Abstract
Leather waste carbonized at 800 °C in an inert atmosphere was coated in situ with the conducting polymer polyaniline. The composition of composites varied from neat carbonaceous to polyaniline. Due to the fibrous collagen structure of the original leather after carbonization, the composites had a bicontinuous conducting morphology. The resistivity of composites determined as a function of applied pressure from 0.1 to 10 MPa fell mainly into the range of units to tens of Ω cm. In contrast to neat polyaniline, the composites maintained a good level of conductivity even under alkaline conditions. The application of a composite as an adsorbent of organic-dye pollutants in water treatment was illustrated using methylene blue and methyl orange with an eye to future functional adsorbents controllable by applied electrical potential.
1. Introduction
Various forms of carbon have widely been used in a variety of applications, but in recent years increasing attention has been paid to solid carbonized organic materials, biochars, due to their simplicity of preparation by pyrolysis of natural organic materials. The environmental aspect has also become important because the carbonization substrates are mainly industrial and agricultural wastes. Most importantly, biochars are used as adsorbents in water-pollution treatment [1] and as the conducting component of electrodes in energy-storage devices [2]. The large, specific surface area of activated carbons is of benefit in both cases. Biochars appear also as compost fertilizers in agriculture [3], additives in construction materials [4], bitumen binders [5] or fuels [6].
Organic conducting polymers such as polyaniline are also easy to prepare by the oxidation of aniline in acidic aqueous media [7]. Their application potential consists in their specific ability to produce submicrometer conducting coatings [8,9,10,11] on any substrate immersed in the reaction mixture. Conducting polymers have been used in supercapacitor and battery electrodes [2,12], and they are also efficient adsorbents of organic dyes or photocatalysts of their decomposition in water-pollution treatment [13,14]. Conducting polymers are rated among functional materials as they participate in redox conversions and salt/base transformations. They also contribute to ionic as well as electronic conduction.
The remediation of wastewater containing organic dyes is typically based on adsorption at various substrates, the photocatalytic decomposition by reactive oxygen species [13,14] or biodegradation [15,16]. There are two reasons for introducing conducting polymers as a component to hybrid composites for dye adsorption. First, the molecular structure of conducting polymers and dyes share some features, such as aromatic rings and nitrogen atoms. The former allows for π–π interactions; the latter for hydrogen bonding, and for adsorption of dyes. They may also contribute to the electrostatic interaction between conducting polycations and anionic dyes. Second, unlike carbonaceous adsorbents, the molecular structure of conducting polymers can be switched between protonated salt and base forms, as well as oxidized and reduced structures, viz. the pernigraniline and leucoemeraldine forms of polyaniline, in response to applied pH or electrical potential. When combined with carbonaceous material, the mutual improvement of the material’s properties—mechanical integrity, specific surface area and porosity—is the goal, as is the enhancement of conductivity, the stability of electrical properties and electrochemical performance. Synergistic effects at the interfaces between the carbonaceous component and conducting polymers is also possible [17,18].
It has recently been demonstrated that carbonized leather waste had a conductivity that increased with carbonization temperature that reached or even exceeded that for conducting polymers [19,20,21]. Instead of a simple mixing of both components, a leather carbonized at 800 °C was coated with polyaniline in situ during preparation to produce a bicontinuous conducting structure. Such morphology is favourable for electrical conduction. The study illustrates the use of composites as dye adsorbents with the possibility of future adsorption control by external stimuli.
2. Experimental
2.1. Materials
Beige flat-lined chrome-plated pigskin leather with a thickness of 0.8 mm was supplied by the Footwear Research Centre, Tomas Bata University in Zlin, Czech Republic. Aniline hydrochloride, hydrochloric acid, ethanol, ammonium peroxydisulfate, ammonium hydroxide, methyl orange and methylene blue were purchased from Sigma Aldrich (St. Louis, MO, USA) and used as delivered.
2.2. Carbonization of Leather
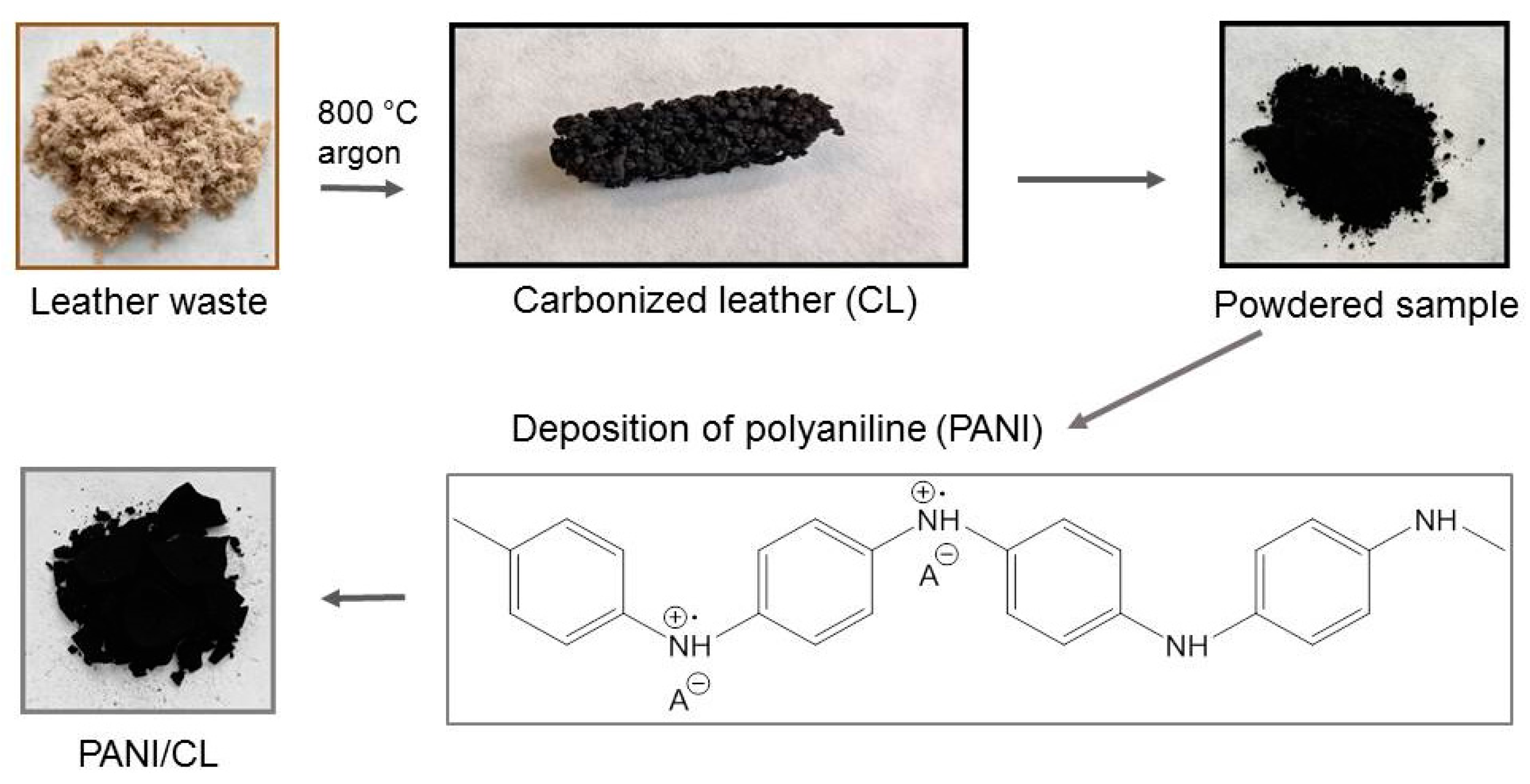
The pieces of leather simulating the waste were shredded into lengths of approximately 4 mm and then transferred into a bench-top horizontal tubular vacuum furnace GSL-1600X (Carbolite Gero, Neuhausen, Germany). The temperature increased at a rate of 5 °C min−1 to the target temperature of 800 °C with a flow rate of argon of 50 mL min−1 and kept there for 1 h. The samples were left to cool under the inert atmosphere. The temperature was selected based on a preliminary study [20] as a compromise between complete carbonization, sufficient yield and good conductivity. Carbonized leather was micronized into fine particles using a ball mill. The product contained 70.7 wt% of carbon, 7.6 wt% of nitrogen, and 12.6 wt% of chromium [19].
