3.1. Characterization of Materials
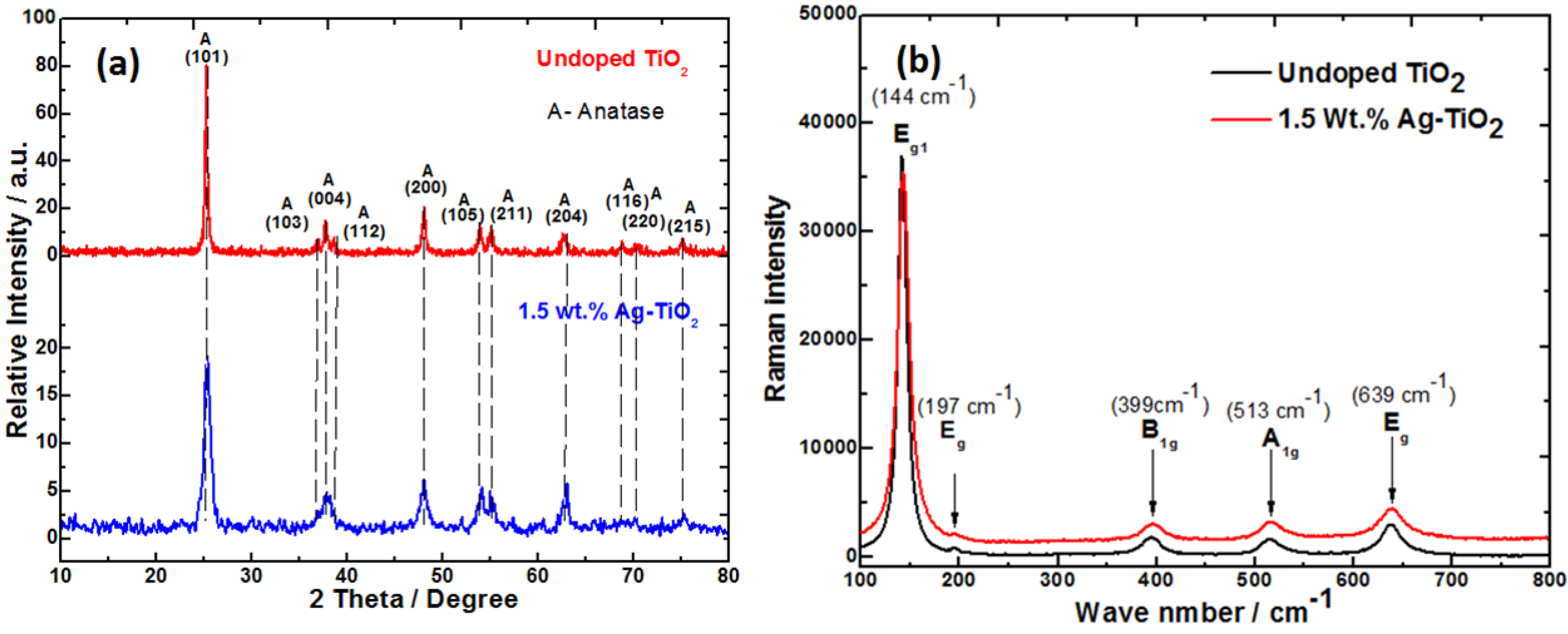
Sol-gel derived nanocrystalline TiO
2 were subjected to the XRD analysis to determine crystalline phase and crystallite size. Titania exists in three crystalline polymorphs–anatase, rutile and brookite forms. Among these, anatase titania has been shown to exhibit higher antimicrobial activity than the other two and thus pure anatase phase content is a desirable feature [
26]. The PXRD of titanias synthesized in this work had the peaks characteristic of anatase phase
Figure 2a. (JCPDS No. 21-1272). From the X-ray diffraction patterns, the size of anatase TiO
2 materials prepared were in the nanometric scale
Table 1. The average crystallite size was determined from the (101) plane in the PXRD pattern using Scherer’s formula. The calculated value of undoped TiO
2 had bigger crystallite size while Ag-doped TiO
2 showed a decrease in the crystallite size. A good correlation between the Raman and PXRD was also observed
Figure 2b. The changes in the crystallite size of TiO
2 nanocrystals upon Ag-doping are closely correlated to the broadening and shifts of the Raman bands with decreasing particle size [
27]. Similar observations were made for the titania sysnthesised in the present work. During annealing process, silver nitrate thermally decomposes into silver. Bigger ionic radii of Ag
+ (0.75 Å) compared to Ti
4+ (0.605 Å) prevents it from entering the crystal lattice of anatase TiO
2 because of a high energy barrier. Thus, it gets distributed uniformly on the surface of TiO
2. However, the PXRD pattern of Ag-TiO
2 did not reveal any Ag or Ag-containing phases. This may be due to the low concentration of Ag incorporated which is below the detection limit of the PXRD analysis.
Doping with Ag
+ ion also resulted in increase in the BET surface area of TiO
2 (48 m
2/g), while that of undoped TiO
2 showed BET surface area of 27 m
2/g. Thus, large surface area to volume ratio of Ag-doped TiO
2 was advantageous for the release of Ag
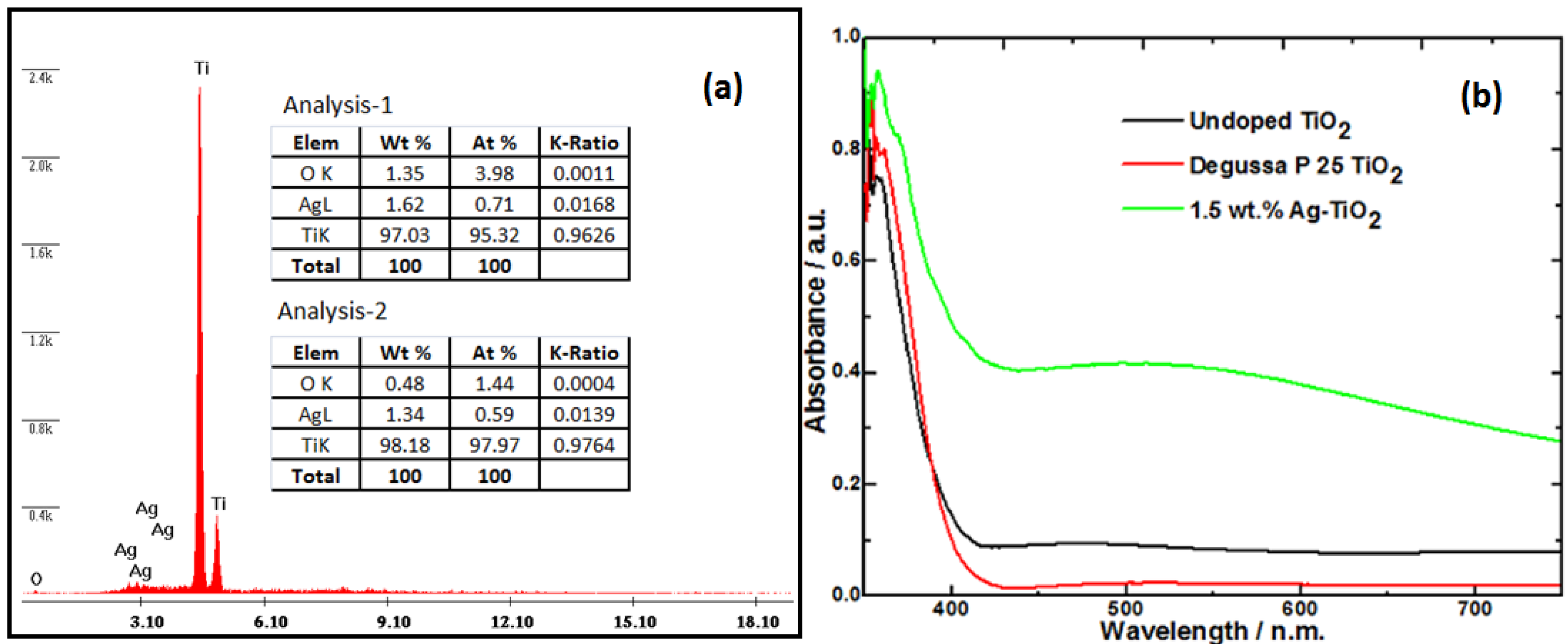
+ ion. From the energy dispersive X-ray (EDS) analysis at two locations (see
Figure 3a), done during the SEM confirms silver is dispersed uniformly in TiO
2 host.
