MOCVD of Noble Metal Film Materials for Medical Implants: Microstructure and Biocompatibility of Ir and Au/Ir Coatings on TiNi
Abstract
1. Introduction
2. Materials and Methods
2.1. Noble Metal Coating Preparation
2.2. Coating Characterization
2.3. Biological Investigation
2.4. Statistical Analysis
3. Results and Discussion
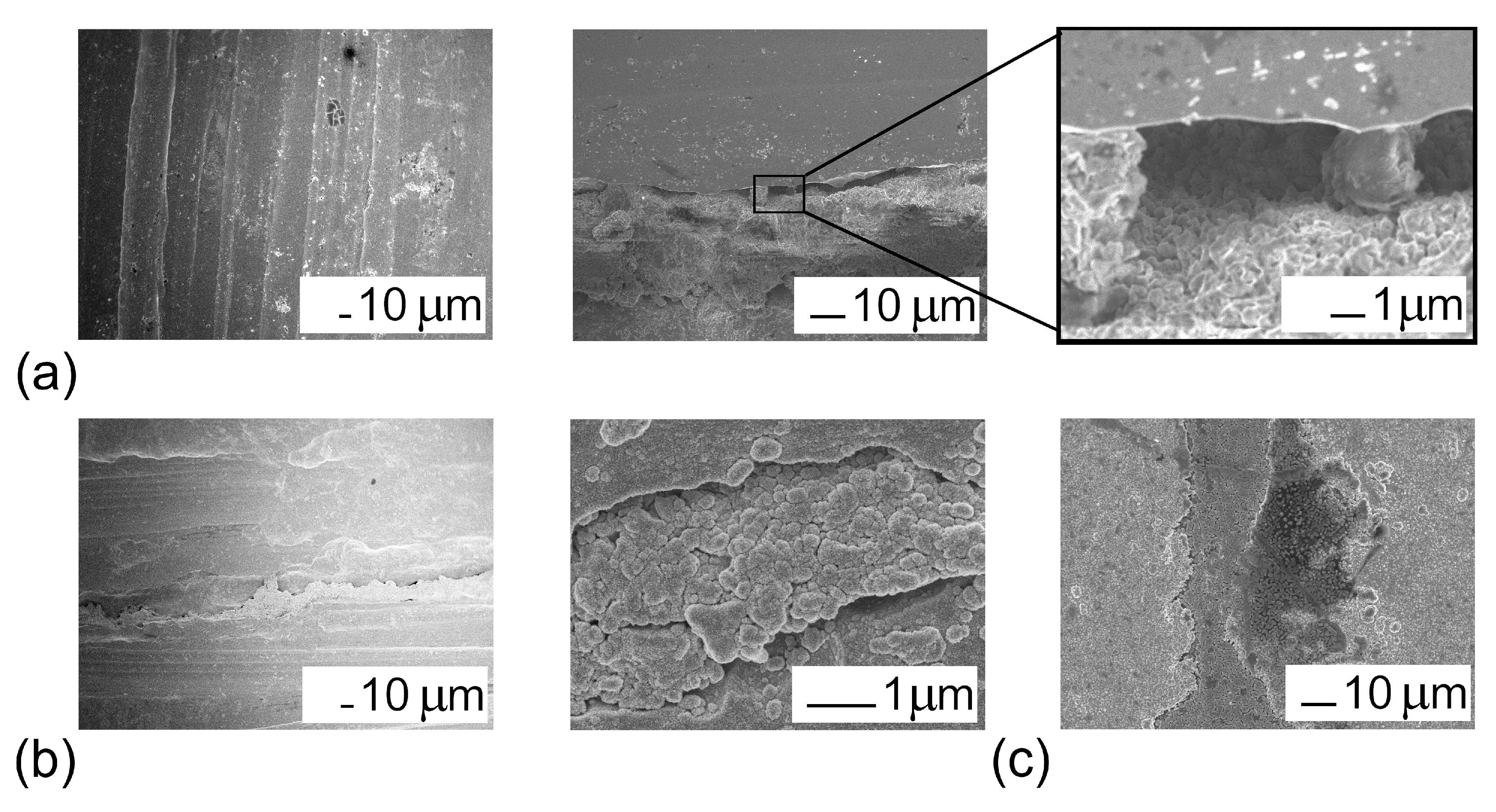
3.1. Composition and Microstructure of Coatings Obtained by MOCVD from [Ir(cod)(acac)] in Oxidizing Atmosphere
3.2. Iridium and Gold-Iridium Coatings on TiNi
3.3. Biological Characteristics of Noble Metal Coatings on TiNi
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shabalovskaya, S.; Anderegg, J.; Van Humbeeck, J. Critical overview of nitinol surfaces and their modifications for medical applications. Acta Biomater. 2008, 4, 447–467. [Google Scholar] [CrossRef] [PubMed]
- Wadood, A. Brief overview on nitinol as biomaterial. Adv. Mater. Sci. Eng. 2016, 4, 1–9. [Google Scholar] [CrossRef]
- Vasudevan, H.; Kottur, V.K.N.; Raina, A.A. Proceedings of International Conference on Intelligent Manufacturing and Automation; Springer Nature: Basingstoke, UK, 2020. [Google Scholar]
- Ivanov, V.E.; Kurilchik, A.A.; Ragulin, Y.A.; Zheravin, A.A.; Starodubtsev, A.L.; Zubarev, A.L. Multimodality therapy for osteosarcoma of the sternum with reconstruction of complex chest wal defects. Sib. J. Oncol. 2017, 16, 96–102. [Google Scholar] [CrossRef][Green Version]
- Rehman, A.U.; Babu, N.K.; Talari, M.K.; Usmani, Y.S.; Al-Khalefah, H. Microstructure and mechanical properties of dissimilar friction welding Ti-6Al-4V alloy to nitinol. Metals 2021, 11, 109. [Google Scholar] [CrossRef]
- Bartolomeu, F.; Costa, M.M.; Alves, N.; Miranda, G.; Silva, F.S. Additive manufacturing of NiTi-Ti6Al4V multi-material cellular structures targeting orthopedic implants. Opt. Lasers Eng. 2020, 134, 106208. [Google Scholar] [CrossRef]
- Basova, T.V.; Vikulova, E.S.; Dorovskikh, S.I.; Hassan, A.; Morozova, N.B. The use of noble metal coatings and nanoparticles for the modification of medical implant materials. Mater. Des. 2021, 204, 109672. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, K. Review on titanium and titanium based alloys as biomaterials for orthopaedic applications. Mater. Sci. Eng. C 2019, 102, 844–862. [Google Scholar] [CrossRef] [PubMed]
- Kwok, D.T.; Schulz, M.; Hu, T.; Chu, C.; Chu, P. Surface treatments of nearly equiatomic NiTi Alloy (nitinol) for surgical implants. Biomed. Eng. Trends Mater. Sci. 2011, 269, 282. [Google Scholar] [CrossRef]
- Lertsapcharoen, P.; Apichai, K.; Vidhavas, L.; Pentip, S.; Phingphol, C. Self-expanding nanoplatinum-coated nitinol devices for atrial septal defect and patent ductus arteriosus closure: A swine model. Indian Heart J. 2006, 58, 315–320. [Google Scholar] [PubMed]
- Lertsapcharoen, P.; Khongphatthanayothin, A.; Srimahachota, S.; Leelanukrom, R. Self-expanding platinum-coated nitinol devices for transcatheter closure of atrial septal defect: Prevention of nickel release. J. Invasive Cardiol. 2008, 20, 279–283. [Google Scholar] [PubMed]
- Lertsapcharoen, P.; Khongphatthanayothin, A.; La-Orkhun, V.; Vithessonthi, K.; Srimahachota, S. Transcatheter closure of patent ductus arteriosus with a self-expanding platinum-coated nitinol device. J. Invasive Cardiol. 2009, 21, 286–289. [Google Scholar]
- Köller, M.; Sengstock, C.; Motemani, Y.; Khare, C.; Buenconsejo, P.J.S.; Geukes, J.; Schildhauer, T.A.; Ludwig, A. Antibacterial activity of microstructured Ag/Au sacrificial anode thin films. Mater. Sci. Eng. C 2015, 46, 276–280. [Google Scholar] [CrossRef]
