Abstract
Antimicrobial stewardship interventions are targeted efforts by healthcare organizations to optimize antimicrobial use in clinical practice. The study aimed to explore effective interventions in improving antimicrobial use in hospitals. Literature was systemically searched for interventional studies through PubMed, CINAHL, and Scopus databases that were published in the period between January 2010 to April 2022. A random-effects model was used to pool and evaluate data from eligible studies that reported antimicrobial stewardship (AMS) interventions in outpatient and inpatient settings. Pooled estimates presented as proportions and standardized mean differences. Forty-eight articles were included in this review: 32 in inpatient and 16 in outpatient settings. Seventeen interventions have been identified, and eight outcomes have been targeted. AMS interventions improved clinical, microbiological, and cost outcomes in most studies. When comparing non-intervention with intervention groups using meta-analysis, there was an insignificant reduction in length of stay (MD: −0.99; 95% CI: −2.38, 0.39) and a significant reduction in antibiotics’ days of therapy (MD: −2.73; 95% CI: −3.92, −1.54). There were noticeable reductions in readmissions, mortality rates, and antibiotic prescriptions post antimicrobial stewardship multi-disciplinary team (AMS-MDT) interventions. Studies that involved a pharmacist as part of the AMS-MDT showed more significant improvement in measured outcomes than the studies that did not involve a pharmacist.
1. Introduction
In 2009, more than 3 million kg of antimicrobials were administered to humans in the US [1]. Despite the undeniable benefits of effective antimicrobial prescribing, there are significant risks associated with use and misuse, and antimicrobial resistance (AMR) is on the rise. Antimicrobial-associated Clostridioides difficile infection (CDI), adverse effects, and increasing antimicrobial and non-antimicrobial healthcare expenses are all major problems [2,3,4,5,6,7,8,9].
Although careful use of antimicrobial agents is widely recommended, their overuse or abuse has become entrenched in diverse contexts across the world [10,11]. AMR-related mortality is expected to exceed 10 million people per year by 2050 with improper antimicrobial usage now regarded as one of the major drivers of AMR [12,13,14].
Antimicrobial stewardship programs (ASPs) are targeted efforts by healthcare organizations or portions of organizations, e.g., inpatient (IP) settings, to optimize antimicrobial use, thus, improving patient outcomes, reducing negative consequences (such as AMR, or toxicity) and providing cost-effective therapy [3,15,16,17]. Such programs are multidisciplinary interventions that include patient-level stewardship (e.g., optimizing antimicrobial therapy for an individual patient based on culture results and clinical syndrome) and population-level stewardship (e.g., reducing overall antimicrobial consumption or consumption of a specific antimicrobial class through interventions) [10].
Between 20% and 50% of antimicrobial prescriptions in acute care hospitals are either unnecessary or inadequately administered [10]. Similarly, in outpatient (OP) settings, where the majority of antimicrobials are dispensed, misuse is unfortunately widespread. For instance, despite studies demonstrating that only 10% of people with sore throat have an antimicrobial-responsive illness, antimicrobials were prescribed for more than 60% of patients with pharyngitis [18]
One single systematic review (without meta-analysis) was published in Cochrane Library between January 2010 and April 2022 that investigated the impact of ASP interventions on improving antibiotic use in hospital settings. It concluded that those interventions have ensured that antibiotics were used more appropriately, the duration of antibiotic treatment was reduced, and length of hospital stay was decreased without increasing the risk of death [10]. By exploring other databases, we identified a few meta-analysis reviews within a similar period with objectives to improve antibiotic use, enhance clinical or microbiological outcomes, and/or decrease antibiotic treatment expenditure [19,20,21,22,23]. Three of those reviews focused on the outpatient setting while two involved inpatient care.
In the present meta-analysis, we have focused on reviewing clinical trials that investigated the impact of antimicrobial stewardship multidisciplinary team (AMS-MDT) interventions on improving clinical, microbiological, or other measured outcomes in two settings, outpatient and inpatient, in order to capture most of the interventions performed by antimicrobial stewardship teams in different clinical trials, and to differentiate effective interventions in each of the two settings.
