Abstract
Bacterial biofilms are a growing problem as it is a major cause of nosocomial infection from urinary catheters to chronic tissue infections and provide resistance to a variety of antibiotics and the host’s immune system. The effect of pectolinarin on the biofilm formation in Enterococcus faecalis, Enterococcus faecium, Escherichia coli, Streptococcus mutans, Streptococcus sobrinus, Staphylococcus aureus, Pseudomonas aeruginosa, Cutibacterium acnes, and Porphyromonas gingivalis was studied in TSBg (tryptic soy broth supplemented with 1% glucose). Pectolinarin inhibited biofilm formation of E. faecalis (IC50 = 0.39 μg/mL), E. faecium (IC50 = 0.19 μg/mL), E. coli (IC50 = 0.25 μg/mL), S. mutans (IC50 = 1.2 μg/mL), S. sobrinus (IC50 = 1.4 μg/mL), S. aureus (IC50 = 0.39 μg/mL), P. aeruginosa (IC50 = 0.9 μg/mL), P. acnes (IC50 = 12.5 μg/mL), and P. gingivalis (IC50 = 9.0 μg/mL) without inhibiting the bacterial growth. Pectolinarin also showed increased susceptibility of antibacterial activity with commercially available antibiotics including ampicillin, vancomycin, streptomycin, and oxytetracyclin against E. faecalis and E. faecium. Finally, pectolinarin dose-dependently reduced the expression of genes including cytolysin genes (cylLS, cylR2 and cylM), quorum sensing (QS) genes (fsrB, fsrC, gelE, ebpA, ebpB, acm, scm and bps), and biofilm virulence genes (esp) of E. faecalis and E. faecium. Pectolinarin reduced the bacterial biofilm formation, activated the antibacterial susceptibility, and reduced the bacterial adherence. These results suggest that bacterial biofilm formation is a good target to develop the antibacterial agents against biofilm-related infections.
1. Introduction
Bacterial biofilms are microbial communities encased within a complex matrix and capable of colonizing natural body surfaces such as the epithelium, lungs, and heart, as well as implanted medical devices such as central venous and urinary catheters, intrauterine devices, and prosthetic heart valves [1]. The biofilm offers many advantages to bacteria, including the ability to acquire increased resistance toward antibiotics, which is of particular importance. This resistance leads to complications in the management of biofilm infections and limits therapeutic options [2,3,4]. In addition, bacterial biofilms pose a challenge to the host immune system [5]. During the past few decades, Enterococcus spp. have emerged as important healthcare-associated pathogens. The continuing progress of modern medical care with the overuse of antibiotics has undoubtedly contributed to increase the emergence of antibiotic resistance among clinical Enterococcus spp. isolates including E. faecium, which is essentially more drug-resistant than E. faecalis, with more than half of the isolates appearing resistant to the antibiotics [6]. Healthcare-associated infections due to E. faecalis and E. faecium more frequently showed resistance to high-level vancomycin and ampicillin, and unsusceptibility to antibiotics [7,8]. Therefore, it is necessary to control bacterial biofilm formation to control the bacterial infection [1,8,9].
Pectolinarin is a flavonoid group, which is present in Cirsium spp. that is commonly known as plume thistle (Figure 1). Cirsium spp. has been reported with bioactive potential including antidiabetic, antioxidant, hepatoprotective, anti-inflammatory, vasorelaxant, and anti-cancer properties [10,11].
Figure 1.
Chemical structure of pectolinarin.
In this work, pectolinarin was tested for anti-biofilm formation of pathogenic bacteria for the candidate as an antibiotic’s adjuvant. Pectolinarin showed inhibitory effect of the biofilm formation and thereby increasing the susceptibility of antibiotics. These results indicate that pectolinarin has the potential as an antibacterial adjuvant to treat the biofilm-related infections.
2. Results
2.1. Pectolinarin Inhibited the Biofilm Formation of Bacteria
The bacterial biofilm is important for bacteria to survive from the treatment of antibiotics or a hard environment. Pectolinarin was tested and showed the dose-dependent inhibition of bacterial biofilm formation by E. faecalis (IC50 = 0.39 μg/mL), E. faecium (IC50 = 0.19 μg/mL), E. coli (IC50 = 0.25 μg/mL), S. mutans (IC50 = 1.2 μg/mL), S. sobrinus (IC50 = 1.4 μg/mL), S. aureus (IC50 = 0.39 μg/mL), P. aeruginosa (IC50 = 0.9 μg/mL), P. acnes (IC50 = 12.5 μg/mL), and P. gingivalis (IC50 = 0.9 μg/mL) (Table 1, Figure 2, Supplementary Figure S1).

