Physicochemical and Antimicrobial Properties of Whey Protein-Based Films Functionalized with Palestinian Satureja capitata Essential Oil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Thymbra Essential Oil Extraction
2.3. Determination of Thymbra Essential Oil Composition
2.4. Antimicrobial Activity of Essential Oils Extracted from Thymbra Leaves
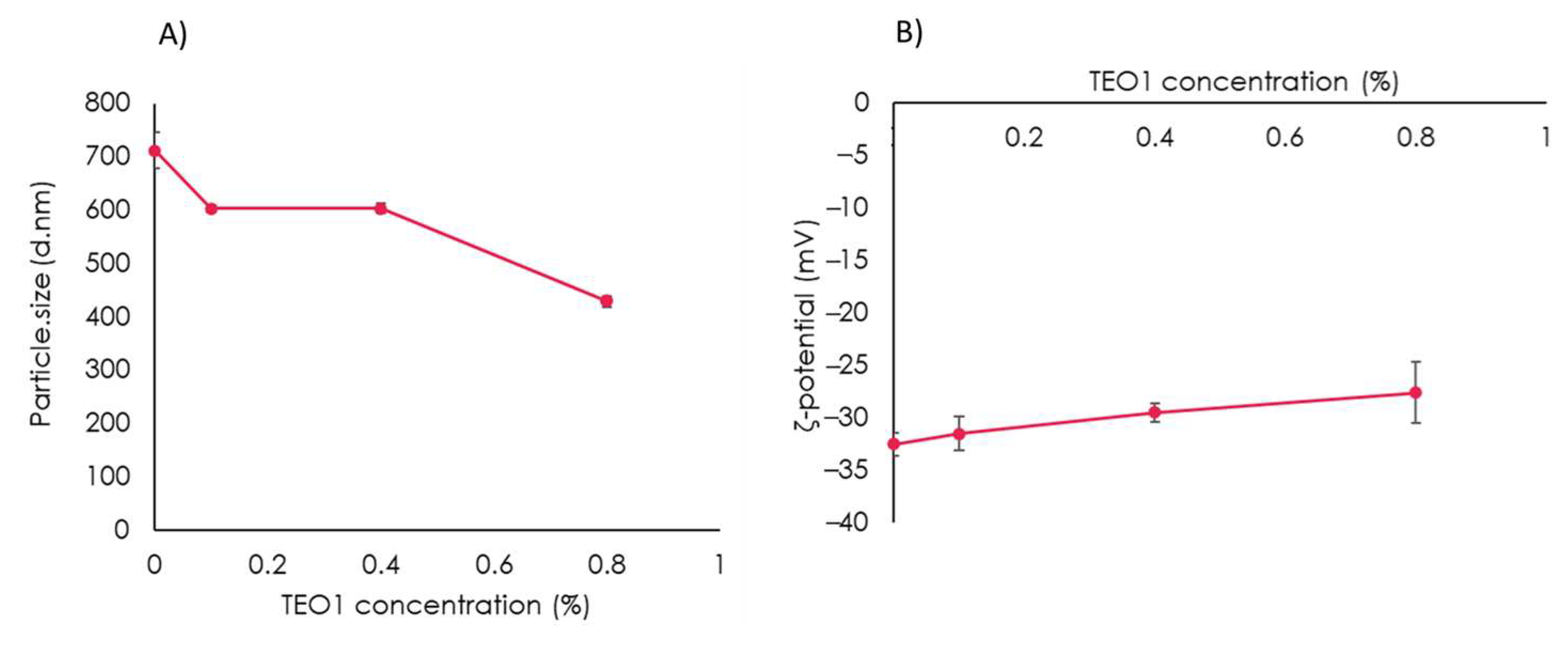
2.5. Zeta Potential and Particle Size Measurements of Whey Protein Film-Forming Solutions (FFSs)
2.6. Antimicrobial Activity of Whey Protein Film-Forming Solutions
2.7. Whey Protein-Based Film Preparation
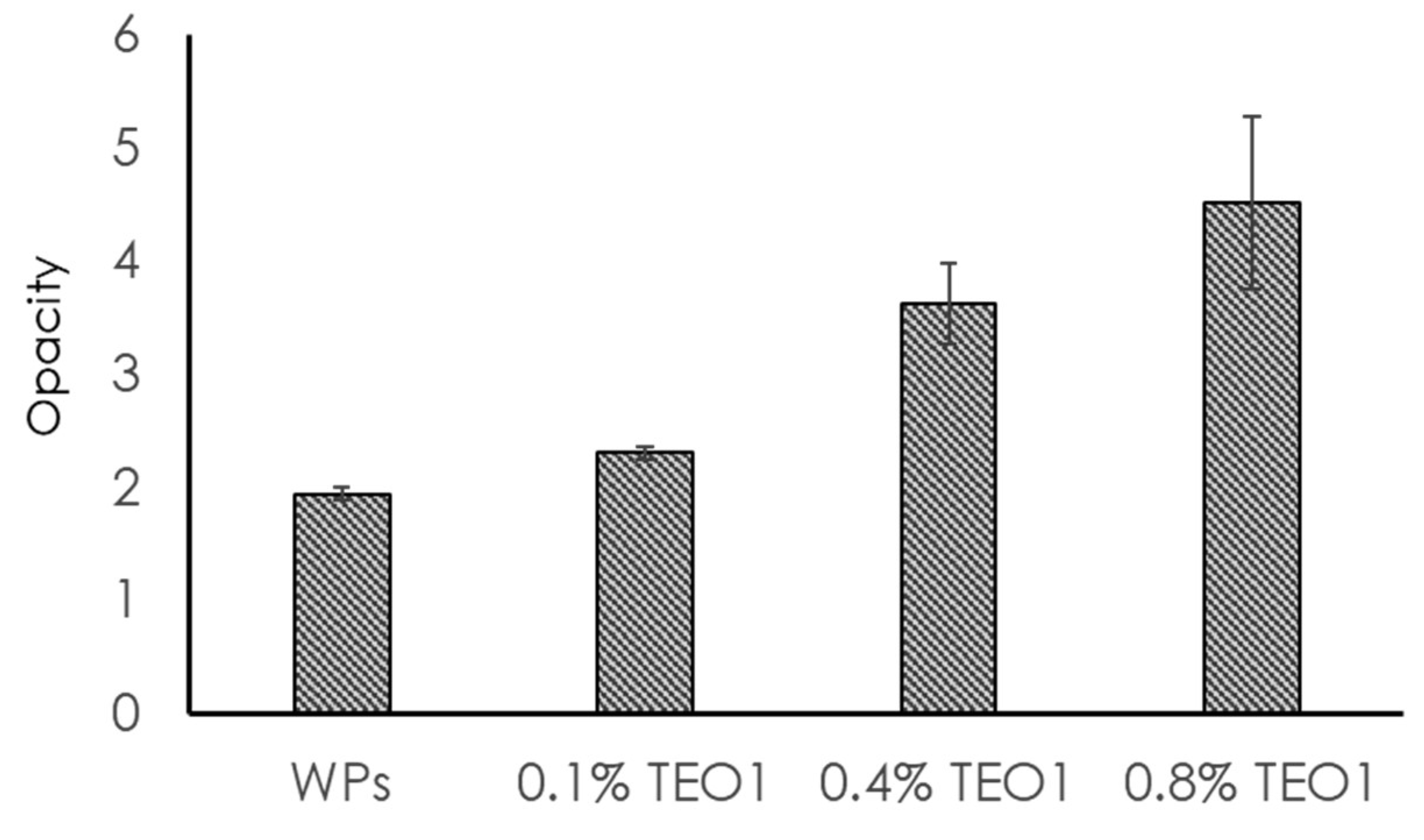
2.8. Film Opacity Determination
2.9. Film Thickness and Mechanical Properties
2.10. Film Moisture Content and Uptake
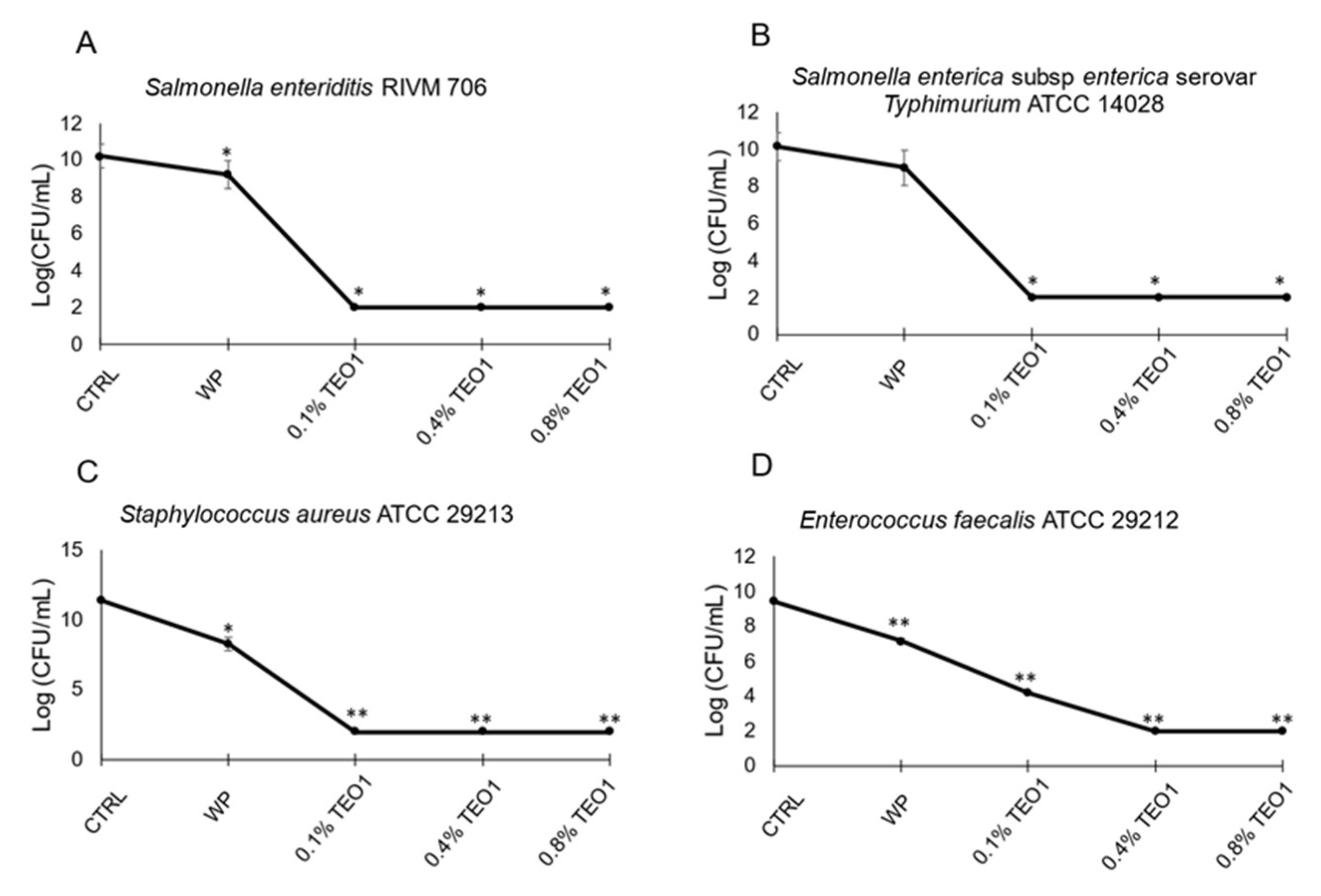
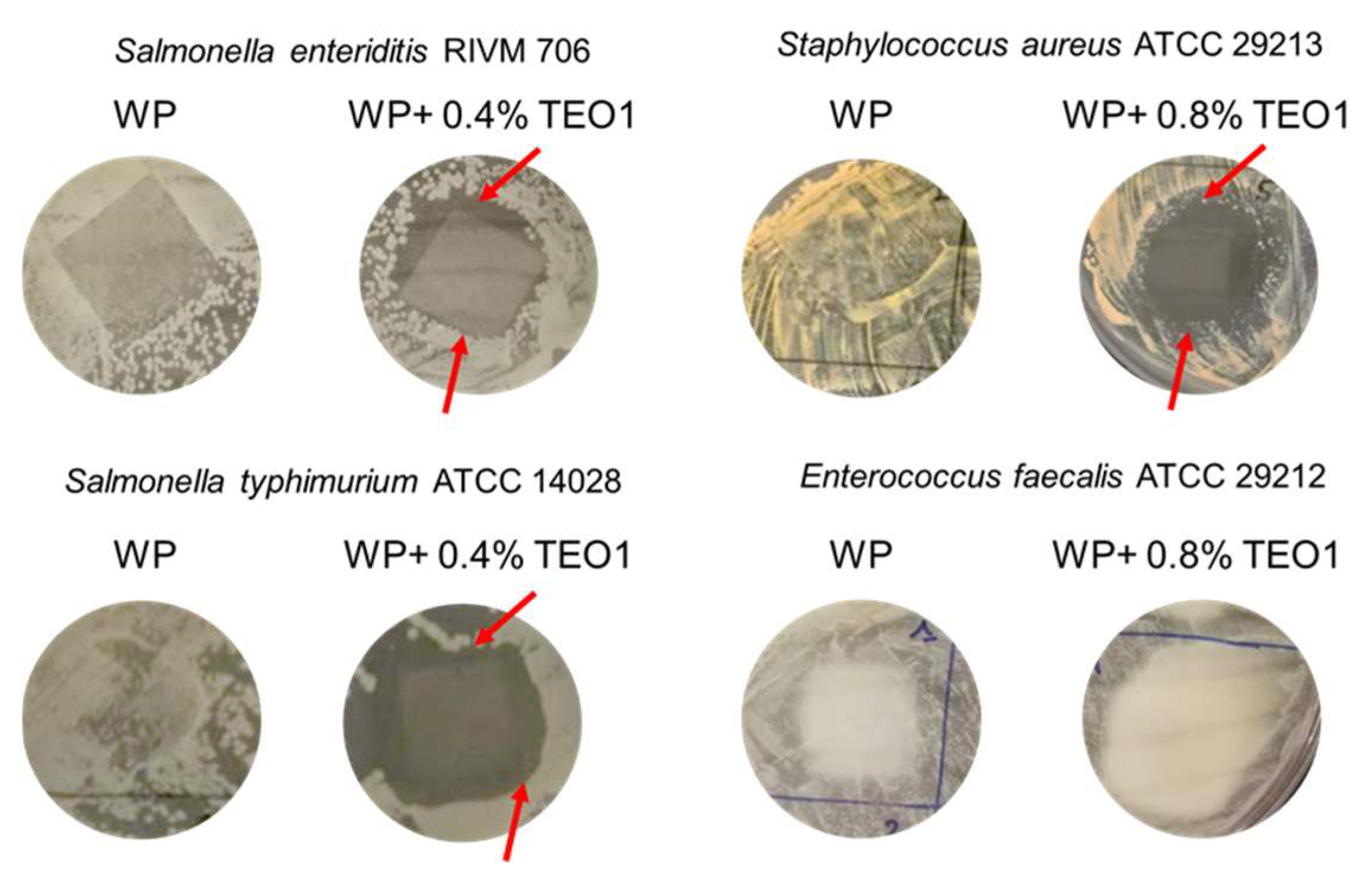
2.11. Antimicrobial Properties of Whey Protein Films
2.12. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition of Thymbra Essential Oils
3.2. Antimicrobial Activity of Thymbra Essential Oil Samples
3.3. Characterization of the Film-Forming Solution Containing Thymbra Essential Oil
3.4. Opacity, Thickness, and Mechanical Properties of Films Functionalized with Thymbra Essential Oil
3.5. Moisture Content and Uptake of Whey Protein Films Containing Thymbra Essential Oil
3.6. Determination of the Antimicrobial Activity of Whey Protein Films Containing Thymbra Essential Oil
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Çakmak, H.; Özselek, Y.; Turan, O.Y.; Fıratlıgil, E.; Güler, F.K. Whey protein isolate edible films incorporated with essential oils: Antimicrobial activity and barrier properties. Polym. Degrad. Stab. 2020, 179, 109285. [Google Scholar] [CrossRef]
