Abstract
Background and Aims: Amoxicillin is one of the most effective antibiotics for treating Helicobacter pylori infections and is widely used in first-line treatment regimens. However, patients with penicillin allergies cannot receive penicillin-based therapies, which significantly limits effective eradication options. This allergy often compels clinicians to choose alternative regimens that may be less effective, thereby increasing the risk of treatment failure. Consequently, therapeutic options for these patients are more restricted, and clinicians must carefully select the most appropriate regimen, taking into account both efficacy and the potential for antimicrobial resistance. This review aims to systematically evaluate the efficacy of penicillin-free treatment regimens for the eradication of H. pylori in patients with penicillin allergies. Specifically, it seeks to identify, analyze, and synthesize current clinical evidence to determine the most effective alternative therapies, thereby supporting evidence-based clinical decision-making. Methods: A literature search was conducted using the PubMed and Scopus databases. We began by reviewing the titles and abstracts of all identified studies to determine eligibility. Next, we assessed the full text of potentially eligible articles according to inclusion and exclusion criteria to establish the eligibility of each study. Results: This review included 26 studies comprising 2713 participants, evaluating penicillin-free therapies for H. pylori eradication in penicillin-allergic patients. Key findings demonstrated high eradication rates with bismuth-based quadruple therapies (88–97%), doxycycline-based regimens (86%), and quinolone-based therapies (75–100%), with Sitafloxacin exceeding 90% efficacy. Minocycline-based regimens also showed promising outcomes, with eradication rates between 80% and 85%. Although the PPI–clarithromycin–metronidazole combination was moderately effective, it was less favored as a first-line option. Overall, bismuth-based and quinolone-based therapies emerged as the most effective alternatives. Conclusions: In patients allergic to penicillin, bismuth quadruple therapy has demonstrated an excellent rate of eradication. Quinolone-based regimens are emerging as a promising alternative in first-line treatment or in cases of treatment failure. Vonoprazan-based therapy is an effective regimen. Combined with clarithromycin and metronidazole, vonoprazan enhances eradication rates and demonstrates effectiveness, including in clarithromycin-resistant strains.
1. Introduction
The Gram-negative, spiral-shaped bacterium Helicobacter pylori (H. pylori) is a widespread and prevalent opportunistic pathogen, most commonly associated with gastritis, peptic ulcers, and various other gastrointestinal disorders. H. pylori infects approximately 50–70% of the global population, with prevalence varying by geography, ethnicity, age, and socioeconomic factors [1]. H. pylori infection is often asymptomatic, with a majority of infected individuals (estimated between 70% and 90%) showing no clinical signs [2]. However, in a significant proportion of cases, it leads to chronic gastritis and peptic ulcer disease [1,3]. Moreover, H. pylori is recognized as a class I carcinogen by the World Health Organization due to its well-established role in the pathogenesis of gastric adenocarcinoma, which develops in approximately 1% of infected individuals [1]. It is also the primary etiological agent of gastric mucosa-associated lymphoid tissue (MALT) lymphoma [1,3]. Beyond these severe complications, H. pylori has been associated with a range of extra-gastric manifestations, including iron-deficiency anemia, idiopathic thrombocytopenic purpura, and vitamin B12 deficiency [3]. These clinical consequences underscore the importance of prompt and effective eradication strategies. The treatment is required due to the long-term complications associated with untreated infection, such as gastritis, gastric ulcers, and malignancies [3]. The goal of H. pylori eradication is to cure peptic ulcer disease and reduce the lifetime risk of gastric cancer [2]. Amoxicillin is one of the most effective antimicrobial agents against H. pylori, and therefore, most eradication regimens include this antibiotic [4]. Penicillin allergy is frequently reported, affecting approximately 10% of patients, although true IgE-mediated hypersensitivity reactions are confirmed in fewer than 1% of cases. The allergy may be immediate or delayed, with clinical manifestations ranging from mild skin rashes to severe reactions [5,6]. In clinical practice, clinicians often avoid all β-lactam antibiotics, including amoxicillin, even in the absence of confirmatory testing. This limits therapeutic options and may lead to the use of broader-spectrum antibiotics, increasing the risk of antimicrobial resistance. Therefore, it is essential to tailor H. pylori eradication strategies while taking this limitation into account [5]. The standard clarithromycin-based triple therapy, with metronidazole replacing amoxicillin, has been commonly used [6]. However, there is growing concern regarding the efficacy of clarithromycin triple therapy, and current guidelines no longer recommend it as a first-line treatment option [7]. In clinical practice, initial eradication therapy—referred to as first-line therapy—generally offers the highest chance of treatment success. Studies have shown that first-line regimens containing amoxicillin achieve eradication rates exceeding 90% in many cases, making them the preferred choice when no allergy is present [8]. Therefore, selecting the most appropriate first-line treatment is crucial for optimizing patient outcomes. Several studies have evaluated different first-line strategies, including standard bismuth-based quadruple therapy, modified bismuth regimens with varying antibiotic combinations, and fluoroquinolone-containing therapies, demonstrating varying degrees of efficacy [9,10,11,12]. However, in penicillin-allergic patients, choosing an effective alternative remains a clinical challenge. While bismuth-based quadruple therapies are widely recommended, their eradication rates have been inconsistent across studies, and fluoroquinolone-based regimens have shown variable efficacy depending on resistance patterns. The need for reliable alternatives is further emphasized by studies indicating that some rescue therapies fail to achieve optimal eradication rates, particularly in the context of antibiotic resistance.
Therefore, identifying the most effective treatment for persistent H. pylori infection in penicillin-allergic patients remains a pressing issue for gastroenterologists [13]. In this systematic review, we analyzed existing studies on penicillin-free therapies to evaluate their clinical effectiveness in both first-line and rescue treatments for H. pylori. Given the lack of standardized eradication guidelines in Morocco and the need for alternative regimens in penicillin-allergic patients, this review aims to provide valuable insights into the success rates and practical application of various treatment options, including bismuth-based quadruple therapies, quinolone-based regimens, and tetracycline alternatives. By addressing the challenges posed by antibiotic resistance, this study seeks to assist clinicians in selecting the most effective and safest therapies for optimal patient outcomes.