Table 1.
Pentoliniums inhibited biofilm formation of bacteria spp. (IC50 value).
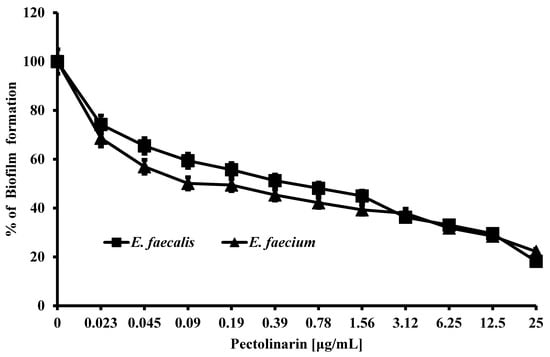
Figure 2.
Pectolinarin inhibited biofilm formation of E. faecalis and E. faecium. Biofilm of E. faecalis and E. faecium was induced in medium supplemented with pectolinarin at the indicated concentrations at 37 °C for 24 h. Data are the mean values ± SD of triplicate experiments.
Pectolinarin exhibited the strongest inhibitory effect of biofilm formation against E. faecalis and E. faecium, so those bacteria were used for further studies.
2.2. Pectolinarin Increased the Susceptibility of E. faecalis and E. faecium to Commercialized Antibiotics
Biofilm formation was performed as described above, and the bacterial growth in the presence or absence of pectolinarin (PEC) with ampicillin (AMP), vancomycin (VAN), oxytetracycline (OXY), or streptomycin (STR) was tested after an additional 24 h. Pectolinarin increased the bacterial susceptibility to antibiotics regardless of whether the bacteria showed resistance against the antibiotics (Figure 3). An amount of 1.56 μg/mL of pectolinarin treatment significantly activated the antibacterial activity of ampicillin, which reduced the viable cells to 0.9% compared with 9% of the only-ampicillin treatment condition. Pectolinarin treatment also reduced the bacterial viability by approximately 10% to 20% compared with only oxytetracycline, streptomycin, or vancomycin treatment (Figure 3a). A total of 0.39 μg/mL of pectolinarin treatment also approximately increased by 10% to 20% the antibacterial activity of ampicillin, oxytetracycline, streptomycin, and vancomycin against E. faecium (Figure 3b).
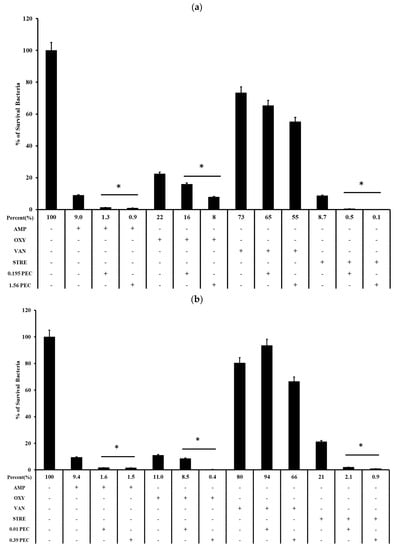
Figure 3.
Pectolinarin increased antibiotic susceptibility. (a) Pectolinarin increased the susceptibility of E. faecalis to conventional antibiotics. Biofilms formed for 24 h by growing E. faecalis in TSBg followed by treatment with OXY (3.125 μg/mL), AMP (6.25 μg/mL), STR (100 μg/mL), or VAN (100 μg/mL) alone or in combination with pectolinarin (0.39 and 1.56 μg/mL) for 24 h. (b) Pectolinarin increased the susceptibility of E. faecium to conventional antibiotics. Biofilms formed for 24 h by growing E. faecium in TSBg followed by treatment with OXY (3.125 μg/mL), AMP (6.25 μg/mL), STR (100 μg/mL), or VAN (100 μg/mL) alone or in combination with pectolinarin (0.01 and 0.39 μg/mL) for 24 h. Data are the mean values ± SD of triplicate experiments. * = p < 0.05 was considered significant.
