Abstract
Metal soaps formation is a well-known issue in oil paintings. Along the lifetime of the painting, carboxylic acids coming from drying oil (free fatty acids, acids from hydrolysis of triglycerides and from oxidation processes) can react with cations of some pigments (in particular, smalt, lead white and zinc white) forming the related carboxylic salts. As observed by many authors, the formation of these carboxylates, with the tendency to migrate and to aggregate, not only modifies the behavior and the aspect of the paint film but also complicates the cleaning approach. In previous works we have demonstrated that a similar pigment reactivity is possible even in presence of natural resins (such as colophony, dammar, mastic, etc) historically used as final varnishes on paintings. In this case, in the reactions the terpenic acids, among the main components of the resins, are involved. In this work, the carboxylates formation kinetics has been studied starting from two representative acids (palmitic and abietic) of painting oils and natural varnishes. Successively, the reactivity of the palmitic acid with the potassium abietate and of the abietic acid with the potassium palmitate has been verified. This investigation aims at clarifying in which way terpenic acids can be involved in the metal soaps reactivity confirming that also surface varnishes may play a significant role in the carboxylates formation and reactivity. It is important to keep in mind that a finishing varnish can be removed and reapplied many times during the lifetime of a painting, thus renewing the provision of reactive terpenic acids at the interface of the painted layers.
1. Introduction
One of the most common decay pattern of oil paintings is the formation, along the lifetime of the work of art, of metal soaps [1,2,3,4,5,6]. Carboxylic acids present in the drying oil can react with cations of some pigments (in particular, smalt, lead white and zinc white) forming the related carboxylic salts. Few free fatty acids are present in the fresh oil but during the ageing many factors such as the hydrolysis of triglycerides can increase drastically the concentration of free acids sites. Moreover, the characteristic of drying oils, linseed oil in particular, is the presence of double bonds that allow the crosslinking process. These reactive sites are also prone to oxidative processes that easily lead to the chain breaking and the formation of new acid sites [7,8,9,10,11,12]. As observed by many authors, these carboxylates, defined metal soaps, with the tendency to migrate in the painted layer and to aggregate, not only modify the chemical-physical properties but also the aesthetic of the painting by generating efflorescences and in the worst cases protrusions [13,14,15,16,17]. Many studies have been carried out in order to understand the chemical structure of these aggregates and in which way the aggregation takes place. Main results suggest that cations migrate from the pigment surface to the bulk of the binder (the drying oil) shifting from an acid site to another. The polar mobile molecules aggregate in three-dimensional structures, a polymeric/ionomeric network, exploiting bifunctional “bricks” such as the azelaic acids molecules coming from the oxidation of the unsaturated fatty acids [18]. The presence on surface of these protrusions and in general of metal soaps make the cleaning approach to the painting definitely complicate and delicate. Metal soaps cannot be easily removed from painting layers and surfaces since they are poorly soluble in traditional solvents used in conservation and, moreover, not all the conservators agree on their removal.
In previous works authors demonstrated that a similar pigment particles reactivity is possible even in presence of natural resins (such as colophony, dammar, mastic, etc) historically used as final varnishes on paintings [19,20,21,22,23,24]. The reaction involves the terpenic acids (mono, di, and triterpenic), the main components of this kind of resins. In a theoretical situation, being “protected” by the binder, very few pigment can come in contact with the natural resins (historical recipes of varnishes are always a mixture of resins) since they are applied as final superficial varnish. It is important to point out that, in the past, artists often used to add some resin in the drying oil recipes and the first varnish application occurs early when the oil is not perfectly dried [25]. Moreover, natural varnishes were applied through the use of a common solvent (the turpentine): factors that can favor the interaction of the two phases. Moreover, the usual conservation treatments of a painting include the varnish substitution [26]: new fresh terpenic varnish in solvent is applied and come in contact with painted layers depleted in the organic component (the binder) as a consequence of previous usual solvent-based removal of the old varnish. It must be considered that this operation could have taken place several times in the life of a painting.
