Simple Summary
Sexual dysfunction, which can result from hormonal imbalances, stress, and chronic health issues, affects a significant portion of the population. This study examines how probiotics, beneficial bacteria that support gut health, can improve sexual and reproductive health. The findings show that probiotics significantly improved sexual function in women, particularly those on antidepressants, and increased pregnancy rates in women undergoing fertility treatments. In men, probiotics improved sperm health, including motility and viability. Additionally, probiotics help reduce menopause symptoms and support hormonal balance. This review highlights the potential of probiotics as an effective treatment for sexual dysfunction and reproductive health, offering promising results that could benefit many individuals. However, further research is needed to fully understand the mechanisms behind these effects.
Abstract
Sexual dysfunction, influenced by hormonal imbalances, psychological factors, and chronic diseases, affects a significant portion of the population. Probiotics, known for their beneficial effects on gut microbiota, have emerged as potential therapeutic agents for improving sexual health. This systematic review evaluates the impact of probiotics on sexual function, hormonal regulation, and reproductive outcomes. A comprehensive search identified 3308 studies, with 12 meeting the inclusion criteria—comprising 10 randomized controlled trials (RCTs) and 2 in vivo and in vitro studies. Probiotic interventions were shown to significantly improve sexual function, particularly in women undergoing antidepressant therapy (p < 0.05). Significant improvements in Female Sexual Function Index (FSFI) scores were observed, with combined treatments such as Lactofem with Letrozole and Lactofem with selective serotonin reuptake inhibitors (SSRIs) demonstrating a 10% biochemical and clinical pregnancy rate compared to 0% in the control group (p = 0.05). Probiotic use was also associated with a 66% reduction in menopausal symptoms, increased sperm motility (36.08%), viability (46.79%), and morphology (36.47%). Probiotics also contributed to favorable hormonal changes, including a reduced luteinizing hormone (LH) to follicle-stimulating hormone (FSH) ratio (from 3.0 to 2.5, p < 0.05) and increased testosterone levels. Regarding reproductive outcomes, probiotic use was associated with higher pregnancy rates in women undergoing fertility treatments and improvements in sperm motility, viability, and morphology in men. This review highlights the promising role of probiotics in addressing sexual dysfunction and reproductive health, suggesting their potential as adjunctive treatments for conditions such as depression and infertility. Further research is needed to better understand the underlying mechanisms of these beneficial effects.
1. Introduction
Sexual dysfunction, affecting approximately 43% of women and 31% of men in the United States, profoundly impacts quality of life [1]. This issue is commonly associated with hormonal imbalances, chronic conditions such as diabetes and hypertension, and psychological factors [2]. The DSM-5 identifies conditions like female sexual interest/arousal disorder and genito-pelvic pain/penetration disorder, with symptoms persisting for at least six months and causing significant distress [3]. Among cancer patients, sexual dysfunction is prevalent, with treatments linked to a roughly three-fold increase in risk for both cervical and breast cancer [2]. Despite its widespread occurrence, sexual dysfunction often goes undiagnosed due to stigma and insufficient clinical training. Diagnostic tools such as the Female Sexual Function Index (FSFI) are instrumental in assessing sexual health [4]. For women, evidence-based treatments include hormone therapies, such as transdermal testosterone, and pelvic floor physical therapy, particularly for hypoactive sexual desire disorder and dyspareunia [3]. Psychological interventions, including mindfulness and cognitive–behavioral therapy, also contribute to effective management [1]. In men, erectile dysfunction is frequently associated with vascular or neurological causes, with first-line treatments like lifestyle modifications and phosphodiesterase type 5 inhibitors demonstrating significant efficacy [5]. The complexity of sexual dysfunction, especially in the context of cancer [2], highlights the critical need for continued research to enhance diagnostic accuracy, optimize treatment strategies, and improve patient outcomes.
Pathophysiological mechanisms involved in sexual dysfunction are closely linked to the gut microbiota, a crucial regulator of metabolism, immunity, and overall health [6,7,8,9]. Dysbiosis, or imbalance in the gut microbiota, is associated with metabolic disorders, including type 2 diabetes [10]. The gut microbiota produces metabolites such as short-chain fatty acids (SCFAs) that interact with the nervous, immune, and metabolic systems, impacting systemic health [11]. Recent research has identified the gut–brain axis as a key pathway through which gut microbiota influences sexual function by regulating neural signaling and hormone metabolism [12]. Specifically, the gut microbiota plays a critical role in modulating sex hormones such as estrogen and testosterone, which are essential for maintaining sexual health [8,13,14]. In diabetic individuals, dysbiosis exacerbates sexual dysfunction through mechanisms including increased inflammation, oxidative stress, and impaired vascular function, all of which are influenced by the gut microbiota [8,15]. Restoring a balanced microbiota may provide promising therapeutic strategies for improving sexual health in patients with diabetes [16].
Probiotics are emerging as a potential solution for sexual dysfunction, especially in patients experiencing medication-induced sexual health issues, such as those caused by selective serotonin reuptake inhibitors (SSRIs). Research has shown that probiotics, including strains like Lactobacillus acidophilus and Bifidobacterium bifidus, not only promote gut microbiome balance but also impact the neuroendocrine systems associated with sexual function. A randomized trial by Hashemi-Mohammadabad et al. (2023) demonstrated that probiotic supplementation improved sexual satisfaction and alleviated depressive symptoms in SSRI-treated patients, suggesting potential beyond gut restoration [17]. Probiotics may exert their beneficial effects through mechanisms such as reduced systemic inflammation, enhanced serotonin production in the gut, and improved hormonal regulation—all of which contribute to sexual health [18]. The gut–brain axis regulates serotonin production, alleviating depression [19,20], a major cause of sexual dysfunction [21,22]. Probiotics modulate key sex hormones like estrogen and testosterone [22,23] and possess antioxidant properties that combat oxidative stress, protecting tissues [24] involved in sexual function. Given that the American Urological Association (AUA) and the International Society for Sexual Medicine (ISSM) have highlighted the role of gut health in sexual function, probiotics are becoming recognized as a promising adjunctive therapy for sexual dysfunction [25,26]. The growing evidence points to the need for more clinical trials and guideline-based recommendations to incorporate probiotics as a therapeutic option, particularly for those affected by drug-induced sexual health disturbances.
