Abstract
The paper describes the research on optical properties of glass/ITO/Ag thin layers obtained on glass/ITO substrates with different properties. The authors will discuss the influence of silver particles and silver layer thickness on the transmission and reflection spectra of the layers and parameters, such as the width of the optical band gap, refractive index, and dieelectric function. For example, the presence of silver leads to a decrease in the transmission of the layers (compared to ITO/glass) regardless of the thickness of the silver layer, and in the case of reflection, both its increase and decrease were observed, depending on the thickness of the silver layer and the type of glass/ITO substrate used. The average transmission value in the visible region depends on the thickness of the silver nanoparticle layer and varies from ~50% to ~90%. The average refractive index takes values from the range ~1.4 to ~1.65 and does not depend on the presence and thickness of the silver layer.
1. Introduction
Metallic nanoparticles have potential applications in many fields e.g., chemistry, biology, engineering, and physics. The plasmonic system is very promising for application in photovoltaics (PV) because of the driving and redirection of light [1], which increases PV efficiency.
The use of metallic nanoparticles in PV devices is a very promising solution, especially in thin-film solar cells, which offer great potential due to their low-cost, low-thickness, and lightweight materials, as well as the flexibility of solar cells. Unfortunately, current thin-film solar cells have lower efficiency compared to silicon PV devices [2]. In addition, it is known that silver or gold nanoparticles can be deposited by simple and inexpensive methods, e.g., magnetron sputtering [1,3].
The properties of nanoparticles depend on their geometry (shape), size, chemical composition environment, etc. [4]. In addition, their optical properties could be programmed by their shape and morphology [5,6,7].
The authors manufactured thin-film solar cells based on layers of copper oxide and titanium dioxide or zinc oxide by reactive DC magnetron sputtering with low efficiency (maximum ~0.9%) [2,8,9]. This construction is not feasible for large-scale fabrication. Metallic nanomodification in TiO2/CuO or TiO2:ZnO/CuO solar cells through Ag particles could be of great prospective importance and lead to higher solar conversion efficiency. For example, in polymer solar cells with silver nanowires, Yang et al. observed an increase of ~30% in short circuit current and power conversion efficiency compared to those without the particles [10]. Oh et al. presented organic solar cells with metal nanodot arrays that increased the power conversion efficiency from 7.52% to 10.11% [11]. Gwóźdź et al. and Placzek-Popko et al. [1,12] demonstrated improved efficiency of ZnO/Si solar cells using Ag and Au nanoparticles of different sizes. The best parameters were achieved for silver nanoparticles with a size of 20–30 nm size on top of a ZnO layer. Tharwat et al. have deposited aluminum nanoparticles on the top of the ITO/GaAs structure. The authors noticed an increase in optical absorption [13,14]
Kumawat et al. [14,15] analyzed thin-film silicon solar cells with Ag nanoparticles within the absorbing layer and found an increase in short circuit current density as well as conversion efficiency from 18.38 to 31.57 mA/cm2 and from 8.4 to 16.18%, respectively.
Mokari et al. manufactured ultra-thin silicon solar cells with plasmonic nanostructures using two or three coupled nanoparticles, and the results obtained indicated an increase in cell performance [14,16]. For example, for solar cells based on a titanium oxide layer with Ag nanoparticles, the conversion efficiency increases from 6.8% to 14%.
Another advantage of solar cells based on titanium dioxide and copper oxide is that it can be deposited on flexible substrates. The problem of flexible all-polymer solar cells (APSCs) was recently studied by, e.g., Ma et al. in [17].
The photovoltaic device must be able to capture as many photons as possible. Transmission through the Ag/ITO/glass thin layers must be very large in the visible range of the light spectrum to allow for absorption of most of the solar spectrum in the absorber layer. More importantly, reflected light is energy loss and should be minimized [3]. Transmission and reflection spectra provide us with information on the choice of the method of nanoparticle application. The morphology of solar cells is the main characteristic for achieving the ultimate PV performance [18].
Silver nanoparticles analyzed in this paper will be deposited on ITO layers to induce columnar growth of TiO2 layers, which will improve the crystal structure and thus increase the efficiency of the PV cells.
Other authors also conducted various experiments to improve the efficiency of PV cells. For example, Luo et al. realized in their work chain modification on small-molecule acceptors in organic solar cells [19], while Gao et al. modified organic solar cells by intramolecular Cl-S interaction [20] and achieved 18.33% and 15.73%, respectively.
In the first step, the authors analyze the optical properties of silver nanoparticles on indium tin oxide (ITO) glass thin layers. To compare the properties of Ag nanoparticles, three types of thickness structures (3, 5, and 10 nm) of nanoparticles were fabricated. The Ag nanoparticles with varied thicknesses were deposited on four types of ITO.
In this work, the influence of silver particles and the thickness of the silver layer on the transmission and reflection spectra of the layers were analyzed. Furthermore, parameters, such as the width of the optical band gap, refractive index, and dielectric function in application in thin film solar cells were calculated.
2. Materials and Methods
Silver nanoparticles were deposited by the magnetron sputtering method. A 99.9% pure Ag target was placed in a vacuum chamber. Substrates (glass coated with ITO) were attached to a rotating table. A very thin layer of metal was deposited (~1 nm). It resulted in the creation of nonhomogeneous “islands” on the substrates, called later nanoparticles. To compare the properties of Ag nanoparticles, nanoparticles of different thicknesses (3, 5, and 10 nm) were fabricated. Additionally, Ag nanoparticles with varied thicknesses were deposited on four types of ITO, named as: ITO1, ITO2, ITO3, and ITO4. Detailed properties of the substrates are shown in Table 1.

Table 1.
Properties of glass substrates coated with ITO.
3. Results and Discussions
3.1. Optical Properties
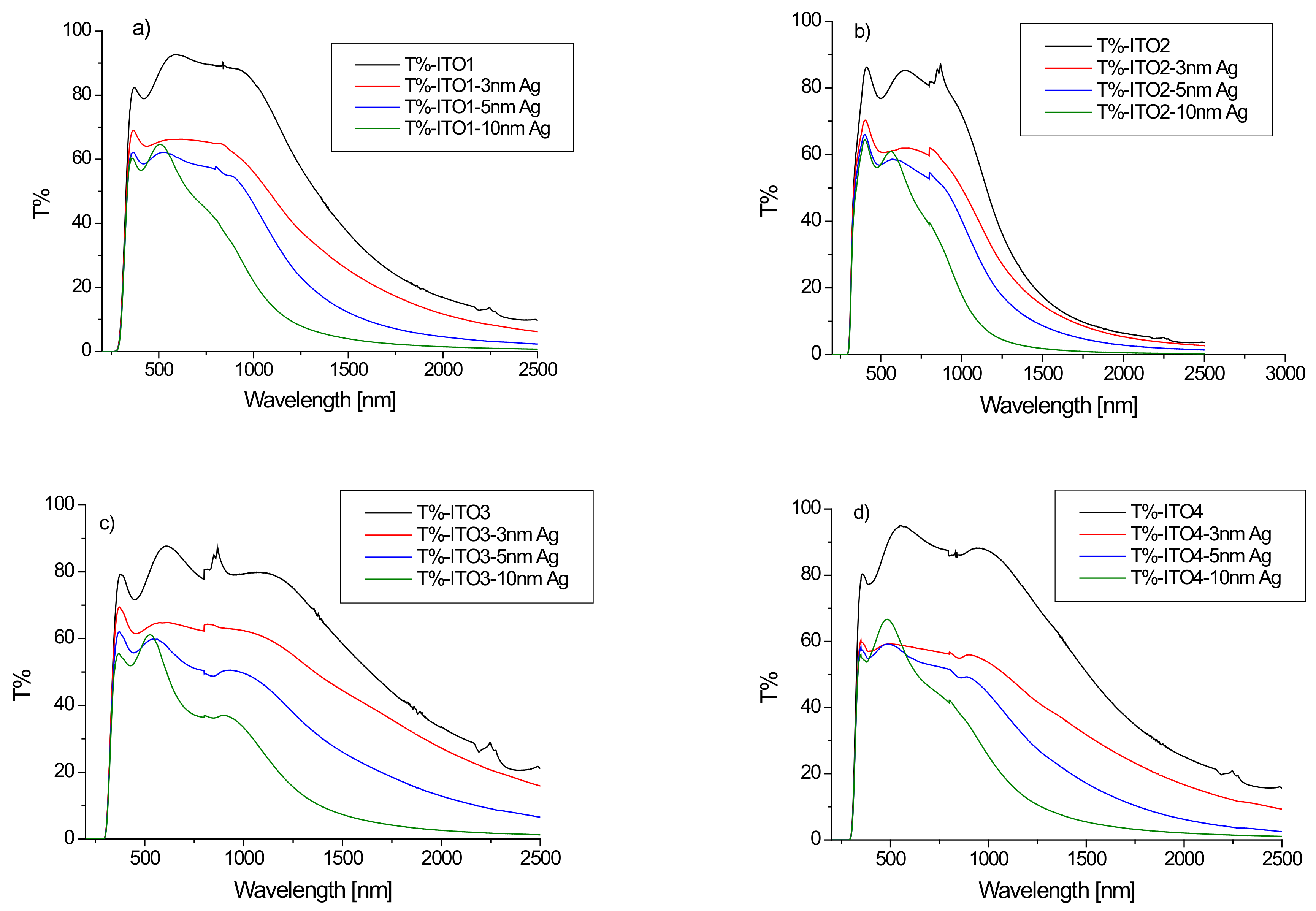
The Cary 5000 spectrophotometer was used to perform the optical measurements. Figure 1 presents the transmission spectra for the conducting transparent indium tin oxide (ITO) films on a glass substrate. The ITO films showed high transmission, higher than 80% in the visible range. Starting from 1000 nm, the ITO/glass transmission intensively decreases. In our case, the interference maxima and minima are poorly developed, exhibited in the visible range being typical for the films deposited by the magnetron sputtering or by PLD method [21,22,23]. These are typical transmission spectra of ITO layers on a glass substrate [23,24]. The shape of the transmission curve and the transmission value depend on the ITO/glass sample (Figure 1). The optical properties of ITO thin films strongly depend on the thickness of the film, the deposition parameters, the substrate temperature, the microstructure, the levels of impurities, and dopants or post-growth treatment [21,22,23,24,25,26,27,28,29,30,31].
