Abstract
Aluminum dross is produced in the process of industrial production and regeneration of aluminum. Currently, the main way to deal with aluminum dross is stacking and landfilling, which aggravates environmental pollution and resource waste. In order to find a green and environmental protection method for the comprehensive utilization, the aluminum dross was used as raw materials to prepare sintered brick. Firstly, the raw material ratio, molding pressure and sintering process were determined by single factor test and orthogonal test, and the mechanism of obvious change of mechanical strength of sintered brick was studied by XRD and SEM. The experimental results show that, the optimal formula of sintered brick is 50% aluminum dross, 37.50% engineering soil and 12.50% coal gangue. The optimum process parameters are molding pressure 10 MPa, heating rate 8 °C/ min, sintering temperature 800 °C, holding time 60 min. The samples prepared under the above formula and process parameters present outstanding performance, and the compressive strength, flexural strength and water absorption rate are 16.21 MPa, 3.42 MPa and 17.12% respectively.
1. Introduction
Globalization and population growth have increased the consumption of natural resources, resulting in a huge amount of waste. Taking the aluminum industry as an example, a large amount of industrial waste is produced every year, in which aluminum dross accounts for a large part. Some scholars have studied the influence of various factors on the harmless treatment of aluminum dross and adopted the aluminum dross after harmless treatment to prepare non-fired bricks [], while some scholars have investigated the use of aluminum dross to prepare inorganic flocculants [], coating material [,], refractory materials [], steelmaking deoxidizers [], concrete [] and other products. However, the existing resource utilization methods have a small amount of disposal and cannot effectively use a large number of aluminum dross. The solid waste disposal method, which uses aluminum dross as raw material for the preparation of sintered brick, has the advantages of large disposal volume and easy to obtain solidified materials. This is an excellent method of solid waste disposal. In current urban construction, concrete is a relatively common building material. It is estimated that 11 billion tons of concrete are consumed every year [], which will inevitably lead to a huge consumption of its raw materials, especially cement. The excellent properties of concrete cannot conceal its shortcomings of causing damage to the environment. According to reports, for every ton of ordinary cement produced, about 900 kg of CO2 is produced, accounting for 7% of the total global CO2 emissions []. Therefore, it is necessary to find a green and environmentally friendly material to deal with global warming and waste disposal problems.
Sintered brick has a long history. After thousands of years of development, different types of sintered brick have been derived. However, the traditional method of making sintered brick destroys land and consumes resources. Therefore, some experts and scholars have studied the use of coal gangue, fly ash, red mud, gold mine tailings, iron ore tailings and other raw materials to prepare sintered brick [,,,,], thus realizing the comprehensive utilization of solid waste. The three raw materials used in this study are all solid wastes, and the preparation process will not cause harm to the surrounding environment.
In 2019, China’s electrolytic aluminum output was 35.75 million tons, and imported bauxite was 100 million tons. Aluminum dross is a kind of industrial waste produced in the process of melting aluminum such as aluminum electrolysis, aluminum alloy production and waste aluminum regeneration [,]. The aluminum–silicon ratio reaches 20–50. It is a very important high-quality aluminum resource with high recycling value. As the AlN in aluminum dross is unstable at room temperature, it is easy to be damp and then produce ammonia []. As a result, the application of aluminum dross has been restricted in terms of resource utilization. However, AlN is oxidized by air during the sintering process to generate Al2O3 and other nitrogen oxides [], so as to realize the harmless treatment of aluminum dross. The harmless treatment process of aluminum dross mainly includes two treatment methods: the pyrometallurgical process and hydrometallurgical process. However, the hydrometallurgical process is not as environmentally friendly as the pyrometallurgical process in terms of waste liquid and exhaust gas emissions []. In addition to aluminum dross, engineering soil and coal gangue are also used as raw materials for making sintered brick. As most countries began to carry out large scale infrastructure construction, a large amount of engineering soil was produced. The main phase of engineering soil is the quartz, and its chemical composition is similar to clay. It can effectively make up for the lack of SiO2 content in aluminum dross. Coal gangue is generated in the coal mining process. At present, China’s coal gangue stockpile has exceeded 5 billion tons, and about 160,000 tons of gangue are discharged for every 1 million tons of coal Because of its own combustible characteristics, it can increase the sintering temperature inside the bricks when preparing sintered brick. Therefore, it is feasible to prepare sintered bricks with aluminum dross as the main component, supplemented by engineering soil and coal gangue.