In this work the carboxylates formation and kinetics have been studied by Fourier Transform Infrared (FTIR) spectroscopy, working in transmission and with an Attenuated Total Reflectance (ATR) accessory. Mid-infrared spectroscopy has been proven to be one of the most effective and sensitive techniques for the detection of metal soaps. They are characterized by a typical signal between 1520 and 1590 cm−1 depending on the cation involved [1,5,6,9,10,12,13,14,15,16,19,21,22,23]. exploiting two representative acids of painting oils and natural varnishes (palmitic and abietic acid respectively) and potassium hydroxide (KOH) as cation source. The potassium cation has been chosen since it is involved in an important degradation reaction in the field of painting conservation: the discoloration of smalt (see below) [27,28,29,30,31,32]. Smalt is an historically widely employed blue pigment constituted by a fine grinded potassium glass and it is well-known that is one of the most reactive pigment in presence of drying oil. This simplified model system was useful for interpreting the results about the reactivity and kinetics, under the same laboratory conditions, of the mixture made with drying oil, natural resins and smalt. Successively, the reactivity of the two acids has been investigated by putting them in contact with the already formed potassium metal soap: palmitic acid with potassium abietate and abietic acid with potassium palmitate. The whole work was aimed at clarifying in which way terpenic acids can be involved in the metal soaps reactivity, and at confirming that also surface varnishes may play a significant role in the carboxylates formation and reactivity.
2. Materials and Methods
Palmitic acid (Hexadecanoic acid, ≥99%), Abietic acid (technical, ~75%) and potassium hydroxide (pellets for analysis EMSURE®) have been supplied by Sigma-Aldrich (Saint Louis, MO, USA.). Linseed stand oil (ref. 73,200) and smalt (ref. 10,000) have been supplied by Kremer Pigmente (average grinding of smalt around 10 microns).
Silicon float zone polished windows (13 mm diameter, by 1mm thickness) have been supplied by Crystran Ltd. (Dorset, UK).
FT-IR transmission spectra (64 scans) have been carried out on a Bruker Vertex 70 spectrophotometer (Bruker, Billerica, MA, USA.), working in the spectral range from 4000 to 400 cm−1 with an average spectral resolution of 4 cm−1.
Mixtures have been applied on the silicon wafer with a plastic spatula (in order to avoid an eventual surface scratching) in a thin layer that allows a good transmission signal with absorbance of the strongest band around 1. Once applied the mixture the recording of spectra started, and a spectrum every 15 min has been recorded for a total of 700 min. Successively, a spectrum every hour has been recorded till 240 h. The kinetic measurements of abietate salts mixed with palmitic acid and of palmitate salts mixed with abietic acid have been carried on a single angle ATR equipment (Harrick, Pleasantville, NJ, USA.) in order to avoid saturation problems (we have the necessity to add material). To prepare the abietate and the palmitate for the reaction with the other acid, the stoichiometric ratio acid/KOH has been calculated and the amount of KOH has been reduced by 10% in order to be sure that there was no KOH unreacted and waited 20 days mixing twice a day. The amount of acid successively added, in order to verify the possibility of cation exchange, is calculated to have almost (the purity of commercially available abietic acid is ~75%) the same number of acid sites.
3. Results and Discussion
Since XVIIth century smalt is a widely used blue pigment. It is a potassium glass with color coordination centers occupied by cobalt cations. During the lifetime of the painting, humidity and acids force the leeching of cations from the glass structure favoring the carboxylates formation. Bibliography [27,28,29,30,31,32] suggests that metal soaps in smalt painted layers are mostly constituted by palmitic acid and potassium extracted from smalt pigment. The reaction can be formalized in this way where R–COOH is a generical fatty acid of the linseed stand oil:
≡Si–O− K+ + R–COO− H3O+→≡Si-OH + R–COO− K+ + H2O
This is a very important reaction in the field of conservation since is the main cause of the discoloration of smalt in ancient paintings. The first step of this work aimed at verifying the reactivity and the kinetics of a stand oil/smalt system. These data are important as reference in order to verify if the simplified modeling of the successive steps is sensible. The behaviour of a mixture of 1:1 (w/w) stand oil/smalt applied on a silicon wafer is therefore compared with the behaviour of a mixture of linseed oil potassium hydroxide (KOH) and palmitic acid with KOH.
