Using Local Ecological Knowledge to Search for Non-Native Species in Natura 2000 Sites in the Central Mediterranean Sea: An Approach to Identify New Arrivals and Hotspot Areas
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
- Sandbanks, which are slightly covered by sea water all the time (Habitat Code: 1110)
- Posidonia beds (Posidonion oceanicae) (Habitat Code: 1120)
- Coastal lagoons (Habitat Code: 1150)
- Reefs (Habitat Code: 1170)
- Submerged or partially submerged sea caves (Habitat Code: 8330).
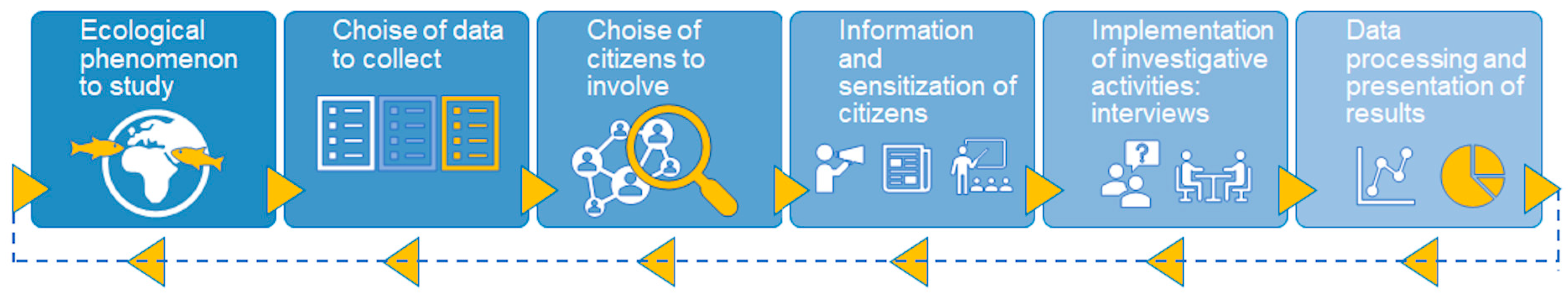
2.2. Data Collection
- data on the interviewee: category (recreational fisher, professional fisher, SCUBA diver); age class; years of experience at sea (starting date of activity); fishing gear/s;
- data on the species: information about the first and subsequent sightings (date/season, site, depth, substrate type and abundance), fishing gear or other sighting method, any available documentation (photo or video);
- data on other species: furthermore, interviewees were also asked to report any other species never captured/seen before.
2.3. Data Management and Analyses
2.3.1. Interviewees and Species Data Analysis
2.3.2. Impact of LEK on the Primary Criterion D2C1 of MSFD
2.3.3. Hotspot Areas of Non-Native Species’ High Impact by LEK Data
- = Index of the state of the invasive alien species population i in the specific N2K site. We used the presence/absence (1/0) data for this state variable.
- = Index of the extent of habitat j in a specific N2K site. We used the habitat presence/absence (1/0) data for this state variable (Table 1).
- = Impact weight for species i on habitat j present in the specific N2K site (Table 4).
- n, m = The numbers of invasive alien species and marine habitats, respectively, that were included in the analysis.
- The total area of occurrences per species as the total number of N2K sites (D1);
- The number of N2K sites with an impact weight score >0 per species (D2);
- The sum of the impact weight score of the species across all N2K sites (D3);
- The average impact weight across the range of occurrence (i.e., estimated across the number of N2K sites where the species was present) (D4).
3. Results
3.1. Data on the Interviewees and Species
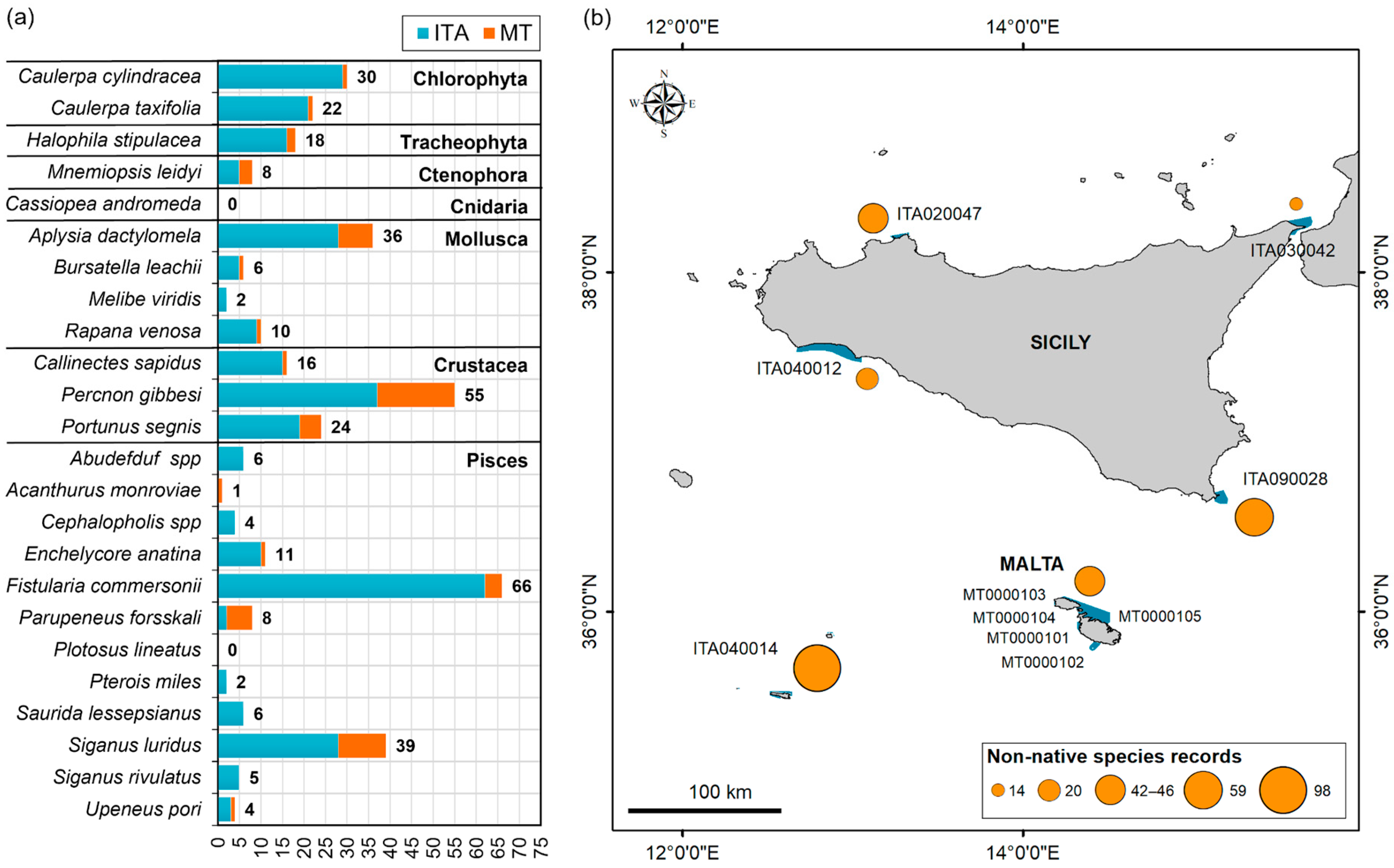
3.2. D2C1, CIMPAL and D1–D4 Indicators
4. Discussion
4.1. LEK Bias: Weaknesses and Strengths
4.1.1. Weaknesses
4.1.2. Strengths
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crooks, J.A.; Soulé, M.E. Lag times in population explosions of invasive species: Causes and implications. In Invasive Species and Biodiversity Management; Sandlund, O.T., Schei, P.J., Viken, Å., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1999; pp. 103–125. [Google Scholar]
- Blackburn, T.M.; Pyšek, P.; Bacher, S.; Carlton, J.T.; Duncan, R.P.; Jarošik, V.; Wilson, J.R.U.; Richardson, D.M. A proposed unified framework for biological invasions. Trends Ecol. Evol. 2011, 26, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Pyšek, P.; Hulme, P.E.; Simberloff, D.; Bacher, S.; Blackburn, T.M.; Carlton, J.T.; Dawson, W.; Essl, F.; Foxcroft, L.C.; Genovesi, P.; et al. Scientists’ warning on invasive alien species. Biol. Rev. 2020, 95, 1511–1534. [Google Scholar] [CrossRef] [PubMed]
- Katsanevakis, S.; Wallentinus, I.; Zenetos, A.; Leppäkoski, E.; Çinar, M.E.; Oztürk, B.; Grabowski, M.; Golani, D.; Cardoso, A.C. Impacts of invasive alien marine species on ecosystem services and biodiversity: A pan-European review. Aquat. Invasions 2014, 9, 391–423. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Tempera, F.; Teixeira, H. Mapping the impact of alien species on marine ecosystems: The Mediterranean Sea case study. Divers. Distrib. 2016, 22, 694–707. [Google Scholar] [CrossRef]
- Ojaveer, H.; Galil, B.S.; Carlton, J.T.; Alleway, H.; Goulletquer, P.; Lehtiniemi, M.; Marchini, A.; Miller, W.; Occhipinti–Ambrogi, A.; Peharda, M.; et al. Historical baselines in marine bioinvasions: Implications for policy and management. PLoS ONE 2018, 13, e0202383. [Google Scholar] [CrossRef]
- Kowarik, I. Time lags in biological invasions with regard to the success and failure of alien species. In Plant Invasions—General Aspects and Special Problems; Pyšek, P., Prach, K., Rejmanek, M., Wade, M., Eds.; SPB Academic Publishing: Amsterdam, The Netherlands, 1995; pp. 15–38. [Google Scholar]
- Crooks, J.A. Lag times. In Encyclopedia of Biological Invasions; Simberloff, D., Rejmanek, M., Eds.; University of California Press: Oakland, CA, USA, 2011; pp. 404–410. [Google Scholar]
- Simberloff, D.; Martin, J.-L.; Genovesi, P.; Maris, V.; Wardle, D.A.; Aronson, J.; Courchamp, F.; Galil, B.; García-Berthou, E.; Pascal, M.; et al. Impacts of biological invasions: What’s what and the way forward. Trends Ecol. Evol. 2013, 28, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Olenin, S.; Alemany, F.; Cardoso, A.C.; Gollasch, S.; Goulletquer, P.; Lehtiniemi, M.; Mccollin, T.; Minchin, D.; Miossec, L.; Occhipinti Ambrogi, A.; et al. Marine Strategy Framework Directive—Task Group 2 Non-Indigenous Species; Piha, H., Ed.; Publications Office of the European Union: Luxembourg, 2010; JRC58108; Available online: https://data.europa.eu/doi/10.2788/87092 (accessed on 23 June 2023).