- Abuayyash, A.; Ziegler, N.; Gessmann, J.; Sengstock, C.; Schildhauer, T.A.; Ludwig, A.; Köller, M. Antibacterial efficacy of sacrifical anode thin films combining silver with platinum group elements within a bacteria-containing human plasma clot. Adv. Eng. Mater. 2017, 20, 1–5. [Google Scholar] [CrossRef]
- Schmidt-Braekling, T.; Streitbuerger, A.; Gosheger, G.; Boettner, F.; Nottrott, M.; Ahrens, H.; Dieckmann, R.; Guder, W.; Andreou, D.; Hauschild, G.; et al. Silver-coated megaprostheses: Review of the literature. Eur. J. Orthop. Surg. Traumatol. 2017, 27, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Steegmueller, R.; Wagner, C.; Fleckenstein, T.; Schuessler, A. Gold coating of nitinol devices for medical applications. Mater. Sci. Forum 2002, 394, 161–164. [Google Scholar] [CrossRef]
- Tripi, T.R.; Bonaccorso, A.; Condorelli, G.G. Fabrication of hard coatings on NiTi instruments. J. Endod. 2003, 29, 132–134. [Google Scholar] [CrossRef]
- Li, J.; Wang, G.; Geng, H.; Zhu, H.; Zhang, M.; Di, Z.; Liu, X.; Chu, P.K.; Wang, X. CVD growth of graphene on NiTi alloy for enhanced biological activity. ACS Appl. Mater. Interfaces 2015, 7, 19876–19881. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, Y.; Chen, X.; Leng, B.; Guo, X.; Zhang, T. ALD mediated heparin grafting on nitinol for self-expanded carotid stents. Colloids Surf. B Biointerfaces 2016, 143, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Vokoun, D.; Klimša, L.; Vetushka, A.; Duchoň, J.; Racek, J.; Drahokoupil, J.; Kopeček, J.; Yu, Y.S.; Koothan, N.; Kei, C.C. Al2O3 and Pt atomic layer deposition for surface modification of NiTi shape memory films. Coatings 2020, 10, 746. [Google Scholar] [CrossRef]
- Liu, Y.; Rath, B.; Tingart, M.; Eschweiler, J. Role of implants surface modification in osseointegration: A systematic review. J. Biomed. Mater. Res. Part A 2020, 108, 470–484. [Google Scholar] [CrossRef] [PubMed]
- Novaes, A.B.; de Souza, S.L.S.; de Barros, R.R.M.; Pereira, K.K.Y.; Iezzi, G.; Piattelli, A. Influence of implant surfaces on osseointegration. Braz. Dent. J. 2010, 21, 471–481. [Google Scholar] [CrossRef]
- Lozanov, V.V.; Il’in, I.Y.; Morozova, N.B.; Trubin, S.V.; Baklanova, N.I. Chemical vapor deposition of an iridium phase on hafnium carbide and tantalum carbide. Russ. J. Inorg. Chem. 2020, 65, 1781–1788. [Google Scholar] [CrossRef]
- Vikulova, E.S.; Karakovskaya, K.I.; Ilyin, I.Y.; Zelenina, L.N.; Sysoev, S.V.; Morozova, N.B. Thermodynamic study of volatile iridium (I) complexes with 1,5-cyclooctadiene and acetylacetonato derivatives: Effect of (O,O) and (O,N) coordination sites. J. Chem. Thermodyn. 2019, 133, 194–201. [Google Scholar] [CrossRef]
- Basova, T.V.; Hassan, A.; Morozova, N.B. Chemistry of gold(I, III) complexes with organic ligands as potential MOCVD precursors for fabrication of thin metallic films and nanoparticles. Coord. Chem. Rev. 2019, 380, 58–82. [Google Scholar] [CrossRef]
- Dorovskikh, S.I.; Klyamer, D.D.; Koretskaya, T.P.; Kal′nyi, D.B.; Morozova, N.B. Studying the microstructure of pt layers prepared by chemical vapor deposition in the presence of hydrogen. J. Struct. Chem. 2020, 61, 1211–1218. [Google Scholar] [CrossRef]
- Krisyuk, V.V.; kyzy, S.U.; Rybalova, T.V.; Korolkov, I.V.; Sysoev, S.V.; Koretskaya, T.P.; Kuchumov, B.M.; Turgambaeva, A.E. Volatile trinuclear heterometallic beta-diketonates: Structure and thermal properties related to the chemical vapor deposition of composite thin films. Polyhedron 2020, 191, 114806. [Google Scholar] [CrossRef]
- Gunther, V.; Marchenko, E.; Chekalkin, T.; Baigonakova, G.; Kang, J.H.; Kim, J.S.; Klopotov, A. Study of structural phase transitions in quinary TiNi(MoFeAg)-based alloys. Mater. Res. Express 2017, 4, 105702. [Google Scholar] [CrossRef]
- Powder Diffraction File; release 2010; International Centre for Diffraction Data: Newtown Square, PA, USA, 2010.