Figure 3b shows the changes in the absorbance of Ag-doped TiO
2 in comparison to undoped TiO
2 and Degussa P 25 titania. Ag doped TiO
2 (calcined in ambient air at 500 °C) was found to have higher visible absorbance. In contrast, pure TiO
2 prepared under similar experimental conditions, had its absorbance slightly shifted towards the visible region as compared to Degussa P25 titania (
Figure 3b). The DRS spectra showed a characteristic absorption band at about 500 nm, due to the surface plasmon resonance of silver [
28]. Using the different absorbance onsets, it was found that the Ag-TiO
2 had a bandgap of ~2.8 eV while both of the undoped titania samples had wider band gaps estimated at ~3.1 eV for TiO
2 and ~3.2 eV for the Degussa P25 TiO
2 sample. Similar observations from previous studies can be confirmed [
29].
Table 1.
Physio-chemical properties of nanofiller, Tg, weight of coated composite material and amount of silver ion released.
Table 1.
Physio-chemical properties of nanofiller, Tg, weight of coated composite material and amount of silver ion released.
| Composite type | Nanocrystalline-TiO2 | Epoxy-TiO2 composite | Amount of Ag released by the composite (µg/mL) * |
|---|
| Crystallite size (nm) | BET surface area (m2/g) | Glass transition temperature Tg (°C) | Weight of the coated composite (gm) |
|---|
| Neat Epoxy | n/a | n/a | 93 | 1.08 | Nil |
| 1.0 wt% Epoxy/TiO2 | 36 | 27 | 90 | 0.99 | Nil |
| 0.5 wt% Epoxy/Ag-TiO2 | 18 | 48 | 94 | 1.09 | 6.6 |
| 1.0 wt% Epoxy/Ag-TiO2 | 18 | 48 | 97 | 0.95 | 10.2 |
| 1.5 wt% Epoxy/Ag-TiO2 | 18 | 48 | 106 | 1.05 | 14.6 |
| 2.0 wt% Epoxy/Ag-TiO2 | 18 | 48 | 97 | 1.10 | 16.8 |
Figure 2.
(a) Powder X-ray diffraction (XRD) and (b) Raman spectra of TiO2 and Ag-TiO2.
Figure 2.
(a) Powder X-ray diffraction (XRD) and (b) Raman spectra of TiO2 and Ag-TiO2.
Figure 3.
(a) Elemental analysis (EDS) of the silver doped TiO2 showing the presence of Ti and Ag species; (b) UV-Vis diffuse reflectance spectra (DRS) of Ag-doped TiO2, TiO2 and Degussa P25 titania.
Figure 3.
(a) Elemental analysis (EDS) of the silver doped TiO2 showing the presence of Ti and Ag species; (b) UV-Vis diffuse reflectance spectra (DRS) of Ag-doped TiO2, TiO2 and Degussa P25 titania.
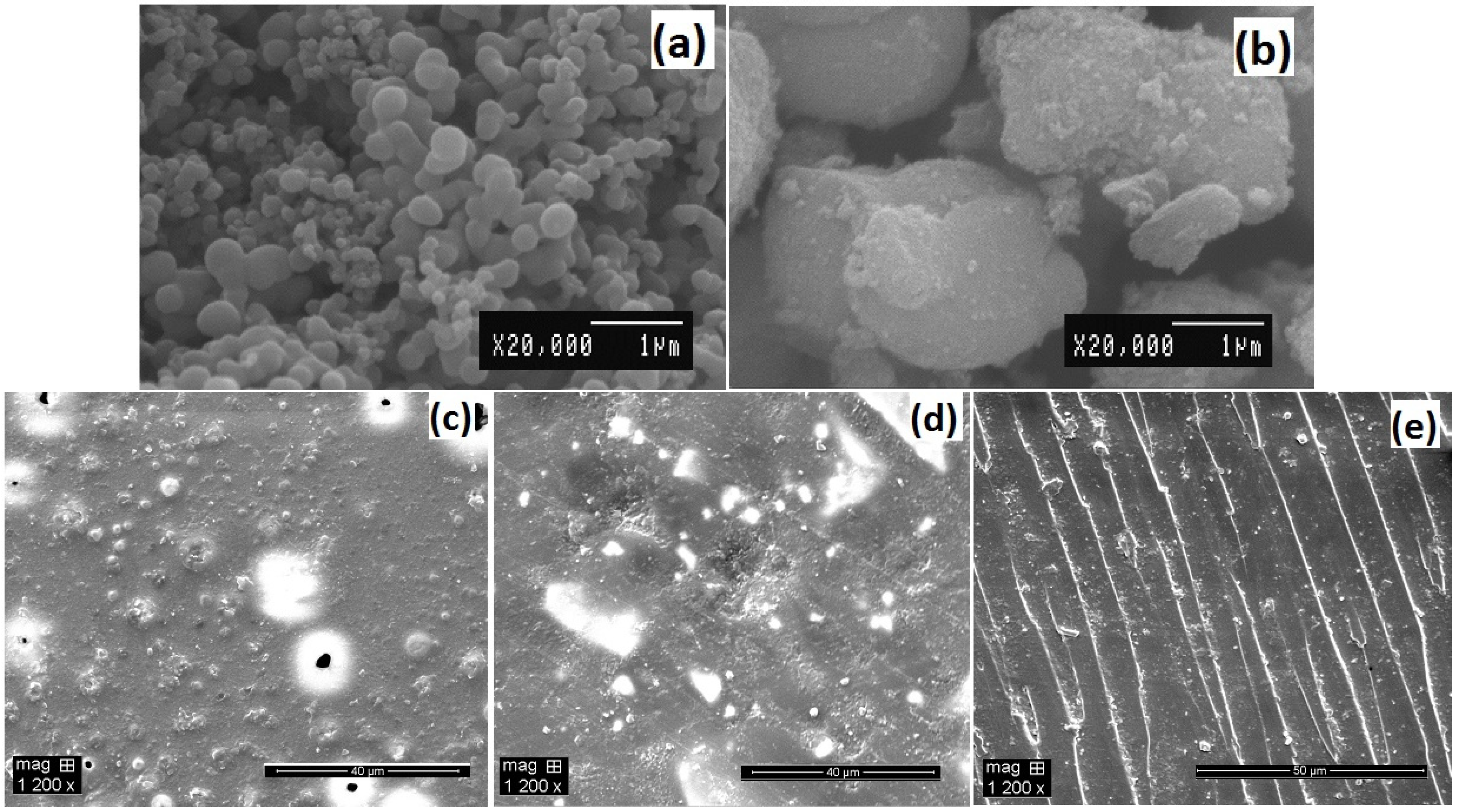
The homogeneous distribution of nano-filler in a polymer matrix has major influence on the composite performance. The morphology of synthesized titania nanoparticles and their dispersion in epoxy matrix were examined by SEM analysis
Figure 4. The primary particle size of undoped and silver doped titania are different, varying from nanometer to micron size for the same magnification as seen in SEM micrographs
Figure 4a,b. The undoped sample exhibited a nanostructure consisting of spherical clusters with a diameter of 50–500 nm, which are extensively agglomerated with an average crystallite size of 36 nm. However, silver doped titania showed bigger aggregates and smaller segregated particles consisting of primary anatase nanocrystals of 18 nm size (
