Abstract
Complex salt solution systems are commonly used as electrolytes for microarc oxidation (MAO). To investigate the MAO phenomenon in simple electrolytes and evaluate the efficacy of MAO coatings, an Na3PO4 monosalt solution was used as the MAO electrolyte. In this study, the effects of the voltage and Na3PO4 concentration on the spark discharge phenomenon, thickness, roughness, and corrosion resistance of the coatings were investigated. The results showed that MAO coatings were effectively synthesised at high concentrations and voltages. Furthermore, the addition of KOH to the Na3PO4 monosalt solution resulted in discernible spark discharge and improved the corrosion resistance of the coatings. Thus, an MAO coating can be efficiently prepared using Na3PO4 solution as the electrolyte. In addition, the addition of KOH to the Na3PO4 monosalt solution reduced the voltage required for coating formation and enhanced the corrosion resistance of the coating.
1. Introduction
Traditionally, microarc oxidation (MAO) has been a crucial surface-strengthening method for Mg alloys in terms of corrosion resistance and wear resistance [1,2,3,4,5,6,7]. Currently, most MAO electrolytes consist of coating-forming agents and additives [8]. The role of the coating-forming agent is to bind Mg ions (Mg+) to form a stable coating. Coating-forming agents include a range of compounds, such as carbonates, borates, phosphates, and silicates. Additives change the process parameters and improve the coating properties. For example, alcohols, carboxylic acids, and organic amines can reduce current density and arc voltage. Al2O3, SiO2, and other nanoparticles can enhance the wear resistance of the coatings. The frequently used electrolytes include silicates, phosphates, and aluminate systems [9].
Previous research has indicated that the composition and concentration of the electrolyte are crucial parameters for regulating the structure and performance of MAO coatings. These parameters affect the surface morphology, thickness, roughness, and corrosion resistance of MAO coatings [10,11,12]. The choice of the coating agent affects the surface states and properties of the coating. The electrolyte systems using Na2SiO3, Na3PO4, NaAlO2, and Na2MoO4 as coating-forming agents were investigated by Chai [13]. This study examined the effect of various coating-forming agents on the performance of the coatings. Seyfoori [14] discovered that the MAO coating obtained on an AZ31B magnesium alloy in a phosphate system was denser than that formed in other systems. Mori [15] et al. [15] investigated the effect of phosphate-to-silicate concentration ratio on the structure and properties of MAO coatings. The optimal concentration ratio for incorporation into the MAO coating was selected to achieve a superior performance.
Electrolyte additives can be classified as organic or inorganic additives. Organic additives, such as alcohols and organosiloxanes, can improve the compactness and corrosion resistance of MAO coatings [16,17,18]. Inorganic additives, such as graphene, SiO2, and similar substances, can be used as coating compounds during the MAO process. The benefits of inorganic additives include improved wear and corrosion resistance of MAO coatings. Therefore, these organic and inorganic additives can improve the anti-corrosion and anti-wear properties of the coatings.
The existing research on microarc oxidation in monosalt electrolytes is limited. Therefore, the single Na3PO4 electrolyte was used as the MAO electrolyte. The effects of concentration and voltage on the formation and performance of the coating were studied. Additionally, the effects of KOH on the microstructure and corrosion performance of MAO coatings were investigated.
2. Materials and Methods
2.1. Materials
All solvents and chemicals supplied by Chengdu Kelong Chemical Reagent Co., Sichuan, China, were of chemically pure grade and used without further purification. AZ31B Mg-alloy samples (50 mm × 25 mm × 2 mm, Shanxi Yinguang Huasheng Magnesium Industry Co., Shanxi, China.) were used as substrates. The primary nominal compositions are listed in Table 1.

Table 1.
Nominal composition of AZ31B magnesium alloy.
2.2. Pretreatment
The AZ31B Mg alloy samples were ground using 600–1200 grit silicon carbide paper, rinsed with deionised water, and ultrasonically cleaned in ethyl alcohol for 5 min before drying in cold air.
2.3. Microarc Oxidation (MAO) Coating
Mg alloy samples were used as the anode, and a stainless-steel sheet was used as the cathode during the MAO process. Various electrolytes were prepared by dissolving 20, 25, 30, 35, and 40 g/L Na3PO4 in deionised water in a glass cylinder. The glass cylinder was placed in a water-cooled pipe to maintain the temperature of the solution below 25 °C during the MAO. The MAO parameters are listed in Table 2. After the MAO treatment, the specimens were cleaned ultrasonically in absolute ethyl alcohol, rinsed with distilled water, and then blow-dried for further analysis.

