Abstract
Antimicrobial stewardship (AMS) programmes in human health and livestock production are vital to tackling antimicrobial resistance (AMR). Data on antimicrobial use (AMU), resistance, and drivers for AMU in livestock are needed to inform AMS efforts. However, such data are limited in Fiji. Therefore, this study aimed to evaluate the association between farmer (socio-economic, demographic) and livestock production and management factors with AMU. Information was collected using purposive and snowball sampling from 236 livestock farmers and managers located in Central and Western divisions, Viti Levu, Fiji. Multinomial logistic regression was used to determine the factors associated with AMU in farms using an aggregated livestock farm model. Farms that raised cattle only for dairy (farm factor) were more likely to use antibiotics and anthelmintics (p = 0.018, OR = 22.97, CI 1.713, 308.075) compared to mixed cattle and poultry farms. Farms that maintained AMU records were more likely to use antibiotics (p = 0.045, OR = 2.65, CI 1.024, 6.877) compared to farms that did not. Other livestock production and management factors had no influence on AMU on the livestock farms. AMU in livestock farms was not influenced by the socio-economic and demographic characteristics of the farmer. There were differences between livestock enterprises regarding their management. The lack of association between management system and AMU could be because there was so much variation in management system, levels of farmer knowledge and awareness of AMU, and in management of farm biosecurity. Future studies exploring farmers’ knowledge and awareness of AMU and livestock management are required to design AMS programmes promoting prudent AMU in all livestock farms locally.
1. Introduction
Antimicrobial resistance (AMR) is a major global threat to human and animal health [1,2,3]. International collaborative efforts by the World Organisation of Animal Health (OIE), the World Health Organization (WHO) and the Food and Agricultural Organization of the United Nations (FAO) have adopted the One Health approach to combat the global risk of AMR [1,2,3]. In doing so, the prudent use of antimicrobials in livestock production systems have been encouraged [1,2]. Additionally, with the increasing risks of transmission of AMR microbes from livestock farms into the environment and agri-food chain, antimicrobial stewardship (AMS) programmes in the human and animal sectors have been advocated [1,2]. Although antimicrobial use (AMU) data are becoming more accessible, the information on drivers of AMU, which are essential in developing AMS programmes, remains unclear.
Socio-economic and demographic factors influence livestock production systems and management practices [4,5,6]. Backyard farmers produce livestock for domestic consumption and at times sell them to buy plant-based food products [7,8]. These backyard and semi-commercial farmers’ management practices are also influenced by other farmers, friends, and neighbours, thus shaping the attitude and intention of the farmers [9,10]. Socio-economic status may also affect farmers’ ability to seek veterinary advice on animal health, production and improve farm biosecurity infrastructure [7,11,12,13]. In principle, veterinary services affect farmers’ decision-making process, since veterinarians may serve as farmers’ knowledge hub [14]. The advice disseminated by veterinarians to farmers also shapes the behaviour of the farmers [15,16].
Fiji is a developing tropical country with backyard and semi-commercial enterprises such as beef, dairy, chickens, and laying hens, predominantly providing food and financial security to many Fijian farmers [17,18]. Shortages in veterinary professionals have been reported in the livestock sector [19] and are similar to other developing countries [20]. In the human health sector, AMR has been reported [19], but AMR in livestock is unknown. Prudent use of antimicrobials has been advocated at global levels using AMS programmes [1,2,3]. However, implementing mitigation policies surrounding prudent AMU in developing countries such as Fiji is challenging, noting the vast difference in livestock production, management practices, socio-economic and demographic factors [6,20,21]. In developing countries, antimicrobials have been used prophylactically and to increase production [22,23]. Additionally, a lack of knowledge and understanding of AMU and AMR have also been reported [13,24,25].
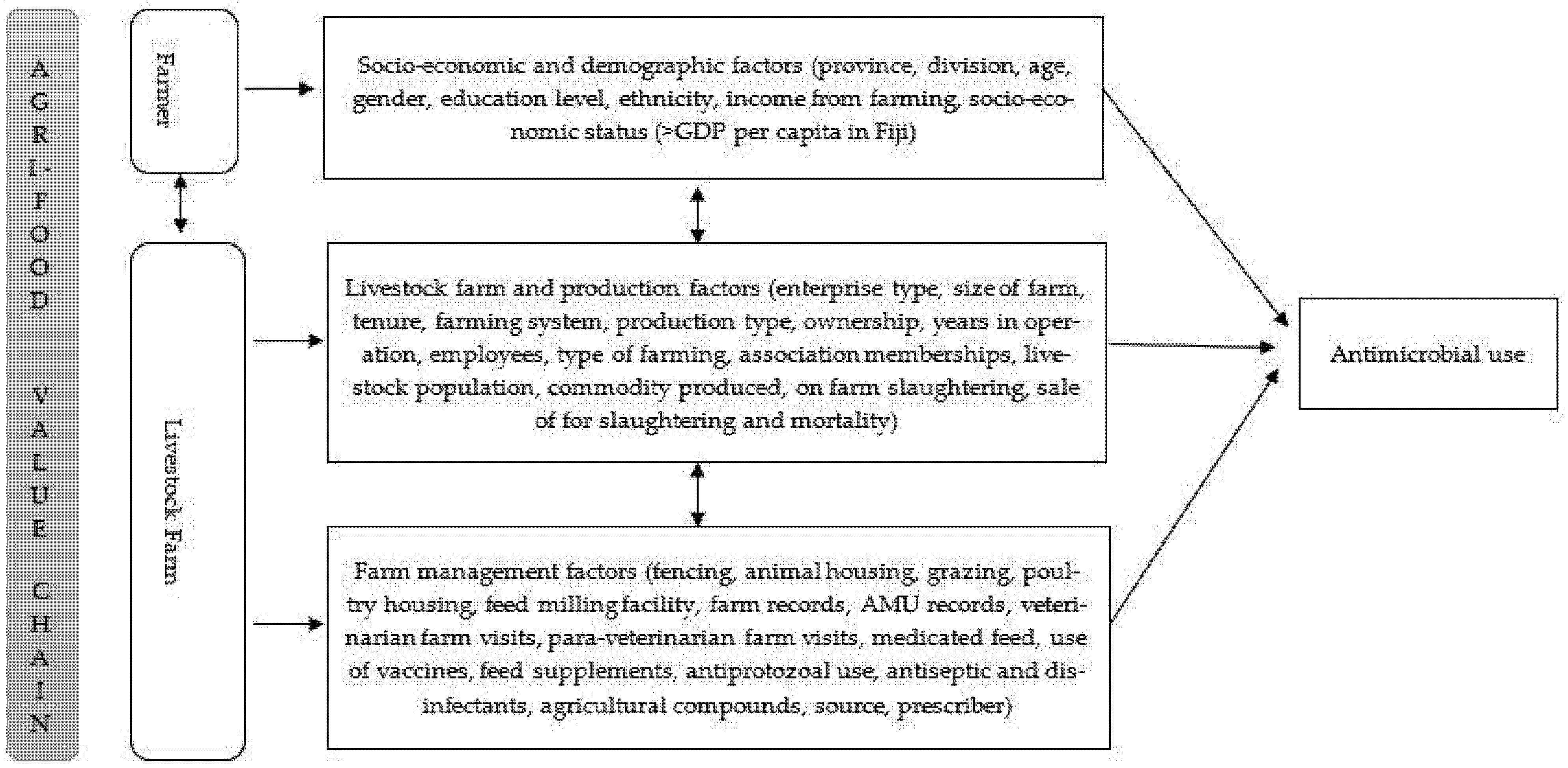
Contextual and socio-psychological factors influence farmers′ attitudes towards farm biosecurity risk management and AMU practice [6,14,26]. However, farm biosecurity risk management strategies differ between farm enterprises [27,28]. Antimicrobials (antibiotics and anthelmintics), nutraceuticals and other medicinal products have been used in livestock production to manage and mitigate farm biosecurity risks [28,29,30]. Nevertheless, the use of these medicinal and non-medicinal products is substantially different in poultry and cattle [29,31]. For instance, antibiotics are administered in flocks of chickens compared to individual animals in cattle herds [31,32,33]. The information about on-farm biosecurity risk management using medicinal and non-medicinal products, including antimicrobials and effects of contextual drivers on AMU practice in Fijian livestock farms, is largely unknown. Our earlier studies have demonstrated the AMU and the patterns of use and lack of knowledge and understanding of AMU and AMR among farmers and para-veterinarians; however, the contextual farmer and farm factors driving AMU remain unexplained. It was hypothesized that farmers′ socio-economic and demographic factors and livestock production and management characteristics influenced the AMU, illustrated in the conceptual framework (see Figure 1). Therefore, this study aimed to investigate the agri-food value chain factors (farmer and livestock farm production and management) that influence AMU in the Central and Western regions of Viti Levu, Fiji.
