Simple Summary
Chagas disease, a vector-borne disease caused by the parasite Trypanosoma cruzi, is a significant threat to human and canine health in the tropics. To control the transmission of T. cruzi, systemic insecticide treatment of dogs with fluralaner has been proposed as an intervention for canine and potentially human Chagas disease. In this study, we evaluated the efficacy of canine treatment regimens with fluralaner to reduce Chagas disease infections (once every three months and once every twelve months) in high and low endemic regions using a data-driven mathematical model. Our study shows that Fluralaner treatment can effectively reduce T. cruzi transmission in humans, but may increase infections in dogs if canine consumption of triatomine increases. The effectiveness of the treatment regimen was shown to vary substantially with the underlying intensity of T. cruzi transmission and the increased rate of canine consumption of dead triatomines. Our study provides new evidence to support further empirical studies on the potential impact of mass treatment of dogs with systemic insecticides as a novel and additional intervention for the control and elimination of Chagas disease in the tropics.
Abstract
Chagas disease, caused by Trypanosoma cruzi and transmitted by triatomines, can lead to severe cardiac issues and mortality in many mammals. Recent studies have shown that systemic insecticide treatment of dogs is highly effective in killing triatomines. Here, we assessed the impact of dog treatment on T. cruzi transmission. We developed a mathematical model of T. cruzi transmission among triatomines, dogs, humans, and rodents. We used the model to evaluate the impact of dog treatment regimens on T. cruzi transmission dynamics to determine their effectiveness in reducing T. cruzi infection among hosts. We show that a 3-month treatment regimen may reduce T. cruzi incidence among humans by 59–80% in a high transmission setting, and 26–82% in a low transmission setting. An annual treatment may reduce incidence among humans by 49–74% in a high transmission setting, and by 11–76% in a low transmission setting. However, dog treatment may substantially increase T. cruzi prevalence among dogs if dog consumption of dead triatomines increases. Our model indicates that dog treatment may reduce T. cruzi infections among humans, but it may increase infections in dogs. Therefore, a holistic approach targeting different hosts is necessary for Chagas elimination.
1. Introduction
Chagas disease is a vector-borne neglected tropical disease that poses a significant health burden in tropical regions [1]. The parasite is primarily transmitted by triatomine insects, commonly known as “kissing bugs”, via contact with their infected fecal material during or after feeding. In addition, transmission can occur when the host ingests the infected bugs or their feces [2,3]. Vertical transmission through transplacental transmission or breast milk, and other routes such as direct contact with infected body fluids, blood transfusion, and organ transplantation, also contribute to parasite transmission [4]. The causative agent of Chagas disease is the parasite Trypanosoma cruzi, and unfortunately, specific treatment options are limited, and viable vaccines are still lacking. Originally endemic in the Americas, spanning from Chile to the United States of America, the disease has now become a global health concern due to human migration, resulting in a significant number of cases in non-endemic regions such as Canada and Europe [5,6]. There are approximately 6 million reported human cases in the Americas alone, but the true disease burden may be even higher due to differences in disease surveillance and reporting practices between countries [1]. For example, recent estimates for Mexico, one of the most affected countries, range from less than 1 million to more than 4 million cases, and there is considerable uncertainty in these figures, possibly influenced by reporting bias [7,8,9]. Chagas disease particularly affects vulnerable communities and can lead to severe cardiac problems. The global annual public health impact of the disease is approximately $627.46 million in healthcare costs and 806,170 disability-adjusted life years (DALYs) lost due to both mortality and morbidity [10]. These statistics underscore the urgent need for effective and comprehensive strategies to control and manage Chagas disease on a global scale.
Triatomines are blood-sucking insects of the family Reduviidae found primarily in the Americas. There are more than 150 species of triatomines distributed across different tribes, with diverse morphological characteristics and unique wing patterns [11,12]. Species such as Rhodnius prolixus, Triatoma infestans, and Triatoma dimidiata are of significant medical importance due to their high prevalence, wide distribution, and efficient transmission of Chagas disease. Understanding the biology, distribution, and vectorial capacity of these triatomine species is critical for effective disease control and prevention strategies [11]. In tropical regions such as Latin America, triatomine species such as T. infestans, T. dimidiata, and R. prolixus are widespread and well-adapted to human dwellings, contributing to Chagas disease transmission [12]. These species exhibit a high degree of domiciliation in and around human habitats [13,14,15]. These triatomine species are commonly found in rural areas with suitable housing conditions and animal reservoirs [14,15].
Efforts to control and eradicate Chagas disease have concentrated on targeting triatomine bugs, the insect vectors [12]. Specifically, T. cruzi transmission to humans occurs via two primary cycles: the domestic and peri-domestic cycles [16]. In the peri-domestic cycle, small wild mammals serve as reservoirs, with peri-domestic bugs introducing the parasite into households and infecting humans and domesticated mammals. The domestic cycle involves the colonization of houses by triatomine bugs, which in certain regions facilitates transmission between humans and domesticated mammals [16]. The epidemiological importance of rodents becomes even more apparent when considering that many species can also enter human dwellings and contribute to the transmission of T. cruzi infections [17]. Given the presence of numerous wild mammalian reservoirs, elimination of the disease in the peri-domestic cycle is very challenging [18,19]. However, within the domestic cycle, dogs serve as more accessible reservoirs than wild animals, providing opportunities for One Health interventions to target T. cruzi transmission and reduce human Chagas disease [20]. Dogs have been identified as a major contributor to T. cruzi transmission [21]. These animals play an increasingly important role in societies, serving as pets, companions, guard dogs, hunting partners, herding assistants, and law enforcement aids [22]. Dogs can contribute to the transmission of T. cruzi in several ways. They may ingest infected triatomine, thereby maintaining the transmission cycle of the parasite as a primary or major reservoir host. In addition, dogs can contribute to the proliferation of triatomine bug populations by serving as an important source of blood meals [23]. Consequently, dogs can establish a link between the peridomestic and domestic transmission cycles, increasing the risk of human T. cruzi infection [24,25,26].
