Multi-Modal Regulation of Circadian Physiology by Interactive Features of Biological Clocks
Simple Summary
Abstract
1. Introduction
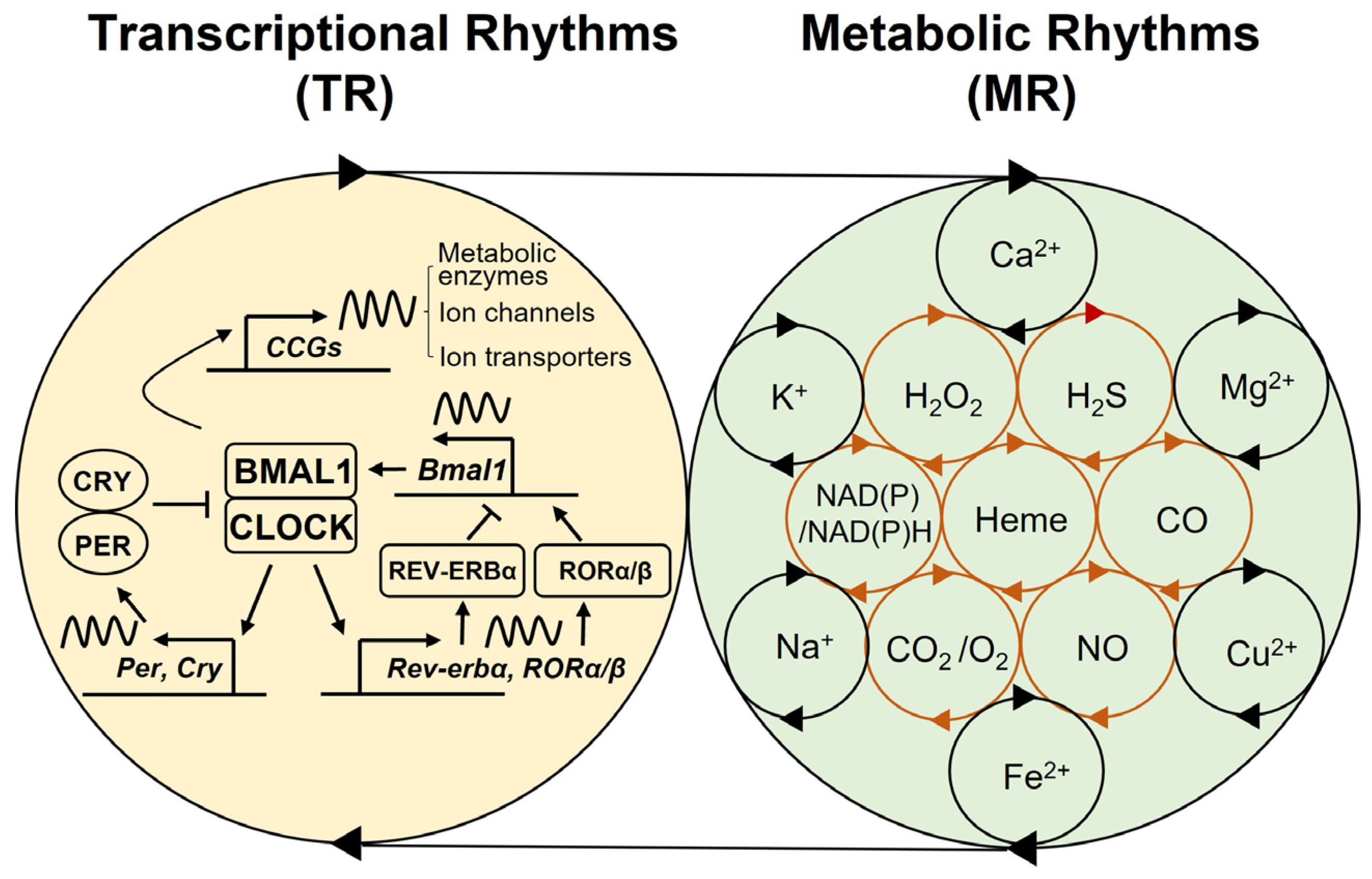
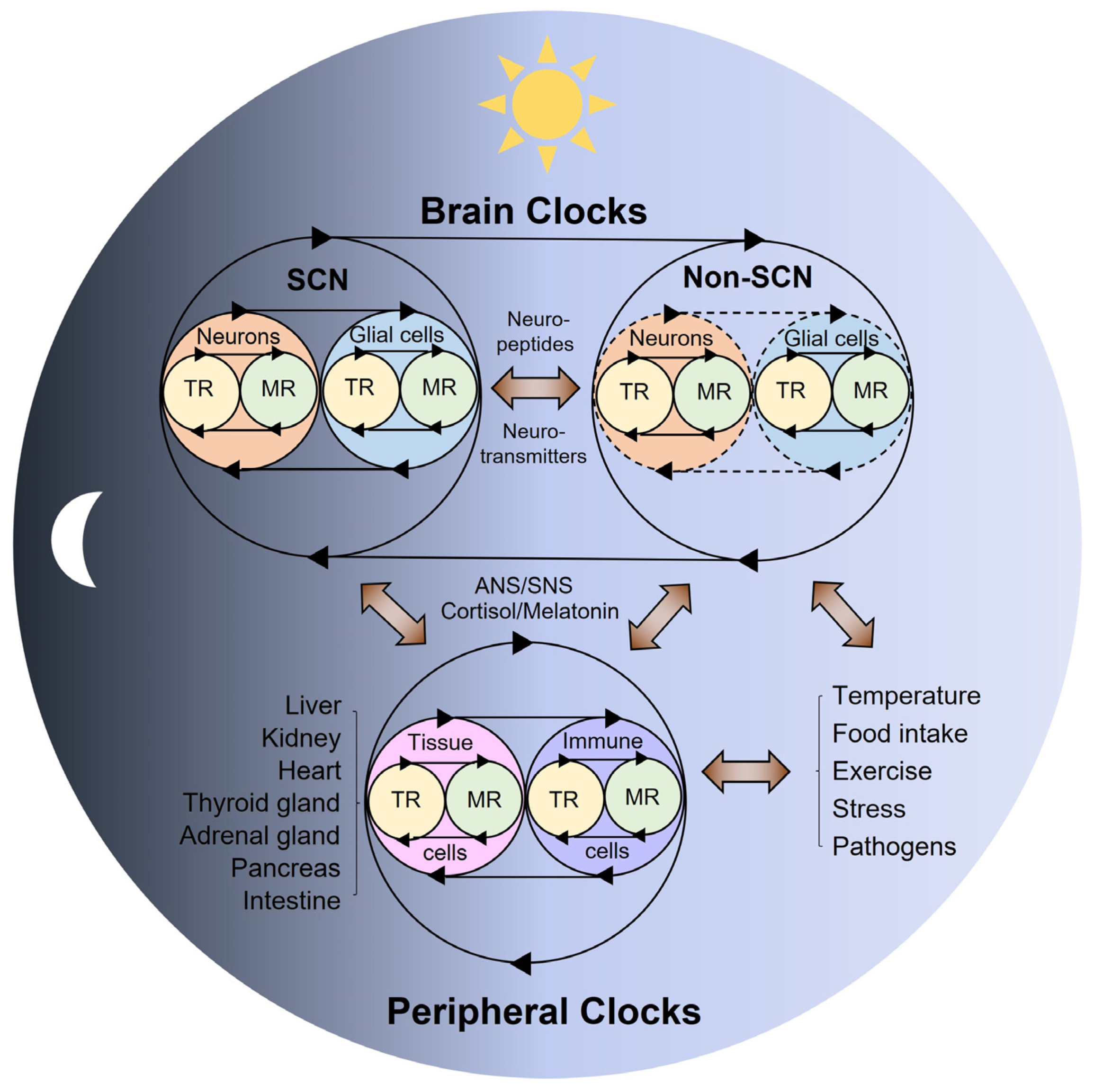
2. Multi-Modal Mechanisms of Circadian Physiology
3. The Role of Brain Clocks in Circadian Rhythms and Disorders
4. The Role of Peripheral Clocks in Circadian Rhythms and Disorders
5. The Role of Feeding in Circadian Rhythms and Disorders
6. The Role of Metabolic Cues in Circadian Rhythms and Disorders
6.1. NAD(P)/NAD(P)H
6.2. Heme
6.3. Carbon Monoxide (CO)
6.4. Nitric Oxide (NO)
6.5. Oxygen (O2)
6.6. Carbon Dioxide (CO2)
6.7. Hydrogen Peroxide (H2O2)
6.8. Hydrogen Sulfide (H2S)
6.9. Minerals and Metal Ions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dunlap, J.C. Molecular bases for circadian clocks. Cell 1999, 96, 271–290. [Google Scholar] [CrossRef]
- Bass, J.; Lazar, M.A. Circadian time signatures of fitness and disease. Science 2016, 354, 994–999. [Google Scholar] [CrossRef] [PubMed]
- Chaix, A.; Zarrinpar, A.; Panda, S. The circadian coordination of cell biology. J. Cell Biol. 2016, 215, 15–25. [Google Scholar] [CrossRef]
- Kalmbach, D.A.; Schneider, L.D.; Cheung, J.; Bertrand, S.J.; Kariharan, T.; Pack, A.I.; Gehrman, P.R. Genetic Basis of Chronotype in Humans: Insights From Three Landmark GWAS. Sleep 2017, 40, zsw048. [Google Scholar] [CrossRef]
- Refinetti, R.; Wassmer, T.; Basu, P.; Cherukalady, R.; Pandey, V.K.; Singaravel, M.; Giannetto, C.; Piccione, G. Variability of behavioral chronotypes of 16 mammalian species under controlled conditions. Physiol. Behav. 2016, 161, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Bloch, G.; Barnes, B.M.; Gerkema, M.P.; Helm, B. Animal activity around the clock with no overt circadian rhythms: Patterns, mechanisms and adaptive value. Proc. Biol. Sci. 2013, 280, 20130019. [Google Scholar] [CrossRef]
- Dominoni, D.M.; Borniger, J.C.; Nelson, R.J. Light at night, clocks and health: From humans to wild organisms. Biol. Lett. 2016, 12, 20160015. [Google Scholar] [CrossRef]
- Cederroth, C.R.; Albrecht, U.; Bass, J.; Brown, S.A.; Dyhrfjeld-Johnsen, J.; Gachon, F.; Green, C.B.; Hastings, M.H.; Helfrich-Förster, C.; Hogenesch, J.B.; et al. Medicine in the Fourth Dimension. Cell Metab. 2019, 30, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Patke, A.; Young, M.W.; Axelrod, S. Molecular mechanisms and physiological importance of circadian rhythms. Nat. Rev. Mol. Cell. Biol. 2020, 21, 67–84. [Google Scholar] [CrossRef]
- Lee, Y.; Field, J.M.; Sehgal, A. Circadian Rhythms, Disease and Chronotherapy. J. Biol. Rhythms 2021, 7487304211044301. [Google Scholar] [CrossRef]
- Logan, R.W.; McClung, C.A. Rhythms of life: Circadian disruption and brain disorders across the lifespan. Nat. Rev. Neurosci. 2019, 20, 49–65. [Google Scholar] [CrossRef]
- Scheiermann, C.; Gibbs, J.; Ince, L.; Loudon, A. Clocking in to immunity. Nat. Rev. Immunol. 2018, 18, 423–437. [Google Scholar] [CrossRef] [PubMed]
- Segers, A.; Depoortere, I. Circadian clocks in the digestive system. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Masri, S.; Sassone-Corsi, P. The emerging link between cancer, metabolism, and circadian rhythms. Nat. Med. 2018, 24, 1795–1803. [Google Scholar] [CrossRef]
- Aiello, I.; Fedele, M.L.M.; Roman, F.; Marpegan, L.; Caldart, C.; Chiesa, J.J.; Golombek, D.A.; Finkielstein, C.V.; Paladino, N. Circadian disruption promotes tumor-immune microenvironment remodeling favoring tumor cell proliferation. Sci. Adv. 2020, 6, eaaz4530. [Google Scholar] [CrossRef] [PubMed]
- Hadadi, E.; Taylor, W.; Li, X.M.; Aslan, Y.; Villote, M.; Riviere, J.; Duvallet, G.; Auriau, C.; Dulong, S.; Raymond-Letron, I.; et al. Chronic circadian disruption modulates breast cancer stemness and immune microenvironment to drive metastasis in mice. Nat. Commun. 2020, 11, 3193. [Google Scholar] [CrossRef]
- Mattis, J.; Sehgal, A. Circadian Rhythms, Sleep, and Disorders of Aging. Trends Endocrinol. Metab. 2016, 27, 192–203. [Google Scholar] [CrossRef]
- Shafi, A.A.; McNair, C.M.; McCann, J.J.; Alshalalfa, M.; Shostak, A.; Severson, T.M.; Zhu, Y.; Bergman, A.; Gordon, N.; Mandigo, A.C.; et al. The circadian cryptochrome, CRY1, is a pro-tumorigenic factor that rhythmically modulates DNA repair. Nat. Commun. 2021, 12, 401. [Google Scholar] [CrossRef]
- Lee, Y.; Lahens, N.F.; Zhang, S.; Bedont, J.; Field, J.M.; Sehgal, A. G1/S cell cycle regulators mediate effects of circadian dysregulation on tumor growth and provide targets for timed anticancer treatment. PLoS Biol. 2019, 17, e3000228. [Google Scholar] [CrossRef]
- Lee, Y.; Fong, S.Y.; Shon, J.; Zhang, S.L.; Brooks, R.; Lahens, N.F.; Chen, D.; Dang, C.V.; Field, J.M.; Sehgal, A. Time-of-day specificity of anticancer drugs may be mediated by circadian regulation of the cell cycle. Sci. Adv. 2021, 7, eabd2645. [Google Scholar] [CrossRef]
- Diallo, A.B.; Coiffard, B.; Leone, M.; Mezouar, S.; Mege, J.L. For Whom the Clock Ticks: Clinical Chronobiology for Infectious Diseases. Front. Immunol. 2020, 11, 1457. [Google Scholar] [CrossRef]
- Gabriel, B.M.; Zierath, J.R. Circadian rhythms and exercise—Re-setting the clock in metabolic disease. Nat. Rev. Endocrinol. 2019, 15, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Lewis, P.; Oster, H.; Korf, H.W.; Foster, R.G.; Erren, T.C. Food as a circadian time cue—Evidence from human studies. Nat. Rev. Endocrinol. 2020, 16, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.H.; Zhao, C.; Xu, Y.; Mori, T. Timing the day: What makes bacterial clocks tick? Nat. Rev. Microbiol. 2017, 15, 232–242. [Google Scholar] [CrossRef]
- Takahashi, J.S. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 2017, 18, 164–179. [Google Scholar] [CrossRef] [PubMed]
- Koronowski, K.B.; Sassone-Corsi, P. Communicating clocks shape circadian homeostasis. Science 2021, 371, eabd0951. [Google Scholar] [CrossRef]
- Anafi, R.C.; Lee, Y.; Sato, T.K.; Venkataraman, A.; Ramanathan, C.; Kavakli, I.H.; Hughes, M.E.; Baggs, J.E.; Growe, J.; Liu, A.C.; et al. Machine learning helps identify CHRONO as a circadian clock component. PLoS Biol. 2014, 12, e1001840. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, J.; Kwon, I.; Nakajima, Y.; Ohmiya, Y.; Son, G.H.; Lee, K.H.; Kim, K. Coactivation of the CLOCK-BMAL1 complex by CBP mediates resetting of the circadian clock. J. Cell Sci. 2010, 123, 3547–3557. [Google Scholar] [CrossRef]
- Lee, J.; Lee, Y.; Lee, M.J.; Park, E.; Kang, S.H.; Chung, C.H.; Lee, K.H.; Kim, K. Dual modification of BMAL1 by SUMO2/3 and ubiquitin promotes circadian activation of the CLOCK/BMAL1 complex. Mol. Cell. Biol. 2008, 28, 6056–6065. [Google Scholar] [CrossRef]
- Lee, Y.; Shen, Y.; Francey, L.J.; Ramanathan, C.; Sehgal, A.; Liu, A.C.; Hogenesch, J.B. The NRON complex controls circadian clock function through regulated PER and CRY nuclear translocation. Sci. Rep. 2019, 9, 11883. [Google Scholar] [CrossRef]
- Lee, Y.; Jang, A.R.; Francey, L.J.; Sehgal, A.; Hogenesch, J.B. KPNB1 mediates PER/CRY nuclear translocation and circadian clock function. eLife 2015, 4, e08647. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Chun, S.K.; Kim, K. Sumoylation controls CLOCK-BMAL1-mediated clock resetting via CBP recruitment in nuclear transcriptional foci. Biochim. Biophys. Acta 2015, 1853, 2697–2708. [Google Scholar] [CrossRef]
- Korge, S.; Maier, B.; Bruning, F.; Ehrhardt, L.; Korte, T.; Mann, M.; Herrmann, A.; Robles, M.S.; Kramer, A. The non-classical nuclear import carrier Transportin 1 modulates circadian rhythms through its effect on PER1 nuclear localization. PLoS Genet. 2018, 14, e1007189. [Google Scholar] [CrossRef]
