and HCPs optimize risk assessment and integrate risk assessment processes into existing workflows and medical records systems. It is a web-based, patient-facing FHH collection and risk assessment technology that provides personalized assessments to patients and HCPs using physician-curated algorithms based on current US clinical guidelines with actionable recommendations to mitigate disease risk [4]. The program collects disease history for 145 conditions to produce risk recommendations for 45 conditions and syndromes based on the current scientific evidence and clinical guidelines from the latest research studies and recommendations across multiple health disciplines, for example, the American Heart Association, the American Diabetes Association, and the American Cancer Association, to name just a few. MeTree provides integrated education on how to identify and collect disease information from their families so patients may create three-generation family trees while in their home settings. Using these data, risk assessment reports are tailored to each user (patient and HCP) to enhance shared decision-making around risk and recommended actions to mitigate that risk. Once risk reports are generated, additional educational resources are provided to the patient through the platform to further explain their recommendations and facilitate sharing information with family members if they choose.
The Office of the National Coordinator (ONC), which oversees health systems’ use of EHRs, strongly encourages interoperability between EHRs and other technology applications (such as MeTree). To ensure streamlined, secure, and standardized data sharing, the ONC strongly encourages applications to connect using SMART on FHIR data standards. MeTree employs SMART on FHIR to integrate with existing EHR systems for seamless single sign-on patient and provider access and streamlined data updates over time. MeTree has been used in research studies by over 35,000 patients in 18 health systems across three countries to test its performance. In these studies, 25% of primary care patients were reported to be at risk for a hereditary condition and 26% met guidelines for enhanced disease screening modalities or frequencies, for example, atherosclerotic cardiovascular disease or breast cancer [4,6].
The 2009 Health Information Technology for Economic and Clinical Health (HITECH) Act, part of the American Recovery and Reinvestment Act of 2009, provided an opportunity for states to build and implement electronic HIEs capable of connecting providers and healthcare facilities within states to share patient healthcare information and provide more comprehensive care [7]. The groundwork for AR’s SHARE was laid prior to the HITECH Act through a Robert Wood Johnson Center for Health Care Strategies’ Regional Quality Improvement Initiative [8]. This initiative was launched in 2006 with the aim of improving chronic care in certain regions of the US by bringing together HCPs and payers around shared quality improvement goals. AR’s Quality Improvement program objectives included the design of a state HIE that could contribute to the evaluation of performance measures for payers and providers. They included the following:
- A business plan and roadmap for statewide HIE;
- A multi-payer quality measurement reporting system; and
- A compendium of quality improvement interventions and educational materials [8].
There are currently more than 3000 AR medical practices, behavioral health facilities, hospitals, long-term post-acute facilities, pharmacies, and other healthcare facilities, including AR Department of Health (ADH) county health units, that participate in the SHARE program.
MeTree has been tested in rural and urban communities, academic and community practices, and low socio-economic and more resourced populations; however, it has never been tested in a public health application using a state HIE as proposed in this project.
2. Materials and Methods
2.1. GMIR Framework
The complexity of factors when integrating genetics and genomics into clinical practice is immense. The GMIR Framework was developed to address the unique challenges in the implementation of genomic medicine, including genomic variation, gene–environmental influences, and social implications in diverse populations [5]. Its conceptual foundation was based on the integration of a model used by the Implementing Genomics in Practice (IGNITE) network with the Consolidated Framework for Implementation Research (CFIR) [9,10]. It was then revised and refined over several prioritization rounds by stakeholders, which included members from groups such as IGNITE, the Clinical Sequencing Evidence-Generating Research consortium [11] (both funded by the National Institutes of Health National Human Genome Research Institute), the Electronic Medical Records and Genomics (eMERGE) network [12], and the Population Architecture using Genomics and Epidemiology network [13,14]. We adapted the GMIR multi-level framework, represented in Figure 1, to arrive at the overarching domains, sub-domains, and concepts used to guide this project (Table 1).