The objective of this study is to systematically examine the potential role of probiotics as a therapeutic intervention for diabetes-related sexual dysfunction. Specifically, the review focuses on understanding how probiotics can modulate key mechanisms such as hormonal regulation and metabolic pathways. By synthesizing findings from in vitro, in vivo, and clinical studies, the research highlights the role of gut microbiota in influencing sexual health and identifies probiotics as a potential adjunct therapy. The study also aims to address knowledge gaps regarding strain-specific effects and long-term safety, paving the way for future research and clinical applications.
2. Materials and Methods
This systematic review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines to explore the potential therapeutic role of probiotics in managing sexual dysfunction and its associated pathophysiological mechanisms. The primary objectives were to address the following research questions:
- What evidence exists from in vitro, in vivo, and clinical studies on the effects of probiotics on sexual dysfunction?
- How do probiotics influence key pathophysiological mechanisms underlying sexual dysfunction, including inflammation, oxidative stress, and hormonal imbalances?
A comprehensive literature search was conducted across multiple electronic databases, including PubMed, Scopus, and Web of Science. The search included all publications available up to August 2024. Search terms included combinations of keywords “probiotics” and “sex” or “sexual function”. Specific terms related to sexual function in MESH terms included “Sexual Dysfunction, Physiological”, “Dyspareunia”, “Ejaculatory Dysfunction”, “Premature Ejaculation”, “Retrograde Ejaculation”, “Erectile Dysfunction”, “Impotence, Vasculogenic” and “Vaginismus”.
2.1. Inclusion and Exclusion Criteria
Studies were included if they investigated the effects of probiotics on sexual dysfunction, were published in peer-reviewed journals, written in English, and conducted as experimental studies (in vivo, in vitro) or epidemiological studies, including clinical trials. Studies lacking original experimental or clinical data, including review articles, meta-analyses, guidelines, protocols, case series, case reports, and conference abstracts, were excluded. Research investigating non-probiotic interventions, such as pharmaceutical agents, herbal extracts, or dietary modifications without a probiotic component, was not considered. Exclusion also applied to studies combining probiotics with other therapeutic modalities without isolating their specific effects. Preclinical animal studies focusing on unrelated conditions and publications in languages other than English or with inaccessible full texts were omitted.
2.2. Study Selection Process
Two independent reviewers, T.T.M.N. and S.J.Y., independently screened the titles and abstracts of identified studies to determine their relevance to the topic of probiotics on sexual function. Each full-text article was systematically evaluated based on the predefined inclusion and exclusion criteria to confirm its eligibility. Any reviewer inconsistencies were addressed through discussion to maintain consistency and reduce selection bias. In cases where consensus could not be reached, a third reviewer was consulted to provide a final determination.
2.3. Data Extraction and Synthesis
Data were extracted from the included studies, focusing on three primary areas. First, sexual function outcomes were assessed using validated tools such as the FSFI and other relevant measures. Second, hormonal markers were analyzed, including changes in hormone levels (e.g., estrogen, testosterone, LH/FSH ratio). Third, reproductive outcomes were evaluated by examining pregnancy rates, sperm parameters, and menopausal symptom relief. Data extraction included clinical assessments, biochemical analyses, and microbiome evaluations, with an emphasis on strain-specific effects. The synthesis aimed to provide a comprehensive understanding of the mechanisms by which probiotics influence sexual function, hormonal balance, and reproductive health.
3. Results
A total of 3308 studies were identified through the initial search (Figure 1) following the PRISMA table (Supplement File S1). After applying inclusion and exclusion criteria, 12 studies were included in the final synthesis on specific parameters (Table 1). The most frequently studied strain was Lactobacillus acidophilus (L. acidophilus), with Iran being the leading contributor to these studies (Table 2). These studies varied in methodology, including 10 randomized controlled trials (RCTs) and two in vivo and in vitro studies exploring the effects of probiotics on sexual dysfunction through (1) improvements in sexual function scores, (2) impacts on hormonal markers, and (3) pregnancy and reproductive outcomes.
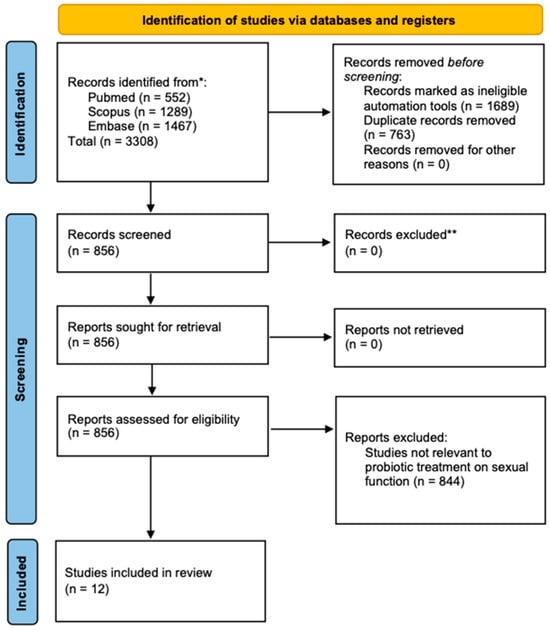
Figure 1.
A diagram outlining the systematic review process, including database searches, abstract screenings, and full-text acquisitions, in compliance with PRISMA guidelines. * Data collected in August 2024; ** Studies with incomplete data were excluded.

Table 1.
Key parameters of sexual and reproductive health influenced by probiotics.

Table 2.
Overview of the characteristics of the studies included in the systematic review.