In the tropics, particularly in Argentina, several studies have demonstrated and documented the significant involvement of dogs in areas endemic to T. cruzi [24,27,28,29]. In the northwestern region, dogs exhibited a notably higher infection rate (65.1%) compared to that of humans (34.2%). In addition, dogs were found to be 18 times more infectious to T. infestans than humans [27]. Furthermore, T. infestans, a major vector of T. cruzi in the tropics, consistently shows a preference for dogs over other domestic animals [22]. This strong preference for triatomine vectors for dogs can be exploited via a targeted vector control approach known as xenointoxication. In this strategy, pesticides are applied to peri-domestic and domestic animals, such as dogs, with the goal of suppressing insect infestations. By specifically targeting dogs with topical insecticides or insecticide-impregnated collars, dogs effectively become baited lethal traps [20,30]. In the context of a pyrethroid shortage, the administration of a safe, long-lasting, and effective insecticide such as fluralaner to dogs could potentially serve as a valuable resource to interrupt the transmission of T. cruzi [20]. Interventions targeting the dog population to interrupt T. cruzi transmission in the domestic cycle have been evaluated. Mathematical models suggest that removal of infected dogs from households with infected humans could interrupt disease transmission but culling the dog population is socially unacceptable [31]. Recent trials of oral or topical insecticides, particularly fluralaner, have shown promising efficacy in killing triatomines that feed on dogs [30,32,33,34]. These interventions have the potential to be cost-effective in reducing T. cruzi infection in humans [35]. Annual treatment of dogs with fluralaner may be effective in reducing infection rates in high transmission settings, but caution is needed in low transmission settings [36]. However, there is a potential counterproductive effect as dogs may ingest treated infected triatomines, increasing infection rates in the dog population.
The Ross-MacDonald theory has made significant contributions to the advancement of quantitative theory and basic principles of epidemiology, particularly in the context of vector-borne diseases [37,38]. Mathematical models have been developed to study and better understand the dynamics of T. cruzi transmission with an emphasis on protecting human health [36,39,40,41,42,43]. Mathematical modeling techniques provide valuable insights into the cycle of T. cruzi transmission and the potential impacts of host-targeted interventions. In this study, we modified the traditional Ross-MacDonald model to examine the dynamics of transmission among triatomines, dogs, rodents, and humans in domestic and peri-domestic settings. We used the model to evaluate the effectiveness of treating dogs with different treatment strategies using the systemic insecticide fluralaner to control triatomine populations and reduce T. cruzi infections among hosts. In addition, we considered the potential impact of increased triatomine consumption and the risk of oral transmission when fluralaner is administered regularly to dogs.
2. Materials and Methods
We conducted a simulation study by modifying the traditional Ross-MacDonald model. Our adapted model made several simplifying assumptions. First, we assumed that the population of hosts (humans, dogs, and other competent hosts, i.e., rodents) was homogeneous and remained constant throughout the study. Similarly, we assumed that the vector population (triatomine) was also homogeneous but differed from the classic Ross-MacDonald model by incorporating a logistic birth rate for triatomine [44]. We based the parameters of the triatomine population on data related to T. infestans, the primary vector of T. cruzi transmission in the tropics. In addition, we assumed that both triatomine bugs and hosts (humans, dogs, and other competent hosts) can be susceptible (S, not infected with T. cruzi and able to become infected) or infectious (I, infected with T. cruzi and able to transmit). We denoted by and , the population sizes of humans, dogs, other competent (rodents), and triatomines, respectively. We denoted and as the number of susceptible and infected triatomines and and are the transmission rate of T. cruzi from humans, dogs, and other competent hosts to triatomine. The number of triatomine births is determined by the birth rate R, carrying capacity and the total number of triatomines in each setting using the following formula:
The model flow diagram is provided in Figure 1.
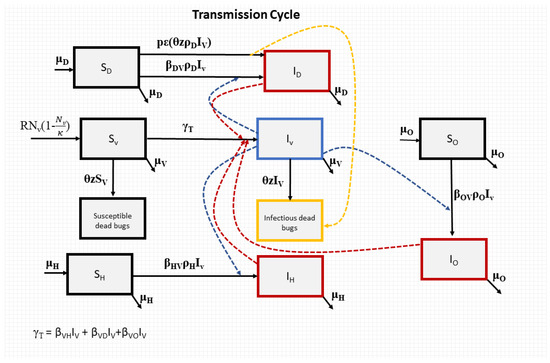
Figure 1.
Flowchart for our model of T. cruzi transmission. The blue, red, and yellow dotted lines represent the transmission processes of T. cruzi.
2.1. Human, Dogs, and Other Transmitters (Rodents)
We denoted and as the transmission rate of T. cruzi from triatomine to humans, dogs, and other competent hosts, respectively. The transition of hosts (humans, dogs, and rodents) from a susceptible state to an infectious state occurs at a rate determined by the force of infection (FOI) resulting from vector-borne transmission. This FOI is calculated as the product of various factors, including the transmission rate of T. cruzi from triatomine to hosts, the ratio of triatomine associated with humans, dogs, and other competent hosts as , and , respectively, and the proportion of infected triatomines present in the system. We further denote by infected hosts by IH, ID, and IO the proportion of humans, dogs, and other competent hosts (rodents) infected with T. cruzi and able to transmit. Prior to dog treatment, disease transmission dynamics between the hosts (humans, dogs, and other competent hosts (rodents)) and the triatomine are described and presented by the following system of nonlinear ordinary differential equations: , the change in the proportion of infected humans, , the change in the proportion of infected dogs, , the change in the proportion of infected other competent hosts (rodents), , the change in the proportion of susceptible triatomines and , the change in the proportion of infectious triatomines:
where .
, and are the ratio of triatomine to humans, dogs, and other competent hosts, and are estimated as 9.3:1; 31.6:1, and 13.7:1, respectively [2,45]. The model’s parameters and corresponding values are provided in Table 1.

Table 1.
Parameter table.
In the absence of T. cruzi infection, triatomine carrying capacity is derived as follows:
At equilibrium, = 0, thus
For our high and low prevalence settings, the endemic prevalence was estimated to be 0.54 and 0.26 for triatomine, 0.48 and 0.23 for dogs, 0.22 and 0.08 for other hosts, and 0.14 and 0.05 for humans, respectively. These estimates are consistent with empirical estimates from the tropics [54,55,56,57,58]. To initialize our simulations at endemic prevalence in each transmission setting, our model was run long enough to reach equilibrium (Figure 2).
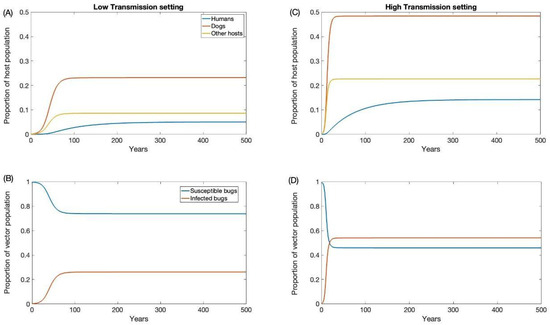
Figure 2.
Endemic equilibrium for low (A,B) and high (C,D) transmission settings.
2.2. Dog Treatment
Fluralaner, an oral systemic insecticide, is used in dogs to prevent tick and flea infestations. Fluralaner-treated dogs have been shown to effectively kill triatomine feeding on them [34,59]. The triatomine mortality rate induced from dog treatment was defined as where is the triatomine contact rate with dogs, with , where is the probability of triatomine infection per feed on T. cruzi infected dogs and is the percentage of bugs that will die after feeding on treated dogs at that given time point. Temporal changes in were informed using empirical data from systematic laboratory studies [34,59].