2. Methods
2.1. Search Strategy
A literature search was conducted in the PubMed and Scopus databases from 2005 to 2023 to identify studies that explored therapeutic options for patients allergic to amoxicillin with H. pylori infection. To obtain relevant results, the following keywords were identified: Helicobacter pylori, treatment, drug therapy, antibacterial agents, penicillin, amoxicillin, and allergy. The boolean terms OR, AND, and NOT were added to gather relevant articles and were used as follows: Helicobacter pylori AND (treatment OR drug therapy combination OR antibacterial agents) AND (penicillin OR amoxicillin) AND allergy.
In PubMed, the following Medical Subject Headings (Mesh) key terms were used: (“Helicobacter pylori” [MeSH Terms] OR (“Helicobacter” [All Fields] AND “pylori” [All Fields]) OR “Helicobacter pylori” [All Fields]) AND (“therapeutics”[MeSH Terms] OR “therapeutics” [All Fields] OR “treatments” [All Fields] OR “therapy” [MeSH Subheading] OR “therapy” [All Fields] OR “treatment” [All Fields] OR “treatment s” [All Fields] OR (“drug therapy, combination” [MeSH Terms] OR (“drug”[All Fields] AND “therapy” [All Fields] AND “combination” [All Fields]) OR “combination drug therapy” [All Fields] OR (“drug”[All Fields] AND “therapy” [All Fields] AND “combination” [All Fields]) OR “drug therapy combination” [All Fields]) OR (“anti-bacterial agents” [Pharmacological Action] OR “anti-bacterial agents” [MeSH Terms] OR (“anti-bacterial” [All Fields] AND “agents” [All Fields]) OR “anti-bacterial agents” [All Fields] OR (“antibacterial” [All Fields] AND “agents” [All Fields]) OR “antibacterial agents” [All Fields])) AND (“benzylpenicillins” [All Fields] OR “penicillin g” [MeSH Terms] OR “penicillin g” [All Fields] OR “benzylpenicillin” [All Fields] OR “penicilline” [All Fields] OR “penicillines” [All Fields] OR “penicillins” [MeSH Terms] OR “penicillins” [All Fields] OR “penicillin” [All Fields] OR (“amoxicillin” [MeSH Terms] OR “amoxicillin” [All Fields] OR “amoxicilline” [All Fields] OR “amoxicillins” [All Fields])) AND (“allergie” [All Fields] OR “hypersensitivity” [MeSH Terms] OR “hypersensitivity” [All Fields] OR “allergies” [All Fields] OR “allergy” [All Fields] OR “allergy and immunology”[MeSH Terms] OR (“allergy” [All Fields] AND “immunology” [All Fields]) OR “allergy and immunology” [All Fields]).
This transparent and reproducible approach aims to identify and select literature to produce an exhaustive analysis and critical synthesis. All suitable published papers were identified and cataloged using the bibliographic management software Zotero 6.0.30.
2.2. Study Selection
The references were analyzed according to predefined inclusion and exclusion criteria to determine the eligibility of each study. The selection criteria are described to ensure transparency and facilitate the objective screening of the literature. The search strategy was applied as follows: initially, titles and abstracts of all identified studies were screened for eligibility. This was followed by a full-text assessment of articles considered potentially eligible. Studies that did not meet the inclusion criteria were excluded.
- Inclusion criteria:
- Study design: Prospective, retrospective, cross-sectional, or case-control studies.
- Language: Studies written in English only.
- Participants:
- All age groups, regardless of gender.
- Patients diagnosed with H. pylori infection, with or without penicillin allergy.
- Intervention:
- First-line and/or rescue therapies.
- Regimens using combinations of antimicrobial agents.
- Penicillin-free regimens.
- Studies involving clinical trials.
- Outcome: Studies reporting on the effectiveness of H. pylori eradication therapy.
- Exclusion criteria:
- Study design: Non-eligible publication types such as review articles, systematic reviews, or meta-analyses.
- Intervention:
- Regimens without antibiotic combinations (i.e., monotherapies).
- Studies evaluating only penicillin-containing therapies.
- Studies without clinical trials (e.g., in vitro or animal research).
- Outcome: Studies not reporting the effectiveness of H. pylori eradication therapy or studies reporting only on the efficacy of probiotics.
- Duplicate records.
- Data Extraction
- The extracted data included the following:
- Study characteristics: study name, year of publication, study type, and country.
- Participant characteristics: participant age, number of subjects enrolled, and prevalence of penicillin allergy.
- Intervention characteristics: H. pylori eradication regimens including drugs, dosages, treatment duration, and eradication rates based on both intention-to-treat (ITT) and per-protocol (PP) analyses.
- Diagnostic methods used to detect H. pylori infection.
- Risk of Bias
To assess the validity and reliability of the studies included in this systematic review, a risk of bias assessment was conducted for each study using the appropriate CASP checklist, depending on the study design. The aim of this assessment was to identify any methodological flaws or potential biases that could affect the findings. This step is essential for evaluating the strength of the evidence and understanding any limitations that may influence the conclusions.
3. Results
3.1. Literature Search
The studies published from 2005 to 2023 involved a total of 2713 subjects, with an average of 104 participants per study. Our search retrieved 106 articles from PubMed and 75 from Scopus, using keywords applied to titles and abstracts in the latter. A total of n = 181 publications were identified. Article selection was conducted in two stages: initial screening of titles and abstracts, followed by full-text review of eligible studies.
At the abstract screening stage, n = 144 articles were excluded. Among these, 35 were duplicate records, 34 were review articles, systematic reviews, or meta-analyses. The remaining articles were excluded primarily due to evaluating only penicillin-containing therapies.
For the full-text review, 37 studies were assessed as potentially eligible. Among them, 11 articles were excluded either because they evaluated monotherapies without antibiotic combinations or did not include clinical trials. Ultimately, n = 26 full-text articles were included in the final synthesis
A flow diagram of the study selection and screening process is presented in Figure 1.
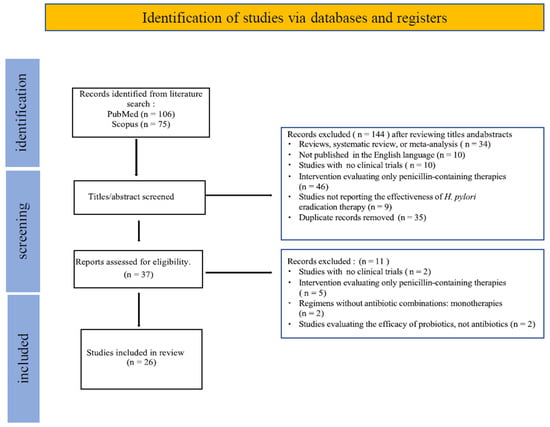
Figure 1.