- Cardoso, A.; Tsiamis, K.; Gervasini, E.; Schade, S.; Taucer, F.; Adriaens, T.; Copas, K.; Flevaris, S.; Galiay, P.; Jennings, E.; et al. Citizen Science and Open Data: A model for Invasive Alien Species in Europe. RIO 2017, 3, e14811. [Google Scholar] [CrossRef]
- Chandler, M.; See, L.; Copas, K.; Bonde, A.M.; López, B.C.; Danielsen, F.; Rosemartin, A. Contribution of citizen science towards international biodiversity monitoring. Biol. Conserv. 2017, 213, 280–294. [Google Scholar] [CrossRef]
- Chase, S.K.; Levine, A. A framework for evaluating and designing citizen science programs for natural resources monitoring. Conserv. Biol. 2016, 30, 456–466. [Google Scholar] [CrossRef]
- Encarnação, J.; Teodósio, M.A.; Morais, P. Citizen science and biological invasions: A review. Front. Environ. Sci. 2021, 8, 602980. [Google Scholar] [CrossRef]
- Beaudreau, A.H.; Levin, P.S. Advancing the use of local ecological knowledge for assessing data-poor species in coastal ecosystems. Ecol. Appl. 2014, 24, 244–256. [Google Scholar] [CrossRef] [PubMed]
- Azzurro, E.; Bolognini, L.; Dragičević, B.; Drakulović, D.; Dulčić, J.; Fanelli, E.; Grati, F.; Kolitari, J.; Lipej, L.; Magaletti, E.; et al. Detecting the occurrence of indigenous and non-indigenous megafauna through fishermen knowledge: A complementary tool to coastal and port surveys. Mar. Pollut. Bull. 2019, 147, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Azzurro, E.; Sbragaglia, V.; Cerri, J.; Bariche, M.; Bolognini, L.; Ben Souissi, J.; Busoni, G.; Coco, S.; Chryssanthi, A.; Fanelli, E.; et al. Climate change, biological invasions, and the shifting distribution of Mediterranean fishes: A large-scale survey based on local ecological knowledge. Glob. Chang. Biol. 2019, 25, 2779–2792. [Google Scholar] [CrossRef] [PubMed]
- Crocetta, F.; Gofas, S.; Salas, C.; Tringali, L.P.; Zenetos, A. Local ecological knowledge versus published literature: A review of non-indigenous Mollusca in Greek marine waters. Aquat. Invasions 2017, 12, 415–434. [Google Scholar] [CrossRef]
- Maggio, T.; Perzia, P.; Falautano, M.; Visconti, G.; Castriota, L. From LEK to LAB: The case of the blue crab Portunus segnis in the Pelagie Islands Marine Protected Area, central Mediterranean Sea. Ocean Coast. Manag. 2022, 219, 106043. [Google Scholar] [CrossRef]
- Grech, D.; Pilloni, Z.; Burton, M.; Serra, E.; Brundu, G.; Baroli, M.; Porporato, E.; Massaro, G.; Ceccherelli, G.; Cerri, J.; et al. A local ecological knowledge approach for a collaborative nis mapping in Sardinia (Italy). In Proceedings of the 2nd Mediterranean Symposium on the Non-Indigenous Species, Genoa, Italy, 22–23 September 2022; pp. 42–47. [Google Scholar]
- Spanò, N.; De Domenico, E. Biodiversity in Central Mediterranean Sea; Fuerst-Bjelis, B., Ed.; 2017; Volume 6, pp. 129–148. Available online: https://www.intechopen.com/chapters/55411 (accessed on 23 June 2023).
- Azzurro, E.; Ben Souissi, S.J.; Boughedir, W.; Castriota, L.; Deidun, A.; Falautano, M.; Ghanem, R.; Zammit-Mangion, M.; Andaloro, F. The Sicily Strait: A Transnational Observatory for Monitoring the Advance of Non Indigenous Species. 2014. Available online: https://www.um.edu.mt/library/oar/handle/123456789/26215 (accessed on 26 July 2023).
- European Environment Agency. Natura 2000, Standard Data Form Site ITA020047. 2021. Available online: natura2000.eea.europa.eu/Natura2000/SDF.aspx?site=ITA020047 (accessed on 20 June 2023).
- European Environment Agency. Natura 2000, Standard Data Form Site ITA030042. 2021. Available online: natura2000.eea.europa.eu/Natura2000/SDF.aspx?site=ITA030042 (accessed on 20 June 2023).
- European Environment Agency. Natura 2000, Standard Data Form Site ITA040012. 2021. Available online: natura2000.eea.europa.eu/Natura2000/SDF.aspx?site=ITA040012 (accessed on 20 June 2023).
- European Environment Agency. Natura 2000, Standard Data Form Site ITA040014. 2021. Available online: natura2000.eea.europa.eu/Natura2000/SDF.aspx?site=ITA040014 (accessed on 20 June 2023).
- European Environment Agency. Natura 2000, Standard Data Form Site ITA090028. 2021. Available online: natura2000.eea.europa.eu/Natura2000/SDF.aspx?site=ITA090028 (accessed on 20 June 2023).
- European Environment Agency. Natura 2000, Standard Data Form Site MT0000101. 2021. Available online: natura2000.eea.europa.eu/Natura2000/SDF.aspx?site=MT0000101 (accessed on 20 June 2023).
- European Environment Agency. Natura 2000, Standard Data Form Site MT0000102. 2021. Available online: natura2000.eea.europa.eu/Natura2000/SDF.aspx?site=MT0000102 (accessed on 20 June 2023).
- European Environment Agency. Natura 2000, Standard Data Form Site MT0000103. 2021. Available online: natura2000.eea.europa.eu/Natura2000/SDF.aspx?site=MT0000103 (accessed on 20 June 2023).
- European Environment Agency. Natura 2000, Standard Data Form Site MT0000104. 2021. Available online: natura2000.eea.europa.eu/Natura2000/SDF.aspx?site=MT0000104 (accessed on 20 June 2023).
- European Environment Agency. Natura 2000, Standard Data Form Site MT0000105. 2021. Available online: natura2000.eea.europa.eu/Natura2000/SDF.aspx?site=MT0000105 (accessed on 20 June 2023).
- Vasquez, M.; Allen, H.; Manca, E.; Castle, L.; Lillis, H.; Agnesi, S.; Al Hamdani, Z.; Annunziatellis, A.; Askew, N.; Bekkby, T.; et al. EUSeaMap 2021. A European Broad-Scale Seabed Habitat Map; D1.13 EASME/EMFF/2018/1.3.1.8/Lot2/SI2.810241–EMODnet Thematic Lot n° 2—Seabed Habitats EUSeaMap 2021—Technical Report. 2017. Available online: https://archimer.ifremer.fr/doc/00723/83528/ (accessed on 23 June 2023).
- European Environment Agency. EUNIS Habitat Classification. Available online: https://www.eea.europa.eu/data-and-maps/data/eunis-habitat-classification-1 (accessed on 20 June 2023).