2.3. Coating with Polyaniline
Various amounts of the carbonized leather (0.2, 0.4, 0.5, 0.6 and 0.8 g) were added to an aqueous solution of 0.2 M aniline hydrochloride (2.59 g in 100 mL) under stirring at room temperature. An oxidant solution of 0.25 M ammonium peroxydisulfate (5.71 g in 100 mL) was added. After 1 h to allow for the polymerization of aniline, the composite precipitates were separated by filtration, rinsed with 0.2 M hydrochloric acid followed by repeated portions of ethanol and left to dry in open air to a constant weight.
The concentrations of reactants after mixing the solutions were 0.1 M aniline hydrochloride and 0.125 M ammonium peroxydisulfate. A 100 mL of mixture produces ca 1 g of polyaniline hydrochloride [7]. As the amount of carbonized leather increased, the volume of the reaction mixture was reduced to generate 1 g of the composite (Table 1). The yield well corresponded to expectations and confirmed that the planned composition was achieved. The part of the composite containing 50% polyaniline was deprotonated by overnight suspension in 1 M ammonium hydroxide, separated, rinsed with ethanol, and dried as above. The components, carbonized leather and polyaniline, were treated in the same manner for comparison.

Table 1.
Polyaniline/carbonized leather composites: preparation conditions and yield.
2.4. Morphology
The scanning electron microscope Nova NanoSEM (FEI, Brno, Czech Republic) was used to depict the surface morphology of the composites and their components. Prior to analysis, the samples were gold sputtered with a JEOL JFC 1300 Auto Fine coater (JEOL, Tokyo, Japan).
2.5. Resistivity
Resistivity was determined by the four-point van der Pauw method using a lab-made press based on a cylindrical glass cell with an inner diameter of 10 mm [22]. The powders were placed between a glass support and a glass piston with four platinum/rhodium wire electrodes on the perimeter. The set-up used a Keithley 220 current source, a Keithley 2010 multimeter and a Keithley 705 scanner with a Keithley 7052 matrix card. The pressure applied to samples up to 10 MPa was recorded by a L6E3 strain gauge cell (Zemic Europe BV, Etten-Leur, The Netherlands). Force was exerted using an E87H4-B05 stepper motor (Haydon Switch & Instrument Inc., Waterbury, CT, USA). Sample thickness was monitored during the compression by a dial indicator Mitutoyo ID-S112X (Mitutoyo Corp., Sakado, Japan). The resistivity was also determined separately on composite pellets prepared after compression at 527 MPa by a manual hydraulic press.
2.6. Dye Adsorption
The performance of the composites in removing anionic and cationic dyes from aqueous solutions by composites was studied. Dye adsorption was carried out in a batch reactor for 24 h at 25 °C,: pH 5.5; initial dye concentration 100 mg L−1; and sample dosage 500 mg L−1. Adsorption efficiency was followed by recording optical absorption using a Varian Cary 100 UV-Vis spectrophotometer (Varian Inc., MA, USA) in a 0.2 cm cell at an absorption maximum of 466 and 594 nm for methyl orange and methylene blue, respectively. The dye removal efficiency was then calculated as R (%) = (A0 − Ae)/A0 × 100 [23], where A0 and Ae were optical absorptions at the beginning and equilibrium, respectively.
3. Results and Discussion
3.1. Composite Preparation
When the open-pore microporous conducting template was coated with another conducting material, it resulted in an internal/external bicontinuous conducting network [24,25] (Figure 1). In the present study, the leather consisted of collagen fibers. The fibrous microstructure was preserved after carbonization and became conductive [21]. Subsequent coating with a conducting polymer, resulted in a bicontinuous conducting morphology with separated conducting phases. Carbonized leather was obtained as a piece of macroporous material, and its integrity confirmed the continuous character of its structure (Figure 2). It had been milled prior to the deposition of polyaniline but the obtained microparticles still retained their fibrous character (Figure 3). When the powder was suspended in the reaction mixture used for preparing the polyaniline, the individual fibers became coated with a thin film of the conducting polymer [26], typically 100 nm thick [8]. A free globular polyaniline precipitate may accompany the coated fibers especially when the content of the carbon substrate to be coated is low but the coating takes places preferentially [27].
Figure 1.
Carbonized collagen fibers coated with a conducting polymer, polyaniline.
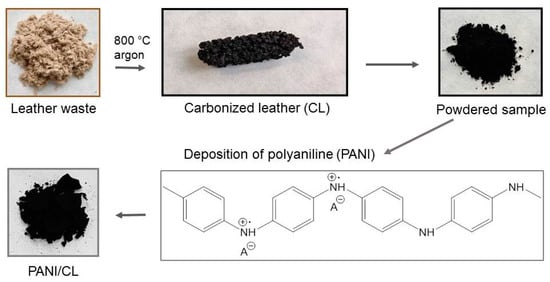
Figure 2.
Schematic presentation of the modification of carbonized leather (CL) with polyaniline (PANI).

Figure 3.
Micrographs of carbonized leather before and after coating with polyaniline (PANI).