- Zhang, R.; Lahens, N.F.; Ballance, H.I.; Hughes, M.E.; Hogenesch, J.B. A circadian gene expression atlas in mammals: Implications for biology and medicine. Proc. Natl. Acad. Sci. USA 2014, 111, 16219–16224. [Google Scholar] [CrossRef]
- Mure, L.S.; Le, H.D.; Benegiamo, G.; Chang, M.W.; Rios, L.; Jillani, N.; Ngotho, M.; Kariuki, T.; Dkhissi-Benyahya, O.; Cooper, H.M.; et al. Diurnal transcriptome atlas of a primate across major neural and peripheral tissues. Science 2018, 359, eaao0318. [Google Scholar] [CrossRef] [PubMed]
- Ruben, M.D.; Wu, G.; Smith, D.F.; Schmidt, R.E.; Francey, L.J.; Lee, Y.Y.; Anafi, R.C.; Hogenesch, J.B. A database of tissue-specific rhythmically expressed human genes has potential applications in circadian medicine. Sci. Transl. Med. 2018, 10, eaat8806. [Google Scholar] [CrossRef] [PubMed]
- Thaiss, C.A.; Zeevi, D.; Levy, M.; Zilberman-Schapira, G.; Suez, J.; Tengeler, A.C.; Abramson, L.; Katz, M.N.; Korem, T.; Zmora, N.; et al. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell 2014, 159, 514–529. [Google Scholar] [CrossRef]
- Dyar, K.A.; Lutter, D.; Artati, A.; Ceglia, N.J.; Liu, Y.; Armenta, D.; Jastroch, M.; Schneider, S.; de Mateo, S.; Cervantes, M.; et al. Atlas of Circadian Metabolism Reveals System-wide Coordination and Communication between Clocks. Cell 2018, 174, 1571–1585.e11. [Google Scholar] [CrossRef]
- Solanas, G.; Peixoto, F.O.; Perdiguero, E.; Jardi, M.; Ruiz-Bonilla, V.; Datta, D.; Symeonidi, A.; Castellanos, A.; Welz, P.S.; Caballero, J.M.; et al. Aged Stem Cells Reprogram Their Daily Rhythmic Functions to Adapt to Stress. Cell 2017, 170, 678–692.e20. [Google Scholar] [CrossRef]
- Wang, J.; Mauvoisin, D.; Martin, E.; Atger, F.; Galindo, A.N.; Dayon, L.; Sizzano, F.; Palini, A.; Kussmann, M.; Waridel, P.; et al. Nuclear Proteomics Uncovers Diurnal Regulatory Landscapes in Mouse Liver. Cell Metab. 2017, 25, 102–117. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Song, L.; Liu, M.; Ge, R.; Zhou, Q.; Liu, W.; Li, R.; Qie, J.; Zhen, B.; Wang, Y.; et al. A proteomics landscape of circadian clock in mouse liver. Nat. Commun. 2018, 9, 1553. [Google Scholar] [CrossRef] [PubMed]
- Robles, M.S.; Humphrey, S.J.; Mann, M. Phosphorylation Is a Central Mechanism for Circadian Control of Metabolism and Physiology. Cell Metab. 2017, 25, 118–127. [Google Scholar] [CrossRef]
- Chiang, C.K.; Xu, B.; Mehta, N.; Mayne, J.; Sun, W.Y.; Cheng, K.; Ning, Z.; Dong, J.; Zou, H.; Cheng, H.M.; et al. Phosphoproteome Profiling Reveals Circadian Clock Regulation of Posttranslational Modifications in the Murine Hippocampus. Front. Neurol. 2017, 8, 110. [Google Scholar] [CrossRef] [PubMed]
- Noya, S.B.; Colameo, D.; Bruning, F.; Spinnler, A.; Mircsof, D.; Opitz, L.; Mann, M.; Tyagarajan, S.K.; Robles, M.S.; Brown, S.A. The forebrain synaptic transcriptome is organized by clocks but its proteome is driven by sleep. Science 2019, 366, eaav2642. [Google Scholar] [CrossRef]
- Malik, D.M.; Paschos, G.K.; Sehgal, A.; Weljie, A.M. Circadian and Sleep Metabolomics Across Species. J. Mol. Biol. 2020, 432, 3578–3610. [Google Scholar] [CrossRef]
- Hofman, M.A.; Fliers, E.; Goudsmit, E.; Swaab, D.F. Morphometric analysis of the suprachiasmatic and paraventricular nuclei in the human brain: Sex differences and age-dependent changes. J. Anat. 1988, 160, 127–143. [Google Scholar] [PubMed]
- Hofman, M.A.; Swaab, D.F. A brain for all seasons: Cellular and molecular mechanisms of photoperiodic plasticity. Prog. Brain Res. 2002, 138, 255–280. [Google Scholar] [CrossRef]
- Hofman, M.A.; Swaab, D.F. Living by the clock: The circadian pacemaker in older people. Ageing Res. Rev. 2006, 5, 33–51. [Google Scholar] [CrossRef]
- Harmar, A.J.; Marston, H.M.; Shen, S.; Spratt, C.; West, K.M.; Sheward, W.J.; Morrison, C.F.; Dorin, J.R.; Piggins, H.D.; Reubi, J.C.; et al. The VPAC(2) receptor is essential for circadian function in the mouse suprachiasmatic nuclei. Cell 2002, 109, 497–508. [Google Scholar] [CrossRef]
- Brown, T.M.; Colwell, C.S.; Waschek, J.A.; Piggins, H.D. Disrupted neuronal activity rhythms in the suprachiasmatic nuclei of vasoactive intestinal polypeptide-deficient mice. J. Neurophysiol. 2007, 97, 2553–2558. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Suzuki, T.; Mizoro, Y.; Kori, H.; Okada, K.; Chen, Y.; Fustin, J.M.; Yamazaki, F.; Mizuguchi, N.; Zhang, J.; et al. Mice genetically deficient in vasopressin V1a and V1b receptors are resistant to jet lag. Science 2013, 342, 85–90. [Google Scholar] [CrossRef]
- Mieda, M.; Ono, D.; Hasegawa, E.; Okamoto, H.; Honma, K.; Honma, S.; Sakurai, T. Cellular clocks in AVP neurons of the SCN are critical for interneuronal coupling regulating circadian behavior rhythm. Neuron 2015, 85, 1103–1116. [Google Scholar] [CrossRef] [PubMed]
- Patton, A.P.; Edwards, M.D.; Smyllie, N.J.; Hamnett, R.; Chesham, J.E.; Brancaccio, M.; Maywood, E.S.; Hastings, M.H. The VIP-VPAC2 neuropeptidergic axis is a cellular pacemaking hub of the suprachiasmatic nucleus circadian circuit. Nat. Commun. 2020, 11, 3394. [Google Scholar] [CrossRef]
- Todd, W.D.; Venner, A.; Anaclet, C.; Broadhurst, R.Y.; De Luca, R.; Bandaru, S.S.; Issokson, L.; Hablitz, L.M.; Cravetchi, O.; Arrigoni, E.; et al. Suprachiasmatic VIP neurons are required for normal circadian rhythmicity and comprised of molecularly distinct subpopulations. Nat. Commun. 2020, 11, 4410. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Ma, D.; Zhao, M.; Xie, L.; Wu, Q.; Gou, L.; Zhu, C.; Fan, Y.; Wang, H.; Yan, J. Spatiotemporal single-cell analysis of gene expression in the mouse suprachiasmatic nucleus. Nat. Neurosci. 2020, 23, 456–467. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Zhu, H.; O’Sullivan, S.; Ogunnaike, B.A.; Weaver, D.R.; Schwaber, J.S.; Vadigepalli, R. Single-Cell Transcriptional Analysis Reveals Novel Neuronal Phenotypes and Interaction Networks Involved in the Central Circadian Clock. Front. Neurosci. 2016, 10, 481. [Google Scholar] [CrossRef]
- Morris, E.L.; Patton, A.P.; Chesham, J.E.; Crisp, A.; Adamson, A.; Hastings, M.H. Single-cell transcriptomics of suprachiasmatic nuclei reveal a Prokineticin-driven circadian network. EMBO J. 2021, 40, e108614. [Google Scholar] [CrossRef]
- Brancaccio, M.; Patton, A.P.; Chesham, J.E.; Maywood, E.S.; Hastings, M.H. Astrocytes Control Circadian Timekeeping in the Suprachiasmatic Nucleus via Glutamatergic Signaling. Neuron 2017, 93, 1420–1435.e5. [Google Scholar] [CrossRef]
- Tso, C.F.; Simon, T.; Greenlaw, A.C.; Puri, T.; Mieda, M.; Herzog, E.D. Astrocytes Regulate Daily Rhythms in the Suprachiasmatic Nucleus and Behavior. Curr. Biol. 2017, 27, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Brancaccio, M.; Edwards, M.D.; Patton, A.P.; Smyllie, N.J.; Chesham, J.E.; Maywood, E.S.; Hastings, M.H. Cell-autonomous clock of astrocytes drives circadian behavior in mammals. Science 2019, 363, 187–192. [Google Scholar] [CrossRef]
- Buijs, R.M.; Guzman Ruiz, M.A.; Mendez Hernandez, R.; Rodriguez Cortes, B. The suprachiasmatic nucleus; a responsive clock regulating homeostasis by daily changing the setpoints of physiological parameters. Auton. Neurosci. 2019, 218, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Gizowski, C.; Zaelzer, C.; Bourque, C.W. Clock-driven vasopressin neurotransmission mediates anticipatory thirst prior to sleep. Nature 2016, 537, 685–688. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Hanna, L.; Harding, C.; Hayter, E.A.; Walmsley, L.; Bechtold, D.A.; Brown, T.M. Output from VIP cells of the mammalian central clock regulates daily physiological rhythms. Nat. Commun. 2020, 11, 1453. [Google Scholar] [CrossRef]
- Collins, B.; Pierre-Ferrer, S.; Muheim, C.; Lukacsovich, D.; Cai, Y.; Spinnler, A.; Herrera, C.G.; Wen, S.; Winterer, J.; Belle, M.D.C.; et al. Circadian VIPergic Neurons of the Suprachiasmatic Nuclei Sculpt the Sleep-Wake Cycle. Neuron 2020, 108, 486–499.e5. [Google Scholar] [CrossRef] [PubMed]
