Hydrogen and Deuterium Incorporation in ZnO Films Grown by Atomic Layer Deposition
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AFM | Atomic force microscopy |
| ALD | Atomic layer deposition |
| CVD | Chemical vapor deposition |
| DEZ | Diethylzinc |
| GPC | Growth per cycle |
| HIM | Helium ion microscopy |
| IR | Infrared |
| MSP | Multiple short pulses |
| OLED | Organic light emitting diode |
| QCM | Quartz crystal microbalance |
| QMS | Quadrupole mass spectrometry |
| RMS | Root-mean-square |
| SIMS | Secondary ion mass spectrometry |
| TMA | Trimethylaluminium |
| ToF-ERDA | Time-of-flight elastic recoil detection analysis |
| XRD | X-ray diffraction |
References
- Choi, J.; Mao, Y.; Chang, J. Development of hafnium based high-k materials—A review. Mater. Sci. Eng. R Rep. 2011, 72, 97–136. [Google Scholar] [CrossRef]
- Li, Y.; Xiong, Y.; Yang, H.; Cao, K.; Chen, R. Thin film encapsulation for the organic light-emitting diodes display via atomic layer deposition. J. Mater. Res. 2020, 35, 681–700. [Google Scholar] [CrossRef]
- Xing, Z.; Xiao, J.; Hu, T.; Meng, X.; Li, D.; Hu, X.; Chen, Y. Atomic Layer Deposition of Metal Oxides in Perovskite Solar Cells: Present and Future. Small Methods 2020, 4, 2000588. [Google Scholar] [CrossRef]
- Ritala, M.; Leskelä, M.; Dekker, J.P.; Mutsaers, C.; Soininen, P.J.; Skarp, J. Perfectly Conformal TiN and Al2O3 Films Deposited by Atomic Layer Deposition. Chem. Vap. Depos. 1999, 5, 7–9. [Google Scholar] [CrossRef]
- Iatsunskyi, I.; Kempíski, M.; Jancelewicz, M.; Załęski, K.; Jurga, S.; Smyntyna, V. Structural and XPS Characterization of ALD Al2O3 Coated Porous Silicon. Vacuum 2015, 113, 52–58. [Google Scholar] [CrossRef]
- Zhao, M.J.; Sun, Z.T.; Hsu, C.H.; Huang, P.H.; Zhang, X.Y.; Wu, W.Y.; Gao, P.; Qiu, Y.; Lien, S.Y.; Zhu, W.Z. Zinc Oxide Films with High Transparency and Crystallinity Prepared by a Low Temperature Spatial Atomic Layer Deposition Process. Nanomaterials 2020, 459. [Google Scholar] [CrossRef]
- Gao, Z.; Banerjee, P. Review Article: Atomic layer deposition of doped ZnO films. J. Vac. Sci. Technol. A 2019, 37, 050802. [Google Scholar] [CrossRef]
- Ingale, P.; Knemeyer, K.; Piernavieja Hermida, M.; Naumann d’Alnoncourt, R.; Thomas, A.; Rosowski, F. Atomic Layer Deposition of ZnO on Mesoporous Silica: Insights into Growth Behavior of ZnO via In-Situ Thermogravimetric Analysis. Nanomaterials 2020, 10, 981. [Google Scholar] [CrossRef]
- Choi, Y.J.; Gong, S.C.; Park, C.S.; Lee, H.S.; Jang, J.G.; Chang, H.J.; Yeom, G.Y.; Park, H.H. Improved Performance of Organic Light-Emitting Diodes Fabricated on Al-Doped ZnO Anodes Incorporating a Homogeneous Al-Doped ZnO Buffer Layer Grown by Atomic Layer Deposition. ACS Appl. Mater. Interfaces 2013, 5, 3650–3655. [Google Scholar] [CrossRef] [PubMed]
- Malm, J.; Sahramo, E.; Perälä, J.; Sajavaara, T.; Karppinen, M. Low-temperature atomic layer deposition of ZnO thin films: Control of crystallinity and orientation. Thin Solid Film. 2011, 519, 5319–5322. [Google Scholar] [CrossRef]
- Cai, J.; Ma, Z.; Wejinya, U.; Zou, M.; Liu, Y.; Zhou, H.; Meng, X. A revisit to atomic layer deposition of zinc oxide using diethylzinc and water as precursors. J. Mater. Sci. 2019, 54, 5236–5248. [Google Scholar] [CrossRef]