3.2. Resistivity
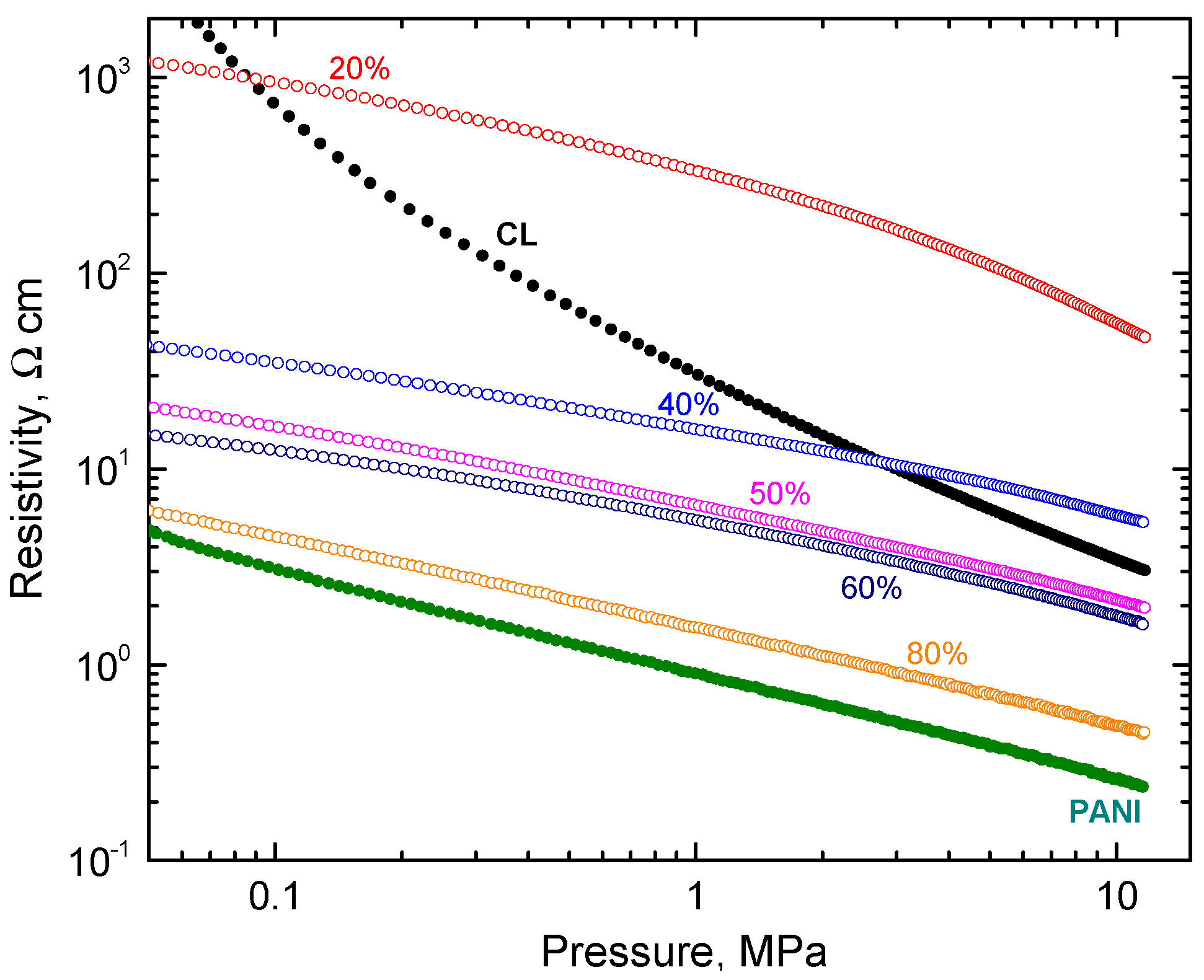
The electrical properties of the conducting materials are of key importance for many applications. Carbonized leather cannot be compressed into pellets, which are used for routine resistivity determination, so the measurement of powders under gradual compression was applied (Figure 4). The resistivity of carbonized leather determined in the same manner was higher compared to that of polyaniline by one or two orders of magnitude during compression.
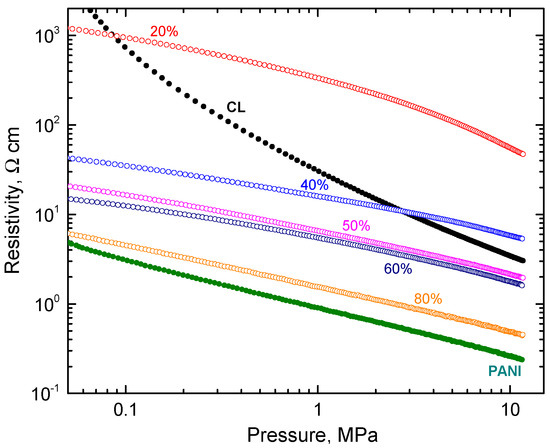
Figure 4.
The pressure dependence of resistivity for carbonized leather (CL) and polyaniline (PANI) (full circles) and their composites with various amounts of polyaniline (open symbols).
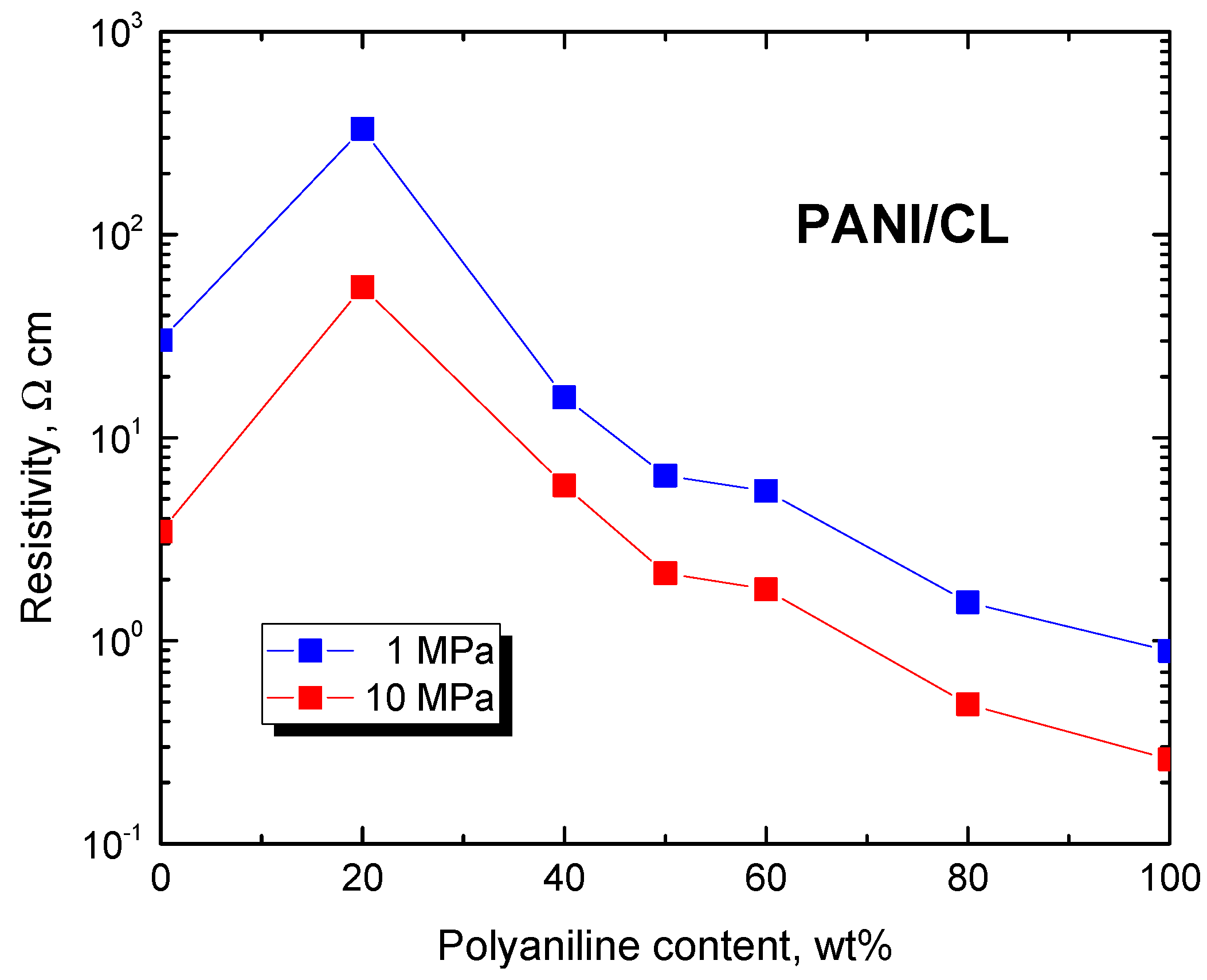
The resistivity of composites decreased with increasing polyaniline content at low pressure below 0.1 MPa as expected (Figure 4). At high pressure, the resistivity decreased only after polyaniline content was above 40 wt% (Figure 5). Above this limit, the electrical properties were clearly dominated by polyaniline, carbonized leather being rather a filler.