2.3. Pectolinarin Reduced Bacterial Adherence to T24 Cells in a Dose-Dependent Manner
Pectolinarin showed significant inhibition of biofilm formation at 0.01–50 μg/mL. Microscopic analysis revealed that pectolinarin inhibited adhesion of the bacteria to the human urinary bladder cancer T24 cells (Figure 4) without inhibition of the growth of T24 cells (data not shown). Especially, 0.01 μg/mL of pectolinarin treatment decreased E. faecium adherence by 26% compared to planktonic bacteria.
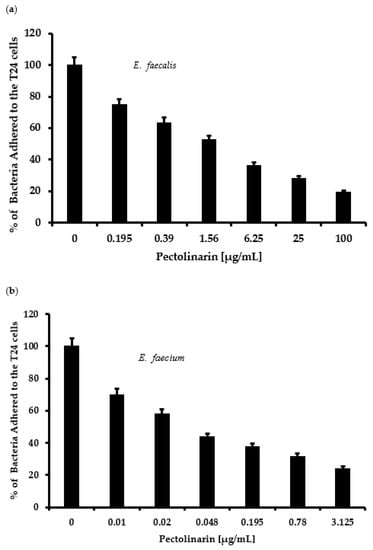
Figure 4.
Pectolinarin reduced bacterial adherence to host cells. (a) Pectolinarin reduced E. faecalis adherence to the host cells. After E. faecalis was allowed to infect T24 cells for 24 h, cultures were washed with PBS, and adhered bacterial cells were counted by plating. (b) Pectolinarin reduced E. faecium adherence to the host cells. After E. faecium was allowed to infect T24 cells for 24 h, cultures were washed with PBS, and adhered bacterial cells were counted by plating. Data are the mean values ± SD of triplicate experiments.
2.4. Pectolinarin Did Not Affect Bacterial Growth
Several antimicrobial compounds also inhibit bacterial biofilm formation because antibiotics kill the bacteria and indirectly decrease biofilm formation [12]. To test whether the biofilm formation inhibition of pectolinarin was due to the antibacterial activity, E. faecalis and E. faecium were treated with 100 μg/mL of pectolinarin for 24 h. Pectolinarin, despite its notable inhibition of biofilm formation, did not show any effect on the bacterial growth (Figure 5).
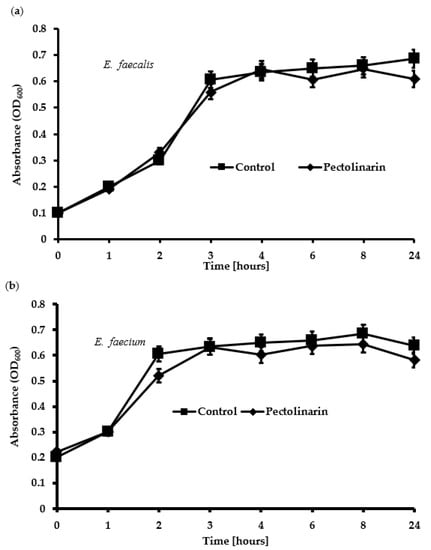
Figure 5.
Pectolinarin did not inhibit the growth of E. faecalis and E. faecium. (a) 100 μg/mL of pectolinarin with OD600 = 0.1 of E. faecalis was incubated at 37 °C for 24 h. (b) 100 μg/mL of pectolinarin with OD600 = 0.1 of E. faecium was incubated at 37 °C for 24 h. The means and standard deviations from at least triplicated determinations are represented. Data are the mean values ± SD of triplicate experiments.