Table 2.
Process parameters of MAO coating.
The solution obtained by adding 35 g/L Na3PO4, as previously stated, was subjected to the addition of 5, 10, or 15 g/L KOH. The corresponding pH and conductivity values are presented in Table 3. The voltage, frequency, duty cycle, and duration remained unchanged, and the experiment was repeated.

Table 3.
Electrical conductivity and pH value of various KOH concentrations at 35 g/L Na3PO4.
2.4. Characterization and Testing
The surfaces of the coatings were examined using a scanning electron microscope (SEM; VEGA 3 SBU, Tescan, Czech Republic) equipped with an energy-dispersive spectrometer (OXFORD, Oxford, England). Porosity was measured using the ImageJ software (Ver.1.51j8) based on the relevant SEM images. The thicknesses of the coatings were measured using a thickness gauge (Mini-Test 4100; EPK, Cologne, Germany). The thickness of the coating was determined by conducting 12 tests on each sample and calculating the average value, excluding the maximum and minimum values obtained from the tests.
All the electrochemical experiments were performed using a 3.5 wt.% NaCl solution at room temperature using an electrochemical test system (Versa STAT 3F, Ametek Trading Co., Ltd, Shanghai, China). A three-electrode electrochemical cell was created in an electrolytic tank with a small hole of 1 cm2. A saturated calomel electrode (SCE) was used as the reference electrode, a platinum sheet was used as the counter electrode, and the coated samples were used as the working electrodes. The open-circuit potentials (OCPs) of the coatings were determined after immersion in a 3.5 wt.% NaCl solution for a duration of approximately 10 min until stabilised values were attained at room temperature. Finally, the potentiodynamic polarisation (PDP) curves of the coatings were obtained. The scanning rate was 1.0 mV·s−1, and the potential region was set to ±1000 mV with respect to the OCP. The icorr of the MAO/Mg coating was confirmed using an anodic polarisation curve.
3. Results and Discussion
3.1. MAO Coating Prepared in Na3PO4 Solution
3.1.1. Spark Condition, Surface Morphologies, and Microstructure
Table 4 and Table 5 present the discharge and surface morphologies of the MAO coatings obtained at various voltages and Na3PO4 concentrations, respectively. No spark discharges were produced at low voltages or concentrations. When the concentration of Na3PO4 was less than 35 g/L, the microarc oxidation process exhibited tiny sparks until the voltage reached 350 V. However, the surfaces of the coatings were neither uniform nor complete. For instance, large uneven wrinkled areas in the middle of the coatings and small white spots were observed. When the voltage exceeded 350 V, the discharge was slightly enhanced, and the coatings became relatively intact. When the concentration of Na3PO4 was 35 g/L, discharge was observed at 300 V during the MAO process. The discharge phenomenon became more evident as voltage increased. This occurred when the surface of the coating was smoother and there were no visible imperfections. When the concentration of Na3PO4 exceeded 35 g/L, an evident spark discharge phenomenon was observed only at 300 V. As the voltage continued to increase, the discharge phenomenon became more intense. However, when the voltage was too high (400 V), an exploding sound and violent arc appeared at the edge of the sample. This resulted in coatings with serious defects because the voltages exceeded the tolerance limit of the coating. At high voltages, the coatings become defective and permeable [19,20,21].

Table 4.
Real-time images of plasma discharges with an oxidation time of 1 min in various Na3PO4 concentrations and voltages.

Table 5.
Optical images of coatings formed for 10 min on the AZ31B magnesium alloy in various Na3PO4 concentrations and voltages.
