Abstract
Antibiotic resistance is an increasing problem, especially in children with urinary tract infections. Rates of drug-specific resistant pathogens were reported, and an easy prediction model to guide the clinical decision-making process for antibiotic treatment was proposed. Data on microbiological isolation from urinoculture, between January 2007–December 2018 at Istituto Gaslini, Italy, in patients aged <19 years were extracted. Logistic regression-based prediction scores were calculated. Discrimination was determined by the area under the receiver operating characteristic curve; calibration was assessed by the Hosmer and Lemeshow test and the Spiegelhalterz test. A total of 9449 bacterial strains were isolated in 6207 patients; 27.2% were <6 months old at the first episode. Enterobacteriales (Escherichia coli and other Enterobacteriales) accounted for 80.4% of all isolates. Amoxicillin-clavulanate (AMC) and cefixime (CFI) Enterobacteriales resistance was 32.8% and 13.7%, respectively, and remained quite stable among the different age groups. On the contrary, resistance to ciprofloxacin (CIP) (overall 9.6%) and cotrimoxazole (SXT) (overall 28%) increased with age. After multivariable analysis, resistance to AMC/CFI could be predicted by the following: sex; age at sampling; department of admission; previous number of bacterial pathogens isolated. Resistance to CIP/SXT could be predicted by the same factors, excluding sex. The models achieved very good calibration but moderate discrimination performance. Specific antibiotic resistance among Enterobacteriales could be predicted using the proposed scoring system to guide empirical antibiotic choice. Further studies are needed to validate this tool.
1. Introduction
Urinary tract infection (UTI) is a common health problem in pediatrics [1]. The main targets in childhood urinary tract infection treatment are rapid recovery and prevention of related complications, such as urosepsis and renal abscess, and permanent renal parenchymal damage that can cause chronic renal failure and hypertension [2,3,4,5]. To achieve these aims, empirical antibiotic prescription is often endorsed already before the culture results are available, since a delay in treatment initiation could be associated with permanent renal scarring [6]. Administration of low-dose antibiotic prophylaxis is a common practice in the presence of recurrent urinary tract infections due to 3rd–5th degree vesico-ureteral reflux [7,8,9]. At the single-patient level, the efficacy of antimicrobial treatment is critically dependent on correctly matching the antibiotic(s) to the specific susceptibilities of pathogens [10,11,12]. However, the antibiotic resistance of urinary tract pathogens isolated in children is increasing, especially for commonly used antimicrobials. This has important implications for treatments [13,14]. For this reason, knowledge of the etiology of UTIs and their resistance patterns in general and in specific geographical locations may aid clinicians in choosing the appropriate antimicrobial treatments, reducing the risk of incorrect initial treatment, especially in cases of recurrent infections [10,14,15,16]. The common etiological agents of pediatric UTIs are Gram-negatives, mainly Escherichia coli, followed by Klebsiella pneumoniae and Proteus mirabilis; and Enterococcus faecalis among Gram-positives [1,17]. Resistance rates exceeding 20% for commonly used drugs have been described for these pathogens [17,18,19]. The risk of resistance to different antibiotics is associated with patient demographics; comorbidities; and patient’s clinical history, including recurrent UTIs, especially in the presence of malformations and prophylaxis administration, hospitalizations and antibiotic resistance in previous infections [11,16,19,20,21].
The aims of the present study were to describe the proportions of drug-specific resistant pathogens and to develop a simple, initial drug-specific risk prediction model of antibiotic resistant UTIs.
2. Materials and Methods
The Istituto Giannina Gaslini (IGG), Genoa-Italy, is a tertiary care pediatric hospital in Northern Italy serving as a local pediatric hospital for the Genoa area but also representing a referral hospital nationwide and for many foreign countries.
Data on microbiological isolation from urinoculture, in the period January 2007–December 2018, in patients <19 years old, were extracted from the IGG Laboratory of Microbiology database and anonymously analyzed. For each episode, the following data were available: age at time of positive culture (considering median and interquartile range, it was categorized as: ≤6 months, 7 months–2 years, 3–7 years and >7 years), sex, number of previous episodes (0, 1 or >1) and department of admission where the UTIs were diagnosed.
All positive urinary cultures were considered, independently from a diagnosis of upper (pyelonephritis) or lower (cystitis) UTI or asymptomatic bacteriuria [1,22]. All urine samples were obtained by midstream clean-catch, catheterization or urine bags, according to patient’s age (continence or not) and clinical condition and international recommendations [23,24,25]. Samples were processed on Columbia agar +5% sheep blood (bioMérieux SA, Marcy-l’Etoile, France) and MacConkey agar (bioMérieux SA, Marcy-l’Etoile, France) and were incubated at 37 °C overnight. Significant growth was considered to be ≥105 colony forming units (CFU)/mL of urine. Urinary culture, independently from gender and isolated pathogen, was considered positive with ≥105 CFU in all samples [22,26], since the method of urine collection was not available in our data [27,28]. The cultures with growth of more than two pathogens were excluded from the analysis because they considered as contaminated.