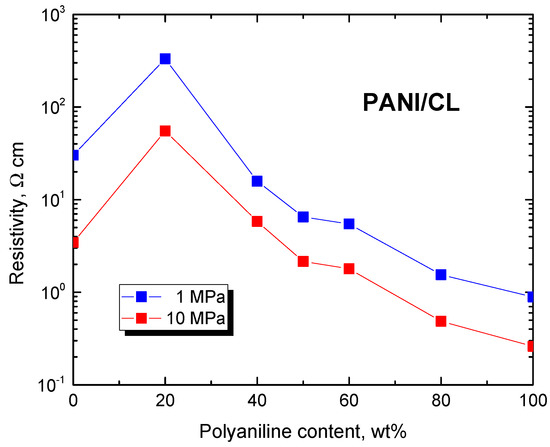
Figure 5.
Resistivity of composites of polyaniline/carbonized leather determined at 1 and 10 MPa pressure.
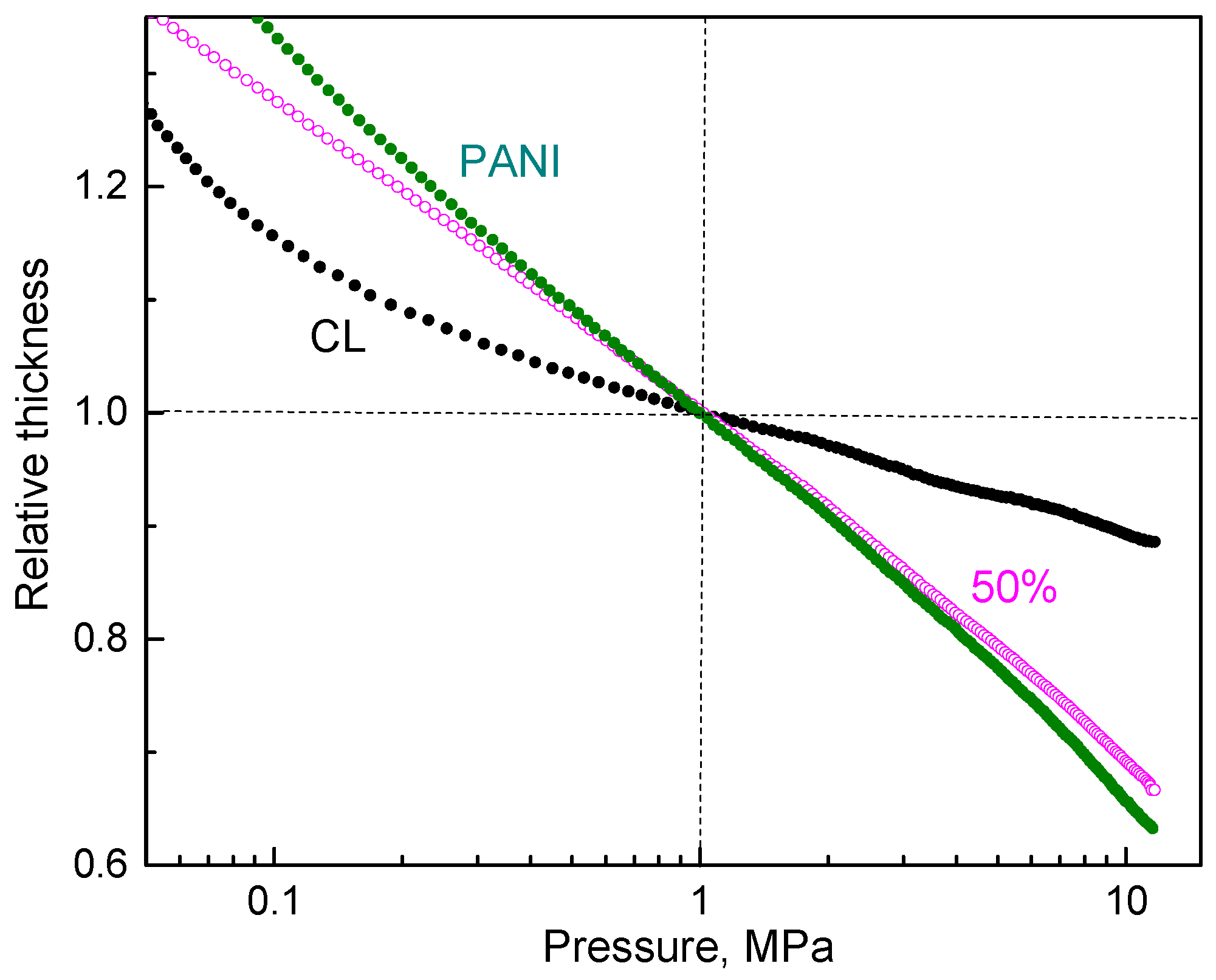
The pressure dependence of resistivity was affected by the morphology and mechanical properties of the particles. This effect was visible from the change in sample dimensions during compression (Figure 6). The steeper these dependences, the more easily the samples yielded to pressure, i.e., they became fluffier and easily compressible.
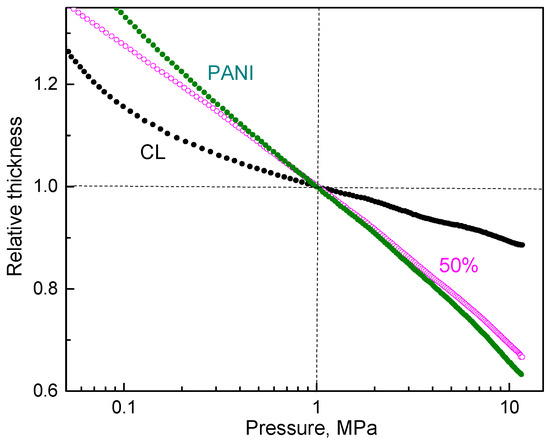
Figure 6.
The pressure dependence of sample thickness normalized to 1 MPa compression for carbonized leather (CL), polyaniline (PANI) and their composite (50 wt% PANI).
