Simple Summary
This study explored how Bacillus proteolyticus (B. proteolyticus) helps the root development of Eleutherococcus senticosus (Rupr. and Maxim.) Maxim. (therm E. senticosus). The researchers used therm E. senticosus seeds and watered the soil with different concentrations of B. proteolyticus suspension. Results showed that after using B. proteolyticus, the seed germination rate, number of root tips, total root length and total root volume of therm E. senticosus first increased and then decreased. The 50% concentration of B. proteolyticus was best for seed germination. Compared with the control group, this concentration led to 16 different substances related to plant metabolism and an increase in the content of six main medicinal components. It also increased two types of nitrogen in the soil around roots and the amount of a specific kind of bacteria, which were all related to better root growth. This study found that 50% B. proteolyticus can promote therm E. senticosus growth, root development, and its medicinal quality. These bacteria can be made into a useful biological product. The study suggests using 50% B. proteolyticus suspension to grow therm E. senticosus, which can increase its yield and medicinal value, providing a sustainable way for its agricultural production.
Abstract
This study aimed to investigate the promoting mechanisms of Bacillus proteolyticus (B. proteolyticus) on Eleutherococcus senticosus (Rupr. and Maxim.) Maxim. (therm E. senticosus) root development. Using therm E. senticosus seeds as experimental material, soil was drenched with B. proteolyticus suspensions at different concentrations v/v (water, 25%; 50%; 75%, and 100%). The results showed that the germination rate, root tip number (52 ± 2.97), total root length (23.7 ± 0.46 cm), and total root volume (57.36 ± 1.64 mm3) exhibited an initial increase, then a decrease after B. proteolyticus treatment (p < 0.05). Principal component analysis (PCA) indicated that the soil of B. proteolyticus at a 50% concentration was conducive to seed germination. Compared with CK, GC-MS analysis revealed that 16 differential primary metabolites were screened, primarily enriched in galactose metabolism, starch and sucrose metabolism, and TCA cycle pathways after 50% B. proteolyticus treatment. LC-MS analysis revealed that the contents of six main medicinal components were higher than those of CK, with the content of eleutheroside E being 2.62 times greater. In rhizosphere soil, the contents of NO3−-N and NH4+-N were promoted, and the abundance of Gemmatimonadetes was increased in bacterial communities. Correlation analysis revealed significant correlations between the abundance of Gemmatimonadetes and the contents of NO3−-N and NH4+-N, as well as between total root length and D-galactose content, suggesting that these relationships may contribute to the root growth. Therefore, the soil of B. proteolyticus at a 50% concentration could enhance both the biomass and medicinal value of cultivated therm E. senticosus. This study provided novel insight that B. proteolyticus would be expected to be developed as an effective microbial preparation, offering a sustainable strategy for its agricultural production.
1. Introduction
Eleutherococcus senticosus (Rupr. and Maxim.) Maxim. (E. senticosus) was a perennial deciduous shrub belonging to the Araliaceae family and the genus Eleutherococcus [1]. Its roots and rhizomes were used medicinally and have been widely utilized as a folk herbal medicine in northeastern China [2]. Therm E. senticosus was one of the Traditional Chinese Medicines (TCM) stipulated in the Pharmacopoeia of the People’s Republic of China, which plays a role in treating and preventing rheumatism, coronary heart disease, hyperlipidemia, and depression [3]. The unrestrained harvesting of wild therm E. senticosus has led to severe destruction of its wild resources and a sharp decline in its population, due to its extensive medicinal and economic value [4]. However, seeds of therm E. senticosus possess innate dormancy characteristics. Under natural conditions, the seeds require two years to achieve a mere 10% germination rate, which makes artificial cultivation challenging in compensating for the shortfalls of wild resources [5]. In recent years, a lot of studies have focused on enhancing the germination rate of ES seeds.
Plant growth-promoting bacteria (PGPB) were emerging as novel practices and successful biological solutions to promote seed germination and boost medicinal and agricultural plant growth, development, and production [6,7]. PGPB inoculation can reshape the plant-associated microbiome, enhancing connectivity between rhizosphere and endophytic microbial communities and potentially improving crop resilience [8]. While the potential of PGPB is well-recognized, studies specifically exploring their application in therm E. senticosus cultivation remain limited. Bacillus thuringiensis CAPE95 and Paenibacillus polymyxa CAPE238 have been shown to increase germination rates and germination indices in nasturtium (Tropaeolum majus) and horsetail casuarina (Casuarina equisetifolia) [9,10]. Furthermore, PGPB can significantly enhance the accumulation of minor metabolites in medicinal plants by modulating their secondary metabolism. The study suggested that the application of Plesiomonas bacteria provides interesting benefits to an increase in crocin and safranal content in Crocus sativus [11]. The Bacillus sphaericus CIMAP-A7, found in Andrographis paniculata (Burm. f.) Nees was involved in the biosynthesis and accumulation of secondary metabolites [12]. Previous research has demonstrated that PGPB can not only promote growth but also significantly enhance the accumulation of bioactive compounds. For instance, the co-application of mycorrhizal fungi and specific PGPB with vermicompost has been shown to substantially increase the total phenolic content and fruit yield in Physalis alkekengi L., highlighting the potential of microbial communities in enhancing both the quality and quantity of medicinal plant production [13]. Therefore, investigating the growth-promoting mechanisms of PGPB from medicinal plants was crucial for formulating effective cultivation strategies and enhancing the quality of its seedlings.
PGPB mediates the modulation of soil nutrient availability. Research reports indicate that PGPB can convert organic phosphorus compounds into phosphate in soil or transform insoluble inorganic phosphorus into soluble phosphorus to promote plant growth [14]. It was well known that PGPB could improve plant growth and change root conformation through direct mechanisms, including the synthesis of phytohormones and growth regulators such as auxin (indole-3-acetic acid (IAA)) [15]. This inoculation with phosphate-solubilizing bacteria ASR16 and ALR332 showed a significant growth improvement in Medicago sativa L. compared to the control, especially in plant height, biomass, and root length [16]. In addition, PGPB can promote soil nitrogen fixation, providing important nutrient elements for plants [17]. The current research was motivated by the isolation of a bacterial strain from the rhizosphere soil of two-year-old E. senticosus, which was later identified as Bacillus proteolyticus. Preliminary functional characterization revealed its promising plant growth-promoting abilities, leading to a systematic evaluation of its effects on the growth and metabolism of its host plant. We studied the effect of B. proteolyticus from therm E. senticosus rhizosphere on its seed germination and analyzed the related physiological mechanisms combined with the rhizosphere soil nutrient and bacterial community structure. Therefore, investigating the growth-promoting mechanisms of PGPB from medicinal plants was crucial for formulating effective cultivation strategies and enhancing the quality of its seedlings.