All antibiotic susceptibility tests were performed with automated systems (BD Phoenix, Becton, Dickinson and Company, Sparks, MD, USA) that were based on Clinical and Laboratory Standard Institute (CLSI) criteria from 2007 to 2010; but from 2011, European Committee on Antimicrobial Susceptibility Testing (EUCAST) criteria for definition of antibiotic susceptibility were adopted. Due to this major change and considering that EUCAST breakpoints could have been changed every year, we decided to report the results in terms of susceptibility or resistance according to the definitions provided by the system used each year, which corresponded to what was performed in everyday practice [19].
Isolated pathogens were grouped as: Escherichia coli, Pseudomonas aeruginosa, other Enterobacteriales, other non-fermenting Gram-negatives, Enterococcus spp. and other Gram-positives (details in Supplementary Table S1). For each isolated strain, antibiotic susceptibility to the following drugs was recorded: amikacin, amoxicillin-clavulanate (AMC), ampicillin, cefixime (CFI), cefuroxime, cefotaxime, ceftazidime, ciprofloxacin (CIP), fosfomycin tromethamine, gentamicin, meropenem, nitrofutantoin, cotrimoxazole (SXT) and piperacillin-tazobactam for Escherichia coli and other Enterobacteriales; amikacin, ceftazidime, ciprofloxacin, fosfomycin tromethamine, meropenem and piperacillin-tazobactam for Pseudomonas aeruginosa; amikacin, ceftazidime, ciprofloxacin, gentamicin, meropenem, and piperacillin-tazobactam for other non-fermenting Gram-negatives; and ampicillin, teicoplanin and vancomycin for Enterococcus spp.
Statistical Analysis
Categorical data are reported in terms of absolute frequencies and percentages. Continuous data are described in terms of median values and interquartile ranges (IQR), due to their non-normal (Gaussian) distribution. Percentages of antibiotic resistant infections by pathogen were calculated, and the 95% confidence interval (CI) is reported with the cluster-robust estimator of variance allowing for intra-group correlation due to possible repeated UTI in the same patient.
The associations between the binary outcome variable (antibiotic resistance) and independent variables were assessed by logistic regression and are reported in terms of odds ratio (OR) and 95% CI. The cluster-robust estimator of variance was used, calculating CI to control for intra-group correlation. Multivariable regressions were focused on the group of Gram-negative (Escherichia coli and other Enterobacteriales) pathogens that are more homogeneous and representative in terms of tested susceptibility and frequency of antibiotic resistance. Available variables as independent factors were entered into the multivariable models, and the possible interactions between factors were also examined. The likelihood-ratio test (LR) was calculated to measure the effect of each predictor.
Prediction models were developed from the final logistic models to predict the risks of resistance to specific antibiotics (AMC, CFI, CIP and SXT) with oral formulations commonly used to treat Gram-negative UTIs in pediatrics. As multivariable analysis showed similar results of risk factors for AMC and CFI and for CIP and SXT, two variables were generated to consider AMC and CFI and CIP and SXT together. To correct for the optimism bias, data were randomized, using a random number generator, into either the training data set (70% of the total) or the validation data set. The models were fitted using the training data sets and then assessed by using the models to score the validation data sets. In addition, internal validation, k-fold cross-validation(k = 10), was performed.
The overall predictive model performance was assessed using the Brier score (that includes component of discrimination and calibration); the predictive accuracy for discrimination was determined by calculating the area under the receiver operating characteristic (ROC) curve (AUC) and the discrimination slope; calibration was assessed by comparing predicted and observed probabilities using the Hosmer and Lemeshow test, the Spiegelhalterz test and the calibration plot [29]. To compare the areas under two independent ROC curves (training and validation) the Chi-square test was applied [30]. To develop the predictive risk score function, a weight was assigned to each risk factor in relation to each β parameter based on the multivariable logistic regression model [31]. Analysis and presentation were based on available data, i.e., no imputation of missing data was performed. All tests were 2-tailed, and a p value < 0.05 was considered statistically significant. All analyses were performed using Stata (Stata Corp. Stata Statistical Software, Release 13.1 College Station, TX, USA, Stata Corporation, 2013) and SAS software Version 9.4 (SAS Institute Inc, Cary, NC, USA).
The TRIPOD statement recommendations for develop prediction models were followed [32].
This study was conducted in accordance with the Helsinki Declaration. According to Italian legislation, the study did not need ethical approval, as it was a purely observational retrospective study on routine collected anonymous data. Moreover, informed consent for participate in the study was not required since retrospective data were obtained by an anonymous microbiology database. In any case, consent to completely anonymous use of clinical data for research/epidemiological purposes is requested by clinical routine at the time of admission/diagnostic procedure.
3. Results
A total of 9449 bacterial strains were isolated in 9356 urine samples, Figure 1, from 6207 patients, and 1432 (23.1%) patients presented having had more than one episode (812 patients with 2 and 620 with >2). Half of patients were females, and the median age at the first positive sample was 1.8 years (IQR, 0.4–6.8); 1685 (27.2%) children were aged <6 months at the first episode. Characteristics of the study population are reported in Supplementary Table S2.