- Guziewicz, E.; Godlewski, M.; Krajewski, T.; Wachnicki, Ł.; Szczepanik, A.; Kopalko, K.; Wójcik-Głodowska, A.; Przeździecka, E.; Paszkowicz, W.; Łusakowska, E.; et al. ZnO grown by atomic layer deposition: A material for transparent electronics and organic heterojunctions. J. Appl. Phys. 2009, 105, 122413. [Google Scholar] [CrossRef]
- Di Mauro, A.; Cantarella, M.; Nicotra, G.; Privitera, V.; Impellizzeri, G. Low temperature atomic layer deposition of ZnO: Applications in photocatalysis. Appl. Catal. B Environ. 2016, 196, 68–76. [Google Scholar] [CrossRef]
- Wang, L.C.; Han, Y.Y.; Yang, K.C.; Chen, M.J.; Lin, H.C.; Lin, C.K.; Hsu, Y.T. Hydrophilic/hydrophobic surface of Al2O3 thin films grown by thermal and plasma-enhanced atomic layer deposition on plasticized polyvinyl chloride (PVC). Surf. Coatings Technol. 2016, 305, 158–164. [Google Scholar] [CrossRef]
- Kekkonen, V.; Hakola, A.; Kajava, T.; Sahramo, E.; Malm, J.; Karppinen, M.; Ras, R.H.A. Self-erasing and rewritable wettability patterns on ZnO thin films. Appl. Phys. Lett. 2010, 97, 044102. [Google Scholar] [CrossRef]
- Kääriäinen, M.L.; Weiss, C.; Ritz, S.; Pütz, S.; Cameron, D.; Mailänder, V.; Landfester, K. Zinc release from atomic layer deposited zinc oxide thin films and its antibacterial effect on Escherichia coli. Appl. Surf. Sci. 2013, 287, 375–380. [Google Scholar] [CrossRef]
- Miikkulainen, V.; Leskelä, M.; Ritala, M.; Puurunen, R. Crystallinity of inorganic films grown by atomic layer deposition: Overview and general trends. J. Appl. Phys. 2013, 113, 021301. [Google Scholar] [CrossRef]
- Fang, M.; Qi, L.; Zhang, Z.; Chen, Q. Effects of thickness and deposition temperature of ALD ZnO on the performance of inverted polymer solar cells. J. Mater. Sci. Mater. Electron. 2016, 27, 10252–10258. [Google Scholar] [CrossRef]
- Guziewicz, E.; Kowalik, I.A.; Godlewski, M.; Kopalko, K.; Osinniy, V.; Wójcik, A.; Yatsunenko, S.; Łusakowska, E.; Paszkowicz, W.; Guziewicz, M. Extremely low temperature growth of ZnO by atomic layer deposition. J. Appl. Phys. 2008, 103, 033515. [Google Scholar] [CrossRef]
- Yousfi, E.B.; Fouache, J.; Lincot, D. Study of atomic layer epitaxy of zinc oxide by in-situ quartz crystal microgravimetry. Appl. Surf. Sci. 2000, 153, 223–234. [Google Scholar] [CrossRef]
- Puurunen, R.L. Surface chemistry of atomic layer deposition: A case study for the trimethylaluminum/water process. J. Appl. Phys. 2005, 97, 121301. [Google Scholar] [CrossRef]
- Vandalon, V.; Kessels, W.M.M. What is limiting low-temperature atomic layer deposition of Al2O3: A vibrational sum-frequency generation study. Appl. Phys. Lett. 2016, 108, 011607. [Google Scholar] [CrossRef]
- Mackus, A.J.M.; MacIsaac, C.; Kim, W.H.; Bent, S.F. Incomplete elimination of precursor ligands during atomic layer deposition of zinc-oxide, tin-oxide, and zinc-tin-oxide. J. Chem. Phys. 2017, 146, 052802. [Google Scholar] [CrossRef]
- Weckman, T.; Laasonen, K. Atomic Layer Deposition of Zinc Oxide: Diethyl Zinc Reactions and Surface Saturation from First-Principles. J. Phys. Chem. C 2016, 120, 21460–21471. [Google Scholar] [CrossRef]