2. Materials and Methods
2.1. Bacterial Strain
Strain identification was performed based on morphological, physiological, and biochemical characteristics following Bergey’s Manual of Determinative Bacteriology [18]. The physiological and biochemical characteristics following standard microbiological methods were employed as follows: Gram staining, catalase test, oxidase test, gelatin hydrolysis test, starch hydrolysis test, methyl red test, Voges–Proskauer (V–P) test, nitrate reduction test, indole production test, and hydrogen sulfide (H2S) production test. The functional traits were assessed using the following assays: the nitrogen-free Jensen’s medium method for nitrogen-fixation, the Pikovskaya’s broth method for phosphate solubilization, and the Salkowski’s reagent method for indole-3-acetic acid (IAA) production quantification.
Genomic DNA extraction was conducted using a proteinase K lysis protocol. PCR amplification was carried out with universal bacterial 16S rDNA primers, forward primer 5′-AGAGTTTGATCCTGGCTCAG-3′ and reverse primer 5′-AAGGAGGTGATCCAGCC-3′. The PCR amplification system and reaction conditions are detailed in Table 1. Amplified 16S rDNA products were purified and subsequently sent to Shanghai Majorbio Bio-pharm Technology Co., Ltd. (Shanghai, China) for sequencing. The obtained sequences were submitted to the NCBI database for Blastn analysis (Table 1).

Table 1.
PCR amplification system and reaction conditions.
2.2. B. proteolyticus Suspension and Soil Preparation
A single colony of B. proteolyticus was inoculated into 200 mL of liquid LB medium and cultured at 37 °C with shaking at 200 rpm for 8–10 h until the OD600 reached 0.8, after which the bacterial cells were collected by centrifugation at 12,000 r/min for 10 min. The pellets were then resuspended in sterile distilled water and diluted to concentrations of 25%, 50%, 75%, and 100% (v/v), designated as T25, T50, T75, and T100. Using sterile distilled water as CK. The resulting suspensions were stored at 4 °C.
The potted soil used in this study was peat soil. The scope was conducted in a pot with an upper diameter of 54.5 mm, a bottom diameter of 28.5 mm, and a depth of 5 cm. Each treatment consisted of 5 pots, with 300 seeds shown per pot. Each pot was irrigated with 825 mL of bacterial suspension after seeding.
2.3. Plant Materials
Therm E. senticosus seeds were purchased from the Northern Medicinal Plant Planting Base, Yichun City, Heilongjiang Province, and cultivated in the greenhouse of the Key Laboratory of Forest Plant Ecology, Ministry of Education at Northeast Forestry University (Harbin City, Heilongjiang Province, China). The different concentrations of suspensions are drenched in soil.
2.4. Determination of Seed Germination and Root Growth
Germination rates ((Germinated seeds/Total tested seeds) × 100%) were recorded at five-day intervals post-sowing until day 50. The germination rate was calculated by dividing the number of germinated seeds by the total number of seeds shown (300). At 50 days post-treatment, leaf area was measured using a portable leaf area meter (CI-203; CID Bio-Science, Inc., Camas, WA, USA). Plant height and stem length were determined using a ruler. Fresh weight and dry weight were measured with an analytical balance (FA224C; LICHEN, Shanghai, China). For root character analysis, five uniformly growing seedlings per treatment were randomly selected. Roots were thoroughly rinsed and scanned using a scanner (IN-GX02; LAIYIN, WeiFang City, China). Root length, surface area, volume, and average diameter were quantified from digital images.
2.5. Determination of Metabolic Characteristics in Roots
The determination of primary metabolites followed the methodology described by Liu [19] using an Agilent 7890A-5975C GC-MS system (7890A-5975C; Agilent, Newark, DE, USA). Fresh roots from the CK and treatment (T50) were selected for metabolic analysis. For primary metabolic profiling, 0.09 g of each sample was used. Plant tissues were snap-frozen in liquid nitrogen and homogenized into a fine powder. Metabolites are then extracted using a mixed solvent system (methanol-water-chloroform), followed by centrifugation. The supernatant was collected, concentrated, and reconstituted in a suitable solvent for subsequent GC-MS analysis. This analysis was conducted using a DB-5MS capillary column in split mode, with a 1 μL sample injection and helium as the carrier gas. After a 6.35 min solvent delay, mass spectra were acquired in full-scan mode (m/z 50–500) at a scan rate of 12.5 spectra/s.
Major medicinal active constituents and phenolic compounds in ES were analyzed according to Fu’s protocol [20] using an ACQUITY UPLCXevoG2-SQToFMS system (6890GCMS; Waters Technologies, Shanghai, China). Fresh roots (0.5 g) were frozen in liquid nitrogen. The material was ground into a fine powder, followed by extraction with a methanol-water solvent system. After centrifugation, the supernatant was collected, concentrated under a nitrogen stream, and reconstituted in appropriate solvents for subsequent LC-MS analysis. Separation was achieved on a C18 column (2.1 mm × 100 mm, 1.6 μm) with mobile phase, 62% water (solvent A) and 38% methanol (solvent B) at 0.25 mL·min−1. Column temperature was maintained at 25 °C with a 5 μL injection volume. Reference standards were purchased from the Aladdin official website.
2.6. Determination of Soil Characteristics
According to Ru’s method [21], Soil pH was determined by using a pH meter (PhSJ-5T; Leici, Shanghai, China), and soil electrical conductivity (EC) was determined by using a conductivity meter (DDS-307; Leimagnet, Shanghai, China). Soil ammonium nitrogen (NH4+-N) was determined by Nanocolorimetry, and nitrate nitrogen (NO3−-N) by phenol-disulphonic acid, and available phosphorus (AP) by the Olsen Method.
2.7. Determination of Rhizosphere Soil Bacterial Communities
Rhizosphere soil DNA from the CK and T50 treatments was extracted and analyzed. Nucleic acid extraction and sequencing were outsourced to Shanghai Personalbio Biotechnology Co., Ltd. (Shanghai, China) Genomic DNA was extracted using the Mag Beads Fast DNA Kit for Soil (116564384; MP Biomedicals, Santa Ana, CA, USA). Sequencing was performed on an Illumina HiSeq 2500 platform (Illumina Inc., San Diego, CA, USA). Raw sequencing reads were processed through paired–end merging, quality filtering, and chimera removal to obtain high-quality sequences (Clean Tags). Subsequently, Clean Tags were clustered into Operational Taxonomic Units (OTUs) at 97% similarity threshold using Usearch9. Analysis of species abundance distribution was visualized through Venn diagrams and QIIME 1 pipelines. All bioinformatic analyses were conducted on the Personalbio Cloud Platform (https://www.genescloud.cn/ accessed on 6 February 2025).