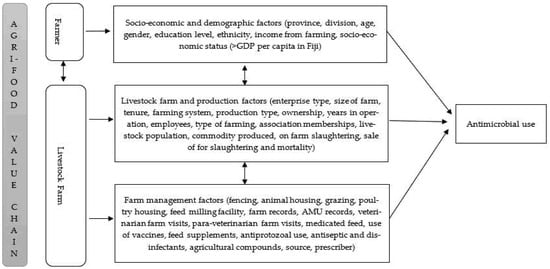
Figure 1.
The conceptual framework illustrating the overarching constructs influencing antimicrobial use.
2. Results
The summary of statistically significant farmer and farm factors associated with AMU are presented in Table 1 (farm model). Refer to Appendix A Table A1 (farm model) for a detailed description of all factors.

Table 1.
Summary of associations between factors (farmer, livestock production and management) and antimicrobial use (antibiotics, anthelmintics, both and no antimicrobial use) on 236 livestock farms located in Central and Western divisions of Viti Levu, Fiji.
2.1. Livestock Farm Model
2.1.1. Characteristics of Fijian Livestock Farmers and Farms
Table A1 shows the characteristics of the 236 livestock farmers and farms. Most participants were farmers (n = 211, 89%) and were from the Western Division of Viti Levu (n = 143, 61%). The majority were from Ba province (n = 84%) and were 40–59 years of age (n = 120, 51%). Most farmers were male (n = 198, 84%) and had obtained secondary education qualifications (n = 142, 60%). Most farmers reported their income from farming comprised between 25–50% of total household income (n = 94, 40%), and their household income was less than gross domestic income per capita (n = 151, 64%). Most respondents were not members of any associations (n = 176, 75%). Most farms were household-owned (n = 162, 69%) with Itaukei Land Trust Board (TLTB) leased tenure (n = 63, 27%). Most farms were medium–large holders with farm sizes greater than 2 ha (n = 185, 78%) raising livestock in semi-commercial farming systems (n = 144, 61%) and classified as organic (n = 101, 43%). Most farms were not mixed (crop and livestock) and raised livestock only (n = 162, 69%) and were in operation for more than 10 years (n = 101, 43%). Most farms employed no farmworkers (n = 134, 57%). The most numerous enterprises were beef, which comprised the only livestock on the farm (n = 57, 24%). Most farms were small–medium sized herds or flocks (n = 171, 72%). Most farms were fenced (n = 133, 56%) and had an animal house (n = 150, 64%). Half the farms had no para-veterinarian farm visits (n = 118, 50%), and the vast majority had no veterinarian visits (n = 223, 94%). Although most farmers had maintained farm records (n = 122, 52%), very few farms maintained AMU records (n = 38, 16%). Most farms had no on-farm milling facility (n = 220, 93%) and had not used medicated feed (n = 125, 53%). Most farms had not used any feed supplements (n = 202, 86%). Most farms had not used antiprotozoal (n = 229, 97%) or herbal preparations (n = 211, 89%). However, most had used vitamins and minerals (n = 122, 52%). Very few farms had used vaccines (n = 11, 5%), and the majority of farms had also not used antiseptics and disinfectants (n = 193, 82%) or other agricultural compounds (herbicides and pesticides) (n = 232, 98%).
2.1.2. Livestock Farm Modelling
Of the 34 variables presented in Table A1, only 15 variables (division, province, gender, association memberships, farm size, years in operations, enterprise type, fencing, animal housing, para-veterinarian farm visits, veterinarian farm visits, AMU records, medicated feed use, feed supplement use, antiseptics and disinfectants use) were associated with AMU (p < 0.05) (see Table 1). Farms that raised cattle only for dairy were more likely to use antibiotics and anthelmintics (p = 0.018, OR = 22.97, CI 1.713, 308.075). Dairy farms were more likely to use antibiotics only (p = 0.097) and anthelmintics only (p = 0.594). There was a tendency (p = 0.848) for beef-only farms to use both anthelmintics and antibiotics. Farms which had both a beef and dairy enterprise used both antibiotics and anthelmintics (p = 0.467). The layer-only (p = 0.917), broiler-only (p = 0.356), and layer and broiler mixed farms (p = 0.698) were most likely to use antibiotics. Smallholder farms were less likely to use a combination of both (p = 0.015 OR = 0.15, CI 0.032, 0.689).
Interestingly, farms that maintained AMU records were more likely to use antibiotics ( p= 0.045, OR = 2.65, CI 1.024, 6.877) and similarly anthelmintics only (p > 0.05). Farms that had not used medicated feeds were more likely to use anthelmintics only (p < 0.001, OR = 11.56, CI 3.456,38.604) and a combination of both (anthelmintics and antibiotics) (p = 0.017, OR =3.10, CI 1.222, 7.882). Farms that had not used feed supplements were also more likely to use anthelmintics only (p = 0.025, OR = 6.37, CI 1.261, 32.155) or both (p = <0.001, OR = 30.41, CI 7.277, 127.081). In contrast, farms that had not used antiseptics and disinfectants were less likely to use antimicrobials (see Table 2).