As dog treatment results in a substantial increase in dead triatomines, this may change the force of infection in dogs due to the potential ingestion of dead infected bugs. We considered this oral force of infection in our model via the additional transmission factor where is the proportion of dead triatomines consumed by dogs and is the probability of dog infection via triatomine ingestion. The system of equations becomes the following:
Using our model, we assessed the effectiveness of both regimens in reducing the prevalence of T. cruzi infection in dogs and triatomines, as well as the density of triatomines in two transmission settings: high and low. The effective outcomes we considered include the reduction in T. cruzi prevalence in various hosts (dogs, humans, and other competent hosts like rodents), the decrease in triatomine density, and the reduction in T. cruzi incidence in humans.
2.3. Treatment Strategies
In this study, we examined two distinct fluralaner treatment approaches: a 3-month regimen, where dogs received treatment once every three months and a 12-month regimen. The efficacy of each treatment and the induced triatomine mortality rate were determined based on empirical data [34]. Our models were utilized to assess the effectiveness of both regimens in reducing T. cruzi infection prevalence among dogs, humans, other hosts (rodents), and triatomine, and reduction in human infection incidence, in two transmission settings (high and low). To evaluate the potential impact of increased oral transmission on the effectiveness of the dogs’ treatment, we considered varying levels of dead triatomine consumption by dogs (low, medium, and high). These dog consumption levels were defined as Low, ; Medium, ; and High, , where is the proportion of dead triatomines consumed by dogs.
All analyses for the figures were performed in MATLAB 2022b. In our model, treatment was initiated once the population of dogs, humans, other competent hosts, and the vector population reached equilibrium.
3. Results
In our study, we investigated two distinct dog treatment regimens targeting the domestic vector of Chagas disease. The domestic vector refers to the triatomine insects that have adapted to living in and around human dwellings and play a significant role in transmitting the T. cruzi parasite responsible for Chagas disease. The two treatment regimens involved administering canine fluralaner treatment every three months and annually for a period of 20 years. The frequent treatment approaches aimed to evaluate the long-term effects of a less frequent intervention strategy on the prevalence of T. cruzi infection in the dog population. Additionally, we explored its potential implications for Chagas disease transmission to humans and other competent hosts.
First, we examined the impact of a 3-month treatment regimen in both low- and high-transmission settings. These scenarios were evaluated for different levels (low, medium, and high) of dead triatomine consumption by dogs. As the percentage of dead triatomines eaten by dogs increases, the effectiveness of dog treatment for reducing T. cruzi infection decreases. The impact on reducing T. cruzi prevalence in the human population is marginal, primarily because humans have a longer lifespan, and our study considered a relatively short period of only 20 years (Figure 3 and Figure 4). In the low-transmission setting, the prevalence of T. cruzi decreased from 0.05 to 0.04, 0.046, and 0.047 in humans for low, medium, and high oral consumption of triatomines over 20 years, respectively (Figure 3), whereas in the high-transmission setting, the prevalence of T. cruzi decreased from 0.14 to 0.11, 0.12, and 0.12 in humans with low, medium, and high oral consumption of triatomines, respectively (Figure 4). Similarly, for other host populations, the prevalence of T. cruzi in the low-transmission setting decreased from 0.08 to 0.005, 0.059, and 0.065 for a low medium, and high oral consumption of triatomines, respectively (Figure 3). On the other hand, in a high transmission setting, T. cruzi prevalence in the other host (rodents) population decreased from 0.22 to 0.03, 0.09, and 0.1 with low, medium, and high oral consumption of triatomines, respectively (Figure 4). T. cruzi prevalence in both the dog and other host population shows a consistent decline for low consumption of dead triatomines in both low and high transmission settings (Figure 3 and Figure 4). In the low transmission setting, when the percentage of dead triatomines consumed is lower (1%), the level of T. cruzi prevalence decreases from 0.23 to 0.05, while in the high transmission setting, it decreases from 0.48 to 0.19 among the dog population.
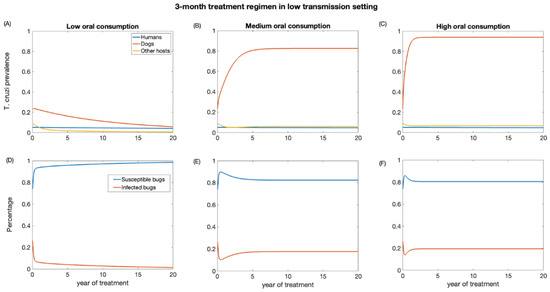
Figure 3.
Impact of systemic insecticide treatment of dogs with fluralaner for the control of Chagas disease in low transmission setting with a 3-month regimen under different levels of dog consumption of dead triatomines. (A,D) Low oral consumption, (B,E) medium oral consumption, and (C,F) high oral consumption.
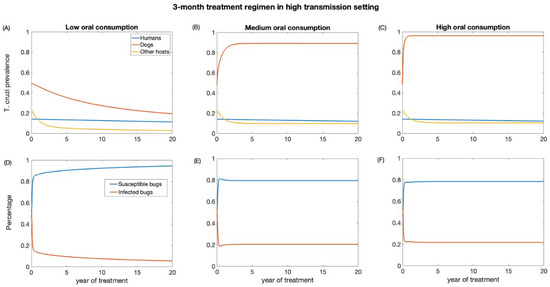
Figure 4.
Impact of systemic insecticide treatment of dogs with fluralaner for the control of Chagas disease in high transmission setting with a 3-month regimen under different levels of dog consumption of dead triatomines. (A,D) Low oral consumption, (B,E) medium oral consumption, and (C,F) high oral consumption.
However, when the consumption of dead triatomines is medium and higher (20% and 60%), the level of T. cruzi prevalence among dogs increases in both transmission settings (Figure 3 and Figure 4). With a medium oral consumption of dead triatomines by dogs, T. cruzi prevalence among dogs rises from 0.23 to 0.82 in a low-transmission setting and from 0.48 to 0.89 in a high-transmission setting. Similarly, for higher oral consumption of dead triatomines, T. cruzi prevalence increases from 0.23 to 0.93 in the low-transmission setting and from 0.48 to 0.96 in the high transmission setting.