2.8. Statistical Analysis
Data processing was performed using SPSS 26.0 and Microsoft Excel 2021. Differences between treatment groups were assessed by one-way analysis of variance (ANOVA) at p < 0.05 significance level. Comprehensive scores across treatments were calculated through principal component analysis (PCA). Figures were generated with Origin 2021, with data presented as mean ± standard deviation (mean ± SD).
3. Results
3.1. Identification of Rhizosphere B. proteolyticus
The B. proteolyticus strain used in this study was previously isolated from the rhizosphere soil of two-year-old therm E. senticosus. Combined with colonial morphology, the colonies exhibited a cream-colored, opaque appearance, predominantly circular with regular margins. Surface characteristics included glossiness without wrinkles, moist texture consistent with typical bacterial colony morphology (Figure 1); the strain was preliminarily identified as B. proteolyticus. PCR amplification and sequencing revealed 100% similarity to B. proteolyticus strain MCCC 1A00365.
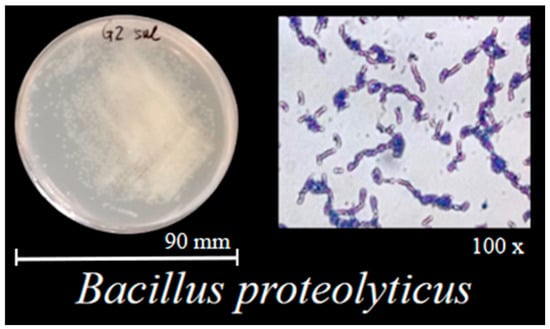
Figure 1.
Morphological characteristics and Gram staining results of B. proteolyticus strain.
Physiological and biochemical assays identified the strain as Gram–positive (Figure 1), positive for catalase, oxidase, gelatin hydrolysis, starch hydrolysis, methyl red test, nitrate reduction, and indole production, but negative for Voges–Proskauer (V–p) reaction and H2S production. Functional characterization might demonstrate the action of its nitrogen-fixing capacity, phosphate solubilization (22.79 ± 0.92 μg/mg), and IAA production (14.99 ± 0.83 mg/L) (Table 2). Based on these findings, the strain was identified as a PGPB with multifunctional activities.

Table 2.
Plant growth-promoting attributes of inoculum strains.
3.2. Promotion of E. senticosus Seed Germination and Root Growth by B. proteolyticus
As the B. proteolyticus concentration increased, the germination rate of therm E. senticosus seeds initially rose, then declined. At day 50 post-treatments, the highest germination rate (27%) was achieved after the T50 treatment (Figure 2A), and the seedlings’ height gradient followed: T50 > T25 > T75 > CK > T100; the maximal height (7.10 ± 0.47 cm) was exhibited significantly (p < 0.05). Both parameters were significantly enhanced compared to all treatments except T25 (p < 0.05), with stem length 1.5-fold greater than CK after T50 treatment (Figure 2B). While germination rate, plant height, stem length, and leaf area were optimally promoted, it showed limited efficacy in increasing leaf number after T50 treatment.
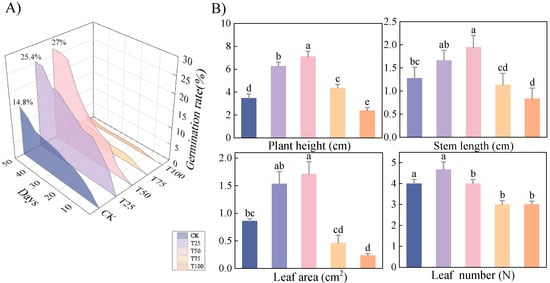
Figure 2.
Therm E. senticosus growth characteristics after different B. proteolyticus treatments. (A) Seed germination rate, (B) growth parameters of shoots. Note: different lowercase letters indicate significant differences (p < 0.05) among treatments. Differences between treatment groups were assessed by one-way analysis of variance (ANOVA) at p < 0.05 significance level.
After T50 treatment, therm E. senticosus exhibited the highest values among all treatments for root tip number (52 ± 2.97), total root length (23.7 ± 0.46 cm), total root volume (57.36 ± 1.64 mm3), and root-to-shoot ratio (20.10 ± 1.54). Compared to CK, T75, and T100 treatments, there was a demonstrated significant enhancement effect (p < 0.05) after T50 treatment. The average root diameter after T25 (0.53 ± 0.04 mm) was significantly greater than other treatments (p < 0.05), while differences among other treatments were non-significant (p < 0.05). Lower concentrations of B. proteolyticus more effectively promoted root system development in ES but exerted minimal effects on root-to-shoot ratio and root diameter (Figure 3).
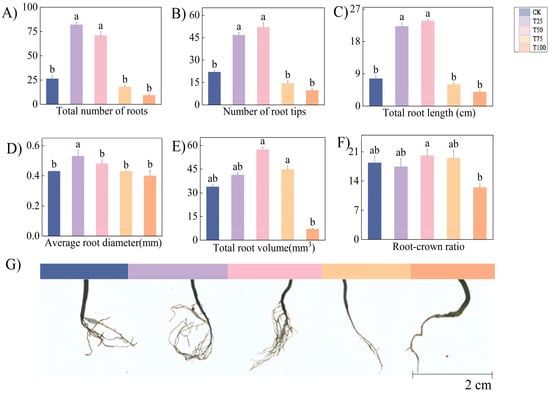
Figure 3.
Root growth characteristics of therm E. senticosus after different B. proteolyticus treatments. (A) Total number of roots, (B) number of root tips, (C) total root length, (D) average root diameter, (E) total root volume, (F) root-crown ratio, (G) root system phenotype images. Note: different lowercase letters indicate significant differences (p < 0.05) among treatments. Differences between treatment groups were assessed by one-way analysis of variance (ANOVA) at p < 0.05 significance level.
Principal component analysis (PCA) was performed on 11 indices across five treatments in this study, and the results are shown in Figure 4 and Table 3. The weights of the 11 measured indices were 10.03%, 9.11%, 10.06%, 9.49%, 6.99%, 8.75%, 9.29%, 9.09%, 8.17%, 10.04%, and 8.97% (Figure 4A). The comprehensive scores of each treatment, calculated by principal component analysis, are presented in Figure 4A, with the ranking of comprehensive scores as follows: T50 > T25 > CK > T75 > T100. The results indicated that the germination and growth status of ES after the T50 treatment were the best among all treatments (Figure 4B).