- Kraus, W.; Nolze, G. PowderCell—A program for the representation and manipulation of crystal structures and calculation of the resulting X-ray powder patterns. J. Appl. Crystallogr. 1996, 29, 301–303. [Google Scholar] [CrossRef]
- Pfeifer, V.; Jones, T.E.; Velasco Vélez, J.J.; Massué, C.; Arrigo, R.; Teschner, D.; Girgsdies, F.; Scherzer, M.; Greiner, M.T.; Allan, J.; et al. The electronic structure of iridium and its oxides. Surf. Interface Anal. 2016, 48, 261–273. [Google Scholar] [CrossRef]
- Grigor'eva, E.V.; Malankhanova, T.B.; Surumbayeva, A.; Pavlova, S.V.; Minina, J.M.; Kizilova, E.A.; Suldina, L.A.; Morozova, K.N.; Kiseleva, E.; Sorokoumov, E.D.; et al. Generation of GABAergic striatal neurons by a novel iPSC differentiation protocol enabling scalability and cryopreservation of progenitor cells. Cytotechnology 2020, 72, 649–663. [Google Scholar] [CrossRef]
- Zhang, B.; Hou, Y.; Wang, X.; Wang, Y.; Geng, L. Mechanical properties, degradation performance and cytotoxicity of Mg-Zn-Ca biomedical alloys with different compositions. Mater. Sci. Eng. C 2011, 31, 1667–1673. [Google Scholar] [CrossRef]
- Medvedev, N.S.; Shaverina, A.V.; Tsygankova, A.R.; Saprykin, A.I. Analysis of high-purity germanium dioxide by ETV-ICP-AES with preliminary concentration of trace elements. Talanta 2016, 155, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.R.V.; Goto, T. Chemical vapor deposition of iridium, platinum, rhodium and palladium. Mater. Trans. 2003, 44, 1717–1728. [Google Scholar] [CrossRef]