This article studies the effects of raw material ratio, molding pressure and sintering process on the properties of sintered brick. After obtaining the best process parameters for preparing sintered brick, the experiment was carried out for phase composition analysis and microscopic scanning analysis, and the formation mechanism of the mechanical strength of sintered brick was studied.
2. Materials and Methods
2.1. Raw Material
The aluminum dross selected for this experiment is from Jiangsu Haiguang Metal Co., Ltd. SuQian, China, specifically the final dross obtained after one-time aluminum dross recovery of part of the metal aluminum; the engineering soil is taken from Haian Zhengcheng New Wall Material Co., Ltd. NanTong, China; coal gangue is taken from Haian Zhengcheng New Wall Material Co., Ltd. NanTong, China; Water: deionized water.
The main components of aluminum dross are Al, Al2O3, AlN and a small amount of SiO2. At high temperatures, Al and AlN can be oxidized to Al2O3 by air, and Al2O3 is the effective component of sintered brick. Aluminosilicate can be formed by Al2O3 combining with SiO2 at a certain temperature to improve the mechanical strength of sintered brick. As the main substance in the engineering soil is SiO2, the engineering soil must be mixed to effectively make up for the deficiency of SiO2 in the aluminum dross. Coal gangue is added to sintered bricks as a kind of combustion-supporting agent. On the one hand, it can increase the content of SiO2 in the raw materials; on the other hand, because of its own combustible characteristics, it effectively reduces the sintering temperature and saves energy. The XRD pattern of each raw material is shown in Figure 1. The mixed three raw materials in proportion for particle size analysis are shown in Figure 2.
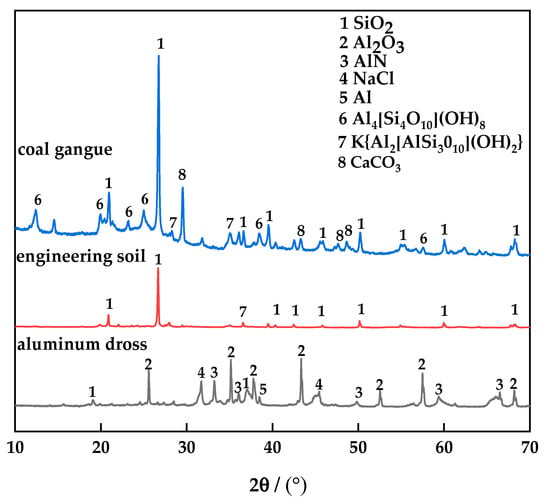
Figure 1.
XRD pattern of the raw material.
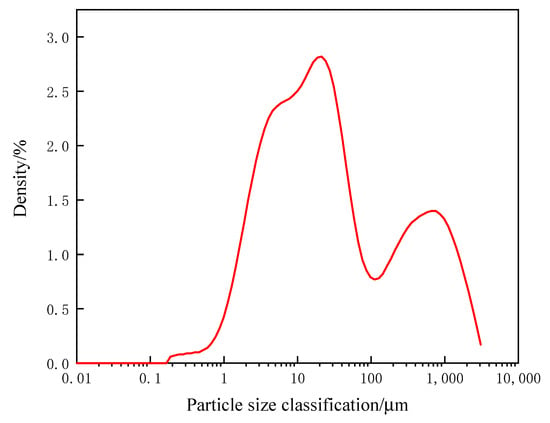
Figure 2.
Raw material particle size.
2.2. Sample Preparation
Firstly, aluminum slag is added according to 30%–70%, engineering soil is added according to 20.73%–55.29%, and coal gangue is added according to 9.27%–14.71%. Then water is added, and the amount of water is 12% of the mass of brick. After sealing, the mixture is placed in a dark place and aged for 72 h to make the water evenly diffuse into the raw material. Coal gangue is added according to the calorific value of aging materials. According to the production practice of brick making enterprises, each kilogram of aging material usually needs about 837 kJ of heat, while the calorific value of coal gangue is about 2000 kJ/kg. The self-made mold is used for compression molding under 6 MPa–14 MPa, and the size of the brick is 40 × 40 × 160 mm3. After natural drying for 15 h, it was dried at 105 °C for 6 h to constant weight. The heating rate is 6~8 °C/min, the roasting temperature is 750~850 °C, and the temperature is maintained for 60~180 min to room temperature. The process flow of sintered brick preparation is shown in Figure 3. Five bricks were prepared for each experimental factor. The final data of compressive strength and flexural strength are the average values after removing maximum and minimum values. The sample is shown in Figure 4. The left side is the unsintered brick and the right side is the sintered brick. In terms of color, the unsintered brick has a darker color, while the color of the sintered brick has changed. In terms of appearance, the shape of unsintered brick is regular without surface damage, while the appearance of sintered brick is basically intact.