2.5. Pectolinarin Inhibited the Expression of Genes Related to the Biofilm Formation and Virulence of Bacteria
To understand the molecular basis of pectolinarin inhibition of biofilm formation, the expression of genes related to biofilm-associated factors, Cytolysin and QS system, was tested by qRT-PCR. After treatment with 0.01 to 25 μg/mL of pectolinarin, the expressions of biofilm-associated factors including ebpB (IC50 = 0.09 μg/mL), esp (IC50 = 0.01 μg/mL), and gelE (IC50 = 0.01 μg/mL) in E. faecalis and acm (IC50 = 0.01 μg/mL), bps (IC50 = 0.01 μg/mL), ebpA (IC50 = 0.01 μg/mL), esp (IC50 = 0.09 μg/mL), gelE (IC50 = 0.09 μg/mL), and scm (IC50 = 0.01 μg/mL) in E. faecium were significantly decreased (Figure 6a and Figure 7a, respectively). The expressions of fsrB (IC50 = 0.01 and 0.09 μg/mL) and fsrC (IC50 = 0.01 μg/mL) genes comprising the Fsr quorum-sensing system were reduced by treatment of pectolinarin (Figure 6b and Figure 7b). The treatment of pectolinarin also dose-dependently reduced the expression of genes related with Enterococcal cytolysin synthesis including cylR2 (IC50 = 0.09 μg/mL), cylM (IC50 = 0.09 μg/mL), and cylLS (IC50 = 0.09 μg/mL) (Figure 6c).
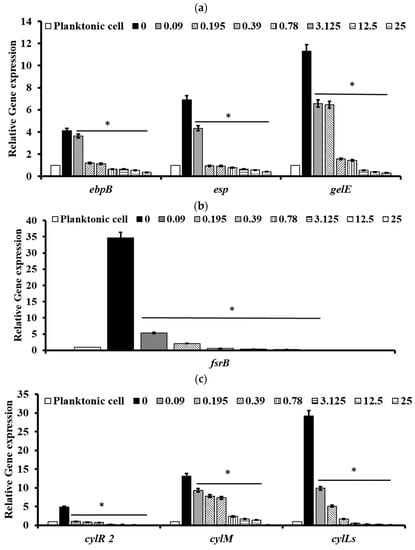
Figure 6.
Pectolinarin inhibited the expression of genes related to the biofilm formation and bacterial virulence in E. faecalis. (a) Pectolinarin inhibited the expression of genes for biofilm formation in E. faecalis. (b) Pectolinarin inhibited the expression of genes for the quorum-sensing system in E. faecalis. (c) Pectolinarin inhibited the expression of genes for the bacterial virulence in E. faecalis. Total RNA was extracted from biofilm control bacteria and the biofilm-induced bacteria that were treated with the indicated concentration of pectolinarin, converted to cDNA, and analyzed by qPCR with the respective primers. The means and standard deviations from at least triplicate determinations are represented. * = p < 0.05 was considered significant.
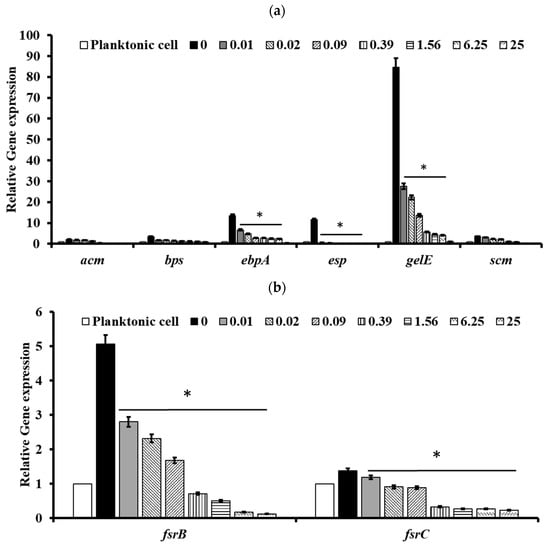
Figure 7.
Pectolinarin inhibited the expression of genes related to the biofilm formation and the bacterial virulence in E. faecium. (a) Pectolinarin inhibited the expression of genes for biofilm formation in E. faecium. (b) Pectolinarin inhibited the expression of genes for the quorum-sensing system in E. faecium. Total RNA was extracted from biofilm control bacteria and the biofilm-induced bacteria that were treated with the indicated concentration of pectolinarin, converted to cDNA, and analyzed by qPCR with the respective primers. The means and standard deviations from at least triplicate determinations are represented. * = p < 0.05 was considered significant.