- Ishida, A.; Mutoh, T.; Ueyama, T.; Bando, H.; Masubuchi, S.; Nakahara, D.; Tsujimoto, G.; Okamura, H. Light activates the adrenal gland: Timing of gene expression and glucocorticoid release. Cell Metab. 2005, 2, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Buijs, R.M.; Kalsbeek, A. Hypothalamic integration of central and peripheral clocks. Nat. Rev. Neurosci. 2001, 2, 521–526. [Google Scholar] [CrossRef]
- Russell, G.; Lightman, S. The human stress response. Nat. Rev. Endocrinol. 2019, 15, 525–534. [Google Scholar] [CrossRef]
- Buijs, F.N.; León-Mercado, L.; Guzmán-Ruiz, M.; Guerrero-Vargas, N.N.; Romo-Nava, F.; Buijs, R.M. The Circadian System: A Regulatory Feedback Network of Periphery and Brain. Physiology 2016, 31, 170–181. [Google Scholar] [CrossRef]
- Firsov, D.; Bonny, O. Circadian rhythms and the kidney. Nat. Rev. Nephrol. 2018, 14, 626–635. [Google Scholar] [CrossRef]
- Ikegami, K.; Refetoff, S.; Van Cauter, E.; Yoshimura, T. Interconnection between circadian clocks and thyroid function. Nat. Rev. Endocrinol. 2019, 15, 590–600. [Google Scholar] [CrossRef]
- Stenvers, D.J.; Scheer, F.; Schrauwen, P.; la Fleur, S.E.; Kalsbeek, A. Circadian clocks and insulin resistance. Nat. Rev. Endocrinol. 2019, 15, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Challet, E. The circadian regulation of food intake. Nat. Rev. Endocrinol. 2019, 15, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Tahara, Y.; Shibata, S. Circadian rhythms of liver physiology and disease: Experimental and clinical evidence. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, S.; Fukushima, H.; Hosoda, H.; Serita, T.; Ishikawa, R.; Rokukawa, T.; Kawahara-Miki, R.; Zhang, Y.; Ohta, M.; Okada, S.; et al. Hippocampal clock regulates memory retrieval via Dopamine and PKA-induced GluA1 phosphorylation. Nat. Commun. 2019, 10, 5766. [Google Scholar] [CrossRef]
- Myung, J.; Schmal, C.; Hong, S.; Tsukizawa, Y.; Rose, P.; Zhang, Y.; Holtzman, M.J.; De Schutter, E.; Herzel, H.; Bordyugov, G.; et al. The choroid plexus is an important circadian clock component. Nat. Commun. 2018, 9, 1062. [Google Scholar] [CrossRef] [PubMed]
- Son, G.H.; Chung, S.; Choe, H.K.; Kim, H.D.; Baik, S.M.; Lee, H.; Lee, H.W.; Choi, S.; Sun, W.; Kim, H.; et al. Adrenal peripheral clock controls the autonomous circadian rhythm of glucocorticoid by causing rhythmic steroid production. Proc. Natl. Acad. Sci. USA 2008, 105, 20970–20975. [Google Scholar] [CrossRef]
- Myung, J.; Wu, M.Y.; Lee, C.Y.; Rahim, A.R.; Truong, V.H.; Wu, D.; Piggins, H.D.; Wu, M.S. The Kidney Clock Contributes to Timekeeping by the Master Circadian Clock. Int. J. Mol. Sci. 2019, 20, 2765. [Google Scholar] [CrossRef]
- Sinturel, F.; Gos, P.; Petrenko, V.; Hagedorn, C.; Kreppel, F.; Storch, K.F.; Knutti, D.; Liani, A.; Weitz, C.; Emmenegger, Y.; et al. Circadian hepatocyte clocks keep synchrony in the absence of a master pacemaker in the suprachiasmatic nucleus or other extrahepatic clocks. Genes Dev. 2021, 35, 329–334. [Google Scholar] [CrossRef]
- Bano-Otalora, B.; Piggins, H.D. Contributions of the lateral habenula to circadian timekeeping. Pharmacol. Biochem. Behav. 2017, 162, 46–54. [Google Scholar] [CrossRef]
- Van Drunen, R.; Eckel-Mahan, K. Circadian Rhythms of the Hypothalamus: From Function to Physiology. Clocks Sleep 2021, 3, 189–226. [Google Scholar] [CrossRef]
- Antle, M.C.; Mistlberger, R.E. Circadian clock resetting by sleep deprivation without exercise in the Syrian hamster. J. Neurosci. 2000, 20, 9326–9332. [Google Scholar] [CrossRef]
- Mendoza, J. Circadian clocks: Setting time by food. J. Neuroendocrinol. 2007, 19, 127–137. [Google Scholar] [CrossRef]
- Marchant, E.G.; Mistlberger, R.E. Morphine phase-shifts circadian rhythms in mice: Role of behavioural activation. Neuroreport 1995, 7, 209–212. [Google Scholar] [CrossRef]
- Guerrero-Vargas, N.N.; Salgado-Delgado, R.; Basualdo, M.e.C.; García, J.; Guzmán-Ruiz, M.; Carrero, J.C.; Escobar, C.; Buijs, R.M. Reciprocal interaction between the suprachiasmatic nucleus and the immune system tunes down the inflammatory response to lipopolysaccharide. J. Neuroimmunol. 2014, 273, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Saderi, N.; Salgado-Delgado, R.; Avendaño-Pradel, R.; Basualdo, M.e.C.; Ferri, G.L.; Chávez-Macías, L.; Roblera, J.E.; Escobar, C.; Buijs, R.M. NPY and VGF immunoreactivity increased in the arcuate nucleus, but decreased in the nucleus of the Tractus Solitarius, of type-II diabetic patients. PLoS ONE 2012, 7, e40070. [Google Scholar] [CrossRef]
- Buijs, F.N.; Cazarez, F.; Basualdo, M.C.; Scheer, F.A.; Perusquía, M.; Centurion, D.; Buijs, R.M. The suprachiasmatic nucleus is part of a neural feedback circuit adapting blood pressure response. Neuroscience 2014, 266, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Shioiri, T.; Takahashi, K.; Yamada, N.; Takahashi, S. Motor activity correlates negatively with free-running period, while positively with serotonin contents in SCN in free-running rats. Physiol. Behav. 1991, 49, 779–786. [Google Scholar] [CrossRef]
- Yi, C.X.; van der Vliet, J.; Dai, J.; Yin, G.; Ru, L.; Buijs, R.M. Ventromedial arcuate nucleus communicates peripheral metabolic information to the suprachiasmatic nucleus. Endocrinology 2006, 147, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Galvan, G.; Yi, C.X.; van der Vliet, J.; Jhamandas, J.H.; Panula, P.; Angeles-Castellanos, M.; Del Carmen Basualdo, M.; Escobar, C.; Buijs, R.M. Interaction between hypothalamic dorsomedial nucleus and the suprachiasmatic nucleus determines intensity of food anticipatory behavior. Proc. Natl. Acad. Sci. USA 2011, 108, 5813–5818. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Levy, M.; Korem, T.; Dohnalova, L.; Shapiro, H.; Jaitin, D.A.; David, E.; Winter, D.R.; Gury-BenAri, M.; Tatirovsky, E.; et al. Microbiota Diurnal Rhythmicity Programs Host Transcriptome Oscillations. Cell 2016, 167, 1495–1510.e12. [Google Scholar] [CrossRef] [PubMed]
- Buhr, E.D.; Yoo, S.H.; Takahashi, J.S. Temperature as a universal resetting cue for mammalian circadian oscillators. Science 2010, 330, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Reinke, H.; Asher, G. Crosstalk between metabolism and circadian clocks. Nat. Rev. Mol. Cell Biol. 2019, 20, 227–241. [Google Scholar] [CrossRef]
- Parmalee, N.L.; Aschner, M. Metals and Circadian Rhythms. Adv. Neurotoxicol. 2017, 1, 119–130. [Google Scholar] [CrossRef]
- Rey, G.; Valekunja, U.K.; Feeney, K.A.; Wulund, L.; Milev, N.B.; Stangherlin, A.; Ansel-Bollepalli, L.; Velagapudi, V.; O’Neill, J.S.; Reddy, A.B. The Pentose Phosphate Pathway Regulates the Circadian Clock. Cell Metab. 2016, 24, 462–473. [Google Scholar] [CrossRef]
- Ch, R.; Rey, G.; Ray, S.; Jha, P.K.; Driscoll, P.C.; Dos Santos, M.S.; Malik, D.M.; Lach, R.; Weljie, A.M.; MacRae, J.I.; et al. Rhythmic glucose metabolism regulates the redox circadian clockwork in human red blood cells. Nat. Commun. 2021, 12, 377. [Google Scholar] [CrossRef]
- Papagiannakopoulos, T.; Bauer, M.R.; Davidson, S.M.; Heimann, M.; Subbaraj, L.; Bhutkar, A.; Bartlebaugh, J.; Vander Heiden, M.G.; Jacks, T. Circadian Rhythm Disruption Promotes Lung Tumorigenesis. Cell Metab. 2016, 24, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, J.D.; Hansen, A.; Ord, T.; Bebas, P.; Chappell, P.E.; Giebultowicz, J.M.; Williams, C.; Moss, S.; Sehgal, A. The circadian clock protein BMAL1 is necessary for fertility and proper testosterone production in mice. J. Biol. Rhythms 2008, 23, 26–36. [Google Scholar] [CrossRef]