Flow diagram of study selection.
3.2. Characteristics of the Included Studies
The 26 included studies (Table 1) were conducted in various regions, predominantly in Asian countries. Among them, 19 were prospective studies, 6 were retrospective, and 1 was a case report. Participants of all age groups and both genders were included, comprising both children and adults. Treatment-naive patients were enrolled for first-line therapies, while those with prior failed eradication attempts were included in rescue regimens (second-line, third-line, or fourth-line therapies).

Table 1.
Overview of strategies for eradicating H. pylori in patients allergic to penicillin across the 26 studies in the review.
3.3. Interventions—Protocols of Treatment
Interventions consisted of penicillin-free regimens using combinations of antimicrobial agents. The identified antibiotic classes included tetracyclines (minocycline, oxytetracycline, doxycycline), 5-nitroimidazoles (metronidazole), beta-lactam cephalosporins (cefuroxime), macrolides (clarithromycin), quinolones (ciprofloxacin, sitafloxacin, levofloxacin), nitrofurans (furazolidone), and rifamycins (rifabutin). Treatment duration ranged from 7 to 10 days for short-term regimens and 14 days for long-term regimens, with 14-day treatments being the most common (10/26 studies—Table 1).
3.4. H. pylori Detection Methods
The assessment of H. pylori infection was performed by various detection methods (Table 2). Some studies diagnosed H. pylori infection in either invasive diagnostic tests (rapid urease test, histological analysis, culture), non-invasive diagnostic tests (13C-urea breath test (13C-UBT), H. pylori stool antigen test (HpSA), anti-H. pylori immunoglobulin G (HpIgG)), or both of them [37].

Table 2.
Overview of the diagnostic methods for H. pylori utilized in the 26 reviewed studies.
3.5. Risk of Bias Assessment
Appendix A summarizes the risk of bias for each study, categorized according to the key questions of the relevant CASP checklists (e.g., Cohort Study, Case-Control Study, Case Report). Each study was assessed for elements such as selection bias, measurement bias, and potential confounding factors. These assessments contribute to evaluating the overall quality of the evidence.
3.6. Tolerability and Compliance
Data on tolerability and compliance were available in a subset of the included studies. The most frequently reported adverse events were mild to moderate and included nausea, dizziness, headache, abdominal discomfort, and metallic taste. Minocycline-containing regimens were occasionally associated with dizziness or light-headedness, while furazolidone-based therapies were linked to gastrointestinal intolerance in some cases. Dropout rates due to adverse effects were generally low (<10%), and overall patient adherence was reported as good in most studies. However, compliance details were inconsistently reported across trials, and only a few studies provided explicit data on treatment completion rates.
4. Discussion
This systematic review investigates eradication strategies for H. pylori infection in penicillin-allergic patients and provides an overview of available penicillin-free therapies, highlighting their success rates to support therapeutic decision-making. The effective therapies are summarized in Appendix B. In Morocco, eradication regimens for H. pylori have not been standardized, and there is no national consensus or clinical practice guideline in place.
The Maastricht VI/Florence consensus report recommends using only regimens that achieve eradication rates ≥90% [7]. However, few currently available regimens meet this threshold. For patients allergic to amoxicillin, bismuth-based quadruple therapies represent an effective option for H. pylori eradication. Our study supports their use, as they show high eradication rates and include antibiotic combinations that serve as alternatives to amoxicillin. The efficacy of the PPI–bismuth–tetracycline–metronidazole quadruple therapy ranges from 88% to 97% as a first-line treatment. It can be used as an initial option for penicillin-allergic patients, as recommended by the Maastricht V and VI guidelines (2017 and 2022) [7,38]. If not used as a first-line therapy, it is widely recommended as an optimal second-line option [39]. Our review confirmed its effectiveness as a second- or third-line therapy, achieving eradication rates ≥ 90% [21,40,41].
Bismuth-based quadruple therapies containing cefuroxime have demonstrated eradication rates exceeding 80%, as reported in recent studies (2023, 2020, and 2019) [23,42,43]. Cefuroxime, a second-generation cephalosporin, is a β-lactam antibiotic with low cross-reactivity to penicillin. Nevertheless, its use in penicillin-allergic patients remains limited due to persistent concerns [44]. Cross-reactivity between penicillins and cephalosporins is primarily attributed to structural similarities, particularly in the beta-lactam ring and the side chains. These shared molecular features can be recognized by the immune system and trigger allergic responses in sensitized individuals. However, second-, third-, and fourth-generation cephalosporins often have distinct side chains, which significantly reduce the risk of cross-reactivity, making them potentially safe options for penicillin-allergic patients [44].
A recent study in 2022 evaluated the efficacy of PPI–bismuth–doxycycline–furazolidone quadruple therapy as a first-line regimen, reporting an eradication rate of 86%—comparable to 85% achieved with PPI–bismuth–amoxicillin–furazolidone [45]. This supports the potential use of doxycycline as an alternative to amoxicillin in penicillin-allergic patients.
Minocycline, a second-generation semi-synthetic tetracycline, has been underutilized in H. pylori treatment. Our review found studies demonstrating its potential: PPI–bismuth–minocycline–metronidazole achieved around 80% efficacy as a first-line regimen [14,22], while PPI–minocycline–metronidazole reached approximately 85% in second-line use. As an alternative to tetracycline, minocycline offers a longer half-life, allowing for once- or twice-daily dosing, compared to four times daily for tetracycline [45], thus reducing the complexity of the treatment schedule. Minocycline is generally well tolerated, but its combination with metronidazole requires close monitoring due to increased incidence of side effects such as dizziness and migraines [46].
Quinolone-based regimens (e.g., levofloxacin and sitafloxacin) represent a promising strategy for H. pylori eradication in penicillin-allergic patients. Studies in our review confirmed their efficacy as both first-line and rescue therapies [18,23,30], consistent with Maastricht V/VI guidelines (2017, 2020), which support their use as second-line options [7,38].
Levofloxacin, a third-generation fluoroquinolone, has demonstrated strong in vitro activity against H. pylori [32,47]. Eradication rates in our review ranged from 80% to 100% in first-line treatment [20,21], around 75% in second-line [21,32,41], and up to 100% in fourth-line regimens [35,41].