In Figure 1 it is possible to observe the growth of a broad band ranging from 1525 to 1600 cm−1, centered at 1572 cm−1, related to the formation of metal soap in the experimental condition (fine grinding of smalt around 10 microns, 22 °C, 45% RH% and applied with a spatula on the silicon wafer) and the corresponding increase of the OH broad band around 3400 cm−1 related to the formation of water molecules. The carbonyl of carboxylic salts bands appears at lower wavenumbers (from ~1700 to ~1550 cm−1) than the carbonyl of the acid in free form or of the esters (see Table 1). In Table 1, the main signals, in the mid-infrared spectral range, of linseed oil, colophony, abietic and palmitic acid, are reported according to literature [6,8,22,23,33,34,35,36]. Many factors can contribute to the broadening of the band even in the experimental conditions; this broadening is related to the presence of different kind of fatty acids (mono and di-functional), the carboxylates partially coordinated around the pigment grains, the free ones and the aggregated ones. The experimental conditions, in particular the fine grinding, aimed, unsuccessfully, to obtain a sharper band of carboxylates. In the real conditions this band is even broader due to the increased complexity of the system induced by the presence of other pigments and the oxidative ageing and crosslinking of the oil. A broad band would not permit to distinguish metal soaps coming from oil or resin acids since they absorb in a range very close as reported in literature [22,23].
KOH + R–COO− H3O+→R–COO− K+ + H2O
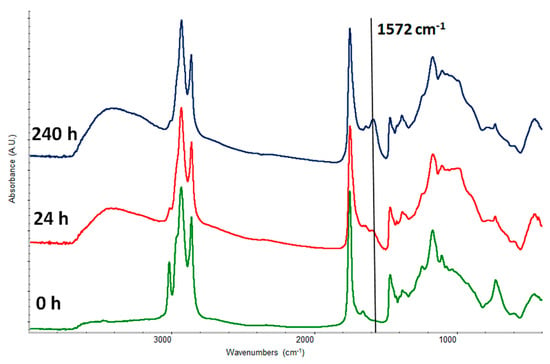
Figure 1.
Kinetics of smalt carboxylate formation from a mixture 1:1 (w/w) of stand oil and smalt. The black arrow shows the carboxylate band.

Table 1.
Table reports the position (in wavenumbers, cm−1) and the assignments of the main bands of linseed oil, colophony, palmitic acid and abietic acid.
As expected, substituting the smalt with KOH the reaction proceeds faster (Figure 2) according to the above reported reaction. The ratio surface/volume is by far more favorable, and the potassium is immediately available as basic hydroxide. Nevertheless, while the reaction proceeds the sharp band at 1564 cm−1 starts broadening. The slight shifting of the maximum from 1572 to 1564 cm−1 and the broadening confirms the fact that we are observing” different” potassium carboxylates: the neo-formed and, over time, the ones involved in more complex interactions with triglycerides and fatty acids (some of them partially cross-linked as pointed out by the decreasing of the double bond signal at 3011 cm−1) that re-organize in new aggregated ionomeric systems [1,9,13,15]. The double bonds are present in the unsaturated fatty acids of linseed oil and they are the responsible of the drying process.
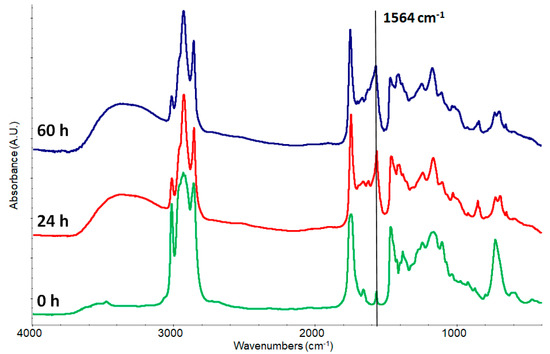
Figure 2.
Kinetics of potassium carboxylate formation from a mixture 1:1 (w/w) of stand oil and KOH. The black arrow shows the carboxylate band.
In order to obtain a definitely sharp band of K carboxylate we need to further simplify the system by substituting the complex natural mix of molecules inside the drying oil with a single representative molecule: palmitic acid. In Figure 3 we may observe that the carboxylate band, centered at 1564 cm−1, remains sharp and perfectly resolved.
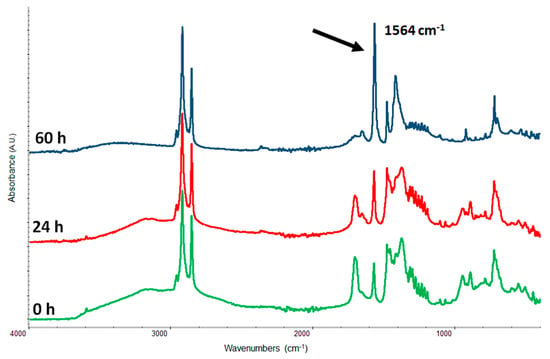
Figure 3.