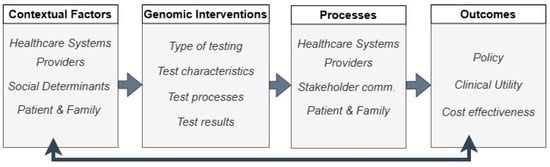
Figure 1.
Representation of the GMIR Framework [5].

Table 1.
Model of the overarching domains, sub-domains, and concepts for the Arkansas—Making History Project based on the GMIR Framework [5].
2.2. Technical Planning
Our team and the SHARE team met over a period of 10 months to develop strategies for on-boarding patient-created MeTree accounts to the SHARE HIE infrastructure. To do this, we opted to (1) match patients by sending MeTree’s patient account identifiers to SHARE and SHARE returning their unique patient identifier to MeTree, and (2) upload MeTree data (both the patient-entered FHH and MeTree-generated risk assessment and clinical decision support) to the patient’s SHARE account via JSON (JavaScript Object Notation). Matching between MeTree and SHARE relied on two of three identifiers (name, birthdate, and/or address). Any remaining unmatched accounts would be reviewed by the project director or research coordinator (RC) to determine how the account needed to be assigned—as a new or existing EHR patient identifier number. Once uploaded, the FHH, the risk level, and the guideline-based risk reducing recommendations would be available for any provider with SHARE access for that patient. Data exchange would occur at least weekly and be overseen by the chief technology officer at SHARE and the MeTree&You (parent company of MeTree) technical support team.
We collaborated with faculty members from the University of Arkansas for Medical Sciences (UAMS) Institute for Digital Health and Innovation (IDHI) team to build the landing spot within the UAMS EHR, Epic, where the MeTree patient record would reside.
2.3. Rural Project Site
We tested system compatibility among MeTree, SHARE, and Epic in the UAMS North Central Regional Program Office clinic in Batesville, AR [15]. The city of Batesville has a population of approximately 11,000 individuals living in around 4100 households. Of these, 73% have broadband Internet subscriptions, and according to US Census data, the percentage of Batesville residents remaining in the same house for successive years is high [16]. The medical director of the UAMS Batesville Regional Clinic is the physician grandson of the clinic’s founding family physician and is well respected in the area. The Batesville clinic’s unique factors—a rural population, established trusting relationships with the clinic and its providers, and the high likelihood of patients having additional healthcare relationships in the area—made it the ideal location to initiate this pilot project. We anticipated additional research studies within the community to assess the longer-term impact of this program on patients’ and their families’ care beyond the primary care clinic.
The UAMS North Central’s mission focuses on wellness and prevention strategies for area residents and the education of healthcare professionals through a medical residency program [15]. The Batesville clinic provides primary care medical services to patients of all ages. The clinic not only offers treatment for acute medical conditions but also ongoing treatment for chronic conditions. We planned onsite training to providers at the Batesville clinic approximately one month prior to beginning recruitment. The Batesville clinic already utilized the SHARE HIE and routinely accessed it for patient information. For this pilot study, providers could access participants’ MeTree data through the SHARE HIE during patient well-care appointments, integrate the FHH into the patients’ EHR, and discuss any actionable recommendations.
We used the UAMS Rural Research Network (RRN) for research recruitment and support. This network was established in January 2020 in response to the high prevalence of health disparities and the lack of research opportunities among rural and minority communities. The RRN, comprising nine regional campuses across the state, leverages the existing clinical and educational infrastructure of UAMS Regional Programs for research and research opportunities to help ensure that AR’s populations have access to community health resources and are included in health research. This partnership allowed us to have an RC who was a member of our project team and who was familiar with the local providers and patients.
2.4. Participants and Recruitment
We submitted our project protocol to the UAMS Institutional Review Board for approval and received a ‘not human subject research’ determination, as this was an assessment of the program on a limited basis before it was expanded across the state and did not meet the regulatory definition of clinical research.
