Abstract
The recent development of several methods for extracting curcumin from the root of the plant Curcuma longa has led to intensified research on the properties of curcumin and its fields of application. Following the studies and the accreditation of curcumin as a natural compound with antifungal, antiviral, and antibacterial properties, new fields of application have been developed in two main directions—food and medical, respectively. This review paper aims to synthesize the fields of application of curcumin as an additive for the prevention of spoilage, safety, and quality of food. Simultaneously, it aims to present curcumin as an additive in products for the prevention of bacterial infections and health care. In both cases, the types of curcumin formulations in the form of (nano)emulsions, (nano)particles, or (nano)composites are presented, depending on the field and conditions of exploitation or their properties to be used. The diversity of composite materials that can be designed, depending on the purpose of use, leaves open the field of research on the conditioning of curcumin. Various biomaterials active from the antibacterial and antibiofilm point of view can be intuited in which curcumin acts as an additive that potentiates the activities of other compounds or has a synergistic activity with them.
1. Introduction
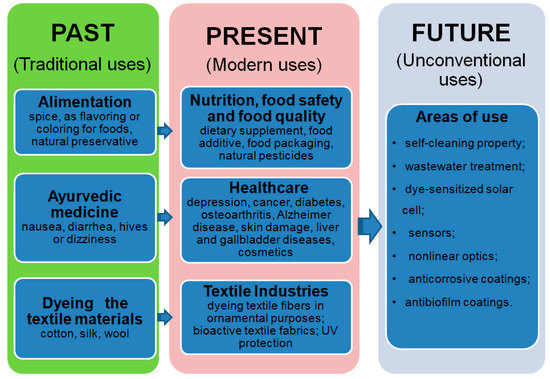
Curcumin has been known since antiquity and used as a spice, preservative, dye for dyeing fabrics, and in traditional medicine. It is one of the three main colored compounds (demethoxy and bisdemethoxy derivatives) which are extracted from Curcuma longa plant roots grown in Asian countries. It is found in the Color Index with the names: Yellow 3 (natural compound) and Gelb 6 (synthetic), it is also known as: turmeric, curcumin, Indian saffron, and saffron of Indians, with the reference number C.A.S. number: 458-37-7 [1].
Considering the epidemiological events caused by the appearance of the COVID-19 virus, in the last two years, there has been an intensification of studies on the methods to obtain [2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20] curcumin and its bioactivity properties [21,22,23,24,25,26,27,28,29,30]. If, in 2017, the global curcumin market was valued at USD 52.45 million, due to the diversification of application areas, it is estimated that the size of the global curcumin market will reach USD 151.9 million by 2027. The estimates were made after an evaluation of the increasing number of articles published (Figure 1) on the antioxidant, anti-inflammatory, or anticancer properties, increasing the availability and accessibility of Ayurvedic medicinal products, cosmetics, food supplements, and the natural additives consumer market in developed countries. In this regard, in North America, which is the largest market followed by Europe, food, nutritional supplements, and cosmetics with the main ingredient as curcumin had the biggest sales in 2019, while India is the most important producer of curcumin extracted from turmeric and represents more than 78% of its global production [31,32,33,34]. However, the forecasts made have a high degree of uncertainty due to operational problems regarding the supply of raw materials [35,36,37] and the distribution of finished products on the consumer market, affected by the COVID-19 pandemic [38,39,40]. Curcumin is used extensively as a phytochemical in studies of various diseases [28,41] and, in particular, in cancer treatments [6] targeting its antioxidant [42,43] and anti-inflammatory properties [43]. The current revision is made to highlight the new fields of application regarding the exploitation of antiviral, antifungal, and antibacterial properties. We will discuss methods of conditioning curcumin to increase its solubility in water and the influence or synergistic action of the compounds found in delivery systems. This study updates the conditioning methods for curcumin to improve the photostability and solubility properties, which are important in medical and food applications. In addition, the advantages and the need to use natural compounds in areas related to the protection of, and contribution to, human health will be discussed.
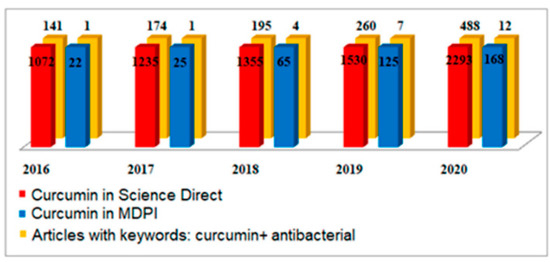
Figure 1.
Total number of articles on “curcumin”, published between 2016 and 2020, in different databases.
3. Additive for the Prevention of Spoilage, Safety and Quality of Food
Curcumin (E100) is a food coloring, yellow-orange, with a hot and bitter taste, approved by the FAO (Food and Agriculture Organization). Restrictions imposed on the products used in food coloring or as a color additives refer to the content of arsenic (calculated as free arsenic) max. 5 mg/kg and lead (calculated as free lead) max. 20 mg/kg. The rhizome extract of the plant, curcumin powder, has over time been used in Southeast Asia in food preservation [1,4]. Recently, the recognition of curcumin as belonging to the category of antifungal and antibacterial agents of natural origin has led to the diversification, on the one hand, in the way of conditioning curcumin [121,122,123,124,125] and, on the other hand, to new sectors of use, for example, as a sensor/pH indicator [126,127,128] in the food field or in packaging [129,130,131,132,133,134,135] (Figure 5).
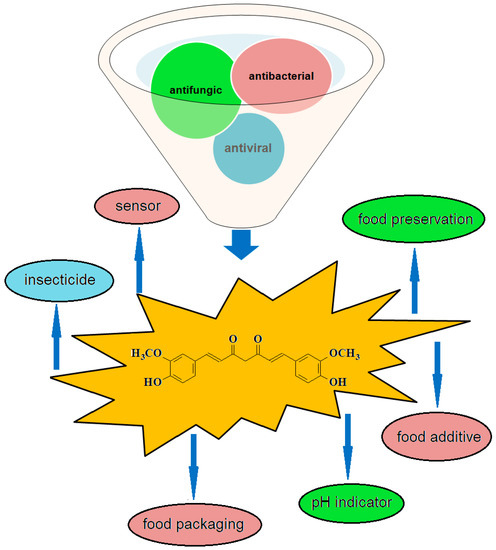
Figure 5.
Applications of curcumin in food, due to its antiviral, antibacterial, and antifungal properties.
Chuacharoen and Sabliov [121] conducted a comparative study of several models of curcumin delivery. In this regard, the encapsulation systems were made in the form of nanosuspensions, zein-based nanoparticles, and nanoemulsions, keeping the same initial amount of curcumin and surfactant concentration for all systems. After evaluating in in vitro systems, it was found that stability depends on certain factors, of which temperature affected the nanoemulsions the most, but the highest antioxidant effect was in curcumin encapsulated in zein. The results of the study showed the advantages and disadvantages of each type of encapsulation, to ease the process of choosing the delivery system of curcumin, depending on the specific application in the food industry (Figure 6).
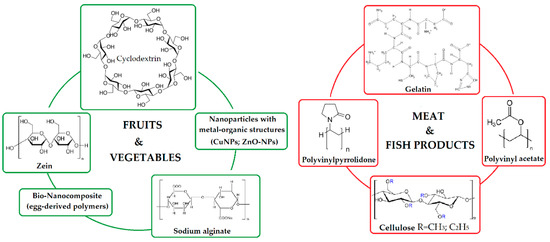
Figure 6.
Types of polymers for the conditioning of curcumin used as an additive in the food industry.
The antifungal properties [42,57] and insecticidal effects [58,123] are capitalized on by using curcumin in the form of natural extracts [42,57], nanocomposites [63,125], or deposited on nanoparticles with metal-organic structures [45,110] to combat a series of pathogens in the process of preserving and preventing fruit and vegetable rot [42,57,62,63] degradation.
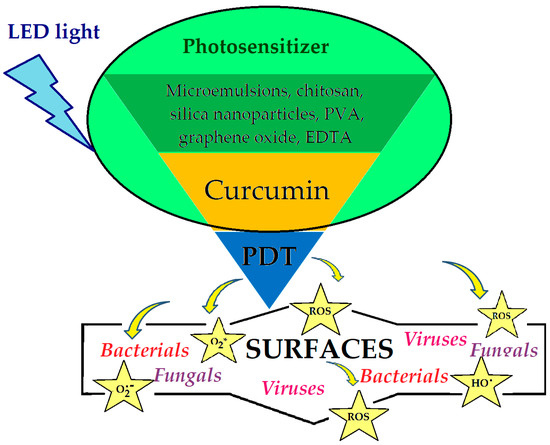
Marchi et al. [54] tested the aqueous curcumin extract on inhibiting the growth of the fungal biomass of some types of fungi, Penicillium paneum, Cladosporiumo xysporum, Cladosporium subliforme, and Aspergillus chevalieri, isolated from bread. The results showed that, due to the antifungal activity, curcumin can be used as a food additive, leading to the extension of the shelf life of food [59]. At the same time, curcumin encapsulated in β-cyclodextrin [122] is used in the cheese-making process or encapsulated in sodium alginate [124] for the edible membranes of matured cheeses, without modifying their initial characteristics. For the preservation and extension of the shelf life of meat and fish products, packaging films are used based on gelatin [125], carboxymethylcellulose [126], or vinyl acetate [107], with curcumin as an antimicrobial agent. Curcumin embedded in polyvinyl acetate films has been used to obtain thin coatings with antimicrobial photodynamic activity under white light irradiation. Embedding curcumin in polymer matrices prevents the isomerization processes, increasing the stability and fluorescence lifetime of curcumin. Photodynamic therapy has shown great efficiency, both in vitro and in vivo, in the process of inactivating planktonic cells. This method can be used as a technique in the control of pathogenic microorganisms and biofilm formation, aiming at decreasing the safety risk of food products [42,102,107,127]. The tendencies to increase durability and improve food safety, as well as to reduce food waste, have resulted in the development of different types of smart packaging. These can monitor the quality of packaged products through data carriers, indicators, or built-in sensors [128,129,130].
Therefore, another property of curcumin, which has found application, is the change in color from yellow to red, as the pH changes from acidic to basic medium. Curcumin has been used as a colorimetric indicator for food packaging [128,131] to detect alkaline compounds produced during food spoilage. Non-woven materials with curcumin made through the electrospinning process have been use for the detection of amines, the main degradation compounds of fish and fishery products. Non-woven materials [128] of polyvinylpyrrolidone (PVP) or ethylcellulose/poly (ethylene oxide) with incorporated curcumin have been exposed to various types of volatile amines. The results showed that, although the loading efficiency with curcumin was lower in the PVP fibers, the detection and quantification limits were higher than in the cellulosic fibers. Intelligent packaging with data carriers (barcode, QR-code) are widespread due to the low cost of production, while packaging with indicators or sensors are uncommon due to the high costs of design and production processes [129,130].
However, to compensate for these disadvantages, active packaging [131,132,133,134,135] has been developed, based on materials with antioxidant [83,132,133], antimicrobial [83,132,133,135], or anti-UV [132,135] properties which protect the packaged products from perishable factors and extend their expiration date. To obtain these packages (Figure 7), cellulose materials were most often used in the form of nanofibers or films, in the composition of which curcumin was integrated (bacterial cellulose-curcumin [131]; carboxymethylcellulose-ZnO-curcumin [133]) or in encapsulated form (cellulose bacterial-protein nanoparticles zein/curcumin [134]; cellulose nanofibers-chitosan-curcumin [135]). The treatment of composite materials with curcumin led to the modification of their physico-chemical structures by improving the elongation properties at break, thermal stability, depending on the concentration and type of curcumin delivery system. At the same time, the antibacterial and antioxidant properties of biocomposites have been improved without changing the properties of the water vapor barrier, which is an important factor for controlling humidity in obtaining an unfavorable environment for the growth and development of mold, yeast, and bacteria. The films based on different polyvinyl polymers in which curcumin was incorporated showed a slight decrease in the properties of the water vapor barrier, but without significantly affecting the other properties. These films have been used to obtain coatings that show antimicrobial photodynamic activity [107,132]. Depending on the concentration of curcumin and temperature, an antibacterial efficiency of 93% was obtained against S. aureus and Salmonella typhimurium (S. typhimurium) [119]. From the point of view of the natural additives used in the food industry, curcumin is the ideal example due to its antioxidant, antifungal, antiviral, and antibacterial properties. However, its disadvantage, as with most phytochemicals, is its sensitivity to photodegradation and its low solubility in aqueous media. To improve these shortcomings, research is continuing to find curcumin delivery systems that are non-toxic and compatible with the use environments.