3.1. Improvement in Sexual Function Scores
Several studies in the reviewed literature demonstrated significant improvements in sexual function scores following probiotic interventions. Kutenaee et al. [27] and Hashemi-Mohammadabad et al. [17] both reported improvements in the FSFI scores, with Kutenaee et al. noting a significant enhancement in the Lactofem plus Letrozole group compared to Letrozole alone (p < 0.05). Similarly, Hashemi-Mohammadabad et al. found that the Lactofem plus SSRIs group showed significant improvements in FSFI domains and total scores compared to SSRIs alone (p < 0.05). Hashemi et al. (Iran) further supported these findings, reporting that the Lactofem group showed better sexual desire, arousal, lubrication, orgasm, satisfaction, and pain dimensions compared to the SSRIs-only group (p < 0.05) [17]. Lim et al. [31] conducted an RCT in Korea with 85 post-menopausal women, evaluating the effects of Lactobacillus acidophilus (L. acidophilus) YT1, showing a 66% reduction in menopausal symptoms, compared to 37% in the placebo group. L. acidophilus YT1 alleviated symptoms such as hot flashes, fatigue, and vaginal dryness, without changes in estrogen levels, suggesting it may improve sexual function by regulating the gut microbiome, immune system, and central nervous system. These findings collectively suggest that probiotics, either alone or in combination with other treatments, can significantly enhance sexual function in women, particularly those with conditions like those undergoing antidepressant therapy.
3.2. Impact on Hormonal Markers
Probiotic interventions were also associated with positive changes in hormonal and inflammatory markers, which may contribute to improved sexual health. Kutenaee [27] reported a significant decrease in the luteinizing hormone (LH) and follicle-stimulating hormone (FSH) ratio in the probiotics group (from 3.0 to 2.5, p < 0.05), indicating improved hormonal balance. Hashemi et al. [17] also noted a significant reduction in depressive symptoms, which are often linked to hormonal imbalances, in the Lactofem group compared to the SSRIs-only group (p < 0.05). Increased serum markers included elevated total antioxidant capacity (TAC), LH, FSH, and testosterone levels (p < 0.05), as reported by Ansari et al. [37]. These findings indicate that probiotics may improve sexual function by modulating hormonal and inflammatory pathways, particularly in individuals with conditions like depression and diabetes.
3.3. Pregnancy and Reproductive Outcomes
Probiotic interventions demonstrated significant improvements in reproductive outcomes. Kutenaee et al. [27] reported higher biochemical and clinical pregnancy rates in the probiotics plus Letrozole group (10%) compared to the Letrozole-alone group (0%) (p = 0.05). Hashemi et al. [17] found that 8 weeks of probiotic consumption improved chemical and clinical pregnancy rates. In male reproductive health, Ansari et al. [37] reported that B. longum and Cynara scolymus L. extract increased sperm motility (36.08%), viability (46.79%), and morphology (36.47%) in diabetic male rats. Similarly, Abbasi et al. [36] showed that the synbiotic product FamiLact significantly improved sperm concentration (44.73 ± 10.02 vs. 23.27 ± 5.19 million/mL), motility (42.2 ± 5.63% vs. 19.4 ± 4.24%), and morphology (48.6 ± 8.56% vs. 25.8 ± 7.05%) while reducing DNA fragmentation (p < 0.05) in men with idiopathic infertility. These findings indicate that probiotics contribute to enhanced pregnancy outcomes, sperm quality, and overall reproductive health, particularly in individuals with underlying reproductive issues.
4. Discussion
This systematic review integrates findings from 12 studies encompassing randomized controlled trials, in vivo experiments, and in vitro analyses to assess the impact of probiotics on sexual dysfunction. The aggregated evidence indicates that probiotics may substantially enhance sexual function scores, regulate hormonal profiles, and improve reproductive outcomes. These results underscore the multifaceted role of probiotics in modulating physiological and psychological factors linked to sexual health, offering promising insights into their therapeutic potential.
4.1. Probiotics and Sexual Function Enhancement
The reviewed studies highlight that probiotics can improve sexual function, especially in individuals experiencing dysfunction due to antidepressant treatment or menopausal symptoms. Probiotic interventions, such as Lactofem in combination with Letrozole or selective serotonin reuptake inhibitors (SSRIs), have shown significant improvements in FSFI scores, with enhanced sexual function and reduced symptoms such as vaginal dryness and fatigue [17,27,31]. The underlying mechanisms appear to be multifactorial, involving modulation of the gut–brain axis [38], regulation of immune responses, and neurochemical pathways that impact mood and sexual health [39,40]. Neurotransmitters such as serotonin, dopamine, gamma-aminobutyric acid, and glutamate [41,42] play vital roles in the connection between the gut and brain, influencing both mental and physical processes [38]. Unlike traditional antidepressants, probiotics do not seem to alter sensitivity to positive or negative emotions [43]. Additionally, probiotics have been found to enhance cognitive adaptability, reduce stress in older adults, and bring about beneficial changes in gut microbial composition [42]. For instance, L. acidophilus YT1 has shown effectiveness in reducing menopausal symptoms without altering estrogen levels, indicating that gut microbiota modulation may work through more indirect pathways [31].
In comparison to conventional interventions such as SSRIs or hormone replacement therapy (HRT), probiotics offer a more natural and integrative alternative. SSRIs are effective in the treatment of depression, but they often induce sexual side effects, including reduced libido and delayed orgasm [44]. While HRT can ameliorate sexual dysfunction in menopausal women, it is frequently associated with long-term health risks [45,46]. In contrast, probiotics provide a promising adjunctive treatment with minimal adverse effects, supporting sexual health through modulation of the gut microbiota, immune regulation, and neurochemical signaling [47,48,49,50]. Emerging research underscores the potential of probiotics, like Lactobacillus plantarum 299v, to enhance cognitive performance, reduce systemic inflammation, and improve sexual well-being, presenting a valuable and safer complementary strategy to traditional pharmacological approaches [47,48,49,50].