- Kondratov, R.V.; Kondratova, A.A.; Gorbacheva, V.Y.; Vykhovanets, O.V.; Antoch, M.P. Early aging and age-related pathologies in mice deficient in BMAL1, the core componentof the circadian clock. Genes Dev. 2006, 20, 1868–1873. [Google Scholar] [CrossRef]
- Walker, W.H., 2nd; Walton, J.C.; DeVries, A.C.; Nelson, R.J. Circadian rhythm disruption and mental health. Transl. Psychiatry 2020, 10, 28. [Google Scholar] [CrossRef]
- Bishehsari, F.; Voigt, R.M.; Keshavarzian, A. Circadian rhythms and the gut microbiota: From the metabolic syndrome to cancer. Nat. Rev. Endocrinol. 2020, 16, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Musiek, E.S.; Lim, M.M.; Yang, G.; Bauer, A.Q.; Qi, L.; Lee, Y.; Roh, J.H.; Ortiz-Gonzalez, X.; Dearborn, J.T.; Culver, J.P.; et al. Circadian clock proteins regulate neuronal redox homeostasis and neurodegeneration. J. Clin. Investig. 2013, 123, 5389–5400. [Google Scholar] [CrossRef]
- Lee, Y. Roles of circadian clocks in cancer pathogenesis and treatment. Exp. Mol. Med. 2021, 53, 1529–1538. [Google Scholar] [CrossRef]
- Walker, W.H., 2nd; Bumgarner, J.R.; Walton, J.C.; Liu, J.A.; Melendez-Fernandez, O.H.; Nelson, R.J.; DeVries, A.C. Light Pollution and Cancer. Int. J. Mol. Sci. 2020, 21, 9360. [Google Scholar] [CrossRef] [PubMed]
- West, A.C.; Smith, L.; Ray, D.W.; Loudon, A.S.I.; Brown, T.M.; Bechtold, D.A. Misalignment with the external light environment drives metabolic and cardiac dysfunction. Nat. Commun. 2017, 8, 417. [Google Scholar] [CrossRef] [PubMed]
- Buxton, O.M.; Cain, S.W.; O’Connor, S.P.; Porter, J.H.; Duffy, J.F.; Wang, W.; Czeisler, C.A.; Shea, S.A. Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci. Transl. Med. 2012, 4, 129ra143. [Google Scholar] [CrossRef] [PubMed]
- Tahkamo, L.; Partonen, T.; Pesonen, A.K. Systematic review of light exposure impact on human circadian rhythm. Chronobiol. Int. 2019, 36, 151–170. [Google Scholar] [CrossRef]
- Husse, J.; Zhou, X.; Shostak, A.; Oster, H.; Eichele, G. Synaptotagmin10-Cre, a driver to disrupt clock genes in the SCN. J. Biol. Rhythms 2011, 26, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Husse, J.; Leliavski, A.; Tsang, A.H.; Oster, H.; Eichele, G. The light-dark cycle controls peripheral rhythmicity in mice with a genetically ablated suprachiasmatic nucleus clock. FASEB J. 2014, 28, 4950–4960. [Google Scholar] [CrossRef]
- Ralph, M.R.; Foster, R.G.; Davis, F.C.; Menaker, M. Transplanted suprachiasmatic nucleus determines circadian period. Science 1990, 247, 975–978. [Google Scholar] [CrossRef]
- Schwartz, W.J.; Zimmerman, P. Lesions of the suprachiasmatic nucleus disrupt circadian locomotor rhythms in the mouse. Physiol. Behav. 1991, 49, 1283–1287. [Google Scholar] [CrossRef]
- Husse, J.; Eichele, G.; Oster, H. Synchronization of the mammalian circadian timing system: Light can control peripheral clocks independently of the SCN clock: Alternate routes of entrainment optimize the alignment of the body’s circadian clock network with external time. BioEssays News Rev. Mol. Cell. Dev. Biol. 2015, 37, 1119–1128. [Google Scholar] [CrossRef]
- Izumo, M.; Pejchal, M.; Schook, A.C.; Lange, R.P.; Walisser, J.A.; Sato, T.R.; Wang, X.; Bradfield, C.A.; Takahashi, J.S. Differential effects of light and feeding on circadian organization of peripheral clocks in a forebrain Bmal1 mutant. eLife 2014, 3. [Google Scholar] [CrossRef]
- Ding, G.; Li, X.; Hou, X.; Zhou, W.; Gong, Y.; Liu, F.; He, Y.; Song, J.; Wang, J.; Basil, P.; et al. REV-ERB in GABAergic neurons controls diurnal hepatic insulin sensitivity. Nature 2021, e04617. [Google Scholar] [CrossRef]
- Yamakawa, G.R.; Basu, P.; Cortese, F.; MacDonnell, J.; Whalley, D.; Smith, V.M.; Antle, M.C. The cholinergic forebrain arousal system acts directly on the circadian pacemaker. Proc. Natl. Acad. Sci. USA 2016, 113, 13498–13503. [Google Scholar] [CrossRef] [PubMed]
- Kolbe, I.; Leinweber, B.; Brandenburger, M.; Oster, H. Circadian clock network desynchrony promotes weight gain and alters glucose homeostasis in mice. Mol Metab 2019, 30, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Quintela, T.; Albuquerque, T.; Lundkvist, G.; Carmine Belin, A.; Talhada, D.; Goncalves, I.; Carro, E.; Santos, C.R.A. The choroid plexus harbors a circadian oscillator modulated by estrogens. Chronobiol. Int. 2018, 35, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Paschos, G.K.; Ibrahim, S.; Song, W.L.; Kunieda, T.; Grant, G.; Reyes, T.M.; Bradfield, C.A.; Vaughan, C.H.; Eiden, M.; Masoodi, M.; et al. Obesity in mice with adipocyte-specific deletion of clock component Arntl. Nat. Med. 2012, 18, 1768–1777. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; Lee, E.J.; Cha, H.K.; Kim, J.; Kim, D.; Son, G.H.; Kim, K. Cooperative roles of the suprachiasmatic nucleus central clock and the adrenal clock in controlling circadian glucocorticoid rhythm. Sci. Rep. 2017, 7, 46404. [Google Scholar] [CrossRef]
- Engeland, W.C.; Massman, L.; Mishra, S.; Yoder, J.M.; Leng, S.; Pignatti, E.; Piper, M.E.; Carlone, D.L.; Breault, D.T.; Kofuji, P. The Adrenal Clock Prevents Aberrant Light-Induced Alterations in Circadian Glucocorticoid Rhythms. Endocrinology 2018, 159, 3950–3964. [Google Scholar] [CrossRef]
- Lamia, K.A.; Storch, K.F.; Weitz, C.J. Physiological significance of a peripheral tissue circadian clock. Proc. Natl. Acad. Sci. USA 2008, 105, 15172–15177. [Google Scholar] [CrossRef]
- Perelis, M.; Marcheva, B.; Ramsey, K.M.; Schipma, M.J.; Hutchison, A.L.; Taguchi, A.; Peek, C.B.; Hong, H.; Huang, W.; Omura, C.; et al. Pancreatic β cell enhancers regulate rhythmic transcription of genes controlling insulin secretion. Science 2015, 350, aac4250. [Google Scholar] [CrossRef]
- Ehlen, J.C.; Brager, A.J.; Baggs, J.; Pinckney, L.; Gray, C.L.; DeBruyne, J.P.; Esser, K.A.; Takahashi, J.S.; Paul, K.N. Bmal1 function in skeletal muscle regulates sleep. eLife 2017, 6. [Google Scholar] [CrossRef]
- Dyar, K.A.; Hubert, M.J.; Mir, A.A.; Ciciliot, S.; Lutter, D.; Greulich, F.; Quagliarini, F.; Kleinert, M.; Fischer, K.; Eichmann, T.O.; et al. Transcriptional programming of lipid and amino acid metabolism by the skeletal muscle circadian clock. PLoS Biol. 2018, 16, e2005886. [Google Scholar] [CrossRef] [PubMed]
- Nikolaeva, S.; Ansermet, C.; Centeno, G.; Pradervand, S.; Bize, V.; Mordasini, D.; Henry, H.; Koesters, R.; Maillard, M.; Bonny, O.; et al. Nephron-Specific Deletion of Circadian Clock Gene Bmal1 Alters the Plasma and Renal Metabolome and Impairs Drug Disposition. J. Am. Soc. Nephrol. 2016, 27, 2997–3004. [Google Scholar] [CrossRef]
- Crislip, G.R.; Douma, L.G.; Masten, S.H.; Cheng, K.Y.; Lynch, I.J.; Johnston, J.G.; Barral, D.; Glasford, K.B.; Holzworth, M.R.; Verlander, J.W.; et al. Differences in renal BMAL1 contribution to Na. Am. J. Physiol. Renal. Physiol. 2020, 318, F1463–F1477. [Google Scholar] [CrossRef]
- Yu, F.; Wang, Z.; Zhang, T.; Chen, X.; Xu, H.; Wang, F.; Guo, L.; Chen, M.; Liu, K.; Wu, B. Deficiency of intestinal Bmal1 prevents obesity induced by high-fat feeding. Nat. Commun. 2021, 12, 5323. [Google Scholar] [CrossRef] [PubMed]
- Young, M.E.; Brewer, R.A.; Peliciari-Garcia, R.A.; Collins, H.E.; He, L.; Birky, T.L.; Peden, B.W.; Thompson, E.G.; Ammons, B.J.; Bray, M.S.; et al. Cardiomyocyte-specific BMAL1 plays critical roles in metabolism, signaling, and maintenance of contractile function of the heart. J. Biol. Rhythms 2014, 29, 257–276. [Google Scholar] [CrossRef] [PubMed]
- Ingle, K.A.; Kain, V.; Goel, M.; Prabhu, S.D.; Young, M.E.; Halade, G.V. Cardiomyocyte-specific Bmal1 deletion in mice triggers diastolic dysfunction, extracellular matrix response, and impaired resolution of inflammation. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H1827–H1836. [Google Scholar] [CrossRef]