Table 2.
Multinomial logistic modelling analysis of factors (farmer, livestock production and management) with antimicrobial use on 236 livestock farms located in Central and Western divisions of Viti Levu, Fiji.
3. Discussion
This study, to our knowledge, is the first study investigating the factors associated with AMU in Fijian livestock farms. Our study demonstrated that AMU in livestock farms was not influenced by the socio-economic and demographic characteristics of the farmer, but influenced by the livestock production and management factors such as species of farmed livestock (enterprise type), and farm management factors (AMU records, medicated feed use and feed supplements) (see Table 2). Despite the differences in farming systems and management practices, most factors were not associated with AMU. Other studies have demonstrated the effects of livestock management and farm biosecurity systems on AMU practice [27,28]. Given that seasonal conditions affect disease burdens such as helminthic and bacterial infections in different livestock enterprises; therefore, the chances of using a type of antimicrobial could be higher. However, the AMU practices in the livestock farms surveyed differed and we could not explain a lack of association between farm factors and AMU [34]. Other studies have demonstrated that socio-economic and demographic factors influence AMU practice [7,14,35], and this may have been the case in our study as well, but we were unable to detect it because of an unequal representation of farmers from all socio-economic and demographic groups and farming systems. Hence, we suggest future studies to consider the sampling strategy to ensure equal representation and larger sample size so that modelling could be executed to better predict the AMU practice in different enterprises and systems.
As flock-level administration of antimicrobials in poultry is more likely compared to an individual animal in cattle [32,33,36], the chances of antimicrobial use in specialist cattle and poultry and mixed cattle and mixed poultry were higher than other mixes of farm enterprises [6,31]. We believe the higher incidence of mammary infections in dairy cows may be the reason for the increased use of antibiotics in cattle [34]; however, we also believe the use may be for prophylaxis and growth promotion, as demonstrated in our previous study [37]. The chances of antibiotic use were higher in poultry enterprises due to flock-level administration, prophylaxis and growth promotion, as demonstrated in our previous study [38]. Our finding of higher chances of using antimicrobials in cattle and poultry enterprises is similar to findings demonstrated in other studies [31,36]. Although flock/herd density is higher in commercial systems than semi-commercial and backyard, there was no influence of the farming system on AMU (p = 0.430). Our earlier study also demonstrated that the farming system did not affect AMU when quantified using different metrics [38]. However, we also believe this similar use between farming systems may be due to a lack of knowledge and understanding of AMU, leading in some cases to an unwitting use of antimicrobials. We could not establish statistical significance between farming systems factors due to modelling inefficiencies. We believe that lower chances of AMU in smallholder farms may be due to a lack of access to antimicrobials [39]. The association between maintenance of AMU records and antibiotic use may be a consequence of farmers producing poultry to contract being required to provide records of AMU to commercial processors [40]. Farmers that were not producing for a contract may be using antimicrobials but have not kept records of AMU because of a lack of knowledge and understanding of the importance of maintaining such records. This has been demonstrated in earlier studies [39,40]. Our earlier study demonstrated that Fijian farmers lacked general knowledge and understanding of medicines and did not differentiate between different types of medicine [39]. We believe farmers considered medicated feed, feed supplements, antiseptics, and disinfectants also as medicines and used them on their livestock [39]. We believe farmers use medicated feed (containing antimicrobials) and feed supplements to prevent animal diseases and promote growth [41] and used antimicrobials as the first line of treatment [37,39]. It was beyond the scope of the current study to explain the motivations behind the use of other medicines (vaccines, topical antimicrobials, antiprotozoals, multivitamins and minerals, feed supplements, herbal preparations, antiseptics and disinfectants and agricultural compounds). Therefore, further studies investigating the drivers of other medicines used are required, so that other medicines used including AMU in Fijian livestock production systems could be better understood.
Our study revealed that farmers used antibiotics, anthelmintics, commercial feed, nutraceuticals, herbal medicines, and vaccines, and these may have been used to mitigate farm biosecurity risks or for other purposes [28,29,42]. Farm biosecurity infrastructure was in place in most farms, but farmers with lower socio-economic status do find it challenging to implement farm biosecurity risk mitigation measures because of the associated costs [14,22,42,43,44]. Nonetheless, our studies showed no association with farm infrastructure factors in most farms.
Maintenance of farm records is another essential part of farm biosecurity assessment [44]. Although most farmers had attained secondary school education, most farms did not maintain farm AMU records. We do not believe that literacy was an issue but understanding the importance of maintaining farm AMU records may be an essential consideration. Hence, we suggest follow up studies exploring attitude and knowledge towards record-keeping and overall biosecurity risk management on farms. Our study revealed that level of education was not associated with AMU practice, but exploring the knowledge and understanding of farmers on AMU and AMR at the enterprise level may be an essential consideration as reported in studies in other settings [24,39]. Additionally, this may inform and assist in developing mitigation strategies adopted as part of AMS programmes.
Veterinarians and para-veterinarians have a critical role in AMS programmes [45], but our study revealed veterinarian and para-veterinarian farm visits were very low. Interaction between farmers with veterinarians and para-veterinarians is therefore low, resulting in imprudent AMU practices as farmers self-prescribe antimicrobials [24,26,39,46]. Also, this may provide a window of opportunity to farmers who may opt to explore other avenues for advice, as is the case in other countries [47]. It must be noted that technical and clinical guidance on managing animal health and farm risks offered by veterinarians are more informed and cannot be compared to other sources [14]; therefore, it is imperative that improving Fijian veterinary services should be considered and incorporated as a critical priority indicator when developing policies in AMS programme so that better farm risk management practices are implemented. Self-prescribing is a common problem in the human health sector; thus, the chances of the same behaviour adopted by farmers in livestock farms is of grave concern as also indicated in our previous studies [37,39,39]; hence such practices need to be further explored [48]. We, therefore, recommend further studies exploring self-prescribing behaviour patterns of farmers so that more informed behavioural change intervention could be recommended.
Although participants were unequally represented by gender, our findings are not extraordinary since farm ownership, and farm decisions are traditionally made by the head of household, usually male, consistent with findings in other studies [49]. Nevertheless, the literature review informed our hypothesized conceptual framework (Figure 1), which assisted in elucidating valuable information about Fijian livestock production and management practices; AMU practices, the nutraceuticals and herbal medicines used, and the feed and feeding systems which were unknown. Therefore, the conceptual framework provided can be used to elucidate information on livestock production systems in other developing countries like Fiji, where information is limited.
4. Materials and Methods
4.1. Study Design and Data Collection
Data on farmer and farm characteristics, livestock production, feed and feeding practices and medicine use, collected from the cross-sectional survey conducted between May to August 2019 in Central and Western Divisions of Viti Levu, Fiji, previously reported in Khan et al. (2021) [38] was evaluated in this present study. Purposive and snowball sampling was used to recruit farmers and farm managers. A total of 236 livestock farms were investigated [38]. Considering the farmer and livestock farm constructs in the conceptual framework (see Figure 1), factors associated with AMU were assessed using the aggregated livestock farm model (see Figure 1 and Table A1 (farm model) for a detailed description of all factors).