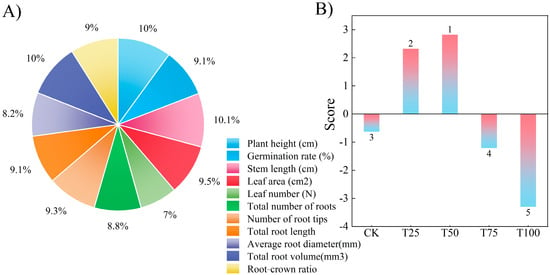
Figure 4.
Principal component analysis. (A) the weights of the 11 measured indices, (B) the comprehensive scores.

Table 3.
The number of bacterial communities in rhizosphere soil.
3.3. Analysis of Metabolic Characteristics of Root
A total of 60 specific primary metabolites were identified. Based on the results of OPLS-DA analysis, metabolites with a VIP value greater than one and a significant difference p-value between groups less than zero point zero five were screened out. A total of 16 differential metabolites between the two treatments were screened out, which were classified into six acids, six sugars, three alcohols, and one phenylpropanoid. The six acids were D-galacturonic acid, quinic acid, citric acid, malic acid, palmitic acid, and stearic acid; the six sugars were d-galactose, maltose, D-psicose, D-fructose, D-glucose, and sucrose; the three alcohols were silanol, inositol, and isopropanol; the one phenylpropanoid was phenylpropionic acid. Compared with CK, the contents of citric acid, silanol, phenylpropionic acid, malic acid, inositol, D-galacturonic acid, sucrose, quinic acid, D-glucose, palmitic acid, stearic acid, D-fructose, D-galactose, and isopropanol in T50 are significantly increased (Figure 5A). After treatment with B. proteolyticus solution, the differences in primary metabolic pathways of ES root were concentrated in galactose metabolism, starch and sucrose metabolism, TCA cycle, glyoxylate and dicarboxylate metabolism, and pyruvate metabolism. Among them, the differential metabolites (citric acid, silanol, phenylpropionic acid, malic acid, and inositol) after T50 treatment were mainly concentrated in the TCA cycle, glyoxylate and dicarboxylate metabolism, and pyruvate metabolism (Figure 5C). The accumulation of carbohydrates in therm E. senticosus was promoted significantly after B. proteolyticus treatment.
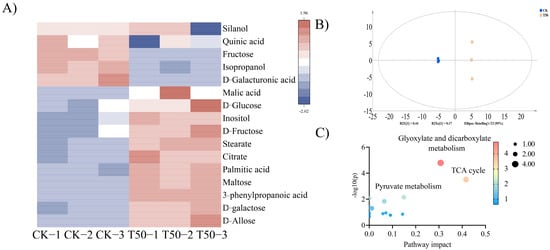
Figure 5.
The primary metabolites analysis after different concentrations of B. proteolyticus treatments in therm E. senticosus roots. (A) cluster analysis plot, (B) OPLS-DA scores plot, and (C) pathway enrichment analysis of differential metabolites.
Metabolomic analysis of the roots revealed distinct accumulation patterns of both primary and secondary metabolites compared to the control. (Figure 6A). Compared with CK, there were higher contents of galangin, quercetin, abscisic acid, apigenin, chlorogenic acid, dihydroquercetin, kaempferol, isoquercitrin, glycyrrhizin, trigonelline, hesperetin, genistein, and catechins (Figure 6A). The contents of eleutheroside E, aucubin, and syringin in roots were significantly higher than those in CK (p < 0.05) (Figure 6B). After treatment, the content of secondary metabolites in ES was significantly (p < 0.05) accumulated.
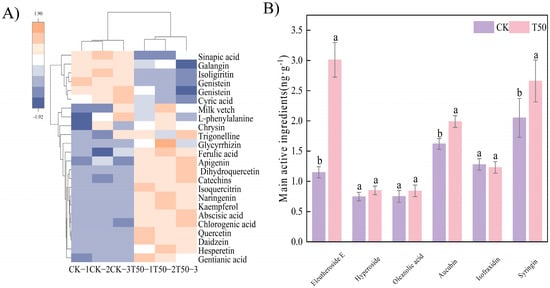
Figure 6.
Analysis of phenolic compounds and main medicinal components after T50 treatment. (A) visualization heatmap of phenolic compounds, (B) contents of main medicinal components. Note: different lowercase letters indicate significant differences (p < 0.05) among treatments. Differences between treatment groups were assessed by one-way analysis of variance (ANOVA) at p < 0.05 significance level.
3.4. Effects on Soil Biochemical Characteristics
As shown in Figure 7A, there were higher contents of soil ammonium nitrogen (NH4+-N), nitrate nitrogen (NO3−-N), and available phosphorus (AP) compared to CK after T50 treatment. The pH of CK was significantly (p < 0.05) higher than that of treatment, while the EC was significantly higher than that of CK (Figure 6B). Treatment with the bacterial suspension significantly (p < 0.05) increased the nutrient content in the soil.
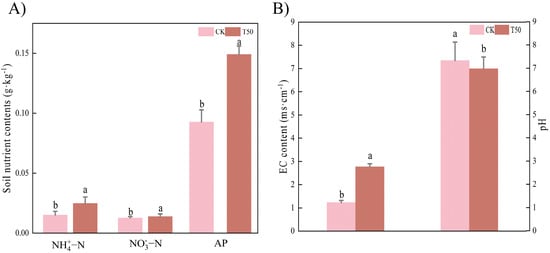
Figure 7.
Soil characteristics analysis after T50 treatment of the rhizosphere soil of therm E. senticosus seedlings. (A) soil nutrient contents, (B) pH, and EC. Note: different lowercase letters indicate significant differences (p < 0.05) among treatments. Differences between treatment groups were assessed by one-way analysis of variance (ANOVA) at p < 0.05 significance level.
3.5. Correlation Analysis
The contents of citrate and D-Glucose were significantly (p < 0.05) positively correlated with NH4+-N content. D-galactose was significantly (p < 0.05) positively correlated with total root length. There was a significant (p < 0.05) positive correlation between NO3−-N content and total root volume. AP content was significantly (p < 0.05) positively correlated with total root length and number of root tips. The content of eleutheroside E, aucubin, and syringin was significantly positively correlated with total root length and number of root tips (p < 0.01) (Figure 8). The contents of AP influenced secondary metabolite accumulation in therm E. senticosus.
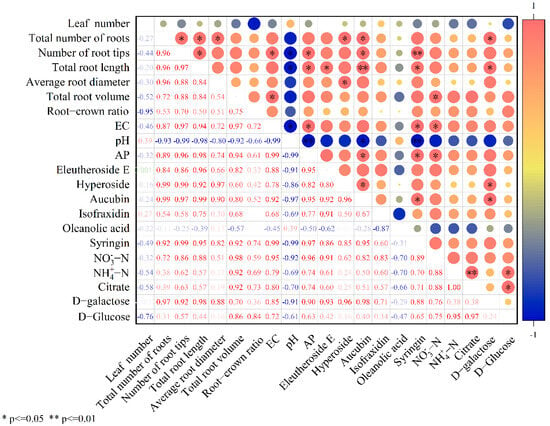
Figure 8.
