Simple Summary
Bottom trawls when fishing move over large areas with different parts of the gears physically impacting the sea bottom, including the trawling wires, doors, ground rope and net. In this way, the trawl nets remove animals from bottom waters, the sediment surface and shallow sub-surface. The animals that live in the sea bottom with their activities and lifestyle play an important role in major ecosystem processes such as nutrient cycling. In this study, we investigated the relationship between species functional characteristics and ecosystem functions under trawling pressure. Our results indicated that under trawling, more opportunistic lifestyles and deposit feeders were associated with the ecosystem processes while in the undisturbed areas these processes were connected with bioturbating and burrowing species. Finding these links helps scientists and policy makers to better predict the impact of fishing disturbance on marine environment and set appropriate thresholds for marine ecosystem impacts.
Abstract
The impact of otter trawling on the relationship between functional traits of benthic invertebrates and specific biogeochemical processes were investigated in the oligotrophic Cretan Sea. The fishery is managed through a seasonal closure during the summer. During two seasons (winter and summer) replicate samples were taken from the field from a commercial trawl ground and an adjacent control area. Environmental parameters related to sediment biogeochemistry were measured including particulate organic carbon, sedimentary organic carbon, bottom water and sedimentary chlorophyll a and phaeopigment concentrations as well as benthic oxygen consumption. A significant impact of trawling was recorded only for bottom water chlorophyll and sedimentary organic carbon. Furthermore, the links between species traits and specific ecosystem processes were affected by trawling, highlighting the importance of unique functional modalities on ecosystem functioning. The traits that mostly influenced benthic biogeochemistry in the control sites were related to bioturbation and burrowing activities. In contrast, in the trawled sites, the associated traits were related to more opportunistic lifestyles and deposit feeding species that do not act as bioturbators. Thus, under trawling disturbance, this shift can decouple the species-sediment relations and affect nutrient cycling.
1. Introduction
Grouping benthic invertebrate species according to their functional identity is a widespread approach that has led to an improved understanding of the role of these species within a community [1]. It also provides a useful tool for linking the ecosystem processes with specific functions as biological traits analysis can provide a direct link to certain functional properties related to life history, behavioral or morphological characteristics of species that drive ecosystem functioning [2]. A better understanding of the link between species functional traits and the processes related to specific ecosystem functions (i.e., nutrient cycling, oxygen consumption, denitrification) could help increase our ability to predict the impact of fishing disturbance on the benthic ecosystem functioning.
Demersal trawls have a large footprint on the seabed [3,4]. They move over large areas, with different parts of the gears physically impacting the sediment, including the trawling wires, doors, ground rope and net [5]. The actual impact on the seabed is therefore complex, partially turning over the sediment, partially reducing spatial heterogeneity and partially increasing it on different scales [6,7,8]. At the biological level, the trawl net removes animals from bottom waters, the sediment surface and shallow sub-surface. Impact studies have shown that the abundance of macrofauna and megafauna (infaunal and epifaunal) is generally reduced with corresponding changes in the community and trophic structure [6,9,10,11,12]. Smaller body-sized fauna, however, with fast life cycles may be more resistant to trawling [8,13,14].
Sediment resuspension and sediment-water nutrient exchanges may also be strongly influenced by the mechanical effects of trawling [15,16,17,18,19,20]. The effects of trawling on biogeochemistry depend on sediment characteristics, with stronger impacts occurring on muddy sediments compared to sand [17,21]. In addition, bioturbating organisms are particularly important as agents for irrigation and the movement of oxygen into the sediment [22,23]. With trawling impacting larger species in the sediment, continually impacted areas have a lower level of bioturbation and consequently different levels of sedimentary fluxes [8,12,13,18]. Trawling was also found to cause changes in oxygen regime, influencing the nitrogen cycle, as oxygen regulates both nitrification and denitrification [15,24].
To date, there are only few studies that have investigated the impact of trawling on the relationship between specific functional traits and biogeochemical processes [17,18,21,25,26,27], primarily in Northern waters (North Sea and Baltic Sea). In the upper few millimeters of the marine sediment, the oxidation of organic carbon controls the fluxes of oxygen and nutrients across the sediment-water interface, ultimately impacting primary productivity in the water column [28]. In the highly oligotrophic Eastern Mediterranean, where regeneration of nutrients from the seabed is likely to be extremely important for pelagic productivity and resultant increases in productivity may be at some distance from regeneration sites [29,30], it is essential to reveal the role of specific traits in biogeochemical processes that take place at the sediment-water interface.
Previous work in the study area has shown that macrofauna are impacted by trawling [6,31]. As a result, it is likely that the changes in the benthic community due to trawling will also have an impact on the community functional composition that regulate the biogeochemical processes in the sediment-water interface and this impact could be chronic, particularly in areas of the seabed that are regularly trawled. The fishery in Heraklion Bay off Crete is regulated through seasonal closures during the summer months when biological activity is likely to be highest. However, it is uncertain whether this closed season is sufficient to restore biological activity at the sediment-water interface. Taking these into account, we carried out a study to test the null hypothesis that trawl fishing affects the sediment biogeochemistry linkage to specific species functional traits. This hypothesis was tested by determining oxygen flux rates, chlorophyl and organic matter concentrations in bottom water and sediment, as well as species composition and functional traits in sediment cores collected inside and outside of trawled areas during both summer (closed season) and winter (open season).
