Abstract
The aim of this study is to evaluate the depth of penetration of an experimental preparation with the characteristics of a dental infiltrant into the decalcified root cementum tissue and observation of the root cementum tissue subjected to a single and repeated twice hydrochloric acid etching process. The study material consisted of 20 human teeth (the study group—12 demineralised teeth, the control group—8 teeth). A commercially available Icon preparation and an experimental preparation were used for the study with addition 2% of YF3 (yttrium trifluoride) added as an indicator to facilitate microscopic observation. Each tooth was divided into two zones, blue (Icon) and red (experimental preparation). The teeth were divided into two subgroups—in the first subgroup, the etching preparation was applied once, in the second subgroup twice, and at the end the teeth were infiltrated with the experimental preparation and the Icon preparation. The study of tooth longitudinal section morphology and chemical composition was performed with the use of a Hitachi S-3400N scanning electron microscope. Microscopic observations show that the applied YF3 tracer in most cases agglomerates and remains in the form of conglomerates on the surface of the root cementum. Single particles of YF3 are visible, penetrating through the cementum tissues into the root dentine structure. The degree of tissue infiltration with the resin (depth of penetration into decalcified tissues) is visible at a depth of approx. 80–120 μm. In the test group subjected to a single etching process, good penetration of both resins was noticeable, however, excessive erosion of the root surface was evident in several of the specimens—indicating that damage occurred as a result of the etching process. In the test group subjected to two etching processes, excessive erosion of the cementum is visible in each deposit.
1. Introduction
The root cementum is mineralised hard tissue of the tooth that covers the outer surface of the root. The thickness of the root cementum is greatest in the periapical region (150–200 μm) and smallest in the cervical region (20–50 μm) [1]. Exposure of the tooth root as a result of recession or marginal periodontal disease uncovers the maladapted root cementum tissue to the negative effects of the oral environment. As the root cementum has approximately half as many minerals as enamel, demineralisation of the root surface already occurs at a higher environmental pH (approx. 6.7). The frequency of diagnosis of root caries increases with age and it often causes tooth loss [2,3,4].
Based on the results of studies published in the last 5 years, caries are still frequent worldwide, and their prevalence varies among countries. The prevalence rate of hard tooth tissue caries ranges from 25% (Australia) to 99% (South Africa), while the prevalence of caries within the roots, among the population of the respective communities, ranged from 8% (Finland) to 74% (Brazil) [5].
The presence of carious bacterial biofilm and fermentable carbohydrates is the main etiological factor for the occurrence of root caries. The process of demineralisation of the cementum and root dentin is similar to caries within the tooth crown, but the process is twice as fast as in the enamel [6]. Another problem that occurs when the root surface is exposed, and a thin layer of cementum is lost is the hypersensitivity to thermal, chemical, and mechanical stimuli [7].
Minimal intervention dentistry is a modern concept of preventive and therapeutic procedures. It is based on the idea of minimally invasive medical procedures in order to preserve maximum patient tissue. One of the procedures of modern minimally invasive dentistry is infiltration of the hard tissues of the tooth. This procedure is a treatment modality that bridges the gap between prevention and restoration of carious lesions of enamel and dentin, microscopically reaching up to 1/3 of the dentin, reducing the visibility of white spots on the enamel. It is a treatment method that fills, strengthens, and stabilises demineralised enamel without losing healthy tooth structure. The procedure involves infiltration of porous, decalcified enamel with a low-viscosity resin by capillary action, thus stopping the progression of lesions by closing microporosities, and preventing further acid diffusion. This technique is designed to create a diffusion barrier within the lesion rather than on the surface. Icon (by DMG) is commonly used for the infiltration process. The preparation that prepares the enamel surface for resin infiltration is 15% hydrochloric acid (Icon Etch).
The infiltration procedure has so far been used in the treatment of initial caries within the enamel. The authors of the following paper assumed that the infiltration method also appears to be feasible for the treatment of initial caries of all hard tissues of the tooth, including the root cementum. In view of the assumption, the authors synthesized an experimental preparation with the characteristics of a dental infiltrant using YF3 (yttrium trifluoride) [8].
The procedure of acid etching of tooth surfaces used during dental procedures has a 60-plus year history. As early as 1955, Buonocore published a study which concluded that the use of an 85% phosphoric acid-based etching agent significantly increased the bonding between acrylic resin and enamel [9]. During the development of adhesion procedures, attempts were made to develop a simplified alternative in the form of successive generations of bonding systems which eliminated the need for an etching agent, although acid etching remains the most effective procedure for stable adhesion of materials to enamel. Other etching agents of different concentrations have been tried, by modification of their contact time with tooth tissue: hydrofluoric acid, citric acid, hydrochloric acid, maleic acid, nitric acid, and phosphoric acid. Suggested digestion times can range from 15 to 60 s. In an in vitro environment, phosphoric acid has been found to be the most effective in improving the adhesion of dental materials to enamel [10]. Silverstone et al. tested acid solutions for their effects on human enamel. The most beneficial effects were produced by an unbuffered solution of 30% phosphoric acid. After etching, clear changes in surface structure were noted—a thin layer of enamel was removed along with plaque, and the enamel surface became porous. This porous area can penetrate the resin and micromechanically bond to the enamel during restorative treatment [11]. Etching enamel with 30–50% phosphoric acid for 60 s was accepted as the preferred method in the early 1980s. It is believed that excessive etching of enamel occurs after 60 s, leading to impairment of tooth structure and adhesion forces [12,13]. Scanning electron microscopy, contact profilo metry, non-contact profilometry, and confocal laser scanning microscopy (CLSM), which combines laser scanning with traditional visible light microscopy to produce a detailed three-dimensional image of the surface, can be used to measure the depth of etching of the hard tissues of the human tooth [14,15].