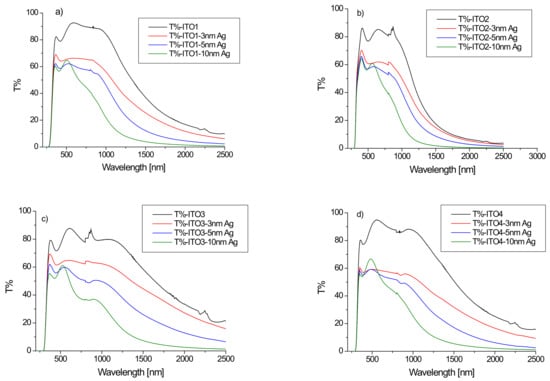
Figure 1.
(a–d) Transmission spectra of ITO/glass substrates and Ag/ITO/glass structures for different ITO layers ((a)-ITO1, (b)-ITO2, (c)-ITO3, (d)-ITO4).
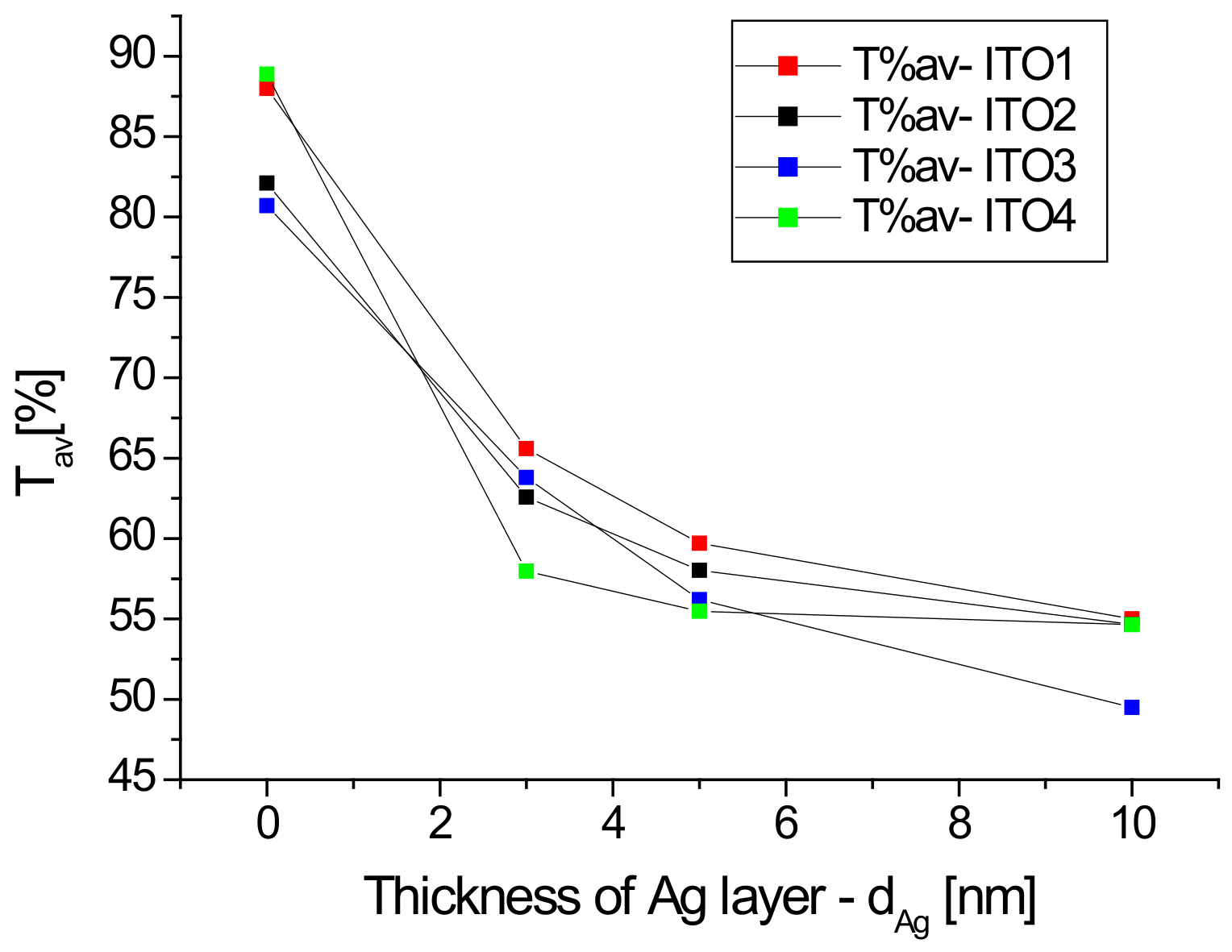
Figure 1 also shows the transmission spectra of the Ag/ITO/glass multilayers. The additional layer of silver nanoparticles causes a decrease in the transmission of the layer depending on the thickness of the layer of Ag nanoparticles, especially in the infrared region, which can be explained by the absorption of the light by silver nanoparticles. The dependence of the average transmission value in the visible region (380–780 nm) on the thickness of the silver nanoparticle layer for the tested samples is shown in Figure 2. It can be seen that the dynamics of the decrease in the average transmission value with the thickness of the Ag layer depends on the initial ITO/glass substrate. The slowest decrease in transmission occurs for the layer deposited on the ITO2/glass substrate.
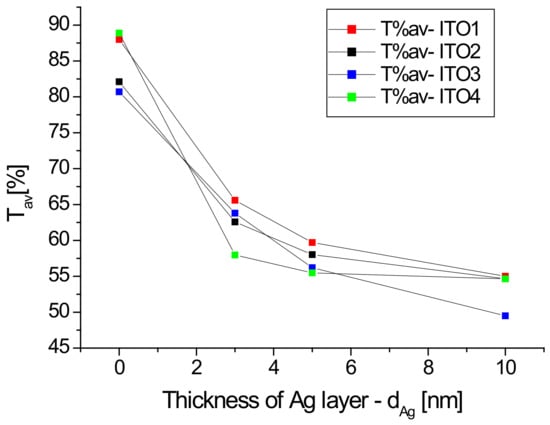
Figure 2.
Dependence of average transmission in the visible region as a function of the Ag nanoparticle layer thickness.
A decrease in mean transmission in the visible region for the ITO/PET layers after the sputtering of the additional silver ion and ITO layers by the continuous roll-to-roll (R2R) sputtering method was also observed by the authors of [32] for the spraying power of 0.05, 0.1, and 0.3 kW. However, the authors [32] obtained an increase in the average ITO/Ag/ITO/PET transmission for 0.15, 0.20 and 0.25 kW sputtering powers. The differences in optical properties of ITO/nano-Ag/ITO/PET multilayers obtained with different sputtering powers authors of [32] explained by the scattering and absorption of the nano-Ag interlayer. According to the authors [32], the optical transparency can be improved at a certain nano-Ag thickness (corresponding to certain DC Ag powers) on the ITO/nano-Ag/ITO structure. The ITO/nano-Ag/ITO/PET multilayer had the highest average optical transmittance at a nano-Ag layer thickness of 6 nm but increasing the nano-Ag thickness above 7 nm reduced light transmittance because the excessively thick Ag layer caused light scattering, particularly in the high wavelength area [32]. In the case of our layers with a thickness of 5 nm, the authors observed deceleration of the decrease in transmission with the layer thickness, excluding the Ag/ITO3/glass sample (Figure 2).
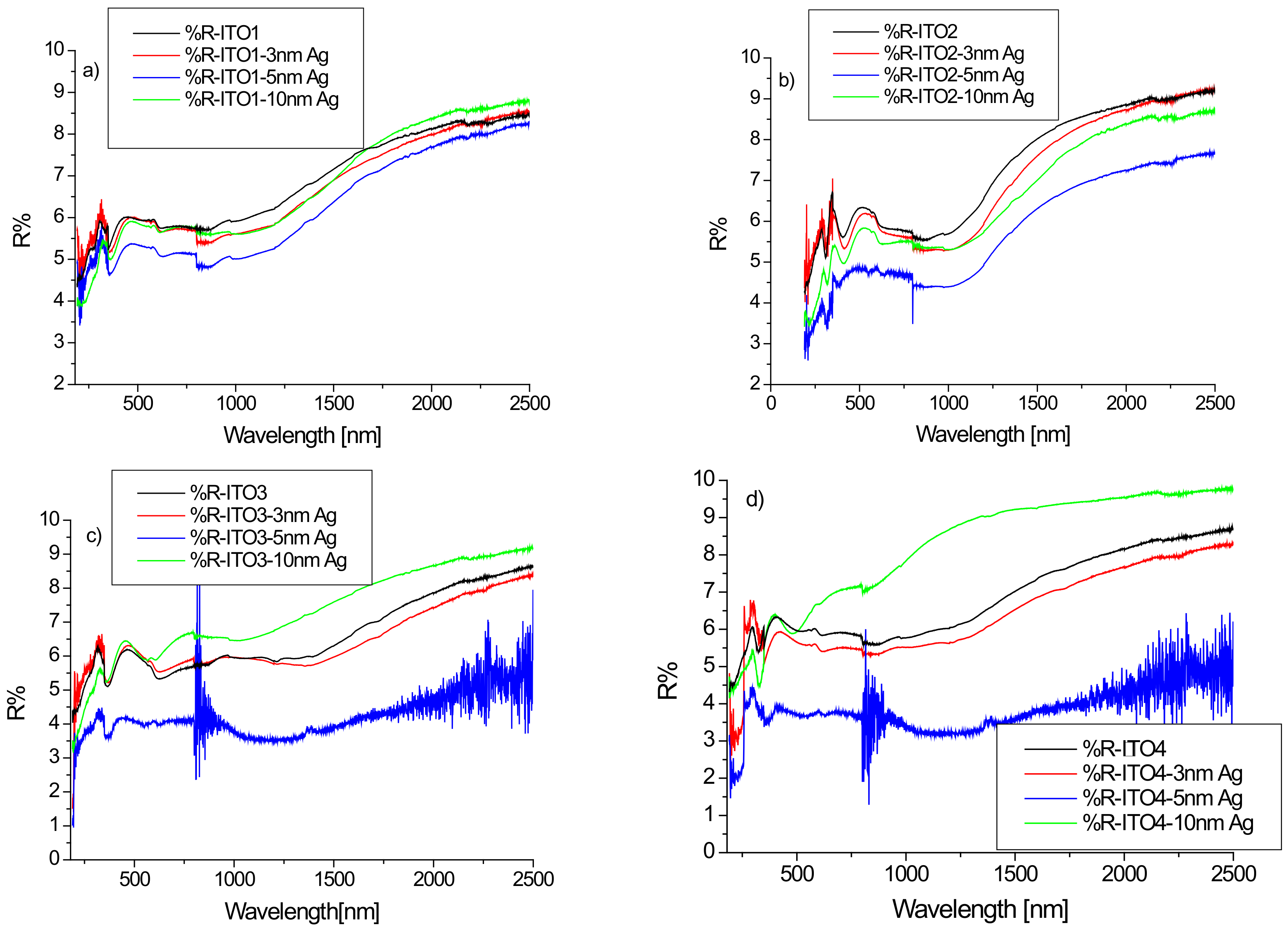
Figure 3 shows the reflection spectra of ITO/glass and Ag/ITO/glass for the wavelength range of 180–2500 nm. The maximum value of the reflectance did not exceed 10% in any case. This result is consistent in the Vis-NIR area with the result presented in [33]. However, the work [34] presents the reflection spectra of ITO deposited by magnetron sputtering on fused silica substrates for which the reflection coefficient increases rapidly from the wavelength of 1000 nm, reaching values on the order of 60% for a wavelength of 2400 nm.