Kinetics of potassium carboxylate formation from a mixture 1:1 (w/w) of palmitic acid and KOH. The black arrow shows the carboxylate band centered at 1564 cm−1.
Even in this case, where the binder is the natural resin, in order to obtain an enough resolved band of carboxylates (see Figure 4) it has not been possible to use a terpenic resin (colophony or dammar) which would form a broad band as assessed in a previous paper, but we used a representative molecule: abietic acid. Abietic acid has been chosen since is one of the few terpenic acids, characteristic of a natural resin, available on the market. Our measurements confirm that even the terpenic resins, the historical varnishes, on paintings can contribute to the formation of metal soaps and then to the smalt decay processes. The certainty of obtaining well resolved spike bands makes it possible the second part of this investigation where the reactivity of palmitic acid with an already formed potassium abietate, and vice versa, have been assessed. Here we want to verify the possibility of cation exchanging between drying oil metal soaps and the acid coming from a terpenic resin. In Figure 5 it is shown what happens when palmitic acid and abietic acid are mixed with an excess of potassium hydroxide. The expected reaction is reported below where P–COOH is the palmitic acid and A–COOH the abietic:
2KOH + P–COO− H3O+ + A–COO− H3O+→P–COO− K+ + A–COO− K+ + 2H2O
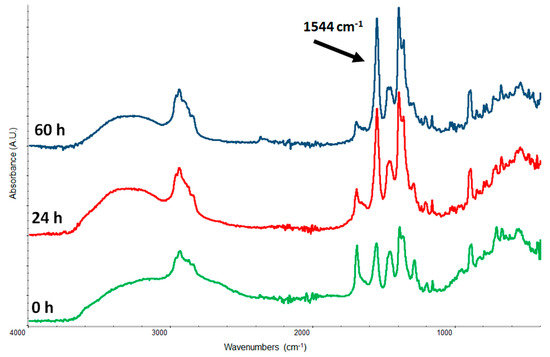
Figure 4.
Kinetics of potassium carboxylate formation from a mixture 1:1 (w/w) of abietic acid and KOH. The black arrow shows the carboxylate band centered at 1544 cm−1.
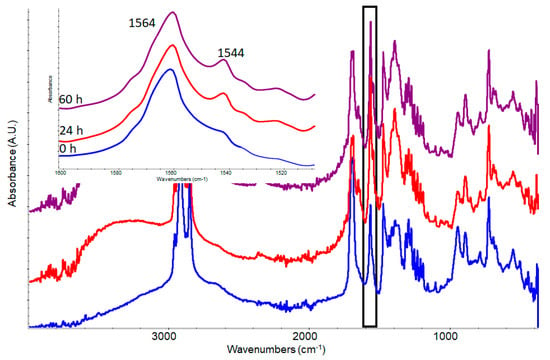
Figure 5.
Kinetics of potassium carboxylates formation from a mixture 1:2:1 (w/w) of palmitic acid, KOH and abietic acid. In the box the spectral range from 1500 to 1600 cm−1 is highlighted.
The simultaneous formation of the two carboxylates occurs at the previously verified wavenumbers of 1564 cm−1 for the palmitate and 1544 cm−1 for the abietate. This means that when KOH is available both acids form the related carboxylate.
When palmitic acid is added to potassium abietate (prepared with a defect of KOH in order to be sure that no KOH was available) no palmitate formation is observed (Figure 6).
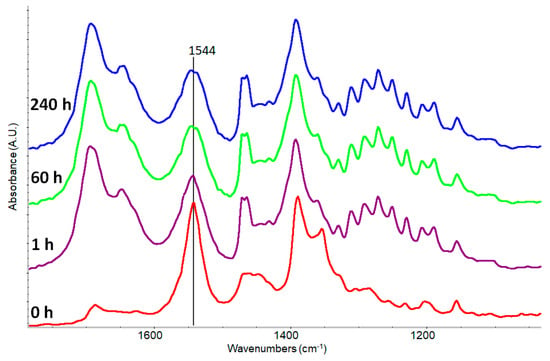
Figure 6.
Kinetics of potassium abietate mixed with fresh added palmitic acid in the spectral range from 1100 to 1800 cm−1. Before the mixing (red line), after 1 h (violet line), after 60 h (green line) and after 240 h (blue line).