- Galus, S.; Kadzińska, J. Gas barrier and wetting properties of whey protein isolate-based emulsion films. Polym. Eng. Sci. 2019, 59, E375–E383. [Google Scholar] [CrossRef]
- Thielen, M. ‘Bioplastics’, Fachagentur Nachwachsende Rohstoffe e. V. (FNR) Agency for Renewable Resources. 2014. Available online: https://mediathek.fnr.de/media/downloadable/files/samples/b/r/brosch.biokunststoffe-web-v01_1.pdf (accessed on 1 August 2020).
- Rajendran, N.; Puppala, S.; Sneha Raj, M.; Ruth Angeeleena, B.; Rajam, C. Seaweeds can be a new source for bioplastics. J. Pharm. Res. 2012, 5, 1476–1479. [Google Scholar]
- Schmid, M.; Müller, K. Whey protein-based packaging films and coatings. In Whey Proteins; Elsevier: London, UK, 2019; pp. 407–437. [Google Scholar]
- Milk and Milk Product Statistics—Statistics Explained. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Milk_and_milk_product_statistics&oldid=511480 (accessed on 7 April 2021).
- Norwood, E.-A.; Croguennec, T.; Le Floch-Fouéré, C.; Schuck, P.; Jeantet, R. Chapter 4: Changes in whey protein powders during storage. In Whey Proteins; Deeth, H.C., Bansal, N., Eds.; Academic Press: New York, NY, USA, 2019; pp. 123–154. [Google Scholar] [CrossRef]
- Abdalrazeq, M.; Giosafatto, C.V.L.; Esposito, M.; Fenderico, M.; Di Pierro, P.; Porta, R. Glycerol-plasticized films obtained from whey proteins denatured at alkaline pH. Coatings 2019, 9, 322. [Google Scholar] [CrossRef] [Green Version]