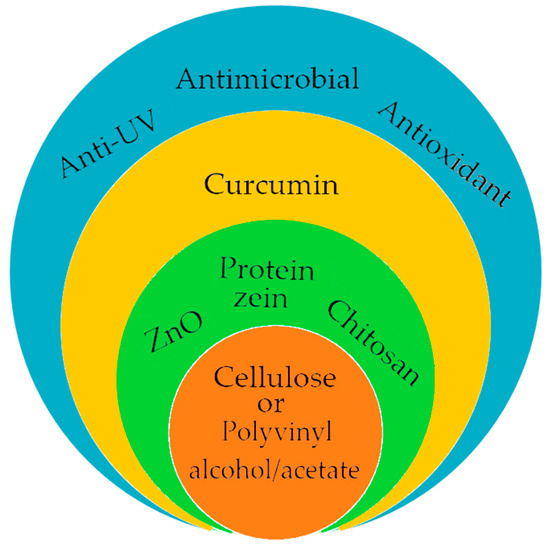
Figure 7.
The main ingredients of active packaging.
4. Additive for Health Care Products
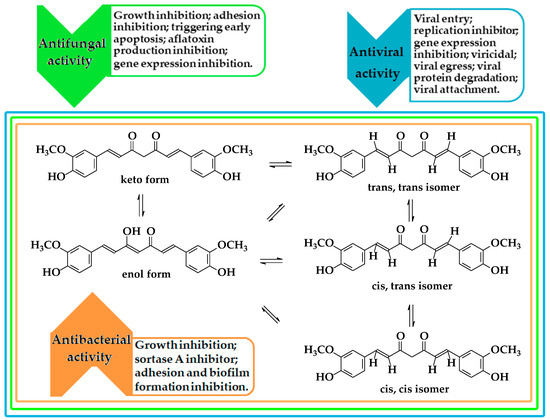
Curcumin is known in traditional medicine, especially in India, for use in treating fever, skin infections, and to facilitate digestion due to its anti-inflammatory [6,7,8], antiviral [26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50], and antibacterial [63,117] properties. In the last two years, because of the COVID-19 pandemic, the congestion of hospitals has led to an increased level of nosocomial infections [136,137,138,139]. This situation has triggered new approaches regarding the dispersion of biofilm and in finding new ways to prevent the initial formation by modifying the surfaces [64,65] (Figure 8). Therefore, a nanocomposite based on metal oxides [82] or chitosan nanoparticles [127] containing curcumin have been tested. The results of the studies showed that curcumin deposited on copper oxide nanoparticles had higher antimicrobial activity against Gram-positive bacteria compared to Gram-negative bacteria, whereas in vitro tests of curcumin-releasing chitosan nanocomposites showed high activity against mono and polymicrobial biofilms of C. albicans and S. aureus, which can proliferate on medical silicone surfaces. Curcumin loaded on polyvinyl materials [91] or silicone rubber [93,95] used in catheters or cosmetic implants showed high antibiofilm activity. The results of tests performed on S. aureus strains showed antibiofilm activity of 99% at a concentration of 500 μg/mL of curcumin nanoparticles. The action of limiting bacterial colonization is achieved by the process of complete lysis of the bacterial cells, which has more effective antibiofilm activity than the drug nystatin and has effects comparable to those of chloramphenicol [116].
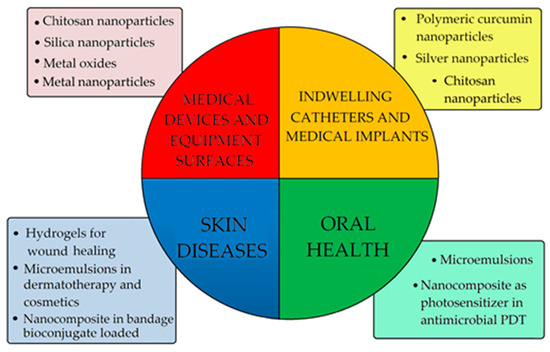
Figure 8.
The main fields of use of curcumin in the medical field and its types of formulations in applications.
Recently, research on the treatment and prevention of dental diseases has made significant progress, especially in treatments involving photodynamic therapy [105]. In this regard, there are many studies with significant results in which curcumin is delivered as microemulsions [106,108,109] or encapsulated as nanoparticles of poly lactic-co-glycolic acid (PLGA) [111], graphene oxide [30], or silver [80]. Composites are used as sensitizers in photodynamic therapy (Figure 4) with antibacterial and antibiofilm effects on several types of bacteria (Enterococcus faecalis (E. faecalis), Streptococcus mutans (S. mutans), Porphyromonas gingivalis (P. gingivalis), and Aggregatibacter actinomycetemcomitans (A. actinomycetemcomitans)) commonly found in periodontitis, tooth decay in children, or endodontic treatments [69,71,84,85,112,113,114]. However, the most intense use of curcumin is found in skin treatments. In the past, it was used in poultices and compresses to cure various skin diseases, and as its antioxidant, antifungal, and antibacterial properties were confirmed, curcumin came to the attention of cosmetic manufacturers [140,141]. Subsequently, loading systems [142,143,144,145,146,147,148,149] were developed to increase the skin penetration effect and the photostability of curcumin. Thus, after conditioning, it was introduced in cosmetics as an active ingredient to protect the quality of the skin [59,142,143] and for the treatment of acne, psoriasis, or eczema [140,141]. Furthermore, studies have been directed to the field of the prevention and treatment of chronic wound infections, especially for diabetic patients where the healing process is slow [90]. However, to improve the solubility of curcumin, o/w nanoemulsions stabilized with PLGA [88] or N-oxide [90] have been made, which were tested against several types of strains (C. albicans, E. coli, and S. aureus) and showed antibacterial and antibiofilm activity. Mirzahosseinipour et al. [110] encapsulated curcumin in silica nanoparticles and used it as a sensitizer in antimicrobial PDT against planktonic systems and the biofilm of P. aeruginosa and S. aureus. The results of in vitro tests demonstrated antimicrobial and antibiofilm activity, without any significant cytotoxic effect of nanocomposites on normal human fibroblasts; while Varaprasad et al. [75] demonstrated the synergistic effect between silver and curcumin by increasing the antimicrobial activity against E. coli as carboxymethylcellulose nanocomposite films. Thus, considering the healing properties of curcumin, which is attributed to the presence of myofibroblast and enhancing fibronectin and collagen expression, the study authors developed composites with increased antibacterial efficacy. The results of the study, in vitro, showed 86% inhibition growth for composites loaded with silver nanoparticles and curcumin, compared to other film composites which showed only 25% inhibition growth of E. coli.
The encouraging results of studies on the action of curcumin in skin treatments has led to the development of another field, that of dressings and biomaterials carrying drugs for treating infected wounds (Table 2). Studies have continued to design different types of structures and platforms which allow them to be loaded with drugs or adjuvants to speed up the healing process.

Table 2.
Types of composite materials used as a support loaded with curcumin, used in bandages or dressings.
Table 2.
Types of composite materials used as a support loaded with curcumin, used in bandages or dressings.
| No. crt | Polymer Support | Target Microorganisms/Potential Application | Ref. |
|---|---|---|---|
| 1. | chitosan/pluronic membranes | S. aureus, P. aeruginosa/healing applications | [150] |
| 2. | chitosan/polycaprolactone | S. aureus/healing properties | [151] |
| 3. | hyaluronic acid modified pullulan polymers | E. coli, S. aureus/accelerating skin wound healing | [152] |
| 4 | lactide-co-glycolide /chitosan/β-cyclodextrin/poly(vinyl alcohol) | blend films | [153,154] |
| 5. | chitosan-collagen, gelatin, sodium alginate | S. aureus, E. coli | [155,156,157] |
| 6. | polyvinyl pyrrolidone(PVP)-cerium nitrate hexahydrate | S. aureus, E. coli/ dressing material-anti-scar property | [156] |
| 7. | cellulose hydroxypropyl-β-cyclodextrin-silver nanoparticle | S. aureus, P. aeruginosa, C. auris/healing properties | [158] |
| 8. | Metal oxides-NPs-cotton | MERSA, S. aureus, E. coli | [159,160,161,162] |
| 9. | cellulose-zinc oxide | S. aureus, T. rubrum/skin infection | [161] |
| 10. | graphene oxide | S. aureus, E. coli | [163] |
| 11. | 3-methyl-1-(hexadecyloxycarbonylmethyl)imidazolium bromide | hydrogel used for the wound healing | [164] |
| 12. | sodium alginate | wound healing applications | [165] |
| 15. | thiocarbohydrazide gelatin nanofibers | E. coli/wound healing applications | [166] |
| 16. | bacterial nanocellulose | S. aureus, E. coli | [167] |
| 17. | oleic acid based polymeric bandage | wound healing | [168] |
Simultaneously, the mechanical properties of resistance, cytotoxicity, absorption capacity, and release of bioactive compounds must be monitored. For this, composites based on curcumin-loaded chitosan have been designed and incorporated into Pluronic [150] or polycaprolactone [151] copolymers that lead to membranes with good thermomechanical properties and sustained release of curcumin. Test results in vitro against S. aureus and P. aeruginosa, most commonly present in chronic wounds, showed that curcumin retention was higher in the epidermis than in the dermis. Pullulan-type polymers [152] modified with hyaluronic acid have been used to make films with specific properties, to speed up the wound healing process and fight infections. The addition of curcumin to the composition of the films and their testing, in vivo, showed an improvement in biocompatibility, and antibacterial and antioxidant activities.
Another type of film obtained from poly nanofibers (lactide-co-glycolide) loaded with curcumin and heparin have been successfully tested for wound healing in diabetic mice [153]. The polyvinyl alcohol-chitosan-curcumin-β-cyclodextrin films also showed antioxidant activity [154]. The patches of polymeric alginate-chitosan-curcumin [155] or PVP-curcumin cerium nitrate [156] have been tested in vivo, showing antimicrobial activity. These bandages are suitable for use in regenerative therapy because the wounds treated with these dressings healed completely without scars. Moreover, other delivery platforms have been developed which, based on their structural design, create the possibility of the controlled release of curcumin. These are then loaded on cellulosic fabrics used as dressings.
Naghshineh et al. [157] obtained and studied three types of composites with spongy structure based on chitosan-collagen/gelatin/alginate-curcumin. Depending on the composition of the mixtures, structures with different degrees of porosity were obtained, which had a direct effect on the ability to release curcumin. Based on the histological tests, of the three types of structures, chitosan-gelatin-curcumin released curcumin the fastest, resulting in the greatest healing effect on wounds and the highest antibacterial action. The nanocomposite with alginate had the most porous structure, thus presenting the best results on biodegradation, while the collagen composite was the most stable.
The structures of metal nanoparticles [158,159], metal oxides (Ag, Zn, or Ti) [159,160,161,162] or graphene oxide [163], were designed as a delivery platform for curcumin. The synergetic action between them was aimed at increasing the regenerating effects of the skin and antibacterial activity. These structures integrated in bacterial cellulose [158,161] or chitosan [160,162] have been used to develop new types of advanced polymeric materials, such as hydrogel [158,161,162,163,164] for bandages with improved wound healing properties in chronic infections [159,165,166,167,168].
Curcumin is used in passive drug delivery systems to treat skin lesions caused by sunburn or erythema. For this, it is integrated in thermosensitive microgels [169,170] deposited on cotton fabrics that can be used efficiently for the treatment of lesions caused by ultraviolet radiation [44]. Furthermore, the dyeing of natural fibers or polyester fabrics by using alum-type mordants, metal sulfates, or biomordants can reduce the risk of developing allergies to fixing compounds, leading to textiles that have antimicrobial activity against Staph aureus, Klebsiella pneumonia, Candida albicans, and Salmonella typhimurium. It has been claimed that in the future, the fabrics will be used in the medical field [22,171], especially designed for a daily breast cancer prevention regimen for healthy women [172].