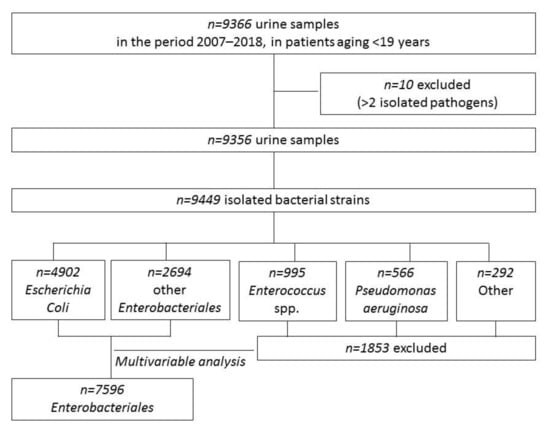
Figure 1.
Flow chart of the study of urine samples and isolated pathogens.
Among the 9449 pathogens isolated, Escherichia coli (n = 4902, 51.9%) was the most frequently isolated, followed by other Enterobacteriales (n = 2694, 28.5%), Enterococcus spp. (n = 995, 10.5%), Pseudomonas aeruginosa (n = 566, 6%), other Gram-positives (n = 231, 2.4%) and other non-fermenting Gram-negatives (n = 61, 0.6%). Proteus mirabilis (n = 988), Klebsiella pneumoniae (n = 633) and Klebsiella oxytoca (n = 307) were the most frequent among the other Enterobacteriales, and Enterococcus faecalis (n = 850) was the most frequent among enterococci. A complete list of the isolated pathogens is available in Supplementary Table S1.
3.1. Antibiotic Resistant UTI by Pathogen
Data on resistance are expressed as proportions of resistant strains over tested strains. Escherichia coli and other Enterobacteriales were the most frequently isolated pathogens and presented similar distributions of antibiotic resistance. Among Escherichia coli, ampicillin resistance was the most frequent (58.8%), followed by SXT (31%) and AMC (29.9%). As for other Enterobacteriales, AMC resistance was 38.3% and SXT was 22.6%. Overall ampicillin resistance was 78.2%, falling to 44.9% among 931 tested Proteus mirabilis and reaching nearly 100% among the remaining other Enterobacteriales. For Pseudomonas aeruginosa, resistance to piperacillin-tazobactam was 8.3%; to ceftazidime, 7.3%; and to ciprofloxacin, 4.3%. Overall ampicillin resistance was 8.3% for the Enterococcus spp. group, 76.5% among 98 Enterococcus faecium and 0.2% among 832 Enterococcus faecalis. Data for specific pathogens and antibiotic resistance are shown in Figure 2 and detailed in Supplementary Table S3.
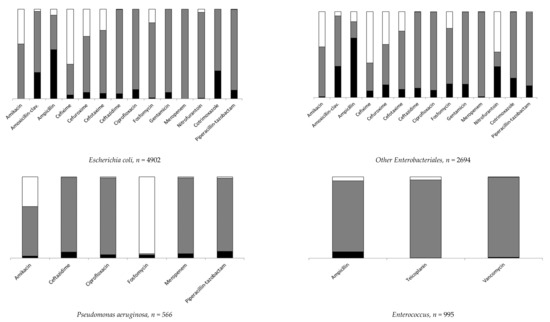
Figure 2.
Distribution of antibiotic resistant strains (details in Supplementary Table S3). A black box represents the percentage of resistant, a gray box the percentage of susceptible and a white box the percentage of not tested.
Escherichia coli and other Enterobacteriales altogether accounted for 80.4% of all isolates (see Figure 1; 7596 strains in 5190 patients; characteristics are reported in Supplementary Table S4), so further analyses were then mainly focused on these groups. AMC and CIF resistance was 32.8% and 13.7%, respectively, and remained quite stable among the different age groups. On the contrary, resistance to CIP and SXT increased with age (Table 1 and Supplementary Table S5).

Table 1.
Distribution antibiotic resistance among 4902 Escherichia coli and 2694 other Enterobacteriales by age at sampling.
In Table 2, risk factors for isolation of antibiotic resistant Enterobacteriales are reported. In general, considering the antibiotics with oral formulations (AMC, CFI, CIP and SXT), similar results were observed for AMC and CFI and for CIP and SXT. Age significantly influenced the risk of resistance to all antibiotics, especially for CIP and SXT, which showed increased risks of resistance with age, whereas for the other drugs, increasing age was a “protective” factor, since compared to children aged <6 months, the risk decreased.

Table 2.
Multivariable logistic regression models for resistance to different antibiotics among 4902 Escherichia coli and 2694 other Enterobacteriales.
As for the risk of resistance according to the department of admission, the risk increased for all antibiotics and for all departments compared to the Emergency Department. Additionally, the previous number of episodes was associated with an increased risk of resistance for all antibiotics for subjects with at least one previous event compared to patients without a history of UTI.