The palmitic acid does not seem able to steal the cation of the salt based on abietic acid or at least not in a significant amount. After 60 h (green line) it may be observed only a splitting of the maximum probably related to the perturbation of COO–Me+ bond by the acid function of the palmitic acid.
The situation is clearly different when abietic acid is added to an already formed potassium palmitate (Figure 7). After 1 h a well-shaped band at 1544 cm−1 appears and after 60 h the band of palmitate almost disappear: the abietic acid “snatches” the cation to palmitate as reported in the reaction below.
P–COO− K+ + A–COOH
→+P–COOH + A–COO− K+
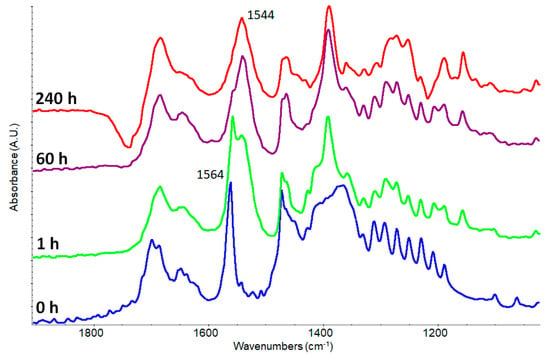
Figure 7.
Kinetics of potassium palmitate mixed with fresh added abietic acid in the spectral range from 1100 to 1800 cm−1. Before the mixing (blue line), after 1 h (green line), after 60 h (violet line) and after 240 h (red line).
After 240 h, the signal of palmitate is practically undetectable while the band of abietate (centered at 1544 cm−1) becomes the main absorption in the critical spectral range. It’s important to point out that all these reactions occur in solid, semi-solid phase trough an intimate but simple mixing and a poorer yield is expected. This behavior is very peculiar since pKa of abietic acid should be very similar to the palmitic acid one or slightly higher due to the methyl in α position. Further investigations are going on, taking in account the solubility of the formed salts vs. acid strength in semi-anhydrous conditions.
This is an interesting result that may change the approach to the metal soaps formation on the painting surfaces. Natural resins not only can form metal soaps interacting with pigments as already previously assessed, but it is shown here that they can also successfully compete and “snatch” the cations from already formed fatty acid carboxylates. From the conservation point of view precise consequences immediately arise. Natural resins are mostly used as varnishes for surface finishing and can be reapplied many times in the life of a painting; fresh and reactive new resin come therefore in contact with already formed and aggregated soaps giving a contribution modifying and influencing the ionomeric structure growth. Ionomeric structures and metal soaps protrusions do not simply grow under the varnish but probably the varnish is deeply involved in the process.
4. Conclusions
The results here presented demonstrate that in the formation and growth of metal soaps the role of natural resins, used as varnishes or co-binder in oleo-resinous media, could be far more important than previously expected. Natural resins in fact are active (though underrated) players in the metal soap formation, growth, and aggregation through the reactivity of their acid constituents. The work confirms that the acid components of natural resins not only can provide “bricks” for the metal soap aggregation reacting with pigments but also can change the composition of already formed aggregates subtracting cations and freeing acids functions of fatty acids. This possibility has been verified with pure reagents (abietic acid, palmitic acid, and potassium hydroxide) since they only can grant spectral bands enough resolved to be detected by FTIR spectroscopy together but there is no reason that a similar behavior could not occur in real conditions. Further studies are going on in order to find appropriate technique able to detect this behavior when drying oil and natural resins (colophony, dammar and sandarac) come in play. This would permit to verify if this behavior of terpenic acids take place even in presence of divalent cations (such as zinc or lead) and overall find evidence of resin soaps in metal soaps protrusion in real paintings.
Author Contributions
Conceptualization, T.P.; methodology, T.P., A.P.; validation, E.D., O.C.; formal analysis, T.P., A.P.; investigation, T.P., A.P.; writing—original draft preparation, T.P.; writing—review and editing, T.P.; O.C. and E.D.; supervision, E.D., O.C.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
This study did not report any data.