- Weckman, T.; Laasonen, K. Atomic Layer Deposition of Zinc Oxide: Study on the Water Pulse Reactions from First-Principles. J. Phys. Chem. C 2018, 122, 7685–7694. [Google Scholar] [CrossRef]
- Macco, B.; Knoops, H.C.; Verheijen, M.A.; Beyer, W.; Creatore, M.; Kessels, W.M. Atomic layer deposition of high-mobility hydrogen-doped zinc oxide. Sol. Energy Mater. Sol. Cells 2017, 173, 111–119. [Google Scholar] [CrossRef]
- Peter, R.; Salamon, K.; Omerzu, A.; Grenzer, J.; Badovinac, I.J.; Saric, I.; Petravic, M. Role of Hydrogen-Related Defects in Photocatalytic Activity of ZnO Films Grown by Atomic Layer Deposition. J. Phys. Chem. C 2020, 124, 8861–8868. [Google Scholar] [CrossRef]
- Przezdziecka, E.; Guziewicz, E.; Jarosz, D.; Snigurenko, D.; Sulich, A.; Sybilski, P.; Jakiela, R.; Paszkowicz, W. Influence of oxygen-rich and zinc-rich conditions on donor and acceptor states and conductivity mechanism of ZnO films grown by ALD—Experimental studies. J. Appl. Phys. 2020, 127, 075104. [Google Scholar] [CrossRef]
- Beh, H.; Hiller, D.; Bruns, M.; Welle, A.; Becker, H.W.; Berghoff, B.; Sürgers, C.; Merz, R.; Zacharias, M. Quasi-metallic behavior of ZnO grown by atomic layer deposition: The role of hydrogen. J. Appl. Phys. 2017, 122, 025306. [Google Scholar] [CrossRef]
- Guerra-Nunẽz, C.; Döbeli, M.; Michler, J.; Utke, I. Reaction and Growth Mechanisms in Al2O3 deposited via Atomic Layer Deposition: Elucidating the Hydrogen Source. Chem. Mater. 2017, 29, 8690–8703. [Google Scholar] [CrossRef]
- Rahtu, A.; Ritala, M. Reaction Mechanism Studies on Titanium Isopropoxide-Water Atomic Layer Deposition Process. Chem. Vap. Depos. 2002, 8, 21–28. [Google Scholar] [CrossRef]
- Juppo, M.; Rahtu, A.; Ritala, M.; Leskelä, M. In Situ Mass Spectrometry Study on Surface Reactions in Atomic Layer Deposition of Al2O3 Thin Films from Trimethylaluminum and Water. Langmuir 2000, 16, 4034–4039. [Google Scholar] [CrossRef]
- Juppo, M.; Rahtu, A.; Ritala, M. In Situ Mass Spectrometry Study on Surface Reactions in Atomic Layer Deposition of TiN and Ti(Al)N Thin Films. Chem. Mater. 2002, 14, 281–287. [Google Scholar] [CrossRef]
- Vandalon, V.; Kessels, W.M.M. Revisiting the Growth Mechanism of Atomic Layer Deposition of Al2O3: A Vibrational Sum-frequency Generation Study. J. Vac. Sci. Technol. A 2017, 35, 05C313. [Google Scholar] [CrossRef]
- Hiraiwa, A.; Saito, T.; Matsumura, D.; Kawarada, H. Isotope Analysis of Diamond-surface Passivation Effect of High-temperature H2O-grown Atomic Layer Deposition-Al2O3 Films. J. Appl. Phys. 2015, 117, 215304. [Google Scholar] [CrossRef]
- Guziewicz, E.; Wozniak, W.; Mishra, S.; Jakiela, R.; Guziewicz, M.; Ivanov, V.Y.; Lusakowska, E.; Schifano, R. Hydrogen in As-Grown and Annealed ZnO Films Grown by Atomic Layer Deposition. Phys. Status Solidi (a) 2021, 218, 2000318. [Google Scholar] [CrossRef]
- Kinnunen, S.; Arstila, K.; Sajavaara, T. Al2O3 ALD films grown using TMA + rare isotope and precursors. Appl. Surf. Sci. 2021, 148909. [Google Scholar] [CrossRef]
- Degen, T.; Sadki, M.; Bron, E.; König, U.; Nénert, G. The HighScore suite (v. 4.9). Powder Diffr. 2014, 29, S13–S18. [Google Scholar] [CrossRef]
- International Centre for Diffraction Data, ICDD-PDF4+ Database. Available online: https://www.icdd.com/ (accessed on 3 May 2021).