Figure 1 shows the surface morphologies of the MAO coatings prepared with various concentrations of Na3PO4 at 350 V. Overall, the coatings had many isolated and unconnected micropores. When the concentration of Na3PO4 was 20 g/L (Figure 1a), numerous micropores were observed on the surfaces of the coatings, and numerous micropores of micrometre size existed with porosities reaching 13.47%. Subsequently, with an increase in Na3PO4 concentration, the size of the micropores increased, but the number of micropores decreased. Some microcracks are shown in Figure 1b–d, and in Figure 1e, the microcracks increase in size and become interconnected. Figure 1f shows that the porosity decreased as the concentration of Na3PO4 increased. Figure 2 shows the surface morphology of MAO at various voltages with 35 g/L of Na3PO4. The micropores on the surface of the coating were small and dense at 250 V (Figure 2a). The micropores were shallow, and their overall distribution was non-uniform. As the voltage increased, the size of the micropores increased; however, the number of micropores decreased. When the voltage reached 400 V, numerous microcracks appeared on the surface of the coating. High voltages can easily cause local breakdown of the coating and damage the coating layer [22,23,24]. In general, an MAO coating can be grown on the surface of an Mg alloy at an appropriate Na3PO4 concentration and voltage. The porosity decreased and subsequently increased, as shown in Figure 2e.
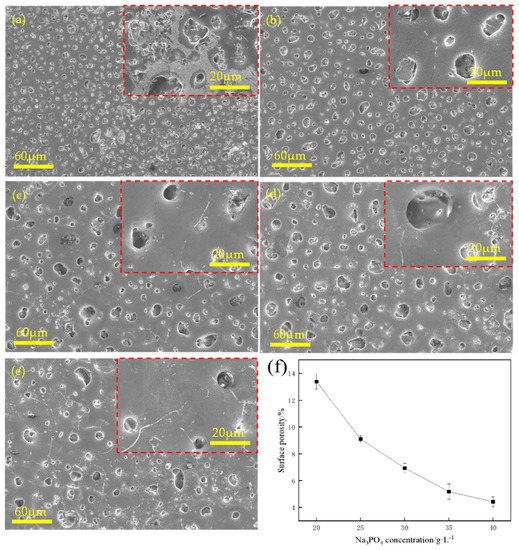
Figure 1.
Surface morphologies of MAO−coated AZ31B Mg alloy in various Na3PO4 concentrations at 350 V: (a) 20 g/L, (b) 25 g/L, (c) 30 g/L, (d) 35 g/L, (e) 40 g/L, and (f) curve of surface porosity vs. Na3PO4 concentration at 350 V.
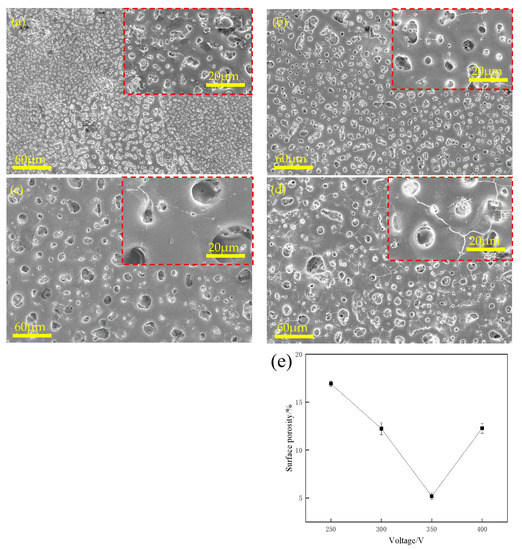
Figure 2.
Surface morphologies of MAO-coated AZ31B Mg alloy in various voltages at 35 g/L Na3PO4: (a) 250 V, (b) 300 V, (c) 350 V, (d) 400 V, and (e) curve of surface porosity vs. voltage at 35 g/L Na3PO4.
3.1.2. Coating Thickness and Roughness
Figure 3 shows the thickness and roughness of the MAO coatings prepared at various Na3PO4 concentrations and voltages. This demonstrates that an increase in the voltage and Na3PO4 concentration results in an increase in the coating thickness because they promote the growth of MAO coatings. The coating thickness reaches a maximum of 24.4 μm. The development of MAO coatings may be facilitated by the stoichiometry of P. This is because P4O3− ions favour the creation of microarcs and pores within the coating, thereby promoting the formation of thick and porous coatings. Although the MAO coating was thick when an Na3PO4 solution was used as the electrolyte, it had a high degree of roughness. In addition, as the voltage or concentration of Na3PO4 increased, the coating became rougher. A minor reduction in roughness was observed when the Na3PO4 concentration was 20–35 g/L and the voltage was 300 V. The surface microstructure of the MAO coating had a significant impact on the surface roughness. The surface roughness of the MAO coating increased because of the increase in the micropore size caused by either an increase in the voltage or an increase in the concentration of Na3PO4.