Correlation coefficients between growth characteristics, soil nutrient contents, and main medicinal components of therm E. senticosus after different concentrations of B. proteolyticus treatments.
3.6. Analysis of the Rhizosphere Soil Bacterial Community Diversity and Community Composition
After filtering the raw sequences, we obtained statistically significant species counts for each taxonomic rank in each sample. There was a higher total number of bacterial groups OTUs in the rhizosphere soil of therm E. senticosus after the T50 treatment than after CK treatment. Statistical tables of bacterial species can be acquired by consulting the Silva and UNITE databases (Table 3). The bacterial diversity after T50 treatment was higher than that after CK at all levels except the domain level.
Proteobacteria were the dominant phylum after both CK and T50 treatments, accounting for 60.51 ± 0.01% and 33.07 ± 0.01% of the communities. There were significantly higher relative abundances of Gemmatimonadetes, Myxococcota, Bacteroidetes, Acidobacteria, and Chloroflexi after T50 treatment (Figure 9A). The abundance of Gemmatimonadetes was 8.33 times higher than that of CK. As a comprehensive measure of the α-diversity index, the Simpson index and Chao index showed a significant difference between CK and T50 treatments (Figure 9B). PICRUST2 was used to predict the potential functions of the bacterial community in rhizosphere soil. The results showed that the primary metabolic functions of the rhizosphere soil bacterial communities in CK and T50 mainly included the following nine categories: interconversion of pentose and glucuronate, degradation of aromatic compounds, lysine biosynthesis, retinol metabolism, flavonoid biosynthesis, folate biosynthesis, amino sugar and nucleotide sugar metabolism, lysine degradation, and glycerophospholipid metabolism, all of which were higher than those in CK (Figure 9C). After T50 treatment, microbial community richness was enhanced in the rhizosphere soil, and primary metabolic functions among microorganisms were altered.
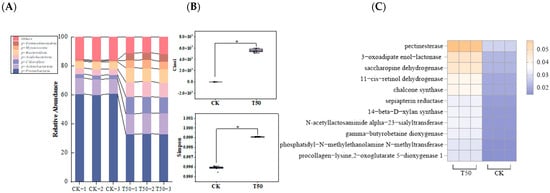
Figure 9.
Analysis of soil bacterial community characteristics in therm E. senticosus after T50 treatment. (A) Primary metabolism of bacterial communities, Simpson and Chao1index, (B) bacterial community composition, and (C) functional divergence. * p ≤ 0.05.
Correlation analysis demonstrated that all bacterial phyla showed differential positive correlations with NO3−-N, NH4+-N, AP, and EC except Proteobacteria after T50 treatment, while exhibiting negative correlations with pH. Consequently, the T50 treatment resulted in significantly higher nutrient content in the soil of therm E. senticosus (Figure 10).
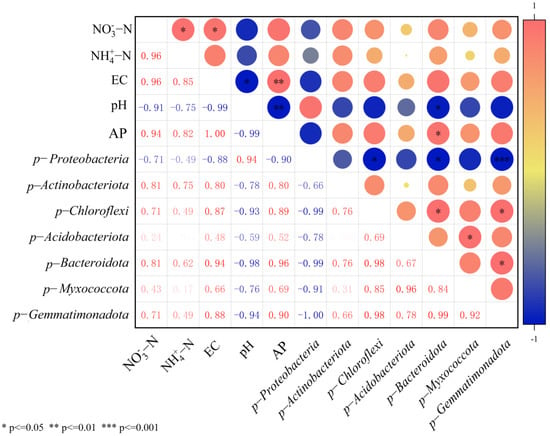
Figure 10.
Correlation coefficients between soil nutrient contents and bacterial community composition of rhizosphere soil in therm E. senticosus seedlings after T50 treatment.
4. Discussion
In recent years, there has been a surge of interest in investigating the promotional effects of plant growth-promoting bacteria (PGPB) on seed germination, growth, and metabolic processes of medicinal plants [22]. PGPB accelerates the growth of medicinal plants and enhances their nutrient absorption efficiency, as they could intervene in and coordinate their growth and nutritional metabolic pathways [23]. Studies have shown that an appropriate bacterial suspension concentration is more conducive to plant growth [24]. Therefore, we established gradient concentrations of the bacterial suspension of B. proteolyticus and conducted a Principal Component Analysis (PCA) based on the growth indicators of therm E. senticosus, which demonstrated that the T50 treatment yielded the most pronounced promoting effect on the plant growth. Studies have shown that PGPB treatment can increase the germination rate of tomato and Seriphidium transiliense seeds, as well as enhance the aboveground biomass and rhizome elongation rate of Zostera marina L. [25]. In our study, inoculation with B. proteolyticus significantly promoted the germination rate and growth indicators of therm E. senticosus, resulting in plant height, stem length, and leaf area that were 2.05, 1.53, and 1.98 times that of CK, respectively, and a 12.2% increase in germination rate (Figure 1). This biosynthesis of IAA can promote plant growth and development and enhance plant metabolic activity [26,27]. We analyzed the PGP properties of B. proteolyticus using in vitro assays to demonstrate its ability to produce IAA, solubilize phosphate (Table 1), indicating that the impact of PGPB on plant development was associated with IAA production [28]. PGPB play a pivotal role in stimulating the production of plant growth regulators, fortifying plant defenses, and mitigating abiotic stress [29,30,31].
The use of microorganisms as green fertilizers to enhance the active ingredients and cultivation yields of medicinal plants is currently a hot research topic [32]. The secondary metabolites of plants were not only crucial to their own defense mechanism but also a source of pharmacologically active components [33,34]. In our study, drenching with B. proteolyticus significantly increased the content of secondary metabolites in therm E. senticosus seedlings, such as eleutheroside E and phenolic compounds. Similar results have been reported in other studies on medicinal plants. LIMA found that inoculation with C. etunicatum or G. albida increased the contents of total leaf phenols and tannins in C. leptophloeos. Actinomadura roseola could promote the upregulation of rutin and hesperetin in Tetrastigma hemsleyanum [35]. Three main active components, including volatile oils in Asarum sieboldii, showed a good correlation with the rhizosphere bacterium Rhodanobacter [36]. The biologics released by microorganisms have also been shown to effectively stimulate the production of key host plant enzymes responsible for secondary metabolite synthesis [37,38,39]. The ability of PGPB to reshape the root-associated microbiome and enhance crosstalk between rhizospheric and endophytic microbial communities may also contribute to the improved metabolic profiles observed in our study, underscoring a potential microbiome-mediated mechanism [4].