Study eligibility included patients 18 years and older who were scheduled to be seen by providers at the Batesville clinic for well-care appointments from 7 August 2023 to 30 May 2024. The RC identified patients with upcoming well-care appointments and, three weeks prior to their appointments, mailed an appointment reminder along with study information, including educational information about how to complete their FHHs, questionnaires to assist them in collecting information from family members, and links to set up their MeTree accounts if they wished to participate. We placed study fliers in the Batesville clinic to prompt patients with sick-patient appointments to make well-care appointments if they wished to participate (Figure 2). As noted earlier, this study was not considered clinical research since its primary goal was to assess the compatibility and feasibility of integrating an HIE, an FHH risk assessment platform, and an EHR in anticipation of clinical implementation; therefore, a power analysis was not required.

Figure 2.
Recruitment marketing.
Patients were able to log into MeTree accounts at home using a study-specific web link. This allowed them time to talk to family members and to gather details about their FHH that they may not have known otherwise. A study laptop was available at the clinic if patients did not have reliable internet access or computers at their homes. Although broadband access has increased in AR, coverage is not 100%, and the cost of computers and Wi-Fi services can be prohibitive, excluding many lower-income individuals in rural areas from full access. Onsite help support from the project director and/or RC was available in the clinic and by phone during regular business. Questions and answers were logged for future reference and evaluated for opportunities to enhance the pilot.
We conducted debriefing sessions at least weekly, both scheduled and informal, with study team members to gain their unique perspectives and insight into how potential problems were identified, how best to address them, and what changes would need to be made for future implementation. We used this information to continuously adapt and update processes to optimize the patient and provider experience.
2.5. Measurement Tools and Data Analysis
We used the 10-item System Usability Scale (SUS) to collect data around patients’ perceptions of the program’s acceptability and usability. The SUS is validated for use in the implementation of FHH tools, with responses collected on a Likert scale from strongly disagree to strongly agree, with choices ranging from 1 to 5, respectively [17].
The SUS survey results were analyzed in REDCap, with no identifiers. REDCap is a secure, web-based database designed to support data captured for research studies and provides the following: (1) an intuitive interface for validated data entry; (2) audit trails for tracking data manipulation and export procedures; (3) automated export procedures to create de-identified analytical datasets; and (4) procedures for importing data from external sources [18,19].
We assessed HCPs’ perceived utility and experiences during the pilot using a web survey administered through REDCap. The survey collected providers’ perspectives on the study’s impact on their patient care, ascertained the degree to which they agreed or disagreed with the determined risk and/or recommendations for their patients, and elicited open-ended ideas about what providers felt would be important to guide the future expansion of the program.
The results from the HCPs survey were summarized and reported descriptively.
3. Results
Fifteen providers and clinic staff attended the face-to-face didactic training, including six medical residents. The session was recorded and available to all front desk clinic staff to review later at their convenience. In addition, the project director and RC communicated at least weekly by phone and email and via scheduled virtual and onsite meetings throughout the project.
Twenty-one patients completed MeTree accounts and received individualized genetic risk assessments based on their FHHs (see Table 2 for patient characteristics). Fourteen of the 21 patients met national clinical guidelines for needing additional screening, testing, or referrals due to increased disease risk because of their FHHs which was previously not in their health records. We facilitated 100% of the genetic counseling visits via telehealth through either the University Cancer Institute or Department of Adult Genetics. Aggregated, these 14 patients met the guidelines for genetic counseling referrals for an increased risk of 15 hereditary cancer syndromes; genetic counseling referrals for an increased risk of 4 inherited conditions other than cancer (thrombophilia, cardiovascular disease, etc.); and 11 recommendations for additional or enhanced PCP-level screening for elevated risk scores of conditions like diabetes, abdominal aortic aneurysm, or primary biliary liver cirrhosis.

Table 2.
Participant characteristics: 21 received individualized genetic risk assessments, with 14 meeting national clinical guidelines for needing additional screening, testing, or referrals due to increased disease risk.
Thirteen patients completed the SUS. As reported in the Methods Section, the scores are on a Likert scale, with 1 being strongly disagree and 5 being strongly agree. Each question should be interpreted independently. Most people rated their experiences with MeTree favorably, indicating that they thought they would use the program frequently. The acceptability and usability of the program scores were ranked highly, with neutral or lower scores indicated on questions related to the technical aspects of the program, perhaps because of our team’s inability to achieve seamless linkages among the FHH program, state HIE, and clinic EHR. The median SUS scores are in Table 3.