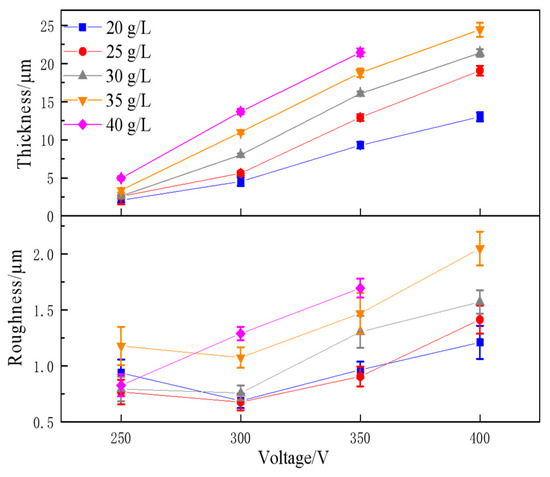
Figure 3.
Thickness and roughness of MAO-treated AZ31B alloy with the Na3PO4 concentration and voltage.
3.1.3. Potentiodynamic Polarisation Test
The polarisation curves obtained for the MAO coatings are shown in Figure 4. The polarisation curves clearly show that the MAO coating substantially slows the pace at which the Mg alloy substrate corrodes in the salt solution (icorr for the AZ31B Mg alloy in 3.5 w.t% NaCl solution is typically approximately 7 × 10−5 μA•cm−2). Figure 4a shows that an increase in the Na3PO4 concentration at 350 V resulted in a decrease in icorr. Ono et al. [23] verified that under the same electrical conditions, the electrolyte concentration determines the thickness and corrosion resistance of the MAO coating. Table 6 shows that when the Na3PO4 concentration reached 30 g/L, icorr of the MAO coating was enhanced by one order of magnitude. However, icorr of the MAO coating did not change significantly at the same voltage when the concentration was increased to 35 and 40 g/L. Figure 4b shows that at the same Na3PO4 content, the corrosion resistance of the MAO coating increased with increasing voltage. At voltages between 250 and 300 V, icorr did not increase significantly (Table 7). When the voltage was increased to 350 V, icorr decreased by an order of magnitude. When the voltage reached 400 V, icorr decreased.
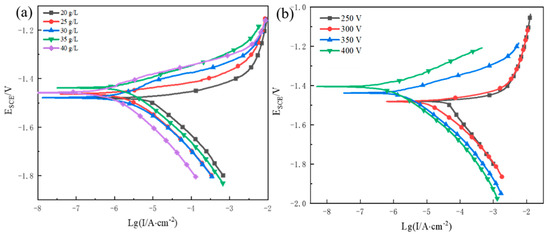
Figure 4.
Polarization curves of MAO−treated AZ31B alloy in 3.5 wt% NaCl solution: (a) various Na3PO4 concentrations at 350 V and (b) various voltages with 35 g/L Na3PO4 concentration.

Table 6.
Tafel polarization parameters of 350 V under various Na3PO4 concentrations.

Table 7.
Tafel polarization parameters of 35 g/L Na3PO4 under various voltages.
In general, the corrosion resistance of a coating is influenced by its thickness and porosity [5,9]. The increase in thickness caused by an increase in concentration increased the corrosion current density at the same voltage, as shown in Figure 3 and Table 6. The same was true for the same concentrations. Therefore, the corrosion resistance of MAO coatings increases with increasing thickness. The corrosion resistance of the MAO coatings increased with decreasing porosity, as shown in Figure 1f and Figure 2e, and Table 6 and Table 7. Even when the porosity increased at voltages up to 400 V at the same concentration, the influence of the thickness on the corrosion resistance was more pronounced.