3.3. Deprotonation
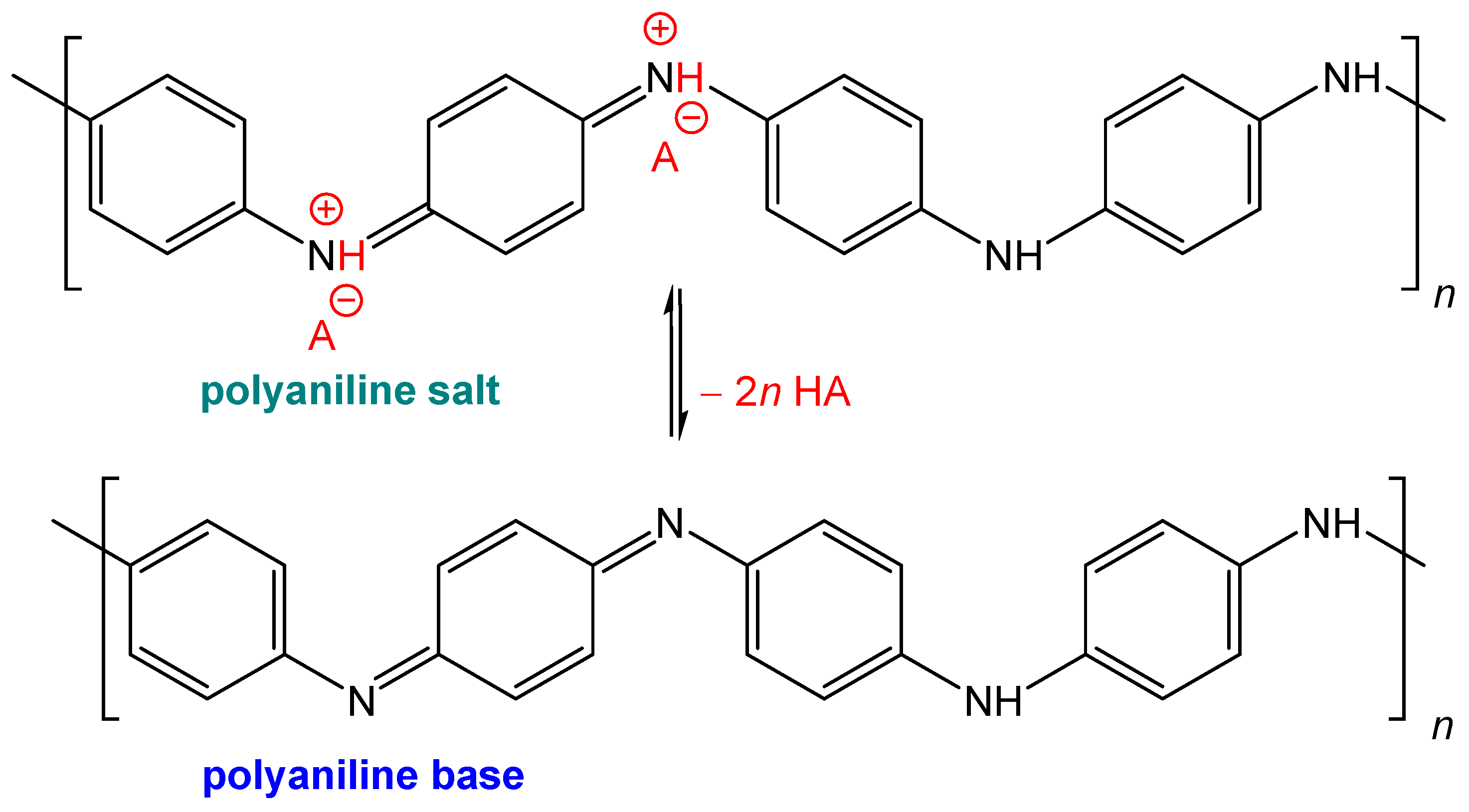
Polyaniline is known for its salt–base transition (Figure 7). Under acidic conditions, green polyaniline salt has a conductivity of ca 4 S cm−1 [7] and converts to a blue non-conducting base with a conductivity < 10−10 S cm−1 under alkaline conditions. If, for example, polyaniline has to be used for any reason under physiological conditions close to pH 7 [10], the reduction or even loss of conductivity may be a problem.
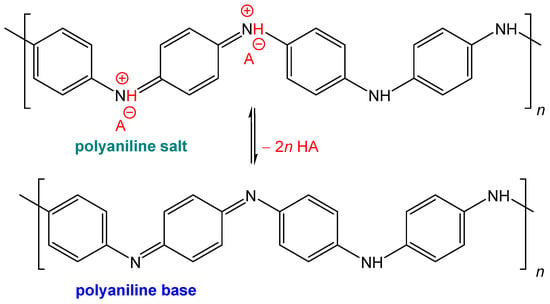
Figure 7.
Conducting green polyaniline salt converts to a non-conducting blue base under alkaline conditions in a pH transition range of 4–6. A is an arbitrary counter-ion, typically chloride.
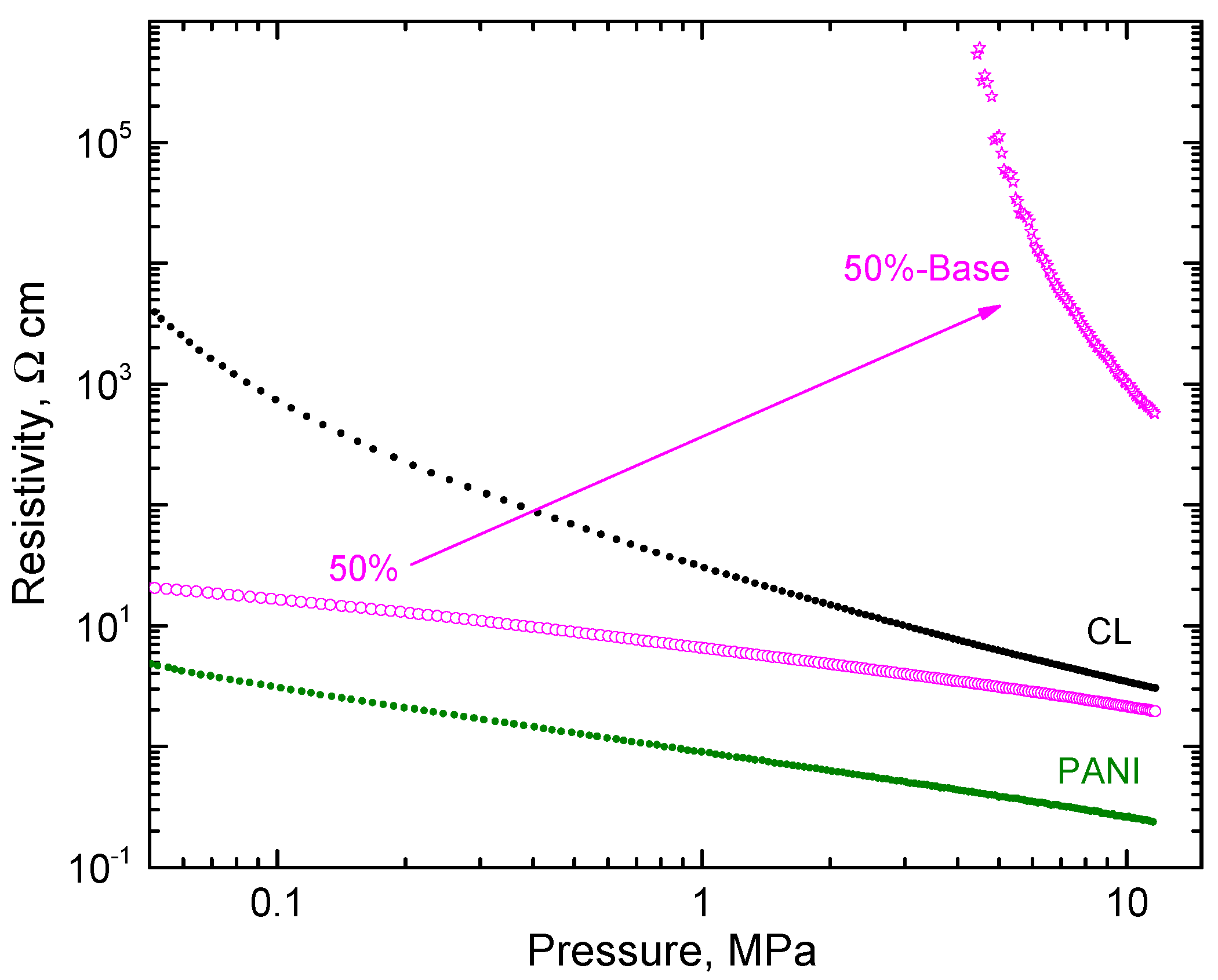
On the hand, carbonized leather has a conductivity that is pH-independent. No change in resistivity was observed after treatment with an ammonia solution. When composite-polyaniline (50 wt%)/carbonized leather was treated with ammonia, the resistivity increased by about three orders of magnitude at 10 MPa pressure (Figure 8) but still retained a good level of conductivity as required by the applications, e.g., in functional dye adsorbents, when dye removal is controlled by applied electrical potential [28,29].