- Mahmoud, R.; Savello, P.A. Mechanical properties of and water vapor transferability through whey protein films. J. Dairy Sci. 1992, 75, 942–946. [Google Scholar] [CrossRef]
- Maté, J.I.; Krochta, J.M. Whey protein coating effect on the oxygen uptake of dry roasted peanuts. J. Food Sci. 1996, 61, 1202–1207. [Google Scholar] [CrossRef]
- Schmid, M.; Krimmel, B.; Grupa, U.; Noller, K. Effects of thermally induced denaturation on technological-functional properties of whey protein isolate-based films. J. Dairy Sci. 2014, 97, 5315–5327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Pierro, P.; Mariniello, L.; Giosafatto, V.L.; Esposito, M.; Sabbah, M.; Porta, R. Dairy whey protein-based edible films and coatings for food preservation. In Food Packaging and Preservation; Elsevier: Cambridge, MA, USA, 2018; pp. 439–456. [Google Scholar]
- Zinoviadou, K.G.; Koutsoumanis, K.P.; Biliaderis, C.G. Physico-chemical properties of whey protein isolate films containing oregano oil and their antimicrobial action against spoilage flora of fresh beef. Meat Sci. 2009, 82, 338–345. [Google Scholar] [CrossRef]
- Shojaee-Aliabadi, S.; Hosseini, H.; Mohammadifar, M.A.; Mohammadi, A.; Ghasemlou, M.; Ojagh, S.M.; Hosseini, S.M.; Khaksar, R. Characterization of antioxidant-antimicrobial κ-carrageenan films containing Satureja hortensis essential oil. Int. J. Biol. Macromol. 2013, 52, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Pranoto, Y.; Salokhe, V.M.; Rakshit, S.K. Physical and antibacte rial properties of alginate-based edible film incorporated with garlic oil. Food Res. Int. 2005, 38, 267–272. [Google Scholar] [CrossRef]