3.2. MAO Coating Prepared by Na3PO4 + KOH Solution
3.2.1. Spark Condition, Surface Morphologies, and Microstructure
Table 8 and Table 9 list the spark discharges and surface morphologies of MAO coatings prepared at various KOH concentrations and voltages, respectively. As shown in Table 8, a clear spark discharge behaviour was observed after the addition of KOH. The spark discharge phenomenon was clearly observed, even at low voltages. The sparks grew and enlarged as the voltage increased. The colour of the sparks changed from yellow, white, and orange as the KOH concentration increased, and the spark density continued to increase. The conductivity of the solution increased with the addition of KOH. According to Stern’s electric double-layer model, the equilibrium OH− density of the compact layer close to the anode surface increases as the solution’s OH− concentration increases. The smaller potential difference may also facilitate the entry of the same amount of OH− ions into the oxide layer. Finally, the induced voltage of the microarc plasma decreased. Table 9 lists the surface morphologies of the MAO coatings obtained at various voltages and KOH concentrations. The surface of the MAO coating exhibited a polished appearance and a noticeable improvement in smoothness after the addition of KOH. However, clear white spots were observed on the surface of MAO when the KOH concentration was either 5 or 10 g/L, and the voltage was set to 300 V. These issues also arise when the voltage exceeded 400 V and the KOH concentration was 15 g/L.

Table 8.
Real-time images of plasma discharges with an oxidation time of 1 min in various concentrations of KOH and voltages.

Table 9.
Optical images of coatings formed for 10 min on the AZ31B magnesium alloy in various concentrations of KOH and voltages.
Figure 5 shows the surface micromorphology of the MAO coatings obtained at various concentrations of KOH. The number of micropores on the surface of the MAO coating increased with increasing KOH concentration and the micropore size decreased. More interconnected microcracks appeared on the surface of the MAO coating when the KOH concentration was 5 g/L than when it was 0 g/L. New structures in the MAO coating emerged at KOH concentrations of 10 and 15 g/L (Figure 6c,d), where the “crater” morphology of the MAO coating was prominent. This led to the conclusion that powerful sparks were responsible for the formation of a protruding structure. The violent breakdown discharge of the coating triggered localised breakdown and melting of the film layer, which resulted in the creation of violent sparks. The old structure was destroyed and replaced with a new one whenever the KOH concentration reached a specified level. The new structure of the MAO surface was denser, the number of micropores increased, and the size of the micropores decreased.
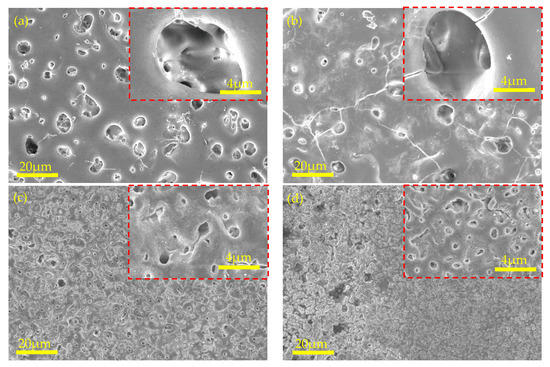
Figure 5.
Surface morphologies of MAO-coated AZ31B Mg alloy in various KOH concentrations at 300 V: (a) 0 g/L, (b) 5 g/L, (c) 10 g/L, and (d) 15 g/L.
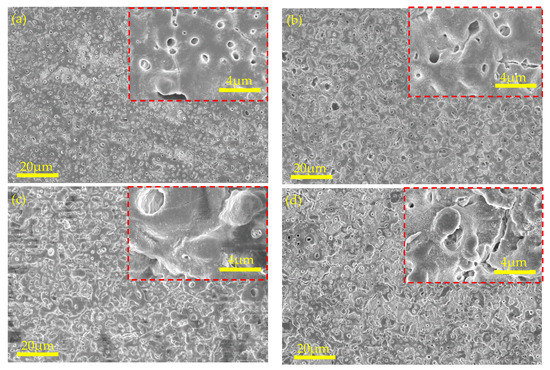
Figure 6.
Surface morphologies of MAO-coated AZ31B Mg alloy in various voltages at 5 g/L KOH: (a) 250 V, (b) 300 V, (c) 350 V, and (d) 400 V.
The surface micromorphologies of the MAO coatings produced using 5 g/L KOH at various voltages are shown in Figure 6. Numerous micropores began to form on the MAO surface when the voltage reached 250 V (Figure 7a), and flat areas began to form because of the dense, tiny spark discharge. The MAO coating exhibited a crater-like morphology as the voltage increased because the spark discharge intensified. Many microcracks emerged on the surface of the MAO coating when the voltage exceeded 400 V (Figure 7d).