A study by Neuhaus et al. compared the penetration of resin after a pre-treatment of the enamel surface with phosphoric and hydrochloric acid on active and inactive carious lesions. The study showed that, after pre-treatment with hydrochloric acid, the depth of penetration was satisfactory and similar for both active and retained lesions. In active lesions, no significant difference in percentage of maximum penetration depth and of mean penetration depth was observed between lesions pre-treated with hydrochloric or phosphoric acid. However, in inactive lesions, pre-treatment with phosphoric acid resulted in significantly lower resin penetration compared to the pre-treatment with hydrochloric acid [16]. A study by Paris et al. evaluated the effect of phosphoric and hydrochloric acid gels on reducing the surface layer of natural carious lesions of deciduous teeth. The study was conducted on 32 extracted or naturally lost deciduous molars. Carious lesions in the white spot stage were etched with phosphoric or hydrochloric acid for 30, 60, 90, or 120 s. Phosphoric acid etching resulted in incomplete reduction of the surface layer in all groups. The use of hydrochloric acid for 120 s led to almost total erosion of the superficial enamel layer [17].
The new recommendations In the ICON application instructions for hard tissue preparation with Icon Etch are very vague. They suggest to visually inspect the infiltrated tissue. If there is no noticeable reduction in enamel discolouration after application of Icon Etch and Icon Dry, the manufacturer recommends repeating the process of etching, rinsing, and application of desiccant two more times.
Dental caries, as mentioned, involves all hard dental tissues, including the root cementum. There are no published reports in the field of micro-invasive dentistry covering the root surface. The authors’ own research in this field covers the area of the root cementum, which in the course of many diseases becomes exposed and undergoes demineralisation under the influence of the oral cavity environment. The lack of a uniform procedure for the preparation of tooth hard tissues for the infiltration process and the lack of any studies describing the preparation of the human tooth root-cementum surface for the application of adhesive procedures in the field of minimally invasive dentistry, prompted the authors to dwell on this topic.
The null hypothesis is the use of a low viscosity resin mixture in the process of infiltration of hard tooth tissues within the root cementum.
The first aim of this study is to evaluate the depth of penetration of an experimental preparation with the characteristics of a dental infiltrant into the decalcified root cementum tissue.
The second objective is to observe the root cementum tissue subjected to a single and repeated twice hydrochloric acid etching process.
2. Materials and Methods
Human teeth:
The study material consisted of 20 human teeth-molars and premolars extracted for periodontal reasons, with preserved anatomical crowns and roots, without cavities or fillings in the cervical region. After extraction, the teeth were stored in a chloramine solution. The study group (1) consisted of 12 demineralised teeth, 8 teeth constituted the control group (2)—which was not demineralised (Table 1, PART ONE). Before the study, the chloramine solution was poured off and the teeth were thoroughly rinsed in distilled water and left there for 24 h. After rinsing and drying, the teeth in the study group (1) were subjected to a chemical decalcification process in a buffer solution which contained calcium chloride (3 mM/dm3), potassium dihydrogen phosphate(V) (3 mM/dm3), acetic acid (50 mM/dm3), methylenehydroxydiphosphonate (MHDP (6 μm/dm3/dm3)), potassium hydroxide (in an amount necessary to ensure that the appropriate pH level of the solution was reached). Demineralisation was carried out at 37 °C for 4 w, with control and maintenance of the pH level below 6.2, which is the critical pH for cementum caries development. If necessary, the pH level was lowered by adding acetic acid. After the decalcification process was completed, the teeth were rinsed three times in distilled water and left there for 24 h and at the end they were dried [18].

Table 1.
Scheme for the preparation of test material.
Etching:
Both the test group and the control group were divided into two subgroups, which differed according to the way of preparation of enamel surface for infiltration. The first subgroup was prepared for the infiltration process according to the traditional protocol (etching with Icon Etch for 120 s was performed once) while the second subgroup was prepared for the infiltration process by using Icon Etch twice-2 times for 120 s each (Table 1—PART ONE), repeating the steps according to the manufacturer’s recommendations (etching, rinsing, and de-glazing).
Infiltration:
Based on the available literature, the infiltration preparation should feature high surface tension, low density, hydrophilicity, lack of toxicity, chemical and mechanical resistance, ability to polymerise, similarity of the colour of the infiltrated area to tooth tissue, no interaction with food and drugs, and ability to inhibit multiplication of microorganisms on the surface. The lack of an antimicrobial component in the Icon preparation led the authors to synthesise an experimental preparation containing the bacteriostatic component called metronidazole. A commercially available Icon preparation (DMG, Hamburg, Germany) and an experimental preparation with the characteristics of a dental infiltrant (Table 2) were used for the study [19,20].

Table 2.
Composition of the experimental preparation.