- Garrabou, J.; Bensoussan, N.; Azzurro, E. Monitoring Climate-Related Responses in Mediterranean Marine Protected Areas and Beyond: Five Standard Protocols; Institute of Marine Sciences, Spanish Research Council ICM-CSIC: Barcelona, Spain, 2018; 36p. [Google Scholar]
- Tsiamis, K.; Palialexis, A.; Stefanova, K.; Ničević Gladan, Ž.; Skejić, S.; Despalatović, M.; Cvitković, I.; Dragičević, B.; Dulčić, J.; Vidjak, O.; et al. Non-indigenous species refined national baseline inventories: A synthesis in the context of the European Union’s Marine Strategy Framework Directive. Mar. Pollut. Bull. 2019, 145, 429–435. [Google Scholar] [CrossRef]
- Piazzi, L.; Balestri, E.; Cinelli, F. Presence of Caulerpa racemosa in the North–Western Mediterranean. Cryptogam. Algol. 1994, 15, 183–189. [Google Scholar]
- Alongi, G.; Cormaci, M.; Furnari, G.; Giaccone, G. Prima segnalazione di Caulerpa racemosa (Chlorophyceae, Caulerpales) per le coste italiane. Boll. Accad. Gioenia Sci. Nat. Catania 1993, 26, 49–53. [Google Scholar]
- Stevens, D.T. Country report—Malta. In Proceeding of the Workshop on Invasive Caulerpa Species in the Mediterranean, MAP Technical Report Series n. 125, Heraklion, Greece, 18–20 March 1998; UNEP Publisher: Heraklion, Greece, 1999; pp. 279–281. [Google Scholar]
- Relini, M.; Torchia, G. Prima segnalazione di Caulerpa taxifolia in acque italiane. Doriana 1992, 6, 1–4. [Google Scholar]
- Fradà Orestano, C.; Calvo, S.; Ferreri, B.M. First record of Caulerpa taxifolia (Vahl) C. Agardh in the southwestern Mediterranean. Giorn. Bot. Ital. 1994, 128, 813–815. [Google Scholar]
- Schembri, P.J.; Barbara, J.; Deidun, A.; Lanfranco, E.; Lanfranco, S. It was only a matter of time: Occurrence of Caulerpa taxifolia (Vahl) C. agardh var. distichophylla (Sonder) Verlaque, Huisman and Procaccini in the Maltese Islands (Chlorophyta, Ulvophyceae, Caulerpaceae). BioInvasions Rec. 2015, 4, 9–16. [Google Scholar] [CrossRef]
- Acunto, S.; Maltagliati, F.; Rindi, F.; Rossi, F.; Cinelli, F. Osservazioni su una prateria di Halophila stipulacea (Forssk.) Aschers. (Hydrocharitaceae) nel mar Tirreno meridionale. Atti Soc. Toscana Sci. Nat. Mem. B 1995, 102, 19–22. [Google Scholar]
- Villari, R. Segnalazioni Floristiche Italiane: 565. Halophila stipulacea (Forssk.) Aschers. Inf. Bot. Ital. 1988, 20, 672. [Google Scholar]
- Lanfranco, E. The occurrence of Halophila stipulacea (Forskaal) Ascherson (Hydrocharitaceae) in Maltese waters. Malt. Naturalist. 1970, 1, 16–17. [Google Scholar]
- Boero, F.; Putti, M.; Trainito, E.; Prontera, E.; Piraino, S.; Shiganova, T.A. First records of Mnemiopsis leidyi (Ctenophora) from the Ligurian, Thyrrhenian and Ionian Seas (Western Mediterranean) and first record of Phyllorhiza punctata (Cnidaria) from the Western Mediterranean. Aquat. Invasions 2009, 4, 675–680. [Google Scholar] [CrossRef]
- Cillari, T.; Andaloro, F.; Castriota, L. First documented record of Cassiopea cfr andromeda (Cnidaria: Scyphozoa) in Italian waters. Cah. Biol. Mar. 2018, 59, 193–195. [Google Scholar] [CrossRef]
- Servello, G.; Andaloro, F.; Azzurro, E.; Castriota, L.; Catra, M.; Chiarore, A.; Crocetta, F.; D’Alessandro, M.; Denitto, F.; Froglia, C.; et al. Marine alien species in Italy: A contribution to the implementation of descriptor D2 of the marine strategy framework directive. Mediterr. Mar. Sci. 2019, 20, 1–48. [Google Scholar] [CrossRef]
- Schembri, P.J.; Deidun, A.; Vella, P.J. First record of Cassiopea andromeda (Scyphozoa: Rhizostomeae: Cassiopeidae) from the central Mediterranean Sea. Mar. Biodivers. Rec. 2010, 3, e6. [Google Scholar] [CrossRef]
- Crocetta, F.; Galil, B.S. The invasive spotted sea hare Aplysia dactylomela (Mollusca: Gastropoda: Aplysiidae)—New records and spread pattern in the Mediterranean. Vie Milieu 2012, 62, 43–46. [Google Scholar]
- Trainito, E. Arlecchini mediterranei. Guida ai Molluschi Opistobranchi del Mediterraneo; Taphros Ed.: Olbia, Italy, 2003; pp. 1–60. [Google Scholar]
- Schembri, P.J. Occurrence of the alien sea hare Aplysia dactylomela Rang, 1828 (Opisthobranchia, Aplysiidae) in Malta. Mediterr. Mar. Sci. 2008, 9, 111–114. Available online: https://www.um.edu.mt/library/oar/handle/123456789/21499 (accessed on 26 July 2023). [CrossRef][Green Version]
- Travaglini, A.; Crocetta, F. Natural history collections and alien species: An overlooked sample of Bursatella leachii Blainville, 1817 (Mollusca: Gastropoda: Aplysiida) backdates its confirmed presence in Italy. Thalassas 2019, 35, 137–141. [Google Scholar] [CrossRef]
- Tortorici, R.; Panetta, P. Notizie ecologiche su alcuni Opistobranchi raccolti nel Golfo di Taranto (Gastropoda). Atti Soc. Ital. Sci. Nat. Mus. Civ. Stor. Nat. Milano 1977, 118, 249–257. [Google Scholar]
- Bebbington, A. Aplysiid species from Malta with notes on the Mediterranean Aplysiomorpha (Gastropoda, Opisthobranchia). Pubbl. Staz. Zool. Napoli 1970, 38, 25–46. [Google Scholar]
- Doneddu, M.; Trainito, E. Melibe viridis (Kelaart, 1858) (Opisthobranchia: Tethydidae): Prime segnalazioni per il Tirreno (Sardegna settentrionale). Boll. Malacol. 2008, 44, 45–47. [Google Scholar]
- Crocetta, F.; Renda, W.; Vazzana, A. Alien Mollusca along the Calabrian shores of the Messina Strait area and a review of their distribution in the Italian Seas. Boll. Malacol. 2009, 45, 15–30. [Google Scholar]
- Borg, J.A.; Evans, J.; Schembri, P.J. Occurrence of the Alien Nudibranch Melibe viridis (Kelaart, 1858) (Opisthobranchia, Tethydidae), in the Maltese Islands. Available online: https://www.um.edu.mt/library/oar/handle/123456789/18153 (accessed on 26 July 2023).
- Terreni, G. Molluschi poco conosciuti dell’Arcipelago Toscano. I Gasteropodi. Boll. Malacol. 1980, 16, 9–17. [Google Scholar]
- Trono, D. Nuovi dati sulla malacofauna del Salento (Puglia meridionale). Boll. Malacol. 2006, 42, 58–84. [Google Scholar]
- Tortonese, E. La comparsa di Callinectes sapidus Rathb. (Decapoda Brachyura) nel Mar Ligure. Doriana 1965, 4, 1–3. [Google Scholar]
- Galil, B.S.; Froglia, C.; Noël, P. CIESM Atlas of Exotic Species in the Mediterranean. Vol. 2. Crustaceans: Decapods and Stomatopods; Briand, F., Ed.; CIESM Publishers: Monaco, 2002; pp. 1–192. [Google Scholar]
- Sparrow, A.; Badalamenti, F.; Pipitone, C. Contribution to the knowledge of Percnon gibbesi (Decapoda, Grapsidae), an exotic species spreading rapidly in sicilian waters. Crustaceana 2001, 74, 1009–1017. Available online: http://www.jstor.org/stable/20105338 (accessed on 26 July 2023). [CrossRef]
- Relini, M.; Orsi, L.; Puccio, V.; Azzurro, E. The exotic crab Percnon gibbesi (H. Milne Edwards, 1853) (Decapoda, Grapsidae) in the Central Mediterranean. Sci. Mar. 2000, 64, 337–340. Available online: https://scientiamarina.revistas.csic.es/index.php/scientiamarina/article/view/772 (accessed on 26 July 2023). [CrossRef]
- Borg, J.J.; Attard–Montalto, J. The grapsid crab Percnon gibbesi (Milne Edwards, 1853) (Crustacea, Decapoda, Brachyura), a new addition to the marine fauna of Malta. Cent. Medit. Natur. 2002, 3, 159–160. [Google Scholar]
- Crocetta, F. First record of Portunus pelagicus (Linnaeus, 1758) (Decapoda, Brachyura, Portunidae) in the northern Tyrrhenian Sea. Crustaceana 2006, 79, 1145–1148. Available online: http://www.jstor.org/stable/20107743 (accessed on 26 July 2023). [CrossRef]
- Torchio, M. Il Callinectes sapidus Rathbun nelle acque siciliane (Crustacea, Decapoda). Natura 1967, 58, 81. [Google Scholar]
- Schembri, P.J.; Lanfranco, E. Marine Brachyura (Crustacea: Decapoda: Brachyura) from the Maltese Islands and surrounding waters (central Mediterranean). Centro 1984, 1, 21–39. Available online: https://www.um.edu.mt/library/oar/handle/123456789/21176 (accessed on 26 July 2023).