- Vasilyev, V.Y.; Morozova, N.B.; Basova, T.V.; Igumenov, I.K.; Hassan, A. Chemical vapour deposition of Ir-based coatings: Chemistry, processes and applications. RSC Adv. 2015, 5, 32034–32063. [Google Scholar] [CrossRef]
- Yang, S.; Yu, X.; Tan, C.; Wang, Y.; Ma, H.; Liu, K.; Cai, H. Growth kinetics and microstructure of MOCVD iridium coating from iridium(III) acetylacetonate with hydrogen. Appl. Surf. Sci. 2015, 329, 248–255. [Google Scholar] [CrossRef]
- Morozova, N.B.; Gelfond, N.V.; Semyannikov, P.P.; Trubin, S.V.; Igumenov, I.K.; Gimeno-Fabra, L. Thermal properties of Ir(I) precupsors: Acetylacetonato(1,5-cyclooctadiene)iridium(I) and (methylcyclopentadienyl)(1,5-cyclooctadiene)itidium (I). Electrochem. Soc. Proc. 2003, 8, 120–125. [Google Scholar]
- Kawano, K.; Takamori, M.; Yamakawa, T.; Watari, S.; Fujisawa, H.; Shimizu, M.; Niu, H.; Oshima, N. A novel iridium precursor for MOCVD. Mater. Res. Soc. Symp. Proc. 2003, 784, 145–150. [Google Scholar] [CrossRef]
- Karakovskaya, K.I.; Dorovskikh, S.I.; Vikulova, E.S.; Ilyin, I.Y.; Zherikova, K.V.; Basova, T.V.; Morozova, N.B. Volatile iridium and platinum MOCVD precursors: Chemistry, thermal properties, materials and prospects for their application in medicine. Coatings 2021, 11, 78. [Google Scholar] [CrossRef]