3.2. AMC/CFI and CIP/SXT Resistance Prediction Models
Since among Enterobacteriales, the resistance ratios were very similar for AMC and CFI (2466/7351, 33.5%) and for CIP and STX (2304/7577, 30.4%), a predictive model was implemented to evaluate the probability of isolating a strain resistant to these two groups of drugs at the time of urine sampling. Seventy percent (n = 5147 for AMC/CFI and n = 5305 for CIP/SXT) of the total number of samples was assigned to the training group and 30% (n = 2204 for AMC/CFI and n = 2272 for CIP/SXT) to the validation group. The results of the final models on the training data set are shown in Table 3 with the weight/point assigned to each one for the score construction. None of the interactions between the variables of the final models were statistically significant.

Table 3.
Multivariable logistic regression models of training data sets. Models and points system to predict risk of specific drug-resistant urinary tract infection due to Enterobacteriales.
The overall predictive model performance was moderate. The Bier scores were 0.216 and 0.199 for AMC/CFI and CIP/SXT, respectively. The predictive accuracy of models was weak for the discrimination performance (the ability of the model to distinguish between events and non-events): For AMC/CFI, the model AUC-ROC was 61.8%, 95% CI (60.1–63.4) in the training cohort; 59.7%, 95% CI (57.2–62.2) in the validation cohort; and 60.9%, 95% CI (59.2–62.1) in the 10-fold cross-validation. For CIP/SXT, AUC-ROC was 64%, 95% CI (62.4–65.6) in the training cohort; 63.3%, 95% CI (60.8–65.8) in the validation cohort; and 63.5%, 95% CI (61.1–64.0) in the 10-fold cross-validation (Supplementary Figures S1–S3). However, the models achieved very good calibration (how close the predicted probabilities are to the actual rate of events/“real” probability), as shown in Figure 3 by comparing predicted and observed probabilities in the calibration plots. In particular, the observed prevalence of resistance to AMC/CFI in the training cohort was 33.5% (1727/5147), and the average estimated risk given by model was 0.334 (i.e., 33.4%); for CIP/SXT the observed prevalence was 30.4% (1613/5305) and the average estimated risk was 0.304 (i.e., 30.4%).
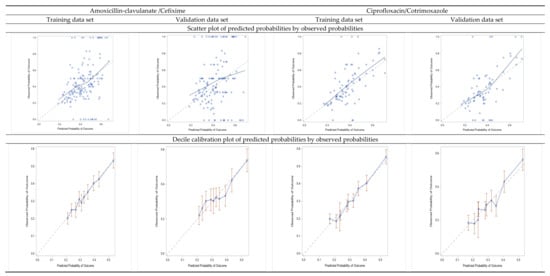
Figure 3.
Calibration plots: scatter plots of predicted probabilities by observed probabilities of resistance, and decile calibration plots for the training and validation data sets.
Table 4 shows the simple risk scoring system and how individual total scores relate to the specific risk of infection due to resistant Enterobacteriales. Considering AMC/CFI, those patients (male aged <6 months admitted in Neonatal or Pediatric ICU and with a history of previous episodes) with total scores of 22 had an estimated risk of over 60%. For CIP/SXT, patients with the maximum total score of 27 (patients aged >7 years, in Hematology/Oncology and with a history of previous episodes) had an estimated risk of over 70%.

Table 4.
Points system and risk estimates of isolating specific drug-resistant Enterobacteriales based on multivariable models reported in Table 3.
4. Discussion
In the present, retrospective, single center study, the proportions of antibiotic resistance among more than 9000 pathogens isolated from urine sample in children were analyzed. Having a large number of cases, we analyzed not only the proportions of isolated pathogens and their patterns of resistance to selected antibiotics; factors associated with a higher probability of antibiotic resistance among Enterobacteriales were identified, especially resistance to antibiotics with oral formulations generally administered for urinary tract infections (AMC, CIF, CIP, SXT). High rates of antibiotic resistances for orally administrated drugs that are commonly prescribed in pediatrics were documented. Overall, Enterobacteriales represented about 80% of isolated strains, and resistance to AMC was the highest (near 33%), followed by SXT (28%); the lowest was for CIP, which was detected in just under 10% of isolated pathogens. These results are in line with other recent data from other Italian regions. In a pediatric cohort, under the age of 18 years, in Emilia-Romagna [26], Enterobacteriales were the most frequently isolated pathogens (82%), and overall, AMC resistance was 34%. Additionally, in a study of children aged 0 to 6 years in Tuscany [33], Enterobacteriales represented the majority of isolates (71%), and isolated uropathogens showed high resistance rates to AMC (26%), particularly in children under one year of age (28%). In this study, a worrisome proportion of resistance in children aged <6 months was observed, especially resistance to AMC (35%). This may be associated with peripartum exposure to maternal antibiotics, which increases the risk of resistant rods in the newborn. Furthermore, the increased resistance rates in children under one year of age could be related to the acquisition of nosocomial microorganisms at the time of birth [33]. In our study, age ≤ 6 months represented a significant risk factor for infection due to strains resistant to AMC/CIF, but not for CIP/SXT, which showed an increasing risk of resistance with increasing age at episode. This observation is particularly worrisome, since this patient group (children ≤ 6 months of age) presented with first UTI episodes due to bacterial strains already resistant to AMC/CIF in a not negligible proportion. These children are frequently affected with urinary tract abnormalities and have a high risk of recurrency for UTIs, and above all vesico-ureteral reflux, and could go through repeated antibiotic courses (prophylaxis and/or therapy), which are a well-known risk factor for antibiotic resistance [11,16]. Our results provide warnings on the feasibility of applying the most recent national recommendations for treatment of the first episode of urinary tract infections in pediatrics, which indicate AMC/CIF as the preferred first-line oral therapy [23]. The choice of use of AMC as an empirical first-line treatment should be carefully evaluated, above all in children under one year of age, and limited to patients in good clinical condition [33].