- Laitinen, M.; Rossi, M.; Julin, J.; Sajavaara, T. Time-of-flight—Energy spectrometer for elemental depth profiling –Jyväskylä design. Nucl. Instruments Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2014, 337, 55–61. [Google Scholar] [CrossRef]
- Arstila, K.; Julin, J.; Laitinen, M.; Aalto, J.; Konu, T.; Kärkkäinen, S.; Rahkonen, S.; Raunio, M.; Itkonen, J.; Santanen, J.P.; et al. Potku—New analysis software for heavy ion elastic recoil detection analysis. Nucl. Instruments Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2014, 331, 34–41. [Google Scholar] [CrossRef]
- Arstila, K.; Sajavaara, T.; Keinonen, J. Monte Carlo simulation of multiple and plural scattering in elastic recoil detection. Nucl. Instruments Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2001, 174, 163–172. [Google Scholar] [CrossRef]
- Guziewicz, E.; Krajewski, T.A.; Przezdziecka, E.; Korona, K.P.; Czechowski, N.; Klopotowski, L.; Terziyska, P. Zinc Oxide Grown by Atomic Layer Deposition: From Heavily n-Type to p-Type Material. Phys. Status Solidi (b) 2020, 257, 1900472. [Google Scholar] [CrossRef]
- Panigrahi, J.; Singh, P.; Gupta, G.; Vandana. Growth and luminescence characteristics of zinc oxide thin films deposited by ALD technique. J. Lumin. 2021, 233, 117797. [Google Scholar] [CrossRef]
- Napari, M.; Lahtinen, M.; Veselov, A.; Julin, J.; Østreng, E.; Sajavaara, T. Room-temperature plasma-enhanced atomic layer deposition of ZnO: Film growth dependence on the PEALD reactor configuration. Surf. Coatings Technol. 2017, 326, 281–290. [Google Scholar] [CrossRef]
- Sønsteby, H.H.; Yanguas-Gil, A.; Elam, J.W. Consistency and Reproducibility in Atomic Layer Deposition. J. Vac. Sci. Technol. A 2020, 38, 020804. [Google Scholar] [CrossRef]
- Gómez-Gallego, M.; Sierra, M. Kinetic Isotope Effects in the Study of Organometallic Reaction Mechanisms. Chem. Rev. 2011, 111, 4857–4963. [Google Scholar] [CrossRef]
- Park, H.K.; Yang, B.S.; Park, S.; Kim, M.S.; Shin, J.C.; Heo, J. Purge-time-dependent growth of ZnO thin films by atomic layer deposition. J. Alloys Compd. 2014, 605, 124–130. [Google Scholar] [CrossRef]