Our analysis shows that canine fluralaner treatment administered every 3 months had an immediate impact in reducing the percentage of infected triatomine population across both transmission settings (Figure 3 and Figure 4). This impact was observed even under various levels of oral consumption of dead triatomines by dogs. In the low transmission setting, when dogs had a low level of oral consumption of triatomines, we observed a continuous decline in the percentage of infected triatomine population over the 20-year period, decreasing from 25% to 1%. Similarly, in the high transmission setting, with a low level of oral consumption of triatomines, the percentage of the triatomine population also exhibited a decreasing trend, declining from 53% to 5% over the 20-year period. On the other hand, under the medium and higher consumption of dead triatomines by dogs in both settings, we see a steep decline in the percentage of the infected triatomine population immediately after treatment, which then gradually increased and eventually stabilized. With a medium oral consumption of dead triatomines by dogs, the infected triatomine population decreases from 25% to 17% in a low-transmission setting and from 53% to 20% in a high transmission setting. Similarly, for higher oral consumption of dead triatomines, T. cruzi prevalence decreases from 25% to 21.6% in the low-transmission setting and from 53% to 19.5% in the high transmission setting.
We also investigate the impact of annual treatment (12-month regimen) in the two transmission settings over a period of 20 years. Similar to the 3-month regimen, there was a prompt decline in T. cruzi prevalence in both dogs and other host population following the initiation of annual treatment when dogs’ consumption of killed triatomine is very low (Figure 5 and Figure 6). The observed annual transient rebound in T. cruzi prevalence was due to the fact that the efficacy of fluralaner for killing triatomines progressively declines at seven months following dog treatment [34,59].
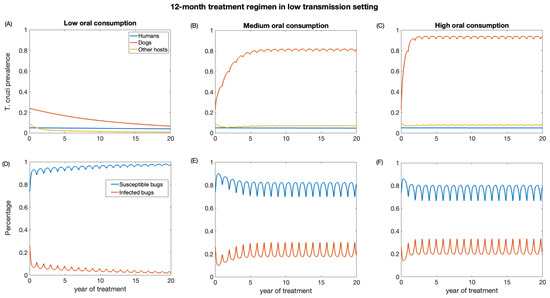
Figure 5.
Impact of systemic insecticide treatment of dogs with fluralaner for the control of Chagas disease in low transmission setting with a 12-month regimen under different levels of dog consumption of dead triatomines. (A,D) Low oral consumption, (B,E) medium oral consumption, and (C,F) high oral consumption.
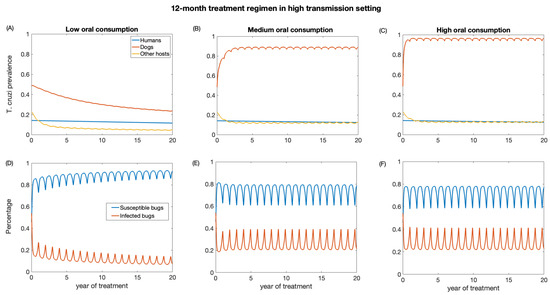
Figure 6.
Impact of systemic insecticide treatment of dogs with fluralaner for the control of Chagas disease in high transmission setting with a 12-month regimen under different levels of dog consumption of dead triatomines. (A,D) Low oral consumption, (B,E) medium oral consumption, and (C,F) high oral consumption.
The reduction in T. cruzi prevalence in humans under annual treatment was similar to that of the 3-month regimen. In the low-transmission setting, the prevalence of T. cruzi in humans was reduced from 0.05 to 0.04, 0.046, 0.047 for low, medium, and high oral consumption of triatomines, respectively (Figure 5), and in a high-transmission setting, the prevalence of T. cruzi in humans was reduced from 0.14 to 0.11, 0.12, 0.13 for low, medium, and high oral consumption of triatomines, respectively (Figure 6). For other host populations, the prevalence of T. cruzi in the low-transmission setting decreased from 0.08 to 0.008 for low, medium, and high oral consumption with the highest reduction observed immediately after treatment initiation. T. cruzi prevalence among dogs decreases from 0.23 to 0.06 in the low transmission setting, while in the high transmission setting, it decreases from 0.48 to 0.24.
With medium and higher consumption of dead triatomines by dogs, the T. cruzi prevalence among dogs increases in both transmission settings (Figure 5 and Figure 6). With a medium oral consumption of dead triatomines by dogs (20%), T. cruzi prevalence among dogs rises from 0.23 to 0.81 in a low-transmission setting and from 0.48 to 0.89 in a high transmission setting. For a higher oral consumption of dead triatomines (60%), T. cruzi prevalence among dogs increases from 0.23 to 0.93 in the low-transmission setting and from 0.48 to 0.96 in the high transmission setting.
Canine fluralaner treatment administered annually was shown to substantially reduce T. cruzi prevalence among triatomines in low and high transmission settings when the oral consumption is low. In the low transmission setting, for low oral consumption of triatomines, the percentage of infected triatomine population decreases from 25% to 2%. Similarly, in the high transmission setting, with a low level of oral consumption of triatomines, the percentage of the triatomine population declines from 53% to 6%. With a medium oral consumption of dead triatomines by dogs, the percentage of infected triatomines in the low transmission setting initially decreases from 25% to 17% and subsequently increases to 33% after 3 years of treatment, whereas in a high transmission setting it reduces from 53% to 20% immediately after the first round of treatment and then rises to 39%. With higher oral consumption of dead triatomines, the percentage of infected triatomines decreases in the low transmission setting from 25% to 19% immediately after treatment initiation and then rises to 33%, whereas in the high transmission setting the percentage of infected triatomines initially decreases from 53% to 22% and subsequently increases to 41%.
In all transmission settings, we observed a prompt decline in vector population density following the initiation of treatment for both regimens (Figure 7). Under the 3-month treatment regimen, the triatomines population was reduced and maintained to 67% of its pre-intervention level in high transmission settings and 79% in low transmission settings (Figure 7A). Under a 12-month regimen, the triatomine population was shown to be initially reduced to 67% of its pre-intervention level in high transmission setting and 79% in low transmission setting after each round of treatment (Figure 7B). However, the triatomine population was shown to recover promptly as fluralaner efficacy wanes (Figure 7B).
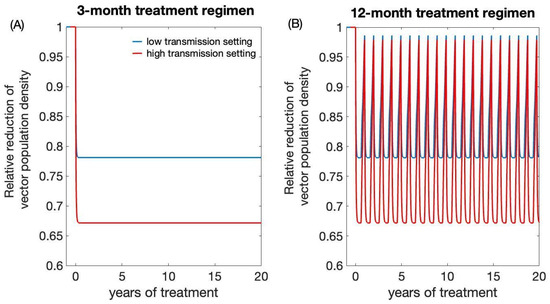
Figure 7.
Impact of systemic insecticide treatment of dogs with fluralaner for reducing triatomines population density. (A) 3-month treatment regimen, (B) 12-month treatment regimen.