2. Materials and Methods
2.1. Study Area
The study was carried out in and adjacent to one of the main commercial trawling lanes in Heraklion Bay (Island of Crete, southern Aegean, Figure 1). The area had been identified previously and work had been carried out there for some time with respect to trawling impacts on sediment characteristics and macrofaunal community structure [6,32]. The trawling lane follows the 200 m contour and narrows with the contours behind Dia Island. This natural constriction allowed for easy identification of the trawling lane and adjacent non-trawled control areas. The surface sediments of all the sampling areas were generally similar in terms of percentage composition. Sediments were predominantly silt (85–90%) with smaller fractions of clay and sand (mostly made up of shell fragments of Turritellinella tricarinata, Aporrhais serresiana and Nucula sp.). At the start of each of the sampling periods, 2 days were spent surveying with side scan sonar to verify the limits of the fishing lane (areas covered with trawl door marks). Four areas were identified for bottom sampling, two control areas to the south of the trawling lane (SOE, South Out East, 190 m depth; SOW, South Out West, 215 m depth) and two areas in the trawling lane (FLE, Fishing Lane East, 185 m depth; FLW, Fishing Lane West, 230 m depth) (Figure 1). Trawling takes place between the beginning of September and the end of May followed by an annual 4 month closed period (June–September). Based on observed trawling activity and knowledge of the gear, it was estimated that the intensity of trawling in the study area was 200% (total coverage 2 times per year). It should be noted that during all the sampling there were no trawlers in the vicinity.
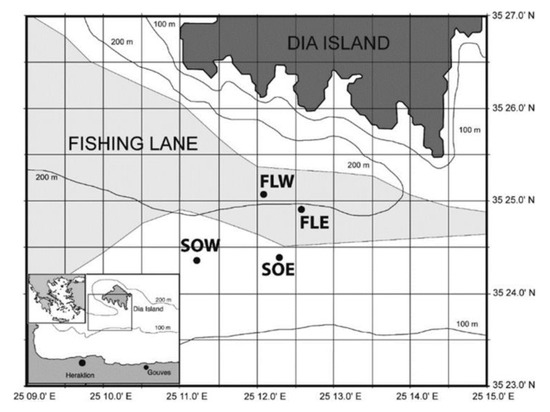
Figure 1.
Study area showing trawling lane and control sites in Heraklion Bay, Crete (FLE, fishing lane east; FLW, fishing lane west, SOE, control area east; SOW, control area west).
2.2. Field Data Collection
Field work was carried out at the sampling sites in Heraklion Bay during July 2002 (summer) and March 2003 (winter). From each of the four sampling areas at Dia Island, 6 individual multicore drops were made (Bowers & Connely, Oban, UK, core diameter 10 cm, core length 40 cm). From each drop, two cores were selected on the basis of good penetration, undisturbed sediment fabric and clear water over the surface. One of these cores was stored in a cool box for use in the oxygen flux determination and the other was processed for sedimentary chemical parameters. This core was sub-sampled with smaller perspex core tubes and sectioned for granulometry (4 cm diam., 0–5 cm depth), organic carbon and chlorophyll concentrations (2 cm diam., 0–2 cm). Summarizing the sampling, there was a total of 6 replicates for an east and a west trawled and untrawled area in summer and winter.
2.3. Physico-Chemical Analyses
Water chlorophyll and phaeopigments were determined according to the fluorometric method of Yentsch and Menzel [33] using a TURNER 112 fluorometer. The analysis of water particulate organic carbon was performed according to Parsons et al. [34] by chromic acid wet oxidation. Sedimentary organic carbon was analysed by the wet oxidation method of Walkley and Black [35]. Chlorophyllous pigments in the sediment were determined according to Strickland and Parsons [36].
2.4. Oxygen Flux Determination
The oxygen flux set-up consisted of a cold sea water recycling system (15 degrees C: stable bottom water temperature at 200 m in the area) with water flowing from a chiller unit through a cooling coil in the bottom water reservoir tank and then into the core incubation tank and back into the chiller (Figure 2). The incubated cores stood upright with the cooling water covering the major part of the core tube. Each core tube was fitted with a special head containing an electrical stirring motor, with an opening allowing overflow, sampling and insertion of an oxygen and temperature probe. The vane of each stirrer was height adjusted for each core tube and was situated near to the top of the water column such that no vortices were formed. Turning speed was approximately 1 revolution per 1.5–2 s. A 12-channel peristaltic pump (Watson-Marlow, UK) fed a constant flow (1 mL per minute) of water from the bottom water reservoir tank to the cores. When the cores were full, they overflowed into the cooling tank. Oxygen concentrations were recorded from each of the cores and the reservoir tank after 24 h of incubation. Oxygen was measured using a dissolved oxygen meter and probe (WTW Oxi-330). Oxygen flux was estimated from:
where:
Fx = (Ci − Co) · Q/A,
- Fx
- flux of nutrient x micromol m−2 h−1)
- CI
- concentration in the reservoir tank (μM)
- Co
- concentration in the Core overlying water (μM)
- Q
- flow of water through the core (l h−1)
- A
- area of the core (m2)
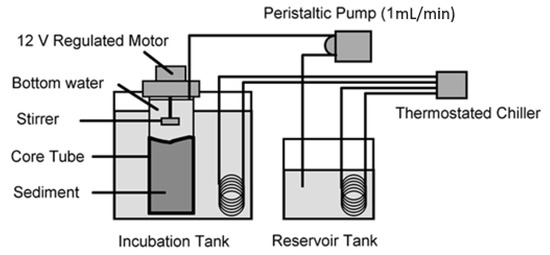
Figure 2.
Experimental set-up for oxygen flux incubation.
Mean rates were estimated from the replicates from each sampling area.
2.5. Macrofaunal Community and Biological Trait Analyses
After the end of the experiment, cores were sieved through a 0.5 mm sieve for the determination of the benthic macroinvertebrate community. The fauna was sorted for species identification, enumeration and biomass measurement. A biological trait analysis (BTA) was conducted on the macrofaunal communities to determine their bioturbation attributes [37]. The biological response and effects traits considered in this study (7 traits, 27 modalities) describe the life history, morphological and behavioral characteristics of the benthic community [38] that may influence oxygen sediment-water exchanges and organic matter degradation (Table 1). Individual taxa were coded for the modalities of each trait using a fuzzy-coding procedure, which allows assessment of the affinity of a taxon to multiple categories. The trait scores were standardized for each species by re-coding the scores as percentage frequencies (Supplementary material Table S1). The species were classified into different functional categories based on information from a variety of literature sources [22,39,40,41] and databases (www.marlin.ac.uk/biotic; www.polytraits.lifewatchgreece.eu; accessed on 28 May 2021).

Table 1.
Description of traits and trait modalities used in the biological trait analysis.