- Papke, J.A.; Stevenson, R.D. Evaluation of metal-organic compounds as materials for chemical vapor deposition. In Proceedings of the Conference on Chemical Vapor Deposition of Refractory Metals, Alloys, and Compounds, Gatlinburg, TN, USA, 12–14 September 1967. [Google Scholar]
- Vanýsek, P. Handbook of Chemistry and Physics, 81th ed.; Electrochemical Series; CRC Press LLC: Boston, MA, USA, 2000. [Google Scholar]
- Chen, R.S.; Chang, H.M.; Huang, Y.S.; Tsai, D.S.; Chiu, K.C. Morphological evolution of the self-assembled IrO2 one-dimensional nanocrystals. Nanotechnology 2005, 16, 93–97. [Google Scholar] [CrossRef]
- Marchenko, E.; Baigonakova, G.; Yasenchuk, Y. Gradient crystalline coating on a biomedical TiNi alloy prepared by magnetron sputtering and annealing. Vacuum 2020, 181, 109652. [Google Scholar] [CrossRef]
- Marchenko, E.S.; Baigonakova, G.A.; Dubovikov, K.M.; Yasenchuk, Y.F.; Gunther, S.V. Reaction synthesis of gradient coatings by annealing of three-layer Ti–Ni–Ti nanolaminate magnetron sputtered on the TiNi substrate. Surf. Interfaces 2021, 24, 101111. [Google Scholar] [CrossRef]