Sitafloxacin, a fourth-generation fluoroquinolone, achieved eradication rates above 90% in both first-line and rescue settings, with no significant difference between short- and long-term therapies. Due to the risk of serious adverse effects, the Maastricht VI/Florence consensus (2017) recommends using fluoroquinolones only when the benefits outweigh the risks [7]. Resistance to quinolones can be easily acquired, and in countries with high quinolone consumption, resistance rates are notably high [47,48].
Mori et al. evaluated the efficacy of PPI–metronidazole–sitafloxacin and found that H. pylori resistance to sitafloxacin did not significantly reduce eradication rates, achieving a 95% success rate [25]. The ACG Clinical Guideline advises avoiding antibiotics previously used in failed eradication regimens to prevent resistance and treatment failure [8]. However, our review included a clinical case where levofloxacin-based therapy successfully eradicated H. pylori in a patient with two prior treatment failures using the same fluoroquinolone [47], suggesting that, in the absence of resistance, antibiotics may still be reused.
Triple therapy with PPI–clarithromycin–amoxicillin remains a standard treatment for H. pylori, achieving approximately 90% eradication [49,50]. In penicillin-allergic patients, guidelines recommend replacing amoxicillin with metronidazole [3,7]. However, our findings indicate that the PPI-clarithromycin-metronidazole regimen, while moderately effective, is not the most suitable first-line option. Some studies reported improved efficacy through the following:
- Adding bismuth to the regimen, significantly increasing eradication rates.
- Extending treatment to 14 days, provided H. pylori is sensitive to clarithromycin and metronidazole.
- Increasing metronidazole dosage, though this may reduce adherence due to side effects.
- Substituting PPIs with vonoprazan, a novel acid suppressant that has shown promising results but is not yet available in all countries.
Despite the strength of the evidence, this review has limitations. Variability in study design, sample sizes, and geographic distribution may introduce heterogeneity, limiting comparability. Regional differences in resistance patterns also affect eradication rates. A major limitation is the lack of standardized resistance testing in clinical practice, forcing clinicians to prescribe empirically rather than based on individual susceptibility.
It is important to note that the strength of the efficacy data varies depending on the number of patients included in each treatment group. While the majority of regimens analyzed in this review were supported by sample sizes exceeding 30 patients—providing a reasonably solid basis for interpreting eradication rates—some regimens were tested in small cohorts. In particular, a few therapies were evaluated in fewer than 10 patients, which significantly limits the generalizability of their reported outcomes. For this reason, these results should be considered exploratory and interpreted with caution. For a reliable evaluation of treatment efficacy, readers are referred to Table 1, where the number of patients is clearly indicated for each regimen summarized in Appendix B.
Moreover, only studies indexed in PubMed and Scopus were included, potentially excluding relevant data from other sources. Selection bias may also be present due to the underreporting of negative findings.
Risk of bias assessment showed that 17 of the 26 included studies had a low risk of bias, indicating reliable and valid findings with strong methodology, clear study populations, and appropriate control for confounding factors.
- Eight studies had a moderate risk of bias, meaning their results are useful but should be interpreted cautiously due to some methodological limitations.
- One study was classified as high risk, with significant methodological issues that may compromise the reliability of its conclusions.
The absence of standardized H. pylori eradication protocols in Morocco underscores the urgent need for a national consensus. Given the high efficacy of bismuth-based and quinolone-based regimens, their inclusion in future national guidelines should be considered, alongside effective antibiotic stewardship policies to prevent resistance. Future research should prioritize large-scale randomized controlled trials (RCTs) comparing different penicillin-free regimens across various regions, with particular attention to resistance patterns. The safety and efficacy of vonoprazan-based regimens and the potential use of second-generation cephalosporins in penicillin-allergic patients should also be further explored.
This review includes numerous clinical trials with various antibiotic combinations and highlights emerging therapies, providing a valuable reference for future clinical research.
5. Conclusions
This systematic review provides a comprehensive synthesis of the literature on the efficacy of penicillin-free therapies used in both first-line and rescue treatments for H. pylori infection. The effectiveness of these regimens has been demonstrated, particularly for bismuth-based quadruple therapies, which consistently achieve high eradication rates [49,51]. The triple therapy combining PPI, metronidazole, and minocycline also appears to be a promising option, especially given its excellent eradication rate in second-line treatment. Regimens containing levofloxacin or sitafloxacin offer encouraging alternatives for both first-line use and in cases of treatment failure.
Given that a significant proportion of the included studies present a low risk of bias, the findings of this review can be considered reliable and well-supported. Nonetheless, the presence of studies with moderate or high risk of bias underscores the need to interpret results in light of study quality and to take methodological limitations into account when drawing conclusions or formulating recommendations for future research.