Both preparations: commercial and experimental had 2% of YF3 (nanopowder, Nanoshel LLC, Wilmington, DE, USA) added as an indicator to facilitate microscopic observation of the depth of penetration of the resin mixture into the root cementum. Teeth from the test and control groups were divided into two zones, blue and red, by a red line drawn along the long axis of the tooth. In the red zone, infiltration was performed with use of the experimental preparation with YF3, in the blue zone-Icon with YF3 (Table 1–PART TWO, Figure 1). The infiltration preparations were then applied. Dentmeate LEDEX WL-070 lamp was used for polymerisation.
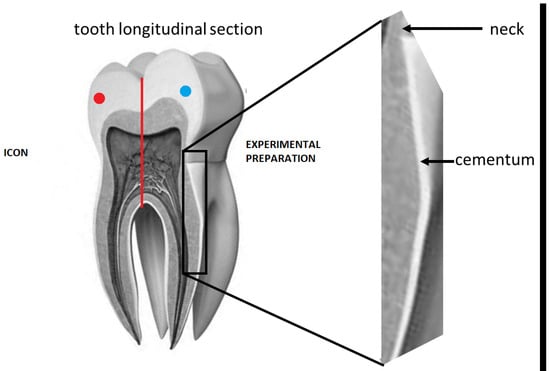
Figure 1.
Diagram of the preparation of a single tooth under observation.
After soaking, the teeth were cold-inlaid in a methylmethacrylate-based two-component resin: Metalogis Opti-Mix (Metalogis s.c., Warsaw, Poland), a methylmethacrylate-based two-component resin with low polymerisation shrinkage. After that, they were cut longitudinally using a Buehler precision cutter (Buehler Holding A.G., Uzwil, Switzerland), and sectioned at the end (Figure 2). The surfaces of the cross-sections were sanded with Metalogis DEMPAX water-resistant sandpaper with decreasing gradations of P320, P500, P800 and P1000 (Metalogis s.c, Warsaw, Poland); and then polished with Struers DP Suspension diamond slurries of 9 μm, 3 μm, 1 μm, 0.25 μm (Struers A/S, Ballerup, Denmark).
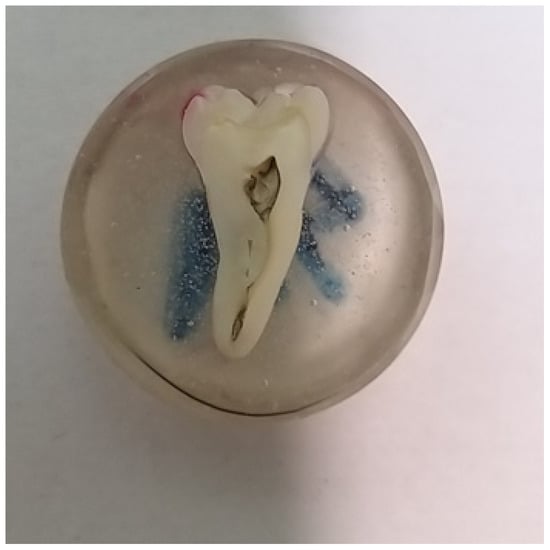
Figure 2.
Specimen prepared for microscopic observation.
The study of tooth longitudinal section morphology and chemical composition was performed with the use of a Hitachi S-3400N scanning electron microscope (Hitachi Ltd., Tokyo, Japan) equipped with a Thermo Noran EDS energy dispersive X-ray spectrometer (Thermo Fisher Scientific Inc., Madison, WI, USA), under low-vacuum conditions (50 Pa) using the backscattered electrons detector (BSE), at an accelerating voltage of 15 kV.
3. Results
Microscopic observations show that the applied YF3 tracer in most cases agglomerates and remains in the form of conglomerates on the surface of the root cementum. Single particles of YF3 are visible, penetrating through the cementum tissues into the root dentine structure. Despite this, the degree of tissue infiltration with the resin (depth of penetration into decalcified tissues) is visible at a depth of approx. 80–120 μm. No differences in penetration were observed between the experimental preparation and the commercial preparation called Icon. (Figure 3A,B).
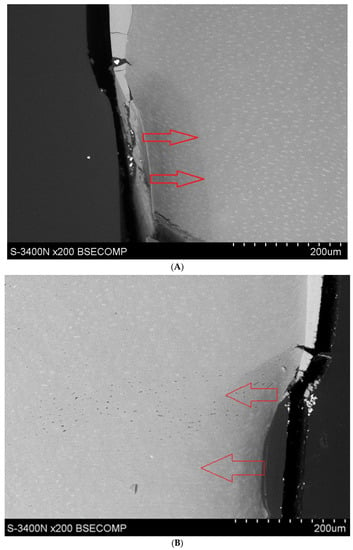
Figure 3.
(A) Study group specimen, single etch, Icon + YF3 (1AI), visible gradual brightening of infiltrated root dentin, arrows showing the depth of the root dentine infiltration. (B) Study group specimen, one etch, experimental preparation + YF3 (1AE), visible infiltration of root dentine.
Yttrium trifluoride particles were present in large amounts in the inlay resin and sporadically on the surface, in cavities of the root cementum, and in dentine. (Figure 4 and Figure 5). Longitudinal sections of the teeth made after embedding them in resin showed large amounts of YF3 particles in the resin used to inlay specimens, sporadically on the surface of root cementum and deep into the cementum and dentin tissue of the root This demonstrates high affinity of the YF3 preparation to the used resin (after completion of the crosslinking process, the resin shrinks slightly, forming a gap between the surface of root cementum and resin) (Figure 4 and Figure 5).