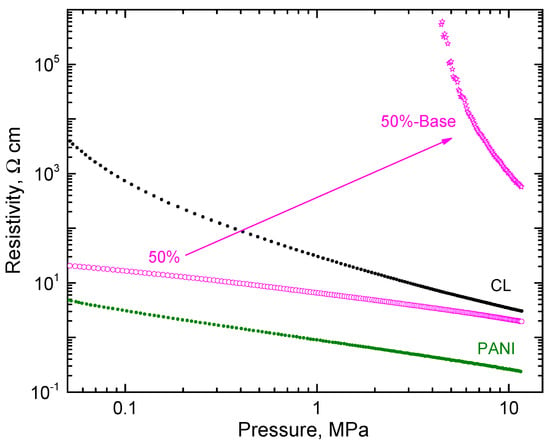
Figure 8.
The pressure dependence of resistivity of the polyaniline/carbonized leather (50/50 wt%) composite and corresponding base. The data for the components are shown for comparison.
3.4. Dye Adsorption
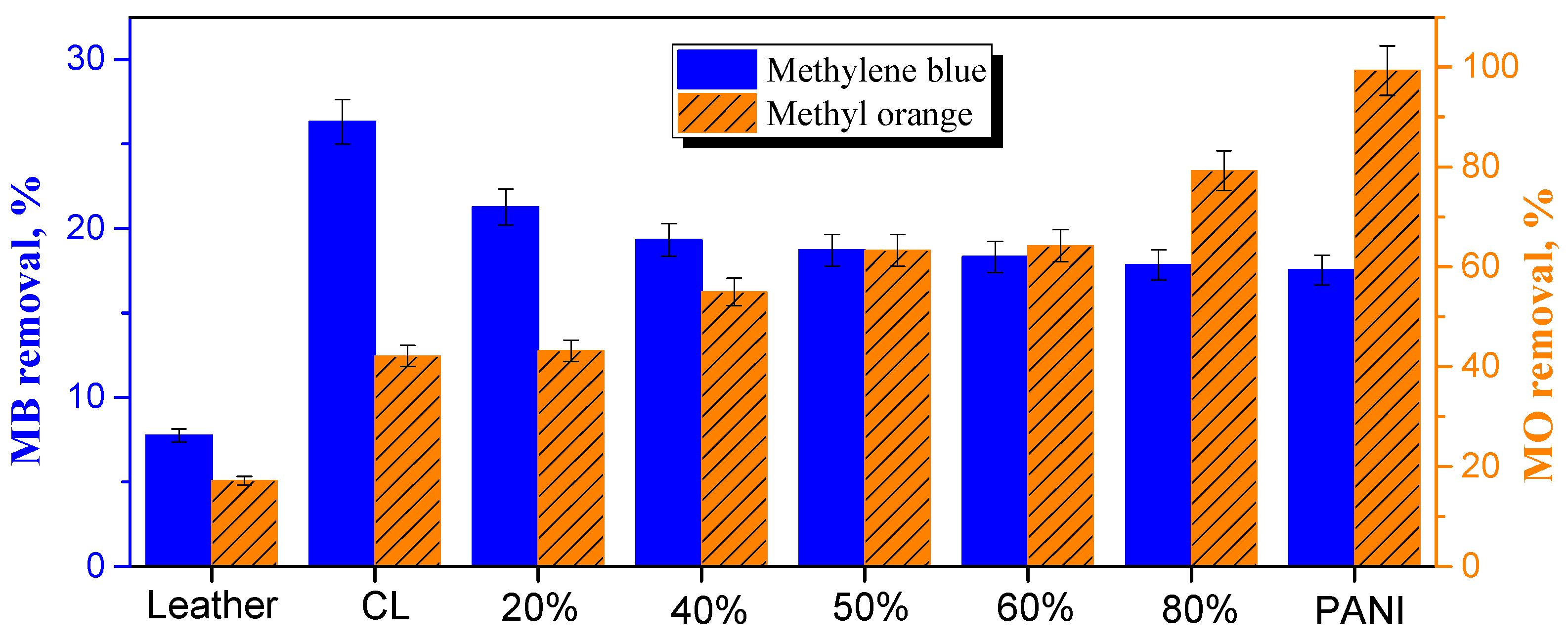
It has to be emphasized that the adsorption tests reported below were not optimized with respect to adsorbent and dye concentrations, contact time, temperature, or pH. They were meant just to demonstrate the potential feasibility of carbonized leather and its composites to be used as dye adsorbents and to reveal trends. Leather waste was tested in the literature for the adsorption of both anionic and cationic dyes [30], the removal of which was found to be dependent on the manner of tanning. In the present study, the removal of cationic methylene blue was only 7.9%, while for anionic methyl orange it reached 17% (Figure 9). The adsorption at carbonized leather waste reported in the literature has recently been reviewed [21]. It has been applied mainly for the adsorption of the cationic dye, methylene blue [31,32,33,34,35,36]. From anionic dyes, the adsorption of Acid Black 210 [37,38] and Acid Brown 414 [30] was investigated. The authors generally concluded that the carbonized leathers were adsorbents competitive with commercial carbons or other carbonized substrates. Compared to original leather waste, the carbonized analog in the present tests performed better than expected: removal of methylene blue 26% and methyl orange 42% (Figure 9).