The objective of this review was to identify antimicrobial stewardship program multi-disciplinary team (AMS-MDT) interventions and their impact on improving clinical and microbiological outcomes, and costs at a hospital level including inpatient and outpatient settings. We also aimed to identify the difference in the outcomes between studies that involved a pharmacist as a part of the AMS multidisciplinary team and those which did not involve a pharmacist.
2. Results
2.1. Search Results
A total of 2056 studies were generated by searching three databases: PubMed, Scopus, and CINAHL. Out of those, 1895 studies were screened by title, and out of those, 116 articles were sought for retrieval. After screening the abstracts for those articles, eighty-nine articles were fully retrieved, and by strictly implementing the inclusion and exclusion criteria, a final number of 48 articles were included in the study. Figure 1 represents the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) flow chart for this review. The included studies have been classified into four groups: 13 articles involved IP settings without a pharmacist as a part of an AMS-MDT intervention [24,25,26,27,28,29,30,31,32,33,34,35,36], 19 were carried out in IP settings with the inclusion of a pharmacist as a part of the intervention team [37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55], eight articles engaged OP without a pharmacist [56,57,58,59,60,61,62,63], while eight involved OP settings with a pharmacist [64,65,66,67,68,69,70,71]. Forty-one articles were from developed countries [24,25,26,27,28,30,31,32,34,35,36,37,38,39,41,42,43,44,46,48,50,51,52,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71], while 7 took place in developing countries [29,33,40,45,47,49,53]. Table 1 represents the data extraction table.
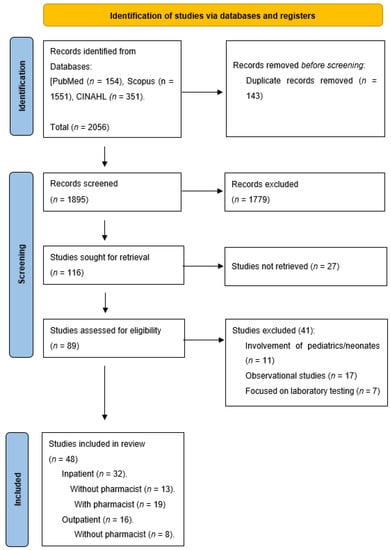
Figure 1.
PRISMA flow diagram of the process of study selection.

Table 1.
Data extraction table.
2.2. Quality Assessment
The risk of bias assessment for the included studies is presented in Figure 2. There were 11 RCT studies assessed using the ROB-2 assessment tool [28,32,33,34,46,57,58,59,60,63,67]. Of those, seven scored high risk [33,34,46,57,59,63,67], three scored low risk [28,32,60], with one article only found to have some concerns [58]. On the other hand, the remaining 37 articles were non-randomized before and after methodology and were assessed using the ROBINS-1 assessment tool [24,25,26,27,29,30,31,35,36,37,38,39,40,41,42,43,44,45,47,48,49,50,51,52,53,54,55,56,61,62,64,65,66,68,69,70,71]. Out of those 37, 29 were at moderate risk of bias [24,25,29,30,35,36,38,39,40,41,42,43,44,47,48,49,50,51,52,53,55,56,62,64,66,69,70,71] and 9 were at serious risk [26,27,31,37,40,45,54,61,68]. No article from those 37 scored low risks.