Figure 4b). Dispersion is an important factor in determining a nanocomposite’s properties. Composites with the same weight percent (1 wt%) of nanofiller showed different degree of dispersion
Figure 4c,d. The unmodified TiO
2 although thoroughly distributed in the matrix, yet particles agglomerated densely as shown in
Figure 4c giving scattered hill lock like appearance on the surface of the composite. The size of these agglomerates varied from nanometers to micrometers. However, the Ag-TiO
2 particles
Figure 4d, showed a lesser degree of agglomeration; interparticle distance are clearly visible between the TiO
2 particles. This indicates that the presence of silver enable good dispersion due to the interaction of oxidized silver ions with surface hydroxyl groups (titanol groups, Ti–OH) of TiO
2 and increase its wettability in apolar media like epoxy (hydrophobic polymer matrix). While
Figure 4e shows the fractured surface of the composite, dispersion in the bulk is similar to distance between agglomerates as on surface. This suggests that the doped nano-fillers have better dispersion due to surface modifications, which improve the interactions between particles and polymer matrix. Use of reactive diluant also significantly reduced viscosity of epoxy resin during preparation and optimized the dispersion along with sonication.
Figure 4.
Scanning electron microscopic (SEM) characterization of (a) sol-gel synthesized TiO2; (b) 1.5 wt% silver doped TiO2; (c) 1 wt% epoxy/TiO2 composite; (d) 1 wt% epoxy/Ag-TiO2 composite; (e) Fractured surface of 1 wt% epoxy/Ag-TiO2 composite.
Figure 4.
Scanning electron microscopic (SEM) characterization of (a) sol-gel synthesized TiO2; (b) 1.5 wt% silver doped TiO2; (c) 1 wt% epoxy/TiO2 composite; (d) 1 wt% epoxy/Ag-TiO2 composite; (e) Fractured surface of 1 wt% epoxy/Ag-TiO2 composite.
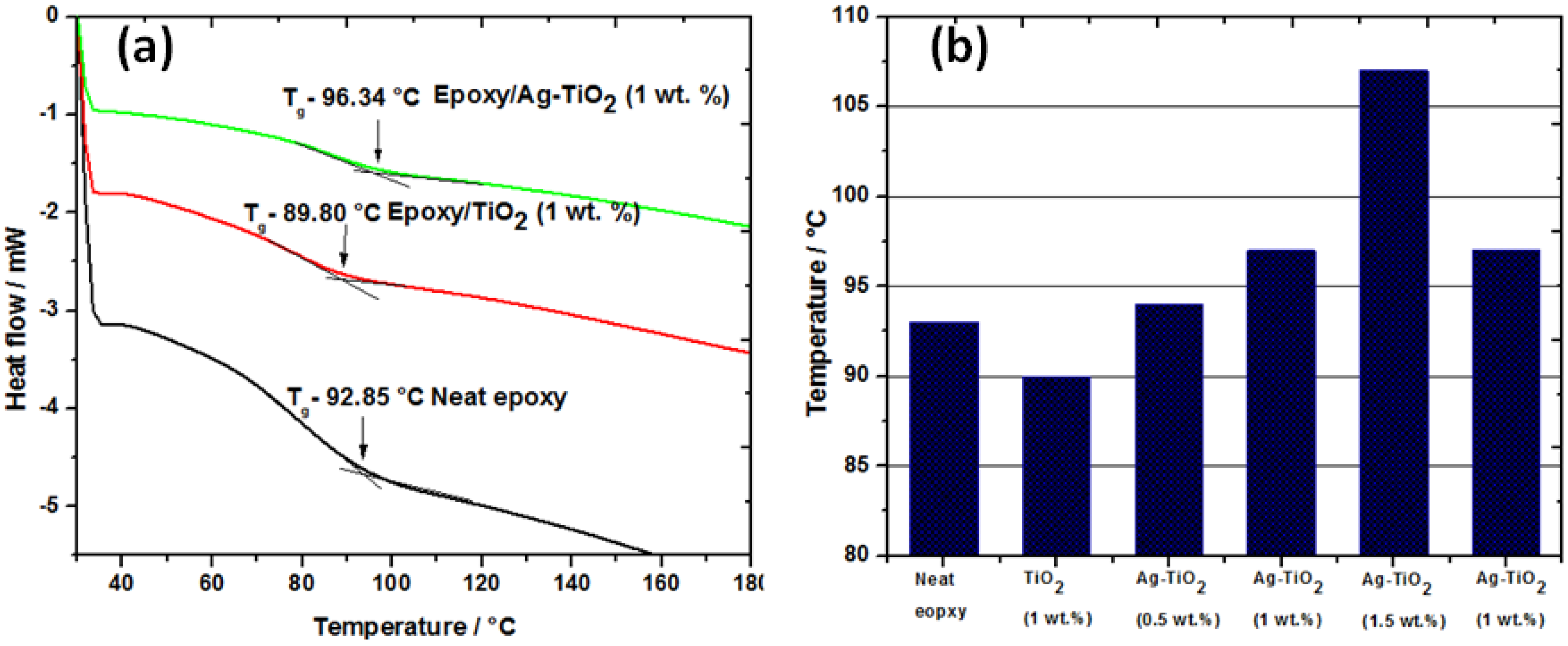
The glass transition temperature (
Tg) of the samples were determined from the tangents of DSC spectra as a function of temperature. The DSC curves of the neat epoxy and nanocomposites with 1 wt% of TiO
2 and Ag-TiO
2 nanofiller from the second run are shown in the
Figure 5a. For thermosetting resin glass transition temperature (
Tg), values can shift due to reasons like cross-linking density, intermolecular interaction and chain length. The addition of nanometer sized TiO
2 particles in epoxy resulted in increase in the
Tg from 93 °C for neat epoxy to 97 °C at 1 wt% loading of Ag-TiO
2. Whereas,
Tg of composite shifts to lower temperature with undoped TiO
2 (1 wt% loading) due to poor dispersion and agglomeration as evident in the SEM micrograph. Nanocomposites with Ag-TiO
2 exhibited maximum
Tg value at 1.5 wt% loading (107 °C) (
Figure 5b). A further increase in the nano-filler content to 2 wt% led to the drop in the
Tg value, this is due to their easy agglomeration arising from van der Waals attraction between particles.