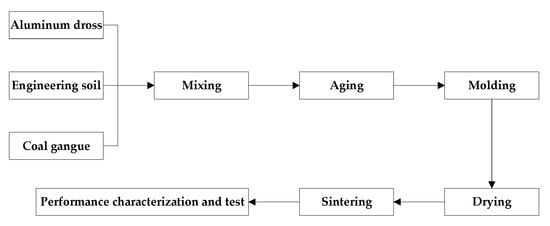
Figure 3.
The process flow of sintered brick preparation.

Figure 4.
Bricks before and after sintering: (a) Unsintered bricks, (b) Sintered brick.
2.3. Specimen Characterization
The measurement of the compressive strength of sintered brick refers to GB/T 5101-2017 “Sintered Ordinary Bricks” [], and the flexural strength and water absorption refers to GB/T 2542-2012 “Testing Methods for Wall Bricks” []. The force loading speed of the compressive strength test is 2.4 KN/s, and the force loading speed of the flexural strength test is 0.05 KN/s. The microscopic morphology of the sample was observed with a field emission scanning electron microscope system (ZEISS Gemini SEM 300, Carl Zeiss AG, Oberkochen, Germany), and the phase composition of the raw materials and the sample was analyzed and tested with an X-ray diffractometer manufactured by Rigaku, Tokyo, Japan. The XRD patterns of raw materials and products are analyzed by Jade6.0, drawn by origin2018, and the mechanical strength change graph is drawn by origin2018.
3. Results and Discussion
3.1. The Influence of the Mixing Amount of Aluminum Dross on the Properties of Sintered Brick
The range of aluminum dross is 30%–70%, and the other raw material admixtures are shown in Table 1.

Table 1.
Raw material composition.
The sintered bricks were naturally placed for 24 h and then tested for mechanical properties and water absorption. The experimental results are shown in Figure 5 and Figure 6.
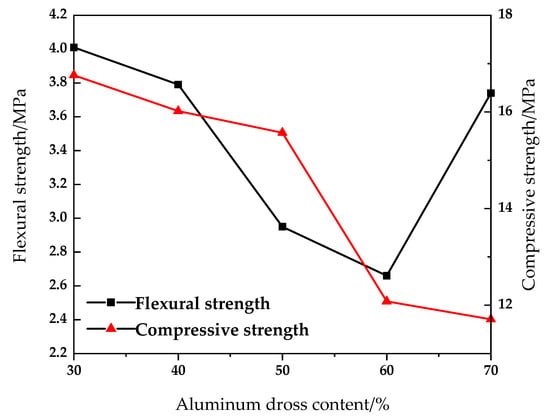
Figure 5.
Effect of aluminum dross content on compressive and flexural properties of sintered brick.
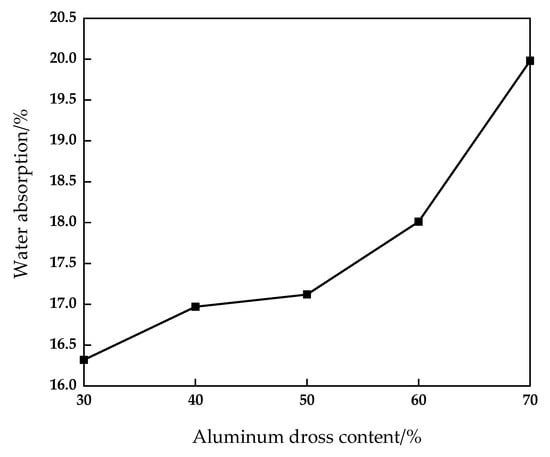
Figure 6.
Effect of aluminum dross content on water absorption of fired brick.