4.2. Data Management and Analysis
Data were analysed using IBM SPSS Software V27. Farmer characteristics (socioeconomic and demographic), livestock farm production and livestock management, including feed and feeding practices, medicinal and non-medicinal product use, and antimicrobial access variables (factors) (see Figure 1 and Table A1 (farm model) for a detailed description of all factors), were descriptively analysed. Frequency and percentages were summarised for categorical factors. Data on other medicines used, excluding antimicrobials administered orally, parenterally and intramammary, were assessed and classified into vaccines, topical antimicrobials, antiprotozoals, multivitamins and minerals, feed supplements, herbal preparations, antiseptics and disinfectants and agricultural compounds [29]. These other categories of medicines used were coded (either used or not used). Continuous factors were reclassified into categories. These were: years of operation (<5 years, 5–10 years, >10 years) [12], number of employees (0, 1, ≥2), farm size (smallholder farm ≤2 hectares (ha), medium–large ≥2 ha) [50], para-veterinary and veterinary visits (no visits, monthly, quarterly) and herd/flock size (as reported in an earlier study [38]) was classified into three categories based on farming system (backyard, semi-commercial = small–medium, commercial = large) [51,52]. From the antimicrobial use data, outcome factor (AMU) was categorised into types of antimicrobial used (antibiotics, anthelmintics, both and none) [38]. Our earlier study demonstrated that antimicrobials were mainly sourced from veterinary clinics and self-prescribed by farmers; hence factors (source and prescriber of antimicrobial) were excluded in this study [37]. A total of 34 variables for the livestock farm model were considered for analysis (see Figure 1 and Table A1 (farm model) for a detailed description of all factors).
4.3. Statistical Analysis
A livestock farm model was developed using the livestock farm data (see Figure 1 for a detailed description of all factors). Chi-square test or Fisher′s exact test as appropriate, were used to investigate the association between hypothesized independent factors (farmer characteristics, farm production and management characteristics) with outcome factor (AMU) [53,54]. Statistically significant independent factors were fitted into multinomial logistic regression models to investigate the relationship between the independent factors and AMU [30,55]. The independent factors with p < 0.2 in univariate analysis were retained, and model reduction was done manually with confounding factors eliminated from the model [56,57]. The ‘no AMU′ outcome category was set as a reference category in livestock farm modelling. Odds ratios with 95% confidence intervals (95% CI) were reported, and p < 0.05 was considered statistically significant.
5. Conclusions
This study suggests that the AMU on livestock farms was not influenced by livestock farmers′ socio-economic and demographic characteristics. AMU was more likely in cattle (dairy) farms, and antibiotic use in poultry (broiler, layer and broiler) farms, compared to mixed cattle and poultry farms. Future studies exploring knowledge and awareness of AMU and livestock management, including farm biosecurity risk management, are required to design and implement AMS programmes to promote prudent AMU in Fijian livestock production systems. Further studies exploring the social and cultural factors driving the AMU are required to better understand the drivers of AMU practice at the national level.
Author Contributions
X.K. contributed to conceptualization, methodology, software, data collection, validation, project administrations, formal analysis, interpretation, writing original draft preparation, writing-review and editing. C.R., R.L., P.R. contributed to conceptualization, methodology, analysis, reviewing and editing, interpretation, and supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical approval for this study was obtained from the University of Reading′s School of Agriculture, Policy, and Development Ethical Committee (Ref #: 00772P).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We extend our acknowledgement to all farmers and farm managers who participated in the survey and assisted in identifying other farmers in their areas. Also, the staff and field officers of the Ministry of Agriculture in Central and Western Divisions who helped in identifying farmers and farms.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Associations between factors (farmer, livestock production and management) and antimicrobial use (antibiotics, anthelmintics, both and no antimicrobial use) on 236 livestock farms located in Central and Western divisions of Viti Levu, Fiji.
Table A1.
Associations between factors (farmer, livestock production and management) and antimicrobial use (antibiotics, anthelmintics, both and no antimicrobial use) on 236 livestock farms located in Central and Western divisions of Viti Levu, Fiji.
| Factor | Total | Antimicrobial Use | p Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Antibiotics | Anthelmintics | Both | No AMU | ||||||||
| n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | ||