Acknowledgments
Authors thanks Vanessa Rosciardi and Giuseppina Afruni that with their thesis work helped in the acquiring data process.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Van den Berg, J.D.J.; Van der Berg, K.J.; Boon, J.J. Chemical changes in curing and ageing oil paints. In Proceedings of the ICOM-CC Triennial 12th Conference, Lyon, France, 29 August–3 September 1999. [Google Scholar]
- Boon, J.J.; Peulvé, S.; Van den Brink, O.F.; Duursma, M.C.; Rainford, D. Molecular aspects of mobile and stationary phases in ageing tempera and oil paint films. In Early Italian Paintings: Techniques and Analysis, Symposium, Maastricht; Bakkenist, T., Hoppenbrouwers, R., Dubois, H., Eds.; Limburg Conservation Institute: Limburg, The Netherlands, 1997. [Google Scholar]
- Van der Weerd, J.; van Loon, A.; Boon, J.J. FTIR studies of the effects of pigments on the aging of oil. Stud. Conserv. 2005, 50, 3–22. [Google Scholar] [CrossRef]
- Boon, J.J.; van der Weerd, J.; Keune, K.; Noble, P.; Wadum, J. Mechanical and chemical changes in old master paintings: Dissolution, metal soap formation and remineralization processes in lead pigmented ground/intermediate paint layers of 17th century painting. In Proceedings of the 13th Triennial Meeting of the ICOM Committee for Conservation, Rio De Janeiro, Brazil, 22–27 September 2002. [Google Scholar]
- Noble, P.; Boon, J.J.; Wadum, J. Dissolution, aggregation and protrusion. Lead soap formation in 17th century grounds and paint layers. Art Matters 2002, 1, 46–61. [Google Scholar]
- Robinet, L.; Corbeil, M.-C. The characterization of metal soaps. Stud. Conserv. 2003, 48, 23–40. [Google Scholar] [CrossRef]
- Meilunas, R.J.; Bentsen, J.G.; Steinberg, A. Analysis of aged paint binders by FTIR spectroscopy. Stud. Conserv. 1990, 35, 33–51. [Google Scholar]
- Lazzari, M.; Chiantore, O. Drying and oxidative degradation of linseed oil. Polym. Degrad. Stab. 1999, 65, 303–313. [Google Scholar] [CrossRef]
- Erhardt, D.; Tumosa, C.S.; Mecklenburg, M.F. Natural and accelerated thermal aging of oil paint films. In Tradition and Innovation: Advances in Conservation; Roy, A., Smith, P., Eds.; International Institute for Conservation: London, UK, 2000. [Google Scholar]
- Van den Berg, J.D.J. Analytical chemical studies on traditional linseed oil paints; (MolArt; 6); University of Amsterdam: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Colombini, M.P.; Modugno, F.; Fuoco, R.; Tognazzi, A. A GC-MS study on the deterioration of lipidic paint binders. Microchem. J. 2002, 73, 175–185. [Google Scholar] [CrossRef]
- Mallégol, J.; Gardette, J.L.; Lemaire, J. Long-Term Behavior of Oil-Based Varnishes and Paints, I. Spectroscopic Analysis of Curing Drying Oils. J. Am. Oil Chem. Soc. 1999, 76, 967–976. [Google Scholar] [CrossRef]
- Platter, M.J.; De Silva, B.; Gelbrich, T.; Hursthouse, M.B.; Higgitt, C.; Saunders, D.R. The characterization of lead fatty acid soaps in “protrusions” in aged traditional oil paint. Polyhedron 2003, 22, 3171–3179. [Google Scholar] [CrossRef]
- Boon, J.J. Processes inside paintings that affect the picture: Chemical changes at, near and underneath the paint surface. In Reporting Highlights of the De Mayerne Programme; Boon, J.J., Ferreira, S.B., Eds.; Netherlands Organisation for Scientific Research (NWO): The Hague, The Netherlands, 2006. [Google Scholar]
- Boon, J.J.; Hoogland, F.; Keune, K. Chemical processes in aged oil paints affecting metal soap migration and aggregation. In Proceedings of the AIC annual meeting, Providence, RI, USA, 16–19 June 2006. [Google Scholar]
- Keune, K.; Boon, J.J. Analytical Imaging studies of cross-sections of paintings affected by lead soap aggregate formation. Stud. Conserv. 2007, 52, 161–176. [Google Scholar] [CrossRef]