- Tardent, P. Capture d’un Abudefduf saxatilis vaigiensis Q. und G. (Pisces, Pomacentridae) dans le Golfe de Naples. Rev. Suisse Zool. 1959, 66, 347–351. [Google Scholar] [CrossRef]
- Deidun, A.; Castriota, L. First Record of Abudefduf cfr saxatilis (Perciformes: Pomacentridae) from the Maltese Islands (Central Mediterranean). BioInvasions Rec. 2014, 3, 53–56. Available online: https://www.um.edu.mt/library/oar/handle/123456789/26170 (accessed on 26 July 2023). [CrossRef]
- Langeneck, J.; Boyer, M.; De Cecco, P.G.; Luciani, C.; Marcelli, M.; Vacchi, M. First record of Acanthurus chirurgus (Perciformes: Acanthuridae) in the Mediterranean Sea, with some distributional notes on Mediterranean Acanthuridae. Mediterr. Mar. Sci. 2015, 16, 427–431. [Google Scholar] [CrossRef][Green Version]
- Guidetti, P.; Giardina, F.; Azzurro, E. A new record of Cephalopholis taeniops in the Mediterranean Sea, with considerations on the Sicily channel as a biogeographical crossroad of exotic fish. Mar. Biodivers. Rec. 2010, 3, e13. [Google Scholar] [CrossRef]
- Schembri, P.J.; Tonna, R. Occurrence of the Malabar grouper Epinephelus malabaricus (Bloch & Schneider, 1801) (Actinopterygii, Perciformes, Serranidae), in the Maltese Islands. Aquat. Invasions 2011, 6, S129–S132. [Google Scholar] [CrossRef]
- Guidetti, P.; Causio, S.; Licchelli, C. The first record of Enchelycore anatina (Muraenidae: Pisces) in the Ionian Sea (Mediterranean basin). Mar. Biodivers. Rec. 2012, 5, e22. [Google Scholar] [CrossRef]
- Deidun, A.; Watson, D.; Castriota, L.; Mazza, G.; Pasolli, L. First record of the fangtooth moray, Enchelycore anatina (Actinopterygii: Anguilliformes: Muraenidae), from Maltese waters, central Mediterranean. Acta Ichthyol. Piscat. 2015, 45, 315–317. [Google Scholar] [CrossRef]
- Pipitone, C.; D’Anna, G.; Coppola, M.; Di Stefano, G.; Badalamenti, F. First record of the lessepsian fish Fistularia commersonii in the Western Mediterranean. Biol. Mar. Mediterr. 2004, 11, 327. [Google Scholar]
- Fiorentino, F.; Giusto, G.B.; Sinacori, G.; Norrito, G. First record of Fistularia commersonii (Fistularidae, Pisces) in the strait of Sicily (Mediterranean Sea). Biol. Mar. Mediterr. 2004, 11, 583–585. [Google Scholar]
- Deidun, A.; Germanà, A. On the increasing occurrence of the Bluespotted Cornetfish Fistularia commersonii (Rüppel, 1838) in the Central Mediterranean (Osteichthyes, Fistulariidae). Biodivers. J. 2011, 2, 19–26. Available online: https://www.um.edu.mt/library/oar/handle/123456789/26304 (accessed on 26 July 2023).
- Sciberras, M.; Schembri, P.J. A critical review of records of alien marine species from the Maltese Islands and surrounding waters (Central Mediterranean). Mediterr. Mar. Sci. 2007, 8, 41–66. Available online: https://www.um.edu.mt/library/oar/handle/123456789/21175 (accessed on 26 July 2023). [CrossRef]
- Azzurro, E.; Stancanelli, B.; Di Martino, V.; Bariche, M. Range expansion of the common lionfish Pterois miles (Bennett, 1828) in the Mediterranean Sea: An unwanted new guest for Italian waters. BioInvasions Rec. 2017, 6, 95–98. [Google Scholar] [CrossRef]
- Castriota, L.; Andaloro, F.; Costa, F. Old findings of the lessepsian immigrants Platycephalus indicus (Platycephalidae) and Saurida undosquamis (Synodontidae) along the Italian coasts. Mar. Biodivers. Rec. 2009, 2, e130. [Google Scholar] [CrossRef]
- Castriota, L.; Andaloro, F. First record of the lessepsian fish Siganus luridus (Osteichthyes: Siganidae) in the Tyrrhenian Sea. Mar. Biodivers. Rec. 2005, 1, e11. [Google Scholar] [CrossRef]
- Azzurro, E.; Andaloro, F. A new settled population of the Lessepsian migrant Siganus luridus (Pisces: Siganidae) in Linosa Island–Sicily Strait. J. Mar. Biol. Assoc. UK 2004, 84, 819–821. [Google Scholar] [CrossRef]
- Karachle, P.K.; Angelidis, A.; Apostolopoulos, G.; Ayas, D.; Ballesteros, M.; Bonnici, C.; Crocetta, F. New Mediterranean Biodiversity Records (March 2016). Mediterr. Mar. Sci. 2016, 17, 230–252. [Google Scholar] [CrossRef]
- Geraci, M.L.; Scannella, D.; Falsone, F.; Colloca, F.; Vitale, S.; Rizzo, P.; Di Maio, G.; Milisenda, G.; Fiorentino, F. Preliminary study on the biological traits of the Por’s goatfish Upeneus pori (Chordata: Actinopterygii) off the southern coast of Lampedusa Island (Central Mediterranean). Eur. Zool. J. 2018, 85, 231–241. [Google Scholar] [CrossRef]
- Doğdu, S.; Uyan, A.; Uygur, N.; Gürlek, M.; Ergüden, D.; Turan, C. First record of the Indo-Pacific striped eel catfish, Plotosus lineatus (Thunberg, 1787), from Turkish marine waters. NESciences 2016, 1, 25–32. [Google Scholar] [CrossRef]
- Amor, K.O.B.; Rifi, Μ.; Ghanem, R.; Draeif, I.; Zaouali, J.; Souissi, J.B. Update of alien fauna and new records from Tunisian marine waters. Mediterr. Mar. Sci. 2016, 17, 124–143. [Google Scholar] [CrossRef]
- Edelist, D.; Golani, D.; Rilov, G.; Spanier, E. The invasive venomous striped eel catfish Plotosus lineatus in the Levant: Possible mechanisms facilitating its rapid invasional success. Mar. Biol. 2012, 159, 283–290. [Google Scholar] [CrossRef]
- European Union. Regulation (EU) No 1143/2014 of the European parliament and of the council of 22 October 2014 on the prevention and management of the introduction and spread of invasive alien species. Off. J. Eur. Union 2014, L 317/35. Available online: http://data.europa.eu/eli/reg/2014/1143/oj (accessed on 23 June 2023).
- European Union. Commission implementing regulation (EU) 2019/1262 of 25 July 2019. Off. J. Eur. Union 2019, L 199/1. Available online: http://data.europa.eu/eli/reg_impl/2019/1262/oj (accessed on 23 June 2023).
- European Union. Commission decision (EU) 2017/848 of 17 May 2017 laying down criteria and methodological standards on good environmental status of marine waters and specifications and standardised methods for monitoring and assessment, and repealing Decision 2010/477/EU. Off. J. Eur. Union 2017, L 125/43. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=COM:2018:0562:FIN:EN:PDF (accessed on 23 June 2023).
- Ojaveer, H.; Galil, B.S.; Campbell, M.L.; Carlton, J.T.; Canning-Clode, J.; Cook, E.J.; Davidson, A.D.; Hewitt, C.L.; Jelmert, A.; Marchini, A.; et al. Classification of Non-Indigenous Species Based on Their Impacts: Considerations for Application in Marine Management. PLoS Biol. 2015, 13, e1002130. [Google Scholar] [CrossRef] [PubMed]
- Zenetos, A.; Tsiamis, K.; Galanidi, M.; Carvalho, N.; Bartilotti, C.; Canning-Clode, J.; Castriota, L.; Chainho, P.; Comas-Gonzalez, R.; Costa, A.C. Status and Trends in the Rate of Introduction of Marine Non-Indigenous Species in European Seas. Diversity 2022, 14, 1077. [Google Scholar] [CrossRef]
- Andaloro, F.; Castriota, L.; Falautano, M.; Azzurro, E.; Deidun, A.; Fenech-Farrugia, A. Public Feedback on Early Warning Initiatives Undertaken for Hazardous Non-Indigenous Species: The Case of Lagocephalus sceleratus from Italian and Maltese Waters). Available online: https://www.um.edu.mt/library/oar/handle/123456789/26204 (accessed on 20 June 2023).