Figure 5.
(a) DSC thermograms of neat epoxy and nanocomposites with 1 wt% of TiO2 and Ag-TiO2; (b) Variations in Tg values of neat resin and nanocomposites at different wt% of TiO2/Ag-TiO2 loading.
Figure 5.
(a) DSC thermograms of neat epoxy and nanocomposites with 1 wt% of TiO2 and Ag-TiO2; (b) Variations in Tg values of neat resin and nanocomposites at different wt% of TiO2/Ag-TiO2 loading.
It can be seen from
Figure 5b that the
Tg value increases steadily then value drops; this corroborates with the trend observed by other investigators [
13,
30]. With our study, the degree of dispersion and nanofiller loading affected the shifts in
Tg for epoxy/Ag-TiO
2 composites. The size, loading and dispersion state of the nanofillers are the factors that impact the glass-transition temperature. The
Tg value increases due to polymer chain-filler (organic-inorganic interfacial contact) that are immobilized by cohesive interactions at the interface of nanofiller in the bulk of the material. On the other hand, higher loading of nanofiller or their agglomeration can result in mobile moieties within the matrix which significantly decrease the glass transition temperature. Very high
Tg values are not achievable by room temperature curing agents, and the composites reported here can find their applications at temperature conditions below their
Tg. These synthesized epoxy composites may be cross linked by means of any conventional hardener at room temperature, without the decomposition of incorporated biocides.
3.2. Antibiofilm Activity on the TiO2 and Ag-TiO2 Nanocomposite Coatings
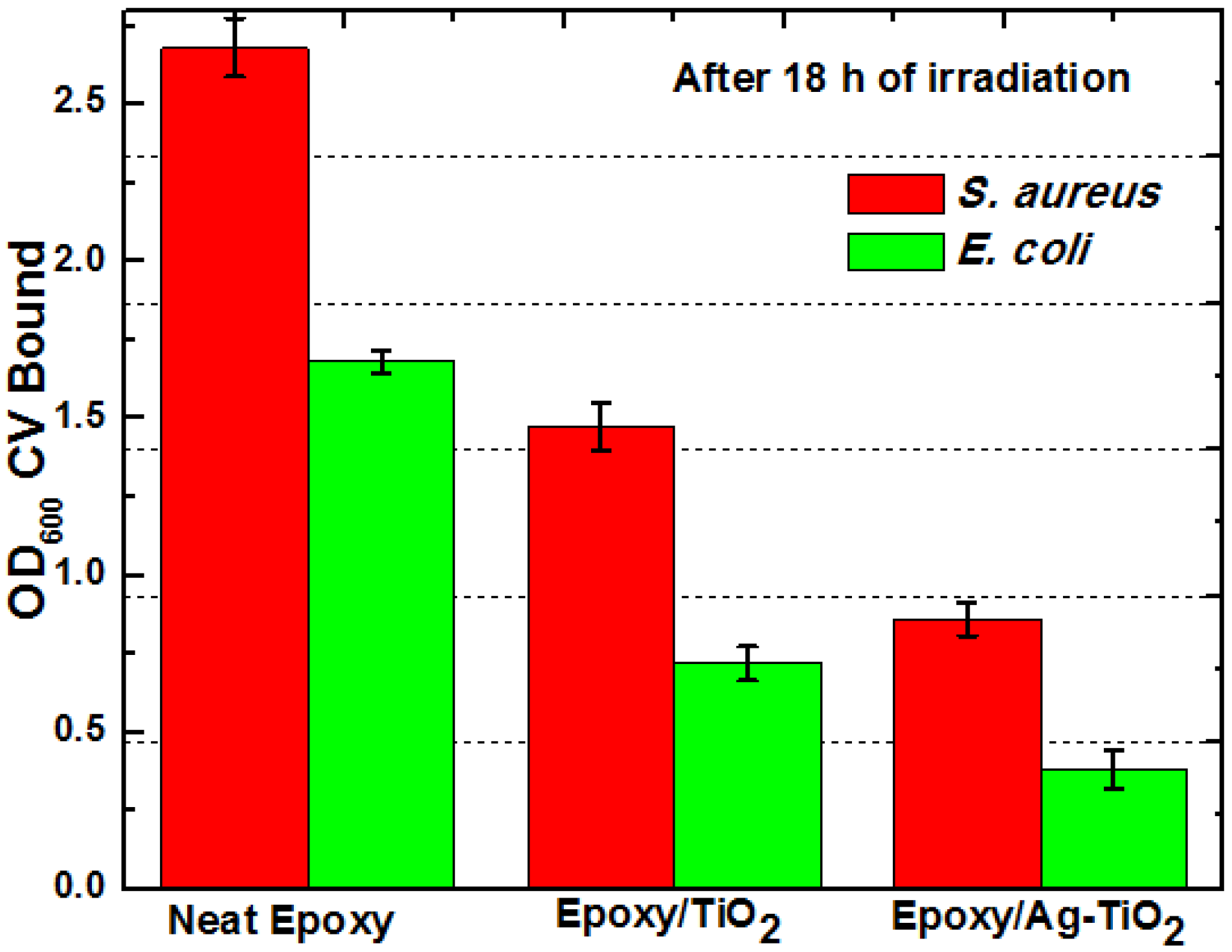
Antibacterial epoxy coatings for antibiofilm properties were tested against S. aureus and E. coli under static conditions in glass petri dish with UV-A irradiation, on the surfaces of TiO2 and Ag-TiO2 composites (both with 1 wt% loading). Both S. aureus and E. coli were able to form biofilm on neat epoxy resin surface (negative control) and composites, i.e., biofilm formation was independent of the underlying composite substrates. In the absence of TiO2, epoxy resin showed higher growth of biofilm than that of epoxy/TiO2 composite. Anti-boifilm activity appeared to increase significantly for Ag-TiO2 composite.