- Jaradat, N.; Al-Masri, M.; Zaid, A.N.; Hussein, F.; Al-Rimawi, F.; Abu Mokh, A.; Mokh, J.A.; Ghonaim, S. Phytochemical, antimicrobial and antioxidant preliminary screening of a traditional Palestinian medicinal plant, Ononis pubescens L. Eur. J. Integr. Med. 2017, 14, 46–51. [Google Scholar] [CrossRef]
- Jaradat, N.; Adwan, L.; K’Aibni, S.; Zaid, A.N.; Shtaya, M.; Shraim, N.; Assali, M. Variability of chemical compositions and antimicrobial and antioxidant activities of Ruta chalepensis leaf essential oils from three Palestinian regions. BioMed Res. Int. 2017, 2017, 2672689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satureja thymbra. Wikipedia. 21 July 2020. Available online: https://en.wikipedia.org/w/index.php?title=Satureja_thymbra&oldid=1013634228 (accessed on 11 April 2021).
- Baydar, H.; Sagdic, O.; Ozkan, G.; Karadoğan, T. Antibacterial activity and composition of essential oils from Origanum, Thymbra and Satureja species with commercial importance in Turkey. Food Control 2004, 15, 169–172. [Google Scholar] [CrossRef]
- Şahin, F.; Karaman, I.; Güllüce, M.; Öğütçü, H.; Şengül, M.; Adiguzel, A.; Öztürk, S.; Kotan, R. Evaluation of antimicrobial activities of Satureja hortensis L. J. Ethnopharmacol. 2003, 87, 61–65. [Google Scholar] [CrossRef]
- Dadalioǧlu, I.; Evrendilek, G.A. Chemical compositions and antibacterial effects of essential oils of turkish oregano (Origanum minutiflorum), bay laurel (Laurus nobilis), Spanish Lavender (Lavandula stoechas L.), and Fennel (Foeniculum vulgare) on Common Foodborne Pathogens. J. Agric. Food Chem. 2004, 52, 8255–8260. [Google Scholar] [CrossRef] [PubMed]