3. Discussion
Pathogenic bacteria such as E. faecalis, E. faecium, E. coli, P. gingivalis, S. mutans, S. sobrinus, S. aureus, C. acnes, and P. aeruginosa contribute to the important global cause of nosocomial infections including community- and hospital-acquired infections [7]. This causes community spreading of pathogenic bacteria that leads to a large increase in the at-risk and high cost to controlling pathogens [7]. The bacterial biofilm reduced the antibacterial susceptibility that make it difficult to eradicate pathogenic bacteria. Enterococcus spp. in biofilms are more resistant to antibiotics than planktonic enterococci are [8,13]. The development of natural products with bioactive ingredients will, therefore, help overcome the issue of drug resistance in bacteria [14]. Cirsium species are considered edible plants and are used as various ailments including hemorrhaging, jaundice, and gastrointestinal disorders [10,11]. Pectolinarin is a secondary metabolite of Cirsium spp. with demonstrated biological activities including antimicrobial, antioxidant, antidiabetic, and anti-inflammatory activity [10,11]. In this study, pectolinarin was identified as an inhibitor of biofilm formation caused by bacteria including E. faecalis, E. faecium, E. coli, P. gingivalis, S. mutans, S. sobrinus, S. aureus, C. acnes, and P. aeruginosa (Figure 2). Pectolinarin also showed increasing susceptibility of an antibacterial effect in combination with antibiotics. Even though E. faecalis and E. faecium showed resistance against vancomycin, pectolinarin decreased their growth by approximately 20%. Pectolinarin dose-dependently decreased bacterial adherence to T24 cells (Figure 4). These results suggest that pectolinarin should be useful as the inhibitor of infection of E. faecalis and E. faecium. Pectolinarin dose-dependently inhibited the QS system and virulence factor expression (Figure 6 and Figure 7). Virulence factors contribute to the pathogenesis of E. faecalis and E. faecium and many of them were involved in bacterial adhesion to host cells or abiotic surfaces, leading to biofilm formation. In addition, genes for biofilm-associated factors and virulence factors including acm, scm, ebpA, ebpB, esp, bps, gelE, cylR2, cylLS, and cylM were significantly down-regulated by the treatment of pectolinarin (Figure 6 and Figure 7). Therefore, the QS system, virulence factor expression, bacterial adherence, and antibiotic resistance were connected with each other, and thus, biofilm inhibition is for not only the biofilm inhibition but also the reduction in the bacterial survival and infection.
In conclusion, pectolinarin inhibited the bacterial QS system (Figure 6 and Figure 7) to block biofilm formation (Figure 2), enhanced the antibiotic susceptibility (Figure 3), and reduced bacterial adherence to the human host cell (Figure 4). Additional studies including the determination of the molecular mechanism, the relation between adherence, biofilm formation, and virulence, and preclinical animal experiments increase the proof for pectolinarin as an agent for a new antibacterial agent or adjuvant.
4. Materials and Methods
4.1. Bacterial Strains
Strains used in this study are listed in Table 1. E. faecalis, E. faecium, E. coli, and S. aureus were maintained in tryptic soy broth (TSB) and tryptic soy broth supplemented with 1% glucose (TSBg). S. mutans and S. sobrinus were maintained in Brain Heart Infusion (BHI) broth. P. gingivalis was maintained in tryptic soy broth supplemented with 10% defibrinated horse blood agar. P. aeruginosa was maintained in nutrient agar, and C. acnes was maintained in Reinforced Clostridial agar. All cultures were incubated at 37 °C [13,15,16].
4.2. Biofilm Formation Assay
Biofilm formation was performed using TSBg as previously described [12,15,17,18,19,20,21]. E. faecalis, E. faecium, E. coli, S. mutans, S. sobrinus, S. aureus, P. aeruginosa, C. acnes, and P. gingivalis were added (OD600 = 0.1) to individual wells of 96-well flat-bottomed plates. Pectolinarin with concentrations ranging from 0.01 to 100 μg/mL was added to respective wells, and the cells were incubated at 37 °C for 24 h. The inhibition of biofilm formation by compound was detected by the crystal violet staining method. Briefly, after 24 h of treatment, the supernatant was removed and the wells were rinsed with physiological saline. In addition, 1% crystal violet (CV) solution was added to each well and incubated for 30 min. The excess of dye was removed by washing the plates under running water. The bound CV was released by adding 125 μL of 30% acetic acid followed by an incubation for 15 min at room temperature. The absorbance was measured at 595 nm using a microplate reader (Bio Tek Instruments, CA, USA) [15,22].