Patani argues that the Bacillus genus can directly stimulate plant growth through enhancing nutrient availability, such as nitrogen fixation and phosphate solubilization [40]. In our study, the incorporation of B. proteolyticus significantly enhanced the physicochemical properties of the soil, particularly augmenting the availability of essential nutrients such as NO3−-N, NH4+-N, and AP (Figure 6). After T50 treatment, Gemmatimonadetes abundance was higher than that of CK. Analysis of soil bacterial α-diversity in this study showed that the richness of the bacterial community in the rhizosphere soil of therm E. senticosus decreased after irrigation with B. proteolyticus, which was similar to the findings of Li [41]. They found that PGPB + SMS treatment significantly increased the abundances of Bacillus and Pseudomonas, which might be attributed to the limited ability of single PGPB irrigation to alter soil microbial community structure, whereas mixed bacterial inoculants enhanced the stability of the soil microbial community in Phyllostachys edulis [42]. Studies have shown that Gemmatimonadetes abundance exhibits a positive correlation with total carbon, nitrogen, and phosphorus in soil. The abundance of Gemmatimonadetes demonstrates strong metabolic and degradation capabilities, playing a crucial role in nutrient cycling and potentially enhancing plant nutrient utilization efficiency [43,44]. Inoculation with PGPB can well fix gaseous nitrogen in the air [16] and simultaneously increase the number of nitrogen-fixing bacteria in the soil, as well as decompose nitrates and nitrites in the soil, thereby increasing soil nitrogen levels [45]. Meanwhile, the contents of NO3−-N, NH4+-N, and AP after irrigation with B. proteolyticus were also significantly increased, which was consistent with the findings of Zhang [46]. They suggested that inoculation with PGPB can effectively convert unavailable forms of soil nutrients into available ones and promote the efficient absorption and utilization of nutrients by plants. The content of AP increased significantly after treatment, which was consistent with the conclusion of Liu [47]. The possible reason was that PGPB secretes inorganic and organic acids to dissolve insoluble phosphorus or upregulate the expression of phosphate transporters to enhance plant absorption and utilization of phosphorus [47]. Such a biofertilizer could serve as an environmentally friendly alternative to partial chemical fertilization, supporting sustainable agricultural practices.
5. Conclusions
The B. proteolyticus strain in this study functioned as a multifunctional PGPB, exhibiting capabilities for nitrogen fixation, phosphorus solubilization, and IAA biosynthesis. This study indicated that B. proteolyticus had a significant promoting effect on seed germination, root growth, and metabolism of therm E. senticosus. It increases the contents of AP, NO3−-N, and NH4+-N in soil through nitrogen fixation and phosphorus solubilization, and secretes IAA to promote the development and growth of therm E. senticosus roots for better nutrient absorption. Differences were observed in primary metabolic pathways, including galactose metabolism, starch and sucrose metabolism, TCA cycle, glyoxylate and dicarboxylate metabolism, and pyruvate metabolism. Additionally, the contents of the main medicinal active components accumulated through secondary metabolism were significantly increased. From a practical perspective, we recommend developing this strain into a specialized microbial inoculant for therm E. senticosus cultivation. Future research should focus on the following: validating the strain’s efficacy under field conditions, elucidating the molecular mechanisms underlying their role in host metabolic reprogramming.
Author Contributions
Y.Z.: writing—original draft, Data curation. X.T.: Investigation. J.Q.: Software, Investigation. W.Z.: Investigation. X.L.: Formal analysis. Z.T.: Investigation. Y.L.: writing—review and editing. D.L.: Supervision, Resources, and Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Key Research and Development Program of China (No. 2022YFF1300503) and the Fundamental Research Funds for the Central Universities (No. 2572023CT11). Heilongjiang Province Seed Industry Innovation Project (ZQTYB251700001).
Institutional Review Board Statement
Not applicable, for studies not involving humans or animals.
Informed Consent Statement
Not applicable, for studies not involving humans or animals.
Data Availability Statement
Data will be made available on request.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Liang, Q.; Han, D.; Jiang, J.; Yan, G.; Kong, L.; Sun, H.; Han, Y.; Zhang, X.; Wang, X.; Wang, X. Extraction, structural characteristics, bioactivities and application of polysaccharides from Acanthopanax senticosus (Rupr. & Maxim.) Harms: A review. Int. J. Biol. Macromol. 2025, 299, 139972. [Google Scholar] [CrossRef]
- Zhang, W.; Gao, R.; Tian, L.; Xu, Z. Integrated microbiome and metabolomics analysis reveal the relationship between plant-specialized metabolites and microbial community in Phellodendron amurense. Front. Plant Sci. 2024, 15, 1363063. [Google Scholar] [CrossRef]
- Wang, F.; Zhao, Z.; Han, Y.; Li, S.; Bi, X.; Ren, S.; Pan, Y.; Wang, D.; Liu, X. The bacterial and fungal compositions in the rhizosphere of Asarum heterotropoides Fr. Schmidt var. mandshuricum (Maxim.) Kitag. in a typical planting region. Microorganisms 2024, 12, 692. [Google Scholar] [CrossRef]
- Huang, Y.; Zhai, L.; Chai, X.; Liu, Y.; Lv, J.; Pi, Y.; Gao, B.; Wang, X.; Wu, T.; Zhang, X.; et al. Bacillus B2 promotes root growth and enhances phosphorus absorption in apple rootstocks by affecting MhMYB15. Plant J. 2024, 119, 1880–1899. [Google Scholar] [CrossRef]