- Parkhomenko, R.G.; Morozova, N.B.; Zharkova, G.I.; Shubin, Y.V.; Trubin, S.V.; Kriventsov, V.V.; Kuchumov, B.M.; Koretskaya, T.P.; Igumenov, I.K. Deposition of au thin films and nanoparticles by MOCVD. Chem. Vap. Depos. 2012, 18, 336–342. [Google Scholar] [CrossRef]
- Holt, L.; Stanyer, J. Carbon inclusion in gold electroplate. Trans. IMF 1972, 50, 24–27. [Google Scholar] [CrossRef]
- Okinaka, Y. Significance of inclusions in electroplated gold films for electronics applications. Gold Bull. 2000, 33, 117–127. [Google Scholar] [CrossRef]
- Lim, Y.H.; Yoo, H.; Choi, B.H.; Lee, J.H.; Lee, H.-N.; Lee, H.K. Atomic-layer-deposited Ir thin film as a novel diffusion barrier layer in Cu interconnection. Phys. Status Solidi C 2011, 8, 891–894. [Google Scholar] [CrossRef]
- Camarasa, M.V.; Kerr, R.W.; Sneddon, S.F.; Bates, N.; Shaw, L.; Oldershaw, R.A.; Small, F.; Baxter, M.A.; McKay, T.R.; Brison, D.R.; et al. Derivation of Man-1 and Man-2 research grade human embryonic stem cell lines. Vitr. Cell. Dev. Biol. Anim. 2010, 46, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Han, M.J.; Kim, B.Y.; Yoon, S.O.; Chung, A.S. Cell proliferation induced by reactive oxygen species is mediated via mitogen-activated protein kinase in Chinese hamster lung fibroblast (V79) cells. Mol. Cells 2003, 15, 94–101. [Google Scholar] [PubMed]