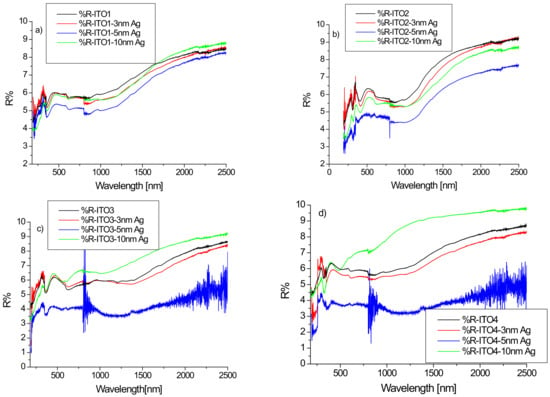
Figure 3.
(a–d) Reflectance spectra of ITO/glass and Ag/ITO/glass layers for various ITO layers.
The silver layer affects the reflection of the structure, with minimal reflectance values for a layer of silver nanoparticles with a thickness of 5 nm. These changes can be best seen in the infrared region. In the case of the ITO2/glass layer, we observe a decrease in infrared reflection after sputtering the layer of silver nanoparticles, regardless of its thickness. For the other substrates (ITO1/glass, ITO3/glass, ITO4/glass) for a silver layer with a thickness of 10 nm, we observe an increase in the reflectance in relation to pure ITO (Figure 3).
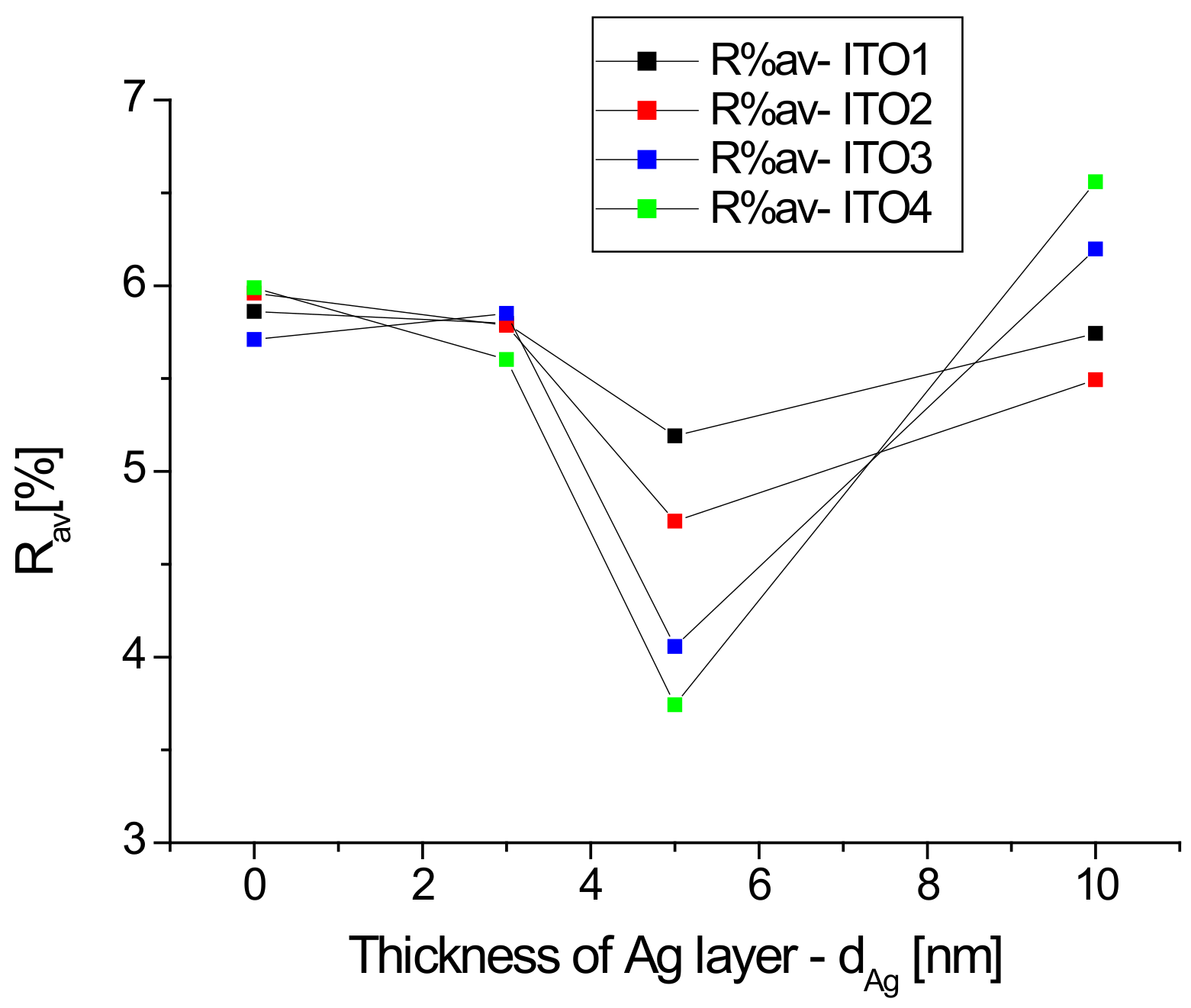
Figure 4 shows the dependence of the average reflectance in the visible spectrum on the thickness of the silver nanoparticle layer.
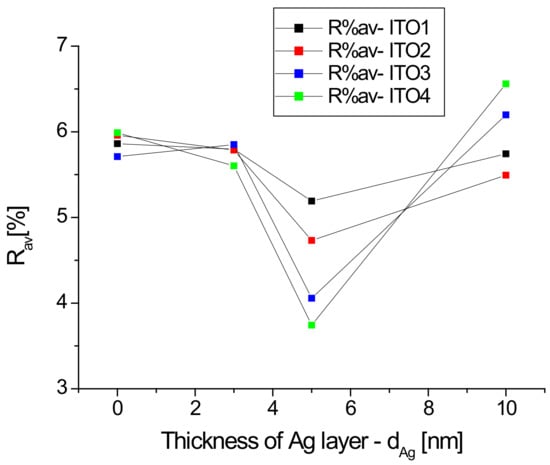
Figure 4.
Dependencies of average reflectance in the visible region as a function thickness of Ag nanoparticle layers.
For all samples with a silver nanoparticle layer thickness equal to 5 nm, we observe a decrease in the average reflectance, which then increases for a thickness of 10 nm, while for Ag/ITO3/glass and Ag/ITO4/glass, the average reflectance at a layer thickness of 10 nm exceeds that for pure ITO (Figure 4). In the case of a thin layer (3 and 5 nm) of silver nanoparticles, light penetrates between the Ag grains and becomes “trapped”, which leads to a decrease in reflection. The increase in the R% av value when changing the thickness from 5 to 10 nm for each sample can be explained by the “mirror effect”, that is, when the layer of Ag nanoparticles is too thick, the layer begins to behave like a mirror. Thus, the previously described minimum reflection for a layer of 5 nm thickness can be explained.
where d is the film thickness, T is the transmission, R is the reflectivity.
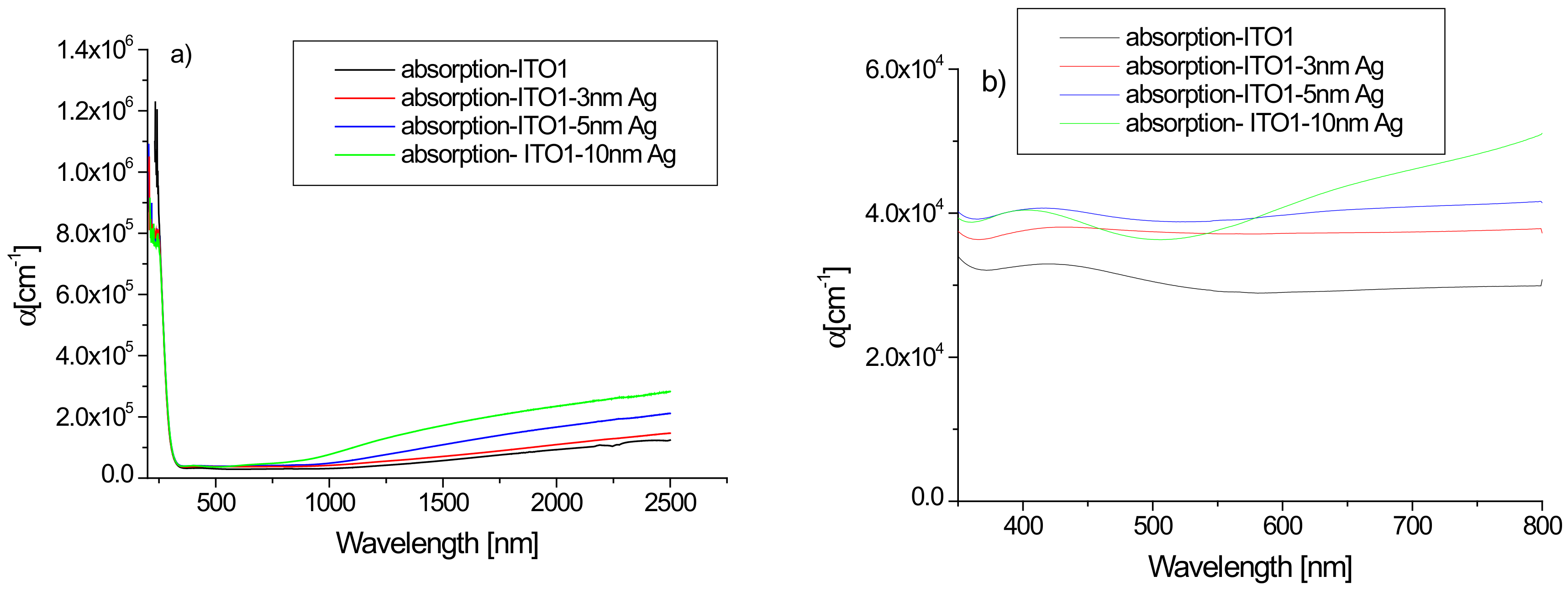
Figure 5 shows the absorption spectra of the tested ITO1/glass samples without and with an additional silver nanoparticle layer. The absorption spectrum of pure ITO1/glass correlates well with those presented in the literature [33]. Analysis of the absorption coefficient spectra of the films reveals the contribution from different absorption processes. In the ultraviolet region, the absorption is strong as a result of excitations across the fundamental band gap. The visible region is the transparency area of the layers. In the IR region, the main origin of the absorption is free carrier absorption. An additional layer of silver affects the absorption of the structure. In the infrared area, we observe an increase in absorption with the thickness of the layer of silver nanoparticles. The absorption coefficient in this wavelength range increases with the increasing thickness of the layer due to the increase of the free carrier density [33]. The changes in absorption caused by an additional silver layer are also visible in the visible area—the additional layer increases the absorption (Figure 5b).