4.2. Hormonal Modulation Through Probiotic Use
Probiotics offer a distinctive and natural approach to hormonal regulation, contrasting favorably with conventional treatments [51,52,53]. While HRT remains the standard for managing sex steroid deficiencies in postmenopausal women, it comes with notable risks, such as cardiovascular complications and breast cancer, with prolonged use [54,55]. Studies have demonstrated that probiotics, such as Lactobacillus rhamnosus GG and Escherichia coli Nissle 1917, modulate the gut microbiome and immune responses, reducing systemic inflammation and improving levels of hormones like LH, FSH, and testosterone [56,57]. Moreover, probiotics address sex steroid deficiency-related issues [56], such as bone loss and metabolic dysfunction, through mechanisms that involve reducing gut permeability and inflammatory cytokines [58,59,60,61], showcasing their multifaceted role in supporting hormonal health. Probiotics support hormonal health by reducing gut permeability, which prevents the translocation of inflammatory cytokines that can disrupt endocrine function [62,63]. This positions probiotics as a promising adjunctive treatment for hormonal regulation, offering a safer, non-pharmacological alternative to HRT and SSRIs.
4.3. Influence on Fertility and Reproductive Health
Probiotics have shown considerable promise in enhancing fertility and reproductive health outcomes [64,65] by modulating the gut microbiota and reducing oxidative stress [66,67,68]. Clinical studies report improved pregnancy rates and sperm parameters when probiotics are combined with conventional treatments [17,27,36,37]. Supplementation with specific probiotic strains has been associated with increased sperm concentration, motility, and morphology, along with reduced DNA fragmentation in men with idiopathic infertility [36]. By restoring gut microbial balance, probiotics help reduce inflammatory cytokines and oxidative markers that negatively impact reproductive function [69]. Unlike antioxidant supplements, which primarily target oxidative stress, probiotics provide comprehensive immune and metabolic regulation [70]. Hormonal therapies, while effective, may have side effects and do not address the systemic imbalances that probiotics can correct [71,72]. Probiotics thus present a multifaceted, non-pharmacological strategy for improving reproductive health, offering distinct advantages over traditional treatments by addressing root causes through gut microbiota modulation and systemic health enhancement [73,74].
4.4. Limitations
While the results are promising, several limitations must be acknowledged. The included studies varied in sample size, probiotic strains, dosages, and treatment durations, which may affect the generalizability of the findings. Heterogeneity in probiotic strains and dosages across studies complicates the comparison of results and makes it difficult to determine the most effective probiotic for sexual function management. Additionally, most studies focused on female populations, with limited research on male populations, making it challenging to assess whether the observed benefits are applicable across sexes. The variable quality of the included studies, particularly concerning their experimental design and controls, limits the reliability of the conclusions drawn. Lastly, there is limited long-term follow-up data, which means the sustainability of any observed effects on sexual function is uncertain.
5. Conclusions
Probiotic interventions have demonstrated promising potential in improving sexual function, modulating hormonal markers, and enhancing reproductive outcomes. These findings underscore the therapeutic value of probiotics as a complementary treatment for sexual dysfunction, particularly among individuals with underlying health conditions such as depression, infertility, and hormonal imbalances. The studies included in this review highlight significant improvements in sexual function, hormonal regulation, and reproductive health following probiotic interventions. While the results indicate that probiotics can be an effective adjunct therapy for improving sexual function and reproductive health, further research is necessary to establish standardized treatment protocols and explore the long-term impact of probiotics on sexual health.
- Probiotics enhance sexual function and satisfaction in Female Sexual Function Index scores.
- Probiotics improve hormonal balance, lowering LH/FSH and increasing testosterone.
- Probiotics enhance reproductive outcomes with respect to pregnancy rates and sperm quality.
- Probiotics are a promising adjunct for sexual dysfunction treatment.
- Future studies are needed to standardize protocols and explore long-term impacts.
Integrating probiotics as part of a multifaceted management approach could provide patients with a non-pharmacological, cost-effective therapeutic option to address sexual dysfunction, hypoandrogenism, and reproductive dysregulation, thereby enhancing overall health-related quality of life.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biology14030286/s1, Supplement File S1: PRISMA 2020 checklist. Reference [75] is cited in the Supplementary Materials.
Author Contributions
Conceptualization, S.-J.Y. and T.-H.Y.; methodology, T.T.M.N.; software, T.T.M.N.; validation, S.-J.Y., Q.Z., G.-S.Y., S.-J.P. and T.-H.Y.; formal analysis, T.T.M.N.; investigation, S.-J.Y.; resources, S.-J.Y.; data curation, S.-J.Y.; writing—original draft preparation, T.T.M.N.; writing—review and editing, T.T.M.N., S.-J.Y. and T.-H.Y.; visualization, X.J.; supervision, T.-H.Y.; project administration, T.-H.Y.; funding acquisition, T.-H.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| FSFI | Female Sexual Function Index |
| BICI | Body Image Concern Inventory |
| TC | Total cholesterol |
| TG | Triglycerides |