It can be seen from Figure 5 and Figure 6 that with the increase of aluminum dross addition, the compressive strength and flexural strength of the sintered brick decrease, and the water absorption increases. When the mixing amount of aluminum dross is 30%, 40% and 50%, the compressive strength of the brick is 16.76 MPa, 16.02 MPa and 15.57 MPa, respectively. The compressive strength decreases significantly after the aluminum dross content exceeds 50%. When the content of aluminum dross increased from 30% to 50%, the flexural strength decreased from 4.01 MPa to 2.95 MPa. When the aluminum dross content was 70%, the flexural strength increased sharply to 3.74 MPa. The water absorption rate increased from 16.32% to 19.98%, and green brick fluctuated from 9.15% to 11.28%. When the additional amount of aluminum dross was continuously increased, the SiO2 content in the brick decreased, and it was difficult to combine with Al2O3 to form aluminosilicate in the roasting process. Aluminosilicate is the main structural framework of the sintered brick, so the mechanical properties were reduced. However, Al2O3 is also an effective component for the formation of mechanical strength of brick. Therefore, when the amount of aluminum dross is increased, it can be found that the downward trend of compressive strength slows down and the flexural strength increases.
The aluminum dross contains a small amount of aluminum. In the process of sintering, the aluminum is melted and filled between various phases, which can improve the overall density and mechanical properties of brick. However, with the increase of aluminum dross content, the content of SiO2 in the product decreases, which is lower than the optimal range of SiO2 content of 55–70%; at the same time, it increases the content of Al2O3. Both SiO2 and Al2O3 are the main components for the mechanical strength of the brick. Therefore, when the aluminum dross content is 70%, the compressive strength decreases slowly and the flexural strength increases significantly. The main reason for the obvious increase of water absorption is that the content of AlN in the brick increases with the increase of aluminum dross addition. During the high temperature sintering process, AlN is converted into nitrogen oxides such as Al2O3 by oxygen. Therefore, there appear pores in the brick, which increases the water absorption. For the purpose of maximizing the utilization of aluminum dross, it is considered that the content of aluminum dross should be 50%, which meets the MU15 grade requirements of GB/T 5101-2017 “Sintered Ordinary Bricks” [].
3.2. Influence of Molding Pressure on Properties of Sintered Brick
The content of aluminum dross is 50%. The molding pressure is set to 6 MPa, 8 MPa, 10 MPa, 12 MPa, 14 MPa, and the experimental results are shown in Figure 7 and Figure 8.
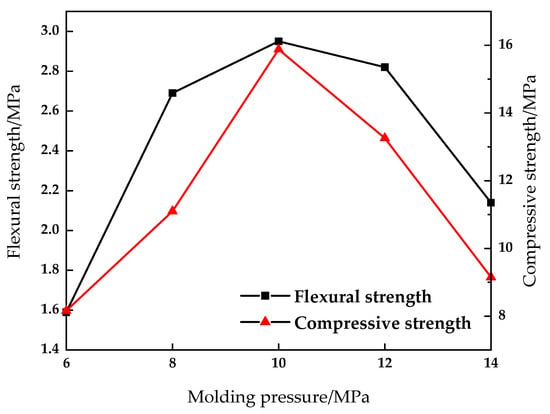
Figure 7.
Effect of molding pressure on compressive and flexural properties of sintered brick.
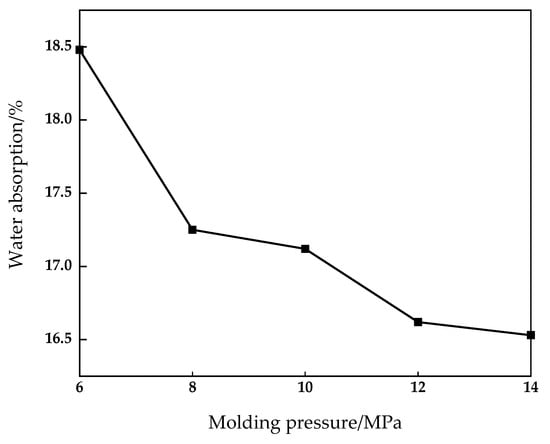
Figure 8.
Effect of molding pressure on water absorption of fired brick.