- Sparkman, O.D. Identification of essential oil components by gas chromatography / mass spectroscopy Robert P. Adams. J. Am. Soc. Mass Spectrom. 1997, 8, 671–672. [Google Scholar] [CrossRef] [Green Version]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential oils’ chemical characterization and investigation of some biological activities: A critical review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaglione, S.; Innac, A.; Carbone, S.P.; Troisi, S.; Angrisano, A. Robust estimation methods applied to GPS in harsh environments. In Proceedings of the 2017 European Navigation Conference (ENC), Lausanne, Switzerland, 9–12 May 2017; pp. 14–25. [Google Scholar]
- Dell’Olmo, E.; Gaglione, R.; Sabbah, M.; Schibeci, M.; Cesaro, A.; Di Girolamo, R.; Porta, R.; Arciello, A. Host defense peptides identified in human apolipoprotein B as novel food biopreservatives and active coating components. Food Microbiol. 2021, 99, 103804. [Google Scholar] [CrossRef]
- Shevkani, K.; Singh, N. Relationship between protein characteristics and film-forming properties of kidney bean, field pea and amaranth protein isolates. Int. J. Food Sci. Technol. 2015, 50, 1033–1043. [Google Scholar] [CrossRef]
- Sartori, T.; Menegalli, F.C. Development and characterization of unripe banana starch films incorporated with solid lipid microparticles containing ascorbic acid. Food Hydrocoll. 2016, 55, 210–219. [Google Scholar] [CrossRef]
- Marotti, M.; Piccaglia, R.; Giovanelli, E.; Deans, S.G.; Eaglesham, E. Effects of variety and ontogenic stage on the essential oil composition and biological activity of fennel (Foeniculum vulgare mill.). J. Essent. Oil Res. 1994, 6, 57–62. [Google Scholar] [CrossRef]