- Saxena, S.; Vekaria, H.; Sullivan, P.G.; Seifert, A.W. Connective tissue fibroblasts from highly regenerative mammals are refractory to ROS-induced cellular senescence. Nat. Commun. 2019, 10, 4400. [Google Scholar] [CrossRef] [PubMed]
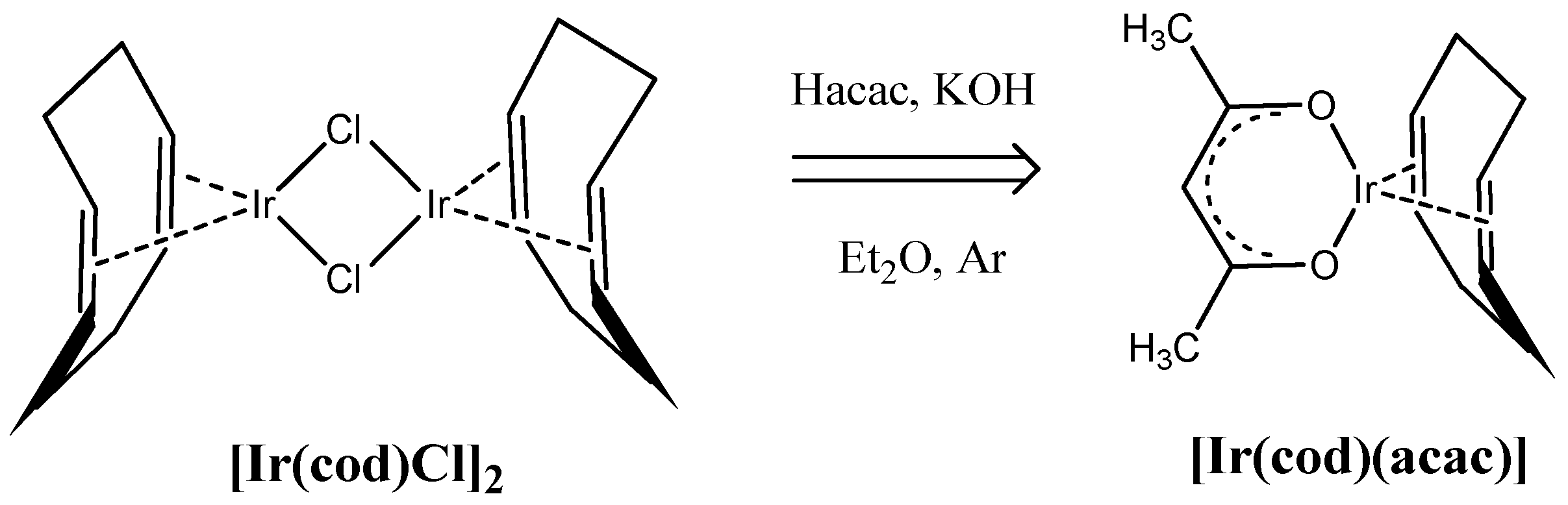
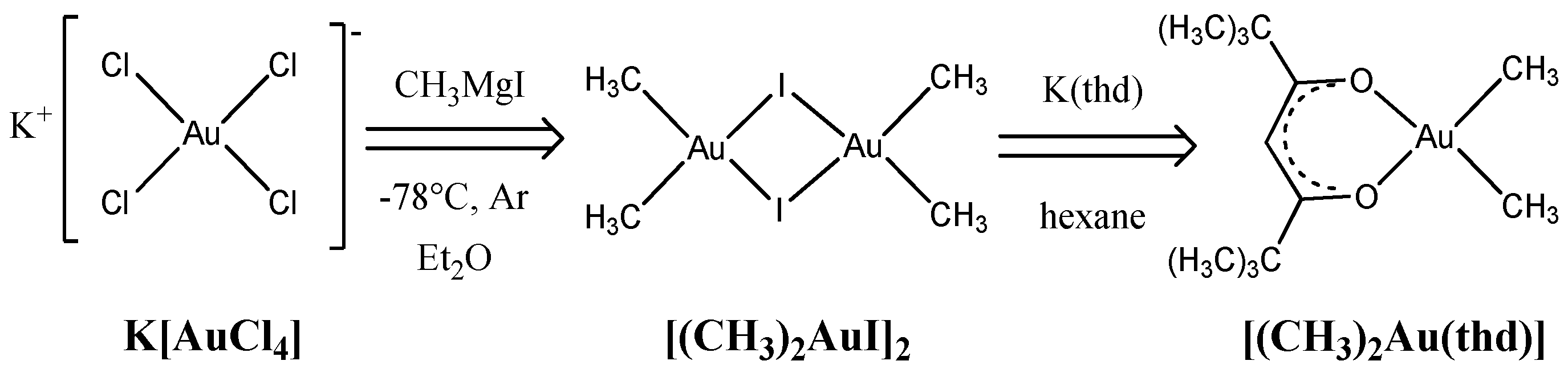
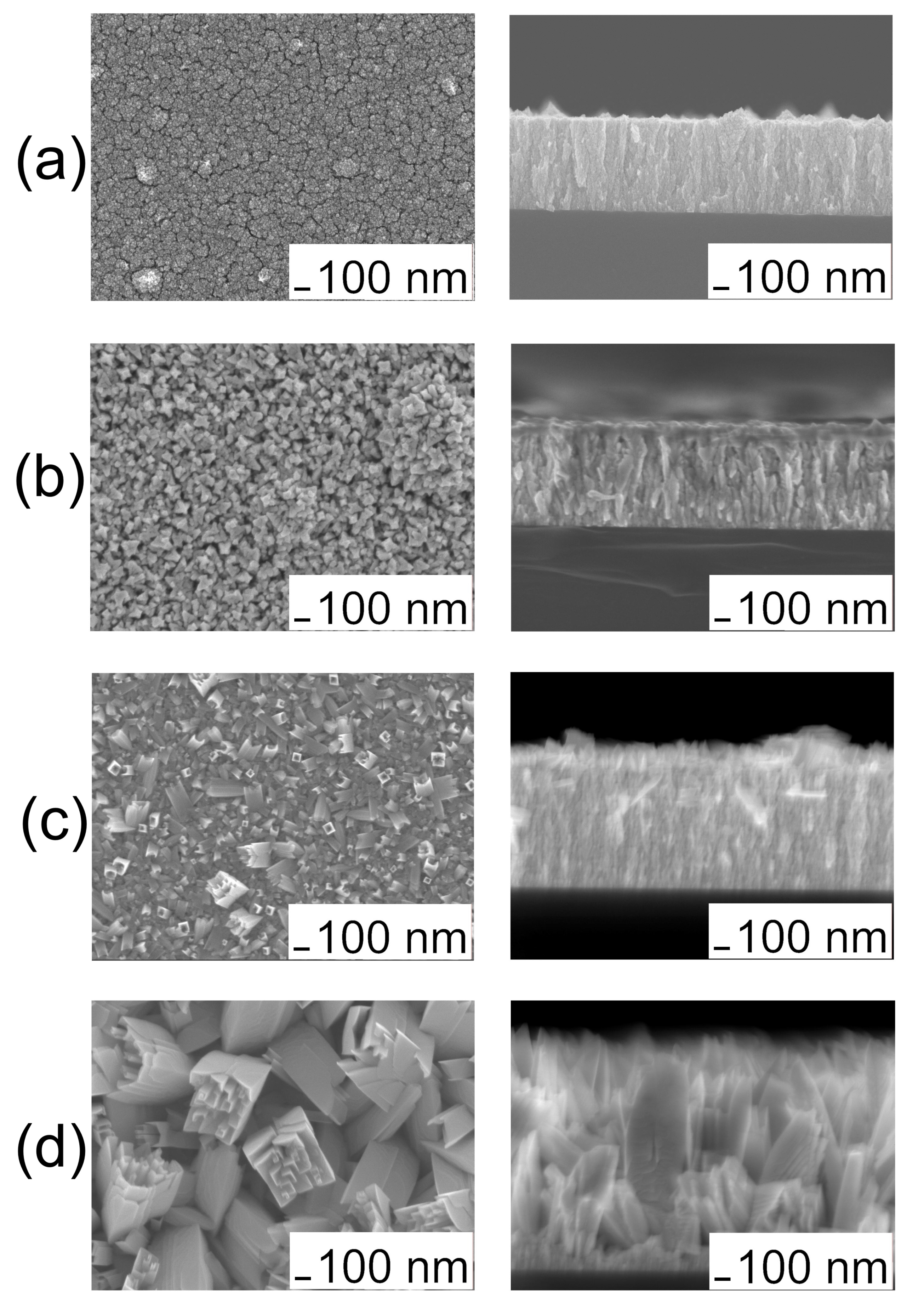
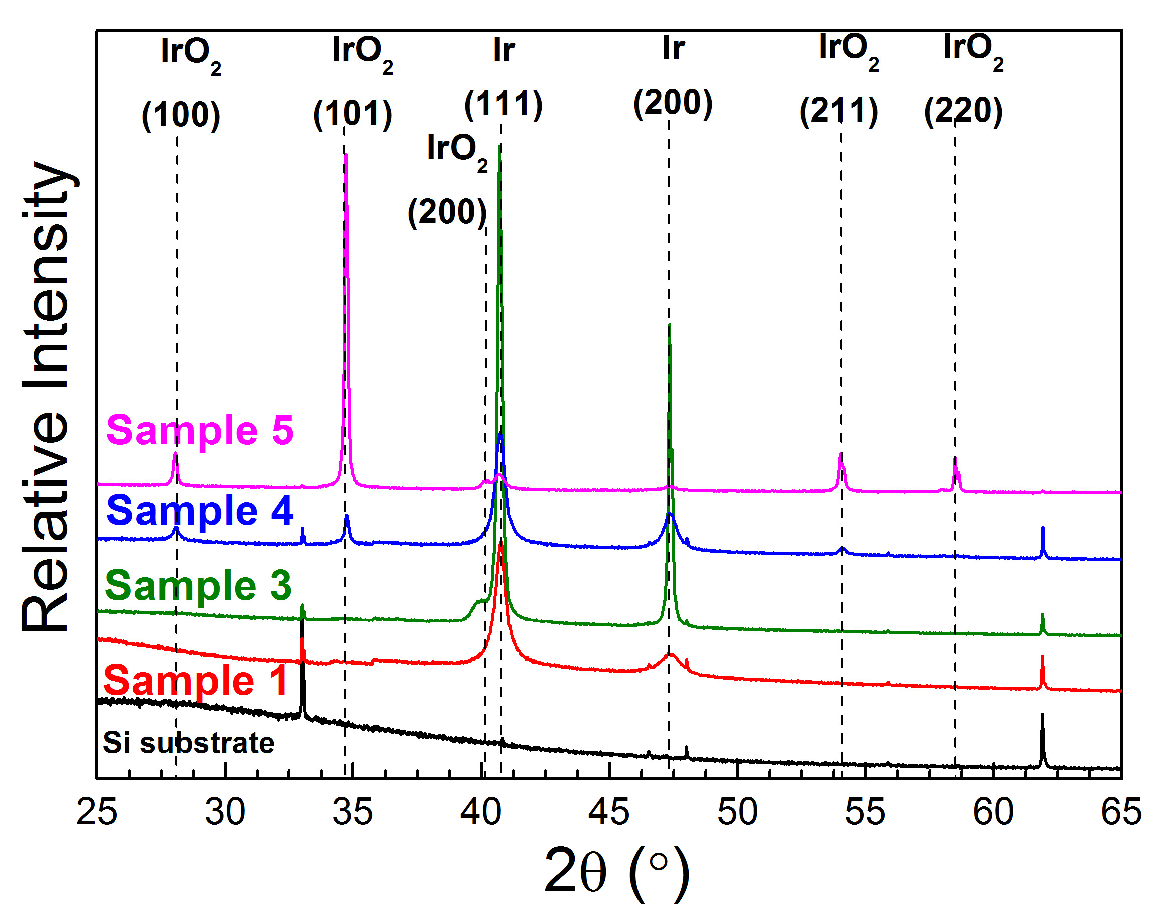
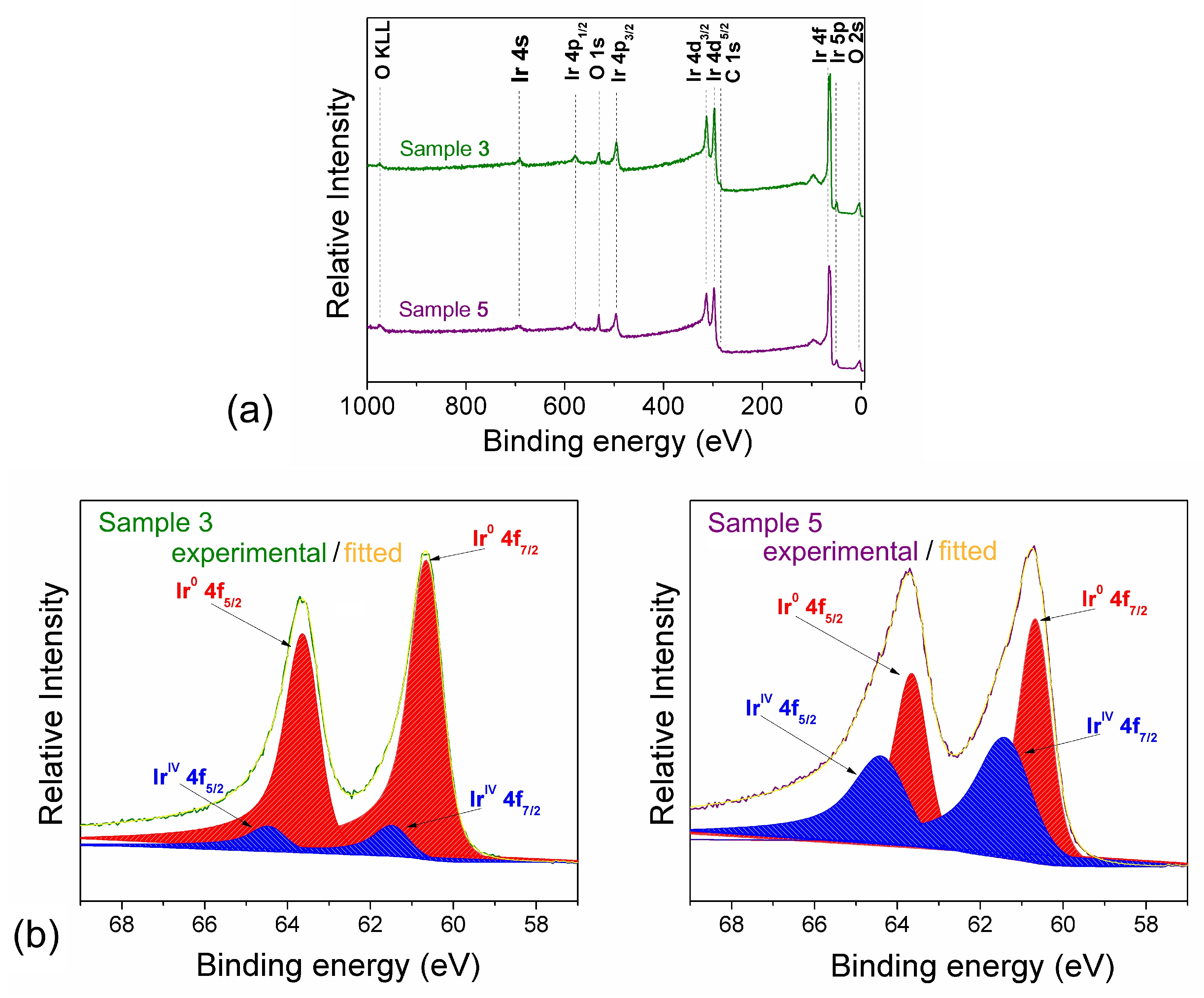
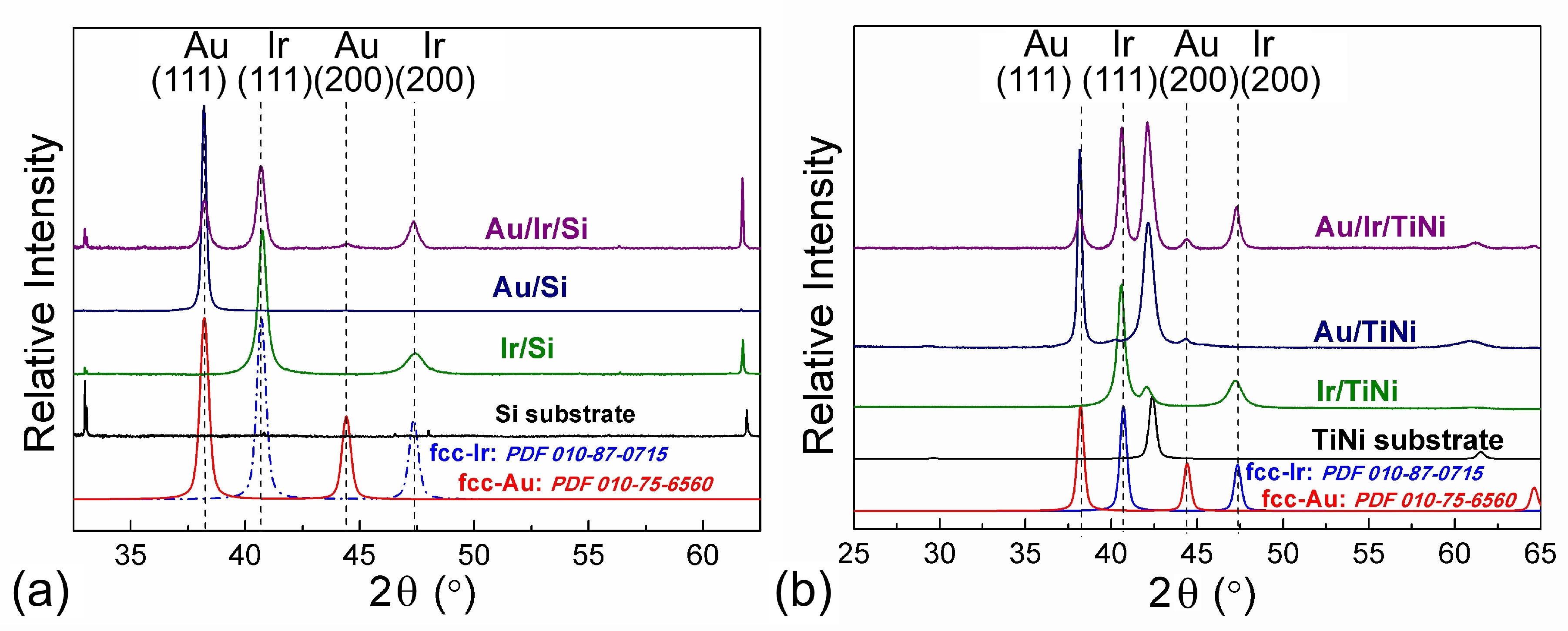
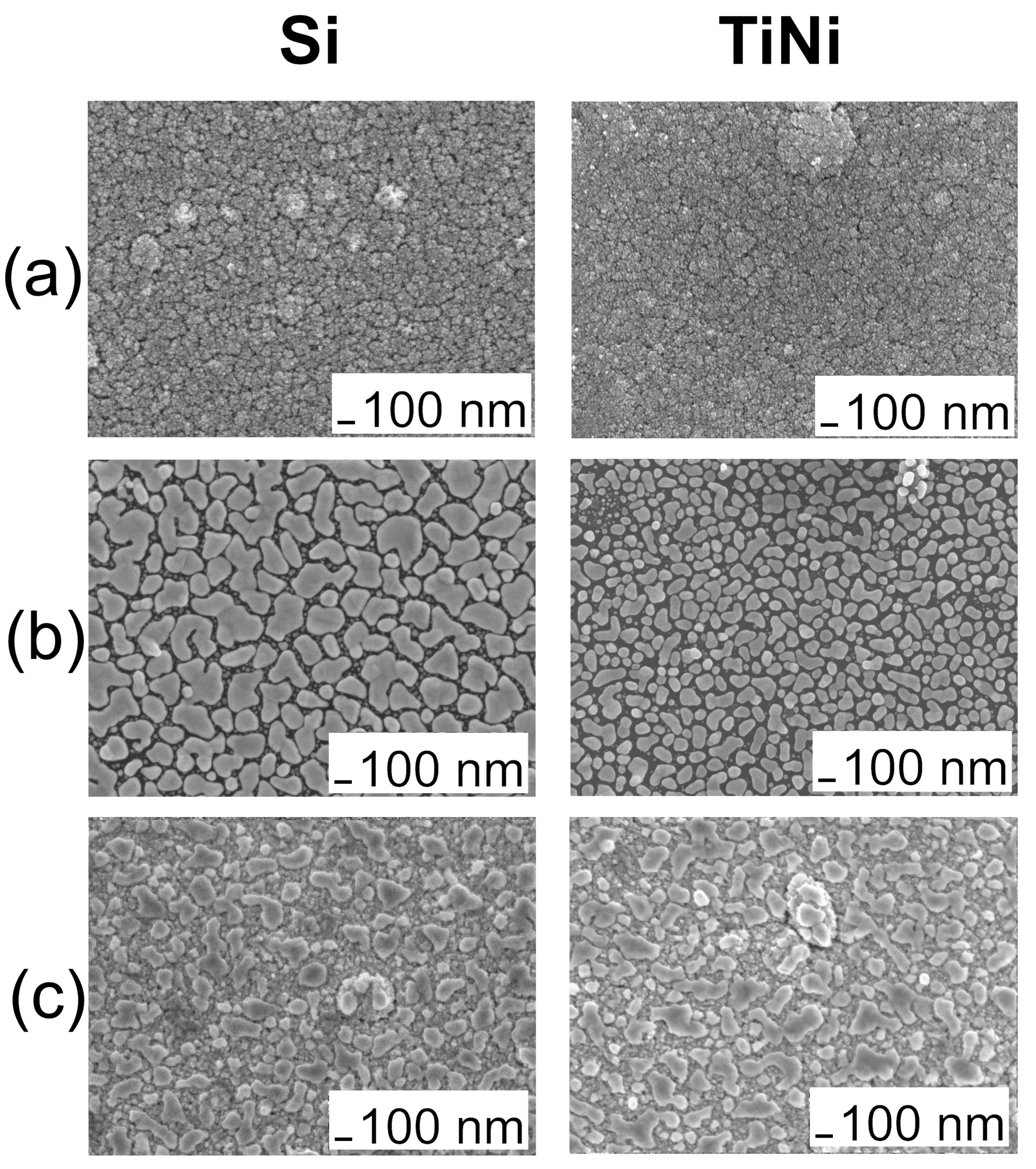
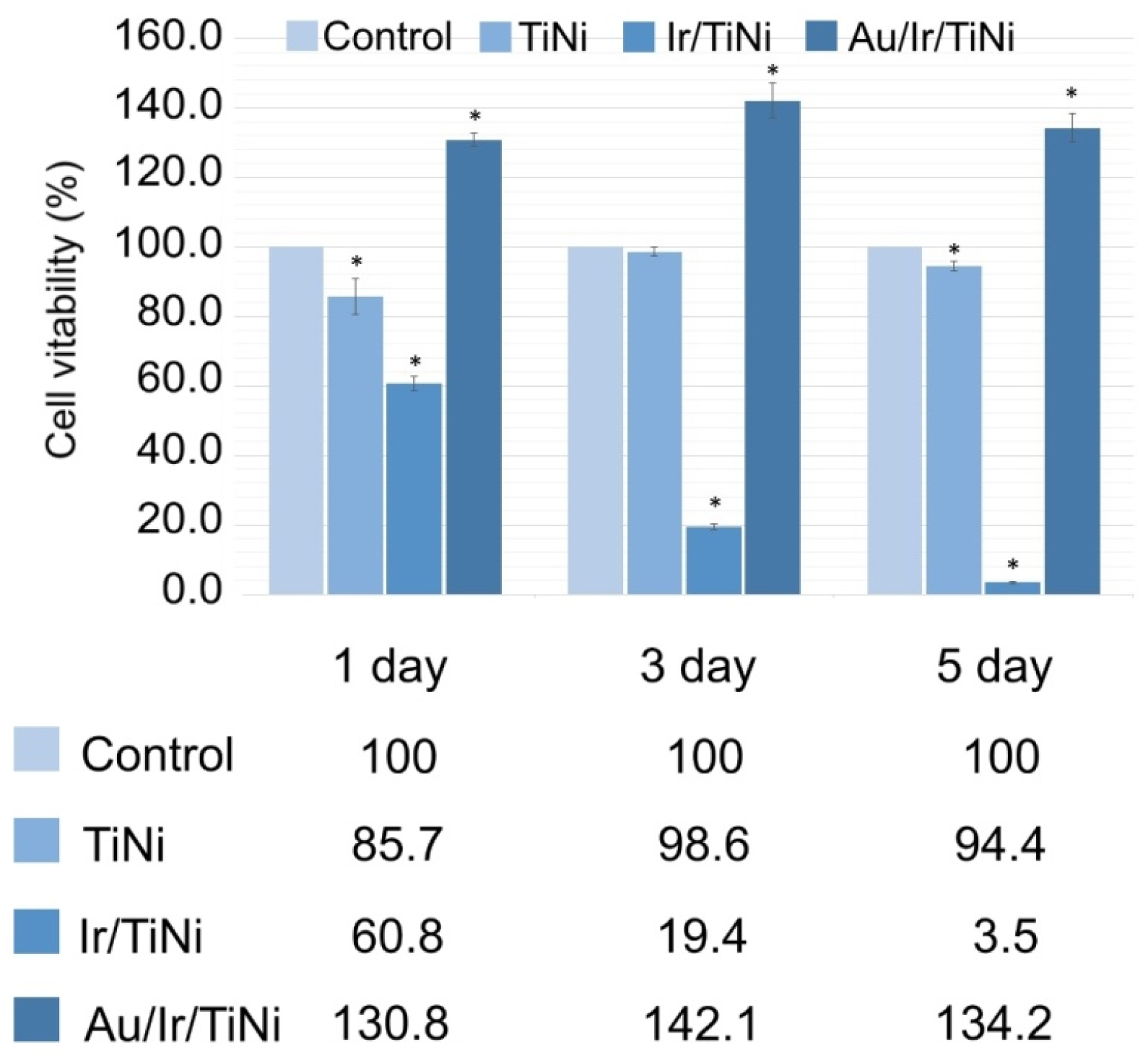

| No. | Tdep., (°C) | v(O2), (mL/min) | Growth Rate, (nm/min) | Composition | Ir Unit Cell Parameter, (nm) | Ir Average Crystallite Size (CSR), (nm) | |
|---|---|---|---|---|---|---|---|
| (111) | (200) | ||||||
| 1 | 290 | 33 | 6.0 | Ir | 3.849(2) | 20(2) | 9(1) |
| 2 | 310 | 33 | 8.5 | Ir | 3.837(2) | 23(2) | 10(1) |
| 3 | 350 | 33 | 12.7 | Ir 1 | 3.839(2) | 73(7) | 84(9) |
| 4 | 310 | 133 | 11.7 | Ir + IrO2 | 3.840(2) | 22(2) | 11(1) |
| 5 | 330 | 67 | 20.4 | IrO2 + Ir | 3.841(2) | 26(3) | 19(2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vikulova, E.S.; Karakovskaya, K.I.; Koretskaya, T.P.; Korolkov, I.V.; Chepeleva, E.V.; Asanov, I.P.; Tsygankova, A.R.; Maksimovskii, E.A.; Marchenko, E.S.; Lantsukhay, Y.A.; et al. MOCVD of Noble Metal Film Materials for Medical Implants: Microstructure and Biocompatibility of Ir and Au/Ir Coatings on TiNi. Coatings 2021, 11, 638. https://doi.org/10.3390/coatings11060638