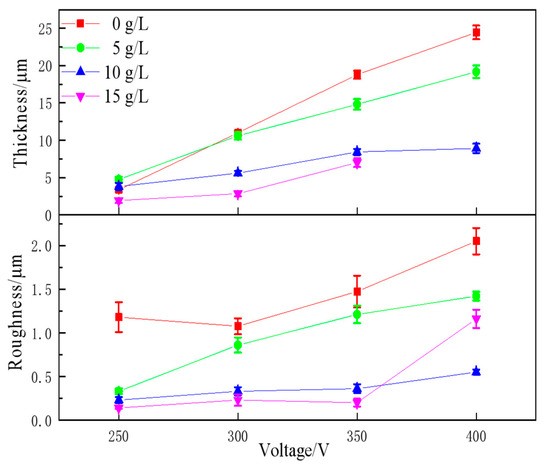
Figure 7.
Thickness and roughness of MAO-treated AZ31B alloy with the KOH concentration.
3.2.2. Coating Thickness and Roughness
Figure 7 illustrates the effect of voltage and KOH concentration on the thickness and roughness. Higher voltages and lower concentrations were helpful in obtaining thicker coatings. As the KOH concentration increases, the MAO coating thickness decreases. The addition of KOH resulted in a denser MAO coating, replacing the MAO coating produced using a pure Na3PO4 solution. Therefore, this could result in a decrease in the coating thickness. The roughness of the MAO coatings increases with increasing voltage and decreasing KOH content. KOH enhances the density of the ceramic layer by reducing the micropore size and depleting the MAO coating. The addition of KOH can reduce the roughness of MAO coatings by reducing the size and density of the micropores, arc-initiating voltage, and termination voltage.
3.2.3. Potentiodynamic Polarisation Test
The potentiodynamic polarisation curve (Figure 8a) shows that the addition of KOH substantially decreased icorr. In addition, as the KOH content increased, icorr continued to decrease. However, the amount of added KOH was limited. The icorr of the coating decreased significantly at a KOH concentration of 5 g/L. As the concentration of KOH increased, the rate of decrease in icorr slowed, and icorr became nearly steady. Consequently, the optimal corrosion resistance of the MAO coating was achieved using 5 g/L of KOH. By altering the pH and conductivity of the electrolyte, the addition of an appropriate amount of KOH facilitated the coating formation and smooth MAO operation. This also increased the density and corrosion resistance of the MAO coating. From Table 10, The coating icorr reduces with increasing voltage. The corrosion resistance of the MAO coating significantly improved when the voltage was increased from 250 V to 300 V. The icorr of MAO exhibited a second major drop at 400 V.
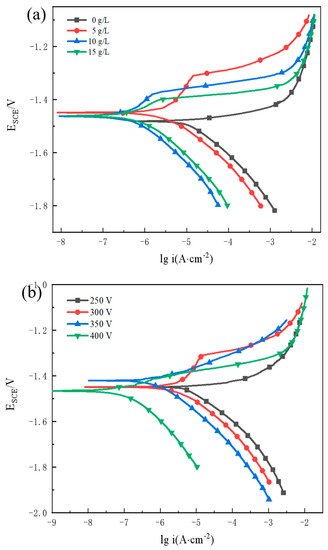
Figure 8.
Polarization curves of MAO-treated AZ31B alloy in 3.5 wt% NaCl solution: (a) various KOH concentrations at 300 V and (b) various voltages with 5 g/L KOH concentration.

Table 10.
Tafel polarization parameters of 5 g/L KOH under various voltages with 35 g/L Na3PO4 concentration.
The coating becomes denser and more corrosion resistant with the addition of KOH [18]. Figure 7 shows that with the addition of KOH, the MAO coating became substantially thinner, especially when the KOH concentration was 10 g/L. Table 11 shows that following the addition of KOH, the thickness of the coating decreased, but its resistance to corrosion increased. The densification of the membrane has positive feedback that balances the negative feedback of thickness reduction.

Table 11.
Tafel polarization parameters of 300 V under various KOH concentrations with 35 g/L Na3PO4.