The biofilm inhibition by composites does not seem to be restricted to specific strains or growth conditions;
E. coli and
S. aureus varied in their ability to produce biofilm on the surface of the composites as shown in
Figure 6. In all assays, the amount of crystal violet eluted from
E. coli biofilms was lower than that of
S. aureus biofilms, because
E. coli, being a Gram negative organism binds lesser dye than Gram positive organisms like
S. aureus. The OD
600 of CV eluates from both biofilms was in the range of 0.121 to 2.8. Among the bacterial pathogens,
E. coli was more susceptible for biofilm inhibition than
S. aureus on these surfaces.
Figure 6.
Spectrophotometric analysis (OD600) of solubilized crystal violet of E. coli and S. aureus biofilm at 18 h irradiation time on the surfaces of TiO2 and Ag-TiO2 composite with similar loading (1 wt%).
Figure 6.
Spectrophotometric analysis (OD600) of solubilized crystal violet of E. coli and S. aureus biofilm at 18 h irradiation time on the surfaces of TiO2 and Ag-TiO2 composite with similar loading (1 wt%).
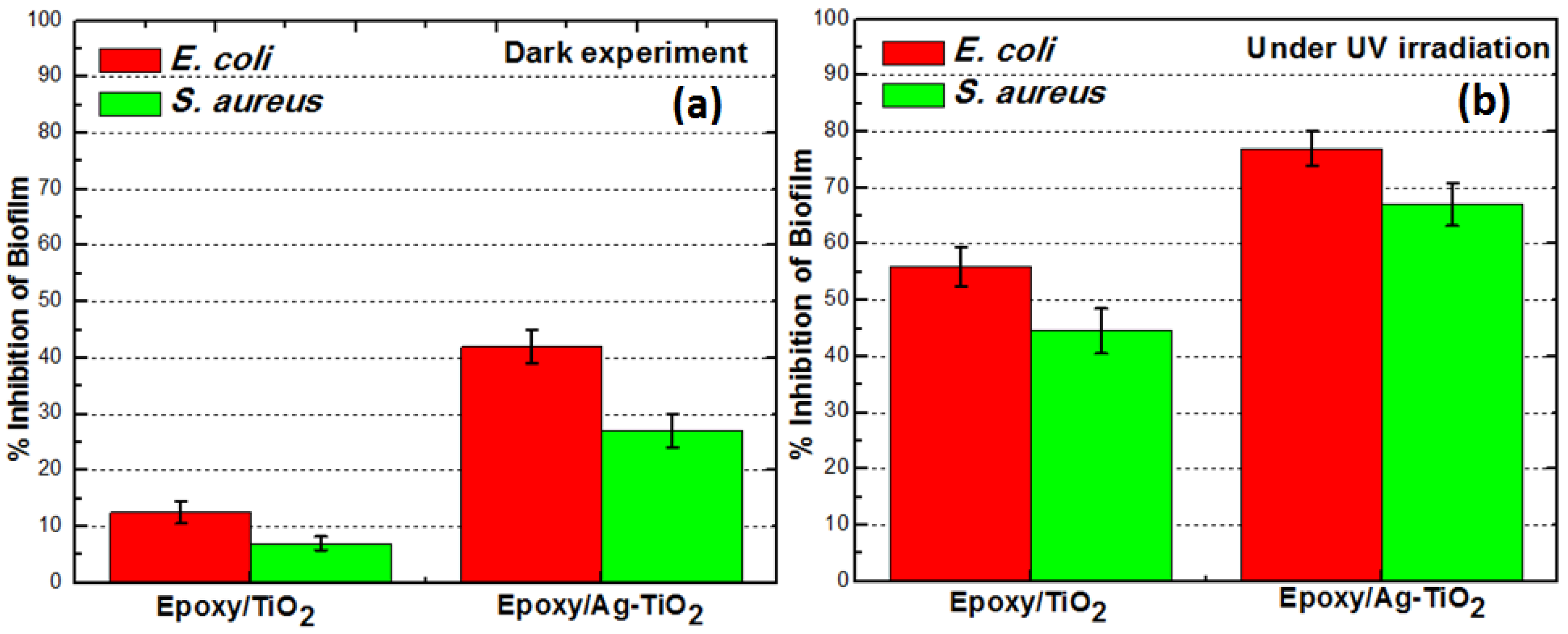
To confirm the activity of TiO
2/Ag-TiO
2 on the surface of nanocomposite for the photokilling, we conducted the experiments under both dark and irradiated conditions as shown in
Figure 7, and we found that higher inhibition of biofilm under irradiated conditions as shown in
Figure 7b. The Ag-TiO
2 composite (1 wt%) showed 24% and TiO
2 composite (1 wt%) showed 6% biofilm inhibition of
E. coli after 18 h of incubation in the dark as shown in
Figure 7a. For the same conditions with UV irradiation
E. coli biofilm showed 56% inhibition for epoxy/TiO
2 and 77% inhibition for epoxy/Ag-TiO
2, while that of
S. aureus biofilm showed 43% and 67% ihibition, for epoxy/TiO
2 and epoxy/Ag-TiO
2 composites respectively. It is, therefore, the bactericidal activity of silver on biofilm that is rendered more likely in the absence of photokilling by Ag-TiO
2 with the dark experiment data. However, enhanced antibiofilm response of Ag-TiO
2 composite under UV irradiation can be attributed to the silver surface plasmon band favoring UV light absorption along with nanometer sized silver particles which exhibited a striking degree of synergy. The antibacterial feature was diminished for epoxy/TiO
2 composite in the dark experiment. However, the bare TiO
2 particles which are non-photo-activated on the surface also supported minor antibacterial activity, even in the dark. This is due to direct attack of cells upon contact with TiO
2 nanoparticles which disrupt the integrity of the bacterial membrane [
31,
32]. This is also in agreement with reported experimental findings by Gogniat
et al. [
33] who also showed a loss of bacterial culturability after contact with TiO
2 nanoparticles even in the dark. These data show that the nature of epoxy resin makes it suitable host for dispersion of photocatalyst like TiO
2 for bacteriacidal activity.