A further important observation is that in any episode observed outside the Emergency Department, in particular, in Hematology/Oncology, Nephrology or Neonatal ICU, there was a significant risk of the presence of a strain resistant to oral antibiotics. This is partially in contrast with our previous study [19], in which, among 4596 positive urine cultures from 2007 to 2014, E. coli represented the majority of isolates (3006, 65.4%) and AMC resistance was 30%. In addition, an increased risk of AMC resistance was significantly associated with hospitalization in Nephrology or other wards managing urinary tract malformation with respect to wards not dealing with this malformation. Instead, a more recent work reported high resistance in Hematology and Oncology departments [34].
Finally, our data confirmed an increased risk of isolation of a resistant strains in case patients who had experienced more than one episode of UTI. Considering repeated same-patient cultures, a personal component of memory-like correlations of resistance can be assumed. These correlations can represent recurrent infections with the same strain or correlations with other patient-specific factors that further contribute to the predictability of resistance [19,21].
As a secondary aim, an initial scoring system was proposed, mainly to guide the clinical decision-making process for antibiotic treatment and not to distinguish between events and non-events. Since antibiotic resistances in pathogens causing UTIs are emerging, research describing risk factors associated with resistances and developing prediction models is of great interest and will help to better target initial antibiotic treatment. The developed models presented a moderate ability (AUC 60%) to discriminate on the basis of the probabilities of those who had an infection with resistant Enterobacteriales from those who did not, and therefore, be able to find the best cut off. However, a well discriminating model may be useless if the decision threshold for clinical decisions is outside the range of predictions provided by the model. Even a poorly discriminating model may be clinically useful if the clinical decision is close to a “toss up.” This implies that the threshold is right in the middle of the distribution of predicted risks, but also that evaluation of calibration is important if model predictions are used to inform patients or physicians to make decisions [29]. Calibration is especially important when the aim is to support decision-making, even when discrimination is moderate [35]. The need for a further evaluations with new data, possibly collected prospectively, is the major limitation of this study, together with its retrospective method that caused a limited number of potential risk factors being included in the statistical model. The data available for this study were extracted from a microbiology laboratory database, so data on patients’ medical history, e.g., antibiotic administration for prophylaxis or treatment of previous UTIs episodes, were not available. However, the department of admission, where the UTIs were diagnosed, played an important role in this analysis, and this factor could be considered as a “proxy variable” for the patient’s clinical condition. This study proposed a weighted scoring model based on simple information available at the time of hospital admission. The easy scoring system also clearly showed that a non-negligible (about 20% and more) risk of resistance to AMC/CIF was present also in patients with no risk factors (i.e., negative or zero scores). This is particularly worrisome if we consider that bacteria causing urinary tract infections are often carried asymptomatically in the human body and are therefore frequently exposed to antibiotics, including those taken to treat other infections, and that AMC is among the most frequently prescribed antibiotics in Italy in pediatrics, and its use can drive also resistance to other antibiotics [36,37,38]. This risk was lower for SXT/CIP, but at least for CIP, use should be restricted not only because of its potential risk of toxicity in pediatrics, but also since it could represent the only oral treatment for Pseudomonas aeruginosa or other Gram-negatives resistant to other antibiotics [39,40,41].
5. Conclusions
Our study provided insight into the predictors associated with a higher probability of antibiotic resistance among Enterobacteriales, especially to antibiotics with oral formulations generally administered in urinary tract infections. The developed scoring system could guide the clinical decision-making process for antibiotic treatment, based on four variables that are easy to define in clinical practice at the time of hospital admission. Proper use of this tool could minimize the time required to manage UTI and could reduce workloads and costs. Further validation works on this novel predictor should be perused to guide appropriate antimicrobial therapy and to improve the prognosis for these infections.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antibiotics11060720/s1, Table S1: Distribution of 9449 bacterial pathogens isolated, Table S2 reports the characteristics of the study population and culture reports of positive samples, Table S3 reports the distribution of isolated pathogens resistant to different antibiotics, Table S4 reports the characteristics of patients with isolation of Enterobacteriales from urine samples and Table S5 reports the distribution of 4902 Escherichia coli and 2694 other Enterobacteriales resistant to different antibiotics by age at sampling. Figure S1 shows ROC curves and AUC of training and validation data sets; Figure S2 shows box plots of predicted probabilities and discrimination slope in the training and validation data sets; Figure S3 shows ROC curves and AUC of 10-fold cross validations and internal validations.