- Castriota, L.; Falautano, M.; Maggio, T.; Perzia, P. The Blue Swimming Crab Portunus segnis in the Mediterranean Sea: Invasion Paths, Impacts and Management Measures. Biology 2022, 11, 1473. [Google Scholar] [CrossRef]
- Deidun, A.; Galdies, J.; Marrone, A.; Sciberras, A.; Zava, B.; Corsini-Foka, M.; Gianguzza, P. The first confirmed record of the Atlantic blue crab Callinectes sapidus Rathbun, 1896 (Decapoda, Brachyura) from Maltese waters. BioInvasions Rec. 2022, 11, 238–243. [Google Scholar] [CrossRef]
- Falzon, M.A. First record of por’s goatfish Upeneus pori (Actinopteri Mulliformes Mullidae) from Malta (central Mediterranean). Nat. Sicil. 2021, 45, 103–108. [Google Scholar] [CrossRef]
- Giakoumi, S. Distribution patterns of the invasive herbivore Siganus luridus (Rüppell, 1829) and its relation to native benthic communities in the central Aegean Sea, Northeastern Mediterranean. Mar. Ecol. 2014, 35, 96–105. [Google Scholar] [CrossRef]
- Sala, E.; Kizilkaya, Z.; Yildirim, D.; Ballesteros, E. Alien Marine Fishes Deplete Algal Biomass in the Eastern Mediterranean. PLoS ONE 2011, 6, e17356. [Google Scholar] [CrossRef] [PubMed]
- Vergés, A.; Tomas, F.; Cebrian, E.; Ballesteros, E.; Kizilkaya, Z.; Dendrinos, P.; Karamanlidis, A.A.; Spiegel, D.; Sala, E. Tropical rabbitfish and the deforestation of a warming temperate sea. J. Ecol. 2014, 102, 1518–1527. [Google Scholar] [CrossRef]
- Yeruham, E.; Shpigel, M.; Abelson, A.; Rilov, G. Ocean warming and tropical invaders erode the performance of a key herbivore. Ecology 2020, 101, e02925. [Google Scholar] [CrossRef]
- Alomar, C.; Deudero, S.; Andaloro, F.; Castriota, L.; Consoli, P.; Falautano, M.; Sinopoli, M. Caulerpa cylindracea Sonder invasion modifies trophic niche in infralittoral rocky benthic community. Mar. Environ. Res. 2016, 120, 86–92. [Google Scholar] [CrossRef]
- Deudero, S.; Box, A.; Alós, J.; Arroyo, N.L.; Marbà, N. Functional changes due to invasive species: Food web shifts at shallow Posidonia oceanica seagrass beds colonized by the alien macroalga Caulerpa racemosa. Estuar. Coast. Shelf Sci. 2011, 93, 106–116. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Issaris, Y.; Poursanidis, D.; Thessalou-Legaki, M. Vulnerability of marine habitats to the invasive green alga Caulerpa racemosa var. cylindracea within a marine protected area. Mar. Environ. Res. 2010, 70, 210–218. [Google Scholar] [CrossRef]
- Piazzi, L.; Ceccherelli, G.; Cinelli, F. Threat to macroalgal diversity: Effects of the introduced green alga Caulerpa racemosa in the Mediterranean. Mar. Ecol. Prog. Ser. 2001, 210, 149–159. [Google Scholar] [CrossRef]
- Ferrer, E.; Ribera, M.A. Effect of Caulerpa taxifolia on the productivity of two Mediterranean macrophytes. Mar. Ecol. Prog. Ser. 1997, 149, 279–287. [Google Scholar] [CrossRef][Green Version]
- Francour, P.; Pellissier, V.; Mangialajo, L.; Buisson, E.; Stadelmann, B.; Veillard, N.; Meinesz, A.; Thibaut, T.; De Vaugelas, J. Changes in invertebrate assemblages of Posidonia oceanica beds following Caulerpa taxifolia invasion. Vie Milieu 2009, 59, 31–38. [Google Scholar]
- Barcelo, A.; Cottalorda, J.M.; Peirache, M.; Abiven, T.; Gomez, M.C.; Viviani, R.A.; Bergere, H.; Baudin, E.; Jullian, E.; Moreau, S.; et al. Définition d’une politique et d’une stratégie globale de gestion concertées du chlorobionte invasif Caulerpa taxifolia à l’échelle des coeurs et de l’aire marine adjacente du Parc national de Port-Cros (Provence, France). Sci. Rep. Port-Cros Natl. Park 2016, 30, 45–64. [Google Scholar]