4. Conclusions
This study demonstrates that MAO coatings can be prepared on Mg alloys in Na3PO4 solution. A desirable coating was obtained at a voltage of 300–400 V and Na3PO4 concentration of 30–40 g/L. The coating thickness reached up to 24.4 μm. The effect of the voltage on the coating properties was greater than that of the Na3PO4 concentration. The spark discharge phenomenon in the MAO process was more visible with the addition of KOH, indicating that the arcing voltage was reduced and coating growth was encouraged. Although the thickness of the MAO coating decreased with the addition of KOH, the corrosion resistance of the coating increased owing to the increase in its density. Based on these results, we conclude that KOH concentrations between 5 and 10 g/L are optimal.
Author Contributions
Conceptualisation, X.C. and H.Y.; methodology, X.C.; software, X.C.; validation, H.Y.; formal analysis, H.Y.; investigation, H.Y.; resources, X.C.; data curation, H.Y.; writing—original draft preparation, H.Y.; writing—review and editing, Y.Q.; visualisation, H.Y.; supervision, Y.Q. and X.C.; project administration, C.L.; funding acquisition, X.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (no. 52275221), the National Natural Science Foundation of Sichuan Province (nos. 2022NSFSC0330 and 2023NSFSC0917), the Opening Project Material Corrosion and Protection Key Laboratory of Sichuan Province (no. 2020CL09), and the Graduate Innovation Fund Program of Sichuan University of Science and Engineering (no. Y2022007).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yao, W.H.; Wu, L.; Wang, J.F.; Jiang, B.; Zhang, D.F.; Serdechnova, M.; Shulha, T.; Blawert, C.; Zheludkevich, M.L.; Pan, F. Micro-arc oxidation of magnesium alloys: A review. J. Mater. Sci. Technol. 2022, 118, 158–180. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.Q.; Chen, C.F.; Gu, Y.H. Advances in microarc oxidation coated AZ31 Mg alloys for biomedical applications. Corros. Sci. 2014, 91, 7–28. [Google Scholar] [CrossRef]
- Kaseem, M.; Fatimah, S.; Nashrah, N. Recent progress in surface modification of metals coated by plasma electrolytic oxidation: Principle, structure, and performance. Prog. Mater. Sci. 2020, 117, 100735. [Google Scholar] [CrossRef]
- Wang, Z.; Cheng, Z.; Zhang, Y.; Shi, X.; Rao, M.; Wu, S. Effect of voltage on the microstructure and high-temperature oxidation resistance of micro-arc oxidation coatings on AlTiCrVZr refractory high-entropy alloy. Coatings 2022, 13, 14. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, Z.; Ai, J.; Bu, S.; Liu, H. Preparation of coating on the titanium surface by micro-arc oxidation to improve corrosion resistance. Coatings 2021, 11, 230. [Google Scholar] [CrossRef]
- Shi, X.; Wang, Y.; Li, H.; Zhang, S.; Zhao, R.; Li, G.; Zhao, Y. Corrosion resistance and biocompatibility of calcium-containing coatings developed in near-neutral solutions containing phytic acid and phosphoric acid on AZ31B alloy. J. Alloys Compd. 2020, 823, 153721. [Google Scholar] [CrossRef]
- Qin, J.; Shi, X.; Li, H.; Zhao, R.; Li, G.; Zhang, S.; Zhang, R. Performance and failure process of green recycling solutions for preparing high degradation resistance coating on biomedical magnesium alloys. Green Chem. 2022, 24, 8113–8130. [Google Scholar] [CrossRef]
- Yan, L.; Zhong, L.W.; Fu, W.Y.; Zhao, Z.; Fa, H.C.; Hui, B.S. Effect of electrolyte constituents on properties of anodic coatings of magnesium alloys. J. Chin. Soc. Corros. Prot. 2011, 31, 255–259. [Google Scholar]
- Cui, X.J.; Ping, J. Research Progress of Microarc Oxidation for Corrosion Prevention of Mg-alloys. J. Chin. Soc. Corros. Prot. 2018, 38, 87–104. [Google Scholar]