| Participant type | |||||||||||
| Farmer | 211 | (89) | 51 | (89) | 35 | (97) | 44 | (92) | 81 | (85) | 0.231 |
| Farm manager | 25 | (11) | 6 | (11) | 1 | (3) | 4 | (8) | 14 | (15) | |
| Division | |||||||||||
| Central | 93 | (39) | 27 | (47) | 12 | (33) | 25 | (52) | 29 | (31) | 0.038 |
| Western | 143 | (61) | 30 | (53) | 24 | (67) | 23 | (48) | 66 | (69) | |
| Province | |||||||||||
| Naitasiri | 26 | (11) | 8 | (14) | 6 | (17) | 7 | (15) | 5 | (5) | 0.001 |
| Namosi | 13 | (6) | 2 | (4) | 1 | (3) | 2 | (4) | 8 | (8) | |
| Rewa | 13 | (6) | 5 | (9) | 1 | (3) | 0 | (0) | 7 | (7) | |
| Serua | 19 | (8) | 5 | (9) | 4 | (11) | 6 | (13) | 4 | (4) | |
| Tailevu | 22 | (9) | 7 | (12) | 0 | (0) | 10 | (21) | 5 | (5) | |
| Ba | 84 | (36) | 15 | (26) | 14 | (39) | 21 | (44) | 34 | (36) | |
| Nadroga-Navosa | 28 | (12) | 5 | (9) | 8 | (22) | 2 | (4) | 13 | (14) | |
| Ra | 31 | (13) | 10 | (18) | 2 | (6) | 0 | (0) | 19 | (20) | |
| Age | |||||||||||
| 10–19 years | 1 | (0) | 0 | (0) | 1 | (3) | 0 | (0) | 0 | (0) | 0.293 |
| 20–39 years | 49 | (21) | 11 | (19) | 7 | (19) | 6 | (13) | 25 | (26) | |
| 40–59 years | 120 | (51) | 27 | (47) | 20 | (56) | 27 | (56) | 46 | (48) | |
| Over 60 years | 66 | (28) | 19 | (33) | 8 | (22) | 15 | (31) | 24 | (25) | |
| Gender | |||||||||||
| Male | 198 | (84) | 48 | (84) | 34 | (94) | 44 | (92) | 72 | (76) | 0.021 |
| Female | 38 | (16) | 9 | (16) | 2 | (6) | 4 | (8) | 23 | (24) | |
| Education level | |||||||||||
| Primary | 31 | (13) | 10 | (18) | 7 | (19) | 3 | (6) | 11 | (12) | 0.850 |
| Secondary | 142 | (60) | 30 | (53) | 19 | (53) | 31 | (65) | 62 | (65) | |
| Tertiary | 39 | (17) | 10 | (18) | 6 | (17) | 9 | (19) | 14 | (15) | |
| Agricultural College | 21 | (9) | 6 | (11) | 4 | (11) | 4 | (8) | 7 | (7) | |
| Never Attended | 3 | (1) | 1 | (2) | 0 | (0) | 1 | (2) | 1 | (1) | |
| Income from farming | |||||||||||
| ≤25% | 71 | (30) | 13 | (23) | 11 | (31) | 12 | (25) | 35 | (37) | 0.213 |
| 25–50% | 94 | (40) | 21 | (37) | 12 | (33) | 21 | (44) | 40 | (42) | |
| 51–75% | 30 | (13) | 10 | (18) | 8 | (22) | 6 | (13) | 6 | (6) | |
| ≥76% | 41 | (17) | 13 | (23) | 5 | (14) | 9 | (19) | 14 | (15) | |
| Household income > GDP (Gross Domestic Product) per capita in Fiji | |||||||||||
| Yes | 85 | (36) | 23 | (40) | 14 | (39) | 19 | (40) | 29 | (31) | 0.552 |
| No | 151 | (64) | 34 | (60) | 22 | (61) | 29 | (60) | 66 | (69) | |
| Association memberships | |||||||||||
| Yes | 60 | (25) | 10 | (18) | 14 | (39) | 22 | (46) | 14 | (15) | <0.001 |
| No | 176 | (75) | 47 | (82) | 22 | (61) | 26 | (54) | 81 | (85) | |
| Farm ownership | |||||||||||
| Individual | 32 | (14) | 14 | (25) | 4 | (11) | 8 | (17) | 6 | (6) | 0.106 |
| Household | 162 | (69) | 30 | (53) | 27 | (75) | 33 | (69) | 72 | (76) | |
| Company | 32 | (14) | 10 | (18) | 3 | (8) | 6 | (13) | 13 | (14) | |
| Cooperative | 7 | (3) | 1 | (2) | 2 | (6) | 1 | (2) | 3 | (3) | |
| Contract farming | 3 | (1) | 2 | (4) | 0 | (0) | 0 | (0) | 1 | (1) | |
| Farm tenure | |||||||||||
| Freehold | 45 | (19) | 15 | (26) | 4 | (11) | 7 | (15) | 19 | (20) | 0.336 |
| Crown Lease | 31 | (13) | 8 | (14) | 4 | (11) | 8 | (17) | 11 | (12) | |
| Agriculture Leased | 43 | (18) | 14 | (25) | 6 | (17) | 5 | (10) | 18 | (19) | |
| TLTB Leased | 63 | (27) | 11 | (19) | 12 | (33) | 19 | (40) | 21 | (22) | |
| Mataqali | 44 | (19) | 8 | (14) | 8 | (22) | 6 | (13) | 22 | (23) | |
| Squatter | 2 | (1) | 0 | (0) | 1 | (3) | 1 | (2) | 0 | (0) | |
| Commercial leased | 8 | (3) | 1 | (2) | 1 | (3) | 2 | (4) | 4 | (4) | |
| Farm size | |||||||||||
| Small holder (<2 ha) | 51 | (22) | 14 | (25) | 2 | (6) | 3 | (6) | 32 | (34) | <0.001 |
| Medium-large holder (>2 ha) | 185 | (78) | 43 | (75) | 34 | (94) | 45 | (94) | 63 | (66) | |
| Farming systems | |||||||||||
| Backyard | 27 | (11) | 8 | (14) | 2 | (6) | 3 | (6) | 14 | (15) | 0.430 |
| Semi commercial | 144 | (61) | 30 | (53) | 23 | (64) | 33 | (69) | 58 | (61) | |
| Commercial | 65 | (28) | 19 | (33) | 11 | (31) | 12 | (25) | 23 | (24) | |
| Production type | |||||||||||
| Organic | 101 | (43) | 24 | (42) | 14 | (39) | 21 | (44) | 42 | (44) | 0.302 |
| Conventional | 70 | (30) | 22 | (39) | 7 | (19) | 13 | (27) | 28 | (29) | |
| Prefer not to comment | 65 | (28) | 11 | (19) | 15 | (42) | 14 | (29) | 25 | (26) | |
| Farming type | |||||||||||
| Livestock only | 162 | (69) | 39 | (68) | 20 | (56) | 36 | (75) | 67 | (71) | 0.270 |
| Mixed (Crop and Livestock) | 74 | (31) | 18 | (32) | 16 | (44) | 12 | (25) | 28 | (29) | |
| Years in operation | |||||||||||
| <5 years | 67 | (28) | 19 | (33) | 4 | (11) | 5 | (10) | 39 | (41) | <0.001 |
| 5–10 years | 68 | (29) | 17 | (30) | 8 | (22) | 15 | (31) | 28 | (29) | |
| >10 years | 101 | (43) | 21 | (37) | 24 | (67) | 28 | (58) | 28 | (29) | |
| Employees | |||||||||||
| 0 | 134 | (57) | 34 | (60) | 21 | (58) | 22 | (46) | 57 | (60) | 0.309 |
| <2 | 25 | (11) | 7 | (12) | 5 | (14) | 8 | (17) | 5 | (5) | |
| >2 | 77 | (33) | 16 | (28) | 10 | (28) | 18 | (38) | 33 | (35) | |
| Enterprise type | |||||||||||
| Beef only | 57 | (24) | 10 | (18) | 17 | (47) | 8 | (17) | 57 | (24) | <0.001 |
| Dairy only | 52 | (22) | 9 | (16) | 11 | (31) | 29 | (60) | 52 | (22) | |
| Beef and dairy | 11 | (5) | 0 | (0) | 2 | (6) | 4 | (8) | 11 | (5) | |