- Villalobos, R.; Chanona, J.; Hernández, P.; Gutierrez, G.; Chiralt, A. Gloss and transparency of hydroxypropyl methylcellulose films containing surfactants as affected by their microstructure. Food Hydrocoll. 2005, 19, 53–61. [Google Scholar] [CrossRef]
- Galus, S.; Kadzińska, J. Moisture Sensitivity, Optical, Mechanical and structural properties of whey protein-based edible films incorporated with rapeseed oil. Food Technol. Biotechnol. 2016, 54, 78–89. [Google Scholar] [CrossRef]
- Zúñiga, R.; Skurtys, O.; Osorio, F.; Aguilera, J.; Pedreschi, F. Physical properties of emulsion-based hydroxypropyl methylcellulose films: Effect of their microstructure. Carbohydr. Polym. 2012, 90, 1147–1158. [Google Scholar] [CrossRef] [PubMed]
- Socaciu, M.-I.; Fogarasi, M.; Semeniuc, C.A.; Socaci, S.A.; Rotar, M.A.; Mureşan, V.; Pop, O.L.; Vodnar, D.C. Formulation and characterization of antimicrobial edible films based on whey protein isolate and tarragon essential oil. Polymers 2020, 12, 1748. [Google Scholar] [CrossRef] [PubMed]
- Salarbashi, D.; Tajik, S.; Ghasemlou, M.; Alibadi, S.S.; Noghabi, M.S.; Khaksar, R. Characterization of soluble soybean polysaccharide film incorporated essential oil intended for food packaging. Carbohydr. Polym. 2013, 98, 1127–1136. [Google Scholar] [CrossRef] [PubMed]
- Sagoo, S.; Board, R.; Roller, S. Chitosan inhibits growth of spoilage micro-organisms in chilled pork products. Food Microbiol. 2002, 19, 175–182. [Google Scholar] [CrossRef]