Table 3.
Patient System Usability Scale scores indicating patients’ perceptions of the MeTree program’s acceptability and usability. Scores from 1–5, with 5 correlating with the strongest agreement.
Six individual PCPs in the clinic, including three resident physicians, completed well-care appointments with patients who completed MeTree accounts. Their PCP feedback about the integration of MeTree with well-care patient appointments included the following: (1) an online FHH and risk assessment platform is better than the current process of collecting, analyzing, and using FHH data in clinical practice; and (2) its use is recommended for peers in other clinics. Overall, the providers recommended using an online FHH and risk assessment platform, such as MeTree, in their clinic. They reported that they had suspicions that their patients were likely to have genetic influences driving some diseases but cited a lack of time in the clinic as a barrier to being able to collect and analyze complex FHHs and wading through the myriad clinical practice guideline algorithms to determine appropriate recommendations on each individual’s risk level.
Several providers noted that the additional time required to educate patients about their results during patient appointments was prohibitive to the integration of genetic screening in rural clinic settings. As part of the study design, a genetic counselor was available via telehealth in the rural clinic, and they expressed a need for ongoing access to this type of resource to support results interpretation and education for the patients (and their families, if desired). In addition, they felt this relationship would be valuable and would enhance the overall patient experience. We asked the genetic counselors who provided telehealth appointments in this pilot program about the impact of MeTree’s risk assessment reports on patient communication. One genetic counselor stated that “patients seemed to be empowered” by having the information, also noting the helpfulness of the tool for the providers.
According to provider consensus, using the online FHH and risk assessment tool and facilitating genetic referrals and/or testing did not negatively impact work or patient flow in the rural clinic and supported provider/patient discussion and shared decision-making. One provider described a patient who had previously been denied a screening test by their insurance coverage but was able to qualify for the screening with the additional FHH evidence from MeTree.
We began this project with the support and collaboration of our University IDHI, the University RRN, the University Translational Research Institute, MeTree, the Batesville rural clinic leadership, and the AR SHARE HIE leadership team. However, despite having cooperatively developed a detailed integration plan, at the beginning of the pilot, as we were readying to send the FHH files, the AR HIE leadership team concluded that they were unable to host or share FHH data within the state’s HIE. There were concerns related to file type and formatting, risk adjustment, and privacy/storage raised by the AR HIE team.
Our intention to integrate the MeTree decision support platform with the AR SHARE HIE repository was to have reliable, comprehensive, high-quality, up-to-date FHH records available to multiple HCPs in real time. Since the HIE leadership’s final decision came several weeks after the project start, multiple patients had already completed MeTree, met with their providers, and discussed their risk recommendations. Feedback from these initial patients and provider appointments was positive and demonstrated clear clinical utility; therefore, we decided to continue the pilot and explore other ways to deliver the intervention on a broader scale.
For the ongoing project, we pivoted to a secure central administrative ‘dashboard’, allowing the project director to monitor and manage patient MeTree accounts. When the patients completed data entry, their risk assessment and clinical decision reports were transmitted by secure email to Batesville clinic providers prior to their well-care appointments.
4. Discussion
We implemented this individualized risk assessment program in primary care well-care appointments in a rural clinic using an adaptation of the GMIR Framework [5]. We engaged with academic institution partners and considered the unique healthcare access barriers in remote areas of a rural state and challenging factors for primary care providers when making decisions around genetic screening, testing, and referrals for their complex patients. We assessed patients’ acceptability and usability of the program and tailored interventions to increase the quality of their experiences, for example, using telehealth and collecting genetic testing locally. Although this was a pilot to test implementation strategies with this small number of patients, our results were similar to those of other larger studies, which have reported that around 70% of patients met national clinical guidelines for further screening, testing, or referrals based on their FHHs [6].