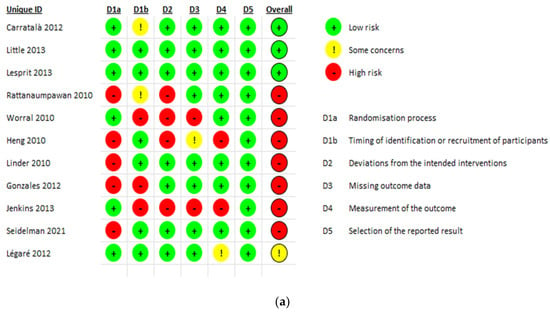
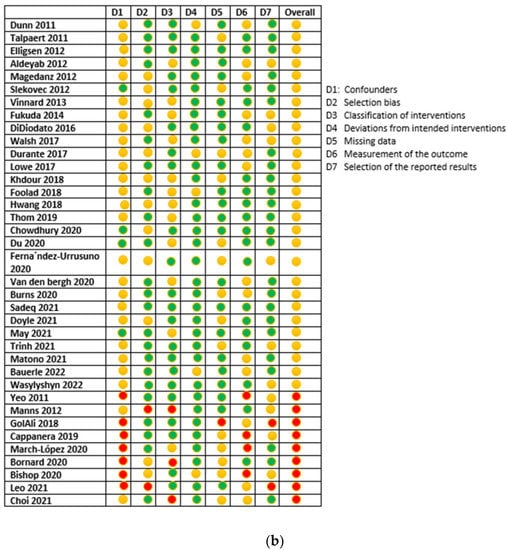
Figure 2.
(a) RCTs risk of bias assessment using ROB-2 tool; (b) ROBIN-1 risk of bias assessment for non-randomized trials.
2.3. Interventions
There were 21 different interventions identified in the included articles as shown in Table 2. Of the identified interventions, twelve were captured in the IP setting [24,25,26,27,28,29,30,31,32,33,37,38,39,40,42,44,45,46,47,48,49,50,53,54,55] while 13 were in the OP setting [56,57,58,59,60,61,62,63,64,65,66,68,69,70,71], with 7 common interventions between the two settings. Those interventions were prospective and audit with direct intervention [37,38,42,44,45,47,48,49,50,53,54], education for health-care professionals (HCP) [25,28,40,43,56,66,68,69,71], antibiotic restriction and pre-authorization [24,30,33,61], use of clinical-decision support systems (CDSS) [31,39,46,66], regular dedicated infectious disease team (IDT) rounds [26,27,29,34], prospective audit and feedback [40,62], delayed antibiotic prescriptions [60,63], clinical decision making algorithm [36], intravenous (IV) to oral guidelines implementation [41], implementation of antibiotic time out [52], shared decision making [58], creating a quality dashboard [59], patient education [71], order sets implementation [69], developing clinical pathways for common OP infections [67], ASP multidisciplinary team escalating approach [55], Multi-faceted IDT visits (rounds, interactive training sessions, meetings) [26], retrospective audit and feedback [65,66], pocket cards containing antimicrobial guidelines [43], HCP education after audit and feedback [64], and soft stop orders [31].

Table 2.
Types of intervention per setting.
2.4. Outcomes
Several common outcomes were identified in the included studies such as length of hospital stay (LOS), days of antibiotic therapy (DOT), 30-day readmission and mortality rate, antimicrobial guidelines’ adherence, CDI and multi-drug resistance (MDR) rates, antibiotic prescription rates, antibiotic consumption, defined daily dose (DDD), and cost-saving.
2.4.1. Length of Hospital Stay
LOS was significantly decreased in five studies that were conducted in the IP setting; three involved a pharmacist as part of an AMS-MDT [45,47,55] and two were without a pharmacist [28,36]. The other 6 studies reported insignificant results [25,32,33,36,37,53]. Interventions described in this meta-analysis were antibiotic restriction and pre-authorization, audit and feedback with direct intervention, clinical decision-making algorithm, HCP education, and ASP MDT escalating approach. The pooled effect size obtained using data from five studies failed to show a significant difference in the length of stay between the intervention and the non-intervention groups in the IP setting (−0.99; 95% CI: −2.38, 0.39) (Figure 3).
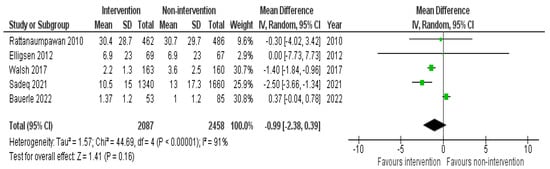
Figure 3.