Finally, we evaluated the impact of the dogs’ treatment regimens on T. cruzi infection incidence among humans (Table 2). We showed that over the first 10 years of treatment, annual dogs’ treatment may reduce T. cruzi infection incidence among humans by 74% and 77% in high and low transmission settings, respectively, whereas a three-month treatment regimen may reduce incidence by more than 80% in both transmission settings. However, the effectiveness of the dog treatment regimen for reducing disease incidence among humans varies significantly with the level of additional dog consumption of dead bugs following treatment initiation (Table 2). For example, if dogs eat 20% of dead triatomines (medium consumption), annual dog treatment will reduce T. cruzi infection incidence by 52% over the first 10 years in high transmission settings, and by 25% in low transmission settings. A three-month treatment regimen will reduce human infection incidence by 62% in high transmission settings, and by 38% in low transmission settings.

Table 2.
Percentage reduction in T. cruzi infection incidence among humans during the first 10 years of treatment. In the absence of dog treatment, the baseline annual endemic incidence was 6.7 cases/10,000 persons per year in low transmission setting and 18.9 cases/10,000 persons per year in high transmission setting.
4. Discussion
The study evaluated the impact of fluralaner treatment for the control of Chagas disease in different T. cruzi transmission settings in the tropics. The effectiveness of treatment was shown to vary significantly with the level of oral consumption of dead triatomine by dogs after treatment initiation. At low oral consumption levels (e.g., 1%), our model showed that dog treatment (once every 3 or 12 months) with fluralaner could substantially reduce T. cruzi infection in most hosts including humans and other hosts (rodents). At moderate or high oral consumption of dead triatomines by dogs (e.g., 5% or more), dog treatment may exacerbate T. cruzi infection in dogs, and moderately reduce infection in triatomines and other hosts (rodents). In humans, Chagas disease incidence could be reduced by 50–60% in high transmission settings, and by less than 40% in low transmission settings, with effectiveness decreasing with increasing oral consumption rates.
The results of our study have important implications for public health strategies aimed at controlling T. cruzi transmission. First, canine fluralaner treatment can effectively reduce T. cruzi infection in dogs if canine treatment does not result in a substantial increase in dog consumption of dead triatomines. The effect of systemic insecticide treatment on canine consumption of triatomine remains unknown. To more accurately estimate the effectiveness of systemic insecticide use on T. cruzi infection in dogs and humans, empirical field studies are needed to better understand canine triatomine feeding behavior and the impact of systemic insecticide use on this feeding behavior. Although canine treatment can be an effective tool for Chagas disease control, it is important to recognize that reliance on canine treatment alone may not be sufficient to achieve elimination of T. cruzi transmission. Additional measures such as insecticide spraying, vaccines, health education, and screening of at-risk human populations are essential components of an integrated approach to Chagas disease control.
As with all mathematical models, our model has some limitations. These limitations are likely to affect our estimates of the impact of dog treatment on reducing T. cruzi infection among humans and other hosts. This is due to several simplifying assumptions made. First, our model does not explicitly account for triatomine migration, which may help to replenish the triatomine population in the area of interest and provide an external source of infection. Second, our model does not explicitly account for the fact that some triatomines may not feed on dogs or may not feed continuously on all hosts. The T. infestans are opportunistic feeders with nocturnal feeding behavior, so their feeding patterns may be highly heterogeneous among hosts and over time. This may reduce the effectiveness of dog treatment for preventing T. cruzi infection among other hosts and humans. Third, our model does not account for triatomine reproductive senescence and seasonality of their feeding patterns, which may also influence the effectiveness of dog treatment for reducing T. cruzi transmission dynamics [60]. Fourth, our model does not account for the impact of possible natural or treatment-induced recovery among infected hosts. The presence of recovered hosts will hinder disease transmission and subsequently reduce the effectiveness of systemic insecticide treatment of dogs for control of Chagas disease. However, low recovery rates are anticipated to have minimal impact on the effectiveness of treatment regimens. In addition to ignoring the potential impact of host recovery, our model did not investigate the impact of dogs’ lifespan on disease transmission. Future studies should investigate the impact of these factors on the effectiveness of systemic insecticide treatment of dogs for control of Chagas disease.
Our model made additional assumptions. We grouped hosts into a single infected class, combining the acute and chronic phases of infection, which have different infectivity. We also ignore disease-induced mortality by assuming that hosts could die via natural death. Although such assumptions are expected to have minimal impact on our results, they remain a simplifying assumption. Finally, we assumed that oral transmission occurred only after the bugs were killed by treatment (fluralaner administration). Thus, we considered that there was no significant oral transmission of T. cruzi in the dog population prior to the administration of the fluralaner.
5. Conclusions
Our study provides valuable insights into the impact of canine fluralaner treatment on T. cruzi infection incidence in different host populations and transmission settings. While canine treatment was shown to be effective in directly reducing infections in the dog population, a potential indirect consequence of treatment via increased oral consumption of dead bugs by dogs may result in increased T. cruzi infection in dogs. The direct impact of dog treatment on human and other hosts (rodents) infection rates has been shown to vary significantly with the underlying transmission setting and the oral infection rate of dogs. Integrated control strategies that include multiple interventions, including vector control and community engagement, are essential to achieve meaningful reductions in T. cruzi transmission and improve public health outcomes. Further research and collaboration between researchers, health authorities, and local communities are essential to develop and implement comprehensive approaches to effectively control Chagas disease in the tropics.
Author Contributions
Conceptualization; M.L.N.-M. methodology, M.L.N.-M. and E.F.; software, M.L.N.-M. and A.D.; formal analysis, E.F. and A.D.; data curation, E.F.; writing—original draft preparation, E.F. and A.D.; writing—review and editing, M.L.N.-M.; visualization, A.D.; supervision, M.L.N.-M.; funding acquisition, M.L.N.-M. All authors have read and agreed to the published version of the manuscript.
Funding
The authors gratefully acknowledge a faculty startup funding from Texas A&M School of Veterinary Medicine and Biomedical Sciences and funding from the Texas A&M AgriLife Research for M.L.N.M. The views, opinions, assumptions, or any other information set out in this article are solely those of the authors.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tibayrenc, M.; Telleria, J. American Trypanosomiasis: Chagas Disease One Hundred Years of Research; Elsevier: Amsterdam, The Netherlands, 2010; Available online: https://books.google.com/books/about/American_Trypanosomiasis_Chagas_Disease.html?hl=&id=WQKeDAEACAAJ (accessed on 1 July 2023).