5. Future Developments of Curcumin-Based Materials
Due to the multitude of properties manifested by curcumin analogs, the diversity of applications of the natural compound will be further developed (Figure 2) as many as the issues related to the extraction, solubility, and stability of the parent compound will be solved. Due to its antioxidant and anti-inflammatory properties, modern applications of curcumin in the medical field targeting the treatment of neurodegenerative diseases [173,174,175,176] and cancer [177,178,179,180,181,182,183,184] will be further developed. Furthermore, studies have shown that, as a result of the metabolism process, curcumin can undergo two types of transformation. Consequently, by reducing the double bonds, hydrogenated derivatives, di, tetra, hexa, and octahydrocurcumin can be obtained, or it can be conjugated to the phenolic groups with sulfate moiety or glucuronol moiety [185]. Each of these compounds manifesting greater solubility and improved bioactivity compared to curcumin. In vivo studies by Zhang et al. [186] showed that tetrahydrocurcumin and octahydrocurcumin demonstrated more pronounced anti-inflammatory activity than curcumin. These studies will be continued to evaluate its antioxidant, anti-cancer, and antiseptic properties, and the mechanisms by which they act. Simultaneously, studies have been conducted on the protective activity of curcumin based on its antioxidant properties against the neurotoxicity produced by methamphetamine [187], organophosphorus insecticides [188], and aluminum [189]. In vivo results have shown that the mechanisms of action of curcumin include the prevention of lipid peroxidation and an increase in the antioxidant capacity of the enzymes superoxide dismutase and glutathione peroxidase that protect cells from damage caused by ROS [187].
In the case of neurological diseases, nano-curcumin treatments have been tested in vitro/in vivo and in clinical trials. For the development of targeted therapy in the treatment of neuronal diseases, curcumin was encapsulated in micelles, liposomes, or polymeric nanoparticles. Thus, the efficacy of neuronal membrane penetration and the mechanisms of action of “nano”-curcumin in the treatment of Parkinson’s disease [173], Huntington’s disease, Alzheimer’s disease [21,176], multiple sclerosis, epilepsy, and amyotrophic lateral sclerosis were evaluated [174,175]. Furthermore, curcumin and curcumin nanoparticles have been tested as a protector on indicators of oxidative stress in cardiovascular disease [190,191,192]. Prathipati et al. evaluated the neuroprotective effects of curcumin-loaded lipid nanoparticles on homocysteine-induced oxidative stress in vascular dementia. In vivo test results showed that 25 mg/kg nano-curcumin demonstrated neuroprotective effects on homocysteine-induced oxidative stress [192].
Studies should be simultaneously continued on the cytotoxicity of nanoparticles, their biodegradation, and the biocompatibility of curcumin delivery systems.
In cancer treatments, curcumin has been approached from several perspectives. It was primarily used as a chemo-protective, reducing the side effects of cytotoxic drugs used in chemotherapy through various mechanisms of reduction, markers of heart damage, or the degree of lipid peroxidation [177,178,179,180]. In vivo studies have shown the hepato-protective and nephrological effect of curcumin administered before and during chemotherapy [178]. The second approach is the use of curcumin as an adjuvant with the effect of the chemo-sensitization of cancer cells resistant to chemotherapeutic agents, through mechanisms of inhibition of the expression of anti-apoptotic proteins or intracellular transcription factors [181]. Thus, the results of studies performed on patients with colorectal cancer refractory to standard chemotherapy established a daily oral dose of 3.6 g curcumin without adverse effects. Encouraging results were obtained after completion of the first stage of clinical trials conducted in patients with breast cancer where the curcumin was used as an adjuvant in combination with the chemotherapeutic agent docetaxel. The final recommendations are that the maximum dose administered should be 6 g/day curcumin [182,183]. To reduce the dose of administration and increase the bioavailability, curcumin was conditioned in the form of nanoparticles, micelles, liposomes, and phospholipid complexes. Thus, curcumin encapsulated in biocompatible polymers or liposomes in combination with chemotherapeutic agents have been tested for breast cancer, cervical cancer, and pancreatic cancer. The results of in vivo tests demonstrated an efficiency of 76–82.5% in inhibiting tumor growth compared to cells treated with individual compounds [182]. Another way in which curcumin has found use in cancer treatments is its use as a photosensitizer in photodynamic therapy to treat skin [193,194], lung [195], prostate [196], breast [197], or cervical cancers [198]. Due to the ability of curcumin to generate reactive oxygen species, the tests in vitro/in vivo have obtained encouraging results for such applications. In vivo/in vitro studies of the delivery of curcumin encapsulated in liposomal structures [193,197] or mesoporous materials [195,199,200] have shown an increase in cytotoxic activity and have suggested the possibility of efficient use of curcumin in lower doses in PDT of cancer.
All these possible applications require further studies on the optimal conditioning conditions to increase the bioactivity of curcumin and the biocompatibility of the delivery systems used.
The use of curcumin in the field of sensors is already known, but research continues in the development of new structures and detection methods depending on its field of use, either in the medical, food [23,24,25], or environmental protection fields [201]. Its application in the development of nanomaterials for antimicrobial and antibiofilm coatings for multiple surfaces, necessary in both the food and medical fields, will continue to be given special attention. In addition, there are new uses in the application of products with self-cleaning properties [202] or anticorrosive coatings [203]. Developing new composite materials with curcumin has started to be addressed in areas such as solar cellules [204], nonlinear optics [205] applications in robotics, and artificial intelligence [206]. All this research on increasing efficiency in the use of curcumin for various fields is conducted with the vision of developing new eco-technologies, based on sustainable methods and principles.
The use of the natural compound has been preferred in many applications due to its known properties and the cytotoxicity tests performed, which considerably reduces the research time regarding the application of curcumin in certain fields, such as medical and food. However, curcumin-derived structures must also be tested, with some studies showing modified structures that have improved properties over the natural compound [16,17,18,19,20].
6. Conclusions
Out of a desire to use as much of the bioactive potential of curcumin as possible, the compound has known and continues to know various applications. Starting from the most diverse extraction methods to conditioning the compound in the form of (nano)emulsions, (nano)particles, and delivery nanoplatforms, all aim to increase the light stability and water solubility of curcumin. The diversity of composite materials that can be designed, depending on the purpose of use, leave the field of curcumin conditioning open to research. Various biomaterials active from an antibacterial and antibiofilm point of view can be intuited in which curcumin plays the role of an additive that potentiates the activities of other compounds or has synergistic activity with them. However, the way in which nanomaterials can influence human health must be taken into account, given that they can be ingested when used for food preservation, or can cross the cell barrier by penetrating the skin from compounds intended for dermatological treatments. Depending on their location inside the cell, nanoparticles, by specific mechanisms, can damage the cell structure or DNA, eventually causing cell death. To this end, in vivo studies should be performed to have a clearer view of the effects of nanocomposites in terms of their cytotoxicity. Moreover, the use of nanocomposites in packaging products or dressings/bandages should not be neglected in terms of the biodegradability properties or the effects that may result after use when released into the environment.
Author Contributions
Conceptualization, F.M.R. and V.R.; formal analysis, A.R.; investigation, F.M.R., V.P.; writing—original draft preparation, F.M.R., V.R.; writing—review and editing, A.R., V.P.; supervision, V.R.; project administration, F.M.R. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a grant of the Romanian Ministry of Research and Innovation, CCCDI–UEFISCDI, project number PN-III-P2-2.1-PED-2019-1471, within PNCDI III.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- FAO. Compendium of Food Additive Specifications. Available online: Fao.org/fileadmin/user_upload/jecfa_additives/docs/Monograph1/Additive-140 (accessed on 1 February 2021).
- Pawar, H.A.; Gavasane, A.J.; Choudhary, P.D. A novel and simple approach for extraction and isolation of curcuminoids from Turmeric rhizomes. Nat. Prod. Chem. Res. 2018, 6, 300. [Google Scholar] [CrossRef]
- Othman, A.F.M.; Rukayadi, Y.; Radu, S. Inhibition of Pseudomonas aeruginosa Quorum Sensing by Curcuma xanthorrhiza Roxb. Extract. J. Pure Appl. Microbiol. 2019, 13, 1335–1347. [Google Scholar] [CrossRef]
- Ibáñez, M.D.; Blázquez, M.A. Curcuma longa L. Rhizome Essential Oil from Extraction to its Agri-Food Applications. A Review. Plants 2020, 10, 44. [Google Scholar] [CrossRef] [PubMed]
- Yadav, D.K.; Sharma, K.; Dutta, A.; Kundu, A.; Awasthi, A. Purity evaluation of curcuminoids in the Turmeric extract obtained by accelerated solvent extraction. J. AOAC Int. 2017, 100, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Panichayupakaranant, P.; Lateh, L.; Yuenyongsawad, S.; Chen, H. A green method for preparation of curcuminoid-rich Curcuma longa extract and evaluation of its anticancer activity. Pharmacogn. Mag. 2019, 15, 730. [Google Scholar] [CrossRef]
- Nagavekar, N.; Singhal, R.S. Supercritical fluid extraction of Curcuma longa and Curcuma amada oleoresin: Optimization of extraction conditions, extract profiling, and comparison of bioactivities. Ind. Crop. Prod. 2019, 134, 134–145. [Google Scholar] [CrossRef]
- Patil, S.S.; Rathod, V.K. Synergistic Effect of Ultrasound and Three Phase Partitioning for the Extraction of Curcuminoids from Curcuma longa and its Bioactivity Profile. Process. Biochem. 2020, 93, 85–93. [Google Scholar] [CrossRef]
- Patil, S.S.; Pathak, A.; Rathod, V.K. Optimization and kinetic study of ultrasound assisted deep eutectic solvent based extraction: A greener route for extraction of curcuminoids from Curcuma longa. Ultrason. Sonochem. 2021, 70, 105267. [Google Scholar] [CrossRef]
- Vijayan, U.K.; Varakumar, S.; Singhal, R.S. A comparative account of extraction of oleoresin from Curcuma aromatica Salisb by solvent and supercritical carbon dioxide: Characterization and bioactivities. LWT 2019, 116, 108564. [Google Scholar] [CrossRef]