- Arciello, A.; Panzella, L.; Dell’Olmo, E.; Abdalrazeq, M.; Moccia, F.; Gaglione, R.; Agustin-Salazar, S.; Napolitano, A.; Mariniello, L.; Giosafatto, C.V.L. Development and characterization of antimicrobial and antioxidant whey protein-based films functionalized with Pecan (Carya illinoinensis) nut shell extract. Food Packag. Shelf Life 2021, 29, 100710. [Google Scholar] [CrossRef]
- Bleoancă, I.; Enachi, E.; Borda, D. Thyme antimicrobial effect in edible films with high pressure thermally treated whey protein concentrate. Foods 2020, 9, 855. [Google Scholar] [CrossRef]


| Component | TEO1 | TEO2 |
|---|---|---|
| α-Phellandrene | 1.65 | 3.16 |
| δ-3-Carene | 1.48 | 1.44 |
| Camphene | 0.36 | 0.36 |
| β-Pinene | 0.41 | 0.36 |
| α-Pinene | 1.10 | – |
| α-Terpinolene | 7.19 | 0.26 |
| p-Cymene | 19.53 | – |
| Ortho-Cymene | – | 0.48 |
| γ-Terpinene | 38.95 | 57.81 |
| ψ-Limonene | 0.69 | – |
| α-Terpinenol | 0.05 | 4.31 |
| Anisole | 0.67 | – |
| Thymol | 1.07 | 0.95 |
| Carvacrol | 22.96 | 23.2 |
| Caryophyllene | 2.63 | 4.40 |
| Linalool | – | 1.06 |
| Endo-Borneol | – | 0.20 |
| α-Humulene | – | 0.14 |
| γ-Elemene | – | 0.10 |
| Total identified | 98.74 | 98.26 |
| Others | 1.26 | 1.74 |
| ATCC # | 25923 | 25922 | 13883 | 8427 | 700221 | 9027 | Clinical Strain | 90028 |
|---|---|---|---|---|---|---|---|---|
| Strains name | Staphylococcus aureus | Escherichia coli | Klebsiella pneumoniae | Proteus vulgaris | Enterococcus faecium | Pseudomonas aeruginosa | Methicillin-Resistant Staphylococcus aureus (MRSA) | Candida albicans |
| MIC100 TEO1 | 1/1600 | 1/800 | 1/800 | 1/1600 | 1/800 | 1/1600 | 1/800 | 1/800 |
| MIC100 TEO2 | 1/800 | 1/800 | 1/400 | 1/800 | 1/400 | 1/800 | 1/400 | 1/400 |
| Film | Moisture Content (%) | Moisture Uptake (%) |