- Moore, R.Y.; Eichler, V.B. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 1972, 42, 201–206. [Google Scholar] [CrossRef]
- Oster, H.; Damerow, S.; Kiessling, S.; Jakubcakova, V.; Abraham, D.; Tian, J.; Hoffmann, M.W.; Eichele, G. The circadian rhythm of glucocorticoids is regulated by a gating mechanism residing in the adrenal cortical clock. Cell Metab. 2006, 4, 163–173. [Google Scholar] [CrossRef]
- Cho, H.; Zhao, X.; Hatori, M.; Yu, R.T.; Barish, G.D.; Lam, M.T.; Chong, L.W.; DiTacchio, L.; Atkins, A.R.; Glass, C.K.; et al. Regulation of circadian behaviour and metabolism by REV-ERB-alpha and REV-ERB-beta. Nature 2012, 485, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Bugge, A.; Feng, D.; Everett, L.J.; Briggs, E.R.; Mullican, S.E.; Wang, F.; Jager, J.; Lazar, M.A. Rev-erbalpha and Rev-erbbeta coordinately protect the circadian clock and normal metabolic function. Genes Dev. 2012, 26, 657–667. [Google Scholar] [CrossRef]
- Guan, D.; Xiong, Y.; Trinh, T.M.; Xiao, Y.; Hu, W.; Jiang, C.; Dierickx, P.; Jang, C.; Rabinowitz, J.D.; Lazar, M.A. The hepatocyte clock and feeding control chronophysiology of multiple liver cell types. Science 2020, 369, 1388–1394. [Google Scholar] [CrossRef]
- Tsang, A.H.; Koch, C.E.; Kiehn, J.T.; Schmidt, C.X.; Oster, H. An adipokine feedback regulating diurnal food intake rhythms in mice. eLife 2020, 9, e55388. [Google Scholar] [CrossRef] [PubMed]
- Calvani, M.; Scarfone, A.; Granato, L.; Mora, E.V.; Nanni, G.; Castagneto, M.; Greco, A.V.; Manco, M.; Mingrone, G. Restoration of adiponectin pulsatility in severely obese subjects after weight loss. Diabetes 2004, 53, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, B.O.; Suchard, M.A.; Wong, M.L.; McCann, S.M.; Licinio, J. Alterations in the dynamics of circulating ghrelin, adiponectin, and leptin in human obesity. Proc. Natl. Acad. Sci. USA 2004, 101, 10434–10439. [Google Scholar] [CrossRef]
- Crosby, P.; Hamnett, R.; Putker, M.; Hoyle, N.P.; Reed, M.; Karam, C.J.; Maywood, E.S.; Stangherlin, A.; Chesham, J.E.; Hayter, E.A.; et al. Insulin/IGF-1 Drives PERIOD Synthesis to Entrain Circadian Rhythms with Feeding Time. Cell 2019, 177, 896–909.e20. [Google Scholar] [CrossRef] [PubMed]
- Kelu, J.J.; Pipalia, T.G.; Hughes, S.M. Circadian regulation of muscle growth independent of locomotor activity. Proc. Natl. Acad. Sci. USA 2020, 117, 31208–31218. [Google Scholar] [CrossRef]
- Masri, S.; Papagiannakopoulos, T.; Kinouchi, K.; Liu, Y.; Cervantes, M.; Baldi, P.; Jacks, T.; Sassone-Corsi, P. Lung Adenocarcinoma Distally Rewires Hepatic Circadian Homeostasis. Cell 2016, 165, 896–909. [Google Scholar] [CrossRef] [PubMed]
- Haspel, J.A.; Chettimada, S.; Shaik, R.S.; Chu, J.H.; Raby, B.A.; Cernadas, M.; Carey, V.; Process, V.; Hunninghake, G.M.; Ifedigbo, E.; et al. Circadian rhythm reprogramming during lung inflammation. Nat. Commun. 2014, 5, 4753. [Google Scholar] [CrossRef]
- Hara, R.; Wan, K.; Wakamatsu, H.; Aida, R.; Moriya, T.; Akiyama, M.; Shibata, S. Restricted feeding entrains liver clock without participation of the suprachiasmatic nucleus. Genes Cells 2001, 6, 269–278. [Google Scholar] [CrossRef]
- Damiola, F.; Le Minh, N.; Preitner, N.; Kornmann, B.; Fleury-Olela, F.; Schibler, U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000, 14, 2950–2961. [Google Scholar] [CrossRef]
- Tognini, P.; Samad, M.; Kinouchi, K.; Liu, Y.; Helbling, J.C.; Moisan, M.P.; Eckel-Mahan, K.L.; Baldi, P.; Sassone-Corsi, P. Reshaping circadian metabolism in the suprachiasmatic nucleus and prefrontal cortex by nutritional challenge. Proc. Natl. Acad. Sci. USA 2020, 117, 29904–29913. [Google Scholar] [CrossRef]
- Eckel-Mahan, K.L.; Patel, V.R.; de Mateo, S.; Orozco-Solis, R.; Ceglia, N.J.; Sahar, S.; Dilag-Penilla, S.A.; Dyar, K.A.; Baldi, P.; Sassone-Corsi, P. Reprogramming of the circadian clock by nutritional challenge. Cell 2013, 155, 1464–1478. [Google Scholar] [CrossRef]
- Yasumoto, Y.; Hashimoto, C.; Nakao, R.; Yamazaki, H.; Hiroyama, H.; Nemoto, T.; Yamamoto, S.; Sakurai, M.; Oike, H.; Wada, N.; et al. Short-term feeding at the wrong time is sufficient to desynchronize peripheral clocks and induce obesity with hyperphagia, physical inactivity and metabolic disorders in mice. Metab. Clin. Exp. 2016, 65, 714–727. [Google Scholar] [CrossRef]
- Garaulet, M.; Gómez-Abellán, P.; Alburquerque-Béjar, J.J.; Lee, Y.C.; Ordovás, J.M.; Scheer, F.A. Timing of food intake predicts weight loss effectiveness. Int. J. Obes. 2013, 37, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Garaulet, M.; Gómez-Abellán, P. Timing of food intake and obesity: A novel association. Physiol. Behav. 2014, 134, 44–50. [Google Scholar] [CrossRef] [PubMed]
- McHill, A.W.; Phillips, A.J.; Czeisler, C.A.; Keating, L.; Yee, K.; Barger, L.K.; Garaulet, M.; Scheer, F.A.; Klerman, E.B. Later circadian timing of food intake is associated with increased body fat. Am. J. Clin. Nutr. 2017, 106, 1213–1219. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Lozano, N.; Tvarijonaviciute, A.; Ríos, R.; Barón, I.; Scheer, F.A.J.L.; Garaulet, M. Late Eating Is Associated with Obesity, Inflammatory Markers and Circadian-Related Disturbances in School-Aged Children. Nutrients 2020, 12, 2881. [Google Scholar] [CrossRef]
- Loh, D.H.; Jami, S.A.; Flores, R.E.; Truong, D.; Ghiani, C.A.; O’Dell, T.J.; Colwell, C.S. Misaligned feeding impairs memories. eLife 2015, 4, e09460. [Google Scholar] [CrossRef]
- Kogevinas, M.; Espinosa, A.; Castello, A.; Gomez-Acebo, I.; Guevara, M.; Martin, V.; Amiano, P.; Alguacil, J.; Peiro, R.; Moreno, V.; et al. Effect of mistimed eating patterns on breast and prostate cancer risk (MCC-Spain Study). Int. J. Cancer 2018, 143, 2380–2389. [Google Scholar] [CrossRef]
- Zarrinpar, A.; Chaix, A.; Yooseph, S.; Panda, S. Diet and feeding pattern affect the diurnal dynamics of the gut microbiome. Cell Metab. 2014, 20, 1006–1017. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Bushman, F.D.; FitzGerald, G.A. Rhythmicity of the intestinal microbiota is regulated by gender and the host circadian clock. Proc. Natl. Acad. Sci. USA 2015, 112, 10479–10484. [Google Scholar] [CrossRef]
- Skarke, C.; Lahens, N.F.; Rhoades, S.D.; Campbell, A.; Bittinger, K.; Bailey, A.; Hoffmann, C.; Olson, R.S.; Chen, L.; Yang, G.; et al. A Pilot Characterization of the Human Chronobiome. Sci. Rep. 2017, 7, 17141. [Google Scholar] [CrossRef] [PubMed]
- Mukherji, A.; Kobiita, A.; Ye, T.; Chambon, P. Homeostasis in intestinal epithelium is orchestrated by the circadian clock and microbiota cues transduced by TLRs. Cell 2013, 153, 812–827. [Google Scholar] [CrossRef]
- Kuang, Z.; Wang, Y.; Li, Y.; Ye, C.; Ruhn, K.A.; Behrendt, C.L.; Olson, E.N.; Hooper, L.V. The intestinal microbiota programs diurnal rhythms in host metabolism through histone deacetylase 3. Science 2019, 365, 1428–1434. [Google Scholar] [CrossRef]
- Makris, A.P.; Karianaki, M.; Tsamis, K.I.; Paschou, S.A. The role of the gut-brain axis in depression: Endocrine, neural, and immune pathways. Hormones 2021, 20, 1–12. [Google Scholar] [CrossRef]
- Ying, W. NAD+/NADH and NADP+/NADPH in cellular functions and cell death: Regulation and biological consequences. Antioxid. Redox Signal. 2008, 10, 179–206. [Google Scholar] [CrossRef]
- Nakahata, Y.; Sahar, S.; Astarita, G.; Kaluzova, M.; Sassone-Corsi, P. Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science 2009, 324, 654–657. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, K.M.; Yoshino, J.; Brace, C.S.; Abrassart, D.; Kobayashi, Y.; Marcheva, B.; Hong, H.K.; Chong, J.L.; Buhr, E.D.; Lee, C.; et al. Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science 2009, 324, 651–654. [Google Scholar] [CrossRef]