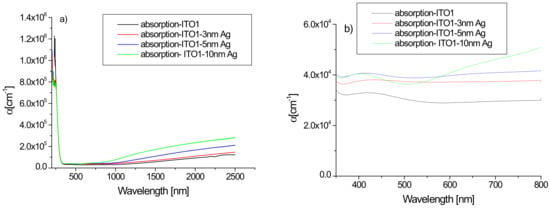
Figure 5.
(a,b) Absorption spectra of ITO1/glass and Ag/ITO1/glass layers in the region 180–2500 nm (a) and in the visible region (b).
The Tauc method was used to determine the optical band gap of the samples. The optical band gap (Eg) for the direct allowed transitions observed for ITO films [33,35,36,37] has been enumerated from the absorption spectra using the following equation [37]:
where A constant, α—absorption coefficient, h—Planck’s constant, ν—incident photon frequency, Eg –optical band gap.
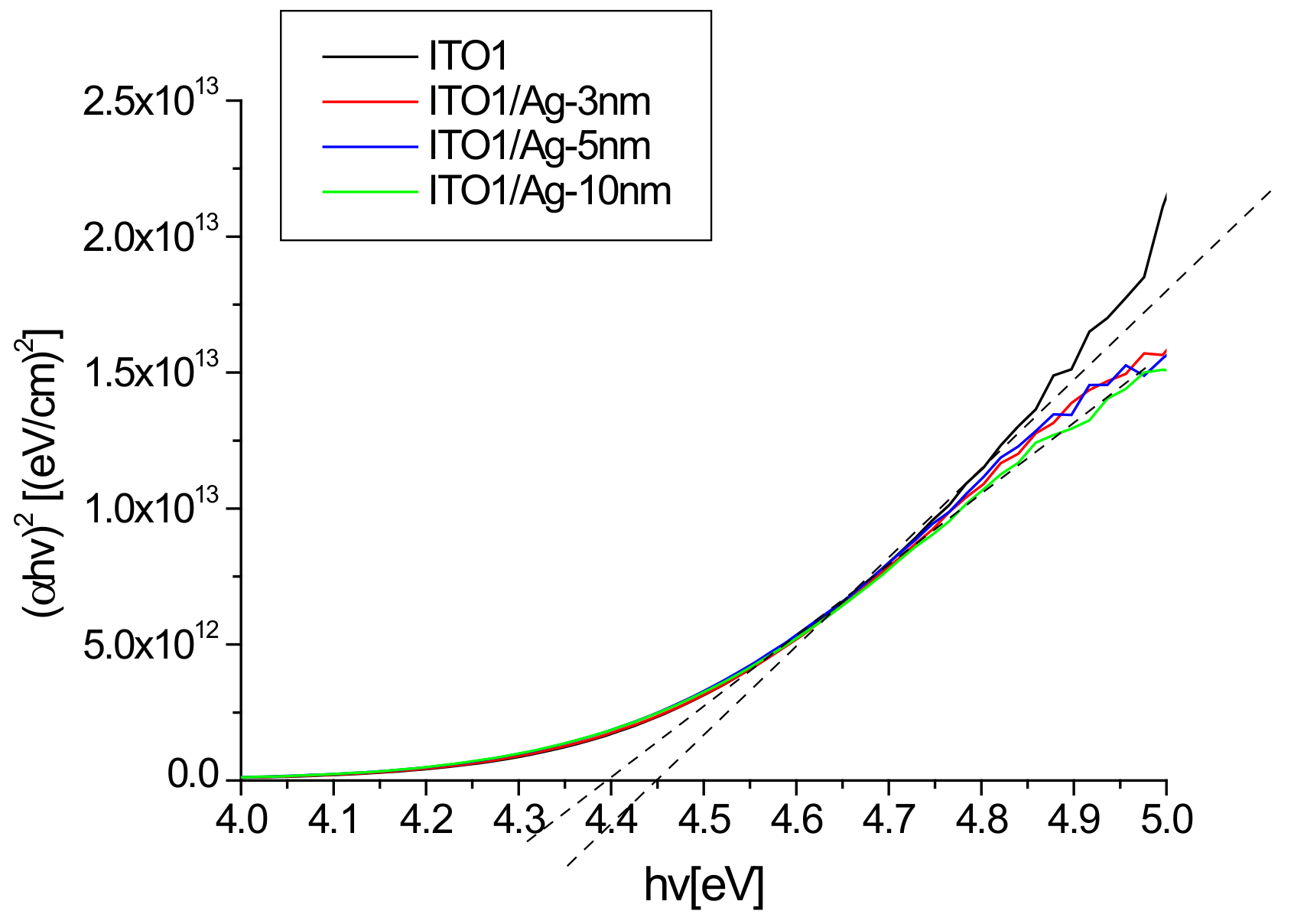
An example of the absorption graphs for ITO1 in Tauc’s coordinates is shown in Figure 6.
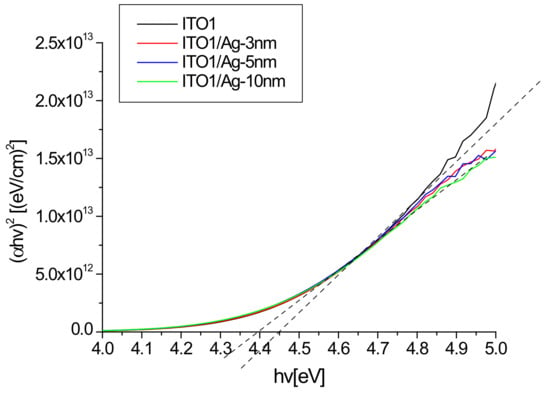
Figure 6.
Absorption spectra of ITO1/glass and Ag/ITO1/glass in Tauc coordinates.
It can be seen that the presence of silver slightly modifies the width of the optical band gap. The optical band gap values for all structures are presented in Table 2.

Table 2.
The values of Eg for different Ag/ITO structures.
The Eg value for individual ITO/glass substrates depends on the batch of samples and ranges from 4.20 to 4.45 eV. We can explain this by the effect of the thickness of the ITO layer [37,38,39,40]. For the ITO2, ITO3, and ITO4 layers, the Eg value does not exceed the upper limit of 4.3 eV reported in the literature [41,42]. The deposition of a layer of silver nanoparticles leads to a slight decrease in the Eg value (Table 1). A reduction of the Eg value due to the presence of silver nanoparticles was also observed for TiO2 layers [43,44].
The authors of [44] explain the reduction in the optical band gap due to the interaction of Ag ions with TiO2 or the formation of an impurity band within the TiO2 band gap.
Knowing the reflectivity and transmission of the layer allows determining the refractive index and the real part of the dielectric function. The refractive index can be determined from the formula [33]:
where k—is the extinction coefficient, α—is the absorption coefficient, λ—is the wavelength.
The real part of the dielectric function can be calculated as [25]:
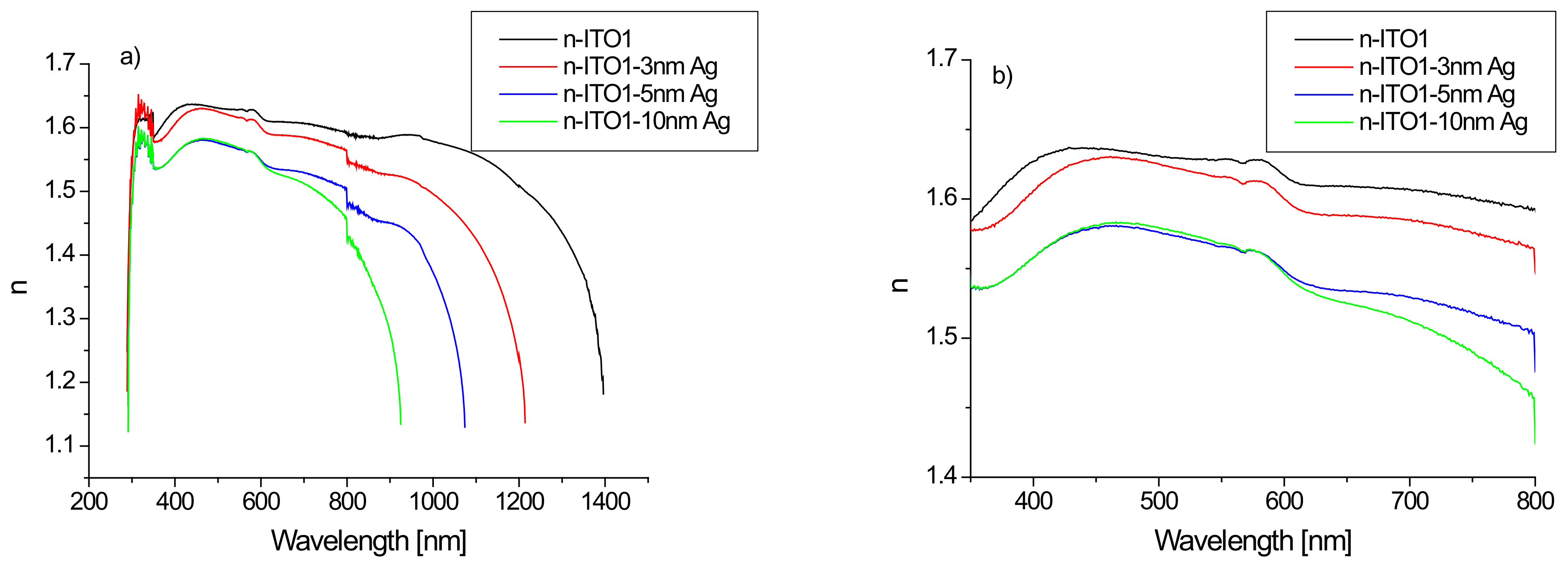
The calculated refractive index for structures based on ITO1/glass substrate as a function of wavelength is presented in Figure 7.