- Chao, I.-C.; Wang, C.-M.; Marcotullio, M.C.; Lin, L.-G.; Ye, W.-C.; Zhang, Q.-W. Simultaneous Quantification of Three Curcuminoids and Three Volatile Components of Curcuma longa Using Pressurized Liquid Extraction and High-Performance Liquid Chromatography. Molecules 2018, 23, 1568. [Google Scholar] [CrossRef]
- Zielińska, A.; Alves, H.; Marques, V.; Durazzo, A.; Lucarini, M.; Alves, T.; Morsink, M.; Willemen, N.; Eder, P.; Chaud, M.; et al. Properties, Extraction Methods, and Delivery Systems for Curcumin as a Natural Source of Beneficial Health Effects. Medicina 2020, 56, 336. [Google Scholar] [CrossRef]
- Thongchai, W.; Fukngoen, P. Synthesis of curcuminoid-imprinted polymers applied to the solid-phase extraction of curcuminoids from turmeric samples. J. Pharm. Anal. 2018, 8, 60–68. [Google Scholar] [CrossRef]
- Mottahedin, P.; Asl, A.H.; Khajenoori, M. Extraction of Curcumin and Essential Oil from Curcuma longa L. by Subcritical Water via Response Surface Methodology. J. Food Process. Preserv. 2016, 41, e13095. [Google Scholar] [CrossRef]
- Degot, P.; Huber, V.; El Maangar, A.; Gramüller, J.; Rohr, L.; Touraud, D.; Zemb, T.; Gschwind, R.M.; Kunz, W. Triple role of sodium salicylate in solubilization, extraction, and stabilization of curcumin from Curcuma longa. J. Mol. Liq. 2021, 329, 115538. [Google Scholar] [CrossRef]
- Theppawong, A.; van de Walle, T.; Grootaert, C.; Bultinck, M.; Desmet, T.; van Camp, J.; D’Hooghe, M. Synthesis of Novel Aza-aromatic Curcuminoids with Improved Biological Activities towards Various Cancer Cell Lines. Chem. Open 2018, 7, 381–392. [Google Scholar] [CrossRef]
- Alneyadi, S.S.; Amer, N.; Thomas, T.G.; Al Ajeil, R.; Breitener, P.; Munawar, N. Synthesis, Characterization, and Antioxidant Activity of Some 2-Methoxyphenols derivatives. Heterocycl. Commun. 2020, 26, 112–122. [Google Scholar] [CrossRef]
- Insuasty, D.; Castillo, J.; Becerra, D.; Rojas, H.; Abonia, R. Synthesis of Biologically Active Molecules through Multicomponent Reactions. Molecules 2020, 25, 505. [Google Scholar] [CrossRef] [PubMed]
- Raduly, M.F.; Raditoiu, V.; Raditoiu, A.; Wagner, L.E.; Amariutei, V.; Darvaru, G.A. Facile Synthesis of Curcumin and Curcuminoid-like Derivatives at Microwaves. Rev. Chim. 2018, 69, 1327–1331. [Google Scholar] [CrossRef]
- Tantriasa, L.D.; Anwar, C.; Astuti, E. Synthesis of Curcumin Analogs under Ultrasound Irradiation for Inhibiting α-Amylase. Mater. Sci. Forum 2019, 948, 115–119. [Google Scholar] [CrossRef]
- Ausili, A.; Gómez-Murcia, V.; Candel, A.M.; Beltrán, A.; Torrecillas, A.; He, L.; Jiang, Y.; Zhang, S.; Teruel, J.A.; Gómez-Fernández, J.C. A comparison of the location in membranes of curcumin and curcumin-derived bivalent compounds with potential neuroprotective capacity for Alzheimer’s disease. Colloids Surf. B Biointerfaces 2021, 199, 111525. [Google Scholar] [CrossRef]
- Li, S.; Lu, M.; Hu, R.; Tang, T.; Hou, K.; Liu, Y. Dyeing ramie fabrics with curcumin in NaOH/Urea solution at low temperature. Cloth. Text. Res. J. 2019, 37, 66–79. [Google Scholar] [CrossRef]
- Mejri, A.; Mars, A.; Elfil, H.; Hamzaoui, A.H. Reduced graphene oxide nanosheets modified with nickel disulfide and curcumin nanoparticles for non-enzymatic electrochemical sensing of methyl parathion and 4-nitrophenol. Microchim. Acta 2019, 186, 704. [Google Scholar] [CrossRef]
- Mejri, A.; Mars, A.; Elfil, H.; Hamzaoui, A.H. Voltammetric simultaneous quantification of p-nitrophenol and hydrazine by using magnetic spinel FeCO2O4 nanosheets on reduced graphene oxide layers modified with curcumin-stabilized silver nanoparticles. Microchim. Acta 2019, 186, 561. [Google Scholar] [CrossRef] [PubMed]
- Prabu, S.; Mohamad, S. Curcumin/beta-cyclodextrin inclusion complex as a new “turn-off” fluorescent sensor system for sensitive recognition of mercury ion. J. Mol. Struct. 2020, 1204, 127528. [Google Scholar] [CrossRef]
- Jennings, M.; Parks, R. Curcumin as an Antiviral Agent. Viruses 2020, 12, 1242. [Google Scholar] [CrossRef]
- Praditya, D.; Kirchhoff, L.; Brüning, J.; Rachmawati, H.; Steinmann, J.; Steinmann, E. Anti-infective Properties of the Golden Spice Curcumin. Front. Microbiol. 2019, 10, 912. [Google Scholar] [CrossRef]
- Wen, C.C.; Kuo, Y.H.; Jan, J.T.; Liang, P.H.; Wang, S.Y.; Liu, H.G.; Lee, C.K.; Chang, S.T.; Kuo, C.J.; Lee, S.S. Specific plant terpenoids and lignoids possess potent antiviral activities against severe acute respiratory syndrome coronavirus. J. Med. Chem. 2007, 50, 4087–4095. [Google Scholar] [CrossRef]
- Ting, D.; Dong, N.; Fang, L.; Lu, J.; Bi, J.; Xiao, S.; Han, H. Multisite Inhibitors for Enteric Coronavirus: Antiviral Cationic Carbon Dots Based on Curcumin. ACS Appl. Nano Mater. 2018, 1, 5451–5459. [Google Scholar] [CrossRef]
- Ghorbanzadeh, R.; Assadian, H.; Chiniforush, N.; Parker, S.; Pourakbari, B.; Ehsani, B.; Alikhani, M.Y.; Bahador, A. Modulation of virulence in Enterococcus faecalis cells surviving antimicrobial photodynamic inactivation with reduced graphene oxide-curcumin: An ex vivo biofilm model. Photodiagn. Photodyn. Ther. 2020, 29, 101643. [Google Scholar] [CrossRef]
- Global Curcumin Market 2021 Covid 19 Analysis with Top Industry. Available online: www.openpr.com/news/2169421/curcumin-market-with-covid-19-impact-analysis-top-industry (accessed on 27 March 2021).
- Curcumin Market Size, Share & Trends Analysis Report by Application (Pharmaceutical, Food, Cosmetics), by Region (North America, Europe, Asia Pacific, Central & South America, Middle East & Africa), and Segment Forecasts, 2020–2027. Available online: www.researchandmarkets.com/reports/4613416 (accessed on 17 January 2021).
- Curcumin Market Size Worth $1.30 Billion by 2025|CAGR 12.3%: Grand View Research, Inc. Available online: www.prnewswire.com/news-releases/curcumin-market-size-worth-1-30-billion-by-2025-cagr-12-3-grand-view-research-inc--811278562 (accessed on 17 January 2021).
- Curcumin Market by Application (Pharmaceutical, Food & Beverage, Cosmetic, Others)—Global Opportunity Analysis and Industry Forecast, 2018–2025. Available online: www.alliedmarketresearch.com/curcumin-market (accessed on 17 January 2021).
- Curcumin Market Growing Rapidly with Prominent Players Update (2022–2031)||Synthite Ind, Sabinsa, Indena. Available online: www.marketwatch.com/press-release/curcumin-market-growing-rapidly-with-prominent-players-update2022-2031-synthite-ind-sabinsa-indena-2021-01-27 (accessed on 17 January 2021).
- Curcumin Market Headed To $104.19 Mn by 2025 at 6.5% CAGR: The Demand is Expected to Surge During the Covid-19 Pandemic. Available online: www.einnews.com/pr_news/529267704/curcumin-market-headed-to-104-19-mn-by-2025-at-6-5-cagr-the-demand-is-expected-to-surge-during-the-covid-19-pandemic (accessed on 17 January 2021).
- Supply of Spices and Herbs Seriously Affected by COVID-19. Available online: www.cbi.eu/news/supply-spices-herbs-seriously-affected-covid-19 (accessed on 27 March 2021).
- Consumer Sentiment and Behavior Continue to Reflect the Uncertainty of the COVID-19 crisis. Available online: www.mckinsey.com/business-functions/marketing-and-sales/our-insights/a-global-view-of-how-consumer-behavior-is-changing-amid-covid-19# (accessed on 27 March 2021).
- Responding to Consumer Trends in the New Reality. Available online: www.assets.kpmg/content/dam/kpmg/xx/pdf/2020/11/consumers-new-reality (accessed on 27 March 2021).
- Hoekstra, J.C.; Leefang, P.S.H. Marketing in the era of COVID 19. Ital. J. Mark. 2020, 249–260. [Google Scholar] [CrossRef]
- Shetty, T.; Dubey, A.; Ravi, G.S.; Hebbar, S.; Shastry, C.S.; Charyulu, N. Antifungal and antioxidant therapy for the treatment of fungal infection with microemulsion gel containing curcumin and vitamin C. Asian J. Pharm. 2017, 11, 717–725. [Google Scholar]
- Sarwar, S.; Netzel, G.; Netzel, M.E.; Mereddy, R.; Phan, A.D.T.; Hong, H.T.; Cozzolino, D.; Sultanbawa, Y. Impact of Curcumin-Mediated Photosensitization on Fungal Growth, Physicochemical Properties and Nutritional Composition in Australian Grown Strawberry. Food Anal. Methods 2021, 14, 465–472. [Google Scholar] [CrossRef]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Sun, X.-Z.; Wang, N. A thermosensitive textile-based drug delivery system for treating UVB-induced damage. Cellulose 2020, 27, 8329–8339. [Google Scholar] [CrossRef]
- Degot, P.; Huber, V.; Hofmann, E.; Hahn, M.; Touraud, D.; Kunz, W. Solubilization and extraction of curcumin from Curcuma Longa using green, sustainable, and food-approved surfactant-free microemulsions. Food Chem. 2021, 336, 127660. [Google Scholar] [CrossRef]
- Degot, P.; Huber, V.; Touraud, D.; Kunz, W. Curcumin extracts from Curcuma Longa—Improvement of concentration, purity, and stability in food-approved and water-soluble surfactant-free microemulsions. Food Chem. 2021, 339, 128140. [Google Scholar] [CrossRef]
- Jeliński, T.; Przybyłek, M.; Cysewski, P. Natural Deep Eutectic Solvents as Agents for Improving Solubility, Stability and Delivery of Curcumin. Pharm. Res. 2019, 36, 1–10. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Fu, R.; Zhang, L.; Wang, D.; Wang, S. Enhanced extraction of natural pigments from Curcuma longa L. using natural deep eutectic solvents. Ind. Crop. Prod. 2019, 140, 111620. [Google Scholar] [CrossRef]
- Doldolova, K.; Bener, M.; Lalikoğlu, M.; Aşçı, Y.S.; Arat, R.; Apak, R. Optimization and modeling of microwave-assisted extraction of curcumin and antioxidant compounds from turmeric by using natural deep eutectic solvents. Food Chem. 2021, 353, 129337. [Google Scholar] [CrossRef]
- Praveen, A.N.K.; Madhyastha, S. Role of cotton and Turmeric smoke as a potential treatment of COVID-19. Int. J. Adv. Res. Innov. Tech. 2020, 6, 287–290. [Google Scholar]
- Saraswat, J.; Singh, P.; Patel, R. A computational approach for the screening of potential antiviral compounds against SARS-CoV-2 protease: Ionic liquid vs. herbal and natural compounds. J. Mol. Liq. 2021, 326, 115298. [Google Scholar] [CrossRef]
- Momo, E.J.; Sen, M.L.; Nguemezi, S.T.; Youassi, O.Y.; Mounbain, F.; Sameza, M.L.; Tchoumbougnang, F.; Jazet, P.M. Chemical Composition of the Essential Oil of Curcuma longa and Evaluation of the Antifungal Activity on Rhizopus stolonifer and Penicillium sp. Responsible Fungi for Post-harvest Rot of Dioscorea rotoundata in Cameroon. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 797–808. [Google Scholar] [CrossRef]
- Gámez-Espinosa, E.; Anaya, M.; Borges, P.; Crespo, D.M.B. Antifungal effects of Curcuma longa L. essential oil against pathogenic strains isolated from indoor air. Aerobiologia 2021, 37, 119–126. [Google Scholar] [CrossRef]
- Marchi, L.B.; Dornellas, F.C.; Polonioc, J.C.; Pamphile, J.A.; Monteiro, A.R.G.; Gonçalves, O.H.; Perdoncini, M.R.F.G. Antifungal activity of Curcuma longa L. (Zingiberaceae) against degrading Filamentous Fungi. Chem. Eng. Trans. 2019, 75, 319–324. [Google Scholar] [CrossRef]
- Kazi, H.A.; Channa, T.; Unarb, A.A.; Unarc, K.; Sabzoib, W.; Perveenc, S.; Mangid, A.A.; Ahmer, A. Pharmaceutical formulation of Garlic and Turmeric dried crude extract and their synergistic antifungal activity and safety. Iran. J. Pharm. Sci. 2018, 14, 75–82. [Google Scholar]