We tested health systems processes, i.e., the integration of direct patient engagement with a state agency HIE using the FHH clinical decision-making support tool, MeTree. Next, we asked both patients and providers about their perceptions of how the program impacted communication and workflow during patient appointments. We assessed follow-through for patients that had recommended actions as a result of their FHHs and reported their perceived utility of the program and the impact of the program based on the increase in telehealth utilization in the rural clinic for genetic counseling consultations.
We identified several critical factors relevant for larger scale clinical implementation in rural populations:
- Many rural residents depend on mobile devices for Internet connectivity, and one of the greatest barriers for patients was limited technology and computer experience and access. Given that the current MeTree format is a web-based software as a service (SaaS), requiring an internet connection and a larger device size (best viewed on a tablet or larger), access and ease of use were limited for some participants.
- Although MeTree supports single sign-on (SSO) via Facebook, Apple, and Google, some patients at the Batesville clinic did not have accounts with these services. In addition, the default method for account creation and access (username and password) requires an email address, and among those unable to use SSO, some did not have an email address or could not remember it.
- Technology facility was also a challenge in some situations. For example, some patients who enrolled in the pilot but did not proceed to the next step reported that difficulty with the initial sign-in process frustrated them enough to not proceed further with setting up their FHH.
4.1. Information Technology Integration
Our intent was to integrate the MeTree decision support program with the AR SHARE HIE as a repository, making FHHs and any subsequent updates by patients to their health history records available to multiple HCPs, eliminating the time-intensive updates of FHHs currently performed at each appointment. The initial sharing of test files was successful, demonstrating the compatibility of the MeTree account file format with patient continuity-of-care documents (CCDs) on SHARE. This was an important finding, although we were unable to move the technology aspect of the project into the clinic setting due to various technical complications, including file type and formatting concerns. Patients and providers were receptive to the model of having FHHs on an HIE in a way that promotes shared decision-making and patient engagement in their own healthcare.
We identified an important barrier to the integration of patient engagement with a state agency HIE. Because the state public health HIE was initially purposed as a tool to monitor community health status (AR Code § 25-43-812 [2020]) [8], the Arkansas Office of Health Information Technology was concerned that data elements from the FHH data integrated onto SHARE could be mislabeled as diagnostic data, falsely elevating the state’s pooled Medicaid risk assessment score with SHARE data. Although our use of the HIE aligned with the vision of the state HIE development by supporting primary care HCP chronic care delivery, the potential for skewed risk analyses ultimately outweighed the potential for chronic disease improvements by correctly identifying existing risk and intervening preemptively.
MeTree can provide full EHR integration via FHIR. Without the ability to connect through the SHARE HIE, we could have set up the connection for the patients and providers directly through their EHR (via FHIR); in which case, it would have been very easy for patients and providers to access MeTree, and much of the patient information could have been populated with data from the EHR. However, this scenario would not have provided access to multiple providers in real time, as was planned. This would also have required additional technical resources from both the university and MeTree.
4.2. Patient Impact
Genetic data create additional ethical, legal, and social implications. Everyone’s genotype, unlike an individual’s fingerprints, is uniquely linked not only to the person but also to that person’s family members. This necessitates additional care around how genetic information is shared and stored and increases the importance of family engagement when considering genomic data. Although we were not able to achieve our goal of having FHHs live on the HIE in this project, a model that places access to FHH risk assessment in patients’ hands by way of a consumer-mediated HIE, for the completion of their FHH before well-patient appointments, eliminates privacy and security concerns by giving patients control over what, how, and with whom data will be shared.
Individual-level FHH risk assessments can not only increase appropriate testing for those at increased risk based on guidelines but can also reduce the overutilization of healthcare services among those who do not meet guideline criteria [2]. The clinical results in our small patient sample mirrored that of larger trials [20]; ~70% of patients who completed MeTree accounts met clinical guidelines for further screenings, testing, or referrals based on their FHH.