- Azzurro, E.; Soto, S.; Garofalo, G.; Maynou, F. Fistularia commersonii in the Mediterranean Sea: Invasion history and distribution modeling based on presence-only records. Biol. Invasions 2012, 15, 977–990. [Google Scholar] [CrossRef]
- Azzurro, E.; Bariche, M.; Cerri, J.; Garrabou, J. The long reach of the Suez Canal: Lagocephalus sceleratus (Gmelin, 1789) an unwanted Indo-Pacific pest at the Atlantic gates. BioInvasions Rec. 2020, 9, 204–208. [Google Scholar] [CrossRef]
- Falautano, M.; Perzia, P.; Castriota, L. First record of the Lessepsian fish Parexocoetus mento in Italian waters and GIS-based spatial and temporal distribution in Mediterranean Sea. J. Mar. Biol. Assoc. UK 2020, 100, 1163–1169. [Google Scholar] [CrossRef]
- Byers, J.E. Impact of non-indigenous species on natives enhanced by anthropogenic alteration of selection regimes. Oikos 2002, 97, 449–458. [Google Scholar] [CrossRef]
- Cacabelos, E.; Gestoso, I.; Ramalhosa, P.; Canning-Clode, J. Role of non-indigenous species in structuring benthic communities after fragmentation events: An experimental approach. Biol. Invasions 2022, 24, 2181–2199. [Google Scholar] [CrossRef]
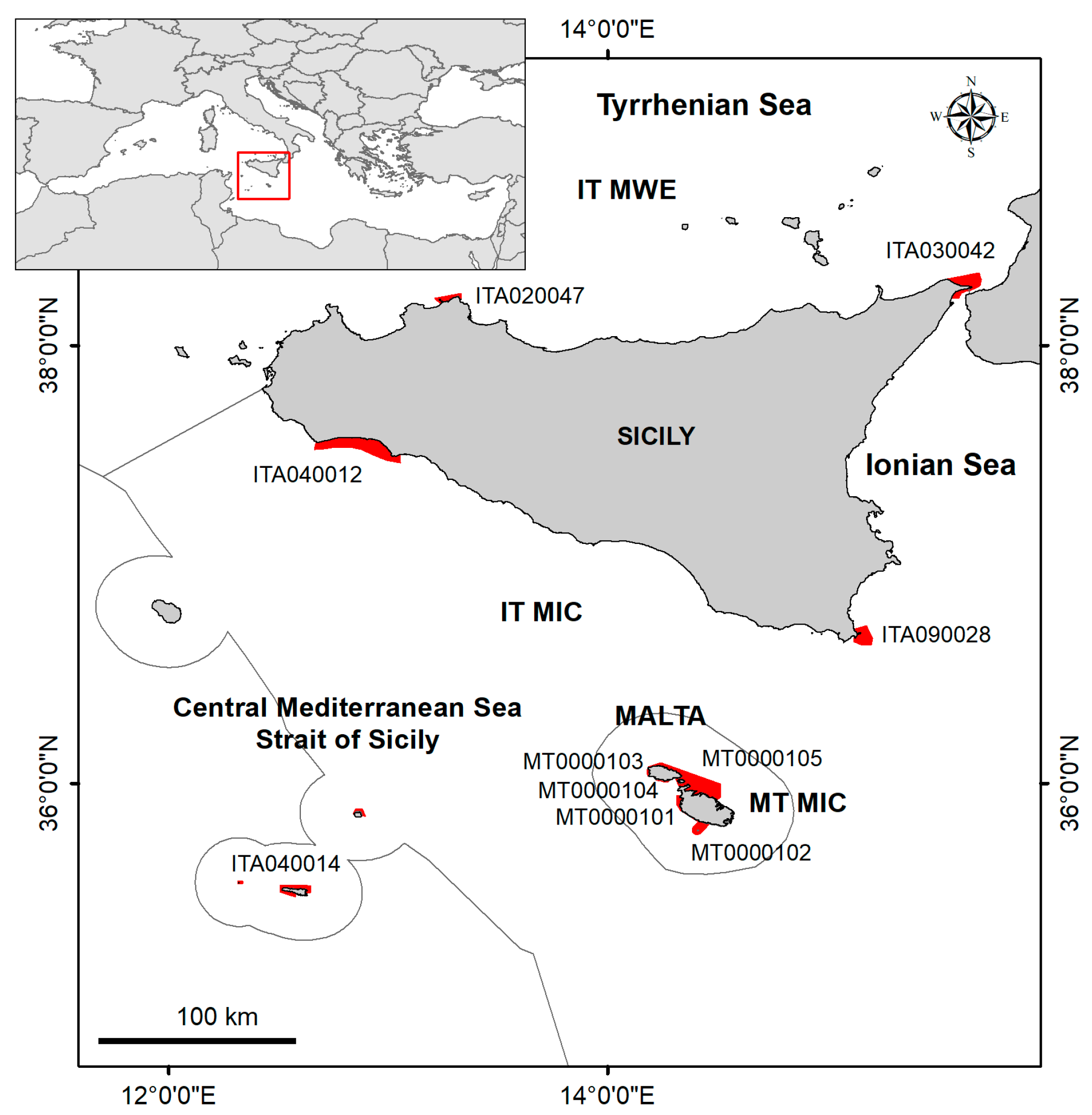
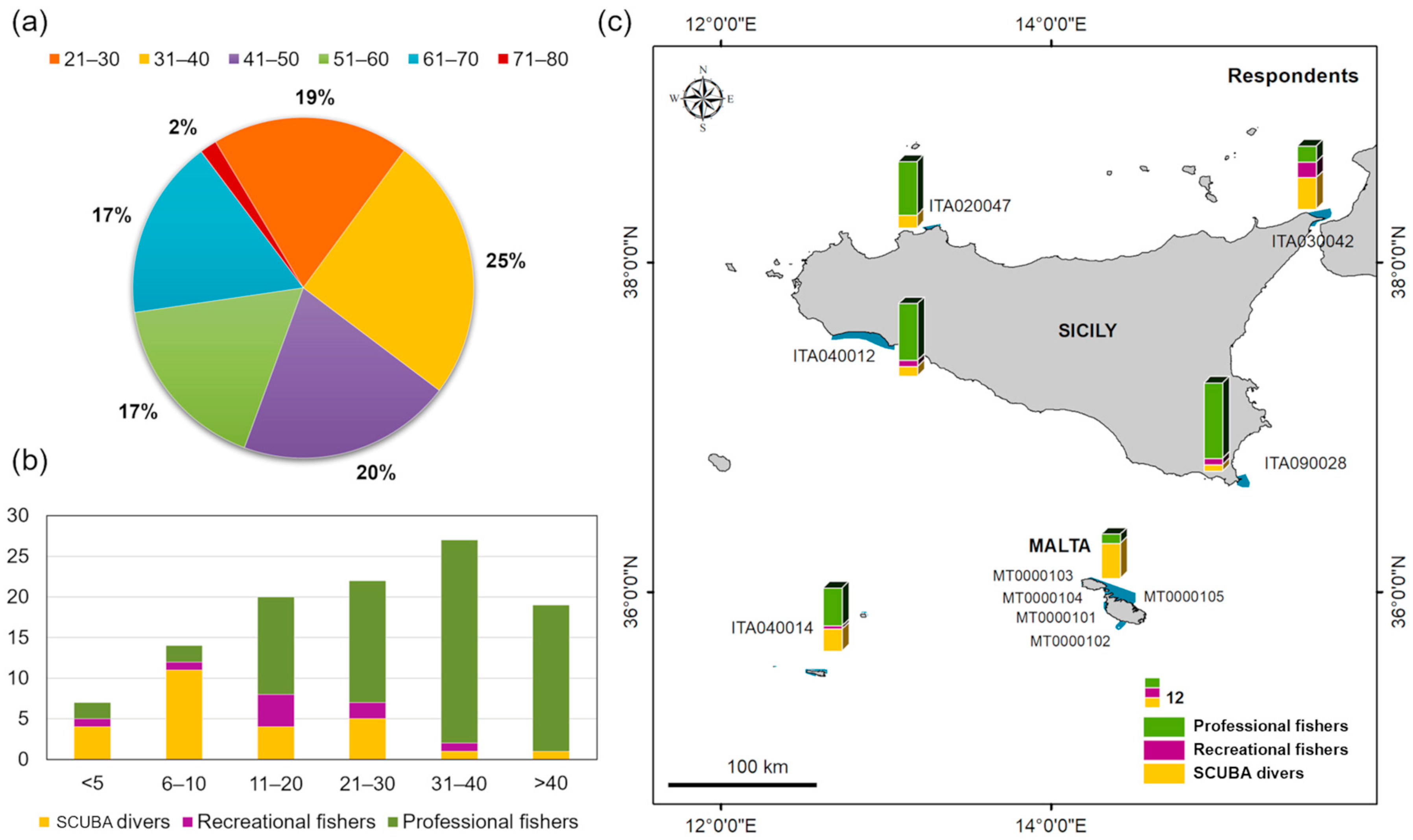
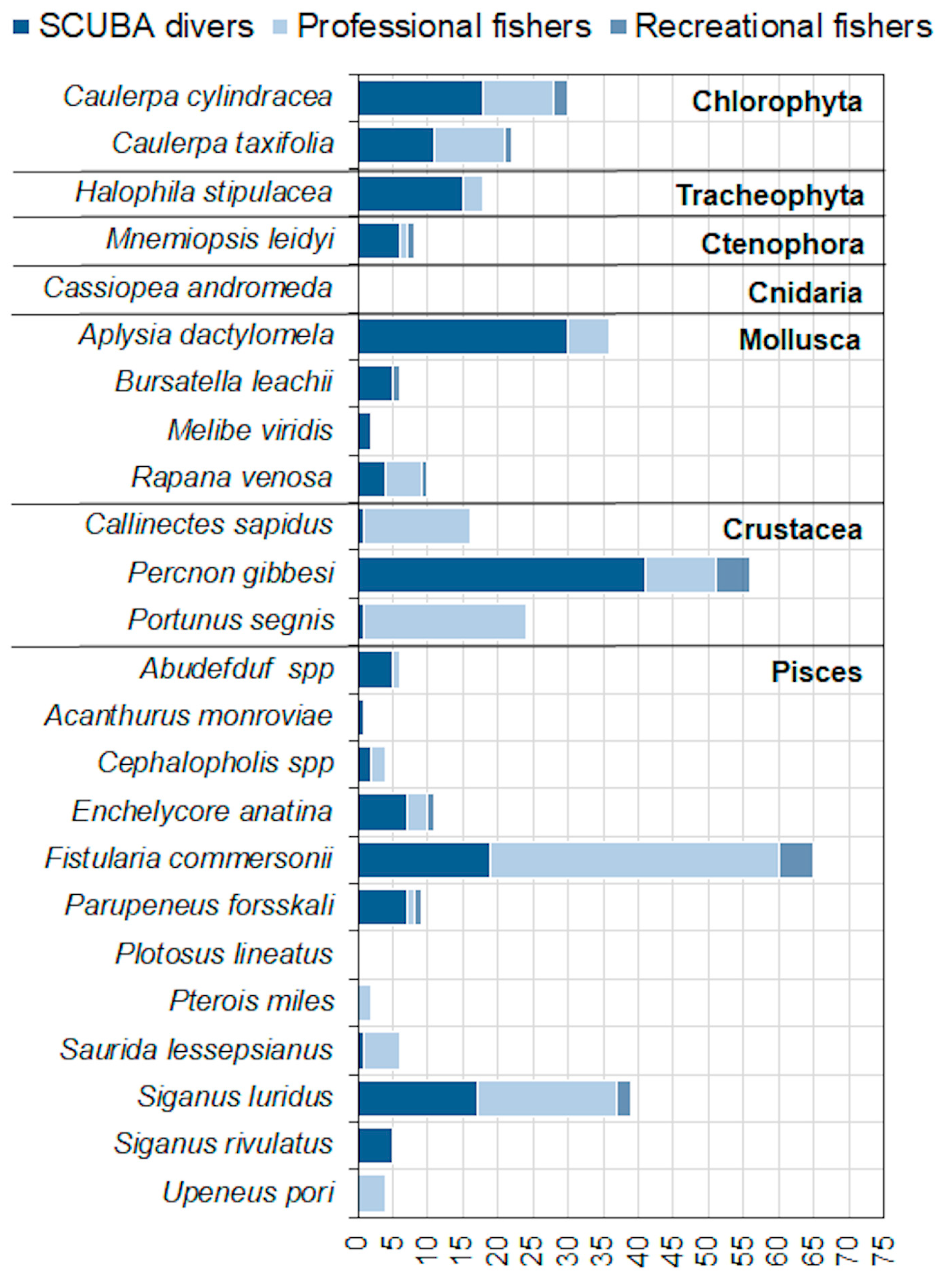
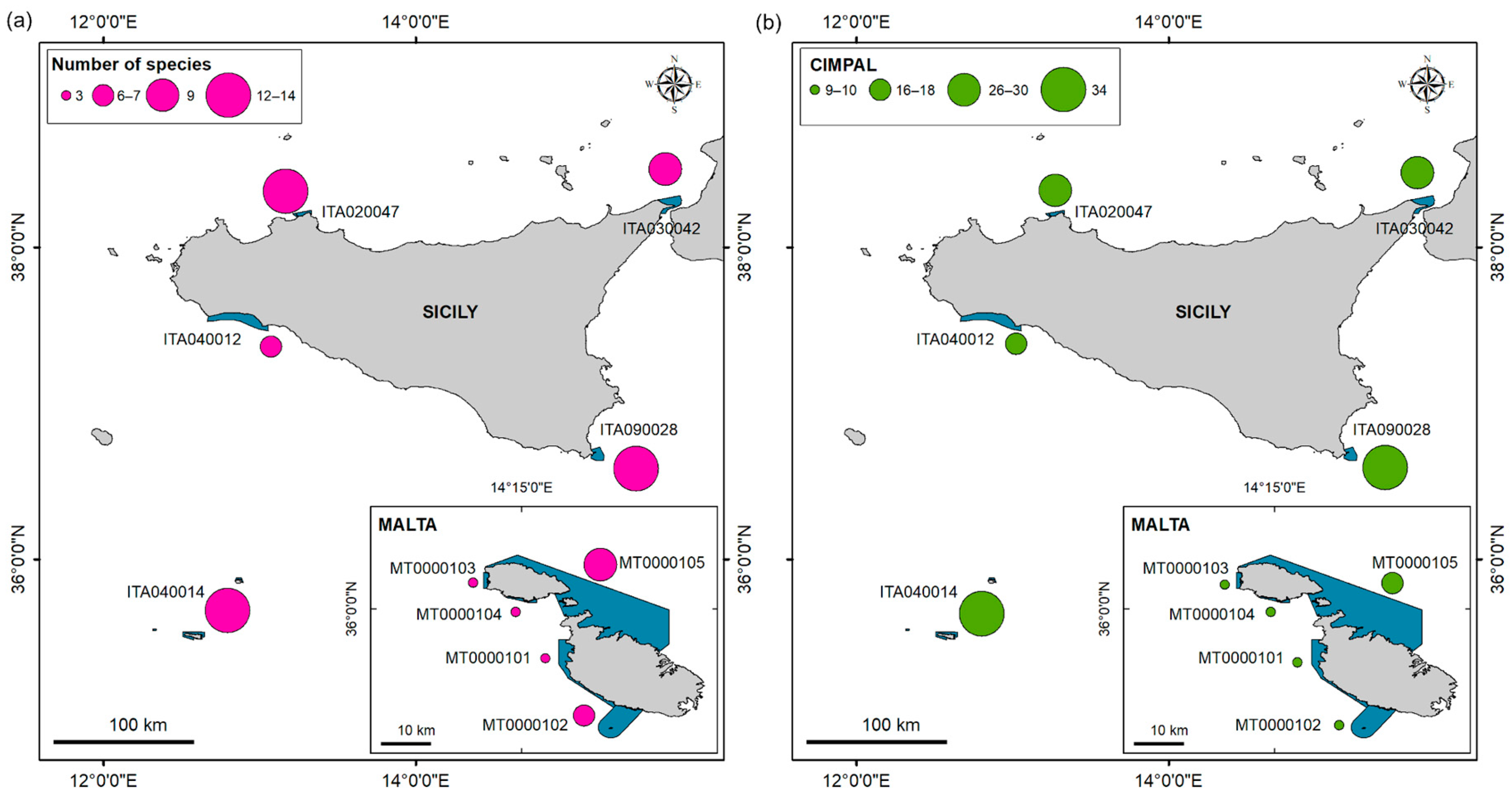
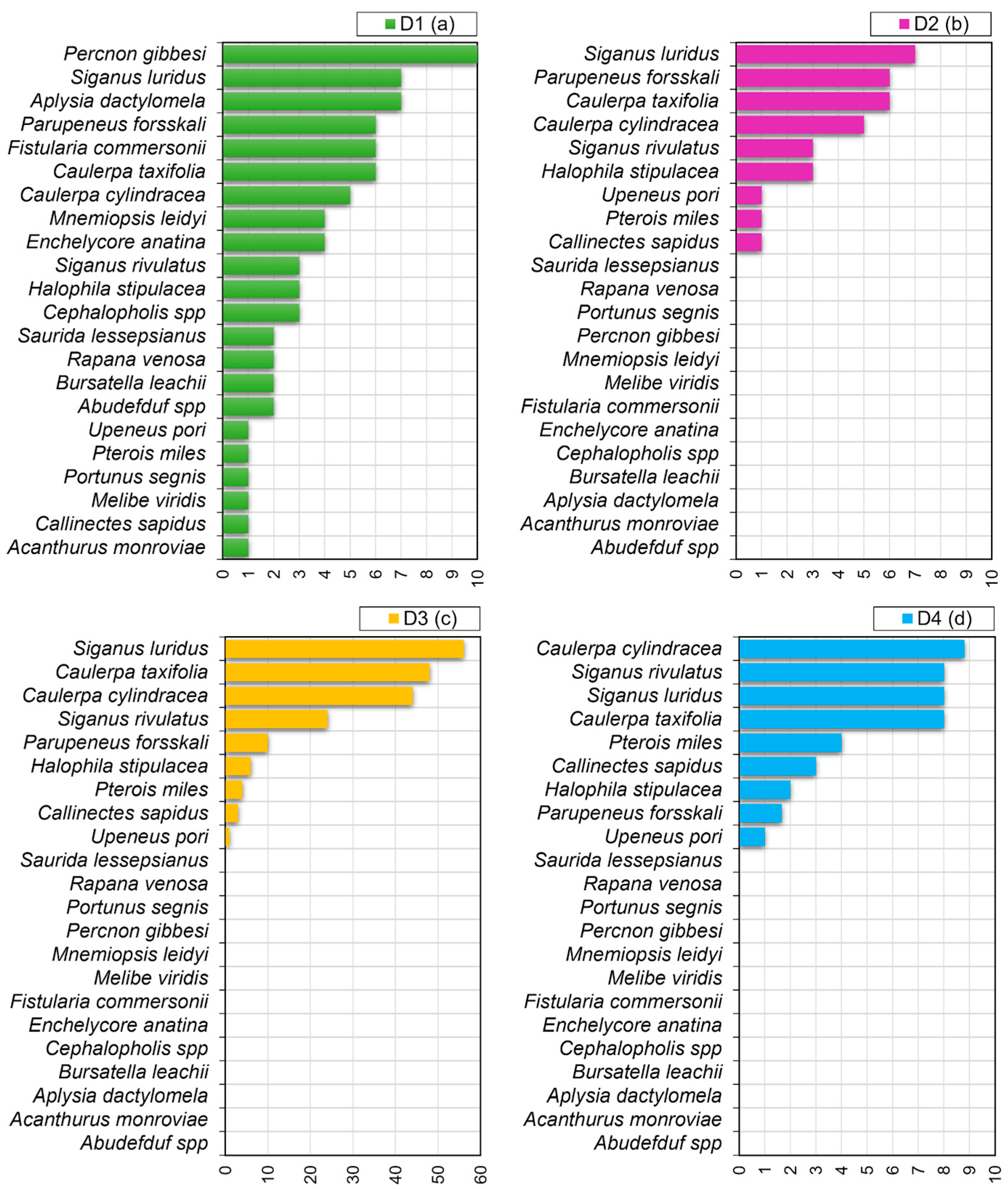
| Country (MSFD Subregion) | Site Type | Site Code | Name | Marine Surface Area (ha) | 1110 Presence | 1120 * Presence | 1150 * Presence | 1170 Presence | 8330 Presence |
|---|---|---|---|---|---|---|---|---|---|
| Italy (IT MWE) | pSCI (MPA) | ITA020047 (EUAP0555) | Fondali di Isola delle Femmine—Capo Gallo | 2155 | x | x | x | x | |
| Italy (IT MWE, IT MIC) | SPA | ITA030042 | Monti Peloritani, Dorsale Curcuraci, Antennamare e area marina dello Stretto di Messina | 8117 | x | x | x | x | |
| Italy (IT MIC) | SAC | ITA040012 | Fondali di Capo San Marco—Sciacca | 18,330 | x | x | x | ||
| Italy (IT MIC) | SAC (MPA) | ITA040014 (EUAP0553) | Fondali delle Isole Pelagie | 4085 | x | x | x | x | |
| Italy (IT MIC) | SAC | ITA090028 | Fondali dell’Isola di Capo Passero | 5367 | x | x | x | x | |
| Malta (MT MIC) | SAC (MPA) | MT0000101 | Żona fil-Baħar Bejn Rdum Majjiesa u Ras ir-Raheb | 1459 | x | x | x | x | |
| Malta (MT MIC) | SAC (MPA) | MT0000102 | Żona fil-Baħar fl-Inħawi ta’ Għar Lapsi u ta’ Filfla | 2629 | x | x | |||
| Malta (MT MIC) | SAC (MPA) | MT0000103 | Żona fil-Baħar fl-Inħawi tad-Dwejra (Għawdex) | 229 | x | x | x | ||
| Malta (MT MIC) | SAC (MPA) | MT0000104 | Żona fil-Baħar fl-Inħawi ta’ Mġarr ix-Xini (Għawdex) | 169 | x | x | x | x | |
| Malta (MT MIC) | SAC (MPA) | MT0000105 | Żona fil-Baħar fil-Grigal ta’ Malta | 15,880 | x | x | x | x |
| Species | IT MWE | IT MIC | MT MIC | |
|---|---|---|---|---|
| Chlorophyta | Caulerpa cylindracea [37,38,39] | 1993 | 1993 | 1999 |
| Caulerpa taxifolia [40,41,42] | 1992 | 1993 | 2013 | |
| Tracheophyta | Halophila stipulacea [43,44,45] | 1995 | 1988 | 1970 |
| Ctenophora | Mnemiopsis leidyi [46] | 2009 | 2009 | – |
| Cnidaria | Cassiopea andromeda [47,48,49] | 2014 | 2014 | 2009 |
| Mollusca | Aplysia dactylomela [50,51,52] | 2009 | 2002 | 2008 |
| Bursatella leachii [53,54,55] | 1969 | 1968 | 1969 | |
| Melibe viridis [56,57,58] | 2007 | 1991 | 2008 | |
| Rapana venosa [59,60] | 1978 | before 1988 | – | |
| Crustacea | Callinectes sapidus [61,62] | 1964 | 1999 | – |