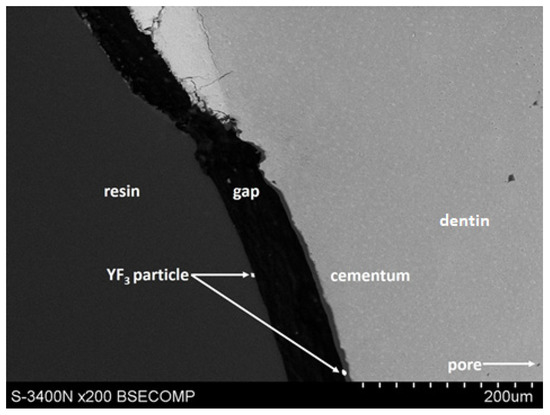
Figure 4.
YF3 particles in the mounting resin, gap formed after resin polymerization.
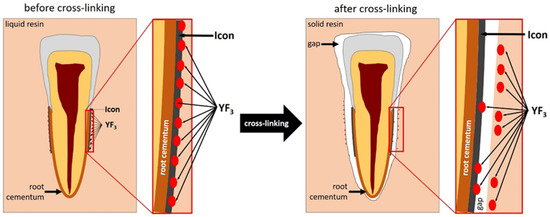
Figure 5.
Diagram of displacement of YF3 particles after polymerisation of the cross-linking resin.
In the test group (1AI, 1AE) subjected to a single etching process, good penetration of both resins was evident, however, excessive erosion of the root surface was manifested in several of the specimens—indicating that damage occurred as a result of the etching process. (Figure 3A,B) In the test group subjected to two etching processes (1BI, 1BE), excessive erosion of the cementum is visible in each deposit. In the control group (2), which was not subjected to the demineralisation process, excessive cementum erosion was also observed, but in this group, infiltration of the root hard tissues treated with both preparations was poorly visible (Figure 6A–D).
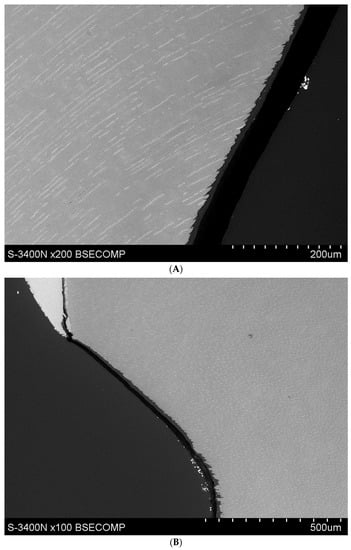
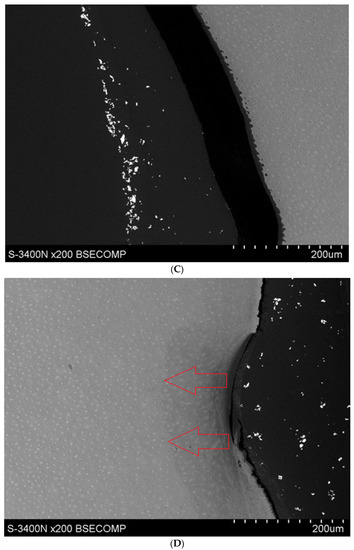
Figure 6.
(A) Study group specimen, double etching, Icon + YF3 (IBI) visible over-etching of root surface. (B) Test specimen, double etched, experimental preparation YF3 (IBE), Excessive etching of the cementum surface extending into the root dentine is visible. (C) Test specimen, double etching, experimental preparation + YF3 (IBE), visible over-etching of the root surface. (D) Specimen from the test group, double etching, experimental preparation + YF3 (IBE), excessive etching of the root surface and penetration of resin into the root dentine visible.
4. Discussion
New recommendations by the manufacturer in the Icon preparation for preparing hard tissue using Icon Etch are not clear. They recommend visual inspection of the infiltrated tissue—if there is no noticeable reduction in enamel discolouration after application of Icon Etch and Icon Dry, the manufacturer recommends repeating the etching process. In the present study, an attempt was made to test and use this method of treatment within the hard root tissue of the human tooth.
The inconvenience of undertaking the following research is the fact that, based on the analysis of the available literature, research in the field of microinvasive dentistry and the etching process most often concerns the crown part of the tooth—the enamel surface. Moreover, not all researchers use human teeth for their studies. Sometimes bovine teeth, which do not always reflect conditions in the human oral cavity, are used as research material [18]. A study conducted by Meyer-Lueckel et al. was aimed at comparing the effectiveness of three different gels—with 37% phosphoric acid, with 5 and 15% hydrochloric acid—for 30–120 s etching to remove the enamel surface layer with different etching times. A gel with phosphoric acid (37%) and hydrochloric acid (5 or 15%) for 30–120 s was used for the study. The samples were examined by confocal microscopy and transverse microradiography. The reduction of the surface layer was significantly increased in lesions etched with 15% HCl gel for 90 and 120 s, as compared to those etched with phosphoric acid for 30–120 s (p < 0.05). The study concluded that an effective reduction of the superficial carious layer of natural enamel can be achieved by etching with 15% hydrochloric acid gel for 90–120 s [21]. In the authors’ own study, it was observed that the use of 15% hydrochloric acid (Icon Etch) can lead to excessive erosion of the root cementum surface tissue already after a single etching for 120 s, while a double etching (2 times for 120 s) virtually causes a complete loss of the root cementum).