- Rutter, J.; Reick, M.; Wu, L.C.; McKnight, S.L. Regulation of clock and NPAS2 DNA binding by the redox state of NAD cofactors. Science 2001, 293, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Nakahata, Y.; Kaluzova, M.; Grimaldi, B.; Sahar, S.; Hirayama, J.; Chen, D.; Guarente, L.P.; Sassone-Corsi, P. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell 2008, 134, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Levine, D.C.; Hong, H.; Weidemann, B.J.; Ramsey, K.M.; Affinati, A.H.; Schmidt, M.S.; Cedernaes, J.; Omura, C.; Braun, R.; Lee, C.; et al. NAD+ Controls Circadian Reprogramming through PER2 Nuclear Translocation to Counter Aging. Mol. Cell 2020, 78, 835–849.e7. [Google Scholar] [CrossRef]
- Asher, G.; Gatfield, D.; Stratmann, M.; Reinke, H.; Dibner, C.; Kreppel, F.; Mostoslavsky, R.; Alt, F.W.; Schibler, U. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell 2008, 134, 317–328. [Google Scholar] [CrossRef]
- Das, A.; Huang, G.X.; Bonkowski, M.S.; Longchamp, A.; Li, C.; Schultz, M.B.; Kim, L.J.; Osborne, B.; Joshi, S.; Lu, Y.; et al. Impairment of an Endothelial NAD+-H2S Signaling Network Is a Reversible Cause of Vascular Aging. Cell 2018, 173, 74–89.e20. [Google Scholar] [CrossRef]
- Shimizu, T.; Huang, D.; Yan, F.; Stranava, M.; Bartosova, M.; Fojtikova, V.; Martinkova, M. Gaseous O2, NO, and CO in signal transduction: Structure and function relationships of heme-based gas sensors and heme-redox sensors. Chem. Rev. 2015, 115, 6491–6533. [Google Scholar] [CrossRef]
- Kaasik, K.; Lee, C.C. Reciprocal regulation of haem biosynthesis and the circadian clock in mammals. Nature 2004, 430, 467–471. [Google Scholar] [CrossRef]
- Yin, L.; Wu, N.; Curtin, J.C.; Qatanani, M.; Szwergold, N.R.; Reid, R.A.; Waitt, G.M.; Parks, D.J.; Pearce, K.H.; Wisely, G.B.; et al. Rev-erbalpha, a heme sensor that coordinates metabolic and circadian pathways. Science 2007, 318, 1786–1789. [Google Scholar] [CrossRef]
- Raghuram, S.; Stayrook, K.R.; Huang, P.; Rogers, P.M.; Nosie, A.K.; McClure, D.B.; Burris, L.L.; Khorasanizadeh, S.; Burris, T.P.; Rastinejad, F. Identification of heme as the ligand for the orphan nuclear receptors REV-ERBalpha and REV-ERBbeta. Nat. Struct. Mol. Biol. 2007, 14, 1207–1213. [Google Scholar] [CrossRef]
- Lukat-Rodgers, G.S.; Correia, C.; Botuyan, M.V.; Mer, G.; Rodgers, K.R. Heme-based sensing by the mammalian circadian protein CLOCK. Inorg. Chem. 2010, 49, 6349–6365. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dioum, E.M.; Rutter, J.; Tuckerman, J.R.; Gonzalez, G.; Gilles-Gonzalez, M.A.; McKnight, S.L. NPAS2: A gas-responsive transcription factor. Science 2002, 298, 2385–2387. [Google Scholar] [CrossRef]
- Freeman, S.L.; Kwon, H.; Portolano, N.; Parkin, G.; Venkatraman Girija, U.; Basran, J.; Fielding, A.J.; Fairall, L.; Svistunenko, D.A.; Moody, P.C.E.; et al. Heme binding to human CLOCK affects interactions with the E-box. Proc. Natl. Acad. Sci. USA 2019, 116, 19911–19916. [Google Scholar] [CrossRef]
- Guenthner, C.J.; Bickar, D.; Harrington, M.E. Heme reversibly damps PERIOD2 rhythms in mouse suprachiasmatic nucleus explants. Neuroscience 2009, 164, 832–841. [Google Scholar] [CrossRef][Green Version]
- Klemz, R.; Reischl, S.; Wallach, T.; Witte, N.; Jurchott, K.; Klemz, S.; Lang, V.; Lorenzen, S.; Knauer, M.; Heidenreich, S.; et al. Reciprocal regulation of carbon monoxide metabolism and the circadian clock. Nat. Struct. Mol. Biol. 2017, 24, 15–22. [Google Scholar] [CrossRef]
- Minegishi, S.; Sagami, I.; Negi, S.; Kano, K.; Kitagishi, H. Circadian clock disruption by selective removal of endogenous carbon monoxide. Sci. Rep. 2018, 8, 11996. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yang, X.; Pan, Z.; Wang, Y.; De La Cruz, L.K.; Wang, B.; Tan, C. Towards “CO in a pill”: Pharmacokinetic studies of carbon monoxide prodrugs in mice. J. Control. Release 2020, 327, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, R.; Schaufler, A.; Calabrese, V.; Fuller, P.M.; Otterbein, L.E. Carbon Monoxide: From Poison to Clinical Trials. Trends Pharmacol. Sci. 2021. [Google Scholar] [CrossRef] [PubMed]
- Correa-Costa, M.; Gallo, D.; Csizmadia, E.; Gomperts, E.; Lieberum, J.L.; Hauser, C.J.; Ji, X.; Wang, B.; Camara, N.O.S.; Robson, S.C.; et al. Carbon monoxide protects the kidney through the central circadian clock and CD39. Proc. Natl. Acad. Sci. USA 2018, 115, E2302–E2310. [Google Scholar] [CrossRef]
- Schallner, N.; Lieberum, J.L.; Gallo, D.; LeBlanc, R.H., 3rd; Fuller, P.M.; Hanafy, K.A.; Otterbein, L.E. Carbon Monoxide Preserves Circadian Rhythm to Reduce the Severity of Subarachnoid Hemorrhage in Mice. Stroke 2017, 48, 2565–2573. [Google Scholar] [CrossRef]
- Emami, Z.; Mesbah Namin, A.; Kojuri, J.; Mashayekhi Jalali, F.; Rasti, M. Expression and Activity of Platelet Endothelial Nitric Oxide Synthase Are Decreased in Patients with Coronary Thrombosis and Stenosis. Avicenna J. Med. Biotechnol. 2019, 11, 88–93. [Google Scholar]
- Ayers, N.A.; Kapas, L.; Krueger, J.M. Circadian variation of nitric oxide synthase activity and cytosolic protein levels in rat brain. Brain Res. 1996, 707, 127–130. [Google Scholar] [CrossRef]
- Mitome, M.; Shirakawa, T.; Oshima, S.; Nakamura, W.; Oguchi, H. Circadian rhythm of nitric oxide production in the dorsal region of the suprachiasmatic nucleus in rats. Neurosci. Lett. 2001, 303, 161–164. [Google Scholar] [CrossRef]
- Tunctan, B.; Weigl, Y.; Dotan, A.; Peleg, L.; Zengil, H.; Ashkenazi, I.; Abacioglu, N. Circadian variation of nitric oxide synthase activity in mouse tissue. Chronobiol. Int. 2002, 19, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Uludag, O.; Tunctan, B.; Guney, H.Z.; Uluoglu, C.; Altug, S.; Zengil, H.; Abacioglu, N. Temporal variation in serum nitrite levels in rats and mice. Chronobiol. Int. 1999, 16, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Machado-Nils, A.V.; de Faria, L.O.; Vieira, A.S.; Teixeira, S.A.; Muscara, M.N.; Ferrari, E.A. Daily cycling of nitric oxide synthase (NOS) in the hippocampus of pigeons (C. livia). J. Circadian Rhythms 2013, 11, 12. [Google Scholar] [CrossRef]
- Denniff, M.; Turrell, H.E.; Vanezis, A.; Rodrigo, G.C. The time-of-day variation in vascular smooth muscle contractility depends on a nitric oxide signalling pathway. J. Mol. Cell. Cardiol. 2014, 66, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Mastronardi, C.A.; Yu, W.H.; McCann, S.M. Resting and circadian release of nitric oxide is controlled by leptin in male rats. Proc. Natl. Acad. Sci. USA 2002, 99, 5721–5726. [Google Scholar] [CrossRef]
- Viswambharan, H.; Carvas, J.M.; Antic, V.; Marecic, A.; Jud, C.; Zaugg, C.E.; Ming, X.F.; Montani, J.P.; Albrecht, U.; Yang, Z. Mutation of the circadian clock gene Per2 alters vascular endothelial function. Circulation 2007, 115, 2188–2195. [Google Scholar] [CrossRef] [PubMed]
- Anea, C.B.; Cheng, B.; Sharma, S.; Kumar, S.; Caldwell, R.W.; Yao, L.; Ali, M.I.; Merloiu, A.M.; Stepp, D.W.; Black, S.M.; et al. Increased superoxide and endothelial NO synthase uncoupling in blood vessels of Bmal1-knockout mice. Circ. Res. 2012, 111, 1157–1165. [Google Scholar] [CrossRef] [PubMed]
- Kanabrocki, E.L.; George, M.; Hermida, R.C.; Messmore, H.L.; Ryan, M.D.; Ayala, D.E.; Hoppensteadt, D.A.; Fareed, J.; Bremner, F.W.; Third, J.L.; et al. Day-night variations in blood levels of nitric oxide, T-TFPI, and E-selectin. Clin. Appl. Thromb. Hemost. 2001, 7, 339–345. [Google Scholar] [CrossRef]
- Kriegsfeld, L.J.; Drazen, D.L.; Nelson, R.J. Circadian organization in male mice lacking the gene for endothelial nitric oxide synthase (eNOS-/-). J. Biol. Rhythms 2001, 16, 142–148. [Google Scholar] [CrossRef]
- Arraj, M.; Lemmer, B. Endothelial nitric oxide is not involved in circadian rhythm generation of blood pressure: Experiments in wild-type C57 and eNOS knock-out mice under light-dark and free-run conditions. Chronobiol. Int. 2007, 24, 1231–1240. [Google Scholar] [CrossRef]