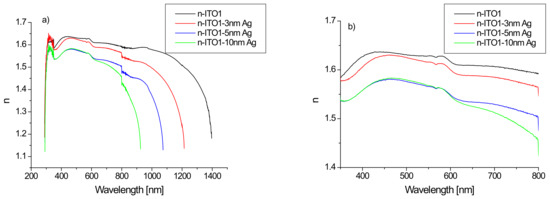
Figure 7.
Dependence of the refractive index on the wavelength of the layer based on ITO1, for the range 200–2500 nm (a) and the visible region (b).
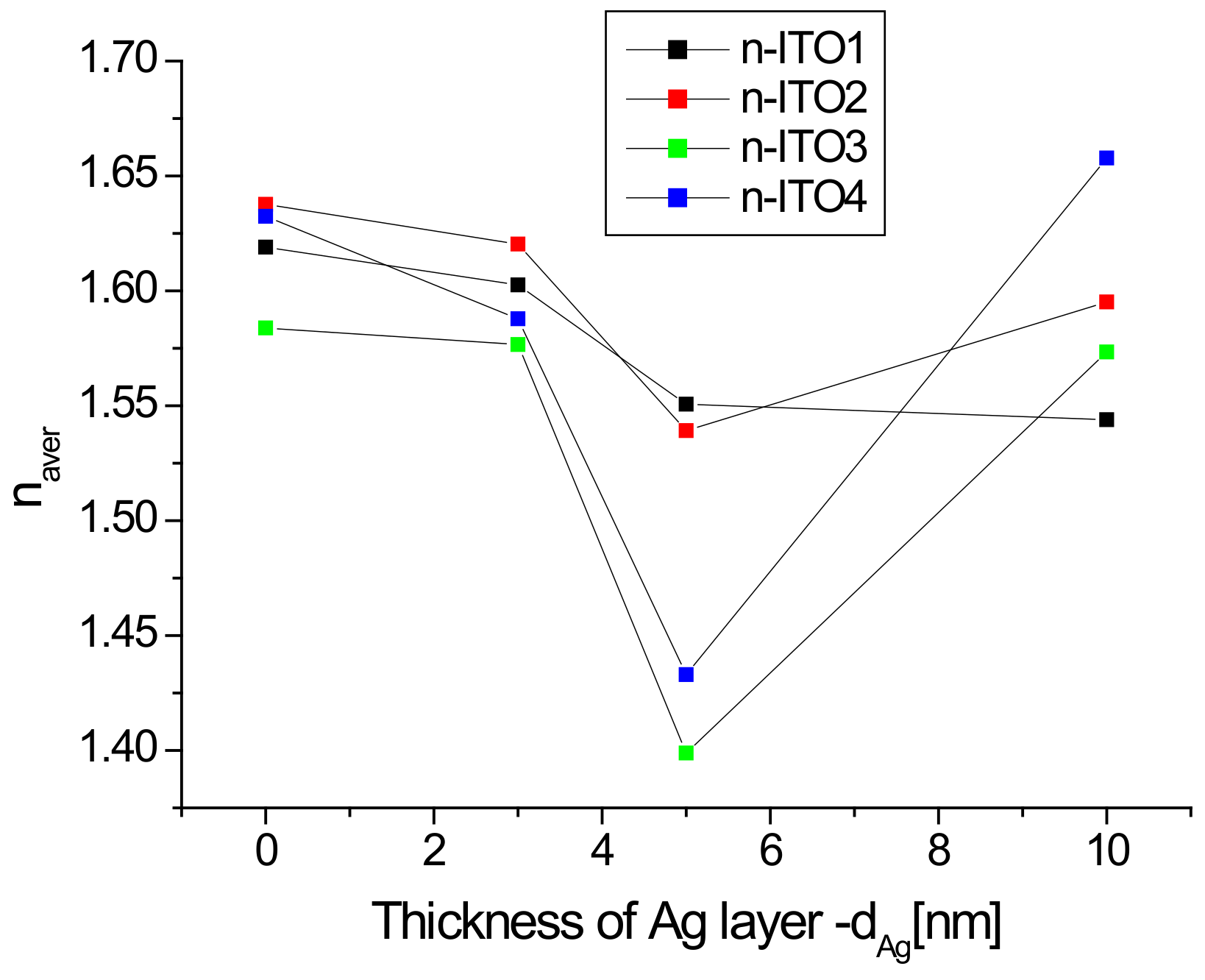
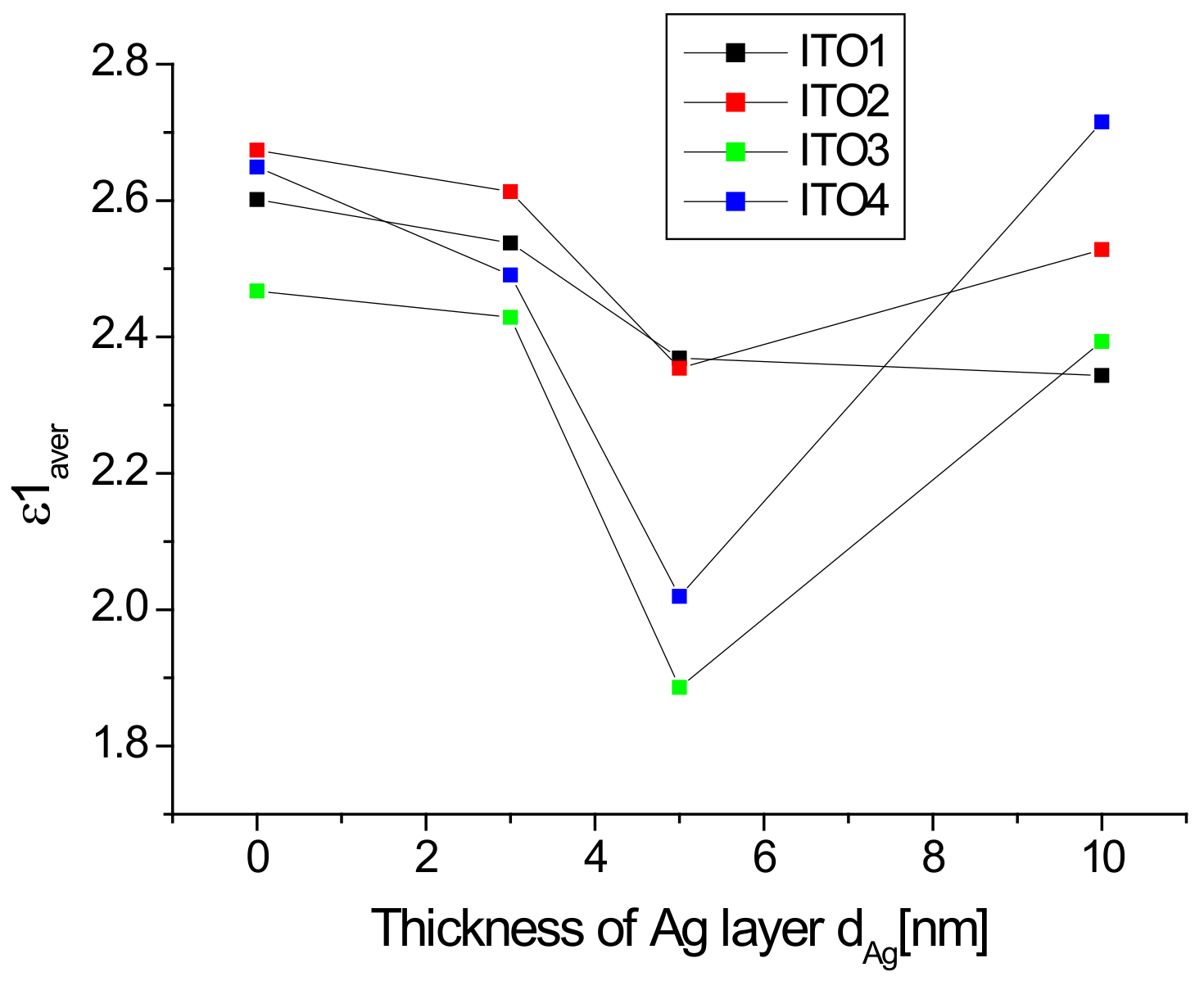
It is clearly visible (Figure 7a) that the refractive index varies in a very wide range of 1.1–1.6, while in the ultraviolet region, there is a rapid increase in its value with a decrease in the infrared region. In the infrared range, the value of the refractive index is significantly dependent on the presence of the silver nanoparticle layer and its thickness. The presence of an additional silver layer leads to a decrease in the refractive index, which decreases as the thickness of the silver layer increases (Figure 7). The refractive index of ITO/glass in the visible range does not differ much from that presented in [44]. The refractive index in the visible region changes slightly with the wavelength, therefore, its representative may be the average refractive index (nav). The calculated values of the average refractive index as a function of the thickness of the Ag layer are presented in Figure 8.
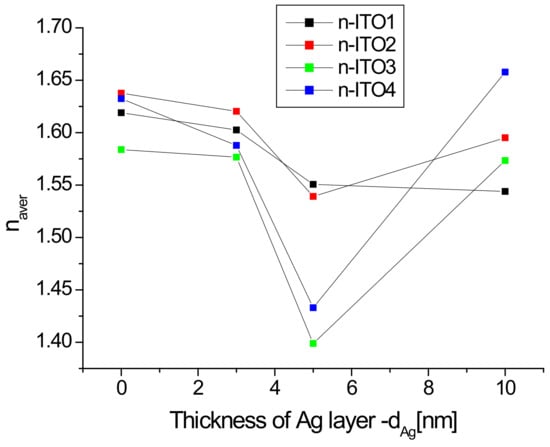
Figure 8.
Dependence of the average refractive index in the visible region as a function of the Ag nanoparticle layer thickness.
The average refractive index is significant in the presence and thickness of the silver layer, and its minimum value occurs for the layer of silver nanoparticles with a thickness of 5 nm (excluding ITO1, for which the minimum occurs at a thickness of 10 nm). The dependence of the refractive index on the thickness of the Al and Ir on fused silica plater layers was also observed in [45]. Reduction of the refractive index with the thickness of the silver layer also occurs for the Ag/glass structure [46]. In turn, the authors of [47] observed that the refractive index of a ZnO thin film decreases after doping with Sn, Mn, or Al ions. A similar effect was presented in [48] in the case of a ZnO thin film doped with aluminum ions. The authors of [48] explain this fact that film doping increases the free carrier concentration in films, which results in the decrease of the refractive index of the films. Thus, in our case, the observed effect can be partially explained by the doping of the ITO layer by silver ions. The increase in the average refractive index for layers Ag/ITO2/glass, Ag/ITO3/glass, and Ag/ITO4/glass with a thickness of 10 nm in comparison to the thickness of 5 nm can be due to the result of an increase in the reflectance of this layer compared to the 5 nm layers, which is caused by the “mirror” effect (a thick layer of silver begins to reflect more intensely like silver nanoparticles). This effect is weaker for the ITO1/glass layer and is compensated by the doping effect of the layer, therefore, we do not observe an increase in the refractive index for the thickness of 10 nm, but only a decrease in the dynamics of its decrease.