- Kribs-Zaleta, C. Estimating contact process saturation in sylvatic transmission of Trypanosoma cruzi in the United States. PLoS Negl. Trop. Dis. 2010, 4, e656. [Google Scholar] [CrossRef] [PubMed]
- Klotz, J.H.; Dorn, P.L.; Logan, J.L.; Stevens, L.; Pinnas, J.L.; Schmidt, J.O.; Klotz, S.A. “Kissing bugs”: Potential disease vectors and cause of anaphylaxis. Clin. Infect. Dis. 2010, 50, 1629–1634. [Google Scholar] [CrossRef] [PubMed]
- Jansen, A.M.; Xavier, S.C.d.C.; Roque, A.L.R. Trypanosoma cruzi transmission in the wild and its most important reservoir hosts in Brazil. Parasit. Vectors 2018, 11, 502. [Google Scholar] [CrossRef]
- Rassi, A., Jr.; Rassi, A.; Marin-Neto, J.A. Chagas disease. Lancet 2010, 375, 1388–1402. [Google Scholar] [CrossRef]
- Schmunis, G.A.; Yadon, Z.E. Chagas disease: A Latin American health problem becoming a world health problem. Acta Trop. 2010, 115, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Arnal, A.; Waleckx, E.; Rico-Chávez, O.; Herrera, C.; Dumonteil, E. Estimating the current burden of Chagas disease in Mexico: A systematic review and meta-analysis of epidemiological surveys from 2006 to 2017. PLoS Negl. Trop. Dis. 2019, 13, e0006859. [Google Scholar] [CrossRef]
- Gómez-Ochoa, S.A.; Rojas, L.Z.; Echeverría, L.E.; Muka, T.; Franco, O.H. Global, Regional, and National Trends of Chagas Disease from 1990 to 2019: Comprehensive Analysis of the Global Burden of Disease Study. Glob Heart 2022, 17, 59. [Google Scholar] [CrossRef] [PubMed]
- Hotez, P.J. Blue Marble Health: An Innovative Plan to Fight Diseases of the Poor Amid Wealth; JHU Press: Baltimore, MD, USA, 2016; Available online: https://books.google.com/books/about/Blue_Marble_Health.html?hl=&id=thXUDAAAQBAJ (accessed on 1 July 2023).
- Lee, B.Y.; Bacon, K.M.; Bottazzi, M.E.; Hotez, P.J. Global economic burden of Chagas disease: A computational simulation model. Lancet Infect. Dis. 2013, 13, 342–348. [Google Scholar] [CrossRef]
- Gil-Santana, H.R.; Martins, D.d.S.; da Silva, J.B.; de Oliveira, J. First report of Microtriatoma borbai Lent & Wygodzinsky, 1979 (Hemiptera, Reduviidae, Triatominae) in the state of Espírito Santo, Brazil: Would M. borbai be living in eucalyptus crops? Rev. Soc. Bras. Med. Trop. 2021, 54, e0147-2021. [Google Scholar] [CrossRef]
- Chagas Disease (American Trypanosomiasis)—Fact Sheet (Revised in August 2012). Wkly. Epidemiol. Rec. 2012, 87, 519–522. Available online: https://www.ncbi.nlm.nih.gov/pubmed/23311009 (accessed on 1 July 2023).
- Gürtler, R.E.; Kitron, U.; Cecere, M.C.; Segura, E.L.; Cohen, J.E. Sustainable vector control and management of Chagas disease in the Gran Chaco, Argentina. Proc. Natl. Acad. Sci. USA 2007, 104, 16194–16199. [Google Scholar] [CrossRef] [PubMed]
- Chagas-Disease-(American-Trypanosomiasis)-Epidemiology-and-Transmission. Available online: https://www.who.int/news-room/fact-sheets/detail (accessed on 3 July 2023).
- Curtis-Robles, R.; Meyers, A.C.; Auckland, L.D.; Zecca, I.B.; Skiles, R.; Hamer, S.A. Parasitic interactions among Trypanosoma cruzi, triatomine vectors, domestic animals, and wildlife in Big Bend National Park along the Texas-Mexico border. Acta Trop. 2018, 188, 225–233. [Google Scholar] [CrossRef] [PubMed]
- WHO Expert Committee on the Control of Chagas Disease, World Health Organization. Control of Chagas Disease: Second Report of the WHO Expert Committee; World Health Organization: Geneva, Switzerland, 2002; Available online: https://books.google.com/books/about/Control_of_Chagas_Disease.html?hl=&id=TaYsDwAAQBAJ (accessed on 3 July 2023).
- Roque, A.L.R.; Xavier, S.C.C.; da Rocha, M.G.; Duarte, A.C.M.; D’Andrea, P.S.; Jansen, A.M. Trypanosoma cruzi transmission cycle among wild and domestic mammals in three areas of orally transmitted Chagas disease outbreaks. Am. J. Trop. Med. Hyg. 2008, 79, 742–749. Available online: https://www.ncbi.nlm.nih.gov/pubmed/18981516 (accessed on 3 July 2023). [CrossRef] [PubMed]
- Finkelman, J. Innovative community-based ecosystem management for dengue and Chagas disease prevention in low and middle income countries in Latin America and the Caribbean. Trans. R. Soc. Trop. Med. Hyg. 2015, 109, 89–90. [Google Scholar] [CrossRef]
- Carcavallo, R.U. Atlas Dos Vetores Da Doença de Chagas Nas Américas. 1998. Available online: https://books.google.com/books/about/Atlas_Dos_Vetores_Da_Doen%C3%A7a_de_Chagas_N.html?hl=&id=ohRgAAAAMAAJ (accessed on 3 July 2023).
- Travi, B.L. Considering Dogs as Complementary Targets of Chagas Disease Control. Vector Borne Zoonotic Dis. 2019, 19, 90–94. [Google Scholar] [CrossRef]
- Cecere, M.C.; Gürtler, R.E.; Canale, D.; Chuit, R.; Cohen, J.E. The role of the peridomiciliary area in the elimination of Triatoma infestans from rural Argentine communities. Rev. Panam. Salud Publica 1997, 1, 273–279. [Google Scholar] [CrossRef]
- Macpherson, C.N.L.; Meslin, F.-X.; Wandeler, A.I. Dogs, Zoonoses and Public Health; CABI: Wallingford, UK, 2013; Available online: https://books.google.com/books/about/Dogs_Zoonoses_and_Public_Health.html?hl=&id=6F0n57jWPioC (accessed on 3 July 2023).
- Guarneri, A.; Lorenzo, M. Triatominae—The Biology of Chagas Disease Vectors; Springer Nature: Berlin/Heidelberg, Germany, 2021; Available online: https://play.google.com/store/books/details?id=Zz83EAAAQBAJ (accessed on 3 July 2023).