2.6. Data Analyses
To detect significant differences in environmental variables and oxygen fluxes between the samples, univariate statistical techniques were used. Specifically, three-way mixed ANOVA with trawling and season as fixed factors and site as nested factor within trawling was conducted to determine the effects of these factors on each of the variables measured (i.e., oxygen flux, organic carbon, chlorophyll a and phaeopigments). Residual analysis was performed to test for the assumptions of the three-way ANOVA. Normality was assessed using Shapiro–Wilk’s normality test and homogeneity of variances was assessed by Levene’s test. Statistical significance was accepted at the p < 0.025 level for simple two-way interactions and effect of trawling at each group of the other factors.
To assess the relationship between species traits and benthic biogeochemical processes as well as the effect of trawling on this relationship, a fourth-corner analysis was performed separately for the disturbed (trawled) and the undisturbed sites and for the two seasons [24,42]. The fourth-corner analysis requires three different data tables: the R table, with measurements of the environmental variables (i.e., oxygen, silt and clay content, chlorophyll a, and organic carbon); the L table, constituted by the biomass of each species in each sample; and the Q table, composed of fuzzy-coded trait data for each species. Prior to fourth-corner analysis, a standardization was applied to environmental data and variables were checked for collinearity. The Hellinger transformation was also applied to species community data. The fourth-corner method evaluates the significance of bivariate associations (i.e., one single trait and one single environmental variable at a time) [43]. For the later analysis, 49,999 permutations were used in all randomization procedures and the false discovery rate method (FDR) was selected to adjust p-values for multiple testing. The fourth-corner method was carried out with the ade4 package in the R program (version 4.0.2) [43,44].
3. Results
3.1. Physico-Chemical Analyses
Figure 3 shows the bottom water and sedimentary parameters (no bottom water data for the easterly sampling sites during summer sampling for technical reasons). For chlorophyll a (Chl a), there was a statistically significant two-way interaction between season and trawling (Table 2). A significant effect of trawling was recorded for the summer sampling (F = 57.1, p = 0.000, lower values in the trawled area) but not for winter (F = 2.29, p = 0.138). No significant changes were recorded for the bottom water phaeopigments (Table 2). Particulate organic carbon in the bottom water varied significantly only between seasons (F = 16.51, p = 0.003, higher in winter).
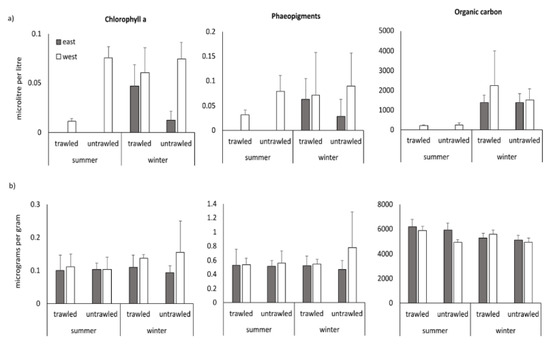
Figure 3.
(a) Bottom water and (b) sedimentary chlorophyll a, phaeopigments and organic carbon from the Heraklion Bay trawled and untrawled sampling sites with mean and standard deviation in summer and winter. Grey bars indicate the eastern sites and white bars the western sites.

Table 2.
Three-way ANOVA results for the comparisons of the bottom water and sedimentary environmental variables as well as oxygen flux for the factor trawling, season and site. Significant differences at p < 0.05 indicated with “*”, at p < 0.01 indicated with “**” and non-significant indicated with “ns”.
Sedimentary Chl values were around 0.1 microg g-1 sediment. There were no significant differences between any of the factors tested (Table 2). Sedimentary phaeopigment values ranged 0.75–1.25 microg g-1 sediment, with the exception of a peak mean value in the westerly fishing lane in winter. Trends were similar to that of Chl a and there were no significant differences between any of the factors tested. A two-way interaction between site and trawling was recorded for sedimentary organic carbon (Table 2). The simple main effect of trawling was significant for the westerly sites (F = 25.6, p = 0.000) but not for the easterly sites (F = 1.72, p = 0.197). Nevertheless, the same trend (higher organic carbon in the trawled area)—even if not statistically significant—was also found in the easterly sites (Figure 3).
Oxygen influxes were recorded in every case (Figure 4). Trawling did not significantly affect oxygen flux rates (Table 3). Significant differences were recorded for the factors site and season (Table 2).
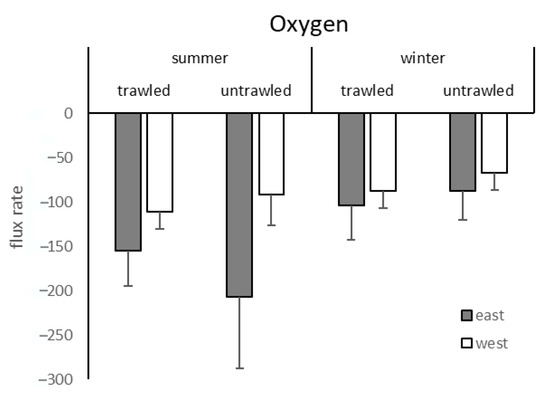
Figure 4.
Oxygen flux rates (micromole per square metre per hour) from the trawled and untrawled sampling sites with mean and standard deviation in summer and winter. Grey bars indicate the eastern sites and white bars the western sites.

Table 3.
Summary of the major functional groups related (positively: + and negatively: −) to specific biogeochemical processes in different trawling intensities and seasons. OC: organic carbon, POC: particulate organic carbon.
3.2. Link between Species Traits and Biogeochemical Processes
A total of 40 species were found in the samples after the end of the experiment. Their functional traits and abundances are shown in Supplementary material (Tables S1 and S2). The most abundant group was Polychaeta, followed by Bivalvia (mainly Abra alba), Sipuncula and Crustacea. Crustacea were not found in the cores from the trawled site nor during the trawling season (winter). The relationships between species functional traits and biogeochemical variables in different sites are summarized in Figure 5.