- Kunieda, T.; Minamino, T.; Miura, K.; Katsuno, T.; Tateno, K.; Miyauchi, H.; Kaneko, S.; Bradfield, C.A.; FitzGerald, G.A.; Komuro, I. Reduced nitric oxide causes age-associated impairment of circadian rhythmicity. Circ. Res. 2008, 102, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Melo, L.; Golombek, D.A.; Ralph, M.R. Regulation of circadian photic responses by nitric oxide. J. Biol. Rhythms 1997, 12, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, A.; Koch, R.; Nagoshi, E. Nitric oxide mediates neuro-glial interaction that shapes Drosophila circadian behavior. PLoS Genet. 2020, 16, e1008312. [Google Scholar] [CrossRef]
- Peek, C.B. Metabolic Implications of Circadian-HIF Crosstalk. Trends Endocrinol. Metab. 2020, 31, 459–468. [Google Scholar] [CrossRef]
- Luers, H.; Hillmann, K.; Litniewski, J.; Bereiter-Hahn, J. Acoustic microscopy of cultured cells. Distribution of forces and cytoskeletal elements. Cell Biophys 1991, 18, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Bosco, G.; Ionadi, A.; Panico, S.; Faralli, F.; Gagliardi, R.; Data, P.; Mortola, J.P. Effects of hypoxia on the circadian patterns in men. High Alt. Med. Biol. 2003, 4, 305–318. [Google Scholar] [CrossRef]
- Coste, O.; Beaumont, M.; Batejat, D.; Beers, P.V.; Touitou, Y. Prolonged mild hypoxia modifies human circadian core body temperature and may be associated with sleep disturbances. Chronobiol. Int. 2004, 21, 419–433. [Google Scholar] [CrossRef]
- Coste, O.; Van Beers, P.; Touitou, Y. Hypoxia-induced changes in recovery sleep, core body temperature, urinary 6-sulphatoxymelatonin and free cortisol after a simulated long-duration flight. J. Sleep Res. 2009, 18, 454–465. [Google Scholar] [CrossRef]
- Adamovich, Y.; Ladeuix, B.; Sobel, J.; Manella, G.; Neufeld-Cohen, A.; Assadi, M.H.; Golik, M.; Kuperman, Y.; Tarasiuk, A.; Koeners, M.P.; et al. Oxygen and Carbon Dioxide Rhythms Are Circadian Clock Controlled and Differentially Directed by Behavioral Signals. Cell Metab. 2019, 29, 1092–1103.e3. [Google Scholar] [CrossRef] [PubMed]
- Adamovich, Y.; Ladeuix, B.; Golik, M.; Koeners, M.P.; Asher, G. Rhythmic Oxygen Levels Reset Circadian Clocks through HIF1alpha. Cell Metab. 2017, 25, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, J.; Canamar, C.P.; Voyageur, C.; Tangpraphaphorn, S.; Lemus, A.; Coffey, C., Jr.; Wald-Dickler, N.; Holtom, P.; Shoenberger, J.; Bowdish, M.; et al. Mortality and Readmission Rates Among Patients With COVID-19 After Discharge From Acute Care Setting With Supplemental Oxygen. JAMA Netw. Open 2021, 4, e213990. [Google Scholar] [CrossRef]
- Sengupta, S.; Ince, L.; Sartor, F.; Borrmann, H.; Zhuang, X.; Naik, A.; Curtis, A.; McKeating, J.A. Clocks, Viruses, and Immunity: Lessons for the COVID-19 Pandemic. J. Biol. Rhythms 2021, 36, 23–34. [Google Scholar] [CrossRef]
- Balaban, R.S.; Nemoto, S.; Finkel, T. Mitochondria, oxidants, and aging. Cell 2005, 120, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Hu, B.; Yu, S.; Pan, X.; Wu, T.; Fu, Z. The effects of hydrogen peroxide on the circadian rhythms of Microcystis aeruginosa. PLoS ONE 2012, 7, e33347. [Google Scholar] [CrossRef]
- Grover, D.; Ford, D.; Brown, C.; Hoe, N.; Erdem, A.; Tavare, S.; Tower, J. Hydrogen peroxide stimulates activity and alters behavior in Drosophila melanogaster. PLoS ONE 2009, 4, e7580. [Google Scholar] [CrossRef]
- Pei, J.F.; Li, X.K.; Li, W.Q.; Gao, Q.; Zhang, Y.; Wang, X.M.; Fu, J.Q.; Cui, S.S.; Qu, J.H.; Zhao, X.; et al. Diurnal oscillations of endogenous H2O2 sustained by p66(Shc) regulate circadian clocks. Nat. Cell Biol. 2019, 21, 1553–1564. [Google Scholar] [CrossRef]
- Harkness, J.H.; Bushana, P.N.; Todd, R.P.; Clegern, W.C.; Sorg, B.A.; Wisor, J.P. Sleep disruption elevates oxidative stress in parvalbumin-positive cells of the rat cerebral cortex. Sleep 2019, 42, zsy201. [Google Scholar] [CrossRef]
- Vaccaro, A.; Kaplan Dor, Y.; Nambara, K.; Pollina, E.A.; Lin, C.; Greenberg, M.E.; Rogulja, D. Sleep Loss Can Cause Death through Accumulation of Reactive Oxygen Species in the Gut. Cell 2020, 181, 1307–1328.e15. [Google Scholar] [CrossRef]
- Fanjul-Moles, M.L.; López-Riquelme, G.O. Relationship between Oxidative Stress, Circadian Rhythms, and AMD. Oxidative Med. Cell Longev. 2016, 2016, 7420637. [Google Scholar] [CrossRef]
- Hine, C.; Mitchell, J.R. Calorie restriction and methionine restriction in control of endogenous hydrogen sulfide production by the transsulfuration pathway. Exp. Gerontol. 2015, 68, 26–32. [Google Scholar] [CrossRef]
- Kimura, H. The physiological role of hydrogen sulfide and beyond. Nitric Oxide 2014, 41, 4–10. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, Z.H.; Ren, Z.; Qu, S.L.; Liu, M.H.; Liu, L.S.; Jiang, Z.S. Hydrogen sulfide, the next potent preventive and therapeutic agent in aging and age-associated diseases. Mol. Cell. Biol. 2013, 33, 1104–1113. [Google Scholar] [CrossRef]
- Shang, Z.; Lu, C.; Chen, S.; Hua, L.; Qian, R. Effect of H2S on the circadian rhythm of mouse hepatocytes. Lipids Health Dis 2012, 11, 23. [Google Scholar] [CrossRef]
- Jin, S.; Tan, B.; Teng, X.; Meng, R.; Jiao, X.; Tian, D.; Xiao, L.; Xue, H.; Guo, Q.; Duan, X.; et al. Diurnal Fluctuations in Plasma Hydrogen Sulfide of the Mice. Front. Pharmacol. 2017, 8, 682. [Google Scholar] [CrossRef] [PubMed]
- Scuffi, D.; Nietzel, T.; Di Fino, L.M.; Meyer, A.J.; Lamattina, L.; Schwarzlander, M.; Laxalt, A.M.; Garcia-Mata, C. Hydrogen Sulfide Increases Production of NADPH Oxidase-Dependent Hydrogen Peroxide and Phospholipase D-Derived Phosphatidic Acid in Guard Cell Signaling. Plant Physiol. 2018, 176, 2532–2542. [Google Scholar] [CrossRef] [PubMed]
- Shaposhnikov, M.; Proshkina, E.; Koval, L.; Zemskaya, N.; Zhavoronkov, A.; Moskalev, A. Overexpression of CBS and CSE genes affects lifespan, stress resistance and locomotor activity in Drosophila melanogaster. Aging 2018, 10, 3260–3272. [Google Scholar] [CrossRef]
- Zhang, H.; Dai, J.; Tian, D.; Xiao, L.; Xue, H.; Guo, Q.; Zhang, X.; Teng, X.; Jin, S.; Wu, Y. Hydrogen Sulfide Restored the Diurnal Variation in Cardiac Function of Aging Mice. Oxidative Med. Cell. Longev. 2021, 2021, 8841575. [Google Scholar] [CrossRef]
- Penarrubia, L.; Andres-Colas, N.; Moreno, J.; Puig, S. Regulation of copper transport in Arabidopsis thaliana: A biochemical oscillator? J. Biol. Inorg. Chem. 2010, 15, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Chen, X.; Rosbash, M. Temporal calcium profiling of specific circadian neurons in freely moving flies. Proc. Natl. Acad. Sci. USA 2017, 114, E8780–E8787. [Google Scholar] [CrossRef] [PubMed]
- Love, J.; Dodd, A.N.; Webb, A.A. Circadian and diurnal calcium oscillations encode photoperiodic information in Arabidopsis. Plant Cell 2004, 16, 956–966. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, Q.R.; Liu, L.L.; Zhang, H.M.; Gao, J.W.; Pei, Z.M. Osmotic stress alters circadian cytosolic Ca2+ oscillations and OSCA1 is required in circadian gated stress adaptation. Plant Signal. Behav. 2020, 15, 1836883. [Google Scholar] [CrossRef]
- Lundkvist, G.B.; Kwak, Y.; Davis, E.K.; Tei, H.; Block, G.D. A calcium flux is required for circadian rhythm generation in mammalian pacemaker neurons. J. Neurosci. Off. J. Soc. Neurosci. 2005, 25, 7682–7686. [Google Scholar] [CrossRef]
- Perea-Garcia, A.; Andres-Borderia, A.; Mayo de Andres, S.; Sanz, A.; Davis, A.M.; Davis, S.J.; Huijser, P.; Penarrubia, L. Modulation of copper deficiency responses by diurnal and circadian rhythms in Arabidopsis thaliana. J. Exp. Bot. 2016, 67, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Perea-Garcia, A.; Sanz, A.; Moreno, J.; Andres-Borderia, A.; de Andres, S.M.; Davis, A.M.; Huijser, P.; Davis, S.J.; Penarrubia, L. Daily rhythmicity of high affinity copper transport. Plant Signal. Behav. 2016, 11, e1140291. [Google Scholar] [CrossRef][Green Version]