| HDL | High-Density Lipoprotein |
| LDL | Low-Density Lipoprotein |
| GC-MS | Gas Chromatography–Mass Spectrometry |
| 16S rRNA | 16S ribosomal RNA |
References
- Christmas, M.M.; Reed, S. Sexual Dysfunction After Menopause. Obstet. Gynecol. Clin. N. Am. 2024, 51, 341–364. [Google Scholar] [CrossRef]
- Sousa Rodrigues Guedes, T.; Barbosa Otoni Gonçalves Guedes, M.; De Castro Santana, R.; Costa Da Silva, J.F.; Almeida Gomes Dantas, A.; Ochandorena-Acha, M.; Terradas-Monllor, M.; Jerez-Roig, J.; Bezerra De Souza, D.L. Sexual Dysfunction in Women with Cancer: A Systematic Review of Longitudinal Studies. Int. J. Environ. Res. Public Health 2022, 19, 11921. [Google Scholar] [CrossRef] [PubMed]
- American College of Obstetricians and Gynecologists. Female Sexual Dysfunction: ACOG Practice Bulletin Clinical Management Guidelines for Obstetrician–Gynecologists, Number 213. Obstet. Gynecol. 2019, 134, e1–e18. [Google Scholar] [CrossRef]
- Clayton, A.H.; Valladares Juarez, E.M. Female Sexual Dysfunction. Med. Clin. N. Am. 2019, 103, 681–698. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Crespo, R.E.; Cordon-Galiano, B.H. Sexual Dysfunction Among Men Who Have Sex with Men: A Review Article. Curr. Urol. Rep. 2021, 22, 9. [Google Scholar] [CrossRef]
- Su, Q.; Long, Y.; Luo, Y.; Jiang, T.; Zheng, L.; Wang, K.; Tang, Q. Specific Gut Microbiota May Increase the Risk of Erectile Dysfunction: A Two-Sample Mendelian Randomization Study. Front. Endocrinol. 2023, 14, 1216746. [Google Scholar] [CrossRef] [PubMed]
- Giatti, S.; Diviccaro, S.; Cioffi, L.; Cosimo Melcangi, R. Post-Finasteride Syndrome And Post-Ssri Sexual Dysfunction: Two Clinical Conditions Apparently Distant, But Very Close. Front. Neuroendocrinol. 2024, 72, 101114. [Google Scholar] [CrossRef]
- Cross, T.-W.L.; Kasahara, K.; Rey, F.E. Sexual Dimorphism of Cardiometabolic Dysfunction: Gut Microbiome in the Play? Mol. Metab. 2018, 15, 70–81. [Google Scholar] [CrossRef]
- Qiao, Y.; Chen, J.; Jiang, Y.; Zhang, Z.; Wang, H.; Liu, T.; Yang, Z.; Fu, G.; Chen, Y. Gut Microbiota Composition May Be an Indicator of Erectile Dysfunction. Microb. Biotechnol. 2024, 17, e14403. [Google Scholar] [CrossRef]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A Metagenome-Wide Association Study of Gut Microbiota in Type 2 Diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef]
- Ikeda, T.; Nishida, A.; Yamano, M.; Kimura, I. Short-Chain Fatty Acid Receptors and Gut Microbiota as Therapeutic Targets in Metabolic, Immune, and Neurological Diseases. Pharmacol. Ther. 2022, 239, 108273. [Google Scholar] [CrossRef]
- Cussotto, S.; Sandhu, K.V.; Dinan, T.G.; Cryan, J.F. The Neuroendocrinology of the Microbiota-Gut-Brain Axis: A Behavioural Perspective. Front. Neuroendocrinol. 2018, 51, 80–101. [Google Scholar] [CrossRef]
- Brettle, H.; Tran, V.; Drummond, G.R.; Franks, A.E.; Petrovski, S.; Vinh, A.; Jelinic, M. Sex Hormones, Intestinal Inflammation, and the Gut Microbiome: Major Influencers of the Sexual Dimorphisms in Obesity. Front. Immunol. 2022, 13, 971048. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, J.; Zhou, Y.; Xiong, S.; Zhou, M.; Wu, L.; Liu, Q.; Chen, Z.; Jiang, H.; Yang, J.; et al. Gut Microbiota Affects the Estrus Return of Sows by Regulating the Metabolism of Sex Steroid Hormones. J. Anim. Sci. Biotechnol. 2023, 14, 155. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Hill, M.A.; Sowers, J.R. Vascular Endothelial Mineralocorticoid Receptors and Epithelial Sodium Channels in Metabolic Syndrome and Related Cardiovascular Disease. J. Mol. Endocrinol. 2023, 71, e230066. [Google Scholar] [CrossRef] [PubMed]
- Mostafavi Abdolmaleky, H.; Zhou, J.-R. Gut Microbiota Dysbiosis, Oxidative Stress, Inflammation, and Epigenetic Alterations in Metabolic Diseases. Antioxidants 2024, 13, 985. [Google Scholar] [CrossRef]
- Hashemi-Mohammadabad, N.; Taghavi, S.-A.; Lambert, N.; Moshtaghi, R.; Bazarganipour, F.; Sharifi, M. Adjuvant Administration of Probiotic Effects on Sexual Function in Depressant Women Undergoing SSRIs Treatment: A Double-Blinded Randomized Controlled Trial. BMC Psychiatry 2024, 24, 44. [Google Scholar] [CrossRef]
- Perez-Lloret, S.; Rey, M.V.; Pavy-Le Traon, A.; Rascol, O. Emerging Drugs for Autonomic Dysfunction in Parkinson’s Disease. Expert Opin. Emerg. Drugs 2013, 18, 39–53. [Google Scholar] [CrossRef]
- Dinan, T.G.; Cryan, J.F. The Microbiome-Gut-Brain Axis in Health and Disease. Gastroenterol. Clin. N. Am. 2017, 46, 77–89. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, Q.; Hou, Y.; Zhang, X.; Yin, Z.; Cai, X.; Wei, W.; Wang, J.; He, D.; Wang, G.; et al. Bacteroides Species Differentially Modulate Depression-like Behavior via Gut-Brain Metabolic Signaling. Brain. Behav. Immun. 2022, 102, 11–22. [Google Scholar] [CrossRef]
- Sramek, J.J.; Murphy, M.F.; Cutler, N.R. Sex Differences in the Psychopharmacological Treatment of Depression. Dialogues Clin. Neurosci. 2016, 18, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Thériault, R.-K.; Perreault, M.L. Hormonal Regulation of Circuit Function: Sex, Systems and Depression. Biol. Sex Differ. 2019, 10, 12. [Google Scholar] [CrossRef]
- Basnet, J.; Eissa, M.A.; Cardozo, L.L.Y.; Romero, D.G.; Rezq, S. Impact of Probiotics and Prebiotics on Gut Microbiome and Hormonal Regulation. Gastrointest. Disord. Basel Switz. 2024, 6, 801–815. [Google Scholar] [CrossRef]
- Helli, B.; Kavianpour, M.; Ghaedi, E.; Dadfar, M.; Haghighian, H.K. Probiotic Effects on Sperm Parameters, Oxidative Stress Index, Inflammatory Factors and Sex Hormones in Infertile Men. Hum. Fertil. 2022, 25, 499–507. [Google Scholar] [CrossRef]
- American Urological Association Recurrent Uncomplicated Urinary Tract Infections in Women: AUA/CUA/SUFU Guideline. 2022. Available online: https://www.auanet.org/guidelines-and-quality/guidelines/recurrent-uti (accessed on 1 August 2024).