- Holt, J.G.; Krieg, N.R.; Sneath, P.H.A.; Staley, J.T.; Williams, S.T. (Eds.) Bergey’s Manual of Determinative Bacteriology, 9th ed.; Williams & Wilkins: Philadelphia, PA, USA, 1994. [Google Scholar]
- Visen, A.; Bohra, M.; Singh, P.N.; Srivastava, P.C.; Kumar, S.; Sharma, A.K.; Chakraborty, B. Two pseudomonad strains facilitate AMF mycorrhization of litchi (Litchi chinensis Sonn.) and improving phosphorus uptake. Rhizosphere 2017, 3, 196–202. [Google Scholar] [CrossRef]
- Zaki, R.M.; Afify, A.H.; Ashour, E.H.; El-Sawah, A.M. Salt-Tolerant Bacteria Support Salinity Stress Mitigating Impact of Arbuscular Mycorrhizal Fungi in Maize (Zea mays L.). Microorganisms 2025, 13, 1345. [Google Scholar] [CrossRef]
- Braglia, C.; Alberoni, D.; Baffoni, L.; Angeli, S.; Di Gioia, D. PGPB Administration Influences the Cross-Talk and Interactions of Rhizosphere and Endophyte Microbial Communities in Sunflower. Curr. Res. Microb. Sci. 2025, 9, 100478. [Google Scholar] [CrossRef]
- Chu, W.; Wang, Y.Y.; Guo, Y.; Peng, Y.H.; Wu, Z.Y.; Lin, W.X. Screening of plant growth-promoting rhizobacteria from Casuarina equisetifolia rhizosphere and their effects on seed germination and seedling growth. Chin. J. Appl. Ecol. 2024, 35, 2159–2166. [Google Scholar]
- Ghayoumi, M.; Emamjomeh, A.; Kavousi, K.; Najafi, A. Rhizosphere soil bacteria community vary and correlate with Crocus sativus (saffron) quality at four locations. Rhizosphere 2022, 24, 100627. [Google Scholar] [CrossRef]
- Tripathi, P.; Yadav, R.; Das, P.; Singh, S.; Singh, A.; Singh, P.; Kandasamy, P.; Kalra, A.; Khare, P. Endophytic bacterium CIMAP-A7 mediated amelioration of atrazine induced phyto-toxicity in Andrographis paniculata. Environ. Pollut. 2021, 287, 117635. [Google Scholar] [CrossRef]
- Khan, W.; Zhu, Y.; Khan, A.; Zhao, L.; Yang, Y.-M.; Wang, N.; Hao, M.; Ma, Y.; Nepal, J.; Ullah, F.; et al. Above-and below-ground feedback loop of Zea mays (maize) is jointly enhanced by plant growth-promoting rhizobacteria and arbuscular mycorrhizal fungi in drier soil. Sci. Total Environ. 2024, 917, 170417. [Google Scholar] [CrossRef]
- Mortezaei, Z.; Ramezan, D.; Mohkami, Z.; Aran, M.; Rahimi, M.; Nejad, M.S.; Zargar, M. Effects of Mycorrhizal Fungi, Plant Growth-Promoting Bacteria, and Vermicompost on Enhance Growth Characteristics of Physalis alkekengi L. Russ. J. Plant Physiol. 2025, 72, 188. [Google Scholar] [CrossRef]
- Kang, H.Y.; Wang, W.; Liu, J.L.; Guo, D.L.; Guo, C.H. Isolation, purification and identification of two endophytes with growth-promoting effects from Medicago sativa. Microbiol. China 2015, 42, 280–288. [Google Scholar] [CrossRef]
- Yang, R.; Meng, X.; Zhao, W.; Xu, S.Q.; Wang, S.Y.; Li, M.M.; Guan, W.; Chen, Q.S.; Zhang, L.L.; Kuang, H.X.; et al. Phenylpropanoids of Eleutherococcus senticosus (Rupr. & Maxim.) Maxim. Alleviate oxidative stress in Alzheimer’s disease in vitro and in vivo models by regulating Mst1 and affecting the Nrf2/Sirt3 pathway. Bioorganic Chem. 2025, 159, 108347. [Google Scholar] [CrossRef]
- Ahmad, M.; Hussain, A.; Dar, A.; Luqman, M.; Ditta, A.; Iqbal, Z.; Ahmad, H.T.; Nazli, F.; Soufan, W.; Almutairi, K.; et al. Combating iron and zinc malnutrition through mineral biofortification in Zea mays (maize) through plant growth promoting Bacillus and Paenibacillus species. Front. Plant Sci. 2023, 13, 1094551. [Google Scholar] [CrossRef] [PubMed]
- Raklami, A.; Quintas-Nunes, F.; Nascimento, F.X.; Jemo, M.; Oufdou, K.; Syed, A.; Bahkali, A.H.; Verma, M.; Nafis, A. Assessing the growth-promoting traits of actinobacteria spp. isolated from Cleome africana: Implications on growth and root enhancement of Medicago sativa. J. King Saud Univ.—Sci. 2023, 35, 102722. [Google Scholar] [CrossRef]
- Liu, Y. Comparative Study on the Metabolic Basis of Two Astragalus membranaceus Species in Response to UV-B and Drought Stress by Metabonomics. Ph.D. Thesis, Northeast Forestry University, Harbin, China, 2018. [Google Scholar]
- Fu, J.Y.; Yang, C.; Li, D.W. Effects of elevated UV-B radiation on photosynthesis and contents of active substance of Eucommia ulmoides plantation. Anhui Agric. Sci. 2017, 45, 6–10. [Google Scholar] [CrossRef]
- Ru, R.K. Soil Agricultural Chemical Analysis Methods; Agricultural Science and Technology Press: Beijing, China, 2000. [Google Scholar]
- Khalediyan, N.; Weisany, W.; Schenk, P.M. Arbuscular mycorrhizae and rhizobacteria improve growth, nutritional status and essential oil production in Ocimum basilicum and Satureja hortensis. Ind. Crops Prod. 2021, 160, 113163. [Google Scholar] [CrossRef]
- Widnyana, I.K.; Javandira, C. Activities of Pseudomonas spp. and Bacillus sp. to Stimulate Germination and Seedling Growth of Solanum lycopersicum (Tomato) Plants. Agric. Agric. Sci. Procedia 2016, 9, 419–423. [Google Scholar] [CrossRef]
- Sun, J.; Zhao, Q.; Gao, Y.N.; Long, Q.G.; Yan, W.J.; Zhang, P.D. Restoration of degraded seagrass meadows: Effects of plant growth-promoting rhizobacteria (PGPR) inoculation on Zostera marina growth, rhizosphere microbiome and ecosystem functionality. J. Environ. Manag. 2024, 371, 123286. [Google Scholar] [CrossRef]
- Keskes, S.; Ben Khedher, S.; Masmoudi, F.; Saadaoui, I.; Tounsi, S. Spore production optimization of a biofertilizer based on Bacillus cabrialesii HB7 for enhancing plant growth under saline stress. Int. Microbiol. 2025. [Google Scholar] [CrossRef]