It can be seen from Figure 7 and Figure 8 that when the molding pressure reaches 10 MPa, the compressive strength of the sintered brick reaches the maximum value of 15.88 MPa. At the same time, the flexural strength of the brick reaches the maximum value of 2.95 MPa. When the molding pressure is 6 MPa and 14 MPa, the compressive strength is 8.16 MPa and 9.15 MPa respectively, which fails to meet the requirements of the MU10 compressive strength grade. When the molding pressure is 8 MPa and 12 MPa, the compressive strength is 11.1 MPa and 13.06 MPa respectively, which meets the requirements of mu10 compressive strength grade, but cannot meet the requirements of Mu15 compressive strength grade. Water absorption of brick is reduced from 18.48% to 16.53%, and that of green brick is reduced from 13.21% to 10.73%. As the molding pressure increases from 6 Mpa to 14 MPa, the density of brick increases from 1.93 g/cm3 to 2.09 g/cm3. As the molding pressure increases, both the compressive strength and the flexural strength of sintered bricks first increase and then decrease, while the water absorption rate decreases. This is mainly because when the molding pressure is low, the contact between the raw material particles of the brick is not close enough to form a larger pore, and its cohesive force and bite force are small. Therefore, good mechanics cannot be formed during the sintering process. When the forming pressure is high, the contact between the particles is closer, which causes the problem that the gas produced in the sintering process cannot be released in time. In this case, the volume of the brick expands and obvious cracks appear on the surface and inside, which reduces the mechanical properties of the brick.
3.3. The Effect of Firing System on the Performance of Sintered Brick
During the firing of the bricks, a series of complex physical and chemical changes take place in the various components inside the brick, which mainly include dehydration, isomorphic phase transformation, decomposition and the formation of new crystalline phases. Under high temperature conditions, Al and AlN in aluminum dross are oxidized by air, and the following Equations (1)–(6) occur.
Al + 3/4O2 = 1/2Al2O3
AlN + 3/4O2 = 1/2Al2O3 + 1/2N2
AlN + 5/4O2 = 1/2Al2O3 + NO
AlN + 7/4O2 = 1/2Al2O3 + NO2
AlN + O2 = 1/2Al2O3 + 1/2N2O
AlN + 2O2 = 1/2Al2O3 + 1/2N2O5
The thermodynamic calculations of Equations (2)–(6) are shown in Figure 9. It can be seen from Figure 9 that the ΔG values of Equations (2)–(6) at 300–1100 °C are all less than 0. AlN in the aluminum dross can convert nitrogen and a variety of nitrogen oxides during the sintering process, and the tendency to convert to nitrogen is even greater. Nitrogen is the main component in the air and is harmless to the environment. Therefore, the preparation of sintered brick with aluminum dross as the main raw material is in line with the concept of green environmental protection.
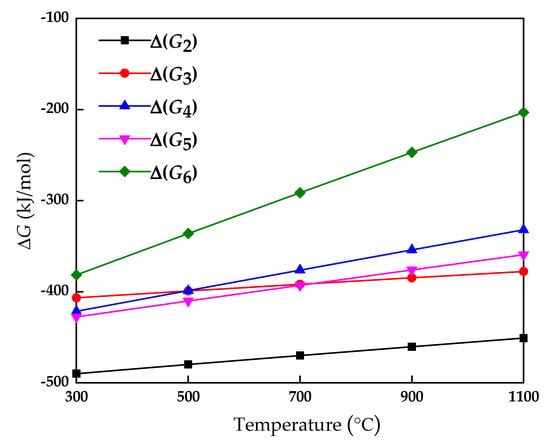
Figure 9.
ΔG changes with temperature.
During the sintering experiment, orthogonal experiments were performed to analyze the significant influence of heating rate, sintering temperature, and holding time on the compressive strength of sintered brick. The best technological parameters of sintering brick were further found. In this orthogonal experiment, the above influencing factors are selected as the orthogonal experiment factors, and the three factors and three levels orthogonal experiment is carried out. Preliminary exploratory test results show that too fast heating speed leads to local burst of brick in furnace cavity. Therefore, considering various factors, the heating rate is 6 °C/min, 7 °C/min and 8 °C/min. A temperature gradient is set for every 100 °C increase and the temperature is maintained for 20 min. The selection of the sintering temperature is mainly based on the temperature of AlN oxidation [] and the sintering temperature of traditional sintered brick. Therefore, the sintering temperature is set at 750 °C, 800 °C and 850 °C. The selection and level of each factor are shown in Table 2, the orthogonal experiment results are displayed in Table 3, and the analysis of variance is presented in Table 4.

Table 2.
Orthogonal factors and standard selection table.

Table 3.
The orthogonal pilot programs and experimental results.

Table 4.
Variance analysis.
The mechanical strength of the sintered brick is formed in the process of sintering, and its strength mainly comes from the silicate phase formed by the combination of SiO2 and Al2O3 in different forms. When the sintering temperature is too low or holding time is too short, underfired brick will be produced. If the sintering temperature is too high, the brick will burn excessively, which will seriously reduce the quality of the brick. If the heating rate is too fast, the gas inside the brick will not have time to overflow, which will cause the volume expansion of the product and produce cracks on the surface or inside of the brick. And in severe cases, it will burst in the furnace cavity.