- Jiang, B.-C.; Shen, J.-Y.; Wu, J.; Lu, R.-Y.; Zheng, W.; Dong, J.-X.; Yan, L.; Jin, Y.-S. In vitro antifungal activity of 163 extracts from traditional Chinese medicine herbs. Eur. J. Integr. Med. 2020, 39, 101213. [Google Scholar] [CrossRef]
- De Paula, R.L.; Maniglia, B.C.; Assis, O.B.G.; Tapia-Blácido, D.R. Evaluation of the turmeric dye extraction residue in the formation of protective coating on fresh bananas (Musa acuminata cv. ’Maçã’). J. Food Sci. Technol. 2018, 55, 3212–3220. [Google Scholar] [CrossRef]
- Abdelgaleil, S.A.M.; Zoghroban, A.A.M.; El-Bakry, A.M.; Kassem, S.M.I. Insecticidal and antifungal activities of crude extracts and pure compounds from rhizomes of Curcuma longa L. (Zingiberaceae). J. Agr. Sci. Tech. 2019, 21, 1049–1061. [Google Scholar]
- Olszewska, M.A.; Gędas, A.; Simões, M. Antimicrobial polyphenol-rich extracts: Applications and limitations in the food industry. Food Res. Int. 2020, 134, 109214. [Google Scholar] [CrossRef]
- Solano, A.A.N.; Aguirre, P.C.; Vargas, L.O.S.; Navarro, J.B.R.; Escalona, J.R.B. Antimicrobial effect of curcumin on Enterococcus faecalis, Escherichia coli, Staphylococcus aureus and Candida albicans. Nova Sci. 2020, 25, 1–12. [Google Scholar]
- Phuna, Z.X.; Yu, J.K.E.; Tee, J.Y. In vitro evaluation of nanoemulsions of curcumin, piperine and tualang honey as antifungal agents for Candida species. J. Appl. Biotechnol. Rep. 2020, 7, 190–198. [Google Scholar] [CrossRef]
- Sathiyabama, M.; Indhumathi, M.; Amutha, T. Preparation and characterization of curcumin functionalized copper nanoparticles and their application enhances disease resistance in chickpea against wilt pathogen. Biocatal. Agric. Biotechnol. 2020, 29, 101823. [Google Scholar] [CrossRef]
- Jung, S.; Cui, Y.; Barnes, M.; Satam, C.; Zhang, S.; Chowdhury, R.A.; Adumbumkulath, A.; Sahin, O.; Miller, C.; Sajadi, S.M.; et al. Multifunctional Bio-Nanocomposite Coatings for Perishable Fruits. Adv. Mater. 2020, 32, e1908291. [Google Scholar] [CrossRef] [PubMed]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial biofilm and associated infections. J. Chin. Med. Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Misba, L.; Khan, A.U. Antibiotics versus biofilm: An emerging battleground in microbial communities. Antimicrob. Resist. Infect. Control. 2019, 8, 1–10. [Google Scholar] [CrossRef]
- Abebe, G.M. The Role of Bacterial Biofilm in Antibiotic Resistance and Food Contamination. Int. J. Microbiol. 2020, 2020, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Raorane, C.J.; Lee, J.-H.; Kim, Y.-G.; Rajasekharan, S.K.; García-Contreras, R.; Lee, J. Antibiofilm and Antivirulence Efficacies of Flavonoids and Curcumin Against Acinetobacter baumannii. Front. Microbiol. 2019, 10, 990. [Google Scholar] [CrossRef] [PubMed]
- Baishya, R.; Banerjee, S. In-Vitro antibiofilm activity of selected medicinal plants against Staphylococcus aureus biofilm on chitin flakes as substrate. Int. J. Res. Pharm. Sci. 2020, 11, 1595–1603. [Google Scholar] [CrossRef][Green Version]
- Samiappan, S.C.; Pandiyan, R.; Palanisamy, S.; Ramalingam, S.; Saravanan, R.; Hameed, S.A. Targeting the Extracellular Polysaccharide Production (EPS) by Biofilm Forming Bacteria from Orthodontic Brackets and Wires through Antiquorum Sensing Action of Bioactive Compounds from Curcuma longa and Zingiber officinale. Biomed. Pharmacol. J. 2020, 13, 1037–1045. [Google Scholar] [CrossRef]
- Bali, E.B.; Türkmen, K.E.; Erdönmez, D.; Sağlam, N. Comparative Study of Inhibitory Potential of Dietary Phytochemicals Against Quorum Sensing Activity of and Biofilm Formation by Chromobacterium violaceum 12472, and Swimming and Swarming Behaviour of Pseudomonas aeruginosa PAO1. Food Technol. Biotechnol. 2019, 57, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Li, X.; Lin, H.; Zhou, Y. Curcumin as a Promising Antibacterial Agent: Effects on Metabolism and Biofilm Formation in S. mutans. BioMed Res. Int. 2018, 2018, 1–11. [Google Scholar] [CrossRef]
- Fakhrullina, G.; Khakimova, E.; Akhatova, F.; Lazzara, G.; Parisi, F.; Fakhrullin, R.F. Selective Antimicrobial Effects of Curcumin@Halloysite Nanoformulation: A Caenorhabditis elegans Study. ACS Appl. Mater. Interfaces 2019, 11, 23050–23064. [Google Scholar] [CrossRef]
- Gawish, S.M.; Mashaly, H.M.; Helmy, H.M.; Ramadan, A.M.; Farouk, R. Effect of Mordant on UV Protection and Antimicrobial Activity of Cotton, Wool, Silk and Nylon Fabrics Dyed with Some Natural Dyes. J. Nanomed. Nanotechnol. 2017, 8. [Google Scholar] [CrossRef]
- Xue, B.; Huang, J.; Zhang, H.; Li, B.; Xu, M.; Zhang, Y.; Xie, M.; Li, X. Micronized curcumin fabricated by supercritical CO2 to improve antibacterial activity against Pseudomonas aeruginosa. Artif. Cells Nanomed. Biotechnol. 2020, 48, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Varaprasad, K.; Vimala, K.; Ravindra, S.; Reddy, N.N.; Reddy, G.V.S.; Raju, K.M. Fabrication of silver nanocomposite films impregnated with curcumin for superior antibacterial applications. J. Mater. Sci. Mater. Med. 2011, 22, 1863–1872. [Google Scholar] [CrossRef]
- Versace, D.-L.; Moran, G.; Belqat, M.; Spangenberg, A.; Meallet-Renault, R.; Abbad-Andaloussi, S.; Brezova, V.; Malval, J.-P. Highly Virulent Bactericidal Effects of Curcumin-Based μ-Cages Fabricated by Two-Photon Polymerization. ACS Appl. Mater. Interfaces 2020, 12, 5050–5057. [Google Scholar] [CrossRef]
- Saha, T.; Kumar, P.; Sepay, N.; Ganguly, D.; Tiwari, K.; Mukhopadhyay, K.; Das, S. Multitargeting Antibacterial Activity of a Synthesized Mn2+ Complex of Curcumin on Gram-Positive and Gram-Negative Bacterial Strains. ACS Omega 2020, 5, 16342–16357. [Google Scholar] [CrossRef]
- Singh, A.K.; Mishra, H.; Firdaus, Z.; Yadav, S.; Aditi, P.; Nandy, N.; Sharma, K.; Bose, P.; Pandey, A.K.; Chauhan, B.S.; et al. MoS2-Modified Curcumin Nanostructures: The Novel Theranostic Hybrid Having Potent Antibacterial and Antibiofilm Activities against Multidrug-Resistant Hypervirulent Klebsiella pneumoniae. Chem. Res. Toxicol. 2019, 32, 1599–1618. [Google Scholar] [CrossRef] [PubMed]
- Angelini, G.; Pasc, A.; Gasbarri, C. Curcumin in silver nanoparticles aqueous solution: Kinetics of keto-enol tautomerism and effects on AgNPs. Colloids Surf. A Physicochem. Eng. Asp. 2020, 603, 125235. [Google Scholar] [CrossRef]
- El Hamid, A.; Hajer, M.; Aziz, A.; Mohamed, S.; Naeem, A.; Fatma, M. Antimicrobial efficacy of nanopropolis coated vs. silver-curcumin nanoparticles coated gutta-percha Points on various microbial species. Egypt. Dent. J. 2020, 66, 1893–1902. [Google Scholar] [CrossRef]
- Gholami, M.; Zeighami, H.; Bikas, R.; Heidari, A.; Rafiee, F.; Haghi, F. Inhibitory activity of metal-curcumin complexes on quorum sensing related virulence factors of Pseudomonas aeruginosa PAO1. AMB Express 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Masoule, S.F.; Pourhajibagher, M.; Safari, J.; Khoobi, M. Base-free green synthesis of copper (II) oxide nanoparticles using highly cross-linked poly(curcumin) nanospheres: Synergistically improved antimicrobial activity. Res. Chem. Intermed. 2019, 45, 4449–4462. [Google Scholar] [CrossRef]
- Huang, G.; Yan, Y.; Xu, D.; Wu, J.; Xu, C.; Fu, L.; Lin, B. Curcumin-loaded nanoMOFs@CMFP: A biological preserving paste with antibacterial properties and long-acting, controllable release. Food Chem. 2021, 337, 127987. [Google Scholar] [CrossRef] [PubMed]
- Kumbar, V.M.; Peram, M.R.; Kugaji, M.S.; Shah, T.; Patil, S.P.; Muddapur, U.M.; Bhat, K.G. Effect of curcumin on growth, biofilm formation and virulence factor gene expression of Porphyromonas gingivalis. Odontology 2021, 109, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Pan, T.; Lin, H.; Zhou, Y. The enhancing antibiofilm activity of curcumin on Streptococcus mutans strains from severe early childhood caries. BMC Microbiol. 2020, 20, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mitsuwan, W.; Sangkanu, S.; Romyasamit, C.; Kaewjai, C.; Jimoh, T.O.; Pereira, M.D.L.; Siyadatpanah, A.; Kayesth, S.; Nawaz, M.; Rahmatullah, M.; et al. Curcuma longa rhizome extract and Curcumin reduce the adhesion of Acanthamoeba triangularis trophozoites and cysts in polystyrene plastic surface and contact lens. Int. J. Parasitol. Drugs Drug Resist. 2020, 14, 218–229. [Google Scholar] [CrossRef]
- Shariati, A.; Asadian, E.; Fallah, F.; Azimi, T.; Hashemi, A.; Sharahi, J.Y.; Moghadam, M.T. Evaluation of Nano-curcumin effects on expression levels of virulence genes and biofilm production of multidrug-resistant Pseudomonas aeruginosa isolated from burn wound infection in Tehran, Iran. Infect. Drug Resist. 2019, 12, 2223–2235. [Google Scholar] [CrossRef]
- Kumari, A.; Guliani, A.; Shukla, A.K.; Kumar, S.; Acharya, A. Green surfactant based synthesis of curcumin loaded poly lactic-co-glycolic acid nanoparticles with enhanced solubility, photo-stability and anti-biofilm activity. J. Drug Deliv. Sci. Technol. 2020, 59, 101884. [Google Scholar] [CrossRef]
- Cui, J.; Zhou, J.; Huang, L.; Jing, J.; Wang, N.; Wang, L. Curcumin encapsulation and protection based on lysozyme nanoparticles. Food Sci. Nutr. 2019, 7, 2702–2707. [Google Scholar] [CrossRef]
- Lewińska, A.; Jaromin, A.; Jezierska, J. Role of architecture of N-oxide surfactants in the design of nanoemulsions for Candida skin infection. Colloids Surf. B Biointerfaces 2020, 187, 110639. [Google Scholar] [CrossRef]
- Soumya, K.R.; Jishma, P.; Dhivya, R.; Annaraj, J.; Sugathan, S.; Mathew, J.; Radhakrishnan, E.K. Role of Nanocurcumin as a Surface Modifying Agent with Excellent Preventive Effect on Device-Related CoNS Infections. Proc. Natl. Acad. Sci. USA India Sect. B Boil. Sci. 2019, 90, 29–35. [Google Scholar] [CrossRef]
- Yakub, G.; Toncheva, A.; Kussovski, V.; Toshkova, R.; Georgieva, A.; Nikolova, E.; Manolova, N.; Rashkov, I. Curcumin-PVP Loaded Electrospun Membranes with Conferred Antibacterial and Antitumoral Activities. Fibers Polym. 2020, 21, 55–65. [Google Scholar] [CrossRef]
- Montoya-Villegasa, K.A.; Ramirez-Jimeneza, A.; Zizumbo-Lopeza, A.; Perez-Sicairosa, S.; Leal-Acevedoc, B.; Bucioc, E.; Licea-Claverie, A. Controlled surface modification of silicone rubber by gamma-irradiation followed by RAFT grafting polymerization. Eur. Polym. J. 2020, 134, 109817. [Google Scholar] [CrossRef]
- Singh, A.K.; Prakash, P.; Singh, R.; Nandy, N.; Firdaus, Z.; Bansal, M.; Singh, R.K.; Srivastava, A.; Roy, J.K.; Mishra, B. Curcumin Quantum Dots mediated degradation of bacterial biofilms. Front. Microbiol. 2017, 8, 1517. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Moser, D.; Han, F.; Leonhard, M.; Schneider-Stickler, B.; Tan, Y. Preparation and antibiofilm studies of curcumin loaded chitosan nanoparticles against polymicrobial biofilms of Candida albicans and Staphylococcus aureus. Carbohydr. Polym. 2020, 241, 116254. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Patlan, D.; Solis-Cruz, B.; Cano-Vega, M.A.; Beyssac, E.; Garrait, G.; Hernandez-Velasco, X.; Lopez-Arellano, R.; Tellez, G.; Rivera-Rodriguez, G.R. Development of Chitosan and Alginate Nanocapsules to Increase the Solubility, Permeability and Stability of Curcumin. J. Pharm. Innov. 2019, 14, 132–140. [Google Scholar] [CrossRef]