- Perea-Garcia, A.; Andres-Colas, N.; Penarrubia, L. Copper homeostasis influences the circadian clock in Arabidopsis. Plant Signal. Behav. 2010, 5, 1237–1240. [Google Scholar] [CrossRef]
- Lifschitz, M.D.; Henkin, R.I. Circadian variation in copper and zinc in man. J. Appl. Physiol. 1971, 31, 88–92. [Google Scholar] [CrossRef]
- Aono, H.; Araki, S. Circadian rhythms in the urinary excretion of heavy metals and organic substances in metal workers in relation to renal excretory mechanism: Profile analysis. Int. Arch. Occup. Environ. Health 1988, 60, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Araki, S.; Murata, K.; Yokoyama, K.; Yanagihara, S.; Niinuma, Y.; Yamamoto, R.; Ishihara, N. Circadian rhythms in the urinary excretion of metals and organic substances in “healthy” men. Arch. Environ. Health 1983, 38, 360–366. [Google Scholar] [CrossRef]
- Kanabrocki, E.L.; Sothern, R.B.; Ryan, M.D.; Kahn, S.; Augustine, G.; Johnson, C.; Foley, S.; Gathing, A.; Eastman, G.; Friedman, N.; et al. Circadian characteristics of serum calcium, magnesium and eight trace elements and of their metallo-moieties in urine of healthy middle-aged men. Clin. Ter. 2008, 159, 329–346. [Google Scholar] [PubMed]
- Flourakis, M.; Kula-Eversole, E.; Hutchison, A.L.; Han, T.H.; Aranda, K.; Moose, D.L.; White, K.P.; Dinner, A.R.; Lear, B.C.; Ren, D.; et al. A Conserved Bicycle Model for Circadian Clock Control of Membrane Excitability. Cell 2015, 162, 836–848. [Google Scholar] [CrossRef]
- Xie, L.; Gao, S.; Alcaire, S.M.; Aoyagi, K.; Wang, Y.; Griffin, J.K.; Stagljar, I.; Nagamatsu, S.; Zhen, M. NLF-1 delivers a sodium leak channel to regulate neuronal excitability and modulate rhythmic locomotion. Neuron 2013, 77, 1069–1082. [Google Scholar] [CrossRef]
- Ding, F.; O’Donnell, J.; Xu, Q.; Kang, N.; Goldman, N.; Nedergaard, M. Changes in the composition of brain interstitial ions control the sleep-wake cycle. Science 2016, 352, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Shim, H.S.; Kim, H.; Lee, J.; Son, G.H.; Cho, S.; Oh, T.H.; Kang, S.H.; Seen, D.S.; Lee, K.H.; Kim, K. Rapid activation of CLOCK by Ca2+-dependent protein kinase C mediates resetting of the mammalian circadian clock. EMBO Rep. 2007, 8, 366–371. [Google Scholar] [CrossRef]
- Brancaccio, M.; Maywood, E.S.; Chesham, J.E.; Loudon, A.S.; Hastings, M.H. A Gq-Ca2+ axis controls circuit-level encoding of circadian time in the suprachiasmatic nucleus. Neuron 2013, 78, 714–728. [Google Scholar] [CrossRef]
- Harrisingh, M.C.; Wu, Y.; Lnenicka, G.A.; Nitabach, M.N. Intracellular Ca2+ regulates free-running circadian clock oscillation in vivo. J. Neurosci. Off. J. Soc. Neurosci. 2007, 27, 12489–12499. [Google Scholar] [CrossRef] [PubMed]
- Palacios-Munoz, A.; Ewer, J. Calcium and cAMP directly modulate the speed of the Drosophila circadian clock. PLoS Genet. 2018, 14, e1007433. [Google Scholar] [CrossRef]
- Yamada, Y.; Prosser, R.A. Copper in the suprachiasmatic circadian clock: A possible link between multiple circadian oscillators. Eur. J. Neurosci. 2020, 51, 47–70. [Google Scholar] [CrossRef]
- Feeney, K.A.; Hansen, L.L.; Putker, M.; Olivares-Yanez, C.; Day, J.; Eades, L.J.; Larrondo, L.F.; Hoyle, N.P.; O’Neill, J.S.; van Ooijen, G. Daily magnesium fluxes regulate cellular timekeeping and energy balance. Nature 2016, 532, 375–379. [Google Scholar] [CrossRef]
- Zhang, S.L.; Yue, Z.; Arnold, D.M.; Artiushin, G.; Sehgal, A. A Circadian Clock in the Blood-Brain Barrier Regulates Xenobiotic Efflux. Cell 2018, 173, 130–139.e10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.L.; Lahens, N.F.; Yue, Z.; Arnold, D.M.; Pakstis, P.P.; Schwarz, J.E.; Sehgal, A. A circadian clock regulates efflux by the blood-brain barrier in mice and human cells. Nat. Commun. 2021, 12, 617. [Google Scholar] [CrossRef]
- Cuddapah, V.A.; Zhang, S.L.; Sehgal, A. Regulation of the Blood-Brain Barrier by Circadian Rhythms and Sleep. Trends Neurosci. 2019, 42, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Henslee, E.A.; Crosby, P.; Kitcatt, S.J.; Parry, J.S.W.; Bernardini, A.; Abdallat, R.G.; Braun, G.; Fatoyinbo, H.O.; Harrison, E.J.; Edgar, R.S.; et al. Rhythmic potassium transport regulates the circadian clock in human red blood cells. Nat. Commun. 2017, 8, 1978. [Google Scholar] [CrossRef]
- Hu, Y.; Spengler, M.L.; Kuropatwinski, K.K.; Comas-Soberats, M.; Jackson, M.; Chernov, M.V.; Gleiberman, A.S.; Fedtsova, N.; Rustum, Y.M.; Gudkov, A.V.; et al. Selenium is a modulator of circadian clock that protects mice from the toxicity of a chemotherapeutic drug via upregulation of the core clock protein, BMAL1. Oncotarget 2011, 2, 1279–1290. [Google Scholar] [CrossRef] [PubMed]
- Simcox, J.A.; Mitchell, T.C.; Gao, Y.; Just, S.F.; Cooksey, R.; Cox, J.; Ajioka, R.; Jones, D.; Lee, S.H.; King, D.; et al. Dietary iron controls circadian hepatic glucose metabolism through heme synthesis. Diabetes 2015, 64, 1108–1119. [Google Scholar] [CrossRef]
- Gizowski, C.; Bourque, C.W. Sodium regulates clock time and output via an excitatory GABAergic pathway. Nature 2020, 583, 421–424. [Google Scholar] [CrossRef] [PubMed]
| Cell type of Selective Bmal1 Ablation | Promoter Controlling Cell-Specific Manipulation | Rhythm(s) Eliminated | Effect on Circadian Behavior | Clinical Manifestation | Citation Number |
|---|---|---|---|---|---|
| Adipocyte | Adipocyte protein 2 (aP2) gene promoter | Adiponectin | Shift in the diurnal rhythm of food intake and energy expenditure | Obesity | [117] |
| Adrenal | Melanocortin 2 Receptor (MC2R) gene promoter, Aldosterone synthase (AS) gene promoter data | Circulating corticosterone, ACTH sensitivity | Attenuated behavioral rhythmicity | Hyperadrenocorticism | [76,118,119] |
| Hepatocyte | Abumin (ABL) gene promoter | Glucoregulatory genes | None | Increase glucose clearance and hypoglycemia restricted to the fasting phase | [120] |
| Pancreatic β cell | Pancreatic Additionally, Duodenal Homeobox 1 (PDX1) gene promoter | Insulin secretion | None | Insulin resistance | [121] |
| Skeletal muscle | Human α-skeletal actin (HSA) gene promoter | Muscle growth and metabolism | Sleep disturbance | Metabolic inefficiency and impaired muscle triglyceride biosynthesis | [122,123] |
| Renal | Kidney-specific cadherin (KSP-Cad) gene promoter | None | None | Altered the plasma metabolome, lowered blood pressure in male mice | [124,125] |
| Intestine | Villin (VIL1) gene promoter | None | None | Prevents obesity induced by high-fat feeding | [126] |
| Cardiomyocyte | Myosin heavy chain α (MHCα) gene promoter | Circadian gene expression in heart | None | Diastolic dysfunction, Impaired resolution of inflammation, Reduced life span | [127,128] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.; Wisor, J.P. Multi-Modal Regulation of Circadian Physiology by Interactive Features of Biological Clocks. Biology 2022, 11, 21. https://doi.org/10.3390/biology11010021
Lee Y, Wisor JP. Multi-Modal Regulation of Circadian Physiology by Interactive Features of Biological Clocks. Biology. 2022; 11(1):21. https://doi.org/10.3390/biology11010021
Chicago/Turabian StyleLee, Yool, and Jonathan P. Wisor. 2022. "Multi-Modal Regulation of Circadian Physiology by Interactive Features of Biological Clocks" Biology 11, no. 1: 21. https://doi.org/10.3390/biology11010021
APA StyleLee, Y., & Wisor, J. P. (2022). Multi-Modal Regulation of Circadian Physiology by Interactive Features of Biological Clocks. Biology, 11(1), 21. https://doi.org/10.3390/biology11010021