Author Contributions
All authors contributed to this article. Methodology, Data Curation, Formal Analysis, Visualization, Writing—Original draft preparation, Writing—Reviewing and Editing: F.B. Conceptualization, Methodology, Resources, Writing—Reviewing and Editing: G.P. Investigation, Writing—Reviewing and Editing: A.M. Resources, Writing—Reviewing and Editing: M.M. Investigation, Writing—Reviewing and Editing: C.R. Resources, Writing—Reviewing and Editing: C.S., G.L. and C.P. Conceptualization, Methodology, Resources, Writing—Original draft preparation, Writing—Reviewing and Editing, Supervision, Project administration: E.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the Ministero della Salute-Ricerca Corrente 2022. The authors have no other relevant financial or non-financial interests to disclose.
Institutional Review Board Statement
The study did not need Internal Review Board approval as it was a purely observational retrospective study on routinely collected anonymous data.
Informed Consent Statement
Informed consent to participate in the study was not required, since retrospective data were obtained from an anonymous microbiology database.
Data Availability Statement
Data are contained within the article or supplementary material. They are available on request from the corresponding author.
Conflicts of Interest
Authors have no conflict of interest to declare for the present study.
References
- Williams, G.J.; Hodson, E.H.; Isaacs, D.; Craig, J.C. Diagnosis and management of urinary tract infection in children. J. Paediatr. Child Health 2012, 48, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Beetz, R.; Westenfelder, M. Antimicrobial therapy of urinary tract infections in children. Int. J. Antimicrob. Agents 2011, 38, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, N.; Haralam, M.A.; Kurs-Lasky, M.; Hoberman, A. Association of Renal Scarring with Number of Febrile Urinary Tract Infections in Children. JAMA Pediatr. 2019, 173, 949–952. [Google Scholar] [CrossRef] [PubMed]
- Simões ESilva, A.C.; Oliveira, E.A.; Mak, R.H. Urinary tract infection in pediatrics: An overview. J. Pediatr. 2020, 96, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Toffolo, A.; Ammenti, A.; Montini, G. Long-term clinical consequences of urinary tract infections during childhood: A review. Acta Paediatr. 2012, 101, 1018–1031. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, N.; Mattoo, T.K.; Keren, R.; Ivanova, A.; Cui, G.; Moxey-Mims, M.; Majd, M.; Ziessman, H.A.; Hoberman, A. Early Antibiotic Treatment for Pediatric Febrile Urinary Tract Infection and Renal Scarring. JAMA Pediatr. 2016, 170, 848–854. [Google Scholar] [CrossRef] [Green Version]
- Craig, J.C.; Simpson, J.M.; Williams, G.J.; Lowe, A.; Reynolds, G.J.; McTaggart, S.J.; Hodson, E.M.; Carapetis, J.R.; Cranswick, N.E.; Smith, G.; et al. Antibiotic prophylaxis and recurrent urinary tract infection in children. N. Engl. J. Med. 2009, 361, 1748–1759, Erratum in: N. Engl. J. Med. 2010, 362, 1250. [Google Scholar] [CrossRef] [Green Version]
- RIVUR Trial Investigators; Hoberman, A.; Greenfield, S.P.; Mattoo, T.K.; Keren, R.; Mathews, R.; Pohl, H.G.; Kropp, B.P.; Skoog, S.J.; Nelson, C.P.; et al. Antimicrobial prophylaxis for children with vesicoureteral reflux. N. Engl. J. Med. 2014, 370, 2367–2376. [Google Scholar] [CrossRef] [Green Version]
- Silay, M.S.; Undre, S.; Nambiar, A.K.; Dogan, H.S.; Kocvara, R.; Nijman, R.J.M.; Stein, R.; Tekgul, S.; Radmayr, C. Role of antibiotic prophylaxis in antenatal hydronephrosis: A systematic review from the European Association of Urology/European Society for Paediatric Urology Guidelines Panel. J. Pediatr. Urol. 2017, 13, 306–315. [Google Scholar] [CrossRef]
- Barbieri, E.; Bottigliengo, D.; Tellini, M.; Minotti, C.; Marchiori, M.; Cavicchioli, P.; Gregori, D.; Giaquinto, C.; Da Dalt, L.; Donà, D. Development of a Weighted-Incidence Syndromic Combination Antibiogram (WISCA) to guide the choice of the empiric antibiotic treatment for urinary tract infection in paediatric patients: A Bayesian approach. Antimicrob. Resist. Infect. Control 2021, 10, 74. [Google Scholar] [CrossRef]