- Dos Reis, P.M.; de Oliveira, A.S.; Pecoraro, E.; Ribeiro, S.J.; Góes, M.S.; Nascimento, C.S.; Gonçalves, R.R.; dos Santos, D.P.; Schiavon, M.A.; Ferrari, J.L. Photoluminescent and structural properties of ZnO containing Eu3+ using PEG as precursor. J. Lumin. 2015, 167, 197–203. [Google Scholar] [CrossRef]
- Shirazi, M.; Elliott, S.D. Cooperation between adsorbates accounts for the activation of atomic layer deposition reactions. Nanoscale 2015, 7, 6311–6318. [Google Scholar] [CrossRef]
- Elam, J.W.; George, S.M. Growth of ZnO/Al2O3 Alloy Films Using Atomic Layer Deposition Techniques. Chem. Mater. 2003, 15, 1020–1028. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Z.; Xu, X.; Liu, Y.; Chen, C.; Chen, P.; Hu, W.; Duan, Y. Multiple short pulse process for low-temperature atomic layer deposition and its transient steric hindrance. Appl. Phys. Lett. 2019, 114, 201902. [Google Scholar] [CrossRef]


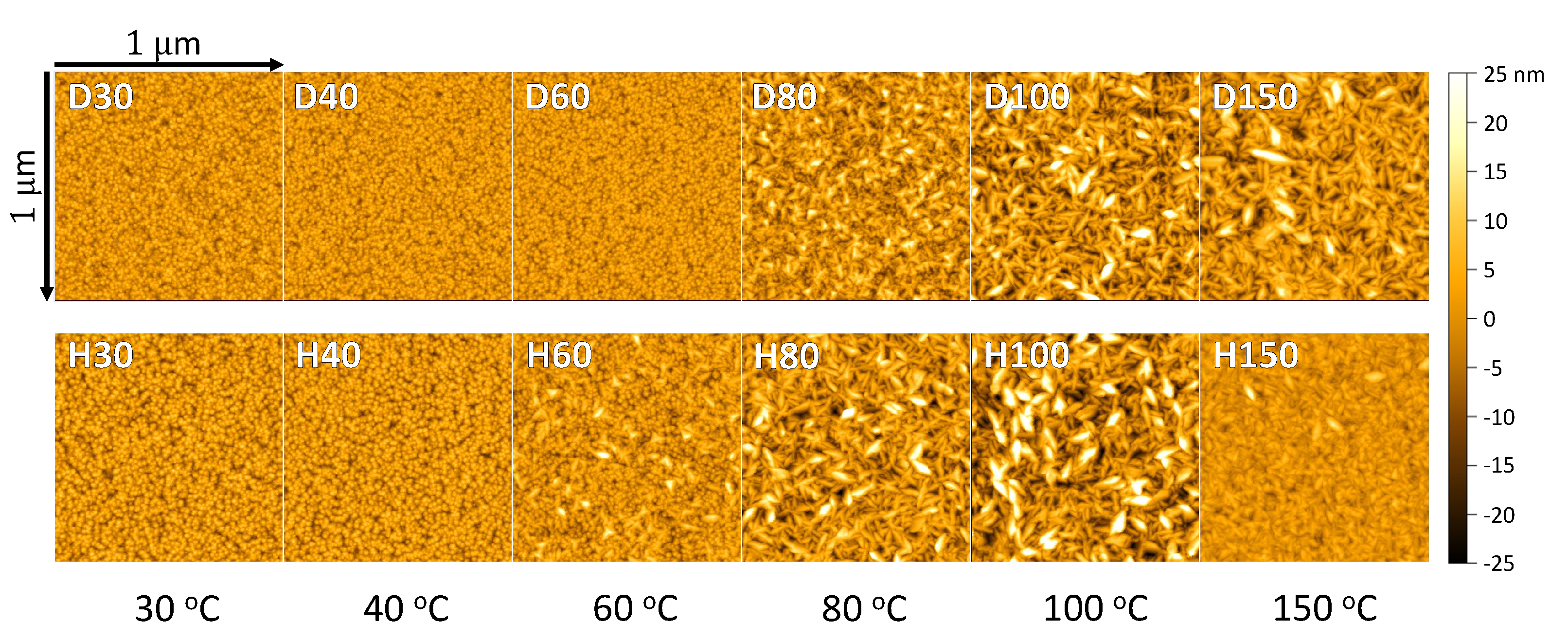
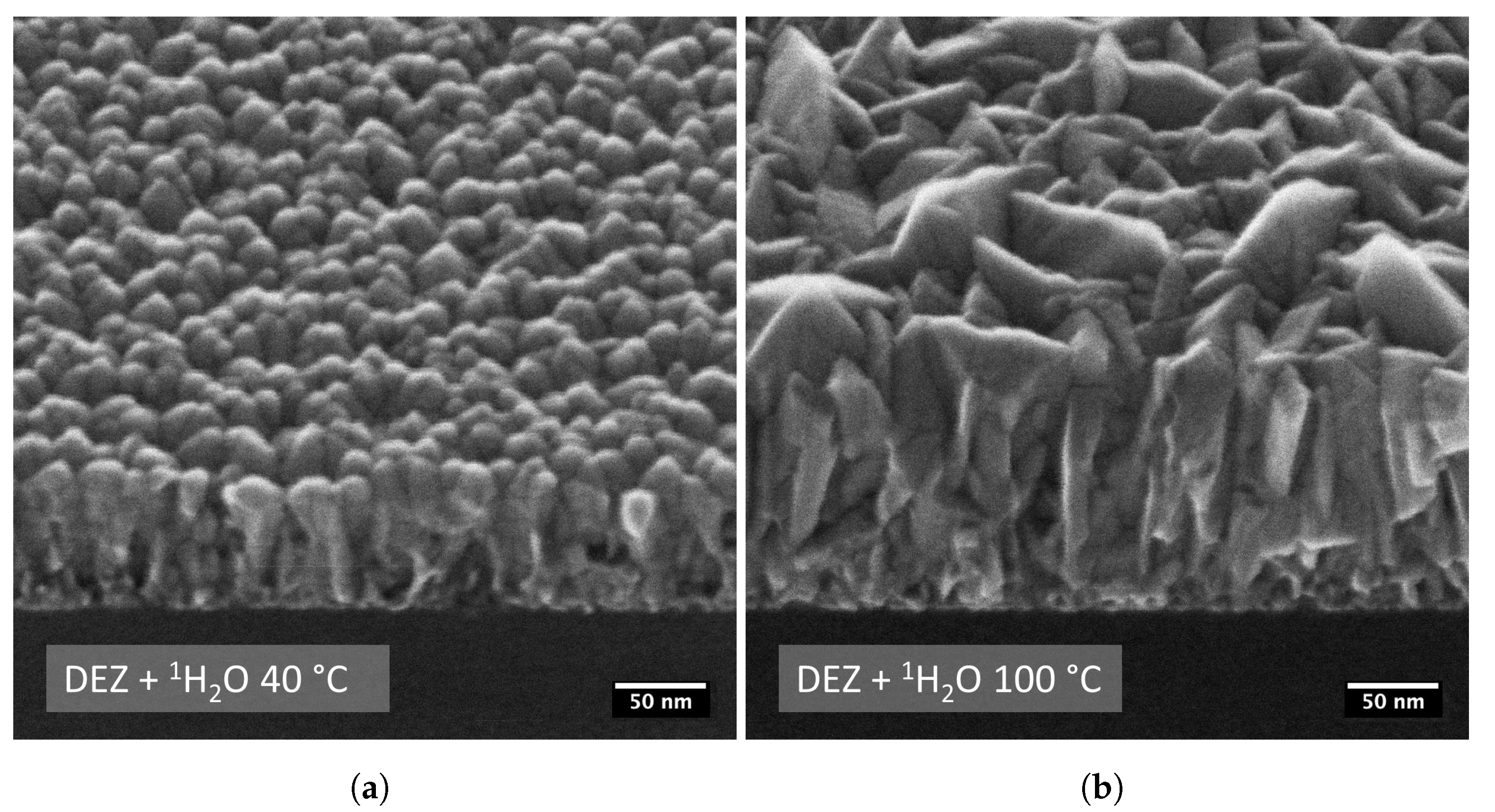


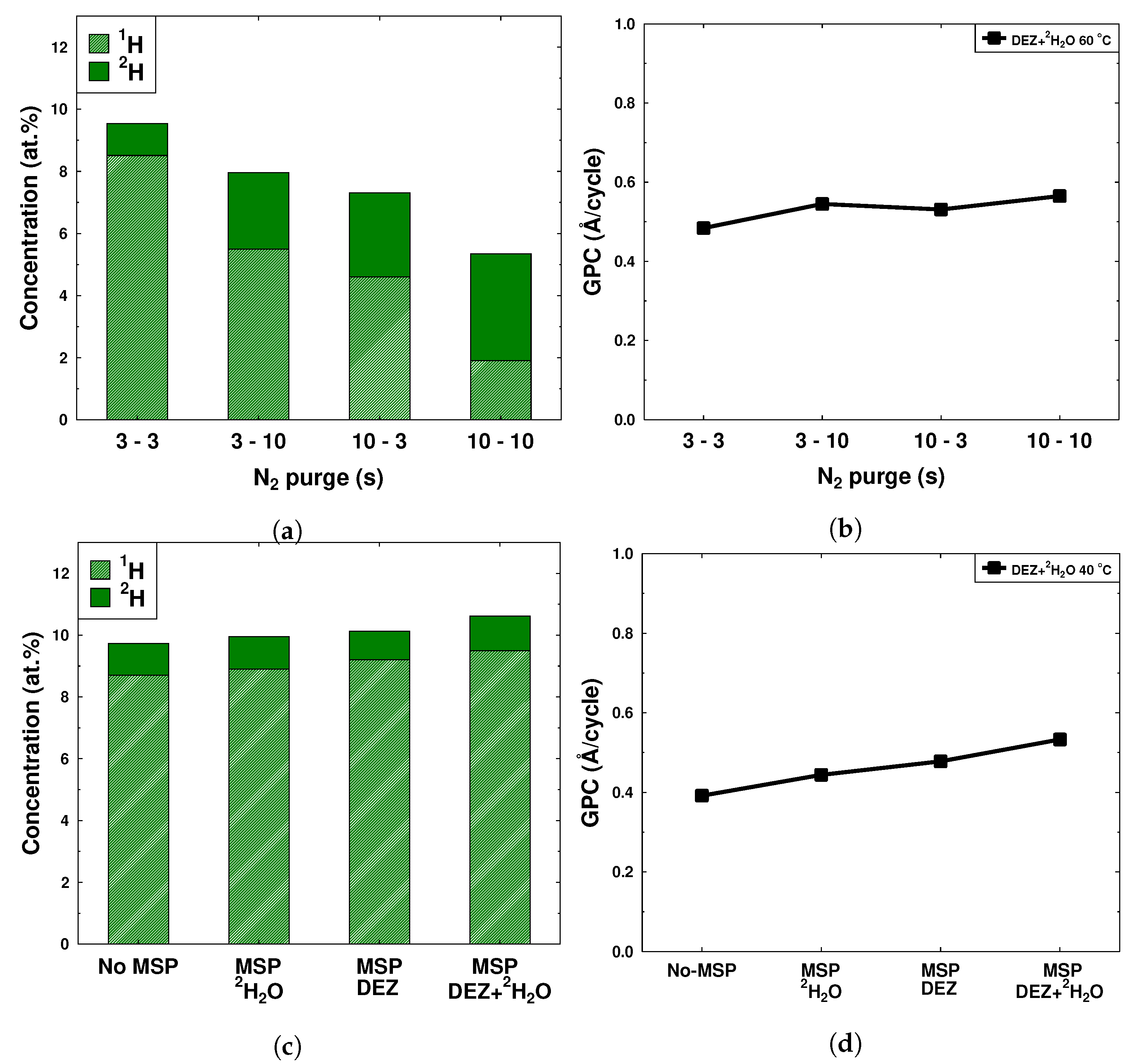
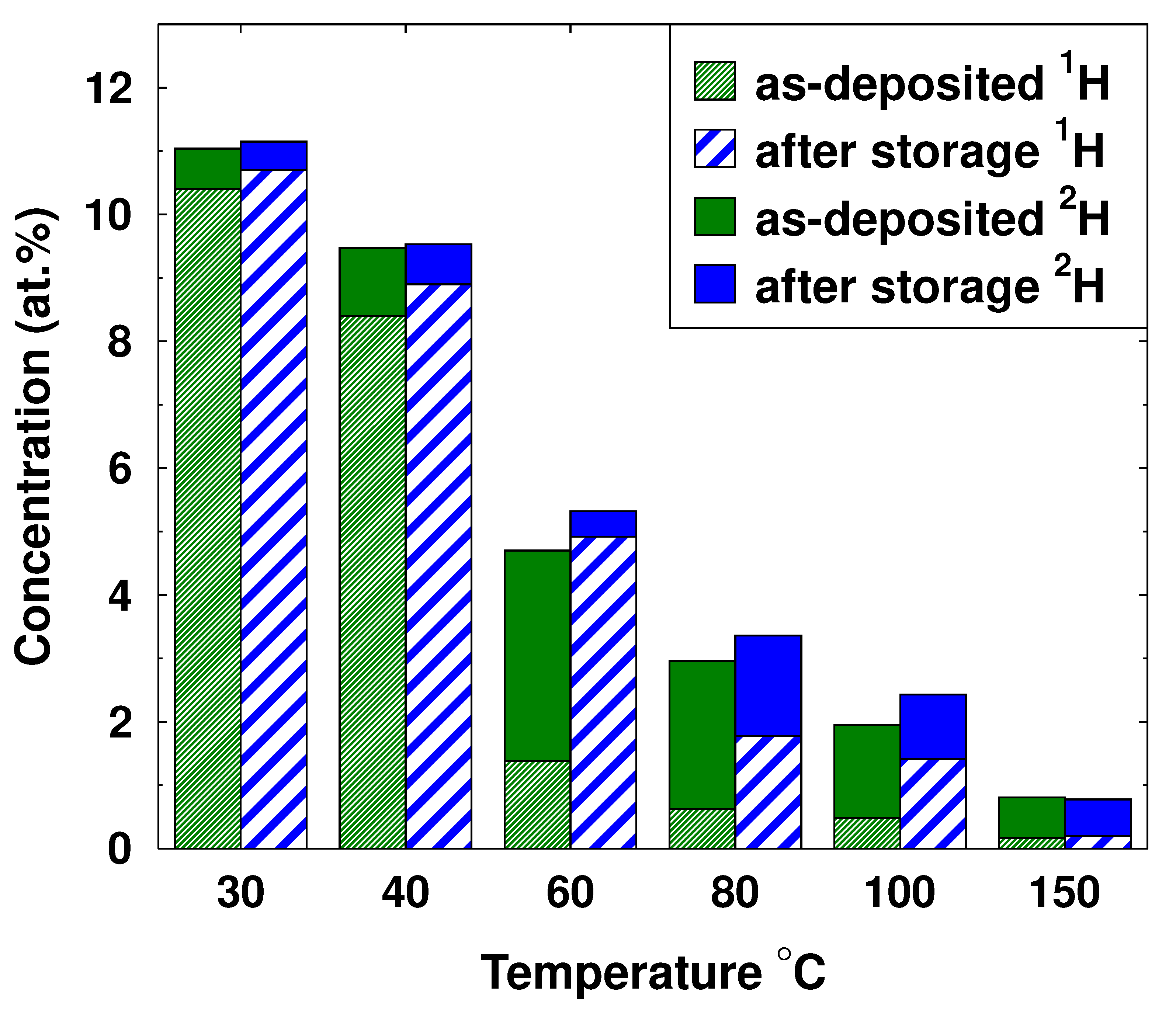
| Sample | T (°C) | 1H (at.%) | 2H (at.%) | C (at.%) | Thickness (nm) | RMS Roughness (nm) | GPC (Å/cycle) |