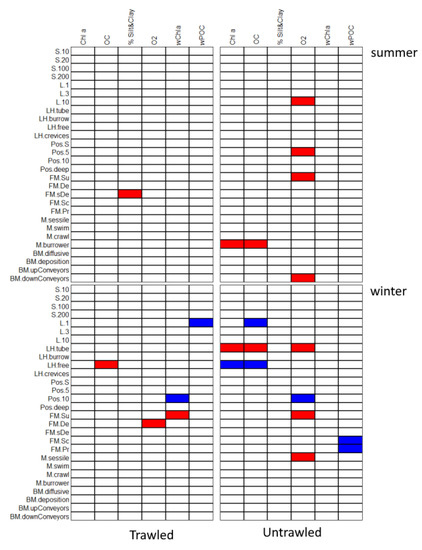
Figure 5.
Representation of significant (p < 0.05) associations identified by the fourth-corner method. The blue shades indicate significant positive associations and red shades significant negative associations between traits and biogeochemical variables. Variables with no significant associations are shown in white. p values were adjusted for multiple comparisons using the FDR procedure. Codes for traits are explained in Table 1. Chla: chlorophyll a, OC: organic carbon, %Silt&Clay: silt and clay percentage, O2: oxygen, wChla: water column chlorophyll, wPOC: particulate organic carbon.
The significant associations (negative and positive) of species traits and biogeochemical variables describing either the bottom water or the sediment, were different in trawled and untrawled sites. More associations were recorded in the untrawled sites. The traits that are linked to biogeochemical processes in trawled sites versus control sites in both seasons are summarized in Table 3.
In the untrawled sites, biogeochemical processes are influenced by the more complex functional traits community consisting of free-living scavengers and predators, by sessile suspension feeders and also by burrowing species with high longevity that are oriented with their heads towards the sediment surface. These species transport sediment from the surface to deeper layers as they feed. In contrast, in the trawled sites biogeochemical processes were only related to short-lived species with high mobility either deposit feeders or suspension feeders.
4. Discussion
This study explored how trawling affects the linkage between functional traits of benthic macrofaunal species and specific biogeochemical processes such as oxygen consumption and organic matter degradation. In general, significant differences were detected only for bottom water Chl a and sediment OC concentrations between the trawled and control areas indicating that there was an impact of bottom fishing on benthic biogeochemistry. Nevertheless, oxygen consumption was not affected. In addition, the traits that are linked to specific ecosystem processes were different between trawled and undisturbed sites, highlighting the importance of unique functional traits in preserving ecosystem functioning.
The sediment and the adjacent bottom water environmental parameters could be directly affected by trawling in two ways, either removal by resuspension or constant exposure at the sediment surface by uncovering deeper sedimentary-locked carbon with continuous trawling, but also indirectly through species loss which can cause changes in ecosystem functioning [17,18,25,45,46]. As a result, differences in bottom water Chl a concentration and sedimentary organic carbon at the different sites can be explained by the aforementioned processes. Pusceddu et al. [47] in the northwestern Aegean, found that sedimentary organic carbon concentrations displayed a significant increase immediately after the initiation of the trawling season, probably from trawling-induced uplift from deeper sediment layers, but no significant short-term changes in sedimentary pigment levels. In accordance, Sciberras et al. [17] have linked bottom trawling to increased sediment Chl a and organic carbon and attributed this enhancement to a considerable reduction in bacterial biomass due to sediment resuspension that leads to a slow-down in the remineralization of the labile portion of organic matter within the sediment rather than to a loss in macrofaunal community bioturbation potential. Despite the findings of Sciberras et al. [17], macrofaunal species play also an important role in controlling the levels of OC within the sediment via bioturbation [48]. Thus, the difference in OC between the trawled and untrawled areas may be related to differences in macrofauna functional traits. Specifically, a decline in community complexity and bioturbation capacity can lead to a decrease in sediment oxygenation and carbon cycling and result in higher sedimentary OC concentrations [48]. On the other hand, oxygen consumption remains unaffected by trawling in our experiment which is in accordance with other studies suggesting that oxygen consumption is either unaffected [15,17] or decreased [18,19,21] by trawling due to the removal of the reactive surface sediment and the consequent reduction in carbon mineralization.
Besides the changes in environmental parameters, trawl fishing causes changes in species composition either through the direct removal of animals or by decreasing the settlement succession of species with pelagic larvae [49] from the control areas in the trawled areas and vice versa. This may result in alterations both in the functional effects and response traits of a community, that in turn may have broad implications for the overall ecosystem functioning [50]. In our study, trawling affected the links between species traits and biogeochemistry. Specifically, the untrawled sites presented more variable associations between specific traits and biogeochemical processes than the trawled sites. This indicated that in the undisturbed area, biogeochemistry was mainly controlled by macrofaunal community. In contrast, trawled sites appeared to have less associations between macrofaunal traits and biogeochemical variables. This could be explained either by lower functional redundancy within the disturbed sites due to fewer species overall or by the stimulation of microbial activity against the macrofaunal-induced metabolism in the disturbed sites [18,24,51]. It is also worth noting that more associations between species functional traits and biochemical processes were recorded in winter than in summer. It was anticipated that higher temperatures in summer would have favored macrofaunal activity [52] and as a result, an increased number of significant associations between functional traits and specific biogeochemical processes. Nevertheless, there is not always a straightforward relation between single species and biogeochemical cycling processes but rather complex interactions of species competition for space and food and habitat characteristics since macrofaunal activities have different effects across different environments [53].
In the undisturbed sites, many traits were found to be related to benthic biogeochemistry indicating a balanced and diverse community. Bioturbation, burrowing activities and feeding mode are some of the traits influencing sediment geochemistry. Specifically, bioturbation and burrowing affect nutrient fluxes across the sediment water interface, organic carbon concentrations, chlorophyll burial and decomposition, oxygen consumption and mediation of nitrogen [22,24,54,55,56,57,58]. In addition, sessile species that live in tubes influence the stability and accumulation rates of sediment, which are key drivers of organic carbon burial and storage [28,59]. Burrowing fauna can increase the stability and accumulation rate of sediment if there is an increase in biogenic materials such as tubes or mucus production [60].