- Selekman, R.E.; Shapiro, D.J.; Boscardin, J.; Williams, G.; Craig, J.C.; Brandström, P.; Pennesi, M.; Roussey-Kesler, G.; Hari, P.; Copp, H.L. Uropathogen Resistance and Antibiotic Prophylaxis: A Meta-analysis. Pediatrics 2018, 142, e20180119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vazouras, K.; Basmaci, R.; Bielicki, J.; Folgori, L.; Zaoutis, T.; Sharland, M.; Hsia, Y. Antibiotics and Cure Rates in Childhood Febrile Urinary Tract Infections in Clinical Trials: A Systematic Review and Meta-analysis. Drugs 2018, 78, 1593–1604. [Google Scholar] [CrossRef] [PubMed]
- Bryce, A.; Hay, A.D.; Lane, I.F.; Thornton, H.V.; Wootton, M.; Costelloe, C. Global prevalence of antibiotic resistance in paediatric urinary tract infections caused by Escherichia coli and association with routine use of antibiotics in primary care: Systematic review and meta-analysis. BMJ 2016, 352, i939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vazouras, K.; Velali, K.; Tassiou, I.; Anastasiou-Katsiardani, A.; Athanasopoulou, K.; Barbouni, A.; Jackson, C.; Folgori, L.; Zaoutis, T.; Basmaci, R.; et al. Antibiotic treatment and antimicrobial resistance in children with urinary tract infections. J. Glob. Antimicrob. Resist. 2020, 20, 4–10. [Google Scholar] [CrossRef]
- Kantamalee, W.; Santanirand, P.; Saisawat, P.; Boonsathorn, S.; Techasaensiri, C.; Apiwattanakul, N. Outcomes of Empirical Antimicrobial Therapy for Pediatric Community-onset Febrile Urinary Tract Infection in the Era of Increasing Antimicrobial Resistance. Pediatr. Infect. Dis. J. 2020, 39, 121–126. [Google Scholar] [CrossRef]
- Delbet, J.D.; Lorrot, M.; Ulinski, T. An update on new antibiotic prophylaxis and treatment for urinary tract infections in children. Expert Opin Pharmacother. 2017, 18, 1619–1625. [Google Scholar] [CrossRef]
- Farrell, D.J.; Morrissey, I.; De Rubeis, D.; Robbins, M.; Felmingham, D. A UK multicentre study of the antimicrobial susceptibility of bacterial pathogens causing urinary tract infection. J. Infect. 2003, 46, 94–100. [Google Scholar] [CrossRef]
- Edlin, R.S.; Shapiro, D.J.; Hersh, A.L.; Copp, H.L. Antibiotic resistance patterns of outpatient pediatric urinary tract infections. J. Urol. 2013, 190, 222–227. [Google Scholar] [CrossRef] [Green Version]
- Calzi, A.; Grignolo, S.; Caviglia, I.; Calevo, M.G.; Losurdo, G.; Piaggio, G.; Bandettini, R.; Castagnola, E. Resistance to oral antibiotics in 4569 Gram-negative rods isolated from urinary tract infection in children. Eur. J. Pediatr. 2016, 175, 1219–1225. [Google Scholar] [CrossRef]
- Alberici, I.; La Manna, A.; Pennesi, M.; Starc, M.; Scozzola, F.; Nicolini, G.; Toffolo, A.; Marra, G.; Chimenz, R.; Sica, F.; et al. First urinary tract infections in children: The role of the risk factors proposed by the Italian recommendations. Acta Paediatr. 2019, 108, 544–550. [Google Scholar] [CrossRef]
- Yelin, I.; Snitser, O.; Novich, G.; Katz, R.; Tal, O.; Parizade, M.; Chodick, G.; Koren, G.; Shalev, V.; Kishony, R. Personal clinical history predicts antibiotic resistance of urinary tract infections. Nat. Med. 2019, 25, 1143–1152. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, J.; Temple-Smith, M.; Sanci, L. Urinary tract infections in children: An overview of diagnosis and management. BMJ Paediatr. Open 2019, 3, e000487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ammenti, A.; Alberici, I.; Brugnara, M.; Chimenz, R.; Guarino, S.; La Manna, A.; La Scola, C.; Maringhini, S.; Marra, G.; Materassi, M.; et al. Updated Italian recommendations for the diagnosis, treatment and follow-up of the first febrile urinary tract infection in young children. Acta Paediatr. 2020, 109, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Labrosse, M.; Levy, A.; Autmizguine, J.; Gravel, J. Evaluation of a New Strategy for Clean-Catch Urine in Infants. Pediatrics 2016, 138, e20160573. [Google Scholar] [CrossRef] [Green Version]
- Urinary Tract Infection in under 16s: Diagnosis and Management; National Institute for Health and Care Excellence (NICE): London, UK, 2018.