Author Contributions
Conceptualization, Supervision, Project Administration, Writing—Review & Editing: M.K.H.; Data Curation, Formal Analysis, Visualization, Writing—Original Draft: K.E.B., I.A., S.O., O.A., H.D., F.A.L., S.I., M.E.J. and I.D.; Validation, Writing—Original Draft: M.A.S.; Writing—Review & Editing: H.B. and Z.B.; Validation: L.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. The Article Processing Charges (APC) were funded by the Fondation Mohammed VI des Sciences et de la Santé, Casablanca, Morocco.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A. Summary of Risk of Bias Assessment for Included Studies
| Study ID | Study Type | Selection Bias | Justification | Measurement Bias | Justification | Confounding Bias | Justification | Overall Risk |
| [14] | Randomized controlled trial | Low | Randomized groups ensure comparability | Low | Objective outcome measure (urea breath test) | Low | Confounders considered, compliance assessed | Low |
| [15] | Retrospective | Moderate | Not randomized, selection bias possible due to patient selection. | Low | 13C-UBT breath test is a reliable outcome measure. | Moderate | Small sample size (n = 53), potential confounders not fully controlled. | Moderate |
| [16] | RCT | Low | Randomized multicenter trial reduces selection bias. | Low | Objective measures (eradication rates, ulcer healing). | Low | Balanced groups, no major confounders noted. | Low |
| [17] | Case Report | High | Single-patient case, no randomization. | Moderate | Use of agar dilution susceptibility testing ensures reliability, but single case limits generalizability. | High | No control group, high risk of confounding. | High |
| [18] | Prospective Intervention | Moderate | Small sample size (n = 17), limited generalizability. | Low | Objective measures used for eradication confirmation. | Moderate | Lack of control group increases confounding risk. | Moderate |
| [19] | RCT | Low | Randomized trial comparing AST-guided therapy vs. Pylera®. | Low | Objective outcome measure (fecal antigen test). | Low | Balanced groups, adherence and side effects analyzed. | Low |
| [12] | Prospective Cohort | Moderate | Not randomized, selection bias possible. | Low | Objective microbiome analysis via 16S rRNA gene sequencing. | Moderate | No control over external factors affecting microbiome. | Moderate |
| [20] | Randomized controlled trial | Moderate | No mention of allocation concealment, which could lead to selection bias. | Low | The E-test method for antimicrobial susceptibility is a standard and reliable measurement. | Low | Randomization controls confounding factors, and the populations were comparable. | Low |
| [21] | Registry-based study | Moderate | No randomization, potential for selection bias in choosing patients from the registry. | Low | The methodology and efficacy outcomes were well-defined, with good validation of the treatments. | Low | The large sample size and use of standard treatment regimens minimize confounding. | Moderate |
| [22] | Randomized controlled trial | Moderate | No mention of allocation concealment or blinding, which could lead to selection bias. | Low | The 13C-UBT breath test was used, which is a standard and reliable method for H. pylori eradication assessment. | Low | Randomization was performed, and baseline characteristics are similar, minimizing confounding. | Low |
| [23] | Cohort study | Moderate | No mention of randomization or allocation concealment, which could lead to selection bias. | Low | Urea breath test was used to confirm H. pylori eradication, which is reliable. | Low | The study does not have confounding variables since it is well controlled with penicillin allergy as the main criterion. | Low |
| [11] | Randomized controlled trial | Moderate | No mention of allocation concealment, which could lead to selection bias. | Low | 13C-UBT breath test was used, which is a reliable measure for H. pylori eradication. | Low | Randomization is performed, and the two groups are comparable, minimizing confounding. | Low |
| [24] | Prospective observational study | Moderate | Small sample size (20 patients) may lead to selection bias. | Low | 13C-UBT breath test is a reliable measure for H. pylori eradication. | Low | Random assignment of patients to VPZ-based and PPI-based regimens, minimizing confounding. | Low |
| [25] | Cohort study | Moderate | No randomization, and patients were not equally distributed between resistant and non-resistant groups. | Low | The use of 13C-UBT breath test and stool antigen test is reliable for confirming eradication. | Low | Resistance to antibiotics (sitafloxacin and metronidazole) could be a confounder, but the study adjusts for this. | Moderate |
| [26] | Retrospective cohort study | Moderate | Retrospective design may lead to selection bias. | Low | 13C-UBT breath test is a reliable method for determining eradication success. | Low | The study considered different regimens and analyzed intention-to-treat vs. per-protocol outcomes, minimizing confounding. | Moderate |
| [27] | Randomized controlled trial | Low | Randomization of patients into 3 groups reduces selection bias. | Low | Standardized methodologies for measuring eradication and evaluating side effects. | Low | Randomization and large sample size minimize potential confounders. | Low |
| [28] | Cohort study | Moderate | No randomization, but a large sample size (650 patients) reduces selection bias. | Low | Serum PG I/II ratios were measured before and after treatment, which is reliable. | Low | No significant confounders mentioned, but the study acknowledges variations in treatment regimens. | Low |
| [29] | Randomized controlled trial | Low | Randomization reduces selection bias. | Low | Use of antimicrobial susceptibility testing and standardized eradication protocols. | Low | Randomization minimizes confounding. The study accounts for resistance factors. | Low |