|---|---|---|
| Control sample | 22.3 ± 2.6 a | 11.3 ± 2.9 a |
| + 0.1 % (v/v) TEO1 | 20.3 ± 1.8 a,b | 11.7 ± 2.5 a |
| + 0.4 % (v/v) TEO1 | 18.5 ± 1.4 a,b | 12.1 ± 1.1 a |
| + 0.8 % (v/v) TEO1 | 17.5 ± 0.7 b | 13.3 ± 0.8 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdalrazeq, M.; Jaradat, N.; Qadi, M.; Giosafatto, C.V.L.; Dell’Olmo, E.; Gaglione, R.; Arciello, A.; Porta, R. Physicochemical and Antimicrobial Properties of Whey Protein-Based Films Functionalized with Palestinian Satureja capitata Essential Oil. Coatings 2021, 11, 1364. https://doi.org/10.3390/coatings11111364
Abdalrazeq M, Jaradat N, Qadi M, Giosafatto CVL, Dell’Olmo E, Gaglione R, Arciello A, Porta R. Physicochemical and Antimicrobial Properties of Whey Protein-Based Films Functionalized with Palestinian Satureja capitata Essential Oil. Coatings. 2021; 11(11):1364. https://doi.org/10.3390/coatings11111364
Chicago/Turabian StyleAbdalrazeq, Manar, Nidal Jaradat, Mohammad Qadi, C. Valeria L. Giosafatto, Eliana Dell’Olmo, Rosa Gaglione, Angela Arciello, and Raffaele Porta. 2021. "Physicochemical and Antimicrobial Properties of Whey Protein-Based Films Functionalized with Palestinian Satureja capitata Essential Oil" Coatings 11, no. 11: 1364. https://doi.org/10.3390/coatings11111364
APA StyleAbdalrazeq, M., Jaradat, N., Qadi, M., Giosafatto, C. V. L., Dell’Olmo, E., Gaglione, R., Arciello, A., & Porta, R. (2021). Physicochemical and Antimicrobial Properties of Whey Protein-Based Films Functionalized with Palestinian Satureja capitata Essential Oil. Coatings, 11(11), 1364. https://doi.org/10.3390/coatings11111364