- Luo, L.; Wu, Y.; Liu, C.; Zou, Y.; Huang, L.; Liang, Y.; Ren, J.; Liu, Y.; Lin, Q. Elaboration and characterization of curcumin-loaded soy soluble polysaccharide (SSPS)-based nanocarriers mediated by antimicrobial peptide nisin. Food Chem. 2021, 336, 127669. [Google Scholar] [CrossRef]
- Vasudevan, S.; Prabhune, A.A. Photophysical studies on curcumin-sophorolipid nanostructures: Applications in quorum quenching and imaging. R. Soc. Open Sci. 2018, 5, 170865. [Google Scholar] [CrossRef]
- Oves, M.; Rauf, M.A.; Ansari, M.O.; Khan, A.A.P.; Qari, H.A.; Alajmi, M.F.; Sau, S.; Iyer, A.K. Graphene Decorated Zinc Oxide and Curcumin to Disinfect the Methicillin-Resistant Staphylococcus aureus. Nanomaterials 2020, 10, 1004. [Google Scholar] [CrossRef] [PubMed]
- Prateeksha; Rao, C.V.; Das, A.K.; Barik, S.K.; Singh, B.N. ZnO/Curcumin Nanocomposites for Enhanced Inhibition of Pseudomonas aeruginosa Virulence via LasR-RhlR Quorum Sensing Systems. Mol. Pharm. 2019, 16, 3399–3413. [Google Scholar] [CrossRef]
- Hami, Z. Coating Iron Oxide Nanoparticles with Chitosan for Targeted Delivery of Nanocurcumin. Ann. Mil. Health Sci. Res. 2020, 18, 103657. [Google Scholar] [CrossRef]
- Yang, Q.-Q.; Farha, A.K.; Kim, G.; Gul, K.; Gan, R.-Y.; Corke, H. Antimicrobial and anticancer applications and related mechanisms of curcumin-mediated photodynamic treatments. Trends Food Sci. Technol. 2020, 97, 341–354. [Google Scholar] [CrossRef]
- Abdulrahman, H.; Misba, L.; Ahmad, S.; Khan, A.U. Curcumin induced photodynamic therapy mediated suppression of quorum sensing pathway of Pseudomonas aeruginosa: An approach to inhibit biofilm in vitro. Photodiagn. Photodyn. Ther. 2020, 30, 101645. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Chen, B.; Li, H.; Zeng, Q.-H.; Wang, J.J.; Liu, H.; Pan, Y.; Zhao, Y. Enhanced antibacterial and antibiofilm functions of the curcumin-mediated photodynamic inactivation against Listeria monocytogenes. Food Control 2020, 108, 106886. [Google Scholar] [CrossRef]
- Pan, H.; Wang, D.; Zhang, F. In vitro antimicrobial effect of curcumin-based photodynamic therapy on Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans. Photodiagn. Photodyn. Ther. 2020, 32, 102055. [Google Scholar] [CrossRef] [PubMed]
- Rocha, M.P.; Santos, M.S.; Rodrigues, P.L.F.; Araújo, T.S.D.; de Oliveira, J.M.; Rosa, L.P.; Bagnato, V.S.; da Silva, F.C. Photodynamic therapry with curcumin in the reduction of enterococcus faecalis biofilm in bone cavity: rMicrobiological and spectral fluorescense analysis. Photodiagn. Photodyn. Ther. 2021, 33, 102084. [Google Scholar] [CrossRef]
- Chen, L.; Song, Z.; Zhi, X.; Du, B. Photoinduced Antimicrobial Activity of Curcumin-Containing Coatings: Molecular Interaction, Stability and Potential Application in Food Decontamination. ACS Omega 2020, 5, 31044–31054. [Google Scholar] [CrossRef]
- Rocha, M.P.; Ruela, A.L.; Rosa, L.P.; Santos, G.P.; Rosa, F.C. Antimicrobial photodynamic therapy in dentistry using an oil-in-water microemulsion with curcumin as a mouthwash. Photodiagnosis Photodyn. Ther. 2020, 32, 101962. [Google Scholar] [CrossRef]
- Nima, G.; Soto-Montero, J.; Alves, L.A.; Mattos-Graner, R.O.; Giannini, M. Photodynamic inactivation of Streptococcus mutans by curcumin in combination with EDTA. Dent. Mater. 2021, 37, e1–e14. [Google Scholar] [CrossRef]
- Mirzahosseinipour, M.; Khorsandi, K.; Hosseinzadeh, R.; Ghazaeian, M.; Shahidi, F.K. Antimicrobial photodynamic and wound healing activity of curcumin encapsulated in silica nanoparticles. Photodiagn. Photodyn. Ther. 2020, 29, 101639. [Google Scholar] [CrossRef]
- Ahmadi, H.; Haddadi-Asl, V.; Ghafari, H.-A.; Ghorbanzadeh, R.; Mazlum, Y.; Bahador, A. Shear bond strength, adhesive remnant index, and anti-biofilm effects of a photoexcited modified orthodontic adhesive containing curcumin doped poly lactic-co-glycolic acid nanoparticles: An ex-vivo biofilm model of S. mutans on the enamel slab bonded brackets. Photodiag. Photodyn. Ther. 2020, 30, 101674. [Google Scholar] [CrossRef]
- Zago, L.H.D.P.; de Annunzio, S.R.; de Oliveira, K.T.; Barbugli, P.A.; Valdes, B.R.; Feres, M.; Fontana, C.R. Antimicrobial photodynamic therapy against metronidazole-resistant dental plaque bactéria. J. Photochem. Photobiol. B Biol. 2020, 209, 111903. [Google Scholar] [CrossRef] [PubMed]
- Pourhajibagher, M.; Plotino, G.; Chiniforush, N.; Bahador, A. Dual wavelength irradiation antimicrobial photodynamic therapy using indocyanine green and metformin doped with nano-curcumin as an efficient adjunctive endodontic treatment modality. Photodiag. Photodyn. Ther. 2020, 29, 101628. [Google Scholar] [CrossRef]
- Pourhajibagher, M.; Hodjat, M.; Bahador, A. Sonodynamic excitation of nanomicelle curcumin for eradication of Streptococcus mutans under sonodynamic antimicrobial chemotherapy: Enhanced anti-caries activity of nanomicelle curcumin. Photodiag. Photodyn. Ther. 2020, 30, 101780. [Google Scholar] [CrossRef] [PubMed]
- Kali, A.; Bhuvaneshwar, D.; Charles, P.M.V.; Seetha, K.S. Antibacterial synergy of curcumin with antibiotics against biofilm producing clinical bacterial isolates. J. Basic Clin. Pharm. 2016, 7, 93–96. [Google Scholar] [CrossRef]
- Hamzah, H.; Hertiani, T.; Pratiwi, S.U.T.; Nuryastuti, T. Inhibitory activity and degradation of curcumin as Anti-Biofilm Polymicrobial on Catheters. Int. J. Res. Pharm. Sci. 2020, 11, 830–835. [Google Scholar] [CrossRef][Green Version]
- Hosseini, A.; Nejadsattari, T.; Zargar, M. In Vitro Anti-Biofilm Activity of Curcumin Nanoparticles in Acinetobacter baumannii: A Culture-Based and Molecular Approach. Arch. Clin. Infect. Dis. 2019, 14, 83263. [Google Scholar] [CrossRef]
- Yadav, S.; Singh, A.K.; Agrahari, A.K.; Sharma, K.; Singh, A.S.; Gupta, M.K.; Tiwari, V.K.; Prakash, P. Making of water soluble curcumin to potentiate conventional antimicrobials by inducing apoptosis-like phenomena among drug-resistant bacteria. Sci. Rep. 2020, 10, 1–22. [Google Scholar] [CrossRef]
- Adlia, A.; Tomagola, I.; Damayanti, S.; Mulya, A.; Rachmawati, H. Antifibrotic Activity and In Ovo Toxicity Study of Liver-Targeted Curcumin-Gold Nanoparticle. Sci. Pharm. 2018, 86, 41. [Google Scholar] [CrossRef] [PubMed]
- Muniyappan, N.; Pandeeswaran, M.; Amalraj, A. Green synthesis of gold nanoparticles using Curcuma pseudomontana isolated curcumin: Its characterization, antimicrobial, antioxidant and anti-inflammatory activities. Environ. Chem. Ecotoxicol. 2021, 3, 117–124. [Google Scholar] [CrossRef]
- Chuacharoen, T.; Sabliov, C.M. Comparative effects of curcumin when delivered in a nanoemulsion or nanoparticle form for food applications: Study on stability and lipid oxidation inhibition. LWT 2019, 113, 108319. [Google Scholar] [CrossRef]
- Marcolino, V.A.; Zanin, G.M.; Durrant, L.R.; Benassi, M.D.T.; Matioli, G. Interaction of Curcumin and Bixin with β-Cyclodextrin: Complexation Methods, Stability, and Applications in Food. J. Agric. Food Chem. 2011, 59, 3348–3357. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.; Liaqat, I.; Hyder, M.Z.; Akhtar, S.; Bhatti, A.H.; Butt, S.B.; Imran, Z.; Yasmin, T.; Abbas, S. Elucidation of larvicidal potential of metallic and environment friendly food-grade nanostructures against Aedes albopictus. Environ. Geochem. Health 2020, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Olivo, P.M.; Scapim, M.R.D.S.; Miazaki, J.; Madrona, G.S.; Maia, L.F.; Rodrigues, B.M.; Pozza, M.S.D.S. Sodium alginate with turmeric coating for ripened cheeses. J. Food Sci. Technol. 2020, 57, 2364–2369. [Google Scholar] [CrossRef] [PubMed]
- Matche, R.S.; Anup, G.J.; Mrudula, G. Development of Biodegradable Films from Marine Ingredients Incorporated with Natural Antimicrobial Agents for Food Packaging. J. Packag. Technol. Res. 2020, 4, 45–55. [Google Scholar] [CrossRef]
- Dalvandi, F.; Almasi, H.; Ghanbarzadeh, B.; Hosseini, H.; Khosroshahi, N.K. Effect of vacuum packaging and edible coating containing black pepper seeds and turmeric extracts on shelf life extension of chicken breast fillets. JFBE 2020, 3, 69–78. [Google Scholar]
- Li, T.; Zhao, Y.; Matthews, K.; Gao, J.; Hao, J.; Wang, S.; Han, J.; Jia, Y. Antibacterial activity against Staphylococcus aureus of curcumin-loaded chitosan spray coupled with photodynamic treatment. LWT 2020, 134, 110073. [Google Scholar] [CrossRef]
- Luo, X.; Lim, L.-T. Curcumin-loaded electrospun nonwoven as a colorimetric indicator for volatile amines. LWT 2020, 128, 109493. [Google Scholar] [CrossRef]
- Balbinot-Alfaro, E.; Craveiro, D.V.; Lima, K.O.; Costa, H.L.G.; Lopes, D.R.; Prentice, C. Intelligent Packaging with pH Indicator Potential. Food Eng. Rev. 2019, 11, 235–244. [Google Scholar] [CrossRef]
- Halonen, N.; Pálvölgyi, P.S.; Bassani, A.; Fiorentini, C.; Nair, R.; Spigno, G.; Kordas, K. Bio-Based Smart Materials for Food Packaging and Sensors—A Review. Front. Mater. 2020, 7, 82. [Google Scholar] [CrossRef]
- Ma, X.; Chen, Y.; Huang, J.; Lv, P.; Hussain, T.; Wei, Q. In Situ formed active and intelligent bacterial cellulose/cotton fiber composite containing curcumin. Cellulose 2020, 27, 1–12. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Antioxidant and antimicrobial poly (vinyl alcohol)-based films incorporated with grapefruit seed extract and curcumin. J. Environ. Chem. Eng. 2021, 9, 104694. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Carboxymethyl cellulose-based antioxidant and antimicrobial active packaging film incorporated with curcumin and zinc oxide. Int. J. Biol. Macromol. 2020, 148, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Gao, R.; Wang, L.; Xu, M.; Yuan, Y.; Ma, L.; Wan, Z.; Yang, X. Nanocomposites of Bacterial Cellulose Nanofibrils and Zein Nanoparticles for Food Packaging. ACS Appl. Nano Mater. 2020, 3, 2899–2910. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Guo, M.; Jin, T.Z.; Arabi, S.A.; He, Q.; Ismail, B.B.; Hu, Y.; Liu, D. Antimicrobial and UV Blocking Properties of Composite Chitosan Films with Curcumin Grafted Cellulose Nanofiber. Food Hydrocoll. 2021, 112, 106337. [Google Scholar] [CrossRef]
- Zhou, Q.; Gao, Y.; Wang, X.; Liu, R.; Du, P.; Wang, X.; Zhang, X.; Lu, S.; Wang, Z.; Shi, Q.; et al. Nosocomial infections among patients with COVID-19, SARS and MERS: A rapid review and meta-analysis. Ann. Transl. Med. 2020, 8, 629. [Google Scholar] [CrossRef] [PubMed]
- Bardi, T.; Pintado, V.; Gomez-Rojo, M.; Escudero-Sanchez, R.; Lopez, A.A.; Diez-Remesal, Y.; Castro, N.M.; Ruiz-Garbajosa, P.; Pestaña, D. Nosocomial infections associated to COVID-19 in the intensive care unit: Clinical characteristics and outcome. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 495–502. [Google Scholar] [CrossRef]
- Wake, R.M.; Morgan, M.; Choi, J.; Winn, S. Reducing nosocomial transmission of COVID-19: Implementation of a COVID-19 triage system. Clin. Med. 2020, 20, e141–e145. [Google Scholar] [CrossRef]
- Abbas, M.; Nunes, T.R.; Martischang, R.; Zingg, W.; Iten, A.; Pittet, D.; Harbarth, S. Nosocomial transmission and outbreaks of coronavirus disease 2019: The need to protect both patients and healthcare workers. Antimicrob. Resist. Infect. Control 2021, 10, 1–13. [Google Scholar] [CrossRef]
- Spice It Up: 7 Turmeric Benefits For Skin. Available online: www.neutrogena.com/the-bar/spice-it-up-7-turmeric-benefits-for-skin.html (accessed on 17 January 2021).