The dependences with a wavelength of the real part of dielectric constants for ITO1/glass with different thicknesses of additional silver nanoparticle layer are presented in Figure 9.
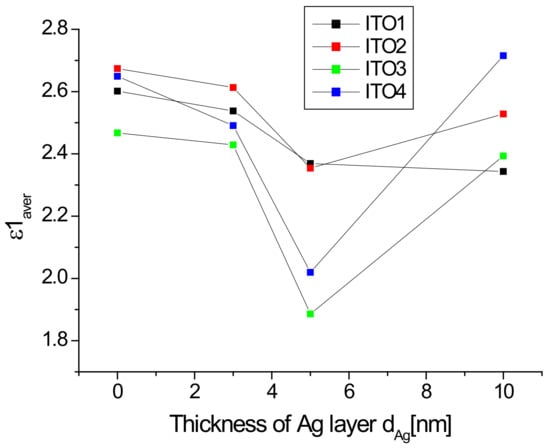
Figure 9.
Real part of the dielectric function for ITO1/glass and Ag/ITO1/glass structures.
The additional silver layer leads to a reduction in the value of the real part of the dielectric function of ITO1/glass, depending on the Ag layer thickness. The values of the real part of the dielectric function in the visible region for the ITO/glass layers correspond well to the results obtained from [33]. The authors observe similar dependencies for the other ITO layers.
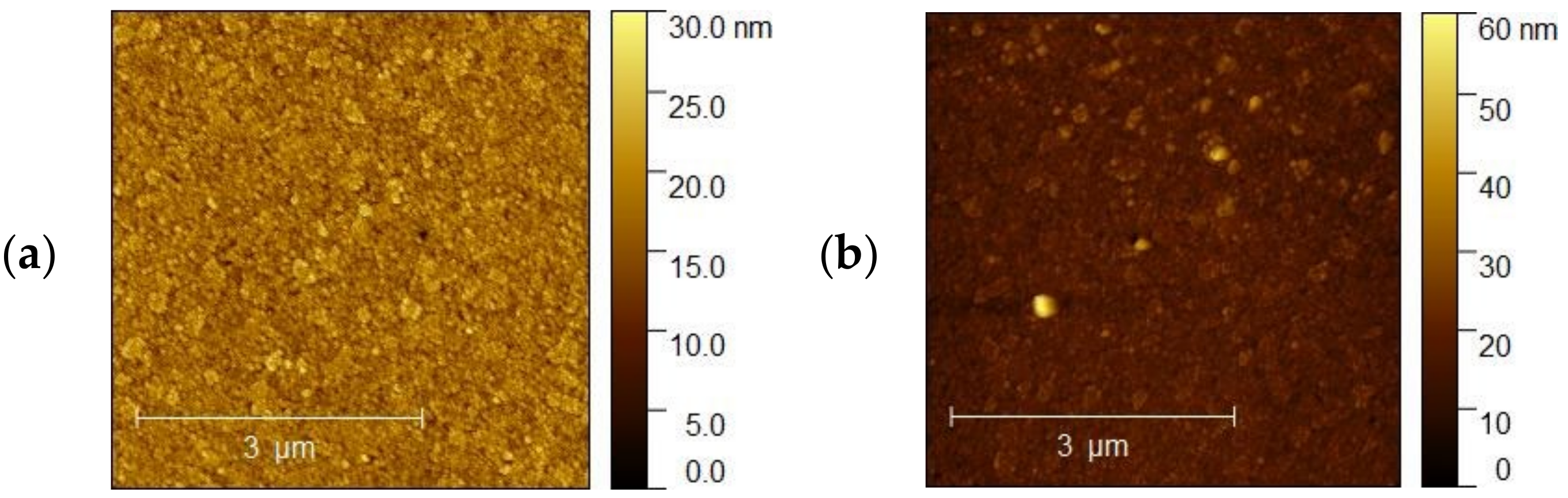
3.2. AFM Analysis
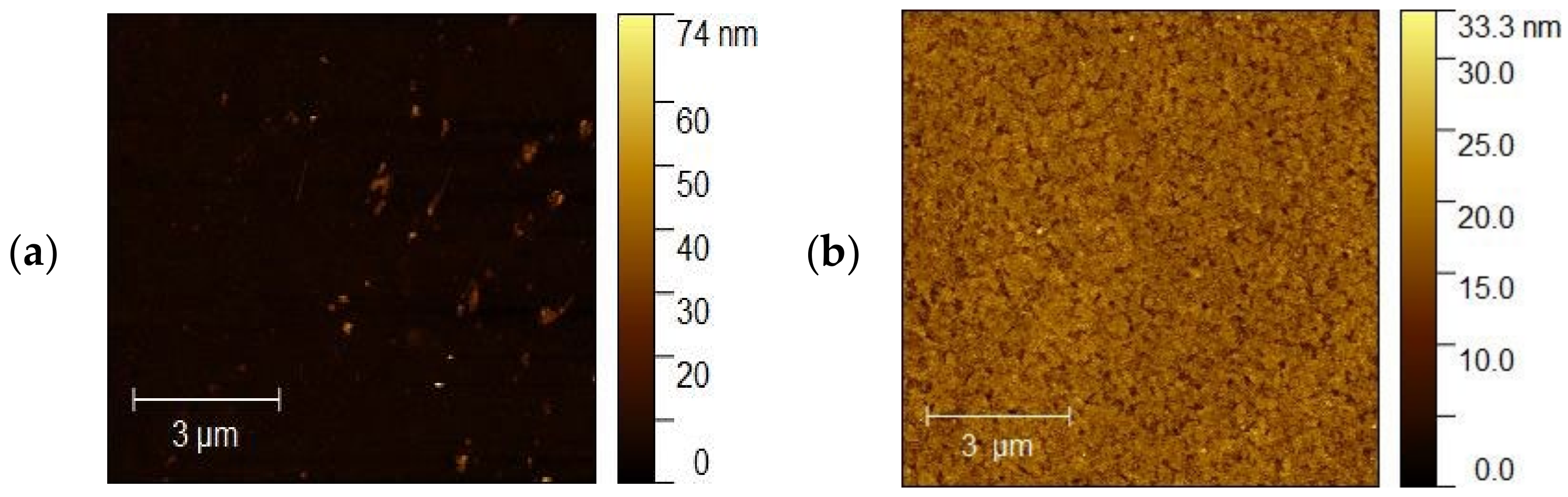
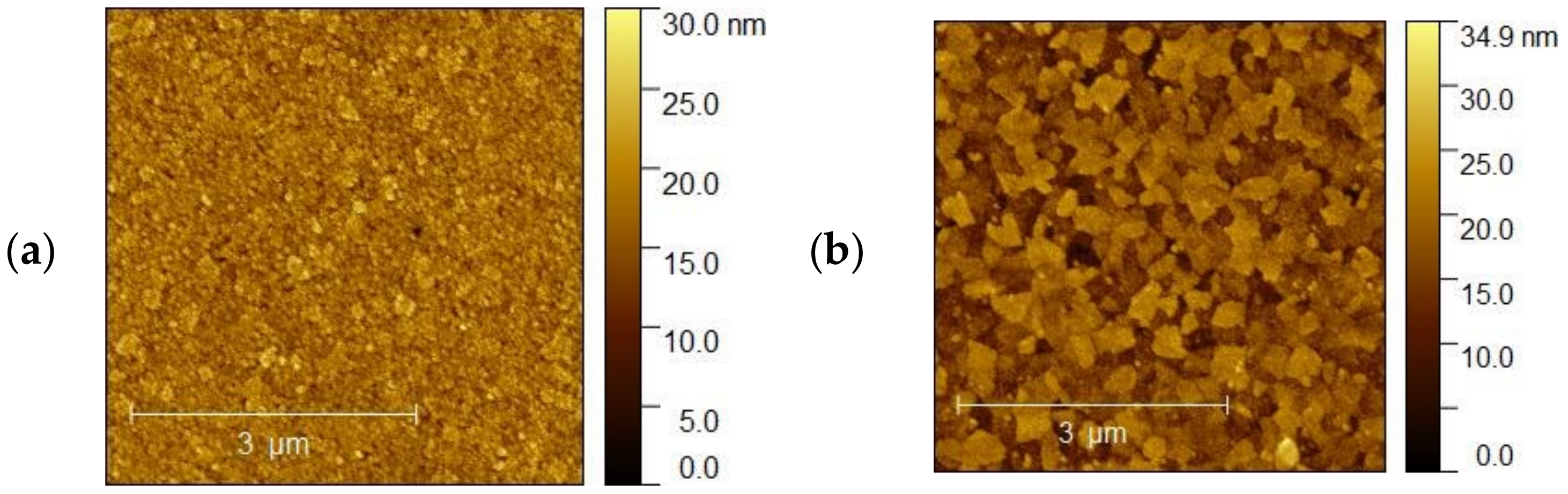
The topography of the selected samples was determined by atomic force microscopy (AFM, Park System XE-70) and was conducted at ambient pressure and room temperature. Figure 10a–d shows AFM images of the ITO1-ITO4 before Ag nanoparticle deposition. It is clearly visible that the structure and shape of grains of ITO substrates are different, which affects different growth mechanisms of the silver nanoparticle layers. It is visible for ITO3 and ITO4 with 5 nm Ag nanoparticles, Figure 11a,b.
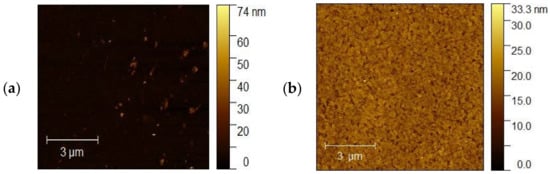
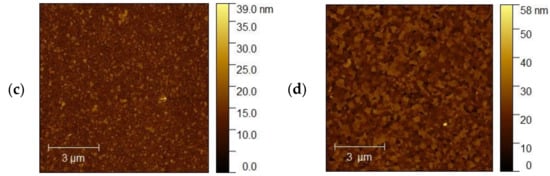
Figure 10.