| Percnon gibbesi [63,64,65] | 2000 | 1999 | 2001 | |
| Portunus segnis [66,67,68] | 2004 | 1966 | 1972 | |
| Pisces | Abudefduf spp. [69,70] | 1957 | – | 2013 |
| Acanthurus monroviae [71] | – | – | 2013 | |
| Cephalopholis spp. [72,73] | – | 2009 | 2008 | |
| Enchelycore anatina [74,75] | – | 2011 | 2013 | |
| Fistularia commersonii [76,77,78] | 2003 | 2002 | 2005 | |
| Parupeneus forsskali [79] | – | – | maybe 1979 | |
| Plotosus lineatus | – | – | – | |
| Pterois miles [80] | – | 2016 | – | |
| Saurida lessepsianus [81] | – | 1978 | – | |
| Siganus luridus [82,83] | 2004 | 2003 | 1990 | |
| Siganus rivulatus [84] | – | 2015 | – | |
| Upeneus pori [85] | – | 2012 | – |
| Key Steps | Strategy |
|---|---|
| Citizen engagement | The respondents were recruited through trusted intermediaries, such as directors of fishing associations and MPA operators, after being informed of the ongoing activities carried out by researchers and the importance of their involvement in such activities. In particular, intermediaries were required to involve a diversified array of fishers in terms of the gear used, in order to obtain more exhaustive information. |
| Local knowledge of marine organisms and environment | Intermediaries were asked to involve mainly citizens with marine-related jobs (e.g., fishers, SCUBA divers), having a certain degree of marine experience. |
| Citizen skill and experience | The questions asked to the participating citizens were straightforward and suited to their competences. The skill level (beginner, basic, or advanced experience) was deducted from the declared experience in terms of years of activity at sea. |
| Identification and description of the species | In order to facilitate the species identification, a poster with 24 photos of selected non-native species was shown (Supplementary File), also specifying their distinctive characters from similar species. |
| Description of the observation | The respondents were invited to provide, whenever possible, supplementary information on the habitat, depth, distance from the coast, etc., of the site where the species was observed. |
| Location of the sighting | Detailed maps of the study area were shown, in order to properly locate the sightings. |
| Observation documentation | In order to validate the sightings, respondents were invited to provide any photographic/video material of the species sighted, as well as of other organisms they considered interesting to report. |
| Non-Native Species | 1110 | 1120 * | 1150 * | 1170 | 8330 | |
|---|---|---|---|---|---|---|
| Chlorophyta | Caulerpa cylindracea | 4 | 0 | 4 | 4 | – |
| Caulerpa taxifolia | 4 | 0 | – | 4 | – | |
| Tracheophyta | Halophila stipulacea | 2 | 0 | 2 | – | – |
| Ctenophora | Mnemiopsis leidyi | – | – | 2 | – | – |
| Mollusca | Aplysia dactylomela | – | 0 | 0 | 0 | – |
| Bursatella leachii | 0 | 0 | 0 | 0 | – | |
| Melibe viridis | 0 | – | 0 | – | – | |
| Rapana venosa | 0 | – | 1 | 0 | – | |
| Crustacea | Callinectes sapidus | 1 | 1 | 1 | 1 | – |
| Percnon gibbesi | – | – | – | 0 | – | |
| Portunus segnis | 0 | 0 | – | 0 | – | |
| Pisces | Abudefduf spp. | – | 0 | – | 0 | – |
| Acanthurus monroviae | – | – | 0 | 0 | – | |
| Cephalopholis spp. | 0 | – | – | 0 | 0 | |
| Enchelycore anatina | – | – | – | 0 | 0 | |
| Fistularia commersonii | 0 | 0 | – | 0 | – | |
| Parupeneus forsskali | 1 | – | – | 1 | – | |
| Pterois miles | – | – | – | 4 | – | |
| Saurida lessepsianus | 0 | – | – | – | – | |