- Turmeric. Available online: www.lorealparisusa.com/ingredient-library/turmeric.aspx (accessed on 17 January 2021).
- Zheng, Y.; Pan, C.; Zhang, Z.; Luo, W.; Liang, X.; Shi, Y.; Liang, L.; Zheng, X.; Zhang, L.; Du, Z. Antiaging effect of Curcuma longa L. essential oil on ultraviolet-irradiated skin. Microchem. J. 2020, 154, 104608. [Google Scholar] [CrossRef]
- Sienkiewicz, N.; Członka, S.; Kairyte, A.; Vaitkus, S. Curcumin as a natural compound in the synthesis of rigid polyurethane foams with enhanced mechanical, antibacterial and anti-ageing properties. Polym. Test. 2019, 79, 106046. [Google Scholar] [CrossRef]
- Vater, C.; Hlawaty, V.; Werdenits, P.; Cichoń, M.A.; Klang, V.; Elbe-Bürger, A.; Wirth, M.; Valenta, C. Effects of lecithin-based nanoemulsions on skin: Short-time cytotoxicity MTT and BrdU studies, skin penetration of surfactants and additives and the delivery of curcumin. Int. J. Pharm. 2020, 580, 119209. [Google Scholar] [CrossRef] [PubMed]
- Rapalli, V.K.; Kaul, V.; Waghule, T.; Gorantla, S.; Sharma, S.; Roy, A.; Dubey, S.K.; Singhvi, G. Curcumin loaded nanostructured lipid carriers for enhanced skin retained topical delivery: Optimization, scale-up, in-vitro characterization and assessment of ex-vivo skin deposition. Eur. J. Pharm. Sci. 2020, 152, 105438. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Z.; Xiao, L.; Ding, Z.; He, J.; Lu, G.; Lu, Q.; Kaplan, D.L. Natural Nanofiber Shuttles for Transporting Hydrophobic Cargo into Aqueous Solutions. Biomacromolecules 2019, 21, 1022–1030. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.T.; Lee, S.; Kim, J.S.; Jeong, J.; Jeon, B.S.; Lee, J.W.; Kim, J.H.; Kim, J. Highly Stable and Fine-Textured Hybrid Microspheres for Entrapment of Cosmetic Active Ingredients. ACS Omega 2020, 5, 29577–29584. [Google Scholar] [CrossRef] [PubMed]
- Al-Akayleh, F.; Al-Naji, I.; Adwan, S.; Al-Remawi, M.; Shubair, M. Enhancement of Curcumin Solubility Using a Novel Solubilizing Polymer Soluplus®. J. Pharm. Innov. 2020, 1–13. [Google Scholar] [CrossRef]
- Wang, F.; Li, Y.; Yu, L.; Zhu, J.; Zhang, F.; Linhardt, R.J. Amphiphilic mPEG-Modified Oligo-Phenylalanine Nanoparticles Chemoenzymatically Synthesized via Papain. ACS Omega 2020, 5, 30336–30347. [Google Scholar] [CrossRef]
- Enumo, A.; Argenta, D.F.; Bazzo, G.C.; Caon, T.; Stulzer, H.K.; Parize, A.L. Development of curcumin-loaded chitosan/pluronic membranes for wound healing applications. Int. J. Biol. Macromol. 2020, 163, 167–179. [Google Scholar] [CrossRef]
- Huang, Y.; Dan, N.; Dan, W.; Zhao, W. Reinforcement of Polycaprolactone/Chitosan with Nanoclay and Controlled Release of Curcumin for Wound Dressing. ACS Omega 2019, 4, 22292–22301. [Google Scholar] [CrossRef]
- Duan, Y.; Li, K.; Wang, H.; Wu, T.; Zhao, Y.; Li, H.; Tang, H.; Yang, W. Preparation and evaluation of curcumin grafted hyaluronic acid modified pullulan polymers as a functional wound dressing material. Carbohydr. Polym. 2020, 238, 116195. [Google Scholar] [CrossRef]
- Liao, H.T.; Lai, Y.-T.; Kuo, C.-Y.; Chen, J.-P. A bioactive multi-functional heparin-grafted aligned poly(lactide-co-glycolide)/curcumin nanofiber membrane to accelerate diabetic wound healing. Mater. Sci. Eng. C 2021, 120, 111689. [Google Scholar] [CrossRef]
- Kaolaor, A.; Phunpee, S.; Ruktanonchai, U.R.; Suwantong, O. Effects of β-cyclodextrin complexation of curcumin and quaternization of chitosan on the properties of the blend films for use as wound dressings. J. Polym. Res. 2019, 26, 43. [Google Scholar] [CrossRef]
- Mohanty, C.; Pradhan, J. A human epidermal growth factor-curcumin bandage bioconjugate loaded with mesenchymal stem cell for in vivo diabetic wound healing. Mater. Sci. Eng. C 2020, 111, 110751. [Google Scholar] [CrossRef]
- Pandey, V.K.; Ajmal, G.; Upadhyay, S.N.; Mishra, P.K. Nano-fibrous scaffold with curcumin for anti-scar wound healing. Int. J. Pharm. 2020, 589, 119858. [Google Scholar] [CrossRef]
- Naghshineh, N.; Tahvildari, K.; Nozari, M. Preparation of Chitosan, Sodium Alginate, Gelatin and Collagen Biodegradable Sponge Composites and their Application in Wound Healing and Curcumin Delivery. J. Polym. Environ. 2019, 27, 2819–2830. [Google Scholar] [CrossRef]
- Gupta, A.; Briffa, S.M.; Swingler, S.; Gibson, H.; Kannappan, V.; Adamus, G.; Kowalczuk, M.M.; Martin, C.; Radecka, I. Synthesis of Silver Nanoparticles Using Curcumin-Cyclodextrins Loaded into Bacterial Cellulose-Based Hydrogels for Wound Dressing Applications. Biomacromolecules 2020, 21, 1802–1811. [Google Scholar] [CrossRef] [PubMed]
- El-Nahhal, I.M.; Salem, J.; Anbar, R.; Kodeh, F.S.; Elmanama, A. Preparation and antimicrobial activity of ZnO-NPs coated cotton/starch and their functionalized ZnO-Ag/cotton and Zn (II) curcumin/cotton materials. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, C.; Varaprasad, K.; Akbari-Fakhrabadi, A.; Hameed, A.S.H.; Sadiku, R. Biomolecule chitosan, curcumin and ZnO-based antibacterial nanomaterial, via a one-pot process. Carbohydr. Polym. 2020, 249, 116825. [Google Scholar] [CrossRef]
- Anagha, B.; George, D.; Maheswari, P.U.; Begum, K.M.M.S. Biomass derived antimicrobial hybrid cellulose hydrogel with green ZnO nanoparticles for curcumin delivery and its kinetic modelling. J. Polym. Environ. 2019, 27, 2054–2067. [Google Scholar] [CrossRef]
- Marulasiddeshwara, R.; Jyothi, M.; Soontarapa, K.; Keri, R.S.; Velmurugan, R. Nonwoven fabric supported, chitosan membrane anchored with curcumin/TiO2 complex: Scaffolds for MRSA infected wound skin reconstruction. Int. J. Biol. Macromol. 2020, 144, 85–93. [Google Scholar] [CrossRef]
- Konwar, A.; Kandimalla, R.; Kalita, S.; Chowdhury, D. Approach to Fabricate a Compact Cotton Patch without Weaving: A Smart Bandage Material. ACS Sustain. Chem. Eng. 2018, 6, 5806–5817. [Google Scholar] [CrossRef]
- Kuddushi, M.; Patel, N.K.; Gawali, S.L.; Mata, J.P.; Montes-Campos, H.; Varela, L.M.; Hassan, P.A.; Malek, N.I. Thermo-switchable de novo ionogel as metal absorbing and curcumin loaded smart bandage material. J. Mol. Liq. 2020, 306, 112922. [Google Scholar] [CrossRef]
- Ahmed, S.S.Z.; Balu, N.; Khader, S.Z.A.; Mahboob, M.R.; Lakshmanan, S.O.; Vetrivel, M. Fabrication and evaluation of bamboo fabric coated with extracts of Curcuma longa, Centella asiatica and Azadirachta indica as a wound dressing material. Adv. Tradit. Med. 2021, 21, 83–95. [Google Scholar] [CrossRef]
- Kulkarni, A.S.; Gurav, D.D.; Khan, A.A.; Shinde, V.S. Curcumin loaded nanofibrous mats for wound healing application. Colloids Surf. B Biointerfaces 2020, 189, 110885. [Google Scholar] [CrossRef]
- Tangsatianpan, V.; Torgbo, S.; Sukyai, P. Release Kinetic Model and Antimicrobial Activity of Freeze-Dried Curcumin-loaded Bacterial Nanocellulose Composite. Polym. Sci. Ser. A 2020, 62, 218–227. [Google Scholar] [CrossRef]
- Mohanty, C.; Das, M.; Sahoo, S.K. Sustained Wound Healing Activity of Curcumin Loaded Oleic Acid Based Polymeric Bandage in a Rat Model. Mol. Pharm. 2012, 9, 2801–2811. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, X.; Pi, C.; Yang, H.; Zheng, X.; Zhao, L.; Wei, Y. Review of Curcumin Physicochemical Targeting Delivery System. Int. J. Nanomed. 2020, 15, 9799–9821. [Google Scholar] [CrossRef]
- Zheng, B.; McClements, D.J. Formulation of More Efficacious Curcumin Delivery Systems Using Colloid Science: Enhanced Solubility, Stability, and Bioavailability. Molecules 2020, 25, 2791. [Google Scholar] [CrossRef]
- Al-Soliemy, A.; Al-Zahrani, F. Synthesis of novel disperse dyes based on curcumin for the creation of antibacterial polyester fabrics. Pigment. Resin Technol. 2019, 48, 502–507. [Google Scholar] [CrossRef]
- Atlan, M.; Neman, J. Targeted Transdermal Delivery of Curcumin for Breast Cancer Prevention. Int. J. Environ. Res. Public Health 2019, 16, 4949. [Google Scholar] [CrossRef]
- Sharma, N.; Nehru, B. Curcumin affords neuroprotection and inhibits α-synuclein aggregation in lipopolysaccharide-induced Parkinson’s disease model. Inflammopharmacology 2018, 26, 349–360. [Google Scholar] [CrossRef]
- Ferreira, N.; Saraiva, M.J.; Almeida, M.R. Uncovering the Neuroprotective Mechanisms of Curcumin on Transthyretin Amyloidosis. Int. J. Mol. Sci. 2019, 20, 1287. [Google Scholar] [CrossRef]