Pooled data from inpatient studies representing the impact of antimicrobial stewardship program intervention on length of hospital stay when comparing non-intervention with intervention groups.
2.4.2. Days of Therapy
DOT was significantly reduced in 9 studies; out of those, four were in an IP setting without the involvement of a pharmacist [30,31,33,36], and four in an IP setting with the presence of a pharmacist [42,43,47,48]. One study was conducted in the OP setting and pharmacists did not take part in the intervention [62]. On the other hand, insignificant changes were reported by all the six studies conducted in the IP setting [29,34,35,38,52,55]. The data from four IP studies have been pooled to produce an overall effect. The overall pooled estimate was significant (−2.73; 95% CI: −3.92, −1.54) when comparing non-intervention with intervention group (Figure 4). Interventions that impacted DOT were HCP education, prospective audit and feedback with or without direct intervention, IV to oral guideline implementation, pocket cards containing antimicrobial guidelines, regular dedicated IDT rounds, and HCP education after audit and feedback.
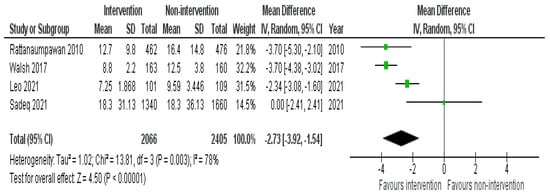
Figure 4.
Pooled data from inpatient studies representing the impact of antimicrobial stewardship program intervention on days of antibiotic therapy when comparing non-intervention with intervention groups.
2.4.3. Thirty-Day Readmission and Mortality
Only two studies reported significant changes in 30-day readmissions [36,55] and both were conducted in the IP setting with one of them involving a pharmacist [36]. In contrast, eight studies found no significant differences [25,28,37,38,43,46,47,48]. Similarly, only three articles in the IP setting reported a significant reduction in mortality, with all of them having a pharmacist playing a role in the intervention [47,48,55], while no significant changes in mortality reported in the remaining studies [30,32,35,37,38,42,43,46,47,53]. The pooled proportion of patients who were re-admitted without AMS intervention was 11% (95% CI: 6%, 18%) and this was reduced to 10% (95% CI: 5%, 16%) with the intervention group as shown in Figure 5, while the pooled proportion of mortality was 11% (95% CI: 7%, 17%) in the non-intervention group compared to 9% (95% CI 5%, 14%) in the intervention group (Figure 6). Types of intervention that were used by the pooled studies with an impact on readmission and mortality were HCP education, clinical decision-making algorithm (only tested readmission), prospective audit and feedback with direct intervention, clinical-decision support system use, and MDT escalating approach.
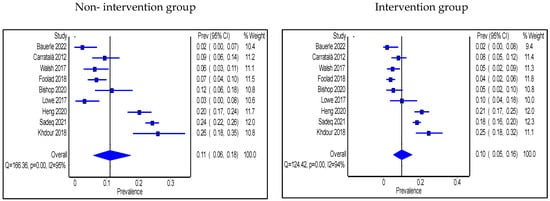
Figure 5.
Prevalence of 30-day readmission within the inpatient setting in non-intervention and intervention groups.
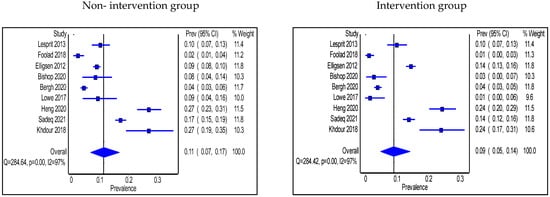
Figure 6.
Prevalence of mortality within the inpatient setting in non-intervention and intervention groups.