| Siganus luridus | – | 4 | – | 4 | – | |
| Siganus rivulatus | – | 4 | – | 4 | – | |
| Upeneus pori | 1 | – | – | – | – |
| Non-Native Species | IT MWE | IT MIC | MT MIC | IT MWE | IT MIC | MT MIC | |
|---|---|---|---|---|---|---|---|
| Already Known | New | ||||||
| Chlorophyta | * Caulerpa cylindracea | 2000 (4) | 2008 (25) | 2019 (1) | – | – | – |
| * Caulerpa taxifolia | 2009 (3) | 2000 (18) | 2019 (1) | – | – | – | |
| Tracheophyta | * Halophila stipulacea | 2009 (7) | 2015 (9) | 2019 (2) | – | – | – |
| Ctenophora | * Mnemiopsis leidyi | 2012 (2) | 2013 (4) | n.r. | – | – | 2018 (2) |
| Mollusca | Aplysia dactylomela | 2000 (8) | 2000 (20) | 2016 (8) | – | – | – |
| Bursatella leachii | 2015 (2) | 2009 (3) | 2018 (1) | – | – | – | |
| * Melibe viridis | n.r. | 2017 (2) | n.r. | – | – | – | |
| * Rapana venosa | 2015 (6) | ≤ 2019 (3) | n.r. | – | – | ≤2019 (1) | |
| Crustacea | * Callinectes sapidus | n.r. | 2008 (14) | n.r. | – | – | 2019 (2) |
| Percnon gibbesi | 2008 (9) | 2000 (28) | 2004 (18) | – | – | – | |
| * Portunus segnis | 2017 (1) | 2009 (17) | 2000 (6) | – | – | – | |
| Pisces | Abudefduf spp. | ≤2019 (2) | n.r. | n.r. | – | 2016 (4) | – |
| Acanthurus monroviae | n.r. | n.r. | 2014 (1) | – | – | – | |
| Cephalopholis spp | n.r. | 2013 (3) | n.r. | ≤2019 (1) | – | – | |
| Enchelycore anatina | n.r. | 2000 (9) | 2019 (1) | 2018 (1) | – | – | |
| * Fistularia commersonii | 2005 (15) | 2003 (47) | 2018 (4) | – | – | – | |
| *Parupeneus forsskali | n.r. | n.r. | 2018 (6) | – | 2017 (2) | – | |
| *Pterois miles | n.r. | 2017 (2) | n.r. | – | – | – | |
| * Saurida lessepsianus | n.r. | 1990 (4) | n.r. | ≤2019 (2) | – | – | |
| * Siganus luridus | n.r. | 2009 (28) | 2000 (11) | – | – | – | |
| * Siganus rivulatus | n.r. | ≤2019 (3) | n.r. | 2014 (2) | – | – | |
| * Upeneus pori | n.r. | 2017 (3) | n.r. | – | – | 2018 (1) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perzia, P.; Cillari, T.; Crociata, G.; Deidun, A.; Falautano, M.; Franzitta, G.; Galdies, J.; Maggio, T.; Vivona, P.; Castriota, L. Using Local Ecological Knowledge to Search for Non-Native Species in Natura 2000 Sites in the Central Mediterranean Sea: An Approach to Identify New Arrivals and Hotspot Areas. Biology 2023, 12, 1158. https://doi.org/10.3390/biology12091158
Perzia P, Cillari T, Crociata G, Deidun A, Falautano M, Franzitta G, Galdies J, Maggio T, Vivona P, Castriota L. Using Local Ecological Knowledge to Search for Non-Native Species in Natura 2000 Sites in the Central Mediterranean Sea: An Approach to Identify New Arrivals and Hotspot Areas. Biology. 2023; 12(9):1158. https://doi.org/10.3390/biology12091158
Chicago/Turabian StylePerzia, Patrizia, Tiziana Cillari, Giuseppe Crociata, Alan Deidun, Manuela Falautano, Giulio Franzitta, Johann Galdies, Teresa Maggio, Pietro Vivona, and Luca Castriota. 2023. "Using Local Ecological Knowledge to Search for Non-Native Species in Natura 2000 Sites in the Central Mediterranean Sea: An Approach to Identify New Arrivals and Hotspot Areas" Biology 12, no. 9: 1158. https://doi.org/10.3390/biology12091158
APA StylePerzia, P., Cillari, T., Crociata, G., Deidun, A., Falautano, M., Franzitta, G., Galdies, J., Maggio, T., Vivona, P., & Castriota, L. (2023). Using Local Ecological Knowledge to Search for Non-Native Species in Natura 2000 Sites in the Central Mediterranean Sea: An Approach to Identify New Arrivals and Hotspot Areas. Biology, 12(9), 1158. https://doi.org/10.3390/biology12091158