We found the mean age of patients in this pilot project was older than that of the general population in the Batesville area—60 years in our pilot compared to 36 years in the Batesville population [16]. It may be that older adults have more chronic conditions and are more likely to be followed-up regularly with well-care appointments or that they are more likely to schedule well-care appointments with Medicare health coverage (at age 65 and greater) compared to other coverages or no coverage at all. Regardless of the cause, knowing this pattern might lead us to ‘market’ our larger statewide scale out as valuable health legacies that older generations can gift to younger generations.
We collected limited data on participants’ acceptability and usability of the program. With larger scaling of this program, we plan to conduct focus groups of both providers and patients as well as concurrent think-aloud user testing with the patients.
This small study may not be generalizable to all populations. Our primary goal with the implementation of this program was to improve healthcare access to specialty services and to higher quality healthcare with less burden for rural primary providers. Patients in urban areas may not have the same set of barriers to healthcare access. We did not collect extensive educational histories from patients to know if educational levels influenced abilities to use the program.
4.3. Equitable Genetic Services Access
Ultimately, the broad implementation of an FHH-based risk assessment program could have widespread implications for improved health and wellness in rural areas by increasing individualized risk assessment for a broad spectrum of diseases to enable large-scale access to appropriate enhanced preventive care, diagnosis, and management. Using a clinically validated program to deliver genetic services through primary care appointments decreases transportation and economic barriers for rural patients who would have to obtain these services at distant brick-and-mortar locations. Further pairing this intervention in rural areas with telehealth technology for genetic referrals compounds the impact to communities that have lacked equitable access to the same interventions experienced in more urban areas.
The MeTree program is delivered with patients’ health literacy in mind, providing patient reports in lay language and provider reports with in-depth background and references to aid providers in educating themselves and their patients. Studies have shown significant differences in levels of genomic understanding when comparing urban and rural cohorts [21]. Providers noted challenges when faced with educating their patients about the complex interactions of genetics with chronic diseases, all within constrictive patient appointment slots. Health disparities with rural populations are well documented and are often attributed, in part, to lower general educational levels [22,23,24]. Additional support for providers and patients in rural areas is necessary to increase patients’ knowledge about important aspects of their health and healthcare, which will empower them to live their healthiest lives possible.
4.4. Future Directions
We noted several areas that could build on the outcomes of this project.
Most people rated their experiences with MeTree favorably, indicating that they felt confident in using MeTree and would use the program frequently. As noted previously, we were unable to complete the technical aim of this project. This may have been reflected in patients’ neutral answers to questions such as the MeTree program being cumbersome to use or needing the support of a technical person to be able to use the program, as there were extra steps to getting MeTree reports to their providers in this project. Even so, more than 75% of patients indicated that most people would learn to use the MeTree program very quickly and did not find the program unnecessarily complex. This is important for future work to explore how tools like MeTree could be used in broad ways to impact patients and their health.
Although it helped to have dedicated support staff in the clinic to answer questions and assist patients with setting up their accounts, for broader scaling, we anticipate that one centralized staff member could support a relatively large clinical footprint and still meet the needs of the clinics without demanding the same level of resources that a dedicated person in each clinic would require. In addition, providers (at least in the beginning) need specific guidance and training related to genetic screening, testing, and referral. MeTree significantly aided providers in identifying patients appropriate for these services; but most providers in rural clinics have little to no experience in performing these services. Therefore, development training on the technical aspects of referring to available genetic services was needed for providers who had not previously ordered genetics referrals or genetics testing as part of their primary care practices. To facilitate this training, we provided EHR screenshots depicting the steps to place appropriate referrals. In the future, to better meet this need for Arkansas providers, we are building a genetics-dedicated web resource that will host not only educational modules for both providers and families but also a directory of available statewide genetics services.
We did not gather qualitative data from patients about their experiences using the MeTree program and how the results of the screening impacted their healthcare decisions. Future focus groups would be valuable to capture those perspectives.