2.4.4. Adherence to Antimicrobial Guidelines/Protocols
Adherence to antimicrobial guidelines was significantly higher in the intervention group than in the non-intervention group (n = 7 studies); two in the IP setting had no pharmacist in the intervention [25,31], three in the IP setting included a pharmacist [37,45,53], and two studies were in the OP setting and involved a pharmacist [64,65]. No significant changes were seen in terms of adherence to guidelines in 2 studies where a pharmacist was engaged in the intervention, one was in the IP [39] and the other one was in the OP setting [69]. The pooled proportion of patients who were prescribed antibiotics in accordance with hospital antimicrobial guidelines in IP groups was 55% (95% CI: 43%, 68%) in the non-intervention group compared with 50% (95% CI: 39–64%) in the intervention group (Figure 7a). Types of intervention used were multi-faceted IDT round visits, pre-configured antibiotics, soft stop order, CDSS use, HCP education, and prospective audit and feedback with direct intervention. On the other hand, the pooled proportion of patients prescribed antibiotics as per the hospital antimicrobial guidelines in OP settings was 53% (95% CI: 8%, 95%) in the non-intervention group compared with 66% (95% CI: 33%, 92%) in the intervention group (Figure 7b), and the types of intervention used were HCP education after audit and feedback, HCP education, guidelines and order set implementations, and retrospective audit and feedback.
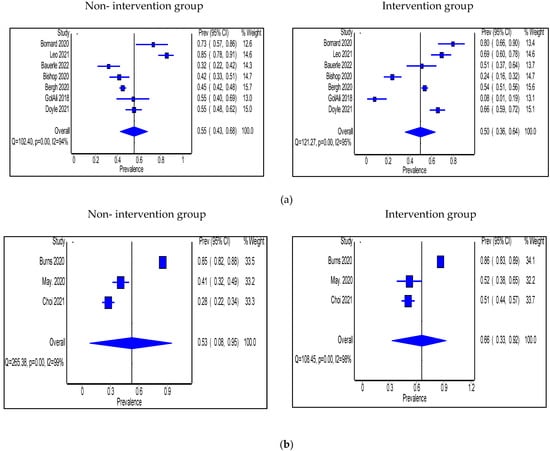
Figure 7.
(a) Prevalence of patients prescribed antibiotics in accordance with hospital guidelines within the inpatient setting in the non-intervention and intervention groups; (b) Prevalence of patients prescribed antibiotics in accordance with hospital guidelines within the outpatient setting in the non-intervention and intervention groups.
2.4.5. Antimicrobial Use
Antimicrobial use was expressed in two ways. First is the antimicrobial prescribing rate. Seven studies expressed antimicrobial use as prescribing rate and were all performed in OP settings [56,57,58,59,62,70,71]. Pharmacists took part in the intervention in only two of these studies [70,71]. The pooled proportion of patients prescribed antibiotics was 45% (95% CI: 32%, 60%) in the non-intervention group compared with 39% (95% CI: 30%, 49%) in the intervention group (Figure 8). Interventions that significantly decreased prescribing rate were shared decision making, prospective audit and feedback, HCP education, and antimicrobial treatment guidelines’ implementation.
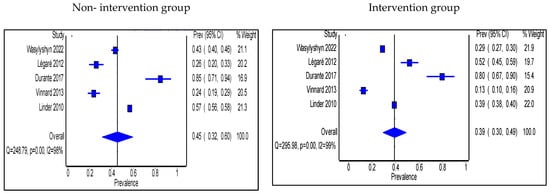
Figure 8.
Prevalence of patients prescribed antibiotics in accordance with hospital guidelines within the outpatient setting in the non-intervention and intervention groups.
Daily defined dose (DDD) either alone, adjusted per patient days, or adjusted per inhabitant days, was the second measure used to express antimicrobial use, and was used in 11 studies. In the IP setting, three studies were without a pharmacist [27,29,33], and the results did not change significantly, while four out of six studies that engaged a pharmacist found a significant reduction [40,49,51,54]. In the OP setting, two studies involved a pharmacist as part of the intervention and concluded a significant reduction in one study [66] while the other did not report a p-value [68]. Interventions that significantly reduced DDD were antibiotic restriction and pre-authorization, prospective audit and feedback with or without direct intervention, HCP education, antimicrobial treatment guidelines’ implementation, retrospective audit and feedback, and CDSS use.