- Juliawan, I.M.P.; Suwana, F.P.; Annas, J.Y.; Akbar, M.F.; Widjiati, W. High Sucrose and Cholic Acid Diet Triggers PCOS-like Phenotype and Reduces Enterobacteriaceae Colonies in Female Wistar Rats. Pathophysiol. Off. J. Int. Soc. Pathophysiol. 2022, 29, 344–353. [Google Scholar] [CrossRef]
- Azizi-Kutenaee, M.; Heidari, S.; Taghavi, S.-A.; Bazarganipour, F. Probiotic Effects on Sexual Function in Women with Polycystic Ovary Syndrome: A Double Blinded Randomized Controlled Trial. BMC Women’s Health 2022, 22, 373. [Google Scholar] [CrossRef] [PubMed]
- Wallace, C.J.K.; Foster, J.A.; Soares, C.N.; Milev, R.V. The Effects of Probiotics on Symptoms of Depression: Protocol for a Double-Blind Randomized Placebo-Controlled Trial. Neuropsychobiology 2020, 79, 108–116. [Google Scholar] [CrossRef]
- Wallace, C.J.K.; Milev, R.V. The Efficacy, Safety, and Tolerability of Probiotics on Depression: Clinical Results From an Open-Label Pilot Study. Front. Psychiatry 2021, 12, 618279. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Chen, Y.; Zhu, X.; Wang, L.; Tian, P.; Jin, X.; Liang, M.; Chen, Z.; Zhang, T.; Qian, L.; et al. A Randomised Double-Blind Placebo-Controlled Trial of a Probiotic Combination for Manipulating the Gut Microbiota and Managing Metabolic Syndrome. Food Biosci. 2024, 59, 104076. [Google Scholar] [CrossRef]
- Lim, E.Y.; Lee, S.-Y.; Shin, H.S.; Lee, J.; Nam, Y.-D.; Lee, D.O.; Lee, J.Y.; Yeon, S.H.; Son, R.H.; Park, C.L.; et al. The Effect of Lactobacillus Acidophilus YT1 (MENOLACTO) on Improving Menopausal Symptoms: A Randomized, Double-Blinded, Placebo-Controlled Clinical Trial. J. Clin. Med. 2020, 9, 2173. [Google Scholar] [CrossRef]
- Rapisarda, A.M.C.; Pino, A.; Grimaldi, R.L.; Caggia, C.; Randazzo, C.L.; Cianci, A. Lacticaseibacillus Rhamnosus CA15 (DSM 33960) Strain as a New Driver in Restoring the Normal Vaginal Microbiota: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Front. Surg. 2023, 9, 1075612. [Google Scholar] [CrossRef]
- Mr, O. Combination Local Therapy of Genitourinary Menopausal Syndrome Symptoms. World J. Gynecol. Women’s Health 2020, 3, 3–9. [Google Scholar] [CrossRef]
- Khodaverdi, S.; Mohammadbeigi, R.; Khaledi, M.; Mesdaghinia, L.; Sharifzadeh, F.; Nasiripour, S.; Gorginzadeh, M. Beneficial Effects of Oral Lactobacillus on Pain Severity in Women Suffering from Endometriosis: A Pilot Placebo-Controlled Randomized Clinical Trial. Int. J. Fertil. Steril. 2019, 13, 178. [Google Scholar] [CrossRef] [PubMed]
- Mollazadeh-Narestan, Z.; Yavarikia, P.; Homayouni-Rad, A.; Samadi Kafil, H.; Mohammad-Alizadeh-Charandabi, S.; Gholizadeh, P.; Mirghafourvand, M. Comparing the Effect of Probiotic and Fluconazole on Treatment and Recurrence of Vulvovaginal Candidiasis: A Triple-Blinded Randomized Controlled Trial. Probiotics Antimicrob. Proteins 2023, 15, 1436–1446. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, B.; Abbasi, H.; Niroumand, H. Synbiotic (FamiLact) Administration in Idiopathic Male Infertility Enhances Sperm Quality, DNA Integrity, and Chromatin Status: A Triple-Blinded Randomized Clinical Trial. Int. J. Reprod. Biomed. IJRM 2021, 19, 235–244. [Google Scholar] [CrossRef]
- Ansari, Z.; Maleki, M.H.; Roohy, F.; Ebrahimi, Z.; Shams, M.; Mokaram, P.; Zamanzadeh, Z.; Hosseinzadeh, Z.; Koohpeyma, F.; Dastghaib, S. Protective Effects of Artichoke Extract and Bifidobacterium Longum on Male Infertility in Diabetic Rats. Biochem. Biophys. Rep. 2024, 40, 101834. [Google Scholar] [CrossRef]
- Samtiya, M.; Dhewa, T.; Puniya, A.K. Probiotic Mechanism to Modulate the Gut-Brain Axis (GBA). In Microbiome-Gut-Brain Axis; Sayyed, R.Z., Khan, M., Eds.; Springer Nature Singapore: Singapore, 2022; pp. 237–259. ISBN 9789811616259. [Google Scholar]
- Jaggar, M.; Rea, K.; Spichak, S.; Dinan, T.G.; Cryan, J.F. You’ve Got Male: Sex and the Microbiota-Gut-Brain Axis across the Lifespan. Front. Neuroendocrinol. 2020, 56, 100815. [Google Scholar] [CrossRef]
- Shobeiri, P.; Kalantari, A.; Teixeira, A.L.; Rezaei, N. Shedding Light on Biological Sex Differences and Microbiota–Gut–Brain Axis: A Comprehensive Review of Its Roles in Neuropsychiatric Disorders. Biol. Sex Differ. 2022, 13, 12. [Google Scholar] [CrossRef]
- Qu, S.; Yu, Z.; Zhou, Y.; Wang, S.; Jia, M.; Chen, T.; Zhang, X. Gut Microbiota Modulates Neurotransmitter and Gut-Brain Signaling. Microbiol. Res. 2024, 287, 127858. [Google Scholar] [CrossRef]