| [4] | Prospective multicenter | Low | Consecutive patients were included, reducing the likelihood of selection bias. | Low | The use of 13C-UBT is objective and reliable for measuring eradication. | Low | No major confounders were identified; treatment regimens were standardized. | Low |
| [30] | Prospective multicenter | Low | 28 consecutive patients were treated, reducing bias in selection. | Low | 13C-UBT is used for accurate measurement of eradication. | Low | The study clearly focused on penicillin-allergic patients with standardized treatment regimens. | Low |
| [31] | Observational cohort | Moderate | Patients who failed previous treatments were included, potentially creating a selection bias. | Moderate | Pre-antibiotic sensitivity testing can lead to misclassification of resistance. | Moderate | Resistance to clarithromycin and metronidazole could influence results, especially in resistant strains. | Moderate |
| [32] | Prospective multicenter | Low | Consecutive patients with penicillin allergy were included, minimizing selection bias. | Low | 13C-UBT is reliable for eradication assessment. | Low | No significant confounding factors were identified; treatment regimens were carefully managed. | Low |
| [33] | Randomized controlled trial | Moderate | Randomization was performed, but may have overlooked some baseline differences. | Moderate | Eradication was measured, but the distinction between metronidazole-sensitive and resistant strains could introduce some bias. | Moderate | The use of metronidazole and amoxicillin can be affected by resistance patterns, introducing potential confounding. | Moderate |
| [34] | Observational cohort | Low | Patients were treated consecutively, minimizing selection bias. | Low | HP eradication was tested using reliable methods, reducing measurement bias. | Low | No significant confounders were identified in the analysis, and treatment regimens were standardized. | Low |
| [35] | Prospective single-center | Low | Consecutive patients with penicillin allergy were included, reducing selection bias. | Low | 13C-UBT used for measuring eradication is accurate and reliable. | Low | No significant confounders identified; therapies were well-defined and standardized. | Low |
| [36] | Prospective single-center | Low | 20 consecutive patients were included, reducing selection bias. | Low | Follow-up panendoscopy and biopsies were used to ensure reliable measurement of HP eradication. | Low | No major confounders were identified; standard treatment regimen used. | Low |
Appendix B. Efficacy of Therapeutic Protocols in Case of Penicillin Allergy *
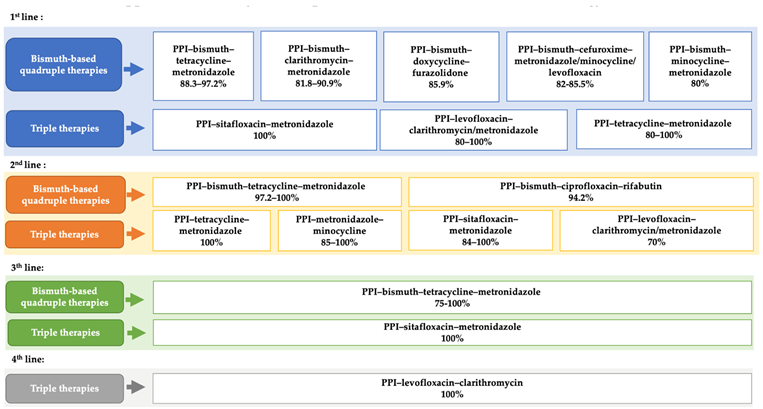 |
| * For detailed information on individual treatment regimens, including sample sizes and eradication rates, please refer to Table 1. It is important to note that several regimens listed in this appendix are based on a small number of participants (n < 10), which may limit the reliability and generalizability of the reported efficacy outcomes. |
References
- Malfertheiner, P.; Camargo, M.C.; El-Omar, E.; Liou, J.-M.; Peek, R.; Schulz, C.; Smith, S.I.; Suerbaum, S. Helicobacter pylori infection. Nat. Rev. Dis. Primers 2023, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Hunt, R.H.; Xiao, S.D.; Megraud, F.; Leon-Barua, R.; Bazzoli, F.; van der Merwe, S.; Vaz Coelho, L.G.; Fock, M.; Fedail, S.; Cohen, H.; et al. Helicobacter pylori in developing countries. World Gastroenterology Organisation Global Guideline. J. Clin. Gastroenterol. 2011, 45, 383–388. [Google Scholar]
- Roberts, L.T.; Issa, P.P.; Sinnathamby, E.S.; Granier, M.; Mayeux, H.; Eubanks, T.N.; Malone, K.; Ahmadzadeh, S.; Cornett, E.M.; Shekoohi, S.; et al. Helicobacter pylori: A Review of Current Treatment Options in Clinical Practice. Life 2022, 12, 2038. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Chung, J.W. Best Helicobacter pylori Eradication Strategy in the Era of Antibiotic Resistance. Antibiotics 2020, 9, 436. [Google Scholar] [CrossRef]
- Bigby, M.; Jick, S.; Jick, H.; Arndt, K. Drug-Induced Cutaneous Reactions: A Report From the Boston Collaborative Drug Surveillance Program on 15 438 Consecutive Inpatients, 1975 to 1982. JAMA 1986, 256, 3358–3363. [Google Scholar] [CrossRef]
- Haouichat, H.; Guénard, L.; Bourgeois, S.; Pauli, G.; De Blay, F. Les tests cutanés dans l’exploration de l’allergie à la pénicilline. Rev. Française Allergol. Immunol. Clin. 2002, 42, 779–792. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Megraud, F.; Rokkas, T.; Gisbert, J.P.; Liou, J.-M.; Schulz, C.; Gasbarrini, A.; Hunt, R.H.; Leja, M.; O’Morain, C.; et al. Management of Helicobacter pylori infection: The Maastricht VI/Florence consensus report. Gut 2022, 71, 1724–1762. [Google Scholar] [CrossRef]
- Chey, W.D.; Leontiadis, G.I.; Howden, C.W.; Moss, S.F. ACG Clinical Guideline: Treatment of Helicobacter pylori Infection. Off. J. Am. Coll. Gastroenterol. ACG 2017, 112, 212–239. [Google Scholar] [CrossRef]
- Liang, X.; Xu, X.; Zheng, Q.; Zhang, W.; Sun, Q.; Liu, W.; Xiao, S.; Lu, H. Efficacy of bismuth-containing quadruple therapies for clarithromycin-, metronidazole-, and fluoroquinolone-resistant Helicobacter pylori infections in a prospective study. Clin. Gastroenterol. Hepatol. 2013, 11, 802–807.e1. [Google Scholar] [CrossRef]
- Sun, Y.; Zhu, M.; Yue, L.; Hu, W. Multiple bismuth quadruple therapy containing tetracyclines combined with other antibiotics and Helicobacter pylori eradication therapy. J. Clin. Med. 2022, 11, 7040. [Google Scholar] [CrossRef]