Figure 7.
Mean values of quadruplicate experiments showing percent inhibition of E. coli and S. aureus bio-film formation on epoxy/TiO2 and epoxy/Ag-TiO2 composite surfaces calculated relative to the neat epoxy (negative control), under (a) dark and (b) UV irradiated conditions.
Figure 7.
Mean values of quadruplicate experiments showing percent inhibition of E. coli and S. aureus bio-film formation on epoxy/TiO2 and epoxy/Ag-TiO2 composite surfaces calculated relative to the neat epoxy (negative control), under (a) dark and (b) UV irradiated conditions.
The release of the antimicrobial species (Ag
+, Ag
0 and ROS) from a composite occurs due to the interaction of the diffused water molecules with TiO
2 and dispersed silver within the matrix during UV exposure; upon submerging it in the culture media [
34,
35]. Silver ions resident within the metal oxide nanofiller can diffuse to the surface of the epoxy matrix. The leaching of Ag
+ ions was confirmed by AAS analysis of the bacterial media from blank experiments (without inoculums as explained in the experimental section). The Ag
+ ion concentration of the same media was determined by atomic absorption spectrophotometer (AAS), which strongly suggests Ag
+/Ag
0 are associated noncovalently with cross-linked polymeric host and has leached to aqueous medium. By AAS analysis, the silver concentration (Ag
+/Ag
0) in the exposed media for the different epoxy/Ag-TiO
2 composite, showed a nonlinear increase that approached a maximum for the composite with 2.0 wt% of Ag-TiO
2 loading
Table 1.
The valence band “electrons” can be excited to the conduction band (
), leaving positive “holes” in the valence band (
) to form an e
−/h
+ couple that react with aqueous environment and oxygen, to generate reactive oxygen speces (ROS) such as OH
.−, HO
2.− and O
2.−, which are responsible for the mechanistic photo-biocidal activity [
36,
37].The photoexcitation of non-leachably associated TiO
2 occurs when it absorbs light equal to or greater than band-gap energy near-ultraviolet light region. While Ag NP and Ag
+ could act as efficient electron scavengers, and significantly enhanced the visible light responsiveness of TiO
2 to generate more oxygen free radicals by improving the quantum efficiency of a charge pair generated [
35]. At the same time, these oxygen species can reduce Ag
+ ions to form Ag nanoparticles. The smaller Ag
+ ions can easily penetrate the cell wall and thus can hasten antimicrobial activity.
The attack of Ag
+ on disulfide or sulfhydryl (thiol) groups present in the membrane protein result in formation of stable S–Ag bond with –SH groups thus inhibiting enzyme-catalyzed reactions and the electron transport chain that are necessary for biofilm formation [
38]. We speculate that the outer membrane of the bacterial cell is attacked by photocatalytic oxidation enabling the antimicrobial metal ions/particles to diffuse to interior of the cell thus becoming much more lethal to the bacterium. Thus, capability of photoactiveTiO
2 and leachable silver in destabilizing the biofilm matrix is enhanced by synergistic approach.
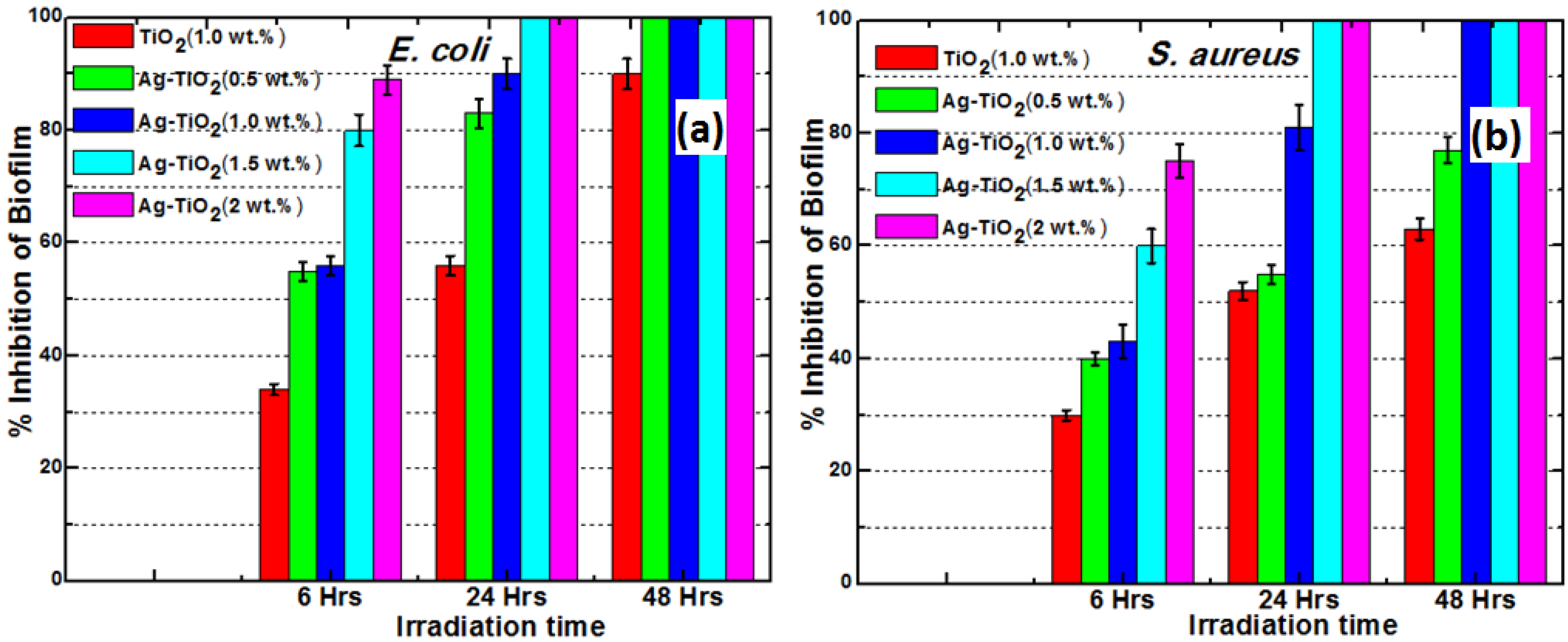
3.4. Effect of Ag-TiO2 Loading on Biofilm Inhibition
The results showed that biofilm formation was highly inhibited in a dose dependent manner as shown in
Figure 9. Increasing the load of Ag-TiO
2 resulted in shorter inhibition time
i.e., antibiofilm activity of composite is directly proportional to Ag-TiO
2 loading. Exposure of the composite with 1.5 wt% Ag-TiO
2 for 24 h. resulted in a inhibition of 100% (as per crystal violet binding assay) of both
E. coli and
S. aureus. The higher activity of these composites against
E. coli a Gram-negative bacterium is attributed to its thinner peptidoglycan cell wall compared to
S. aureus a Gram-positive bacterium. Complete inhibition of biofilm was achieved with 24 h of irradiation time with composite of Ag-TiO
2 with 1.5 wt% loading, in case of both
E. coli and
S. aureus (see
Figure 9a,b). The antibacterial activity could also have effect on planktonic bacteria due to silver that has diffused to media from the matrix. The bactericidal efficacy of these composite is through the diffusion of photogenerated ROS and Ag
+ particles (acting as a leaching biocide) to the surface from the bulk of the polymer where such species/particles attack proteins and membrane lipids in bacterial cell wall. The driving force for silver particle diffusion is determined by a concentration gradient, which forms between the bulk of the composite material and the surface. The diffusion behavior depends on several factors including the structure of the material, environmental osmolarity and temperature.