- Strandwitz, P. Neurotransmitter Modulation by the Gut Microbiota. Brain Res. 2018, 1693, 128–133. [Google Scholar] [CrossRef]
- Baião, R.; Capitão, L.P.; Higgins, C.; Browning, M.; Harmer, C.J.; Burnet, P.W.J. Multispecies Probiotic Administration Reduces Emotional Salience and Improves Mood in Subjects with Moderate Depression: A Randomised, Double-Blind, Placebo-Controlled Study. Psychol. Med. 2023, 53, 3437–3447. [Google Scholar] [CrossRef] [PubMed]
- Reisman, Y. Sexual Consequences of Post-SSRI Syndrome. Sex. Med. Rev. 2017, 5, 429–433. [Google Scholar] [CrossRef]
- González, M.; Viáfara, G.; Caba, F.; Molina, E. Sexual Function, Menopause and Hormone Replacement Therapy (HRT). Maturitas 2004, 48, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Pearce, M.J.; Hawton, K. Psychological and Sexual Aspects of the Menopause and HRT. Baillieres Clin. Obstet. Gynaecol. 1996, 10, 385–399. [Google Scholar] [CrossRef] [PubMed]
- Rayan, N.A.; Aow, J.; Lim, M.G.L.; Arcego, D.M.; Ryan, R.; Nourbakhsh, N.; de Lima, R.M.S.; Craig, K.; Zhang, T.Y.; Goh, Y.T.; et al. Shared and Unique Transcriptomic Signatures of Antidepressant and Probiotics Action in the Mammalian Brain. Mol. Psychiatry 2024, 29, 3653–3668. [Google Scholar] [CrossRef]
- Godzien, J.; Kalaska, B.; Rudzki, L.; Barbas-Bernardos, C.; Swieton, J.; Lopez-Gonzalvez, A.; Ostrowska, L.; Szulc, A.; Waszkiewicz, N.; Ciborowski, M.; et al. Probiotic Lactobacillus Plantarum 299v Supplementation in Patients with Major Depression in a Double-Blind, Randomized, Placebo-Controlled Trial: A Metabolomics Study. J. Affect. Disord. 2025, 368, 180–190. [Google Scholar] [CrossRef]
- Rudzki, L.; Ostrowska, L.; Pawlak, D.; Małus, A.; Pawlak, K.; Waszkiewicz, N.; Szulc, A. Probiotic Lactobacillus Plantarum 299v Decreases Kynurenine Concentration and Improves Cognitive Functions in Patients with Major Depression: A Double-Blind, Randomized, Placebo Controlled Study. Psychoneuroendocrinology 2019, 100, 213–222. [Google Scholar] [CrossRef]
- Forth, E.; Buehner, B.; Storer, A.; Sgarbossa, C.; Milev, R.; Chinna Meyyappan, A. Systematic Review of Probiotics as an Adjuvant Treatment for Psychiatric Disorders. Front. Behav. Neurosci. 2023, 17, 1111349. [Google Scholar] [CrossRef]
- Tyagi, P.; Tasleem, M.; Prakash, S.; Chouhan, G. Intermingling of Gut Microbiota with Brain: Exploring the Role of Probiotics in Battle against Depressive Disorders. Food Res. Int. Ott. Ont 2020, 137, 109489. [Google Scholar] [CrossRef]
- Loh, J.S.; Mak, W.Q.; Tan, L.K.S.; Ng, C.X.; Chan, H.H.; Yeow, S.H.; Foo, J.B.; Ong, Y.S.; How, C.W.; Khaw, K.Y. Microbiota-Gut-Brain Axis and Its Therapeutic Applications in Neurodegenerative Diseases. Signal Transduct. Target. Ther. 2024, 9, 37. [Google Scholar] [CrossRef]
- Guo, M.; Liu, H.; Yu, Y.; Zhu, X.; Xie, H.; Wei, C.; Mei, C.; Shi, Y.; Zhou, N.; Qin, K.; et al. Lactobacillus Rhamnosus GG Ameliorates Osteoporosis in Ovariectomized Rats by Regulating the Th17/Treg Balance and Gut Microbiota Structure. Gut Microbes 2023, 15, 2190304. [Google Scholar] [CrossRef] [PubMed]
- Goddard, M.K. Hormone Replacement Therapy and Breast Cancer, Endometrial Cancer and Cardiovascular Disease: Risks and Benefits. Br. J. Gen. Pract. J. R. Coll. Gen. Pract. 1992, 42, 120–125. [Google Scholar]
- Hickey, M.; Elliott, J.; Davison, S.L. Hormone Replacement Therapy. BMJ 2012, 344, e763. [Google Scholar] [CrossRef]
- Li, J.-Y.; Chassaing, B.; Tyagi, A.M.; Vaccaro, C.; Luo, T.; Adams, J.; Darby, T.M.; Weitzmann, M.N.; Mulle, J.G.; Gewirtz, A.T.; et al. Sex Steroid Deficiency-Associated Bone Loss Is Microbiota Dependent and Prevented by Probiotics. J. Clin. Investig. 2016, 126, 2049–2063. [Google Scholar] [CrossRef]
- Luo, M.; Chen, Y.; Pan, X.; Chen, H.; Fan, L.; Wen, Y.E. Coli Nissle 1917 Ameliorates Mitochondrial Injury of Granulosa Cells in Polycystic Ovary Syndrome through Promoting Gut Immune Factor IL-22 via Gut Microbiota and Microbial Metabolism. Front. Immunol. 2023, 14, 1137089. [Google Scholar] [CrossRef] [PubMed]