| Layer only | 50 | (21) | 13 | (23) | 3 | (8) | 2 | (4) | 50 | (21) | |
| Broiler only | 38 | (16) | 18 | (32) | 0 | (0) | 1 | (2) | 38 | (16) | |
| Broiler and layer | 12 | (5) | 4 | (7) | 0 | (0) | 1 | (2) | 12 | (5) | |
| Mixed cattle and poultry | 16 | (7) | 3 | (5) | 3 | (8) | 3 | (6) | 16 | (7) | |
| Flock/herd size | |||||||||||
| Small-medium | 171 | (72) | 38 | (67) | 25 | (69) | 36 | (75) | 72 | (76) | 0.614 |
| Large | 65 | (28) | 19 | (33) | 11 | (31) | 12 | (25) | 23 | (24) | |
| Fencing | |||||||||||
| Yes | 133 | (56) | 28 | (49) | 24 | (67) | 36 | (75) | 45 | (47) | 0.005 |
| No | 103 | (44) | 29 | (51) | 12 | (33) | 12 | (25) | 50 | (53) | |
| Animal housing | |||||||||||
| Yes | 150 | (64) | 43 | (75) | 13 | (36) | 22 | (46) | 72 | (76) | <0.001 |
| No | 86 | (36) | 14 | (25) | 23 | (64) | 26 | (54) | 23 | (24) | |
| Para-veterinarian farm visits | |||||||||||
| No visits | 118 | (50) | 21 | (37) | 14 | (39) | 20 | (42) | 63 | (66) | 0.004 |
| quarterly | 74 | (31) | 20 | (35) | 15 | (42) | 19 | (40) | 20 | (21) | |
| monthly | 44 | (19) | 16 | (28) | 7 | (19) | 9 | (19) | 12 | (13) | |
| Veterinarian farm visits | |||||||||||
| No visits | 223 | (94) | 46 | (81) | 35 | (97) | 48 | (100) | 94 | (99) | <0.001 |
| quarterly | 4 | (2) | 2 | (4) | 1 | (3) | 0 | (0) | 1 | (1) | |
| monthly | 9 | (4) | 9 | (16) | 0 | (0) | 0 | (0) | 0 | (0) | |
| Farm records | |||||||||||
| Yes | 122 | (52) | 28 | (49) | 23 | (64) | 24 | (50) | 47 | (49) | 0.469 |
| No | 114 | (48) | 29 | (51) | 13 | (36) | 24 | (50) | 48 | (51) | |
| AMU records | |||||||||||
| Yes | 38 | (16) | 16 | (28) | 8 | (22) | 4 | (8) | 10 | (11) | 0.010 |
| No | 198 | (84) | 41 | (72) | 28 | (78) | 44 | (92) | 85 | (89) | |
| Feed milling on farm | |||||||||||
| Yes | 16 | (7) | 1 | (2) | 3 | (8) | 3 | (6) | 9 | (9) | |
| No | 220 | (93) | 56 | (98) | 33 | (92) | 45 | (94) | 86 | (91) | 0.317 |
| Medicated feed used | |||||||||||
| Not used | 125 | (53) | 22 | (39) | 32 | (89) | 35 | (73) | 36 | (38) | <0.001 |
| Used | 111 | (47) | 35 | (61) | 4 | (11) | 13 | (27) | 59 | (62) | |
| Feed supplements | |||||||||||
| Not used | 202 | (86) | 53 | (93) | 30 | (83) | 27 | (56) | 92 | (97) | <0.001 |
| Used | 34 | (14) | 4 | (7) | 6 | (17) | 21 | (44) | 3 | (3) | |
| Antiprotozoal | |||||||||||
| Not used | 229 | (97) | 55 | (96) | 36 | (100) | 46 | (96) | 92 | (97) | 0.703 |
| Used | 7 | (3) | 2 | (4) | 0 | (0) | 2 | (4) | 3 | (3) | |
| Herbal preparations | |||||||||||
| Not used | 211 | (89) | 50 | (88) | 34 | (94) | 45 | (94) | 82 | (86) | 0.384 |
| Used | 25 | (11) | 7 | (12) | 2 | (6) | 3 | (6) | 13 | (14) | |
| Vitamins and minerals | |||||||||||
| Not used | 114 | (48) | 33 | (58) | 14 | (39) | 19 | (40) | 48 | (51) | 0.170 |
| Used | 122 | (52) | 24 | (42) | 22 | (61) | 29 | (60) | 47 | (49) | |
| Vaccines | |||||||||||
| Not used | 225 | (95) | 54 | (95) | 36 | (100) | 46 | (96) | 89 | (94) | 0.490 |
| Used | 11 | (5) | 3 | (5) | 0 | (0) | 2 | (4) | 6 | (6) | |
| Antiseptics and disinfectants | |||||||||||
| Not used | 193 | (82) | 44 | (77) | 30 | (83) | 31 | (65) | 88 | (93) | <0.001 |
| Used | 43 | (18) | 13 | (23) | 6 | (17) | 17 | (35) | 7 | (7) | |
| Agricultural compounds (herbicides and pesticides) | |||||||||||
| Not used | 232 | (98) | 57 | (100) | 35 | (97) | 47 | (98) | 93 | (98) | 0.711 |
| Used | 4 | (2) | 0 | (0) | 1 | (3) | 1 | (2) | 2 | (2) | |
Note: zero (0) indicates no participant of that category participated, n denotes frequency, and % denotes percentage observed, both denote (antibiotics and anthelmintics used), AMU denotes antimicrobials used and, p-value denotes probability of association obtained using Chi-square test or Fisher’s exact test as appropriate between antimicrobial use (antibiotic, anthelmintic, both and no AMU) and factors(farmer, livestock production and management).
References
- OIE. Antimicrobial Resistance. Available online: http://www.oie.int/en/for-the-media/amr/ (accessed on 10 January 2022).
- WHO; FAO; OIE. Taking a Multisectoral, One Health Approach: A Tripartite Guide to Addressing Zoonotic Diseases in Countries. Available online: http://www.fao.org/ag/againfo/resources/en/publications/TZG/TZG.htm (accessed on 5 January 2022).
- WHO. Antimicrobial Resistance. Available online: http://www.who.int/en/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 10 January 2022).
- Krishnasamy, V.; Otte, J.; Silbergeld, E. Antimicrobial use in Chinese swine and broiler poultry production. Antimicrob. Resist. Infect. Control 2015, 4, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kristensen, E.; Jakobsen, E.B. Challenging the myth of the irrational dairy farmer; understanding decision-making related to herd health. N. Z. Vet. J. 2011, 59, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Loi, F.; Laddomada, A.; Coccollone, A.; Marrocu, E.; Piseddu, T.; Masala, G.; Bandino, E.; Cappai, S.; Rolesu, S. Socio-economic factors as indicators for various animal diseases in Sardinia. PLoS ONE 2019, 14, e0217367. [Google Scholar] [CrossRef]
- Woodhill, J.; Hasnain, S.; Griffith, A. Farmers and Food Systems: What Future for Small Scale Agriculture? Available online: https://www.foresight4food.net/wp-content/uploads/2020/01/Farming-food-WEB.pdf (accessed on 10 January 2022).