- Long, X.; Chen, Q.; Yu, L.; Liang, X.; Liu, W.; Lu, H. Bismuth improves efficacy of proton-pump inhibitor clarithromycin, metronidazole triple Helicobacter pylori therapy despite a high prevalence of antimicrobial resistance. Helicobacter 2018, 23, e12485. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Ye, Z.; Lu, J.; Miao, S.; Lu, X.; Sun, H.; Wu, J.; Wang, Y.; Huang, Y. Long-term changes in the gut microbiota after 14-day bismuth quadruple therapy in penicillin-allergic children. Helicobacter 2020, 25, e12721. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-J.; Jung, H.-K.; Kang, S.J.; Lee, Y.C.; Park, S.-Y.; Shin, C.M.; Kim, S.E.; Lim, H.C.; Kim, J.-H.; Nam, S.Y.; et al. Salvage regimens after failure of previous Helicobacter pylori eradication therapy: A systematic review and meta-analysis. Korean J. Helicobacter Up. Gastrointest. Res. 2021, 21, 59–71. [Google Scholar] [CrossRef]
- Zhang, Y.; Suo, B.; Tian, X.; Zhang, H.; Lu, H.; Yao, X.; Li, C.; Ren, X.; Zhou, L.; Song, Z. New regimens as first-line eradication therapy for Helicobacter pylori infection in patients allergic to penicillin: A randomized controlled trial. Helicobacter 2023, 28, e12956. [Google Scholar] [CrossRef]
- Kazunori, A.; Shunsuke, K.; Akira, K.; Kazuhiro, N.; Tomoya, S.; Takashi, Y. A Vonoprazan, Clarithromycin, and Metronidazole Regimen as Helicobacter pylori Eradication Therapy for Patients with Penicillin Allergy in Light of Clarithromycin Resistance. Jpn. J. Med. 2023, 62, 2301–2306. [Google Scholar]
- Chi, J.; Xu, C.; Liu, X.; Wu, H.; Xie, X.; Liu, P.; Li, H.; Zhang, G.; Xu, M.; Li, C.; et al. A comparison of doxycycline and amoxicillin containing quadruple eradication therapy for treating Helicobacter pylori-infected duodenal ulcers: A multicenter, open-label, randomized controlled trial in China. Pathogens 2022, 11, 1549. [Google Scholar] [CrossRef]
- Kong, S.; Chen, H.; Huang, K.; Jin, D.; Zhang, G.; Ye, F. Antibiotic susceptibility guided reuse of levofloxacin-based therapy in a penicillin-allergic patient for Helicobacter pylori infection: A case report. Medicine 2021, 100, e24915. [Google Scholar] [CrossRef]
- Sue, S.; Sasaki, T.; Kaneko, H.; Irie, K.; Kondo, M.; Maeda, S. Helicobacter pylori rescue treatment with vonoprazan, metronidazole, and sitafloxacin in the presence of penicillin allergy. JGH Open 2021, 5, 307–311. [Google Scholar] [CrossRef]
- Bonoso Criado, R.; Pérez Citores, L.; Pérez Millán, A.G.; Montero Moretón, Á.; González de Castro, E.; Cabezudo Molleda, L.; García Castro, M.A.; Moreira Da Silva, B.A.; Maestro Antolín, S.; Santos Santamarta, F.; et al. Prospective comparative study of the treatment of Helicobacter pylori with antibiotic susceptibility testing-guided triple therapy compared to quadruple therapy with bismuth-metronidazole-tetracycline subcitrate. Rev. Española Enfermedades Dig. 2021, 113, 597–601. [Google Scholar]
- Luo, L.; Huang, Y.; Liang, X.; Ji, Y.; Yu, L.; Lu, H. Susceptibility-guided therapy for Helicobacter pylori-infected penicillin-allergic patients: A prospective clinical trial of first-line and rescue therapies. Helicobacter 2020, 25, e12699. [Google Scholar] [CrossRef]
- Nyssen, O.P.; Pérez-Aisa, Á.; Tepes, B.; Rodrigo-Sáez, L.; Romero, P.M.; Lucendo, A.; Castro-Fernández, M.; Phull, P.; Barrio, J.; Bujanda, L.; et al. Helicobacter pylori first-line and rescue treatments in patients allergic to penicillin: Experience from the European Registry on H. pylori management (Hp-EuReg). Helicobacter 2020, 25, e12686. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lan, Y.; Wang, Q.; Zhang, Y.; Si, X. Application of minocycline-containing bismuth quadruple therapies as first-line regimens in the treatment of Helicobacter pylori. Gastroenterol. Res. Pract. 2019, 2019, 9251879. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Fu, W.; Zhou, L. Cefuroxime, levofloxacin, esomeprazole, and bismuth as first-line. therapy for eradicating Helicobacter pylori in patients allergic to penicillin. BMC Gastroenterol. 2019, 19, 132. [Google Scholar] [CrossRef]
- Sue, S.; Suzuki, N.; Shibata, W.; Sasaki, T.; Yamada, H.; Kaneko, H.; Tamura, T.; Ishii, T.; Kondo, M.; Maeda, S. First-Line Helicobacter pylori Eradication with Vonoprazan, Clarithromycin, and Metronidazole in Patients Allergic to Penicillin. Gastroenterol. Res. Pract. 2017, 2017, 2019802. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Suzuki, H.; Matsuzaki, J.; Masaoka, T.; Kanai, T. Antibiotic resistance and gyrA mutation affect the efficacy of 10-day sitafloxacin-metronidazole-esomeprazole therapy for Helicobacter pylori in penicillin allergic patients. United Eur. Gastroenterol. J. 2017, 5, 796–804. [Google Scholar] [CrossRef]
- Ono, S.; Kato, M.; Nakagawa, S.; Mabe, K.; Sakamoto, N. Vonoprazan improves the efficacy of Helicobacter pylori eradication therapy with a regimen consisting of clarithromycin and metronidazole in patients allergic to penicillin. Helicobacter 2017, 22, e12374. [Google Scholar] [CrossRef]
- Kahramanoğlu Aksoy, E.; Pirinçci Sapmaz, F.; Göktaş, Z.; Uzman, M.; Nazlıgül, Y. Comparison of Helicobacter pylori eradication rates of 2-week levofloxacin-containing triple therapy, levofloxacin-containing bismuth quadruple therapy, and standard bismuth quadruple therapy as a first-line regimen. Med. Princ. Pract. 2018, 26, 523–529. [Google Scholar] [CrossRef]
- Osumi, H.; Fujisaki, J.; Suganuma, T.; Horiuchi, Y.; Omae, M.; Yoshio, T.; Ishiyama, A.; Tsuchida, T.; Miki, K. A significant increase in the pepsinogen I/II ratio is a reliable biomarker for successful Helicobacter pylori eradication. PLoS ONE 2017, 12, e0183980. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, W.; Fu, Q.; Liang, X.; Liu, W.; Xiao, S.; Lu, H. Rescue Therapy for Helicobacter pylori Eradication: A Randomized Non-Inferiority Trial of Amoxicillin or Tetracycline in Bismuth Quadruple Therapy. Am. J. Gastroenterol. 2016, 111, 1736–1742. [Google Scholar] [CrossRef]
- Furuta, T.; Sugimoto, M.; Yamade, M.; Uotani, T.; Sahara, S.; Ichikawa, H.; Kagami, T.; Yamada, T.; Osawa, S.; Sugimoto, K.; et al. Eradication of H. pylori infection in patients allergic to penicillin using triple therapy with a PPI, metronidazole and sitafloxacin. Intern. Med. Tokyo Jpn. 2014, 53, 571–575. [Google Scholar] [CrossRef]