4.3. The Combinatorial Antibacterial Effects of Pectolinarin with Commercialized Antibiotics
The antibacterial effects by the combinatorial treatment of pectolinarin with antibiotics were evaluated using the plate-counting method. The bacterial culture at an absorbance of OD600 = 0.1 was prepared, and 1 mL of the bacterial suspension with pectolinarin (final concentrations of 1.56, 0.39, 0.09, and 0.01 μg/mL) was added. Planktonic bacteria were removed using sterile saline and the medium (TSBg and Pectolinarin) was incubated for 24 h. The bacteria in the biofilm were enumerated using the plate-counting method [12].
4.4. Bacterial Adherence Assays
The human urinary bladder carcinoma T24 cell was purchased from the Korean Cell Line Bank (Seoul, Korea). T24 cells were maintained in RPMI 1640 media supplemented with 10% fatal bovine serum in a 5% CO2 incubator at 37 °C [12,23,24]. T24 cells were added to 24-well plates (0.5 × 106 cells per well) for 24 h prior and co-incubated with 0.01–50 µg/mL of pectolinarin-treated E. faecium or E. faecalis (100 MOI, 5 × 107 CFU per well) for 24 h. Cells were then washed three times with PBS. Gram iodine mordant was applied for 1 min and briefly washed with PBS. To remove any nonspecific crystal violet, a Gram decolorizer solvent was added to the plate for 30 s. After washing with PBS until the PBS ran clear, safranin was applied for 30 min. Samples were assessed under a microscope [25,26].
4.5. Time Kill Assay
Growth curves were obtained as previously described with slight modification [22]. E. faecalis and E. faecium cultures were prepared with tryptic soy broth at an OD600 = 0.1. Pectolinarin (100 μg/mL) was added and then the cells were incubated at 37 °C. Growth was evaluated by measuring the optical density of OD600 using a microplate reader after 0, 1, 2, 4, 8, 12, 24, and 72 h [11].
4.6. Quantitative RT-PCR Analysis
Bacterial cultures and the pectolinarin treatment condition was the same as the method to check the biofilm formation inhibition. Total RNA was isolated using TRIzol reagent (Life Technology, Thermo Fisher Scientific, MA, USA) according to the manufacturer’s instructions, and the reverse transcriptase (NanoHelix, Daejeon, Korea) reaction was prepared using 1 µg of RNA to obtain cDNA. qRT-PCR was carried out using the 2X Sybr Green qPCR Mater Mix (CellSafe, Yongin, Korea). Primer sets for the fsrB, fsrC, gelE, acm, scm, ebpA, ebpB, esp, bps, cylM, cylR2, and cylLS genes are listed in Table 2. Genes encoding 23 sRNA and tufA were used as endogenous controls.

Table 2.
Gene-specific primers used for real-time RT-PCR.
4.7. Statistical Analysis
All experiments were performed at least three times, and all data are represented as the mean ± S.D.
5. Conclusions
Pectolinarin showed the reduced bacterial biofilm formation, activated the susceptibility of commercially available antibacterial agents, and reduced the bacterial adherence to host cells. These results recommend the bacterial biofilm formation as a good target to develop the antibacterial agents against biofilm-related infections with low cytotoxicity, and pectolinarin should be a promising candidate.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/antibiotics11050598/s1, Figure S1. Pectolinarin inhibited biofilm formation of (a) E. coli, (b) S. mutans, (c) S. sobrinus, (d) S. aureus, (e) P. aeruginosa, (f) C. acnes and (g) P. gingivalis. Biofilm of E. coli, S. mutans, S. sobrinus, S. aureus, P. aeruginosa, C. acnes and P. gingivalis was formed in medium supplemented with pectolinarin at the indicated concentrations at 37 °C for 24 h.