- Esposito, S.; Maglietta, G.; Di Costanzo, M.; Ceccoli, M.; Vergine, G.; La Scola, C.; Malaventura, C.; Falcioni, A.; Iacono, A.; Crisafi, A.; et al. Retrospective 8-Year Study on the Antibiotic Resistance of Uropathogens in Children Hospitalised for Urinary Tract Infection in the Emilia-Romagna Region, Italy. Antibiotics 2021, 10, 1207. [Google Scholar] [CrossRef]
- Okarska-Napierała, M.; Wasilewska, A.; Kuchar, E. Urinary tract infection in children: Diagnosis, treatment, imaging—Comparison of current guidelines. J. Pediatr. Urol. 2017, 13, 567–573. [Google Scholar] [CrossRef]
- Shaikh, N.; Hoberman, A. Urinary Tract Infections in Infants and Children Older than One Month: Clinical Features and Diagnosis; UpToDate: Waltham, MA, USA, 2021. [Google Scholar]
- Steyerberg, E.W.; Vickers, A.J.; Cook, N.R.; Gerds, T.; Gonen, M.; Obuchowski, N.; Pencina, M.J.; Kattan, M.W. Assessing the performance of prediction models: A framework for traditional and novel measures. Epidemiology 2010, 21, 128–138. [Google Scholar] [CrossRef] [Green Version]
- Gönen, M. Analyzing Receiver Operating Characteristic Curves with SAS; SAS Institute Inc.: Cary, NC, USA, 2007. [Google Scholar]
- Sullivan, L.M.; Massaro, J.M.; D’Agostino Sr, R.B. Presentation of multivariate data for clinical use: The Framingham Study risk score functions. Stat. Med. 2004, 23, 1631–1660. [Google Scholar] [CrossRef]
- Moons, K.G.; Altman, D.G.; Reitsma, J.B.; Ioannidis, J.P.; Macaskill, P.; Steyerberg, E.W.; Vickers, A.J.; Ransohoff, D.F.; Collins, G.S. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): Explanation and elaboration. Ann. Intern. Med. 2015, 162, W1–W73. [Google Scholar] [CrossRef] [Green Version]
- Montagnani, C.; Tersigni, C.; D’Arienzo, S.; Miftode, A.; Venturini, E.; Bortone, B.; Bianchi, L.; Chiappini, E.; Forni, S.; Gemmi, F.; et al. Resistance Patterns from Urine Cultures in Children Aged 0 to 6 Years: Implications for Empirical Antibiotic Choice. Infect. Drug Resist. 2021, 14, 2341–2348. [Google Scholar] [CrossRef]
- Landi, F.; Bandettini, R.; Rotulo, G.A.; Mesini, A.; Saffioti, C.; Amoroso, L.; Pierri, F.; Guardo, D.; Castagnola, E. Resistance to Antibiotics of Uropathogen Bacteria Isolated from Urine and Blood in Pediatric Cancer Patients: A Single Center, 12-year Study. Pediatr. Infect. Dis. J. 2020, 39, 1106–1110. [Google Scholar] [CrossRef]
- Van Calster, B.; McLernon, D.J.; van Smeden, M.; Wynants, L.; Steyerberg, E.W.; Topic Group ‘Evaluating diagnostic tests and prediction models’ of the STRATOS initiative. Calibration: The Achilles heel of predictive analytics. BMC Med. 2019, 17, 230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pouwels, K.B.; Freeman, R.; Muller-Pebody, B.; Rooney, G.; Henderson, K.L.; Robotham, J.V.; Smieszek, T. Association between use of different antibiotics and trimethoprim resistance: Going beyond the obvious crude association. J. Antimicrob. Chemother. 2018, 73, 1700–1707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Martino, M.; Lallo, A.; Kirchmayer, U.; Davoli, M.; Fusco, D. Prevalence of antibiotic prescription in pediatric outpatients in Italy: The role of local health districts and primary care physicians in determining variation. A multilevel design for healthcare decision support. BMC Public Health 2017, 17, 886. [Google Scholar] [CrossRef] [PubMed]
- Youngster, I.; Avorn, J.; Belleudi, V.; Cantarutti, A.; Díez-Domingo, J.; Kirchmayer, U.; Park, B.J.; Peiró, S.; Sanfélix-Gimeno, G.; Schröder, H.; et al. Antibiotic Use in Children—A Cross-National Analysis of 6 Countries. J. Pediatr. 2017, 182, 239–244.e1. [Google Scholar] [CrossRef]
- Jackson, M.A.; Schutze, G.E.; Committee on Infectious Diseases. The Use of Systemic and Topical Fluoroquinolones. Pediatrics 2016, 138, e20162706. [Google Scholar] [CrossRef] [Green Version]
- Adefurin, A.; Sammons, H.; Jacqz-Aigrain, E.; Choonara, I. Ciprofloxacin safety in paediatrics: A systematic review. Arch. Dis. Child. 2011, 96, 874–880. [Google Scholar] [CrossRef] [Green Version]
- Patel, K.; Goldman, J.L. Safety Concerns Surrounding Quinolone Use in Children. J. Clin. Pharmacol. 2016, 56, 1060–1075. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).