In contrast to the undisturbed sites, long-lived bioturbators, sessile and burrowing fauna that are particularly vulnerable to damage from mobile demersal fishing, in the trawled sites showed reductions due to the resuspension and the following deposition of the surface sediments caused by trawling [8,18,61]. Subsequently, small opportunistic deposit feeders with low bioturbation ability may take advantage of the aforementioned reduction and appeared to be linked with the biogeochemical parameters in the trawled sites. Thus, under trawling disturbance, the shift towards opportunistic lifestyles can decouple the macrofauna-sediment relations that facilitate nutrient cycling and lead to a microbially driven metabolism [17,51,60].
5. Conclusions
Trawling had an impact on the links between macrofaunal functional traits and biogeochemical processes. Nevertheless, this impact was not adequately reflected in all the biogeochemical variables studied. As a result, in the trawled sites, either there was an uncoupling of species-sediment relations towards a microbially induced metabolism or the benthic community, and specifically ephemeral deposit feeders, took advantage of the organic matter availability due to sediment resuspension and preserved benthic biogeochemistry. The differences recorded in the associations of species functional traits and biogeochemical processes in both trawled and untrawled sites underlined the importance of unique functional traits on ecosystem functioning. In addition, in such an experimental design, quantitative effects of trawling are likely to be underestimated due to reduced suitable settling area for species also within the undisturbed area due to its spatial vicinity to the trawled sites.
Finding the links and relating species functional traits to important specific ecosystem processes will help scientists and policymakers to better predict and communicate the impact of fishing disturbance on benthic ecosystem functioning and set appropriate thresholds for adverse effects. Exceeding these thresholds should trigger management response actions. To this end, successful management measures could include confining the trawling footprint within historically trawled areas and/or allocating a longer closed period for trawling to restore the area [62].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biology11101378/s1, Table S1: Species trait scores, Table S2: Species abundance per sampling core, Table S3: ANOVA results.
Author Contributions
I.T.: Conceptualization, Methodology, Formal analysis, Investigation, Writing—Original draft, Visualization. C.J.S.: Methodology, Formal analysis, Investigation, Resources, Visualization, Supervision, Funding acquisition, Writing—review and editing. K.N.P.: Investigation, Methodology, Formal analysis, Writing—review and editing, Funding acquisition. M.C.A.: Investigation, Writing—review and editing, Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
The work was carried out under the EU-funded project Cost-Impact: Costing the impact of demersal fishing on marine ecosystem processes and biodiversity. FP5, Quality of Life: Q5RS-2001-00993. Co-financing was provided by the General Secretariat for Research and Technology, Ministry of Development, Greece. The authors acknowledge the support by the MarBEF Network of Excellence ‘Marine Biodiversity and Ecosystem Functioning’ which is funded by the Sustainable Development, Global Change and Ecosystems Programme of the European Community’s Sixth Framework Programme (contract no. GOCE-CT-2003-505446).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data on the benthic community can be found in the Supplementary Materials.
Acknowledgments
The authors would like to thank their colleagues at HCMR for help with sampling, construction of the incubation equipment and analyses, the Captain and crew of the RV Philia, and the partners of the Cost-Impact project for their inputs into the work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bolam, S.G.; Garcia, C.; Eggleton, J.; Kenny, A.J.; Buhl-Mortensen, L.; Gonzalez-Mirelis, G.; van Kooten, T.; Dinesen, G.; Hansen, J.; Hiddink, J.G.; et al. Differences in Biological Traits Composition of Benthic Assemblages between Unimpacted Habitats. Mar. Environ. Res. 2017, 126, 1–13. [Google Scholar] [CrossRef]
- Bremner, J. Species’ Traits and Ecological Functioning in Marine Conservation and Management. J. Exp. Mar. Biol. Ecol. 2008, 366, 37–47. [Google Scholar] [CrossRef]
- Amoroso, R.O.; Pitcher, C.R.; Rijnsdorp, A.D.; McConnaughey, R.A.; Parma, A.M.; Suuronen, P.; Eigaard, O.R.; Bastardie, F.; Hintzen, N.T.; Althaus, F.; et al. Bottom Trawl Fishing Footprints on the World’s Continental Shelves. Proc. Natl. Acad. Sci. USA 2018, 115, E10275–E10282. [Google Scholar] [CrossRef] [PubMed]