Wilson et al. conducted a study which compares the etching depth of a traditional 34% phosphoric acid etchant and a self-limiting phosphoric acid etchant by measuring the etching depth at multiple intervals. The study was conducted on 25 bovine teeth with two different etches: a standard 34% phosphoric acid etchant and a self-limiting 35% phosphoric acid etchant, applying the etches for 15, 30, 60, 90, and 120 s. Teeth were scanned using a 3D laser confocal scanning microscope before etching and scanned again after etching to determine the depth of the etched enamel compared to the initial enamel surface before etching. It was observed that phosphoric acid 34% etched significantly deeper than the self-limiting etchant. Etching times which exceeded 30 s also caused significantly deeper changes (for both etch types), but the etching depth at 120 s was not different from that at 60 s, indicating that both etches tend to be self-limiting in terms of depth over time [22].
Zafar and Ahmed evaluated the effect of etching time on the surface properties of dental hard tissues. For this purpose, specimens from extracted human teeth were prepared and treated with 37% phosphoric acid for different times using an established protocol. The effect of etching time on surface roughness was evaluated using a non-contact surface roughness profilometer, and surface hardness was measured using the nano-indentation technique. This study found that etching time affects the surface properties of hard tooth tissues, especially enamel. Prolonged etching time can increase the surface roughness and decrease the surface hardness, which consequently can lead to a deterioration of the bonding strength of adhesive materials [23].
Meng Li et al. evaluated the prophylactic use of enamel infiltration resins—the resistance of healthy enamel surfaces of permanent teeth to demineralisation. Enamel blocks from 80 premolars were prepared, and samples with an initial microhardness of 320–370 were selected. An infiltrant was applied to samples from the experimental group, and no infiltrant was applied to samples from the control group, after which both groups were artificially demineralised, and the microhardness values of the enamel surface were measured again. Confocal laser scanning microscopy was used to measure demineralisation depth and penetration. The samples were subjected to a simulated toothbrush abrasion test. Scanning electron microscopy was used to observe changes in the surface morphology of the samples after each of these procedures. The researchers observed no significant differences between the experimental and control groups in baseline microhardness values or, in case of the experimental group, after resin infiltration, as compared to the baseline conditions. After artificial demineralisation, the microhardness value in the control group was significantly lower (266.0 (±34.5)) than in the experimental group 304.0 (±13.0), p = 0.017). Scanning confocal microscopy results showed that the demineralisation depth in the control group was significantly deeper (97.9 (±22.8) µm) than in the experimental group (50.4 (±14.3) µm), and the resin infiltrate completely penetrated the acid-etched demineralised area of tooth enamel with an average penetration depth of 31.6 (±9.0) µm. Scanning electron microscopy showed that the surface morphology was more uniform and smoother after simulated tooth brushing. Enamel surface structure was more severely damaged in the control group, as compared to the experimental group. These studies provide an experimental basis for the prophylactic application of infiltrations to healthy enamel surfaces in order to protect tooth enamel from demineralisation [24]. In the opinion of the authors of this paper, the continuation of our own research should concern root cementum tissues, as Meng Li’s study, subjected to prophylactic infiltration followed by demineralisation and assessment of the degree of tissue microhardness.
Recent studies recommend use of bonding systems (multi-mode) which are self-etch systems and can therefore be used without prior etching of the mineralised tooth substance. Numerous studies have shown that the bond between the composite and enamel is weaker, which is why the so-called selective enamel etching with 37% orthophosphoric acid for 5–15 s is recommended. The cavity is then rinsed, dried, and, according to the manufacturer’s instructions, a universal bonding system is applied to the dentin and enamel. Phosphoric acid should not be applied to the dentin surface, as additional etching of the dentin during application of some universal bonding systems may result in faster degradation of the hybrid layer and future loss of the bond [25]. In an experimental study on 52 bovine incisors conducted by Diniz et al. it was found that self-etching and universal bonding systems in which acid conditioning of the enamel was carried out, achieved a higher bond strength than the same systems preceded by acid application. This is consistent with literature data and manufacturers’ recommendations [26]. A study by Stape et al. assessed whether selective dentin etching protocols using shortened phosphoric acid etching times would affect the resin–dentin interaction of a universal bonding system to improve long-term bond performance. The researchers found that selective dentin etching for 3 s with conventionally used phosphoric acid improves the binding efficiency to dentin, however, longer etching times should be strictly avoided [27]. The chemical affinity between 10-methacryloyloxydecyl dihydrogen phosphate (MDP) and hydroxyapatite (HA) is an important factor in the enamel bond provided by MDP-based self-etching bonding systems, so Sauag et al. investigated how pre-etching with phosphoric acid affects the formation of MDP-Ca salts when using MDP-based bonding systems. The study showed that pre-etching increased the wettability of the enamel surface and the formation of MDP-Ca salts was higher in samples etched with phosphoric acid [28].