Author Contributions
Conceptualization, L.H., M.G., N.B., B.B. and L.A.O.; Data curation, J.W. and A.S.; Formal analysis, L.H.; Funding acquisition, L.H.; Methodology, L.H., M.G., N.B. and L.A.O.; Project administration, L.H., J.W. and A.S.; Resources, L.H.; Supervision, L.H.; Validation, M.G. and N.B.; Writing—original draft, L.H. and L.A.O.; Writing—review and editing, J.W., M.G., N.B., B.B. and A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the University of Arkansas for Medical Sciences Translational Research Institute award 2023-02 and the University of Arkansas Translational Research Institute under grant UL1 RR029884 from the National Center for Advancing Translational Sciences/National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Institutional Review Board Statement
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 2000. Ethical review and approval were waived by the University of Arkansas for Medical Sciences Institutional Review Board prior to project implementation due to a determination of ‘clinical implementation pilot project—not human subject research’.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
Laura Hays, Jordan Weaver, Brett Bailey, and Ashley Stone declare that they have no conflicts of interest. Nickie Buckner and Matt Gauger are co-owners of MeTree&You. Lori A. Orlando is the president and founder of MeTree&You.
References
- Lander, E.S.; Linton, L.M.; Birren, B.; Nusbaum, C. Initial sequencing and analysis of the human genome. Nature 2001, 409, 860–921. [Google Scholar] [PubMed]
- Ginsburg, G.S.; Wu, R.R.; Orlando, L.A. Family health history: Underused for actionable risk assessment. Lancet 2019, 394, 596–603. [Google Scholar] [CrossRef] [PubMed]
- CDC. Stats of the State of Arkansas; CDC: Atlanta, GA, USA, 2023. [Google Scholar]
- Orlando, L.A.; Wu, R.R.; Myers, R.A.; Buchanan, A.H.; Henrich, V.C.; Hauser, E.R.; Ginsburg, G.S. Clinical utility of a Web-enabled risk-assessment and clinical decision support program. Genet. Med. 2016, 18, 1020–1028. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, C.R.; Orlando, L.A.; Slavotinek, A.M.; Peterson, J.; Angelo, F.; Biesecker, B.; Bonham, V.L.; Cameron, L.D.; Fullerton, S.M.; Gelb, B.D.; et al. The Genomic Medicine Integrative Research Framework: A Conceptual Framework for Conducting Genomic Medicine Research. Am. J. Hum. Genet. 2019, 104, 1088–1096. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, A.H.; Christianson, C.A.; Himmel, T.; Powell, K.P.; Agbaje, A.; Ginsburg, G.S.; Henrich, V.C.; Orlando, L.A. Use of a patient-entered family health history tool with decision support in primary care: Impact of identification of increased risk patients on genetic counseling attendance. J. Genet. Couns. 2015, 24, 179–188. [Google Scholar] [CrossRef] [PubMed]
- HITECH Act: Competitive Grants to States and Indian Tribes for the Development of Loan Programs to Facilitate the Widespread Adoption of Certified EHR Technology, in Part 2, Subtitle C, Sec 13301, Subtitle B, Sec 3014. 2009. Available online: https://www.healthit.gov/sites/default/files/hitech_act_excerpt_from_arra_with_index.pdf (accessed on 23 November 2024).