Author Contributions
Data curation, D.K.; funding acquisition, K.-Y.K.; investigation, D.K.; methodology, D.K.; supervision, K.-Y.K.; writing—original draft, D.K.; writing—review and editing, D.K. and K.-Y.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the GRRC program of Gyeonggi province (GRRC-kyunghee2020(B04)).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
This research was funded by the GRRC Program of Gyeonggi province (GRRCKyungHee2020(B04)), Republic of Korea.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Donlan, R.M.; Costerton, J.W. Biofilm: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, D. Understanding biofilm resistance to antibacterial agents. Nat. Rev. Drug Discov. 2003, 2, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Mah, T.F.; O’Toole, G.A. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001, 9, 34–39. [Google Scholar] [CrossRef]
- Tomita, H.; Ike, Y. Tissue-specific Adherent Enterococcus faecalis stains that show highly efficient adhesion to human bladder carcinoma T24 cells also adhere to extracellular matrix proteins. Infect. Immun. 2004, 72, 5877–5885. [Google Scholar] [CrossRef] [Green Version]
- Guenther, F.; Stroh, P.; Wagner, C.; Obst, U.; Hansch, G.M. Phagocytosis of staphylococci biofilms by polymorph nuclear neutrophils: S. aureus and S. epidermidis differ with regard to their susceptibility towards the host defense. Int. J. Artif. Organs 2009, 32, 565–573. [Google Scholar] [CrossRef]
- Strickertsson, J.A.B.; Desler, C.; Bertelsen, J.M.; Machado, A.M.D.; Wadstrom, T.; Winther, O.; Rasmussen, L.J.; Hansen, L.F. Enterococcus faecalis infection causes inflammation, intracellular oxphos-independent ROS production, and DNA damage in human gastric cancer cells. PLoS ONE 2013, 8, e63147. [Google Scholar] [CrossRef] [Green Version]
- Hidron, A.I.; Edwards, J.R.; Patel, J.; Horan, T.C.; Sievert, D.M.; Pollock, D.A.; Fridkin, S.K. Antimicrobial-resistant pathogens associated with healthcare-associated infection: Annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect. Control Hosp. Epidemiol. 2008, 29, 996–1011. [Google Scholar] [CrossRef] [Green Version]
- Arias, C.A.; Murray, B.E. The rise of the Enterococcus: Beyond vancomycin resistance. Nat. Rev. Microbiol. 2012, 10, 266–278. [Google Scholar] [CrossRef] [Green Version]
- Alejandro, T.A.; Valle, J.; Solano, C.; Arrizubieta, M.J.; Cucarella, C.; Lamata, M.; Amorena, B.; Leiva, J.; Penades, J.R.; Lasa, I. The entrococcal surface protein, Esp, is involved in Enterococcus faecalis biofilm formation. Appl. Environ. Microbiol. 2001, 67, 4538–4545. [Google Scholar]
- Lim, H.; Soo, K.H.; Chang, H.W.; Bae, K.; Kang, S.S.; Kim, H.P. Anti-inflammatory activity of pentolinarigenin and pectolinarin isolated form Cirsium chanroenicum. Biol. Pharm. Bull. 2008, 31, 2063–2067. [Google Scholar] [CrossRef] [Green Version]
- Luo, H.; Jing, B.H.; King, S.M.; Chen, Y.C. Inhibition of cell growth and VEGF expression in ovarian cancer cells by flavonoids. Nutr. Cancer 2008, 60, 800–809. [Google Scholar] [CrossRef] [PubMed]
- Top, J.; Paganelli, F.L.; Zhang, X.; Schaik, W.V.; Leavis, H.L.; Asbroek, M.V.L.; Poll, T.V.D.; Leendertse, M.; Bonten, M.J.M.; Willems, R.J.L. The Enterococcus faecium Enterococcal biofilm regulator, EbrB, regulates the esp operon and is implicated in biofilm formation and intestinal colonization. PLoS ONE 2013, 8, e65224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, C.; Tangjuan, L.; Ke, W.; Hou, C.; Cai, S.; Huang, Y.; Du, Z.; Huang, H.; Kong, J.; Chen, Y. Baicalein inhibits Staphylococcus aureus biofilm formation and the quorum sensing system in vitro. PLoS ONE 2016, 11, e0153469. [Google Scholar]