- Eigaard, O.R.; Bastardie, F.; Hintzen, N.T.; Buhl-Mortensen, L.; Buhl-Mortensen, P.; Catarino, R.; Dinesen, G.E.; Egekvist, J.; Fock, H.O.; Geitner, K.; et al. The Footprint of Bottom Trawling in European Waters: Distribution, Intensity, and Seabed Integrity. ICES J. Mar. Sci. 2017, 74, 847–865. [Google Scholar] [CrossRef]
- Eigaard, O.R.; Bastardie, F.; Breen, M.; Dinesen, G.E.; Hintzen, N.T.; Laffargue, P.; Mortensen, L.O.; Nielsen, J.R.; Nilsson, H.C.; O’Neill, F.G.; et al. Estimating Seabed Pressure from Demersal Trawls, Seines, and Dredges Based on Gear Design and Dimensions. ICES J. Mar. Sci. J. Cons. 2016, 73, i27–i43. [Google Scholar] [CrossRef]
- Smith, C.J.; Papadopoulou, K.N.; Diliberto, S. Impact of Otter Trawling on an Eastern Mediterranean Commercial Trawl Fishing Ground. ICES J. Mar. Sci. 2000, 57, 1340–1351. [Google Scholar] [CrossRef]
- Hinz, H.; Prieto, V.; Kaiser, M.J. Trawl Disturbance on Benthic Communities: Chronic Effects and Experimental Predications. Ecol. Appl. 2009, 19, 761–773. [Google Scholar] [CrossRef]
- Tillin, H.M.; Hiddink, J.G.; Jennings, S.; Kaiser, M.J. Chronic Bottom Trawling Alters the Functional Composition of Benthic Invertebrate Communities on a Sea-Basin Scale. Mar. Ecol. Prog. Ser. 2006, 318, 31–45. [Google Scholar] [CrossRef]
- Jennings, S.; Kaiser, M.J. The Effects of Fishing on Marine Ecosystems. In Advances in Marine Biology; Blaxter, J.H.S., Southward, A.J., Tyler, P.A., Eds.; Academic Press: London, UK, 1998; Volume 34, pp. 1–475. [Google Scholar]
- Kaiser, M.J.; Clarke, K.R.; Hinz, H.; Austen, M.C.V.; Somerfield, P.J.; Karakassis, I. Global Analysis of Response and Recovery of Benthic Biota to Fishing. Mar. Ecol. Prog. Ser. 2006, 311, 1–14. [Google Scholar] [CrossRef]
- Queirós, A.M.; Hiddink, J.G.; Kaiser, M.J.; Hinz, H. Effects of Chronic Bottom Trawling Disturbance on Benthic Biomass, Production and Size Spectra in Different Habitats. J. Exp. Mar. Biol. Ecol. 2006, 335, 91–103. [Google Scholar] [CrossRef]
- de Juan, S.; Thrush, S.F.; Demestre, M. Functional Changes as Indicators of Trawling Disturbance on a Benthic Community Located in a Fishing Ground (NW Mediterranean Sea). Mar. Ecol. Prog. Ser. 2007, 334, 117–129. [Google Scholar] [CrossRef]
- Olsgard, F.; Schaanning, M.T.; Widdicombe, S.; Kendall, M.A.; Austen, M.C. Effects of Bottom Trawling on Ecosystem Functioning. J. Exp. Mar. Biol. Ecol. 2008, 366, 123–133. [Google Scholar] [CrossRef]
- Van Denderen, P.D.; Bolam, S.G.; Hiddink, J.G.; Jennings, S.; Kenny, A.; Rijnsdorp, A.D.; Van Kooten, T. Similar Effects of Bottom Trawling and Natural Disturbance on Composition and Function of Benthic Communities across Habitats. Mar. Ecol. Prog. Ser. 2015, 541, 31–43. [Google Scholar] [CrossRef]
- Trimmer, M.; Petersen, J.; Sivyer, D.B.; Mills, C.; Young, E.; Parker, E.R. Impact of Long-Term Benthic Trawl Disturbance on Sediment Sorting and Biogeochemistry in the Southern North Sea. Mar. Ecol. Prog. Ser. 2005, 298, 79–94. [Google Scholar] [CrossRef]
- Mayer, L.M.; Schick, D.F.; Findlay, R.H.; Rice, D.L. Effects of Commercial Dragging on Sedimentary Organic Matter. Mar. Environ. Res. 1991, 31, 249–261. [Google Scholar] [CrossRef]
- Sciberras, M.; Parker, R.; Powell, C.; Robertson, C.; Kröger, S.; Bolam, S.; Geert Hiddink, J. Impacts of Bottom Fishing on the Sediment Infaunal Community and Biogeochemistry of Cohesive and Non-Cohesive Sediments. Limnol. Oceanogr. 2016, 61, 2076–2089. [Google Scholar] [CrossRef]
- Morys, C.; Brüchert, V.; Bradshaw, C. Impacts of Bottom Trawling on Benthic Biogeochemistry in Muddy Sediments: Removal of Surface Sediment Using an Experimental Field Study. Mar. Environ. Res. 2021, 169, 105384. [Google Scholar] [CrossRef]
- Tiano, J.C.; Witbaard, R.; Bergman, M.J.N.; Van Rijswijk, P.; Tramper, A.; van Oevelen, D.; Soetaert, K. Acute Impacts of Bottom Trawl Gears on Benthic Metabolism and Nutrient Cycling. ICES J. Mar. Sci. 2019, 76, 1917–1930. [Google Scholar] [CrossRef]
- Bradshaw, C.; Jakobsson, M.; Brüchert, V.; Bonaglia, S.; Mörth, C.M.; Muchowski, J.; Stranne, C.; Sköld, M. Physical Disturbance by Bottom Trawling Suspends Particulate Matter and Alters Biogeochemical Processes on and Near the Seafloor. Front. Mar. Sci. 2021, 8, 1127. [Google Scholar] [CrossRef]
- De Borger, E.; Tiano, J.; Braeckman, U.; Rijnsdorp, A.D.; Soetaert, K. Impact of Bottom Trawling on Sediment Biogeochemistry: A Modelling Approach. Biogeosciences 2021, 18, 2539–2557. [Google Scholar] [CrossRef]
- Wrede, A.; Beermann, J.; Dannheim, J.; Gutow, L.; Brey, T. Organism Functional Traits and Ecosystem Supporting Services—A Novel Approach to Predict Bioirrigation. Ecol. Indic. 2018, 91, 737–743. [Google Scholar] [CrossRef]
- Tsikopoulou, I.; Lampa, M.; Tsiola, A.; Pitta, P.; Tsapakis, M.; Karakassis, I. Functional Adaptations of Benthic Communities to Organic Matter Enrichment at the Edge of an Allowable Zone of Effect (AZE). Estuar. Coast. Shelf Sci. 2021, 262, 107596. [Google Scholar] [CrossRef]