- Keune, K.; van Loon, A.; Boon, J.J. SEM backscattered-electron images of paint cross sections as information source for the presence of the Lead white pigment and lead-related degradation and migration phenomena in oil paintings. In Microscopy and Microanalysis; Microscopy Society of America: Reston, VA, USA, 2011. [Google Scholar]
- Hermans, J.J.; Keune, K.; Van Loon, A.; Iedema, P.D. Toward a complete molecular model for the formation of metal soaps in oil paints. In Metal Soaps in Art; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Domenech-Carbo, M.T.; Kuckova, S.; de la Cruz-Canizares, J. Osete-Cortina, Study of the influencing effect of pigments on the photoageing of terpenoid resins used as pictorial media. J. Chromatogr. A 2006, 1121, 248–258. [Google Scholar] [CrossRef]
- Gunn, M.; Chottard, G.; Riviere, E.; Girerd, J.-J.; Chottard, J.-C. Chemical reaction between copper pigments and oleoresinous media. Stud. Conserv. 2002, 47, 12–23. [Google Scholar]
- Poli, T.; Piccirillo, A.; Zoccali, A.; Conti, C.; Nervo, M.; Chiantore, O. The role of Zinc white pigment on the degradation of shellac resin in artworks. Polym. Degrad. Stab. 2014, 102, 138–144. [Google Scholar] [CrossRef]
- Poli, T.; Piccirillo, A.; Nervo, M.; Chiantore, O. Aging of Natural Resins in Presence of Pigments: Metal Soap and Oxalate Formation. In Metal Soaps in Art; Springer: Cham, Switzerland, 2019; pp. 141–152. [Google Scholar]
- Poli, T.; Piccirillo, A.; Nervo, M.; Chiantore, O. Interactions of natural resins and pigments in works of art. J. Colloid Interface Sci. 2017, 503, 1–9. [Google Scholar] [CrossRef]
- Higgs, S.; Burnstock, A. An investigation into metal ions in varnish coatings. In Metal Soaps in Art; Springer: Cham, Switzerland, 2019; pp. 123–140. [Google Scholar]
- Carlyle, L. The artist’s assistant: Oil painting instruction manuals and handbooks in Britain 1800-1900, with reference to selected Eighteenth-century sources; Archetype: London, UK, 2001. [Google Scholar]
- Hedley, G. Solubility parameters and varnish removal: A survey. conservator 1980, 4, 12–18. [Google Scholar] [CrossRef]
- Plesters, J. A preliminary note on the incidence of discolouration of Smalt in oil media. Stud. Conserv. 1969, 14, 62–74. [Google Scholar]
- Giovanoli, R.; Muhlethaler, B. Investigation of discoloured Smalt. Stud. Conserv. 1970, 15, 37–44. [Google Scholar]
- Boon, J.J.; Keune, K.; van der Weerd, J.; Geldof, M.; van Asperen de Boer, J.R.J. Imaging microspectroscopic, secondary ion mass spectrometric and electron microscopic studies on discoloured and partially discoloured Smalt in cross-sections of 16 th century paintings. Chimia 2001, 55, 952–960. [Google Scholar]
- Spring, M.; Higgitt, C.; Sauders, D. Investigation of pigment-medium interaction processes in oil paint containing degraded Smalt; National Gallery Technical Bulletin 26; Yale University Press: London, UK, 2005. [Google Scholar]
- Muhlethaler, B.; Thissen, J. Smalt. Stud. Conserv. 1969, 14, 47–61. [Google Scholar]
- Tournie, A.; Ricciardi, P.; Colomban, P. Glass corrosion mechanisms: A multiscale analysis. Solid State Ion. 2008, 179, 2142–2154. [Google Scholar] [CrossRef]
- Sinclair, R.G.; McKay, A.F.; Jones, R.N. The infrared absorption spectra of saturated fatty acids and esters. J. Am. Chem. Soc. 1952, 74, 2570–2575. [Google Scholar] [CrossRef]
- Jones, R.N.; McKay, A.F.; Sinclair, R.G. Band progressions in the infrared spectra of fatty acids and related compounds. J. Am. Chem. Soc. 1952, 74, 2575–2578. [Google Scholar] [CrossRef]
- Ren, F.; Zheng, Y.F.; Liu, X.M.; Yue, X.Y.; Ma, L.; Li, W.G.; Guan, W.L. An investigation of the oxidation mechanism of abietic acid using two-dimensional infrared correlation spectroscopy. J. Mol. Struct. 2015, 1084, 236–243. [Google Scholar] [CrossRef]
- Nong, W.J.; Chen, X.P.; Liang, J.Z.; Wang, L.L.; Tong, Z.F.; Huang, K.L.; Li, K.X. Isolation and characterization of abietic acid. In Advanced Materials Research; Trans Tech Publications Ltd.: Stafa-Zurich, Switzerland, 2014. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).