F test is performed on the data obtained in Table 4, and the query threshold value is f0.05(2,2) = 19, f0.01(2,2) = 99. Therefore, it can be seen that the heating rate and the calcination temperature have significant impact on the experimental results. Without considering the interaction, the optimal plan should take the level corresponding to the maximum K value of each factor, namely, A3B2. Since the holding time has no significant effect on the compressive strength of the sintered brick. When the heating rate is 8 °C/min and the sintering temperature is 800 °C, the holding time is 60, 120 and 180 min, respectively. It is found that different holding time has little effect on the mechanical strength of sintered brick. Therefore, from the perspective of energy saving, it is reasonable to set the heat preservation time as 60 min. Finally, A3B2C1 is the best solution. The heating rate was 8 °C/min, the sintering temperature was 800 °C and the holding time was 60 min. The results show that the compressive strength, flexural strength and water absorption of the sintered brick are 16.21 MPa, 3.42 MPa and 17.12%, respectively.
3.4. Microanalysis
The samples of the best group in the orthogonal test were detected by XRD, and analyze the phase composition inside the sintered bricks. The XRD pattern of the best experimental group of orthogonal test is shown in Figure 10.
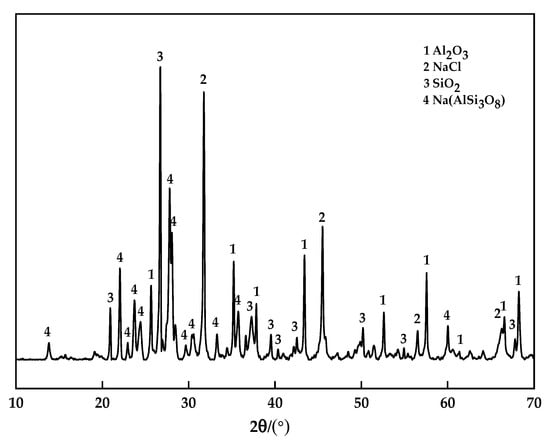
Figure 10.
XRD pattern of the sample.
It is believed that the main materials of sintered brick after sintering are quartz, alumina, albite and soluble salts. Compared with the XRD patterns of raw materials, it can be seen that the AlN and Al in the aluminum dross disappear and transform into corresponding oxides after sintering. More importantly, albite is formed during the sintering process. At high temperatures, albite can form eutectic with quartz and aluminosilicate, fill between the crystal grains of the brick, and draw the particles closer under the effect of surface tension, making the brick more compact. The main crystalline phases are alumina, quartz and albite. These crystalline phases constitute the main framework of the sintered brick. The formation of new crystalline phase albite plays a key role in promoting sintering.
Figure 11a–c show the micro morphology of samples containing 30%, 50% and 70% aluminum dross, and Figure 11d shows the micro morphology of samples without aluminum dross. Then, it can be clearly seen that a glass phase appears inside the sintered brick without aluminum dross, which causes the particles to begin to melt and bond. It can be seen from Figure 11d that the glass phase is evenly distributed on the surface of the sample and connected with each other, which improves the mechanical strength of the sintered brick. Figure 11a–c show that with the increase of aluminum dross content, the glass phase inside the sintered brick becomes less and the porosity increases. This leads to a significant decrease in the mechanical properties of sintered bricks. However, with the addition of aluminum dross, the shape of crystal particles in the brick becomes more regular. As shown in Figure 11c, crystal particles mostly exist in the form of rods, strips and flakes. These are intertwined with each other, which helps to improve the mechanical properties of bricks to a certain extent.
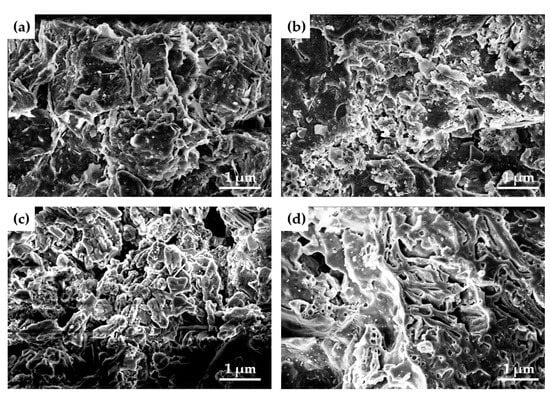
Figure 11.