The most common ways to prevent and treat initial root caries are remineralisation with fluoride-containing preparations and use of antimicrobial preparations which contain chlorhexidine or triclosan. Another way to prevent root caries is to seal the root surface with glutaraldehyde, which is a fixative and a component of some adhesive systems. It has been shown that sealing the root surface with two types of a dentin adhesive systems which contained glutardialdehyde led to a significant reduction or even complete inhibition of root cavity formation [29]. Observations in the authors’ own study indicate that it is also possible to use infiltration to treat initial root caries.
A study by Walter et al. evaluated the ability of different adhesive systems to inhibit root caries formation in in vitro conditions. The lesions in the groups were significantly shallower after the use of One-up Bond F (Tokuyama), iBond (Heraeus Kulzer), and Gluma Comfort Bond + Desensitizer (Heraeus Kulzer) preparations than in the control group [30].
Zhou et al. in their study evaluated the effect of infiltration on root caries induced by Streptococcus mutans biofilm. Clearfil SE Bond (SEB), Icon-etch120s+Icon-infiltrant (HA120), Icon-etch10s+Icon-infiltrant (HA10), and K-etchant10s+Icon- infiltrant (PA10) were used for the study. The resin after 120s-HCl root pre-treatment was found to have good penetration ability and prevent root caries, but an additional risk factor for enamel loss in the cervical region was identified [31]. The authors, according to their own observations, confirm the conclusions of Zhou et al.
Other researchers on human tooth root caries have compared the durability of a resin-based root dentin surface coating material and a universal self-etch adhesive on the root surface in vitro. In the experiments by Tian et al., PRG Barrier Coat (PRG) or Clearfil S3 Bond (CS3) was used for the study. The sample preparation was subjected to a demineralisation process and then longitudinal sections were examined with the use of a scanning electron microscope. The thickness of the coating material was measured in SEM images. With regard to the toothbrush wear test, coronal tooth discs were prepared and coated with PRG and CS3, respectively. Coating the root dentin with PRG Barrier Coat gave a coating layer of (47.1 ± 27.3) μm thickness, while CS3 presented a thin layer of (5.7 ± 2.1) μm thickness. The exposed dentin was hermetically sealed and no clear gap was observed at the interface in both PRG and CS3 groups. No dentin demineralisation was observed in both groups [32].
A study by Ke-le Liu et al. aimed to evaluate the effect of different acid etching modes on the bond strength between composite resin and non-carious sclerotic dentin. The use of a total-etch adhesion system increasing the etching time could increase the bond strength. In the case of a self-etching adhesive system, both double the adhesive treatment time and the use of phosphoric acid can improve the bond strength. The use of phosphoric acid to etch for 15 s and coating with a self-etching adhesive system for 20 s achieved the highest bond strength. In both the self-etching and fully etching systems, the use of phosphoric acid to etch for 15 s and coating with a self-etching adhesive system for 20 s achieved optimal bond strength, the lowest bond strength was observed for the self-etching system used at the recommended time [33]. It has been observed that the application of 37% phosphoric acid to dentin, in some cases, can promote excessive demineralisation by preventing proper infiltration and impregnation of the resin between the collagen fibres [34]. Consequently, it can lead to bond failure, contrary to what happens when self-etching adhesive systems are used [35].
Root cement infiltration appears to be a promising method for the treatment of initial root caries. The results of the authors’ own research have shown that the use of YF3 as an additive to the resin greatly facilitates microscopic observation, however, the size of its particles, which tend to form conglomerates on the surface of the root cementum, is very important. The study also showed that the indicator (YF3) may have insufficient adhesion to the outer surface of the tooth root cementum. On longitudinal sections of the teeth made after embedding them into the inlay resin, the authors observed presence of YF3 particles in large quantities in the inlay resin and sporadically on the surface of the root cementum and deep into the cementum and root dentin tissue. This demonstrates the high affinity of the YF3 infiltrant to the used encapsulation resin (after completion of the resin crosslinking process, a slight shrinkage of the resin occurs, resulting in the formation of a gap between the root cementum surface and the resin).
The lack of reports by other authors and the results of our own research make it necessary to continue observations in this field and try to answer the following questions: how to prepare the surface of the human tooth root for the infiltration procedure, what acid should be used and how long should its application last? Due to the porous structure of the root cementum, it also seems advisable to compare the penetration of the preparation without prior preparation of the surface in case of teeth that have had previous contact with the oral environment. In case of periodontal diseases (e.g., recessions), further investigation should focus on the preparation of shielded root dentine. In the opinion of the authors of the present study, the continuation of own research should concern the assessment of the degree of microhardness of root cementum tissues before and after prophylactic infiltration, and then after demineralisation, followed by a comparison of these results. The technique of preparing teeth for testing needs to be improved. Due to the high affinity of YF3 molecules for the resin used to inlay specimens, subsequent studies should be performed with a different resin without inlaying the samples.
5. Conclusions
- On the basis of the observations made, it has been shown that an infiltration procedure is possible within the exposed tissues of the tooth root;
- The degree of infiltration is satisfactory and is about 80–120 μm;
- Re-etching of hard root tissues causes excessive erosion of the root cementum tissue; and
- On the basis of the observations to date, there is a need for further research to refine the methodology for preparing the decalcified root cementum tissue for the infiltration procedure.
6. Patents
Experimental preparation patent was reported in 2021 under number P.439851.