- Forno, A.D.; Bestetti, M. Effect of the electrolytic solution composition on the performance of micro-arc anodic oxidation films formed on AM60B magnesium alloy. Surf. Coat. Technol. 2010, 205, 1783–1788. [Google Scholar] [CrossRef]
- Sun, S.N.; Ye, G.; Lu, Z.T.; Weng, Y.M.; Ma, G.F.; Liu, J.T. Surface Treatment of Zn-Mn-Mg Alloys by Micro-Arc Oxidation in Silicate-Based Solutions with Different NaF Concentrations. Materials 2021, 14, 4289. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Y.; Cai, Z.B.; Cui, X.J.; Liu, R.R.; Yang, Z.B.; Zhu, M.H. Influence of nanoparticle additions on structure and fretting corrosion behavior of micro-arc oxidation coatings on zirconium alloy. Surf. Coat. Technol. 2021, 410, 126949. [Google Scholar] [CrossRef]
- Chai, L.Y.; Yu, X.; Yang, Z.H.; Wang, Y.Y.; Masazumi, O. Anodizing of magnesium alloy AZ31 in alkaline solutions with silicate under continuous sparking. Corros. Sci. 2008, 50, 3274–3279. [Google Scholar] [CrossRef]
- Seyfoori, A.; Mirdamadi, S.; Khavandi, A.; Raufi, Z.S. Biodegradation behavior of micro-arc oxidized AZ31 magnesium alloys formed in two different electrolytes. Appl. Surf. Sci. 2012, 261, 92–100. [Google Scholar] [CrossRef]
- Mori, Y.; Koshi, A.; Liao, J.S.; Ason, H.; Ono, S. Characteristics and corrosion resistance of plasma electrolytic oxidation coatings on AZ31B Mg alloy formed in phosphate-silicate mixture electrolytes. Corros. Sci. 2014, 88, 254–262. [Google Scholar] [CrossRef]
- Cui, X.J.; Dai, X.; Zheng, B.Y.; Zhang, Y.J. Effect of KH-550 on the structure and properties of micro-arc oxide films on the surface of AZ31B magnesium alloy. J. Chin. Soc. Corros. Prot. 2017, 37, 227–232. [Google Scholar]
- Feng, L.; Zheng, L.G.; Li, S.Z.; Zheng, D.J.; Lin, C.J.; Dong, S.G. Effect of Ferric Citrate on Microstructure and Corrosion Resistance of Micro-arc Oxidation Black Film on Mg-alloy AZ40M. J. Chin. Soc. Corros. Prot. 2017, 37, 360–365. [Google Scholar]
- Bai, A.; Chen, Z.J. Effect of electrolyte additives on anti-corrosion ability of micro-arc oxide coatings formed on magnesium alloy AZ91D. Surf. Coat. Technol. 2009, 203, 1956–1963. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, Z.L.; Yang, F.W.; Zhang, Z. Anodizing of AZ91D Magnesium Alloy in Borate-Terephthalic Acid Electrolyte. Acta Phys. Chim. Sin. 2011, 27, 2385–2392. [Google Scholar]
- Gu, Y.H.; Cai, X.J.; Ning, C.Y.; Xiong, W.M.; Yue, W.; He, B.H. Effects of voltage on the microstructure and corrosion performance of microarc oxidation coatedAZ31 magnesium alloys. China Surf. Eng. 2012, 25, 21. [Google Scholar]
- Cui, X.J.; Wang, R.; Wei, J.S.; Bai, C.B.; Lin, X.Z. Effect of electrical parameters on micromorphology and corrosion Resistance of micro-arc oxidation coating on AZ31B Mg alloy. J. Chin. Soc. Corros. Prot. 2014, 34, 495–501. [Google Scholar]
- Simchcn, F.; Sicber, M.; Lampke, T. Electrolyte influence on ignition of plasma electrolytic oxidation processes on light metals. Surf. Coat. Technol. 2017, 315, 205–213. [Google Scholar] [CrossRef]
- Sachiko, O.; Shuichi, M.; Yoichi, M.; Akihiko, K.; Jinsun, L.; Hidetaka, A. Effect of Electrolyte Concentration on the Structure and Corrosion Resistance of Anodic Films Formed on Magnesium through Plasma Electrolytic Oxidation. Electrochim. Acta 2017, 240, 415–423. [Google Scholar]
- Wu, X.R.; Jia, Z.J.; Ma, H.Y.; Liao, S.D.; Wang, B.G. Fundamentals of electrochemistry(III)-Electrical double layer model and its development. Energy Storage Sci. Technol. 2013, 2, 152–156. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).































