AFM images of the (a) ITO1, (b) ITO2, (c) ITO3, (d) ITO4 before Ag nanoparticle deposition.
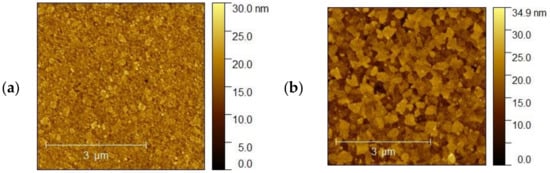
Figure 11.
(a,b). AFM images of the (a) ITO3 for 5 nm and (b) ITO4 for 5 nm.
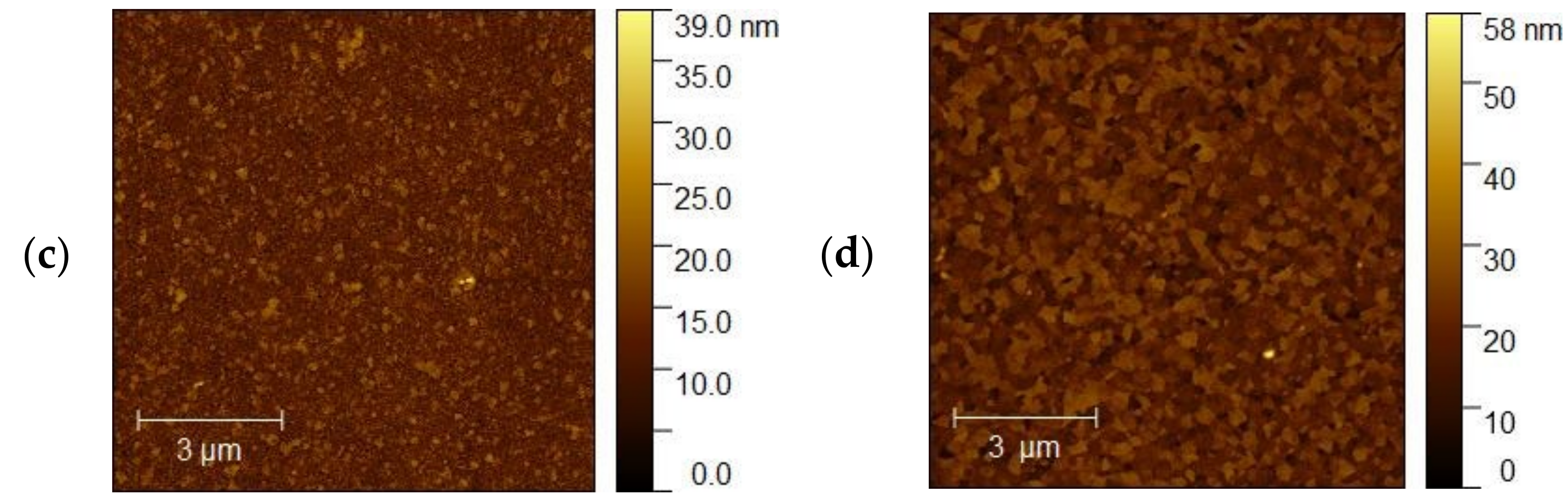
Figure 12a,b shows AFM images of the ITO 3 for 5 and 10 nm thickness of Ag nanoparticles, respectively. Based on our analysis, the surface morphologies for 5 nm have a more porous and granular structure than for 10 nm. However, for sample 10 nm, smooth and homogeneous morphology is becoming a ‘mirror-like’ structure, which is not desirable. Table 3 summarizes the roughness parameters for the initial ITO surfaces and surfaces covered with silver nanoparticles.
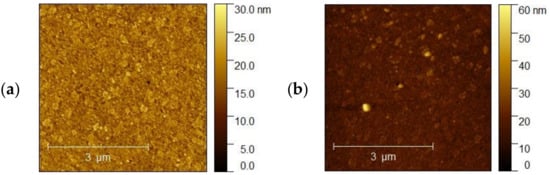
Figure 12.
(a,b) AFM images of the (a) ITO3 5 nm and (b) ITO3 for 10 nm.

Table 3.
AFM roughness analysis of ITO1, ITO2, ITO3, ITO4 surface and ITO3/3 nm, ITO3/5 nm, ITO3/10 nm, ITO4/5 nm Ag NPs.
4. Conclusions
In this study, the authors analyze the optical properties of silver nanoparticles Ag/ITO/glass thin layers. Three types of Ag nanoparticle thickness (3, 5, and 10 nm) were fabricated. In addition, Ag nanoparticles with different thicknesses of four types in ITO (ITO1, ITO2, ITO3, ITO4) were fabricated.
To summarize, substrates ITO3 and ITO4 showed high transmission, higher than 80% in the visible range compared to ITO-1 and ITO-2. Furthermore, ITO-4 showed transmission greater than 90% at 600 nm. However, for ITO-4, after depositing the layer of Ag nanoparticles, the fastest and the highest value of the decrease in the transmission is visible, see Figure 2.
Reflection spectra of all substrates ITO with silver nanoparticles are similar, therefore, the authors will choose a substrate with ITO for thin film solar cells by the maximum transmittance.
When we only take into account the optical properties of the ITO coatings for their application in photovoltaic devices, ITO1 shows the highest transmittance, which is optimal (see Figure 2). However, currently, we cannot determine which type of ITO and which Ag thickness will allow obtaining maximum efficiency for photovoltaic solar cells based on titanium dioxide and copper oxide. This will be verified by the next experiment.
Author Contributions
Conceptualization, G.W., P.S.-C., K.G. and P.P.; methodology, K.G.; software, P.P. and K.G.; validation, G.W. and P.P.; formal analysis, P.S.-C.; investigation, K.G. and P.P.; resources, G.W. and K.G.; data curation, G.W. and P.S.-C.; writing—original draft preparation, P.S.-C.; writing—review and editing, G.W. and P.P.; visualization, G.W., P.S.-C., K.G. and P.P.; supervision, G.W.; project administration, G.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gwóźdź, K.; Płaczek-Popko, E.; Zielony, E.; Gumienny, Z.; Pietruszka, R.; Witkowski, B.S.; Kopalko, K.; Godlewski, M.K. Parametry konwersji fotowoltaicznej dla fotoogniw plazmonicznych na bazie ZnO z nanocząstkami srebra i złota. Przegląd Elektrotechniczny 2016, 9, 38–40. [Google Scholar] [CrossRef]
- Wisz, G.; Sawicka-Chudy, P.; Sibiński, M.; Starowicz, Z.; Płoch, D.; Goral, A.; Bester, M.; Cholewa, M.; Woźny, J.; Sosna-Głębska, A. Solar cells based on copper oxide and titanium dioxide prepared by reactive direct-current magnetron sputtering. Opto-Electron. Rev. 2021, 29, 97–104. [Google Scholar]
- Jacak, W.; Popko, E.; Henrykowski, A.; Zielony, E.; Gwozdz, K.; Luka, G.; Pietruszka, R.; Witkowski, B.; Wachnicki, L.; Godlewski, M.; et al. On the size dependence and spatial range for the plasmon effect in photovoltaic efficiency enhancement. Sol. Energy Mater. Sol. Cells 2016, 147, 1–16. [Google Scholar] [CrossRef]
- Culchac, F.J.; Granada, J.C.; Porras-Montenegro, N. Electron ground state in concentric GaAs-(Ga, Al)As single and double quantum rings. Phys. Status Solidi 2007, 4, 4139–4144. [Google Scholar] [CrossRef]
- Fan, Z.; Lin, Q. Reducing reflection losses in solar cells. In SPIE Newsroom; SPIE: Bellingham, WA, USA, 2014. [Google Scholar] [CrossRef]
- Lin, Q.; Leung, S.-F.; Tsui, K.-H.; Hua, B.; Fan, Z. Programmable nanoengineering templates for fabrication of three-dimensional nanophotonic structures. Nanoscale Res. Lett. 2013, 8, 268. [Google Scholar] [CrossRef]
- Yeh, L.K.; Lai, K.Y.; Lin, G.J.; Fu, P.H.; Chang, H.C.; Lin, C.A.; He, J.H. Giant efficiency en-hancement of GaAs solar cells with graded antireflection layers based on syringelike ZnO nanorod arrays. Adv. Energy Mater. 2011, 1, 506–510. [Google Scholar] [CrossRef]
- Wisz, G.; Sawicka-Chudy, P.; Sibiński, M.; Płoch, D.; Bester, M.; Cholewa, M.; Woźny, J.; Yavorskyi, R.; Nykyruy, L.; Ruszała, M. TiO2/CuO/Cu2O Photovoltaic Nanostructures Prepared by DC Reactive Magnetron Sputtering. Nanomaterials 2022, 12, 1328. [Google Scholar] [CrossRef]
- Wisz, G.; Sawicka-Chudy, P.; Wal, A.; Potera, P.; Bester, M.; Płoch, D.; Sibiński, M.; Cholewa, M.; Ruszała, M. TiO2:ZnO/CuO thin film solar cells prepared via reactive direct-current (DC) magnetron sputtering. Appl. Mater. Today 2022, 29, 101673. [Google Scholar] [CrossRef]
- Yang, Y.; Qing, J.; Ou, J.; Lin, X.; Yuan, Z.; Yu, D.; Zhou, X.; Chen, X. Rational design of metallic nanowire-based plasmonic architectures for efficient inverted polymer solar cells. Sol. Energy 2015, 122, 231–238. [Google Scholar] [CrossRef]
- Oh, Y.; Lim, J.W.; Kim, J.G.; Wang, H.; Kang, B.-H.; Park, Y.W.; Kim, H.; Jang, Y.J.; Kim, J.; Kim, D.H.; et al. Plasmonic Periodic Nanodot Arrays via Laser Interference Lithography for Organic Photovoltaic Cells with >10% Efficiency. ACS Nano 2016, 10, 10143–10151. [Google Scholar] [CrossRef]
- Placzek-Popko, E.; Gwozdz, K.; Gumienny, Z.; Zielony, E.; Pietruszka, R.; Witkowski, B.S.; Wachnicki, Ł.; Gieraltowska, S.; Godlewski, M.; Jacak, W.; et al. Si/ZnO nanorods/Ag/AZO structures as promising photovoltaic plasmonic cells. J. Appl. Phys. 2015, 117, 193101. [Google Scholar] [CrossRef]
- Mandal, P. Application of Plasmonics in Solar Cell Efficiency Improvement: A Brief Review on Recent Progress. Plasmonics 2022, 17, 1247–1267. [Google Scholar] [CrossRef]
- Parashar, P.K.; Komarala, V.K. Engineered optical properties of sil-ver-aluminum alloy nanoparticles embedded in the SiON matrix for maximizing light diffusion in plasmonic silicon solar cells. Sci. Rep. 2017, 7, 12520. [Google Scholar] [CrossRef]
- Kumawat Kumar, K.; Mishra, S.; Dhawa, A. Plasmonic-enhanced micro-crystalline silicon solar cells. JOSA B 2020, 37, 495–504. [Google Scholar] [CrossRef]
- Mokari, G.; Heidarzadeh, H. Efficiency Enhancement of an Ultra-Thin Silicon Solar Cell Using Plasmonic Coupled Core-Shell Nanoparticles. Plasmonics 2019, 14, 1041–1049. [Google Scholar] [CrossRef]
- Ma, R.; Zhou, K.; Sun, Y.; Liu, T.; Kan, Y.; Xiao, Y.; Peña, T.A.D.; Li, Y.; Zou, X.; Xing, Z.; et al. Achieving high efficiency and well-kept ductility in ternary all-polymer organic photovoltaic blends thanks to two well miscible donors. Matter 2022, 5, 725–734. [Google Scholar] [CrossRef]
- Ma, R.; Yan, C.; Fong, P.W.K.; Yu, J.; Liu, H.; Yin, J.; Huang, J.; Lu, X.; Yan, H.; Li, G. In situ and ex situ investigations on ternary strategy and co-solvent effects towards high-efficiency organic solar cells. Energy Environ. Sci. 2022, 15, 2479–2488. [Google Scholar] [CrossRef]