- Sciberras, M.; Tait, K.; Brochain, G.; Hiddink, J.G.; Hale, R.; Godbold, J.A.; Solan, M. Mediation of Nitrogen by Post-Disturbance Shelf Communities Experiencing Organic Matter Enrichment. Biogeochemistry 2017, 135, 135–153. [Google Scholar] [CrossRef]
- Pilskaln, C.H.; Churchill, J.H.; Mayer, L.M. Resuspension of Sediment by Bottom Trawling in the Gulf of Maine and Potential Geochemical Consequences. Conserv. Biol. 1998, 12, 1223–1229. [Google Scholar] [CrossRef]
- Duplisea, D.E.; Jennings, S.; Malcolm, S.J.; Parker, R.; Sivyer, D.B. Modelling Potential Impacts of Bottom Trawl Fisheries on Soft Sediment Biogeochemistry in the North Sea. Geochem. Trans. 2001, 2, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Widdicombe, S.; Austen, M.C.; Kendall, M.A.; Olsgard, F.; Schaanning, M.T.; Dashfield, S.L.; Needham, H.R. Importance of Bioturbators for Biodiversity Maintenance: Indirect Effects of Fishing Disturbance. Mar. Ecol. Prog. Ser. 2004, 275, 1–10. [Google Scholar] [CrossRef]
- LaRowe, D.E.; Arndt, S.; Bradley, J.A.; Estes, E.R.; Hoarfrost, A.; Lang, S.Q.; Lloyd, K.G.; Mahmoudi, N.; Orsi, W.D.; Shah Walter, S.R.; et al. The Fate of Organic Carbon in Marine Sediments—New Insights from Recent Data and Analysis. Earth Sci. Rev. 2020, 204, 103146. [Google Scholar] [CrossRef]
- Dounas, C.; Davies, I.; Triantafyllou, G.; Koulouri, P.; Petihakis, G.; Arvanitidis, C.; Sourlatzis, G.; Eleftheriou, A. Large-Scale Impacts of Bottom Trawling on Shelf Primary Productivity. Cont. Shelf Res. 2007, 27, 2198–2210. [Google Scholar] [CrossRef]
- Petihakis, G.; Smith, C.J.; Triantafyllou, G.; Sourlantzis, G.; Papadopoulou, K.-N.; Pollani, A.; Korres, G. Scenario Testing of Fisheries Management Strategies Using a High Resolution ERSEM–POM Ecosystem Model. ICES J. Mar. Sci. 2007, 64, 1627–1640. [Google Scholar] [CrossRef]
- Tsikopoulou, I.; Smith, C.J.; Papadopoulou, N.K.; Eleftheriadou, E.; Karakassis, I. A Fishing Ground Benthic Ecosystem Improved during the Economic Crisis. ICES J. Mar. Sci. 2019, 76, 402–409. [Google Scholar] [CrossRef]
- Smith, C.J.; Rumohr, H.; Karakassis, I.; Papadopoulou, K.N. Analysing the Impact of Bottom Trawls on Sedimentary Seabeds with Sediment Profile Imagery. J. Exp. Mar. Biol. Ecol. 2003, 285–286, 479–496. [Google Scholar] [CrossRef]
- Yentsch, C.S.; Menzel, D.W. A Method for the Determination of Phytoplankton Chlorophyll and Phaeophytin by Fluorescence. Deep Sea Res. Oceanogr. Abstr. 1963, 10, 221–231. [Google Scholar] [CrossRef]
- Parsons, T.R.; Maita, Y.; Lalli, C.M. A Manual of Chemical & Biological Methods for Seawater Analysis; Pergamon Press: Oxford, UK, 1984. [Google Scholar]
- Walkley, A.; Black, I.A. An Examination of the Degtjareff Method for Determining Soil Organic Matter, and a Proposed Modification of the Chromic Acid Titration Method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Strickland, J.D.H.; Parsons, T.R. A Practical Handbook of Seawater Analysis. In Bulletin/Fisheries Research Board of Canada, 2nd ed.; Fisheries and Marine Service: Ottawa, ON, Canada, 1972; p. 167. [Google Scholar]
- Bremner, J.; Rogers, S.; Frid, C. Assessing Functional Diversity in Marine Benthic Ecosystems: A Comparison of Approaches. Mar. Ecol. Prog. Ser. 2003, 254, 11–25. [Google Scholar] [CrossRef]
- Bolam, S.G.; McIlwaine, P.; Garcia, C. Marine Macrofaunal Traits Responses to Dredged Material Disposal. Mar. Pollut. Bull. 2021, 168, 112412. [Google Scholar] [CrossRef] [PubMed]
- Jumars, P.A.; Dorgan, K.M.; Lindsay, S.M. Diet of Worms Emended: An Update of Polychaete Feeding Guilds. Ann. Rev. Mar. Sci. 2015, 7, 497–520. [Google Scholar] [CrossRef]
- Queirós, A.M.; Birchenough, S.N.R.; Bremner, J.; Godbold, J.A.; Parker, R.E.; Romero-Ramirez, A.; Reiss, H.; Solan, M.; Somerfield, P.J.; Van Colen, C.; et al. A Bioturbation Classification of European Marine Infaunal Invertebrates. Ecol. Evol. 2013, 3, 3958–3985. [Google Scholar] [CrossRef]
- Renz, J.R.; Powilleit, M.; Gogina, M.; Zettler, M.L.; Morys, C.; Forster, S. Community Bioirrigation Potential (BIPc), an Index to Quantify the Potential for Solute Exchange at the Sediment-Water Interface. Mar. Environ. Res. 2018, 141, 214–224. [Google Scholar] [CrossRef]
- Dray, S.; Legendre, P. Testing the Species Traits Environment Relationships: The Fourth-Corner Problem Revisited. Ecology 2008, 89, 3400–3412. [Google Scholar] [CrossRef]
- Dray, S.; Choler, P.; Dolédec, S.; Peres-Neto, P.R.; Thuiller, W.; Pavoine, S.; Ter Braak, C.J.F. Combining the Fourth-Corner and the RLQ Methods for Assessing Trait Responses to Environmental Variation. Ecology 2014, 95, 14–21. [Google Scholar] [CrossRef]
- Dray, S.; Dufour, A.-B.; Chessel, D. The Ade4 Package—II: Two-Table and K-Table Methods. R News 2007, 7, 47–52. [Google Scholar]