2.4.6. Microbiological Outcomes
Microbiological outcomes were also measured in twelve of the included articles. Ten studies measured the difference in CDI rate between non-intervention and intervention groups; two were in the IP setting without the involvement of a pharmacist and reported a significant reduction in the rate [24,35], six in the IP setting with a pharmacist with 3 reporting a significant reduction [37,42,51]; the other three studies did not report significant changes in CDI rate [39,43,55]. Conversely, two studies investigated OP with a pharmacist and did not report significant changes [65,70]. Interventions that significantly decreased CDI rates were antibiotic restriction and pre-authorization, antimicrobial treatment guidelines’ implementation, and prospective audit and feedback with direct intervention.
Multidrug-resistant (MDR) organisms were investigated in two studies; one was in the IP setting [44] and resulted in a significant reduction in MDR rate, while the other was in OP setting [70] and reported insignificant changes. Both studies involved a pharmacist as a part of the intervention team. Interventions performed were prospective audit and feedback with direct intervention, HCP education, and prospective audit and feedback.
2.4.7. Antimicrobial Therapy Cost
Cost-saving was analyzed in 5 studies; two of them were conducted in the IP setting without the presence of a pharmacist and three included a pharmacist in the intervention team and all of them reported a cost reduction [25,33,39,44,55]. Interventions conducted were HCP education, antibiotic restriction and pre-authorization, CDSS use, and MDT escalating approach.
2.5. Funnel Plots
To evaluate for publication bias, bias assessment in the form of funnel plots has been conducted (Figure S1–S4).
3. Discussion
Inappropriate use of antimicrobials could increase the development of AMR, necessitating the need for effective AMS interventions to optimize it [72,73]. Our review has summarized multi-disciplinary AMS interventions in two settings within hospitals, the outpatient and the inpatient settings. Our review has also been able to identify the interventions that have resulted in significant changes in the targeted outcomes. In addition, we have classified types of the intervention performed into four categories, i.e., inpatient with a pharmacist as a part of AMS-MDT intervention, inpatient without a pharmacist, outpatient with a pharmacist, and outpatient without a pharmacist. This allowed us to identify the impact of the presence of a pharmacist as a part of intervention in both outpatient and inpatient settings. Furthermore, the focus was on interventional studies to gain robust evidence with exclusion of any observational studies.
Length of hospital stay achieved significant reduction in more studies when a pharmacist was included in the intervention in the inpatient settings [45,47,55] compared to studies that did not involve a pharmacist. When data were pooled, AMS interventions resulted in lower LOS when compared with an opposing group without the intervention. This supports a previous study which proved that the implementation of hospital-based AMS reduced LOS by 8.9% [21]. In addition, the number of studies that applied multi-disciplinary interventions and achieved significant reductions in DOT were more than those which did not [30,31,33,36,42,43,47,48,70], and pooled data analysis showed a reduction of 2.73 mean days when AMS-MDT interventions were applied. The significant reduction in DOT was also found in a previous study that implemented a pharmacist-led ASP [74].
A significant reduction in thirty-day readmission was not observed in most of our included studies when using AMS interventions. On the other hand, only studies that included a pharmacist as a part of the multi-disciplinary intervention team achieved a significant reduction in mortality [47,48,55]. No previous meta-analysis has been found in the literature investigating the impact of ASP on readmission. Meanwhile, mortality decreased significantly in IP studies that involved a pharmacist, a result which was also concluded by two previous studies [11,74].
Another major outcome used to measure the impact the stewardship intervention studies was adherence to antimicrobial guidelines. In our review, studies that involved a pharmacist as part of the AMS-MDT indicated significant improvement in guidelines adherence [37,45,53]. This result is in line with a systematic review that included 57 articles from both IP and OP settings, and showed that clinical-decision-support system intervention improved adherence to antimicrobial guidelines twice as much in the intervention group than the non-intervention group [75].