We have quantified the silver release characteristics at 37 °C for the composites loaded with the 0.5 wt% to 2.0 wt% Ag-TiO
2 filler
Table 1. And observed that non linear increase in the release of silver on increase of Ag-TiO
2 loading. The total released silver from the coatings was 6.6 to 16.8 μg/mL (16.8 ppm) after 48 h by epoxy/Ag-TiO
2 composites in the culture media without inoculum. From this observation it can be concluded that all the Ag-TiO
2 containing composites can have antibacterial activity even in the dark due to release of silver. However, presence of UV light will hasten the bactericidal activity of the composite due to photogeneration of ROS. Similar observations were made by Akhavan and Ghaderi [
41] who investigated bactericidal activity of the anatase-TiO
2, the Ag thin film and the Ag-TiO
2/anatase-TiO
2 nanocomposite thin film against
E. coli at dark and under UV exposure. In addition, they found superior antibacterial activity of Ag-TiO
2/anatase-TiO
2 nanocomposite thin film under the UV irradiation due its photocatalytic capability when compared to non-photocatalytic bare Ag and TiO
2 films and the silver ions released by Ag-TiO
2/anatase-TiO
2 nanocomposite thin film became saturated after 20 days at ~2 nM/mL. It is also possible to regulate the release of silver to the desired concentration by varying the nano-filler load incorporated into polymer composites and by tuning Ag-TiO
2 structure/composition during the sol-gel incorporation process. Antibiofilm activity of these composite remained unchanged at least for 5–6 cycles when we challenged during experiment through replications, this is due to continuous and uniform diffusion of the antimicrobial agents (ROS and silver species).
Figure 9.
Biofilm inhibitory effect of Ag-TiO2 loading (dose response) after 6, 24 and 48 h of irradiation on (a) E. coli and (b) S. aureus.
Figure 9.
Biofilm inhibitory effect of Ag-TiO2 loading (dose response) after 6, 24 and 48 h of irradiation on (a) E. coli and (b) S. aureus.
3.5. Quantitative Comparisions
There is no general consensus evolved for the comparison of efficiency of antibacterial activity of polymers surfaces between the research groups. However, most studies on antibacterial activity are interpreted by the number of surviving colony forming unit CFU/mL
−1 or per unit area. Kubacka
et al. [
42], studied the antibacterial effect of isotactic polypropylene (iPP) polymeric matrix incorporated with anatase-TiO
2 against
Pseudomonas aeruginosa (Gram negative) and
Enterococcus faecalis (Gram positive). They reported a maximum reduction by
ca. 8–9 log in 30 min in case of
P. aeruginosa. Francolini
et al. [
19] evaluated the effect of (+)-usnic acid incorporated into modified polyurethane surfaces on the biofilm forming ability of
S. aureus. After three days postinoculation, they found culturable biofilm cell concentration of
S. aureus on the untreated polymer was 7.3 log
10 CFU/cm
2 compared to 0.9 log
10 CFU/cm
2 on the (+)-usnic acid-containing polymer. Cen
et al. [
20] introduced pyridinium groups at 15 nmol/cm
2 on the surface of poly(ethylene terephthalate) (PET) film and demonstrated its bactericidal effect against
Escherichia coli. Jansen
et al. introduced silver ions by plasma-induced grafting onto polyurethane films which was found to reduce adherent viable bacteria from initial 10
4 cells/cm
2 to zero within 48 h [
43]. Jiang
et al. [
44] coated silver on silicon rubber substrates and showed decline in number of
L. monocytogenes cells post 6 h. After 12 h, there was a reduction of over 2-log
10 CFU/chip, and no viable bacteria were detected on both types of silver-coated SR after 18 and 24 h. Sambhy
et al. [
21] demonstrated antibacterial activity of composites consisting of poly(4-vinyl-N-hexylpyridinium bromide) (NPVP) embedded with silver bromide nanoparticles. They observed no biofilm formation on 1:1 AgBr/21% NPVP-coated surfaces after 72 h when incubated for 24–72 h with
P. aeruginosa suspension (10
7 CFU/mL) in LB broth. Pant
et al. [
45] have demonstrated the ability to eliminate up to 99.9% of pathogenic bacteria on the surface of siloxane epoxy system containing quaternary ammonium moieties. In another work involving epoxy system, Perk
et al. [
46] observed fungicide, carbendazim supported on poly (ethylene-
co-vinyl alcohol) and epoxy resin coating showed the antifungal activity contingent upon release from their polymer supports.