- Gürtler, R.E.; Cardinal, M.V. Reservoir host competence and the role of domestic and commensal hosts in the transmission of Trypanosoma cruzi. Acta Trop. 2015, 151, 32–50. [Google Scholar] [CrossRef]
- Ordóñez-Krasnowski, P.C.; Lanati, L.A.; Gaspe, M.S.; Cardinal, M.V.; Ceballos, L.A.; Gürtler, R.E. Domestic host availability modifies human-triatomine contact and host shifts of the Chagas disease vector Triatoma infestans in the humid Argentine Chaco. Med. Vet. Entomol. 2020, 34, 459–469. [Google Scholar] [CrossRef]
- Gürtler, R.E.; Cecere, M.C.; Vázquez-Prokopec, G.M.; Ceballos, L.A.; Gurevitz, J.M.; Fernández, M.d.P.; Kitron, U.; Cohen, J.E. Domestic animal hosts strongly influence human-feeding rates of the Chagas disease vector Triatoma infestans in Argentina. PLoS Negl. Trop. Dis. 2014, 8, e2894. [Google Scholar] [CrossRef]
- Gurtler, R.E.; Canale, D.; Lauricella, M.A.; Cecere, M.C.; Castanera, M.B.; Segura, E.L.; Chuit, R.; Cohen, J.E. Probability of infection with Trypanosoma cruzi of the vector Triatoma infestans fed on infected humans and dogs in northwest Argentina. Am. J. Trop. Med. Hyg. 1996, 55, 24–31. Available online: https://www.ncbi.nlm.nih.gov/pubmed/8702018 (accessed on 3 July 2023). [CrossRef]
- Gürtler, R.E.; Cecere, M.C.; Lauricella, M.A.; Cardinal, M.V.; Kitron, U.; Cohen, J.E. Domestic dogs and cats as sources of Trypanosoma cruzi infection in rural northwestern Argentina. Parasitology 2007, 134, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Lauricella, M.A.; Castañera, M.B.; Gürtler, R.E.; Segura, E.L. Immunodiagnosis of Trypanosoma cruzi (Chagas’ disease) infection in naturally infected dogs. Mem. Inst. Oswaldo Cruz. 1998, 93, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Laiño, M.A.; Cardinal, M.V.; Enriquez, G.F.; Alvedro, A.; Gaspe, M.S.; Gürtler, R.E. An oral dose of Fluralaner administered to dogs kills pyrethroid-resistant and susceptible Chagas disease vectors for at least four months. Vet. Parasitol. 2019, 268, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.E.; Gürtler, R.E. Modeling household transmission of American trypanosomiasis. Science 2001, 293, 694–698. [Google Scholar] [CrossRef] [PubMed]
- Loza, A.; Talaga, A.; Herbas, G.; Canaviri, R.J.; Cahuasiri, T.; Luck, L.; Guibarra, A.; Goncalves, R.; Pereira, J.A.; Gomez, S.A.; et al. Systemic insecticide treatment of the canine reservoir of Trypanosoma cruzi induces high levels of lethality in Triatoma infestans, a principal vector of Chagas disease. Parasit. Vectors 2017, 10, 344. [Google Scholar] [CrossRef]
- Reithinger, R.; Ceballos, L.; Stariolo, R.; Davies, C.R.; Gürtler, R.E. Chagas disease control: Deltamethrin-treated collars reduce Triatoma infestans feeding success on dogs. Trans. R. Soc. Trop. Med. Hyg. 2005, 99, 502–508. [Google Scholar] [CrossRef]
- Queiroga, T.B.D.; Gomez, L.C.P.; de Sena, E.R.; dos Santos, W.V.; Ferreira, H.R.P.; de Araújo-Neto, V.T.; Barbosa-Silva, A.N.; Brito, C.R.D.N.; Lima, R.K.d.R.; Fagundes-Neto, J.C.; et al. Insecticidal efficacy of fluralaner (Bravecto) against Triatoma brasiliensis, a major vector of Trypanosoma cruzi in Brazil. Parasit. Vectors. 2021, 14, 456. [Google Scholar] [CrossRef]
- Gürtler, R.E.; Lauricella, M.A.; Cecere, M.C.; Cohen, J.E.; Segura, E.L.; Chuit, R.; Petersen, R.M. Incidence of trypanosoma cruzi infection among children following domestic reinfestation after insecticide spraying in rural northwestern Argentina. Am. J. Trop. Med. Hyg. 2005, 73, 95–103. Available online: https://www.ncbi.nlm.nih.gov/pubmed/16014842 (accessed on 5 July 2023). [CrossRef]
- Rokhsar, J.L.; Raynor, B.; Sheen, J.; Goldstein, N.D.; Levy, M.Z.; Castillo-Neyra, R. Modeling the impact of xenointoxication in dogs to halt Trypanosoma cruzi transmission. PLoS Comput. Biol. 2023, 19, e1011115. [Google Scholar] [CrossRef]
- Smith, D.L.; Battle, K.E.; Hay, S.I.; Barker, C.M.; Scott, T.W.; McKenzie, F.E. Ross, macdonald, and a theory for the dynamics and control of mosquito-transmitted pathogens. PLoS Pathog. 2012, 8, e1002588. [Google Scholar] [CrossRef]
- Reiner, R.C., Jr.; Perkins, T.A.; Barker, C.M.; Niu, T.; Chaves, L.F.; Ellis, A.M.; George, D.B.; Le Menach, A.; Pulliam, J.R.C.; Bisanzio, D.; et al. A systematic review of mathematical models of mosquito-borne pathogen transmission: 1970–2010. J. R. Soc. Interface 2013, 10, 20120921. [Google Scholar] [CrossRef] [PubMed]
- Nouvellet, P.; Cucunubá, Z.M.; Gourbière, S. Ecology, evolution and control of Chagas disease: A century of neglected modelling and a promising future. Adv. Parasitol. 2015, 87, 135–191. [Google Scholar] [CrossRef] [PubMed]
- Fabrizio, M.C.; Schweigmann, N.J.; Bartoloni, N.J. Modelling inter-human transmission dynamics of Chagas disease: Analysis and application. Parasitology 2014, 141, 837–848. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Steindorf, V.; Maidana, N.A. Modeling the Spatial Spread of Chagas Disease. Bull. Math. Biol. 2019, 81, 1687–1730. [Google Scholar] [CrossRef]
- Cucunubá, Z.M.; Nouvellet, P.; Peterson, J.K.; Bartsch, S.M.; Lee, B.Y.; Dobson, A.P.; Basáñez, M.-G. Complementary Paths to Chagas Disease Elimination: The Impact of Combining Vector Control with Etiological Treatment. Clin. Infect. Dis. 2018, 66, S293–S300. [Google Scholar] [CrossRef]
- Bartsch, S.M.; Peterson, J.K.; Hertenstein, D.L.; Skrip, L.; Ndeffo-Mbah, M.; Galvani, A.P.; Dobson, A.P.; Lee, B.Y. Comparison and validation of two computational models of Chagas disease: A thirty year perspective from Venezuela. Epidemics 2017, 18, 81–91. [Google Scholar] [CrossRef]
- Anderson, R.M.; May, R.M. Population Biology of Infectious Diseases: Report of the Dahlem Workshop on Population Biology of Infectious Disease Agents, Berlin 1982, March 14–19; Springer: Berlin/Heidelberg, Germany, 1982; Available online: https://books.google.com/books/about/Population_Biology_of_Infectious_Disease.html?hl=&id=MZ5rAAAAMAAJ (accessed on 11 July 2023).