Author Contributions
Conceptualisation A.N.-W., M.S.-N. and A.K.-W.; methodology A.N.-W., M.S.-N., A.K.-W. and B.C.; software, B.C., K.W., M.Ł. and S.M.; validation, A.N.-W., M.S.-N., A.K.-W., B.C., K.W. and M.W.; formal analysis, A.N.-W., M.S.-N., A.K.-W., B.C., K.W., M.W. and M.Ł.; investigation, A.N.-W., M.S.-N., A.K.-W. and B.C.; resources, A.N.-W., K.W. and T.M.; data curation, A.N.-W., M.S.-N., B.C. and A.K.-W.; writing—original draft preparation, A.N.-W., M.S.-N., B.C. and A.K.-W.; writing—review and editing, A.N.-W., M.S.-N., B.C. and A.K.-W.; visualisation, A.N.-W., M.S.-N., B.C., A.K.-W. and K.W.; supervision, M.S.-N., B.C. and A.K.-W.; project administration, A.N.-W. and M.S.-N.; funding acquisition, A.N.-W. and M.S.-N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Medical University of Silesia in Katowice, research number: PCN-2-070/N/0/K and PCN-1-016/N/1/K.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available from the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yamamoto, T.; Hasegawa, T.; Yamamoto, T.; Hongo, H.; Amizuka, N. Histology of human cementum: Its structure, function, and development. Jpn. Dent. Sci. Rev. 2016, 52, 63–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinakaran, S.; Gopinathan, A.S. Root caries: A geriatric challenge. Dent Med. Probl. 2017, 54, 403–408. [Google Scholar] [CrossRef] [Green Version]
- Gavriilidou, N.N.; Belibasakis, G.N. Root caries: The intersection between periodontal disease and dental caries in the course of ageing. Br. Dent. J. 2019, 227, 1063–1067. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Richards, L.; Walsh, T.; Worthington, H.V.; Clarkson, J.E.; Wang, L.; de Amoedo Campos Velo, M.M. Interventions for managing root caries. Cochrane Database Syst Rev. 2017, 2017, CD012750. [Google Scholar] [CrossRef] [Green Version]
- Chan, A.K.Y.; Tamrakar, M.; Jiang, C.M.; Lo, E.C.M.; Leung, K.C.M.; Chu, C.H. A Systematic Review on Caries Status of Older Adults. Int. J. Environ. Res. Public Health 2021, 18, 10662. [Google Scholar] [CrossRef]
- Ekstrand, K.R.; Cordeschi, T.; Abreu-Placeres, N. ICCMS™ root caries lesions stages and their underlying depth towards the pulp: An in vitro study with histologic evaluation. Clin. Oral Investig. 2021, 26, 2597–2605. [Google Scholar] [CrossRef]
- Tanasiewicz, M.; Gibas, M.; Skucha-Nowak, M.; Twardawa, H.; Machorowska-Pieniążek, A. Concept of experimental preparation for treating dentin hypersensitivity. Open Med. 2016, 11, 387–393. [Google Scholar] [CrossRef]
- Nowak-Wachol, A.; Korytkowska-Wałach, A.; Chmiela, B.; Wachol, K.; Łopaciński, M.; Wyszyńska, M.; Al-Dulaimi, Y.; Skucha-Nowak, M. Yttrium Trifluoride as a Marker of Infiltration Rate of Decalcified Root Cementum: An In Vitro Study. Polymers 2022, 14, 780. [Google Scholar] [CrossRef]
- Buonocore, M.G. A simple method of increasing the adhesion of acrylic filling materials to enamel surfaces. J. Dent. Res. 1955, 34, 849–853. [Google Scholar] [CrossRef]
- Gwinnett, A.J. Histologic changes in human enamel following treatment with acidic adhesive conditioning agents. Arch. Oral Biol. 1971, 16, 731–738. [Google Scholar] [CrossRef]
- Silverstone, L.M.; Saxton, C.A.; Dogon, I.L.; Fejerskov, O. Variation in the pattern of acid etching of human dental enamel examined by scanning electron microscopy. Caries Res. 1975, 9, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.J.; Tang, A.T.; Matinlinna, J.P.; Hägg, U. Acid etching of human enamel in clinical applications: A systematic review. J. Prosthet. Dent. 2014, 112, 122–135. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.N.; Lu, T.C. Bond strength with various etching times on young permanent teeth. Am. J. Orthod. Dentofac. Orthop. 1991, 100, 72–79. [Google Scholar] [CrossRef]
- Paepegaey, A.M.; Barker, M.L.; Bartlett, D.W.; Mistry, M.; West, N.X.; Hellin, N.; Brown, L.J.; Bellamy, P.G. Measuring enamel erosion: A comparative study of contact profilometry, non-contact profilometry and confocal laser scanning microscopy. Dent. Mater. 2013, 29, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Legler, L.R.; Retief, D.H.; Bradley, E.L. Effects of phosphoric acid concentration and etch duration on enamel depth of etch: An in vitro study. Am. J. Orthod. Dentofac. Orthop. 1990, 98, 154–160. [Google Scholar] [CrossRef]
- Neuhaus, K.W.; Schlafer, S.; Lussi, A.; Nyvad, B. Infiltration of natural caries lesions in relation to their activity status and acid pretreatment in vitro. Caries Res. 2013, 47, 203–210. [Google Scholar] [CrossRef] [Green Version]