|---|---|---|---|---|---|---|---|
| DEZ+2H2O | |||||||
| D30 | 30 | 10.7 | 0.7 | 1.3 | 45 | 3.6 | 0.32 |
| D40 | 40 | 8.7 | 1.0 | 0.8 | 47 | 3.5 | 0.39 |
| D60 | 60 | 1.4 | 3.3 | 0.3 | 59 | 3.4 | 0.59 |
| D80 | 80 | 0.7 | 2.4 | 0.2 | 87 | 5.7 | 0.87 |
| D100 | 100 | 0.5 | 1.6 | 0.2 | 113 | 7.5 | 1.13 |
| D150 | 150 | 0.1 | 0.7 | 0.2 | 156 | 6.2 | 1.56 |
| D200 | 200 | >0.1 | 0.4 | >0.1 | 139 | - | 1.39 |
| DEZ+1H2O | |||||||
| H30 | 30 | 7.8 | - | 0.5 | 82 | 4.5 | 0.82 |
| H40 | 40 | 5.3 | - | 0.3 | 89 | 4.3 | 0.89 |
| H60 | 60 | 3.2 | - | 0.1 | 107 | 4.6 | 1.07 |
| H80 | 80 | 2.1 | - | 0.2 | 118 | 6.8 | 1.18 |
| H100 | 100 | 1.6 | - | 0.2 | 155 | 9.2 | 1.55 |
| H150 | 150 | 1.0 | - | 0.2 | 133 | 3.1 | 1.66 |
| H200 | 200 | 0.4 | - | 0.1 | 137 | - | 1.37 |
| Sample | N2 Purge (s) | 1H (at.%) | 2H (at.%) | C (at.%) | Thickness (nm) | RMS Roughness (nm) | GPC (Å/cycle) |
|---|---|---|---|---|---|---|---|
| N3 | 3 | 8.5 | 1.0 | 0.7 | 48 | 3.3 | 0.48 |
| N5 | 5 | 3.4 | 4.2 | 0.7 | 52 | 2.7 | 0.52 |
| N10 | 10 | 1.9 | 3.4 | 0.3 | 57 | 3.3 | 0.57 |