Antibiotic consumption, represented by DDD, was reduced significantly only in studies that involved a pharmacist as part of the AMS-MDT [40,49,51,54,66], while it did not change significantly in other studies not involving a pharmacist. Antibiotic cost was reduced in all five studies that investigated expenditure, with three of them involving a pharmacist in the intervention [39,44,55]. This outcome was consistent with the findings of two past meta-analysis articles [76,77].
CDI and MDRO rates showed significant improvement in multiple studies conducted in the IP setting, and more than half of those studies had a pharmacist as part of the implemented intervention.
Our review has some limitations. Firstly, it included non-randomized trials in addition to randomized trials; this was due to the number of studies in the literature using non-randomized before and after designs. Secondly, classification of the site of infection or type of infectious disease was not possible because most studies either tested only respiratory tract infections or did not specify an infection site. Third, some outcomes have a few numbers of studies, and this is probably because we included only articles that fit our study’s inclusion criteria. Finally, publication bias should be considered while interpreting our results.
4. Materials and Methods
4.1. Search Strategy
This systematic review and meta-analysis followed the guidelines of the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) protocol, an evidence-based set of items for reporting systematic reviews and meta-analyses [78].
We systemically searched PubMed, CINAHL and Scopus databases for related articles that were published in the period between January 2010 to April 2022. All articles investigating the impact of AMS-MDT interventions in hospitals and primary care were included for screening and review. The search strategy followed PIO (Population, Intervention, and Outcome) model and the keywords chosen for the search strategy were: P (hospital OR hospitals OR inpatient OR inpatients OR outpatients OR outpatient OR primary care) AND I ((antibiotic stewardship) OR (antimicrobial stewardship) OR (antibacterial stewardship)) AND O (outcome OR outcomes OR use OR utilization OR implementation OR prescribing OR prescription OR consumption OR mortality OR hospital stay OR therapy days OR difficile OR MDR OR MRSA OR ESBL OR Appropriate OR infection OR infections). Table 3 illustrates the full search strategy.

Table 3.
Search Strategy.
4.2. Study Selection
Two independent investigators (A. A. Sadeq and S. S. Hasan) examined titles and abstracts appearing in the database results to find potentially suitable publications. Any disagreements (e.g., including different articles by the two investigators) between the two authors were resolved by discussion and consensus.
For a study to be eligible for further screening and retrieval, the title or the abstract should have indicated an AMS-MDT intervention process that affected one or more of the outcomes of interest. The inclusion criteria for articles to be included in our review included interventional studies (whether randomized or non-randomized) that were conducted in hospitals or primary health care centers and investigated the impact of AMS-MDT interventions on improving clinical and microbiological outcomes, and cost.
Observational studies and articles that involved children or infants, discharge practice, antimicrobial surgical prophylaxis, long-term and nursing home facilities, interventions using rapid diagnostic tests, infection control practice, antifungals or antivirals, interventions conducted by nurses, special populations (e.g., renal disease), and online stewardship programs were all excluded from the final review. Exclusion criteria did not omit studies with high risk of bias. All inclusion and exclusion criteria assessments were carried out by two reviewers (A. A. Sadeq and S. S. Hasan).
4.3. Classification of Outcomes
The selected articles for our review were discussed in detail by two reviewers (A. A. Sadeq, S. S. Hasan), then agreed upon independently and then by consensus. The outcomes of interest were classified as clinical outcomes (days of therapy [DOT], length of hospital stay [LOS], 30-day readmission rate and mortality rate), microbiological outcomes (multi-drug resistant organisms [MDRO] resistance rates and CDI rates) and other outcomes including antibiotic prescribing rates, antibiotic consumption, and cost.
Days of therapy are the number of days in which a patient has received antibiotic therapy, while length hospital stay is the difference in days between patient hospital admission and discharge.