- Ortega-Pacheco, A.; Rodriguez-Buenfil, J.C.; Bolio-Gonzalez, M.E.; Sauri-Arceo, C.H.; Jiménez-Coello, M.; Forsberg, C.L. A Survey of Dog Populations in Urban and Rural Areas of Yucatan, Mexico. Anthrozoös 2007, 20, 261–274. [Google Scholar] [CrossRef]
- Catala, S.S.; Gorla, D.E.; Basombrio, M.A. Vectorial transmission of Trypanosoma cruzi: An experimental field study with susceptible and immunized hosts. Am. J. Trop. Med. Hyg. 1992, 47, 20–26. [Google Scholar] [CrossRef]
- Levy, M.Z.; Tustin, A.; Castillo-Neyra, R.; Mabud, T.S.; Levy, K.; Barbu, C.M.; Quispe-Machaca, V.R.; Ancca-Juarez, J.; Borrini-Mayori, K.; Naquira-Velarde, C.; et al. Bottlenecks in domestic animal populations can facilitate the emergence of Trypanosoma cruzi, the aetiological agent of Chagas disease. Proc. Biol. Sci. 2015, 282, 20142807. [Google Scholar] [CrossRef]
- Lee, B.Y.; Bartsch, S.M.; Skrip, L.; Hertenstein, D.L.; Avelis, C.M.; Ndeffo-Mbah, M.; Tilchin, C.; Dumonteil, E.O.; Galvani, A. Are the London Declaration’s 2020 goals sufficient to control Chagas disease? Modeling scenarios for the Yucatan Peninsula. PLoS Negl. Trop. Dis. 2018, 12, e0006337. [Google Scholar] [CrossRef]
- Rabinovich, J.E.; Leal, J.A.; de Piñero, D.F. Domiciliary biting frequency and blood ingestion of the Chagas’s disease vector Rhodnius prolixus Ståhl (Hemiptera: Reduviidae), in Venezuela. Trans. R. Soc. Trop. Med. Hyg. 1979, 73, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Cordovez, J.M.; Rendon, L.M.; Gonzalez, C.; Guhl, F. Using the basic reproduction number to assess the effects of climate change in the risk of Chagas disease transmission in Colombia. Acta Trop. 2013, 129, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Romero-Lopez, J.A.; Jaramillio-Arango, C.J.; Martinez-Maya, J.J.; Alvarez Peralta, E.; Terrones, C.R. Study of the population structure of dogs in a political district in Mexico City. J. Anim. Vet. Adv. 2008, 7, 1352–1357. Available online: https://hero.epa.gov/hero/index.cfm/reference/details/reference_id/515774 (accessed on 11 July 2023).
- Cruz-Pacheco, G.; Esteva, L.; Vargas, C. Control measures for Chagas disease. Math. Biosci. 2012, 237, 49–60. [Google Scholar] [CrossRef]
- Arévalo, A.; Carranza, J.C.; Guhl, F.; Clavijo, J.A.; Vallejo, G.A. Comparison of the life cycles of Rhodnius colombiensis Moreno, Jurberg & Galvão, 1999 and R. prolixus Stal, 1872 (Hemiptera, Reduviidae, Triatominae) under laboratory conditions. Biomedica 2007, 27 (Suppl. S1), 119–129. Available online: https://www.ncbi.nlm.nih.gov/pubmed/18154252 (accessed on 13 July 2023).
- Jiménez-Coello, M.; Guzmán-Marín, E.; Ortega-Pacheco, A.; Acosta-Viana, K.Y. Serological survey of American trypanosomiasis in dogs and their owners from an urban area of Mérida Yucatàn, México. Transbound. Emerg. Dis. 2010, 57, 33–36. [Google Scholar] [CrossRef]
- Estrada-Franco, J.G.; Bhatia, V.; Diaz-Albiter, H.; Ochoa-Garcia, L.; Barbabosa, A.; Vazquez-Chagoyan, J.C.; Martinez-Perez, M.A.; Guzman-Bracho, C.; Garg, N. Human Trypanosoma cruzi infection and seropositivity in dogs, Mexico. Emerg. Infect. Dis. 2006, 12, 624–630. [Google Scholar] [CrossRef]
- Jimenez-Coello, M.; Poot-Cob, M.; Ortega-Pacheco, A.; Guzman-Marin, E.; Ramos-Ligonio, A.; Sauri-Arceo, C.H.; Acosta-Viana, K.Y.; Bárcenas-Irabién, A.; López-Cancino, S.A.; Tun-Ku, E.; et al. American trypanosomiasis in dogs from an urban and rural area of Yucatan, Mexico. Vector Borne Zoonotic Dis. 2008, 8, 755–761. [Google Scholar] [CrossRef]
- Gürtler, R.E.; Ceballos, L.A.; Ordóñez-Krasnowski, P.; Lanati, L.A.; Stariolo, R.; Kitron, U. Strong host-feeding preferences of the vector Triatoma infestans modified by vector density: Implications for the epidemiology of Chagas disease. PLoS Negl. Trop. Dis. 2009, 3, e447. [Google Scholar] [CrossRef]
- Coronado, X.; Rozas, M.; Botto-Mahan, C.; Ortíz, S.; Cattan, P.E.; Solari, A. Molecular epidemiology of Chagas disease in the wild transmission cycle: The evaluation in the sylvatic vector Mepraia spinolai from an endemic area of Chile. Am. J. Trop. Med. Hyg. 2009, 81, 656–659. [Google Scholar] [CrossRef]
- Ortega-Pacheco, A.; Poot-Ramos, A.; Chan-Pérez, J.I.; Gutiérrez-Blanco, E.; Acevedo-Arcique, C.M.; Baak-Baak, C.M.; Jiménez-Coello, M. Evaluation of the effectiveness of fluralaner against adult stages of Rhodnius prolixus in dogs. Parasitol. Int. 2022, 87, 102508. [Google Scholar] [CrossRef] [PubMed]
- Fiatsonu, E.; Busselman, R.E.; Hamer, G.L.; Hamer, S.A.; Ndeffo-Mbah, M.L. Effectiveness of fluralaner treatment regimens for the control of canine Chagas disease: A mathematical modeling study. PLoS Negl. Trop. Dis. 2023, 17, e0011084. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).