- Lin, X.; Watanabe, K.; Kuragano, M.; Tokuraku, K. Aggregation of Mouse Serum Amyloid A Protein Was Promoted by Amyloid-Enhancing Factors with the More Genetically Homologous Serum Amyloid A. Int. J. Mol. Sci. 2021, 22, 1036. [Google Scholar] [CrossRef]
- Gao, C.; Chu, X.; Gong, W.; Zheng, J.; Xie, X.; Wang, Y.; Yang, M.; Li, Z.; Gao, C.; Yang, Y. Neuron tau-targeting biomimetic nanoparticles for curcumin delivery to delay progression of Alzheimer’s disease. J. Nanobiotechnol. 2020, 18, 1–23. [Google Scholar] [CrossRef]
- Mansouri, K.; Rasoulpoor, S.; Daneshkhah, A.; Abolfathi, S.; Salari, N.; Mohammadi, M.; Shabani, S. Clinical effects of curcumin in enhancing cancer therapy: A systematic review. BMC Cancer 2020, 20, 1–11. [Google Scholar] [CrossRef]
- Rizeq, B.; Gupta, I.; Ilesanmi, J.; Alsafran, M.; Rahman, M.; Ouhtit, A. The Power of Phytochemicals Combination in Cancer Chemoprevention. J. Cancer 2020, 11, 4521–4533. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, J.; Jiang, B.; Guo, J. The roles of curcumin in regulating the tumor immunosuppressive microenvironment (Review). Oncol. Lett. 2020, 19, 3059–3070. [Google Scholar] [CrossRef]
- Willenbacher, E.; Khan, S.Z.; Mujica, S.C.A.; Trapani, D.; Hussain, S.; Wolf, D.; Willenbacher, W.; Spizzo, G.; Seeber, A. Curcumin: New Insights into an Ancient Ingredient against Cancer. Int. J. Mol. Sci. 2019, 20, 1808. [Google Scholar] [CrossRef]
- Gbenga, A.A.; Mwakikunga, A. Lopinavir and curcumin directly alters BAX/BCL2 and VEGF165b mRNA levels to suppress human squamous cervical carcinoma cell growth. Int. J. Morphol. 2019, 37, 584–591. [Google Scholar] [CrossRef]
- Shaikh, S.; Shaikh, J.; Naba, Y.S.; Doke, K.; Ahmed, K.; Yusufi, M. Curcumin: Reclaiming the lost ground against cancer resistance. Cancer Drug Resist. 2021, 4. [Google Scholar] [CrossRef]
- Tomeh, M.A.; Hadianamrei, R.; Zhao, X. A Review of Curcumin and Its Derivatives as Anticancer Agents. Int. J. Mol. Sci. 2019, 20, 1033. [Google Scholar] [CrossRef] [PubMed]
- Manzanares-Guevara, L.A.; Licea-Claverie, A.; Oroz-Parra, I.; Bernaldez-Sarabia, J.; Diaz-Castillo, F.; Licea-Navarro, A.F. Smart Nanoformulation Based on Stimuli-Responsive Nanogels and Curcumin: Promising Therapy against Colon Cancer. ACS Omega 2020, 5, 9171–9184. [Google Scholar] [CrossRef]
- Pandey, A.; Chaturvedi, M.; Mishra, S.; Kumar, P.; Somvanshi, P.; Chaturvedi, R. Reductive metabolites of curcumin and their therapeutic effects. Heliyon 2020, 6, e05469. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-B.; Luo, D.-D.; Xie, J.-H.; Xian, Y.-F.; Lai, Z.-Q.; Liu, Y.-H.; Liu, W.-H.; Chen, J.-N.; Lai, X.-P.; Lin, Z.-X.; et al. Curcumin’s Metabolites, Tetrahydrocurcumin and Octahydrocurcumin, Possess Superior Anti-inflammatory Effects in vivo Through Suppression of TAK1-NF-κB Pathway. Front. Pharmacol. 2018, 9, 1181. [Google Scholar] [CrossRef]
- Hadizadeh-Bazaz, M.; Vaezi, G.; Khaksari, M.; Hojati, V. Curcumin attenuates spatial memory impairment by anti-oxidative, anti-apoptosis, and anti-inflammatory mechanism against methamphetamine neurotoxicity in male Wistar rats: Histological and biochemical changes. NeuroToxicology 2021, 84, 208–217. [Google Scholar] [CrossRef]
- Mandal, M.; Jaiswal, P.; Mishra, A. Curcumin loaded nanoparticles reversed monocrotophos induced motor impairment and memory deficit: Role of oxidative stress and intracellular calcium level. J. Drug Deliv. Sci. Technol. 2020, 56, 101559. [Google Scholar] [CrossRef]
- Laabbar, W.; Abbaoui, A.; Elgot, A.; Mokni, M.; Amri, M.; Masmoudi-Kouki, O.; Gamrani, H. Aluminum induced oxidative stress, astrogliosis and cell death in rat astrocytes, is prevented by curcumin. J. Chem. Neuroanat. 2021, 112, 101915. [Google Scholar] [CrossRef]
- Hussein, Y.A.; Al-Sarraf, A.M.; Alfalluji, W.L. Modulation of oxidative stress, inflammatory and apoptotic response by curcumin against cerebral ischemia reperfusion injury in a mouse model. Interdiscip. Neurosurg. 2020, 21, 100741. [Google Scholar] [CrossRef]
- Ahmadabady, S.; Beheshti, F.; Shahidpour, F.; Khordad, E.; Hosseini, M. A protective effect of curcumin on cardiovascular oxidative stress indicators in systemic inflammation induced by lipopolysaccharide in rats. Biochem. Biophys. Rep. 2021, 25, 100908. [Google Scholar] [CrossRef]
- Prathipati, B.; Rohini, P.; Kola, P.K.; Danduga, R.C.S.R. Neuroprotective effects of curcumin loaded solid lipid nanoparticles on homocysteine induced oxidative stress in vascular dementia. CRBS 2021, 2, 100029. [Google Scholar]
- Fadeel, D.A.A.; Kamel, R.; Fadel, M. PEGylated lipid nanocarrier for enhancing photodynamic therapy of skin carcinoma using curcumin: In-Vitro/In-Vivo studies and histopatho-logical examination. Sci. Rep. 2020, 10, 10435. [Google Scholar] [CrossRef]
- Baghdan, E.; Duse, L.; Schüer, J.J.; Pinnapireddy, S.R.; Pourasghar, M.; Schäfer, J.; Schneider, M.; Bakowsky, U. Development of inhalable curcumin loaded Nano-in-Microparticles for bronchoscopic photodynamic therapy. Eur. J. Pharm. Sci. 2019, 132, 63–71. [Google Scholar] [CrossRef]
- Su, W.; Wei, T.; Lu, M.; Meng, Z.; Chen, X.; Jing, J.; Li, J.; Yao, W.; Zhu, H.; Fu, T. Treatment of metastatic lung cancer via inhalation administration of curcumin composite particles based on mesoporous silica. Eur. J. Pharm. Sci. 2019, 134, 246–255. [Google Scholar] [CrossRef]
- Kazantzis, C.; Koutsonikoli, K.; Mavroidi, B.; Zachariadis, M.; Alexiou, P.; Pelecanou, M.; Politopoulos, K.; Alexandratou, E.; Sagnou, M. Curcumin derivatives as photosensitizers in photodynamic therapy: Photophysical properties and in vitro studies with prostate cancer cells. Photochem. Photobiol. Sci. 2020, 19, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Kamel, A.E.; Fadel, M.; Louis, D. Curcumin-loaded nanostructured lipid carriers prepared using Peceol™ and olive oil in photodynamic therapy: Development and application in breast cancer cell line. Int. J. Nanomed. 2019, 14, 5073–5085. [Google Scholar] [CrossRef] [PubMed]
- Ambreen, G.; Duse, L.; Tariq, I.; Ali, U.; Ali, S.; Pinnapireddy, S.R.; Bette, M.; Bakowsky, U.; Mandic, R. Sensitivity of Papilloma Virus-Associated Cell Lines to Photodynamic Therapy with Curcumin-Loaded Liposomes. Cancers 2020, 12, 3278. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.-H.; Yu, K.-H.; Huang, Y.-C.; Lee, C.-I. EGFR-targeted photodynamic therapy by curcumin-encapsulated chitosan/TPP nanoparticles. Int. J. Nanomed. 2018, 13, 903–916. [Google Scholar] [CrossRef]
- Kuang, G.; Zhang, Q.; He, S.; Liu, Y. Curcumin-loaded PEGylated mesoporous silica nanoparticles for effective photodynamic therapy. RSC Adv. 2020, 10, 24624–24630. [Google Scholar] [CrossRef]
- Bhuyan, T.; Singh, A.K.; Ghosh, S.S.; Bandyopadhyay, D. Magnetotactic curcumin iButtonbots as efficient bactericidal agents. Bull. Mater. Sci. 2020, 43, 1–10. [Google Scholar] [CrossRef]
- Singh, R.; Verma, K.; Kumar, R. Core–shell Ag-ZnO/Curcumin nanocomposite having optically active, thermally stable, hydrophilic surfaces for self cleaning applications. Appl. Phys. A 2020, 126, 1–15. [Google Scholar] [CrossRef]
- Ishak, N.A.; Hamidon, T.S.; Zi-Hui, T.; Hussin, M.H. Extracts of curcumin-incorporated hybrid sol–gel coatings for the corrosion mitigation of mild steel in 0.5 M HCl. J. Coatings Technol. Res. 2020, 17, 1515–1535. [Google Scholar] [CrossRef]
- Kashif, M.; Ngaini, Z.; Harry, A.V.; Vekariya, R.L.; Ahmad, A.; Zuo, Z.; Sahari, S.K.; Hussain, S.; Khan, Z.A.; Alarifi, A. An experimental and DFT study on novel dyes incorporated with natural dyes on titanium dioxide (TiO2) towards solar cell application. Appl. Phys. A 2020, 126, 1–13. [Google Scholar] [CrossRef]
- Sakshi; Pathak, N.K.; Swain, B.C.; Tripathy, U. Analyzing nonlinear trends in curcumin: A comparative study. Opt. Laser Technol. 2020, 121, 105822. [Google Scholar] [CrossRef]
- Zhu, C.; Li, R.; Chen, X.; Chalmers, E.; Liu, X.; Wang, Y.; Xu, B.B.; Liu, X. Ultraelasticyarns from curcumin-assisted ELD toward wearable human–machine interface textiles. Adv. Sci. 2020, 7, 2002009. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).