- Sreedhar, R.N.; Kavindra, V.S.; Jouko, S.; Danielle, A.G.; Magnus, H.; Stanley, L.E.; Barbara, E.M. Endocarditis and biofilm-associated pili of Enterococcus faecalis. J. Clin. Investig. 2006, 116, 2799–2807. [Google Scholar]
- Ken-ichi, A.M.; Kunitoshi, Y.; Yoshimitsu, M.; Tanaka, Y.; Ogura, T.; Sugimoto, S. Inhibitory effects of Myricetin derivatives on curli-dependent biofilm formation in Escherichia coli. Sci. Rep. 2018, 8, 8452. [Google Scholar]
- Ong, T.H.; Chitra, E.; Ramamurthy, S.; Siddalingam, R.P.; Yen, K.H.; Ambu, S.P.; Davamani, F. Chitosan-propolis nanoparticle formulation demonstrates anti-bacterial activity against Enterococcus faecalis biofilm. PLoS ONE 2017, 12, e0174888. [Google Scholar]
- Chien, Y.C. Surface sensing for biofilm formation in Pseudomonas aeruginosa. Front. Microbiol. 2017, 8, 2671. [Google Scholar]
- Hahnel, S.; Muhlbauer, G.; Hoffmann, J.; Ionescu, A.; Burgers, R.; Rosentritt, M.; Handel, G.; Haberlein, I. Streptococcus mutans and Streptococcus sobrinus biofilm formation and metabolic activity on dental materials. Acta Odontol. Scand. 2012, 70, 114–121. [Google Scholar] [CrossRef]
- He, L.; Wang, H.; Zhang, R.; Li, H. The regulation of Porphyromonas gingivalis biofilm formation by ClpP. Biochem. Biophys. Res. Commun. 2019, 509, 335–340. [Google Scholar] [CrossRef]
- Holmberg, A.; Lood, R.; Morgelin, M.; Soderquist, B.; Holst, E.; Collin, M.; Christensson, B.; Rasmussen, M. Biofilm formation by Propionibacterium acnes is a characteristic of invasive isolates. Clin. Microbiol. Infect. 2009, 15, 787–795. [Google Scholar] [CrossRef] [Green Version]
- Mok, J.Y.; Kang, H.J.; Cho, J.K.; Jeon, I.H.; Kim, H.S.; Park, J.M.; Jeong, S.I.; Shim, J.S.; Jang, S.I. Antioxidative and anti-inflammatory effects of extracts from different organs of Cirsium japonicum var. ussuriense. Korea J. Herbol. 2011, 26, 39–47. [Google Scholar]
- Kim, J.G.; Ha, Q.B.T.; Shin, Y.K.; Kim, K.Y. Antifungal activity of magnoflorine against Candida strains. World J. Microbiol. Biotechnol. 2018, 34, 167. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.S. Mechanisms of antibiotic resistance in bacterial biofilms. Int. J. Med. Microbiol. 2002, 292, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Thomas, V.C.; Thurlow, L.R.; Boyle, D.; Hancock, L. Regulation of autolysis-dependent extracellular DNA release by Enterococcus faecalis extracellular proteases influences biofilm development. J. Bacteriol. 2008, 190, 5690–5698. [Google Scholar] [CrossRef] [Green Version]
- Becerra, S.C.; Roy, D.C.; Sanchez, C.J.; Christy, R.J.; Burmeister, D.M. An optimized staining technique for the detection of Gram positive and Gram-negative bacteria within tissue. BMC Res. Notes 2016, 9, 216. [Google Scholar] [CrossRef] [Green Version]
- Daw, K.; Baghdayan, A.S.; Awasthi, S.; Shankar, N. Biofilm and planktonic Enterococcus faecalis elicit different responses from host phagocytes in vitro. FEMS Immunol. Med. Microbiol. 2012, 65, 270–282. [Google Scholar] [CrossRef] [Green Version]
- Sillanpää, J.; Nallapareddy, S.R.; Singh, K.V.; Prakash, V.P.; Fothergill, T.; Ton-That, H.; Murray, B.E. Characterization of the ebpfm pilus-encoding operon of Enterococcus faecium and its role in biofilm formation and virulence in a murine model of urinary tract infection. Virulence 2010, 1, 236. [Google Scholar] [CrossRef] [Green Version]
- Hendrickx, A.P.A.; Luit-Asbroek, M.V.; Schapendonk, C.M.E.; Wamel, W.J.B.; Braat, J.C.; Wijnands, L.M.; Bonten, M.J.M.; Willems, R.J.L. SgrA, a Nidogen-Binding LPXTG Surface Adhesin Implicated in Biofilm Formation, and EcbA, a Collagen Binding MSCRAMM, Are Two Novel Adhesins of Hospital-Acquired Enterococcus faecium. Infect Immun. 2009, 77, 5097. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).