SEM images. (a) 30% aluminum dross (b) 50% aluminum dross (c) 70% aluminum dross (d) No aluminum dross.
3.5. TG Analysis
The samples with 40%, 50% and 60% aluminum dross content were selected for thermogravimetric analysis to study the variation of sample weight during the sintering process of sintered brick, as shown in Figure 12. It can be seen from Figure 11 that with the increase of temperature, the weight of the sample decreases. The weight change of 40% and 50% aluminum slag content is basically the same. However, when the aluminum dross content is 60%, the weight of the sample increases at low temperature. The reason is that the content of aluminum and AlN in the sample increases with the increase of aluminum dross content, and the weight of the sample increases during the oxidation process. When the temperature increases, the weight of the sample decreases in varying degrees, which is mainly due to the evaporation of the adsorbed water on the sample surface or the decomposition of other surface materials and impurities.
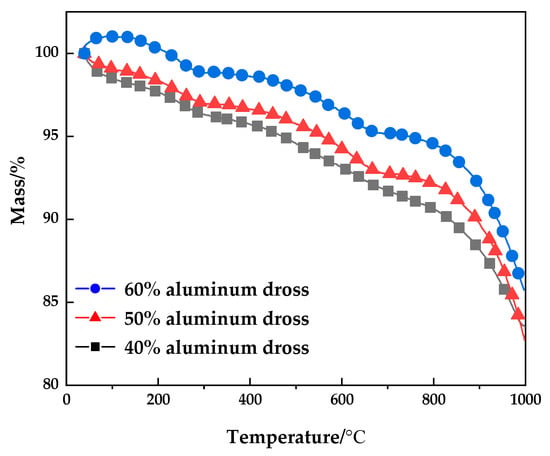
Figure 12.
TG analysis.
4. Conclusions
(1) The best raw material ratio for the preparation of sintered brick is 50% aluminum dross, 37.5% engineering soil, 12.5% coal gangue. The best molding pressure is 10 MPa, the best sintering process: the heating rate is 8 °C/min, the temperature is 800 °C and the holding time is 60 min. According to the best preparation process, the sintered brick meets the requirements of Mu15 grade in GB/T 5101-2017 “fired ordinary brick”.
(2) The sintering process of sintered brick is mainly affected by sintering temperature, holding time and heating rate. From the orthogonal test results, the heating rate and sintering temperature have a more significant impact on the overall mechanical strength of the sintered brick.
(3) After sintering, AlN and Al disappear and albite phase is formed. At high temperature, albite forms grain boundary with quartz and aluminosilicate, which is filled between the grains of the brick and improves the mechanical properties of the brick.
(4) Using aluminum dross, engineering soil and coal gangue to prepare sintered brick does not produce waste gas and waste liquid. This not only makes comprehensive use of solid waste, but also does not affect the surrounding environment in the production process, realizing the concept of green environmental protection and sustainable development.
Author Contributions
Conceptualization, H.N. and X.W.; methodology, Y.Z.; validation, Y.Z.; formal analysis, H.N. and S.L. (Shuaishuai Lv); investigation, Y.Z. and X.W.; resources, H.N. and S.L. (Shuaishuai Lv); writing—original draft preparation, Y.Z.; writing—review and editing, H.N. and S.L. (Songyuan Li); visualization, Y.Z. and J.Z.; supervision, H.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was Supported by the Priority Academic Program Development of Jiangsu Higher Education Institutions, grant number PAPD.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, J.H.; Zhong, Y.Q.; Tong, Y.; Xu, X.L.; Lin, G.Y. Removal of AlN from secondary aluminum dross by pyrometallurgical treatment. J. Cent. South. Univ. 2021, 28, 386–397. [Google Scholar] [CrossRef]
- Lin, Q.T.; Peng, H.L.; Zhong, S.X.; Xiang, J.X. Synthesis, characterization and secondary sludge dewatering performance of a novel combined silicon–aluminum–iron–starch flocculant. J. Hazard. Mater. 2015, 285, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.S.; Zhang, J.Q.; Ni, H.J.; Wang, X.X.; Zhu, Y.; Gu, T. Study on Preparation of Aluminum Ash Coating Based on Plasma Spray. Appl. Sci. 2019, 9, 4980. [Google Scholar] [CrossRef] [Green Version]