- Martey, E.; Etwire, P.M.; Wiredu, A.N.; Ahiabor, B.D.K. Establishing the link between market orientation and agricultural commercialization: Empirical evidence from Northern Ghana. Food Secur. 2017, 9, 849–866. [Google Scholar] [CrossRef]
- Ajzen, I. The theory of planned behaviour: Reactions and reflections. Psychol. Health 2011, 26, 1113–1127. [Google Scholar] [CrossRef]
- Jones, P.J.; Marier, E.A.; Tranter, R.B.; Wu, G.; Watson, E.; Teale, C.J. Factors affecting dairy farmers’ attitudes towards antimicrobial medicine usage in cattle in England and Wales. Prev. Vet. Med. 2015, 121, 30–40. [Google Scholar] [CrossRef]
- Alawneh, J.I.; Barnes, T.S.; Parke, C.; Lapuz, E.; David, E.; Basinang, V.; Baluyut, A.; Villar, E.; Lopez, E.L.; Blackall, P.J. Description of the pig production systems, biosecurity practices and herd health providers in two provinces with high swine density in the Philippines. Prev. Vet. Med. 2014, 114, 73–87. [Google Scholar] [CrossRef]
- Williams, L.J.; van Wensveen, M.; Dahlanuddin; Grünbühel, C.M.; Puspadi, K. Adoption as adaptation: Household decision making and changing rural livelihoods in Lombok, Indonesia. J. Rural Stud. 2022, 89, 328–336. [Google Scholar] [CrossRef]
- Lekagul, A.; Tangcharoensathien, V.; Liverani, M.; Mills, A.; Rushton, J.; Yeung, S. Understanding antibiotic use for pig farming in Thailand: A qualitative study. Antimicrob. Resist. Infect. Control 2021, 10. [Google Scholar] [CrossRef]
- Raj Singh, S.; Kumar Datta, K.; Singh Shekhawat, S. Importance of Socio-Economic and Institutional Factors in the Use of Veterinary Services by the Smallholder Dairy Farmers in Punjab. In Veterinary Medicine and Pharmaceuticals; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef] [Green Version]
- Om, C.; McLaws, M.L. Antibiotics: Practice and opinions of Cambodian commercial farmers, animal feed retailers and veterinarians. Antimicrob. Resist. Infect. Control 2016, 5, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Ajzen, I.; Madden, T.J. Prediction of goal-directed behavior: Attitudes, intentions, and perceived behavioral control. J. Exp. Soc. Psychol. 1986, 22, 453–474. [Google Scholar] [CrossRef]
- MOA. 2018 GDP Release. Available online: https://agriculture.gov.fj/stats.php (accessed on 10 January 2022).
- Fiji Agriculture Census. Available online: https://www.agriculture.gov.fj/documents/census/VOLUMEI_DESCRIPTIVEANALYSISANDGENERALTABLEREPORT.pdf (accessed on 15 July 2020).
- Loftus, M.; Stewardson, A.J.; Naidu, R.; Coghlan, B.; Jenney, A.; Kepas, J.; Lavu, E.; Munamua, A.; Peel, T.; Sahai, V.; et al. Antimicrobial resistance in the Pacific Island countries and territories. BMJ Global Health 2020, 5, e002418. [Google Scholar] [CrossRef]
- Ayukekbong, J.A.; Ntemgwa, M.; Atabe, A.N. The threat of antimicrobial resistance in developing countries: Causes and control strategies. Antimicrob. Resist. Infect. Control 2017, 6, 1–8. [Google Scholar] [CrossRef]
- Acharya, K.P.; Karki, S.; Shrestha, K.; Kaphle, K. One health approach in Nepal: Scope, opportunities and challenges. One Health 2019, 8, 100101. [Google Scholar] [CrossRef]
- Kiambi, S.; Mwanza, R.; Sirma, A.; Czerniak, C.; Kimani, T.; Kabali, E.; Dorado-Garcia, A.; Eckford, S.; Price, C.; Gikonyo, S.; et al. Understanding Antimicrobial Use Contexts in the Poultry Sector: Challenges for Small-Scale Layer Farms in Kenya. Antibiotics 2021, 10, 106. [Google Scholar] [CrossRef]
- Manyi-Loh, C.; Mamphweli, S.; Meyer, E.; Okoh, A. Antibiotic Use in Agriculture and Its Consequential Resistance in Environmental Sources: Potential Public Health Implications. Molecules 2018, 23, 795. [Google Scholar] [CrossRef] [Green Version]
- Benavides, J.A.; Streicker, D.G.; Gonzales, M.S.; Rojas-Paniagua, E.; Shiva, C. Knowledge and use of antibiotics among low-income small-scale farmers of Peru. Prev. Vet. Med. 2021, 189, 105287. [Google Scholar] [CrossRef]
- Dankar, I.; Hassan, H.; Serhan, M. Knowledge, attitudes, and perceptions of dairy farmers regarding antibiotic use: Lessons from a developing country. J. Dairy Sci. 2022, 105, 1519–1532. [Google Scholar] [CrossRef]
- Redding, L.E.; Brooks, C.; Georgakakos, C.B.; Habing, G.; Rosenkrantz, L.; Dahlstrom, M.; Plummer, P.J. Addressing Individual Values to Impact Prudent Antimicrobial Prescribing in Animal Agriculture. Front. Vet. Sci. 2020, 7, 297. [Google Scholar] [CrossRef]
- Ruvalcaba-Gómez, J.M.; Villagrán, Z.; Valdez-Alarcón, J.J.; Martínez-Núñez, M.; Gomez-Godínez, L.J.; Ruesga-Gutiérrez, E.; Anaya-Esparza, L.M.; Arteaga-Garibay, R.I.; Villarruel-López, A. Non-Antibiotics Strategies to Control Salmonella Infection in Poultry. Animals 2022, 12, 102. [Google Scholar] [CrossRef]
- Yoo, D.; Lee, K.; Chun, B.-C.; Lee, H.; Park, H.; Kim, J. Preventive effect of on-farm biosecurity practices against highly pathogenic avian influenza (HPAI) H5N6 infection on commercial layer farms in the Republic of Korea during the 2016-17 epidemic: A case-control study. Prev. Vet. Med. 2022, 199, 105556. [Google Scholar] [CrossRef]
- Cuong, N.V.; Phu, D.H.; Van, N.T.B.; Dinh Truong, B.; Kiet, B.T.; Hien, B.V.; Thu, H.T.V.; Choisy, M.; Padungtod, P.; Thwaites, G.; et al. High-Resolution Monitoring of Antimicrobial Consumption in Vietnamese Small-Scale Chicken Farms Highlights Discrepancies Between Study Metrics. Front. Vet. Sci. 2019, 6, 174. [Google Scholar] [CrossRef] [Green Version]
- Alhaji, N.B.; Aliyu, M.B.; Ghali-Mohammed, I.; Odetokun, I.A. Survey on antimicrobial usage in local dairy cows in North-central Nigeria: Drivers for misuse and public health threats. PLoS ONE 2019, 14, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Hosain, M.Z.; Kabir, S.M.L.; Kamal, M.M. Antimicrobial uses for livestock production in developing countries. Vet. World 2021, 14, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.L.; Caffrey, N.P.; Nóbrega, D.B.; Cork, S.C.; Ronksley, P.E.; Barkema, H.W.; Polachek, A.J.; Ganshorn, H.; Sharma, N.; Kellner, J.D.; et al. Restricting the use of antibiotics in food-producing animals and its associations with antibiotic resistance in food-producing animals and human beings: A systematic review and meta-analysis. Lancet Planet. Health 2017, 1, e316–e327. [Google Scholar] [CrossRef]