Vikulova ES, Karakovskaya KI, Koretskaya TP, Korolkov IV, Chepeleva EV, Asanov IP, Tsygankova AR, Maksimovskii EA, Marchenko ES, Lantsukhay YA, et al. MOCVD of Noble Metal Film Materials for Medical Implants: Microstructure and Biocompatibility of Ir and Au/Ir Coatings on TiNi. Coatings. 2021; 11(6):638. https://doi.org/10.3390/coatings11060638
Chicago/Turabian StyleVikulova, Evgeniia S., Ksenya I. Karakovskaya, Tatyana P. Koretskaya, Ilya V. Korolkov, Elena V. Chepeleva, Igor P. Asanov, Alphiya R. Tsygankova, Eugene A. Maksimovskii, Ekaterina S. Marchenko, Yuriy A. Lantsukhay, and et al. 2021. "MOCVD of Noble Metal Film Materials for Medical Implants: Microstructure and Biocompatibility of Ir and Au/Ir Coatings on TiNi" Coatings 11, no. 6: 638. https://doi.org/10.3390/coatings11060638
APA StyleVikulova, E. S., Karakovskaya, K. I., Koretskaya, T. P., Korolkov, I. V., Chepeleva, E. V., Asanov, I. P., Tsygankova, A. R., Maksimovskii, E. A., Marchenko, E. S., Lantsukhay, Y. A., Zheravin, A. A., & Morozova, N. B. (2021). MOCVD of Noble Metal Film Materials for Medical Implants: Microstructure and Biocompatibility of Ir and Au/Ir Coatings on TiNi. Coatings, 11(6), 638. https://doi.org/10.3390/coatings11060638