- Ni, H.J.; Zhang, J.Q.; Lv, S.S.; Gu, T.; Wang, X.X. Performance Optimization of Original Aluminum Ash Coating. Coatings 2020, 10, 831. [Google Scholar] [CrossRef]
- Yoshimura, H.N.; Abreu, A.P.; Molisani, A.L.; de Camargo, A.C.; Portela, J.C.S.; Narita, N.E. Evaluation of aluminum dross waste as raw material for refractories. Ceram. Int. 2006, 34, 581–591. [Google Scholar] [CrossRef]
- Zhan, D.P.; Zhang, H.S.; Jiang, Z.H. Effects of AIMnCa and AIMnFe Alloys on Deoxidization of Low Carbon and Low Silicon Aluminum Killed Steels. J. Iron Steel Res. Int. 2008, 15, 15–18. [Google Scholar] [CrossRef]
- Elseknidy, M.H.; Salmiaton, A.; Nor, S.I.; Saad, A.H. A Study on Mechanical Properties of Concrete Incorporating Aluminum Dross, Fly Ash, and Quarry Dust. Sustainability 2020, 12, 9230. [Google Scholar] [CrossRef]
- Kizito, P.M.; Revocatus, L.M.; Yusufu, A.C.J. Effect of Elevated Temperature on Compressive Strength and Physical Properties of Neem Seed Husk Ash Concrete. Materials 2020, 13, 1198. [Google Scholar]
- Turner, L.K.; Collins, F.G. Carbon dioxide equivalent (CO2-e) emissions: A comparison between geopolymer and OPC cement concrete. Constr. Build. Mater. 2013, 43, 125–130. [Google Scholar] [CrossRef]
- Wei, Z.A.; Zhao, J.K.; Wang, W.S.; Yang, Y.H.; Zhuang, S.N.; Lu, T.; Hou, Z.K. Utilizing gold mine tailings to produce sintered bricks. Constr. Build. Mater. 2021, 282, 122655. [Google Scholar] [CrossRef]
- Luo, L.Q.; Li, K.Y.; Weng, F.; Liu, C.; Yang, S.Y. Preparation, characteristics and mechanisms of the composite sintered bricks produced from shale, sewage sludge, coal gangue powder and iron ore tailings. Constr. Build. Mater. 2020, 232, 117250. [Google Scholar] [CrossRef]
- Chiraporn, A.; Ryan, C.; McCuiston, T.; Prasartseree, P.; Pungpipat, S.O. Properties of Sintered Brick Containing Lignite Bottom Ash Substitutions. Mech. Corros. Prop. 2015, 4122, 138–142. [Google Scholar]
- Ran, F.Z.; Gao, X.D.; Le, F.M.; Wei, J.G.; Jing, H.L. Preparation and Properties of Sintering Brick from Iron Tailings. Mech. Corros. Prop. 2012, 1809, 1023–1027. [Google Scholar]
- Yang, H.L.; Liu, H.M. Performance Analysis on the Raw Material of Nantong Silt Sintered Brick. Adv. Mater. Res. 2012, 1616, 743–748. [Google Scholar] [CrossRef]
- He, L.Q.; Shi, L.; Huang, Q.Z.; Hayat, W.; Shang, Z.B.; Ma, T.F.; Wang, M.; Yao, W.D.; Huang, H.Y.; Chen, R. Extraction of alumina from aluminum dross by a non-hazardous alkaline sintering process: Dissolution kinetics of alumina and silica from calcined materials. Sci. Total Env. 2021, 777, 146123. [Google Scholar] [CrossRef] [PubMed]
- Meshram, A.; Jain, A.; Gautam, D.; Singh, K.K. Synthesis and characterization of tamarugite from aluminium dross: Part, I. J. Environ. Manag. 2019, 232, 978–984. [Google Scholar] [CrossRef] [PubMed]
- Kristoffer, K.; Tomaž, K. Protection of AlN powder against hydrolysis using aluminum dihydrogen phosphate. J. Eur. Ceram. Soc. 2001, 21, 2075–2079. [Google Scholar]
- Mostafa, M.; Ali, A. Hazardous aluminum dross characterization and recycling strategies: A critical review. J. Environ. Manag. 2018, 223, 452–468. [Google Scholar]
- Shen, H.L.; Liu, B.; Ekberg, C.; Zhang, S.G. Harmless disposal and resource utilization for secondary aluminum dross: A review. Sci. Total Env. 2021, 760. [Google Scholar]
- GB/T 5101-2017. Fired Common Bricks; General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 2017. (In Chinese) [Google Scholar]
- GB/T 2542-2012. Test Methods for Wall Bricks; General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 2012. (In Chinese) [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).