| N20 | 20 | 1.4 | 3.3 | 0.3 | 59 | 3.4 | 0.59 |
| N30 | 30 | 1.3 | 3.1 | 0.3 | 61 | 3.1 | 0.61 |
| N60 | 60 | 1.0 | 2.7 | 0.2 | 65 | 3.4 | 0.65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kinnunen, S.; Lahtinen, M.; Arstila, K.; Sajavaara, T. Hydrogen and Deuterium Incorporation in ZnO Films Grown by Atomic Layer Deposition. Coatings 2021, 11, 542. https://doi.org/10.3390/coatings11050542
Kinnunen S, Lahtinen M, Arstila K, Sajavaara T. Hydrogen and Deuterium Incorporation in ZnO Films Grown by Atomic Layer Deposition. Coatings. 2021; 11(5):542. https://doi.org/10.3390/coatings11050542
Chicago/Turabian StyleKinnunen, Sami, Manu Lahtinen, Kai Arstila, and Timo Sajavaara. 2021. "Hydrogen and Deuterium Incorporation in ZnO Films Grown by Atomic Layer Deposition" Coatings 11, no. 5: 542. https://doi.org/10.3390/coatings11050542
APA StyleKinnunen, S., Lahtinen, M., Arstila, K., & Sajavaara, T. (2021). Hydrogen and Deuterium Incorporation in ZnO Films Grown by Atomic Layer Deposition. Coatings, 11(5), 542. https://doi.org/10.3390/coatings11050542