- Luo, Z.; Gao, Y.; Lai, H.; Li, Y.; Wu, Z.; Chen, Z.; Sun, R.; Ren, J.; He, F.; Woo, H.; et al. Asymmetric side-chain substitution enables a 3D network acceptor with hydrogen bond assisted crystal packing and enhanced electronic cou-pling for efficient organic solar cells. Energy Environ. Sci. 2022, 15, 4601–4611. [Google Scholar] [CrossRef]
- Gao, W.; Jiang, M.; Wu, Z.; Fan, B.; Jiang, W.; Cai, N.; Xie, H.; Lin, F.R.; Luo, J.; An, Q.; et al. Intramolecular Chloro–Sulfur Interaction and Asymmetric SideChain Isomeriza-tion to Balance Crystallinity and Miscibility in AllSmall-Molecule Solar Cells. Angew. Chem. Int. Ed. 2022, 61, e202205168. [Google Scholar] [CrossRef]
- Kim, H.; Gilmore, C.M.; Pique, A.; Horwitz, J.S.; Mattoussi, H.; Murata, H.; Kafafi, Z.H.; Chrisey, D.B. Electrical, optical, and structural properties of indium-tin-oxide thin films for organic light-emitting devices. J. Appl. Phys. 1999, 86, 6451–6461. [Google Scholar] [CrossRef]
- Prepelita, P.; Filipescu, M.; Stavarache, I.; Garoi, F.; Craciun, D. Transparent thin films of indium tin oxide: Morphology–optical investigations, inter dependence analyzes. Appl. Surf. Sci. 2017, 424, 368–373. [Google Scholar] [CrossRef]
- Socol, M.; Preda, N.; Rasoga, O.; Costas, A.; Stanculescu, A.; Breazu, C.; Gherendi, F.; Socol, G. Pulsed Laser Deposition of Indium Tin Oxide Thin Films on Nanopatterned Glass Substrates. Coatings 2018, 9, 19. [Google Scholar] [CrossRef]
- Zhang, J.; Chia, A.C.E.; Lapierre, R.R. Low resistance indium tin oxide contact to n-GaAs nanowires. Semicond. Sci. Technol. 2014, 29, 054002. [Google Scholar] [CrossRef]
- Thirumoorthi, M.; Prakash, J.T.J. Structure, optical and electrical properties of indium tin oxide ultra thin films prepared by jet nebulizer spray pyrolysis technique. J. Asian Ceram. Soc. 2016, 4, 124–132. [Google Scholar] [CrossRef]
- Kang, M.; Kim, I.; Chu, M.; Kim, S.W.; Ryu, J.W. Optical Properties of Sputtered Indium-tin-oxide. Thin Film. J. Korean Phys. Soc. 2011, 59, 3280–3283. [Google Scholar] [CrossRef]
- Gwamuri, J.; Marikkannan, M.; Mayandi, J.; Bowen, P.K.; Pearce, J.M. Influence of Oxygen Concentration on the Performance of Ultra-Thin RF Magnetron Sputter Deposited Indium Tin Oxide Films as a Top Electrode for Photovoltaic Devices. Materials 2016, 9, 63. [Google Scholar] [CrossRef]
- Dong, L.; Zhu, G.; Xu, H.; Jiang, X.; Zhang, X.; Zhao, Y.; Yan, D.; Yuan, L.; Yu, A. Fabrication of Nanopillar Crystalline ITO Thin Films with High Transmittance and IR Reflectance by RF Magnetron Sputtering. Materials 2019, 12, 958. [Google Scholar] [CrossRef]
- Pokaipisit, A.; Horprathum, M.; Limsuwan, P. Effect of Films Thickness on the Properties of ITO Thin Films Prepared by Electron Beam Evaporation. Kasetsart J. (Nat. Sci.) 2007, 41, 255–261. [Google Scholar]
- Mazur, M.; Kaczmarek, D.; Domaradzki, J.; Wojcieszak, D.; Song, S.; Placido, F. Influence of thickness on transparency and sheet resistance of ITO thin films. In Proceedings of the Eighth International Conference on Advanced Semiconductor Devices and Microsystems, Smolenice, Slovakia, 25–27 October 2010; pp. 65–68. [Google Scholar]
- Ibrahem, H.; Moghdad, M. Preparation of ITO thin film by Sol-Gel method. Int. J. Electr. Eng. 2013, 1, 18–22. [Google Scholar]
- Cheng, Y.-T.; Lu, T.-L.; Hong, M.-H.; Ho, J.-J.; Chou, C.-C.; Ho, J.; Hsieh, T.-P. Evaluation of Transparent ITO/Nano-Ag/ITO Electrode Grown on Flexible Electrochromic Devices by Roll-to-Roll Sputtering Technology. Coatings 2022, 12, 455. [Google Scholar] [CrossRef]
- Khusayfan, N.M.; El-Nahass, M.M. Study of Structure and Electro-Optical Characteristics of Indium Tin Oxide Thin Films. Adv. Condens. Matter Phys. 2013, 2013, 408182. [Google Scholar] [CrossRef]
- Maniyara, R.A.; Graham, C.; Paulillo, B.; Bi, Y.; Chen, Y.; Herranz, G.; Baker, D.E.; Mazumder, P.; Konstantatos, G.; Pruneri, V. Highly transparent and conductive ITO substrates for near infrared applications. APL Mater. 2021, 9, 021121. [Google Scholar] [CrossRef]
- Her, S.-C.; Chang, C.-F. Fabrication and Characterization of Indium Tin Oxide Films. J. Appl. Biomater. Funct. Mater. 2017, 15, 170–175. [Google Scholar] [CrossRef]
- Saeed, U.; Abdel-Wahab, M.S.; Sajith, V.K.; Ansari, M.S.; Ali, A.M.; Al-Turaif, H.A. Characterization of an amorphous indium tin oxide (ITO) film on a polylactic acid (PLA) substrate. Bull. Mater. Sci. 2019, 42, 175. [Google Scholar] [CrossRef]
- Sofi, A.H.; Shah, M.A.; Asokan, K. Structural, Optical and Electrical Properties of ITO Thin Films. J. Electron. Mater. 2017, 47, 1344–1352. [Google Scholar] [CrossRef]
- Mohamed, S.H.; El-Hossary, F.M.; Gamal, G.A.; Kahlid, M.M. Properties of In-dium Tin Oxide Thin Films Depositedon Polymer Substrates. Acta Phys. Pol. A 2009, 115, 704–708. [Google Scholar] [CrossRef]
- Amalathas, A.P.; Alkaisi, M.M. Effects of film thickness and sputtering power on properties of ITO thin films deposited by RF magnetron sputtering without oxygen. J. Mater. Sci. Mater. Electron. 2016, 27, 11064–11071. [Google Scholar] [CrossRef]
- Hamberg, I.; Granqvist, C.G.; Berggren, K.F.; Sernelius, B.E.; Engström, L. Band-gap widening in heavily Sn-doped In2O3. Phys. Rev. B 1984, 30, 3240–3249. [Google Scholar] [CrossRef]
- Kim, J.; Shrestha, S.; Souri, M.; Connell, J.G.; Park, S.; Seo, A. High-temperature optical properties of indium tin oxide thin-films. Sci. Rep. 2020, 10, 12486. [Google Scholar] [CrossRef]
- Ivanova, T.; Harizanova, A.; Koutzarova, T.; Vertruyen, B. Characterization of nanostructured TiO2:Ag films: Structural and optical properties. J. Phys. Conf. Ser. 2016, 764, 012019. [Google Scholar] [CrossRef]
- Zhao, C.; Krall, A.; Zhao, H.; Zhang, Q.; Li, Y. Ultrasonic spray pyrolysis synthesis of Ag/TiO2 nanocomposite photocatalysts for simultaneous H2 production and CO2 reduction. Int. J. Hydrog. Energy 2012, 37, 9967–9976. [Google Scholar] [CrossRef]
- Baum, M.; Alexeev, I.; Latzel, M.; Christiansen, S.H.; Schmidt, M. Determination of the effective refractive index of nanoparticulate ITO layers. Opt. Express 2013, 21, 22754–22761. [Google Scholar] [CrossRef] [PubMed]
- Lehmuskero, A.; Kuittinen, M.; Vahimaa, P. Refractive index and extinction coefficient dependence of thin Al and Ir films on deposition technique and thickness. Opt. Express 2007, 15, 10744. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.; Clavero, C.; Schweigert, D.; Le, M. Thickness and microstructure effects in the optical and electrical properties of silver thin films. AIP Adv. 2015, 5, 117234. [Google Scholar] [CrossRef]
- Amroun, M.N.; Salim, K.; Kacha, A.H.; Khadraoui, M. Effect of TM (TM= Sn, Mn, Al) Doping on the PhysicalProperties of ZnO Thin Films Grown by Spray Pyrol-ysis Technique: A comparative Study. Int. J. Thin. Film. Sci. Technol. 2020, 9, 7–19. [Google Scholar]
- Zhai, C.-H.; Zhang, R.-J.; Chen, X.; Zheng, Y.-X.; Wang, S.-Y.; Liu, J.; Dai, N.; Chen, L.-Y. Effects of Al Doping on the Properties of ZnO Thin Films Deposited by Atomic Layer Deposition. Nanoscale Res. Lett. 2016, 11, 407. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).