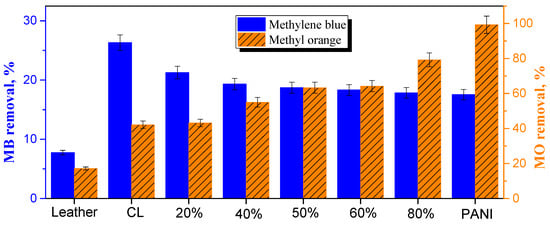
Figure 9.
The adsorptive removal of cationic methylene blue or anionic methyl orange at original leather, carbonized leather (CL) and the composites with deposited fraction of polyaniline (PANI). N.B. The scales for each dye are different!
Conducting polymers, such as polyaniline and polypyrrole, have been extensively used in the removal of organic dyes from the aqueous media by adsorption or photocatalytic decomposition as demonstrated by recent reviews [13,14]. The combination of carbonaceous materials with conducting polymers is thus of potential interest in the design of tunable functional adsorbents. As the content of polyaniline in the composite with carbonized leather increased, the removal of cationic methyl orange dropped mildly (Figure 9). The adsorption of methyl orange, however, steadily increased with growing polyaniline content and neat polyaniline removed methyl orange practically completely. This was expected due to the electrostatic interaction between an anionic dye and a polyaniline polycation [39] (Figure 7).
4. Conclusions and Future Prospects
Carbonized leather waste was coated with a conducting polymer, polyaniline. Due to the fibrous nature of carbonized leather, the composites had a bicontinuous conducting morphology. The resistivity of both components (carbonized leather and polyaniline) on powders under applied pressure was close to each other. For that reason, the composites had a resistivity on the order of units to tens Ω cm, i.e., only a little dependent on polyaniline content. The composites of this type offer some potential benefits. Polyaniline lost conductivity under alkaline conditions while the composite retained conductivity even under such a condition. This may be of advantage in applications operating under physiological conditions. Due to the responsivity of polyaniline to external stimuli, the bicontinuous composites could be used in sensing, as heating elements or in electromagnetic interference shielding. Their application as dye adsorbents in treating polluted water is offered as another prospect. As an example, the removal of anionic methyl orange and cationic methylene blue from aqueous media was provided. The adsorption of the former dyes increased along with the content of polyaniline while the adsorption of the latter slightly decreased. Composites of this type pave the way to functional adsorbents controlled by applied electrical potential.
Author Contributions
J.S.: Conceptualization, Writing—Original Draft. F.A.N.: Investigation, Formal Analysis. T.S.: Funding Acquisition, Resources. J.P.: Methodology, Data Curation. All authors have read and agreed to the published version of the manuscript.
Funding
The authors thank the Ministry of Education, Youth, and Sports of the Czech Republic (RP/CPS/2022/005) for financial support.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rossi, D.; Cappello, M.; Antognoli, M.; Brunazzi, E.; Seggiani, M. Pyrolyzed tannery sludge as adsorbent of volatile organic compounds from tannery air emissions. Chem. Eng. J. 2023, 454, 140320. [Google Scholar] [CrossRef]
- Ul-Hoque, M.I.; Holze, R. Intrinsically conducting polymer composites as active masses in supercapacitors. Polymers 2023, 15, 730. [Google Scholar] [CrossRef]
- Kuligowski, K.; Cenian, A.; Konkol, I.; Swierczek, L.; Chojnacka, K.; Izydorczyk, G.; Skrzypczak, D.; Bandrow, P. Application of leather waste fractions and their biochars as organic fertilisers for ryegrass growth: Agri-environmental aspects and plants response modelling. Energies 2023, 16, 3883. [Google Scholar] [CrossRef]
- Sivaprakash, K.; Maharaja, P.; Pavithra, S.; Boopathy, R.; Sekaran, G. Preparation of light weight constructional materials from chrome containing buffing dust solid waste generated in leather industry. J. Mater. Cycles Waste Manag. 2017, 19, 928–938. [Google Scholar] [CrossRef]
- Murugan, K.P.; Balaji, M.; Kar, S.S.; Swarnalatha, S.; Sekaran, G. Nano fibrous carbon produced from chromium bearing tannery solid waste as the bitumen modifier. J. Environ. Manag. 2020, 270, 110882. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Tang, W.; Gu, Q.B.; Li, L.; Duan, Y.Q.; Chen, Y.Q.; Lu, X.Y.; Sun, Z.K.; Qian, X.D.; Duan, L.B. Reaction mechanisms and N-containing compound formation during shoe manufacturing waste pyrolysis. Fuel Process. Technol. 2023, 244, 107699. [Google Scholar] [CrossRef]
- Stejskal, J.; Gilbert, R.G. Polyaniline. Preparation of a conducting polymer (IUPAC technical report). Pure Appl. Chem. 2002, 74, 857–867. [Google Scholar] [CrossRef]
- Stejskal, J.; Sapurina, I. Polyaniline: Thin films and colloidal dispersions (IUPAC technical report). Pure Appl. Chem. 2005, 77, 815–826. [Google Scholar] [CrossRef]
- Elyashevich, G.K.; Gerasimov, D.I.; Kuryndin, I.S.; Lavrentyev, V.K.; Rosova, E.Y.u.; Vylegzhanina, M.E. Evolution of the surface structure and functional properties of the electroconducting polymer coatings onto porous films. Coatings 2022, 12, 51. [Google Scholar] [CrossRef]
- Gablech, I.; Glowacki, E.D. State-of-the-art electronic materials for thin films in bioelectronics. Adv. Electron. Mater. 2023, 9, 2300258. [Google Scholar] [CrossRef]
- Soukupová, G.; Bautkinová, T.; Mazúr, P.; Vilčáková, J.; Prokeš, J.; Dendisová, M.; Lhotka, M.; Hassouna, F. Enhanced specific capacity and cycling stability of flexible nanocellulose-based pseudocapacitative electrodes by controlled nanostructuring of polyaniline. Electrochim. Acta 2023, 441, 141830. [Google Scholar] [CrossRef]
- Heme, H.N.; Alif, M.S.N.; Rahat, S.M.S.M. Recent progress in polyaniline composites for high capacity energy storage: A review. J. Energy Storage 2021, 42, 103018. [Google Scholar] [CrossRef]
- Stejskal, J. Interaction of conducting polymers, polyaniline and polypyrrole, with organic dyes: Polymer morphology control, dye adsorption and photocatalytic decomposition. Chem. Pap. 2020, 74, 1–54. [Google Scholar] [CrossRef]