- Jung, B.K.; Ibal, J.C.; Pham, H.Q.; Kim, M.C.; Park, G.S.; Hong, S.J.; Jo, H.W.; Park, C.E.; Choi, S.D.; Jung, Y.; et al. Quorum Sensing System Affects the Plant Growth Promotion Traits of Serratia fonticola GS2. Front. Microbiol. 2020, 11, 536865. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Singh, P.C.; Chaudhry, V.; Shirke, P.A.; Chakrabarty, D.; Farooqui, A.; Nautiyal, C.S.; Sane, A.P.; Sane, V.A. PGPR-induced OsASR6 improves plant growth and yield by altering root auxin sensitivity and the xylem structure in transgenic Arabidopsis thaliana. J. Plant Physiol. 2019, 240, 153010. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Guo, L.P.; Chen, B.D.; Hao, Z.P.; Wang, J.Y.; Huang, L.Q.; Yang, G.; Cui, X.M.; Yang, L.; Wu, Z.X.; et al. Arbuscular mycorrhizal symbiosis for sustainable cultivation of Chinese medicinal plants: A promising research direction. Am. J. Chin. Med. 2013, 41, 1199–1221. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.R. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol. Res. 2014, 169, 30–39. [Google Scholar] [CrossRef]
- Mora, M.R.; Gómez, P.A.J.; Reguero, D.G.; Lobo, A.P. Effect of plant Growth-Promoting bacteria on biometrical parameters and antioxidant enzymatic activities of Lupinus albus var. orden dorado under mercury stress. Front. Microbiol. 2022, 13, 891882. [Google Scholar] [CrossRef]
- Corrales-Ramírez MSc, L.C.; Caycedo-Lozano, L.; Gómez-Méndez, M.A.; Ramos-Rojas, S.J.; Rodríguez-Torres, J.N. Bacillus spp: Una alternativa para la promoción vegetal por dos caminos enzimáticos. Nova 2017, 15, 46–65. [Google Scholar] [CrossRef]
- González-Reguero, D.; Robas-Mora, M.; Probanza, A.; Jiménez, P.A. Evaluation of the oxidative stress alleviation in Lupinus albus var. Orden dorado by the inoculation of four plant growth-promoting bacteria and their mixtures in Mercury-polluted soils. Front. Microbiol. 2022, 13, 907557. [Google Scholar]
- Lv, J.; Yang, S.; Zhou, W.; Liu, Z.; Tan, J.; Wei, M. Microbial regulation of plant secondary metabolites: Impact, mechanisms and prospects. Microbiol. Res. 2024, 283, 127688. [Google Scholar] [CrossRef]
- Wang, G.; Ren, Y.; Bai, X.; Su, Y.; Han, J. Contributions of Beneficial Microorganisms in Soil Remediation and Quality Improvement of Medicinal Plants. Plants 2022, 11, 3200. [Google Scholar] [CrossRef]
- Huang, Y.; Hu, H.; Yue, E.; Ying, W.; Niu, T.; Yan, J.; Lu, Q.; Ruan, S. Role of plant metabolites in the formation of bacterial communities in the rhizosphere of Tetrastigma hemsleyanum. Front. Microbiol. 2023, 14, 1292896. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.L. Optimization of Extraction Process and Preliminary Evaluation of Activity of Main Active Components from Acanthopanax senticosus. Ph.D. Thesis, Northeast Forestry University, Harbin, China, 2022. [Google Scholar]
- Çakmakçı, R.; Mosber, G.; Milton, A.H.; Alatürk, F.; Ali, B. The Effect of Auxin and Auxin-Producing Bacteria on the Growth, Essential Oil Yield, and Composition in Medicinal and Aromatic Plants. Curr. Microbiol. 2020, 77, 564–577. [Google Scholar] [CrossRef]
- Kumar, P.; Singh, B.; Thakur, V.; Thakur, A.; Thakur, N.; Pandey, D.; Chand, D. Hyper-production of taxol from Aspergillus fumigatus, an endophytic fungus isolated from Taxus sp. of the Northern Himalayan region. Biotechnol. Rep. 2019, 24, e00395. [Google Scholar] [CrossRef] [PubMed]
- Shweta, S.; Zuehlke, S.; Ramesha, B.T.; Priti, V.; Mohana Kumar, P.; Ravikanth, G.; Spiteller, M.; Vasudeva, R.; Uma Shaanker, R. Endophytic fungal strains of Fusarium solani, from Apodytes dimidiata E. Mey. exArn (Icacinaceae) produce camptothecin, 10-hydroxycamptothecin and 9-methoxycamptothecin. Phytochemistry 2010, 71, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Patani, A.; Patel, M.; Islam, S.; Yadav, V.K.; Prajapati, D.; Patel, A. Recent advances in Bacillus-mediated plant growth enhancement: A paradigm shift in redefining crop resilience. World J. Microbiol. Biotechnol. 2024, 40, 77. [Google Scholar] [CrossRef]
- Li, H.; Qiu, Y.; Yao, T.; Ma, Y.; Zhang, H.; Yang, X. Effects of PGPR microbial inoculants on the growth and soil properties of Avena sativa, Medicago sativa, and Cucumis sativus seedlings. Soil Tillage Res. 2020, 199, 104577. [Google Scholar] [CrossRef]
- Li, N.; Wen, J.; Wu, R.; Hu, D.; Zhang, L.; Zhang, W.; Zhang, M. Dual effects of plant growth-promoting rhizobacteria (PGPR) on the Moso bamboo-soil system: Plant growth promotion and microbial community stability. Ind. Crops Prod. 2023, 203, 117151. [Google Scholar] [CrossRef]
- Deng, J.; Bai, X.; Zhou, Y.; Zhu, W.; Yin, Y. Variations of soil microbial communities accompanied by different vegetation restoration in an open-cut iron mining area. Sci. Total Environ. 2020, 704, 135243. [Google Scholar] [CrossRef]
- Liu, M.L.; Li, X.R.; Zhu, R.Q.; Chen, N.; Ding, L.; Chen, C.Y. Vegetation richness, species identity and soil nutrients drive the shifts in soil bacterial communities during restoration process. Environ. Microbiol. Rep. 2021, 13, 411–424. [Google Scholar] [CrossRef]
- Wei, J.L.; Qin, Y.; Xie, X.Q.; Chen, J.Y.; Dong, D.F.; Xing, Y.X.; Li, Y.R. Effects of plant growth-promoting rhizobacteria on soil nutrients, enzyme activities and functional diversity of bacterial communities. J. South. Agric. 2020, 51, 2348–2357. [Google Scholar]
- Zhang, X.; Zhang, Q.; Yu, M.; Zhang, Y.; He, T.; Qiu, Z.; Qiu, Y.; Wang, W. Integrating serum pharmacochemistry and network pharmacology to explore the molecular mechanisms of Acanthopanax senticosus (Rupr. & Maxim.) Harms on attenuating doxorubicin-induced myocardial injury. J. Ethnopharmacol. 2024, 319 Pt 3, 117349. [Google Scholar] [CrossRef]
- Liu, Z.G. Effects of Phosphate-Solubilizing Bacteria and Controlled Release Fertilizer on Phosphorus Activities of Soil. Ph.D. Thesis, Shandong Agricultural University, Taian, China, 2014. [Google Scholar]
- Huang, L.Z.; Huang, B.K.; Ye, Q.; Qin, L.P. Bioactivity-guided fractionation for anti-fatigue property of Acanthopanax senticosus. J. Ethnopharmacol. 2011, 133, 213–219. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).