- Raff, H.; Hainsworth, K.R.; Woyach, V.L.; Weihrauch, D.; Wang, X.; Dean, C. Probiotic and High-Fat Diet: Effects on Pain Assessment, Body Composition, and Cytokines in Male and Female Adolescent and Adult Rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2024, 327, R123–R132. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Jeong, J.-W.; Kim, J.-Y.; Shim, J.-J.; Lee, J.-H. Lactobacillus helveticus HY7801 Improves Premenstrual Syndrome Symptoms by Regulating Sex Hormones and Inflammatory Cytokines in a Mouse Model of Metoclopramide-Induced Hyperprolactinemia. Nutrients 2024, 16, 3889. [Google Scholar] [CrossRef]
- Lee, J.; Yang, W.; Hostetler, A.; Schultz, N.; Suckow, M.A.; Stewart, K.L.; Kim, D.D.; Kim, H.S. Characterization of the Anti-Inflammatory Lactobacillus Reuteri BM36301 and Its Probiotic Benefits on Aged Mice. BMC Microbiol 2016, 16, 69. [Google Scholar] [CrossRef]
- Aygun, H.; Akin, A.T.; Kızılaslan, N.; Sumbul, O.; Karabulut, D. Probiotic Supplementation Alleviates Absence Seizures and Anxiety- and Depression-like Behavior in WAG/Rij Rat by Increasing Neurotrophic Factors and Decreasing Proinflammatory Cytokines. Epilepsy Behav. 2022, 128, 108588. [Google Scholar] [CrossRef]
- Ait-Belgnaoui, A.; Durand, H.; Cartier, C.; Chaumaz, G.; Eutamene, H.; Ferrier, L.; Houdeau, E.; Fioramonti, J.; Bueno, L.; Theodorou, V. Prevention of Gut Leakiness by a Probiotic Treatment Leads to Attenuated HPA Response to an Acute Psychological Stress in Rats. Psychoneuroendocrinology 2012, 37, 1885–1895. [Google Scholar] [CrossRef]
- Tian, X.; Yu, Z.; Feng, P.; Ye, Z.; Li, R.; Liu, J.; Hu, J.; Kakade, A.; Liu, P.; Li, X. Lactobacillus Plantarum TW1-1 Alleviates Diethylhexylphthalate-Induced Testicular Damage in Mice by Modulating Gut Microbiota and Decreasing Inflammation. Front. Cell. Infect. Microbiol. 2019, 9, 221. [Google Scholar] [CrossRef]
- Hashem, N.M.; Gonzalez-Bulnes, A. The Use of Probiotics for Management and Improvement of Reproductive Eubiosis and Function. Nutrients 2022, 14, 902. [Google Scholar] [CrossRef] [PubMed]
- Akram, M.; Ali, S.A.; Behare, P.; Kaul, G. Dietary Intake of Probiotic Fermented Milk Benefits the Gut and Reproductive Health in Mice Fed with an Obesogenic Diet. Food Funct. 2022, 13, 737–752. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zou, H.; Xu, H.; Cao, R.; Zhang, H.; Zhang, Y.; Zhao, J. The Potential Influence and Intervention Measures of Gut Microbiota on Sperm: It Is Time to Focus on Testis-Gut Microbiota Axis. Front. Microbiol. 2024, 15, 1478082. [Google Scholar] [CrossRef]
- Khan, A.; Kango, N.; Srivastava, R. Impact of Dietary Probiotics on the Immune and Reproductive Physiology of Pubertal Male Japanese Quail (Coturnix coturnix Japonica) Administered at the Onset of Pre-Puberty. Probiotics Antimicrob. Proteins 2024. [Google Scholar] [CrossRef]
- Liu, D.; Han, X.; Zou, W.; Yang, Z.; Peng, J.; Li, Y.; Liu, Y.; Jia, M.; Liu, W.; Li, H.; et al. Probiotics Combined with Metformin Improves Sperm Parameters in Obese Male Mice through Modulation of Intestinal Microbiota Equilibrium. Reprod. Sci. 2025, 32, 116–130. [Google Scholar] [CrossRef] [PubMed]
- Cristofori, F.; Dargenio, V.N.; Dargenio, C.; Miniello, V.L.; Barone, M.; Francavilla, R. Anti-Inflammatory and Immunomodulatory Effects of Probiotics in Gut Inflammation: A Door to the Body. Front. Immunol. 2021, 12, 578386. [Google Scholar] [CrossRef]
- Feng, T.; Wang, J. Oxidative Stress Tolerance and Antioxidant Capacity of Lactic Acid Bacteria as Probiotic: A Systematic Review. Gut Microbes 2020, 12, 1801944. [Google Scholar] [CrossRef]
- Martinez Guevara, D.; Vidal Cañas, S.; Palacios, I.; Gómez, A.; Estrada, M.; Gallego, J.; Liscano, Y. Effectiveness of Probiotics, Prebiotics, and Synbiotics in Managing Insulin Resistance and Hormonal Imbalance in Women with Polycystic Ovary Syndrome (PCOS): A Systematic Review of Randomized Clinical Trials. Nutrients 2024, 16, 3916. [Google Scholar] [CrossRef]
- Wu, L.-Y.; Yang, T.-H.; Ou, Y.-C.; Lin, H. The Role of Probiotics in Women’s Health: An Update Narrative Review. Taiwan. J. Obstet. Gynecol. 2024, 63, 29–36. [Google Scholar] [CrossRef]
- Bodke, H.; Jogdand, S. Role of Probiotics in Human Health. Cureus 2022, 14, e31313. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, M.; Azcarate-Peril, M.A.; Barnard, A.; Benoit, V.; Grimaldi, R.; Guyonnet, D.; Holscher, H.D.; Hunter, K.; Manurung, S.; Obis, D.; et al. Shaping the Future of Probiotics and Prebiotics. Trends Microbiol. 2021, 29, 667–685. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).