- Habib, I.; Mohamed, M.Y.I. Foodborne infections in the Middle East. In Food Safety in the Middle East; Elsevier: Amsterdam, The Netherlands, 2022; pp. 71–107. [Google Scholar]
- Sinha, R.; Sinha, B.; Kumari, R.; Vineeth, M.R.; Verma, A.; Gupta, I.D. Effect of season, stage of lactation, parity and level of milk production on incidence of clinical mastitis in Karan Fries and Sahiwal cows. Biol. Rhythm Res. 2021, 52, 593–602. [Google Scholar] [CrossRef]
- Mujyambere, V.; Adomako, K.; Olympio, S.O.; Ntawubizi, M.; Nyinawamwiza, L.; Mahoro, J.; Conroy, A. Local chickens in East African region: Their production and potential. Poult. Sci. 2022, 101, 101547. [Google Scholar] [CrossRef] [PubMed]
- Imam, T.; Gibson, J.S.; Foysal, M.; Das, S.B.; Gupta, S.D.; Fournié, G.; Hoque, M.A.; Henning, J. A Cross-Sectional Study of Antimicrobial Usage on Commercial Broiler and Layer Chicken Farms in Bangladesh. Front. Vet. Sci. 2020, 7, 6113. [Google Scholar] [CrossRef]
- Khan, X.; Rymer, C.; Ray, P.; Lim, R. Categorisation of antimicrobial use in Fijian livestock production systems. Antibiotics 2022, 11, 294. [Google Scholar] [CrossRef]
- Khan, X.; Rymer, C.; Ray, P.; Lim, R. Quantification of antimicrobial use in Fijian livestock farms. One Health 2021, 13, 100326. [Google Scholar] [CrossRef]
- Khan, X.; Lim, R.; Rymer, C.; Ray, P. Fijian farmers’ attitude and knowledge towards antimicrobial use and antimicrobial resistance in livestock production systems -a qualitative study. Front. Vet. Sci. 2022, 9, 8457. [Google Scholar] [CrossRef]
- Lekagul, A.; Tangcharoensathien, V.; Mills, A.; Rushton, J.; Yeung, S. How antibiotics are used in pig farming: A mixed-methods study of pig farmers, feed mills and veterinarians in Thailand. BMJ Global Health 2020, 5, e001918. [Google Scholar] [CrossRef] [Green Version]
- Willson, N.L.; Van, T.T.H.; Bhattarai, S.P.; Courtice, J.M.; McIntyre, J.R.; Prasai, T.P.; Moore, R.J.; Walsh, K.; Stanley, D. Feed supplementation with biochar may reduce poultry pathogens, including Campylobacter hepaticus, the causative agent of Spotty Liver Disease. PLoS ONE 2019, 14, e0214471. [Google Scholar] [CrossRef]
- Scott, A.B.; Singh, M.; Groves, P.; Hernandez-Jover, M.; Barnes, B.; Glass, K.; Moloney, B.; Black, A.; Toribio, J.A. Biosecurity practices on Australian commercial layer and meat chicken farms: Performance and perceptions of farmers. PLoS ONE 2018, 13, 1–17. [Google Scholar] [CrossRef]
- Malusi, N.; Falowo, A.B.; Idamokoro, E.M. Herd dynamics, production and marketing constraints in the commercialization of cattle across Nguni Cattle Project beneficiaries in Eastern Cape, South Africa. Pastoralism 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Can, M.F.; Altug, N. Socioeconomic implications of biosecurity practices in small-scale dairy farms. Vet. Q. 2014, 34, 67–73. [Google Scholar] [CrossRef] [Green Version]
- More, S.J. European perspectives on efforts to reduce antimicrobial usage in food animal production. Irish Vet. J. 2020, 73, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Gemeda, B.A.; Amenu, K.; Magnusson, U.; Dohoo, I.; Hallenberg, G.S.; Alemayehu, G.; Desta, H.; Wieland, B. Antimicrobial Use in Extensive Smallholder Livestock Farming Systems in Ethiopia: Knowledge, Attitudes, and Practices of Livestock Keepers. Front. Vet. Sci. 2020, 7, 55. [Google Scholar] [CrossRef]
- Mangesho, P.E.; Caudell, M.A.; Mwakapeje, E.R.; Ole-Neselle, M.; Kabali, E.; Obonyo, M.; Dorado-Garcia, A.; Valcarce, A.; Kimani, T.; Price, C.; et al. “We are doctors”: Drivers of animal health practices among Maasai pastoralists and implications for antimicrobial use and antimicrobial resistance. Prev. Vet. Med. 2021, 188, 105266. [Google Scholar] [CrossRef]
- Malik, B.; Bhattacharyya, S. Antibiotic drug-resistance as a complex system driven by socio-economic growth and antibiotic misuse. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Mikecz, O.; Pica-Ciamarra, U.; Felis, A.; Nizeyimana, G.; Okello, P.; Brunelli, C. Data on antimicrobial use in livestock: Lessons from Uganda. One Health 2020, 10, 100165. [Google Scholar] [CrossRef]
- Lowder, S.K.; Skoet, J.; Raney, T. The Number, Size, and Distribution of Farms, Smallholder Farms, and Family Farms Worldwide. World Dev. 2016, 87, 16–29. [Google Scholar] [CrossRef] [Green Version]
- FAO. Handbook on the Agricultural Integrated Survey (AGRIS). Available online: http://www.fao.org/in-action/agrisurvey/resources/methodological-toolkit/en/ (accessed on 10 January 2022).
- FAO. Guidelines for the Preparation of Livestock Sector Reviews. Available online: https://www.fao.org/3/i2294e/i2294e00.htm (accessed on 5 January 2022).
- Adekanye, U.O.; Ekiri, A.B.; Galipó, E.; Muhammad, A.B.; Mateus, A.; La Ragione, R.M.; Wakawa, A.; Armson, B.; Mijten, E.; Alafiatayo, R.; et al. Knowledge, Attitudes and Practices of Veterinarians Towards Antimicrobial Resistance and Stewardship in Nigeria. Antibiotics 2020, 9, 453. [Google Scholar] [CrossRef]
- Hosmer Jr, D.W.; Lemeshow, S.; Sturdivant, R.X. Applied Logistic Regression, 3rd ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2013; Volume 3, pp. 153–168. [Google Scholar]
- Xu, J.; Sangthong, R.; McNeil, E.; Tang, R.; Chongsuvivatwong, V. Antibiotic use in chicken farms in northwestern China. Antimicrob. Resist. Infect. Control 2020, 9, 10. [Google Scholar] [CrossRef] [Green Version]
- Berge, A.C.B.; Atwill, E.R.; Sischo, W.M. Animal and farm influences on the dynamics of antibiotic resistance in faecal Escherichia coli in young dairy calves. Prev. Vet. Med. 2005, 69, 25–38. [Google Scholar] [CrossRef]
- Manyahi, J.; Kibwana, U.; Mgimba, E.; Majigo, M. Multi-drug resistant bacteria predict mortality in bloodstream infection in a tertiary setting in Tanzania. PLoS ONE 2020, 15, e0220424. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).