- Paris, S.; Dörfer, C.E.; Meyer-Lueckel, H. Surface conditioning of natural enamel caries lesions in deciduous teeth in preparation for resin infiltration. J. Dent. 2010, 38, 65–71. [Google Scholar] [CrossRef]
- Skucha-Nowak, M.; Gibas, M.; Tanasiewicz, M.; Twardawa, H.; Szklarski, T. Natural and Controlled Demineralization for Study Purposes in Minimally Invasive Dentistry. Adv. Clin. Exp. Med. 2015, 24, 891–898. [Google Scholar] [CrossRef] [Green Version]
- Skucha-Nowak, M.; Mertas, A.; Tanasiewicz, M. Using an Electron Scanning Microscope to Assess the Penetrating Abilities of an Experimental Preparation with Features of a Dental Infiltrant: Preliminary Study. Adv. Clin. Exp. Med. 2016, 25, 1293–1301. [Google Scholar] [CrossRef]
- Skucha-Nowak, M. Attempt to assess the infiltration of enamel made with experimental preparation using a scanning electron microscope. Open Med. 2015, 10, 238–248. [Google Scholar] [CrossRef]
- Meyer-Lueckel, H.; Paris, S.; Kielbassa, A.M. Surface layer erosion of natural caries lesions with phosphoric and hydrochloric acid gels in preparation for resin infiltration. Caries Res. 2007, 41, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.M.; Lien, W.; Lee, D.P.; Dunn, W.J. Confocal microscope analysis of depth of etch between self-limiting and traditional etchant systems. Angle Orthod. 2017, 87, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Zafar, M.S.; Ahmed, N. The effects of acid etching time on surface mechanical properties of dental hard tissues. Dent. Mater. J. 2015, 34, 315–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Yang, Z.; Huang, Y.; Li, Y.; Zhou, Z. In vitro effect of resin infiltrant on resistance of sound enamel surfaces in permanent teeth to demineralization. PeerJ 2021, 9, e12008. [Google Scholar] [CrossRef]
- Cuevas-Suárez, C.E.; da Rosa, W.L.O.; Lund, R.G.; da Silva, A.F.; Piva, E. Bonding Performance of Universal Adhesives: An Updated Systematic Review and Meta-Analysis. J. Adhes. Dent. 2019, 21, 7–26. [Google Scholar] [CrossRef]
- Diniz, A.C.S.; Bandeca, M.C.; Pinheiro, L.M.; dos Santos Almeida, L., Jr.; Torres, C.R.G.; Borges, A.H.; Pinto, S.C.S.; Tonetto, M.R.; De Jesus Tavarez, R.R.; Firoozmand, L.M. Influence of Different Etching Modes on Bond Strength to Enamel using Universal Adhesive Systems. J. Contemp. Dent. Pract. 2016, 17, 820–825. [Google Scholar] [CrossRef]
- Stape, T.H.S.; Wik, P.; Mutluay, M.M.; Al-Ani, A.A.S.; Tezvergil-Mutluay, A. Selective dentin etching: A potential method to improve bonding effectiveness of universal adhesives. J. Mech. Behav. Biomed. Mater. 2018, 86, 14–22. [Google Scholar] [CrossRef]
- Han, F.; Sun, Z.; Xie, H.; Chen, C. Improved bond performances of self-etch adhesives to enamel through increased MDP-Ca salt formation via phosphoric acid pre-etching. Dent. Mater. 2021, 38, 133–146. [Google Scholar] [CrossRef]
- Hahn, P.; Schaller, H.G.; Gernhardt, C.; Hellwig, E. Influence of two dentin bonding systems on the demineralization of the root surface. Oper. Dent. 1999, 24, 344–350. [Google Scholar]
- Walter, R.; Duarte, W.R.; Pereira, P.N.; Swift, E.J., Jr.; Heymann, H.O.; Arnold, R.R. Effect of resin adhesive systems on root caries formation in vitro. Quintessence Int. 2008, 39, 33–37. [Google Scholar]
- Zhou, Y.; Matin, K.; Shimada, Y.; Sumi, Y.; Tagami, J. Evaluation of resin infiltration on demineralized root surface: An in vitro study. Dent. Mater. J. 2017, 36, 195–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, H.Y.; Yu, P.; Yuan, C.Y.; Zhang, W.; Qiu, Y.X.; Li, D.H.; Liang, X.J.; Wang, X.Y. Durability of protective effect of resin-based coating material on root surface. Beijing Da Xue Xue Bao Yi Xue Ban 2016, 48, 889–893. (In Chinese) [Google Scholar] [PubMed]
- Liu, K.L.; Zhang, X.F.; Wei, X. Influence of different acid etching modes on bond strengths to non-carious sclerotic dentin. Shanghai Kou Qiang Yi Xue 2016, 25, 38–41. (In Chinese) [Google Scholar] [PubMed]
- Perdigão, J.; Reis, A.; Loguercio, A.D. Dentin adhesion and MMPs: A comprehensive review. J. Esthet. Restor. Dent. 2013, 25, 219–241. [Google Scholar] [CrossRef] [PubMed]
- Jacques, P.; Hebling, J. Effect of dentin conditioners on the microtensile bond strength of a conventional and a self-etching primer adhesive system. Dent. Mater. 2005, 21, 103–109. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).