Coatings and thin films based on titania photoctalysts (Ag
+-doped TiO
2/Ag-TiO
2/TiO
2) that kills microbes under UV and visible light illumination, also have been actively investigated in recent years. Studies by Necula
et al. [
47], with TiO
2-Ag composite coating prepared by plasma electrolytic oxidation on implantable titanium substrate, showed the ability to completely kill methicillin-resistant
S. aureus (MRSA) within 24 h. In yet another investigation by Necula
et al. [
25], they examined the ion release and antibacterial activity of porous TiO
2-Ag coating on biomedical alloy disk. Each evaluated samples could release 20.82 and 127.75 µg of Ag
+ per disk and showed markedly enhanced killing of the MRSA inoculums with 98% and >99.75% respectively within 24 h of incubation, while their silver free counterpart sample allowed the bacteria to grow up to 1000-fold. The non-cumulative release of silver ions of 0.4 ppm, 0.26 ppm and 0.005 ppm for 1 h, 24 h and 7 days respectively after immersion in water, from nanometer scale Ag-TiO
2 composite film was demonstrated by Yu
et al. [
34] and they also reported that 0.4 ppm released silver from Ag-TiO
2 composite film is sufficient to cause almost 100% killing of
E. coli when exposed to UV for 1 h. Studies by Jamuna-Thevi
et al. [
48], reported nanostuctured Ag
+ doped TiO
2 coatings deposited by RF magnetron on stainless steel, with overall Ag
+ ion release measured between 0.45 and 122 ppb. They also noted that at least 95 ppb Ag
+ ion released in buffered saline was sufficient for 99.9% of reduction against
S. aureus after 24 h of incubation. Biological activity of silver-incorporated bioactive glass studies conducted by Balamurugan
et al. [
49] assessed
in vitro antibacterial bioactive glass system elicited a rapid bactericidal action. Antimicrobial efficacy of these silver-incorporated bioglass suspension at 1 mg/mL for
E. coli was estimated to be >99% killing, and the amount of Ag
+ released from silver-incorporated glass was up to 0.04 mM after 24 h. In yet another study involving silver ions release by Liu
et al. [
35], the amount of silver released form the mesoporous TiO
2 and Ag/TiO
2 composites was measured to be 1.6 × 10
−8 mol after 20 days. The photo-bactericidal activity on composite films was extremely high and displayed bactericidal activity even in the dark; they further reported that the survival rate was only 9.2% in the dark, and the
E. coli cells were totally killed in UV light. Sun
et al. [
50] reported killing of bacteria on Ag-TiO
2 thin film, even in the absence of UV irradiation against
S. aureus and
E. coli with significant antibacterial rate about 91% and 99% after 24 h respectively due to release of silver, and the concentration of silver ions released from the Ag-TiO
2 film was 0.118 μg/mL during 192 h. Akhavan [
51], reported that a concentration of 2.8 to 2.5 nM/mL completely killed 10
7 CFU/mL
E. coli with visible light response photocatalytic Ag-TiO
2/Ag/a-TiO
2 material in 110 min. However, in most of the cases reports are based on planktonic studies and the release of silver is dependent upon the method employed for coating, thickness, conditions for gradient formation and silver source used. Nevertheless, release of silver ions frombare Ag/TiO
2 composite layers reported above, obtained by methods
viz., impregnation, deposition and nano-coatings gradually diminish over the time.
Bacterial biofilms are often more difficult to eradicate unlike planktonic cells. Until now, there have been very few reports that shown to resist biofilm formation on titania based polymer-nanocomposites. In one such study, Kubacka
et al. [
52] have demonstrated photocatalysis using ethylene-vinyl alcohol copolymer (EVOH) embedded with Ag-TiO
2 nanoparticles (
ca. 10
−2 wt%) that showed outstanding resistance to biofilm formation by bacteria and yeast, upon ultraviolet (UV) light activation. In the present study, although the release kinetics of silver was not established but comparing to above studies which established the antimicribial threshold concentration of silver and efficacy of killing with different bare Ag-TiO
2 (Ag/Ag-TiO
2 nanofilms), the polymer composite system reported here which released 6.4 to 16.8 μg/mL of silver seems adequate [
53], when the overall biocidal ability (to prevent bacterial attachment) of the composite during 48 h period in combination with radical-mediated photocatalytic action. Practically, the added strengths of the polymer-based Ag-TiO
2 nanocomposite coatings as compared to bare TiO
2/Ag-TiO
2 coatings are its wear stability, flexibility, permeability and optical properties.
But the main objective of the disinfection technology in ensuring microbiological safety is to; set a standard for achieving a required logarithm of reduction of the microbial consortia. The microbial cells, which are not inactivated by the antimicrobial coatings adhering onto the testing surface over the different irradiation time, were able to grow on the agar plates. Quantifying their reduction in number (for quantitative assessment) of surviving CFU on a bactericidal surface compared with a non-bactericidal (neat epoxy) surface revealed reduction of microbial cells. In the present study, epoxy/Ag-TiO
2 with 1.0 wt% loading was found to cause a reduction of CFU on agar plates by approximately 6-log in case of
E. coli and the same effected
ca. 4-log reduction in case of
S. aureus after 48 h of incubation, while epoxy/TiO
2 with 1.0 wt% loading exhibited lesser inhibition of biofilm formation, see
Table 2.
There was an initial slower decrease in bacterial load by all the composites,
i.e., below 1-log reduction observed up to 18 h exposure followed by a rapid microbial decrease up to 6-log in 48 h for both 1.0 wt% of TiO
2 and Ag-TiO
2 loaded epoxy composites. Incomplete inhibition of biofilm formation was observed with lesser Ag-TiO
2 loading, but complete inhibition of both
E. coli and
S. aureus was possible for composites with above 1.5 wt% of Ag-TiO
2 after 24 h with UV irradiation. Strikingly, for the composite coating with 2.0 wt% epoxy/Ag-TiO
2 showed highest antibiofilm effectiveness with 1-log reduction in 18 h,
i.e., the shortest period with maximum inhibition. In addition, after 48 h of irradiation against both
S. aureus and
E. coli with very few surviving CFUs and complete inhibition (biofilm formation) and 7-log reduction was observed, relative to that in control plates as shown in
Table 2. However, the present study results take into consideration only biofilm phase inhibition, and the obtained concentrations of range 6.4–16.8 µg/mL (ppm) Ag
+ is very high (many times above minimum biocidal concentration levels) to radically prevent microbial cell viability. The polymer-based nanocomposite reported here obtained by dispersion of the Ag-TiO
2 nanoparticles into epoxy manifest a real potential as photobiocidal coatings in a wide variety of settings that prevents biofilm formation by a wide range of Gram-positive and Gram-negative bacteria.
Table 2.
Different nanocomposite materials and their antibiofilm efficacy for 18 and 48 h irradiation time.
Table 2.
Different nanocomposite materials and their antibiofilm efficacy for 18 and 48 h irradiation time.
| Composite type | E. coli (G−ve) | S. aureus (G+ve) |
|---|
| % Inhibition a | Log10 Reduction b | % Inhibition a | Log10 Reduction b |
|---|
| % Biofilm inhibition and Log CFU reduction after 18 h |
| 1wt% Epoxy/TiO2 | 57.2 (±1.5) | ˂1.0 (±0.03) | 46.0 (±1.4) | ˂1.0 (±0.02) |
| 1wt% Epoxy/AgTiO2 | 77.0 (±1.4) | ˂1.0 (±0.02) | 68.5 (±2.0) | ˂1.0 (±0.02) |
| 2wt% Epoxy/AgTiO2 | 90.0 (±1.3) | 1.0 (±0.2) | 90.0 (±1.4) | 1.0 (±0.03) |
| % Biofilm inhibition and Log CFU reduction after 48 h |
| 1wt% Epoxy/TiO2 | 90.0 (±1.0) | 1.0 (±0.2) | 63 (±0.9) | 1.0 (±0.2) |
| 1wt% Epoxy/AgTiO2 | 100 | 6.0 (±0.18) | 99.9 (±0.1) | 4.0 (±0.11) |
| 2wt% Epoxy/AgTiO2 | 100 | 7.0 (±0.19) | 100 | 7.0 (±0.2) |