- De Madron, X.D.; Ferré, B.; Le Corre, G.; Grenz, C.; Conan, P.; Pujo-Pay, M.; Buscail, R.; Bodiot, O. Trawling-Induced Resuspension and Dispersal of Muddy Sediments and Dissolved Elements in the Gulf of Lion (NW Mediterranean). Cont. Shelf Res. 2005, 25, 2387–2409. [Google Scholar] [CrossRef]
- Paradis, S.; Pusceddu, A.; Masqué, P.; Puig, P.; Moccia, D.; Russo, T.; Lo Iacono, C. Organic Matter Contents and Degradation in a Highly Trawled Area during Fresh Particle Inputs (Gulf of Castellammare, Southwestern Mediterranean). Biogeosciences 2019, 16, 4307–4320. [Google Scholar] [CrossRef]
- Pusceddu, A.; Fiordelmondo, C.; Polymenakou, P.; Polychronaki, T.; Tselepides, A.; Danovaro, R. Effects of Bottom Trawling on the Quantity and Biochemical Composition of Organic Matter in Coastal Marine Sediments (Thermaikos Gulf, Northwestern Aegean Sea). Cont. Shelf Res. 2005, 25, 2491–2505. [Google Scholar] [CrossRef]
- Solan, M.; Scott, F.; Dulvy, N.K.; Godbold, J.A.; Parker, R. Incorporating Extinction Risk and Realistic Biodiversity Futures: Implementation of Trait-Based Extinction Scenarios. In Marine Biodiversity and Ecosystem Functioning; Oxford University Press: Oxford, UK, 2012; Volume 15, pp. 127–148. [Google Scholar]
- McLaverty, C.; Dinesen, G.; Gislason, H.; Brooks, M.; Eigaard, O. Biological Traits of Benthic Macrofauna Show Size-Based Differences in Response to Bottom Trawling Intensity. Mar. Ecol. Prog. Ser. 2021, 671, 1–19. [Google Scholar] [CrossRef]
- Hooper, D.U.; Chapin, F.S.; Ewel, J.J.; Hector, A.; Inchausti, P.; Lavorel, S.; Lawton, J.H.; Lodge, D.M.; Loreau, M.; Naeem, S.; et al. Effects of Biodiversity on Ecosystem Functioning: A Consensus of Current Knowledge. Ecol. Monogr. 2005, 75, 3–35. [Google Scholar] [CrossRef]
- Hale, R.; Godbold, J.A.; Sciberras, M.; Dwight, J.; Wood, C.; Hiddink, J.G.; Solan, M. Mediation of Macronutrients and Carbon by Post-Disturbance Shelf Sea Sediment Communities. Biogeochemistry 2017, 135, 121–133. [Google Scholar] [CrossRef]
- Braeckman, U.; Provoost, P.; Gribsholt, B.; Van Gansbeke, D.; Middelburg, J.J.; Soetaert, K.; Vincx, M.; Vanaverbeke, J. Role of Macrofauna Functional Traits and Density in Biogeochemical Fluxes and Bioturbation. Mar. Ecol. Prog. Ser. 2010, 399, 173–186. [Google Scholar] [CrossRef]
- Toussaint, E.; De Borger, E.; Braeckman, U.; De Backer, A.; Soetaert, K.; Vanaverbeke, J. Faunal and Environmental Drivers of Carbon and Nitrogen Cycling along a Permeability Gradient in Shallow North Sea Sediments. Sci. Total Environ. 2021, 767, 144994. [Google Scholar] [CrossRef]
- Birchenough, S.N.R.; Parker, R.E.; McManus, E.; Barry, J. Combining Bioturbation and Redox Metrics: Potential Tools for Assessing Seabed Function. Ecol. Indic. 2012, 12, 8–16. [Google Scholar] [CrossRef]
- Gogina, M.; Morys, C.; Forster, S.; Gräwe, U.; Friedland, R.; Zettler, M.L. Towards Benthic Ecosystem Functioning Maps: Quantifying Bioturbation Potential in the German Part of the Baltic Sea. Ecol. Indic. 2017, 73, 574–588. [Google Scholar] [CrossRef]
- Gogina, M.; Zettler, M.L.; Vanaverbeke, J.; Dannheim, J.; Van Hoey, G.; Desroy, N.; Wrede, A.; Reiss, H.; Degraer, S.; Van Lancker, V.; et al. Interregional Comparison of Benthic Ecosystem Functioning: Community Bioturbation Potential in Four Regions along the NE Atlantic Shelf. Ecol. Indic. 2020, 110, 105945. [Google Scholar] [CrossRef]
- Braeckman, U.; Foshtomi, M.Y.; Van Gansbeke, D.; Meysman, F.; Soetaert, K.; Vincx, M.; Vanaverbeke, J. Variable Importance of Macrofaunal Functional Biodiversity for Biogeochemical Cycling in Temperate Coastal Sediments. Ecosystems 2014, 17, 720–737. [Google Scholar] [CrossRef]
- Josefson, A.B.; Norkko, J.; Norkko, A. Burial and Decomposition of Plant Pigments in Surface Sediments of the Baltic Sea: Role of Oxygen and Benthic Fauna. Mar. Ecol. Prog. Ser. 2012, 455, 33–49. [Google Scholar] [CrossRef][Green Version]
- Middelburg, J.J. Reviews and Syntheses: To the Bottom of Carbon Processing at the Seafloor. Biogeosciences 2018, 15, 413–427. [Google Scholar] [CrossRef]
- Epstein, G.; Middelburg, J.J.; Hawkins, J.P.; Norris, C.R.; Roberts, C.M. The Impact of Mobile Demersal Fishing on Carbon Storage in Seabed Sediments. Glob. Chang. Biol. 2022, 28, 2875–2894. [Google Scholar] [CrossRef]
- Rijnsdorp, A.D.; Bolam, S.G.; Garcia, C.; Hiddink, J.G.; Hintzen, N.T.; van Denderen, P.D.; van Kooten, T. Estimating Sensitivity of Seabed Habitats to Disturbance by Bottom Trawling Based on the Longevity of Benthic Fauna. Ecol. Appl. 2018, 28, 1302–1312. [Google Scholar] [CrossRef]
- McConnaughey, R.A.; Hiddink, J.G.; Jennings, S.; Pitcher, C.R.; Kaiser, M.J.; Suuronen, P.; Sciberras, M.; Rijnsdorp, A.D.; Collie, J.S.; Mazor, T.; et al. Choosing Best Practices for Managing Impacts of Trawl Fishing on Seabed Habitats and Biota. Fish Fish. 2020, 21, 319–337. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).