- CHCS. Case Study: Arkansas Charts a Course for HIE and Quality Reporting (Arkansas Profile); CHCS: Hamilton, NJ, USA, 2010. [Google Scholar]
- Network, O.B.O.T.I.; Weitzel, K.W.; Alexander, M.; Bernhardt, B.A.; Calman, N.; Carey, D.J.; Cavallari, L.H.; Field, J.R.; Hauser, D.; Junkins, H.A.; et al. The IGNITE network: A model for genomic medicine implementation and research. BMC Med. Genom. 2016, 9, 1. [Google Scholar]
- Damschroder, L.J.; Aron, D.C.; Keith, R.E.; Kirsh, S.R.; Alexander, J.A.; Lowery, J.C. Fostering implementation of health services research findings into practice: A consolidated framework for advancing implementation science. Implement. Sci. 2009, 4, 50. [Google Scholar] [CrossRef] [PubMed]
- Amendola, L.M.; Berg, J.S.; Horowitz, C.R.; Angelo, F.; Bensen, J.T.; Biesecker, B.B.; Biesecker, L.G.; Cooper, G.M.; East, K.; Filipski, K.; et al. The Clinical Sequencing Evidence-Generating Research Consortium: Integrating Genomic Sequencing in Diverse and Medically Underserved Populations. Am. J. Hum. Genet. 2018, 103, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Gottesman, O.; Kuivaniemi, H.; Tromp, G.; Faucett, W.A.; Li, R.; Manolio, T.A.; Sanderson, S.C.; Kannry, J.; Zinberg, R.; Basford, M.A.; et al. The Electronic Medical Records and Genomics (eMERGE) Network: Past, present, and future. Genet. Med. 2013, 15, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Matise, T.C.; Ambite, J.L.; Buyske, S.; Carlson, C.S.; Cole, S.A.; Crawford, D.C.; Haiman, C.A.; Heiss, G.; Kooperberg, C.; Le Marchand, L.; et al. The Next PAGE in Understanding Complex Traits: Design for the Analysis of Population Architecture Using Genetics and Epidemiology (PAGE) Study. Am. J. Epidemiol. 2011, 174, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Wojcik, G.L.; Graff, M.; Nishimura, K.K.; Tao, R.; Haessler, J.; Gignoux, C.R.; Highland, H.M.; Patel, Y.M.; Sorokin, E.P.; Avery, C.L.; et al. Genetic Diversity Turns a New PAGE in Our Understanding of Complex Traits. bioRxiv 2017, 188094. [Google Scholar] [CrossRef]
- Family Medical Center in Batesville. UAMS Health: Little Rock, AR, USA, 2025. Available online: https://uamshealth.com/location/fmc-batesville/ (accessed on 23 November 2024).
- US_Census_Bureau. QuickFacts Batesville City, Arkansas. 2023. Available online: https://www.census.gov/quickfacts/fact/table/batesvillecityarkansas/SEX255222 (accessed on 23 November 2024).
- Brooke, J. SUS: A ‘Quick and Dirty’ Usability Scale, in Usability Evalution in Industry; Jordan, P.W., Thomas, B., McClelland, I.L., Weerdmeester, B., Eds.; CRC Press: London, UK, 1996; p. 252. [Google Scholar]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef] [PubMed]
- Orlando, L.A.; Wu, R.R.; Myers, R.A.; Neuner, J.; McCarty, C.; Haller, I.V.; Harry, M.; Fulda, K.G.; Dimmock, D.; Rakhra-Burris, T.; et al. At the intersection of precision medicine and population health: An implementation-effectiveness study of family health history based systematic risk assessment in primary care. BMC Health Serv. Res. 2020, 20, 1015. [Google Scholar] [CrossRef] [PubMed]
- DiBiase, J.F.; Scharnetzki, E.; Edelman, E.; Lucas, F.L.; Helbig, P.; Rueter, J.; Han, P.K.; Ziller, E.; Jacobs, E.A.; Anderson, E.C.; et al. Urban-Rural and Socioeconomic Differences in Patient Knowledge and Perceptions of Genomic Tumor Testing. JCO Precis. Oncol. 2023, 7, e2200631. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Peesay, T.; Krishnan, V.; Wilcox, J.; Wilsbacher, L.; Khan, S.S. Prioritizing the primary prevention of heart failure: Measuring, modifying and monitoring risk. Prog. Cardiovasc. Dis. 2024, 82, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Harrington, R.A.; Califf, R.M.; Balamurugan, A.; Brown, N.; Benjamin, R.M.; Braund, W.E.; Hipp, J.; Konig, M.; Sanchez, E.; Maddox, K.E.J. Call to Action: Rural Health: A Presidential Advisory From the American Heart Association and American Stroke Association. Circulation 2020, 141, E615–E644. [Google Scholar] [CrossRef] [PubMed]
- Turecamo, S.E.; Xu, M.; Dixon, D.; Powell-Wiley, T.M.; Mumma, M.T.; Joo, J.; Gupta, D.K.; Lipworth, L.; Roger, V.L. Association of Rurality With Risk of Heart Failure. JAMA Cardiol. 2023, 8, 231–239. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).