- Stejskal, J. Recent advances in the removal of organic dyes from aqueous media with conducting polymers, polyaniline and polypyrrole, and their composites. Polymers 2022, 14, 4243. [Google Scholar] [CrossRef] [PubMed]
- Porri, A.; Baroncelli, R.; Guglielminetti, L.; Sarrocco, S.; Guazzelli, L.; Forti, M.; Catelani, G.; Valentini, G.; Bazzichi, A.; Franceschi, M.; et al. Fusarium oxysporum degradation and detoxification of a new textile-glycoconjugate azo dye (GAD). Fungal Biol. 2011, 115, 30–37. [Google Scholar] [CrossRef]
- Jamal, M.; Awadasseid, A.; Su, X.M. Exploring potential bacterial populations for enhanced anthraquinone dyes biodegradation: A critical review. Biotech. Lett. 2022, 44, 1011–1025. [Google Scholar] [CrossRef] [PubMed]
- Tchmutin, I.A.; Ponomarenko, A.T.; Krinichnaya, E.P.; Kozub, G.I.; Efimov, O.N. Electrical properties of composites based on conjugated polymers and conductive fillers. Carbon 2003, 41, 1391–1395. [Google Scholar] [CrossRef]
- do Nascimento, G.M.; Silva, T.B.; Corio, P. Charge-transfer behavior of polyaniline single wall carbon nanotubes nanocomposites monitored by resonance Raman spectroscopy. J. Raman Spectrosc. 2010, 41, 1587–1593. [Google Scholar] [CrossRef]
- Grycová, B.; Klemencová, K.; Leštinský, P.; Stejskal, J.; Sáha, T.; Trchová, M.; Prokeš, J. Conductivity of carbonized and activated leather waste. Sustain. Chem. Pharm. 2023, 35, 101172. [Google Scholar] [CrossRef]
- Ngwabebhoh, F.A.; Sáha, T.; Stejskal, J.; Prokeš, J.; Kolská, Z.; Trchová, M. Conductivity of leather waste carbonized at various temperature: A challenge to conducting polymers. J. Anal. Appl. Pyrolysis 2023, 173, 106056. [Google Scholar] [CrossRef]
- Stejskal, J.; Ngwabebhoh, F.A.; Sáha, P.; Prokeš, J. Carbonized leather waste: A review and conductivity outlook. Polymers 2023, 15, 1028. [Google Scholar] [CrossRef]
- Stejskal, J.; Vilčáková, J.; Jurča, M.; Fei, H.J.; Trchová, M.; Kolská, Z.; Prokeš, J.; Křivka, I. Polypyrrole-coated melamine sponge as a precursor for conducting macroporous nitrogen nitrogen-containing carbons. Coatings 2022, 12, 324. [Google Scholar] [CrossRef]
- Ngwabebhoh, F.A.; Bazi, M.; Oladipo, A. Adsorptive removal of multi-azo dye from aqueous phase using a semi-IPN superabsorbent chitosan-starch hydrogel. Chem. Eng. Res. Des. 2016, 112, 274–288. [Google Scholar] [CrossRef]
- Ul Haq, A.; Lim, J.; Yun, J.M.; Lee, W.J.; Han, T.H.; Kim, S.O. Direct growth of polyaniline chains from N-doped sites of carbon nanotubes. Small 2013, 9, 3829–3833. [Google Scholar] [CrossRef] [PubMed]
- Shi, A.R.; Song, X.M.; Wei, L.; Ma, H.Y.; Pang, H.J.; Li, W.W.; Liu, X.W.; Tan, L.C. Design of an internal/external bicontinuous conductive network for high-performance asymmetrical supercapacitors. Molecules 2023, 27, 8168. [Google Scholar] [CrossRef] [PubMed]
- Beygisangchin, M.; Rashid, S.A.; Shafie, S.; Sadrolhosseini, A.R.; Lim, H.N. Preparations, properties, and applications of polyaniline and polyaniline thin films—A review. Polymers 2021, 13, 2003. [Google Scholar] [CrossRef]
- Fedorova, S.; Stejskal, J. Surface and precipitation polymerization of aniline. Langmuir 2002, 18, 5630–5632. [Google Scholar] [CrossRef]
- Haque, M.M.; Wong, D.K.Y. Improved dye entrapment-liberation performance at electrochemically synthesized polypyrrole-reduced graphene oxide nanocomposite films. J. Appl. Electrochem. 2017, 47, 777–788. [Google Scholar] [CrossRef]
- Yu, H.B.; Che, M.; Zhao, B.; Lu, Y.; Zhu, S.Y.; Wang, X.H.; Qin, W.C.; Huo, M.X. Enhanced electrosorption of rhodamine B over porous copper-nickel foam electrodes modified with graphene oxide/polypyrrole. Synth. Met. 2021, 262, 116332. [Google Scholar] [CrossRef]
- Pinheiro, N.S.C.; Perez-Lopez, O.W.; Gutterres, M. Solid leather wastes as adsorbents for cationic and anionic dye removal. Environ. Technol. 2022, 43, 1285–1293. [Google Scholar] [CrossRef]
- Yılmaz, O.; Kantarli, I.; Yuksel, M.; Saglam, M.; Yanik, J. Conversion of leather wastes to useful products. Resour. Conserv. Recycl. 2007, 49, 436–448. [Google Scholar] [CrossRef]
- Roy, C.; Chowdhury, D.; Sanfui, M.H.; Roy, J.S.D.; Mitra, M.; Dutta, A.; Chattopadhyay, P.K.; Singha, N.R. Solid waste collagen-associated fabrication of magnetic hematite nanoparticle@collagen nanobiocomposite for emission-adsorption of dyes. Int. J. Biol. Macromol. 2023, 242, 124774. [Google Scholar] [CrossRef]
- Putshak'a, J.D.; Akpabio, I.O. Adsorption performance of activated carbon from leather buffing waste. J. Am. Leather Chem. Assoc. 2010, 105, 313–319. [Google Scholar]
- Oliveira, L.C.A.; Coura, C.V.; Guimaraes, L.R.; Goncalves, M. Removal of organic dyes using Cr-containing activated carbon prepared from leather waste. J. Hazard. Mater. 2011, 192, 1094–1099. [Google Scholar] [CrossRef] [PubMed]
- Putshaka, J.D.; Adamu, K.I.; Jauro, A.; Tanko, S.F. Effect of pyrolysis temperature on adsorbent properties of carbon from leather buffing dust and sawdust. J. Test. Eval. 2014, 42, 593–600. [Google Scholar] [CrossRef]
- Ke, L.; Zhao, K.; Yan, X.Y.; Cao, X.J.; Wu, X.Y.; Zhang, C.; Luo, T.T.; Ding, T.; Yan, N. Facile mineralization and valorization of Cr-containing leather shavings for electrocatalytic H2O2 generation and organic pollutant removal. Chem. Eng. J. 2022, 437, 135036. [Google Scholar] [CrossRef]
- Puchana-Rosero, M.J.; Lima, E.C.; Mella, B.; Da Costa, D.; Poll, E.; Gutterres, M. A coagulation-flocculation process combined with adsorption using activated carbon obtained from sludge for dye removal from tannery wastewater. J. Chilean Chem. Soc. 2018, 63, 3867–3874. [Google Scholar] [CrossRef]
- Arcibar-Orozco, J.A.; Barajas-Elias, B.S.; Caballero-Briones, F.; Nielsen, L.; Rangel-Mendez, J.R. Hybrid carbon nanochromium composites prepared from chrome-tanned leather shavings for dye adsorption. Water Air Soil Pollut. 2019, 230, 142. [Google Scholar] [CrossRef]
- Lyu, W.; Li, J.Q.; Trchová, M.; Wang, G.; Liao, Y.Z.; Bober, P.; Stejskal, J. Fabrication of polyaniline/poly(vinyl alcohol)/montmorillonite hybrid aerogels toward efficient adsorption of organic dye pollutants. J. Hazard. Mater. 2022, 435, 129004. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).