- Tay, C.Y.; Windsor, H.M.; Thirriot, F.; Lu, W.; Conway, C.; Perkins, T.T.; Marshall, B.J. Helicobacter pylori eradication in Western Australia using novel quadruple therapy combinations. Aliment. Pharmacol. Ther. 2012, 36, 1076–1083. [Google Scholar] [CrossRef] [PubMed]
- Gisbert, J.P.; Perez-Aisa, A.; Castro-Fernández, M.; Barrio, J.; Rodrigo, L.; Cosme, A.; Marcos, S.; Moreno-Otero, R.; Minutolo, R.; Aghemo, A.; et al. Helicobacter pylori first-line treatment and rescue option containing levofloxacin in patients allergic to penicillin. Dig. Liver Dis. 2010, 42, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Sato, R.; Okimoto, T.; Watanabe, K.; Nasu, M.; Fujioka, T.; Kodama, M.; Abe, T.; Sato, S.; Arita, T. Effectiveness of minocycline-based triple therapy for eradication of Helicobacter pylori infection. J. Gastroenterol. Hepatol. 2006, 21, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Matsushima, M.; Suzuki, T.; Kurumada, T.; Watanabe, S.; Watanabe, K.; Kobayashi, K.; Deguchi, R.; Masui, A.; Takagi, A.; Shirai, T.; et al. Tetracycline, metronidazole and amoxicillin-metronidazole combinations in proton pump inhibitor-based triple therapies are equally effective as alternative therapies against Helicobacter pylori infection. J. Gastroenterol. Hepatol. 2006, 21 1 Pt 2, 232–236. [Google Scholar] [CrossRef]
- Gisbert, J.P.; Gisbert, J.L.; Marcos, S.; Olivares, D.; Pajares, J.M. Helicobacter pylori first-line treatment and rescue options in patients allergic to penicillin. Aliment. Pharmacol. Ther. 2005, 22, 1041–1046. [Google Scholar] [CrossRef]
- Rodríguez-Torres, M.; Salgado-Mercado, R.; Ríos-Bedoya, C.F.; Aponte-Rivera, E.; Marxuach-Cuétara, A.M.; Rodríguez-Orengo, J.F.; Fernández-Carbia, A. High eradication rates of Helicobacter pylori infection with first- and second-line combination of esomeprazole, tetracycline, and metronidazole in patients allergic to penicillin. Dig. Dis. Sci. 2005, 50, 634–639. [Google Scholar] [CrossRef]
- Bordin, D.S.; Voynovan, I.N.; Andreev, D.N.; Maev, I.V. Current Helicobacter pylori Diagnostics. Diagnostics 2021, 11, 1458. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Megraud, F.; O’Morain, C.A.; Atherton, J.; Axon, A.T.; Bazzoli, F.; Gensini, G.F.; Gisbert, J.P.; Graham, D.Y.; Rokkas, T.; et al. Management of Helicobacter pylori infection—The Maastricht IV/Florence consensus report. Gut 2012, 61, 646–664. [Google Scholar] [CrossRef]
- Kim, S.E.; Hwang, J.H. Management of Helicobacter pylori infection: A comparison between Korea and the United States. Gut Liver 2022, 16, 503–514. [Google Scholar] [CrossRef]
- Bang, C.S.; Lim, H.; Jeong, H.M.; Shin, W.G.; Choi, J.H.; Soh, J.S.; Kang, H.S.; Yang, Y.J.; Hong, J.T.; Shin, S.P.; et al. Amoxicillin or tetracycline in bismuth-containing quadruple therapy as first-line treatment for Helicobacter pylori infection. Gut Microbes 2020, 11, 1314–1323. [Google Scholar] [CrossRef]
- Gisbert, J.P.; Barrio, J.; Modolell, I.; Molina-Infante, J.; Aisa, A.P.; Castro-Fernández, M.; Rodrigo, L.; Cosme, A.; Gisbert, J.L.; Fernández-Bermejo, M.; et al. Helicobacter Pylori First-Line and Rescue Treatments in the Presence of Penicillin Allergy. Dig. Dis. Sci. 2014, 60, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Marin, A.C.; Nyssen, O.P.; McNicholl, A.G.; Gisbert, J.P. Efficacy and Safety of Quinolone-Containing Rescue Therapies After the Failure of Non-Bismuth Quadruple Treatments for Helicobacter pylori Eradication: Systematic Review and Meta-Analysis. Drugs 2017, 77, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Kong, Q.; Zhang, Q.; Zhang, L.; Li, R.; Zhang, T.; Guo, L.; Wang, X.; Li, X.; Zhao, H.; et al. Efficacy and Safety of Cefuroxime–Tetracycline-Containing Bismuth Quadruple Therapy for Helicobacter pylori Eradication in Penicillin-Allergic Patients: A Multicenter Randomized Controlled Trial. Helicobacter 2025, 30, e70033. [Google Scholar] [CrossRef] [PubMed]
- Campagna, J.D.; Bond, M.C.; Schabelman, E.; Hayes, B.D. The Use of Cephalosporins in Penicillin- allergic Patients: A Literature Review. J. Emerg. Med. 2012, 42, 612–620. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, J.; Ding, Z.; Chen, X.; Liang, X.; Zeng, X.; Xu, F.; Han, Y.; Lu, H. Minocycline vs. tetracycline in bismuth-containing quadruple therapy for Helicobacter pylori rescue treatment: A multicentre, randomized controlled trial. J. Gastroenterol. 2023, 58, 633–641. [Google Scholar] [CrossRef]
- Ali, N.S.; Long, B.D.; Manzoor, N.F.; Sismanis, A.; Coelho, D.H. Doxycycline-Induced Intracranial Hypertension Presenting as Unilateral Pulsatile Tinnitus. Otol. Neurotol. Open 2023, 3, e043. [Google Scholar] [CrossRef]
- Gisbert, J.P. Optimization Strategies Aimed to Increase the Efficacy of Helicobacter pylori Eradication Therapies with Quinolones. Molecules 2020, 25, 5084. [Google Scholar] [CrossRef]
- Zullo, A.; Perna, F.; Hassan, C.; Ricci, C.; Saracino, I.; Morini, S.; Vaira, D. Primary antibiotic resistance in Helicobacter pylori strains isolated in northern and central Italy. Aliment. Pharmacol. Ther. 2007, 25, 1429–1434. [Google Scholar] [CrossRef]
- Liu, L.; Nahata, M.C. Treatment of Helicobacter pylori Infection in Patients with Penicillin Allergy. Antibiotics 2023, 12, 737. [Google Scholar] [CrossRef]
- Goh, K.; Lee, Y.Y.; Leow, A.H.; Ali, R.A.R.; Ho, S.H.; Mahadeva, S.; Said, R.H.M.; Chettiar, R.M.; Tee, H.P. A Malaysian consensus report on the diagnosis and treatment of Helicobacter pylori infection. JGH Open 2023, 7, 261–271. [Google Scholar] [CrossRef]
- Ishibashi, F.; Suzuki, S.; Nagai, M.; Mochida, K.; Morishita, T. Optimizing Helicobacter pylori Treatment: An Updated Review of Empirical and Susceptibility Test-Based Treatments. Gut Liver 2023, 17, 684–697. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).