4.4. Data Extraction Process
The primary investigators established a standard data extraction form using Microsoft Excel®. This data extraction sheet was divided into four tables: Inpatient settings (IP) with a pharmacist as part of the AMS-MDT, IP settings without a pharmacist, outpatient settings (OP) without a pharmacist, and OP settings with a pharmacist. The following data were gathered from the identified studies: author name, year, country, sample size, study design, infection site, intervention type, outcomes, and findings. Data extraction was undertaken by two investigators (A. A. Sadeq and N. AbouKhater).
4.5. Risk of Bias/Quality Assessment
The risk of bias was assessed using Version 2 of Cochrane risk-of-bias tool (RoB2) for randomized control trials [79]. Based on the responses to the signaling questions, an algorithm generated a proposed judgment regarding the risk of bias resulting from each area as ’Low risk of bias’, ’High risk of bias’, or ‘Some concerns’. The overall risk of bias generally corresponds to the worst risk of bias in any of the domains.
For non-randomized trials, Risk of Bias in Non-Randomized Studies of Interventions (ROBINS-1) was used for bias risk assessment [80]. The overall risk of bias was judged depending on the scoring of the criteria; if the risk of bias for all domains was low then the overall risk was low, if there is a low or moderate risk of bias for all domains then it is moderate, while if there was a serious risk of bias or critical risk of bias in at least one domain, then the overall risk of judgment was serious or critical, respectively.
The process of risk of bias assessment was performed independently by two investigators (A. Sadeq and N. AbouKhater) and any disagreements were resolved by discussion and consensus.
4.6. Study Registration
This review has been recorded in PROSPERO (The International Prospective Register of Systematic Reviews) under the code CRD42022302431.
4.7. Statistical Analysis
RevMan® software version 5.4.1 and MetaXL software version 5.2 were used to conduct the analyses with random-effects model to pool and evaluate data from eligible studies that reported the same outcomes. Pooled estimates were represented as a forest plot with a 95 percent confidence interval (CI) range for risk differences and mean differences. The I2 statistic was used to look at heterogeneity as it calculates the percentage of overall variation that can be attributed to between-study heterogeneity. I2 values of 25%, >50%, and >75% refer, respectively, to low, substantial, and considerable degrees of heterogeneity. Funnel plots were generated using inverse variance methods to examine the publication bias.
5. Conclusions
The present review has identified influential antimicrobial stewardship multidisciplinary team interventions in both inpatient and outpatient settings. Twenty-one interventions have been recognized with the most common interventions being prospective audit and feedback with direct intervention, antibiotic restriction and pre-authorization, regular dedicated ID rounds, HCP education, use of clinical decision support system, antimicrobial guidelines implementation, and retrospective audit and feedback. The inclusion of a pharmacist as a part of the multidisciplinary team increased the chances of achieving statistically significant changes in the outcomes.
Those interventions were able to improve clinical (LOS, DOT, guidelines’ adherence, morbidity and mortality, and antibiotic prescription rate), microbiological, and cost outcomes when AMS-MDT interventions were applied and compared to non-intervention groups.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antibiotics11101306/s1, Figure S1: Funnel plot assessing the risk of publication bias for mortality; Figure S2: Funnel plot assessing the risk of publication bias for readmission rate; Figure S3: Funnel plot assessing the risk of publication bias for the prevalence of antibiotics prescribed; Figure S4: Funnel plot assessing the risk of publication bias for the percentage of adherence to antimicrobial guidelines.
Author Contributions
Conceptualization, all authors; methodology, A.A.S., S.S.H., N.A., A.E.A. and M.A.A.; software, A.A.S. and S.S.H.; validation, A.A.S., J.M.S., Z.O.E.B., S.E.B. and M.A.A.; formal analysis, A.A.S., S.S.H., N.A. and M.A.A.; resources, B.R.C. and M.A.A.; writing—original draft preparation, A.A.S.; writing—review and editing, all authors; supervision, B.R.C. and M.A.A.; project administration, A.A.S., J.M.S., Z.O.E.B. and E.F.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data were analyzed and presented in this paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Spellberg, B.; Bartlett, J.G.; Gilbert, D.N. The future of antibiotics and resistance. N. Engl. J. Med. 2013, 368, 299–302. [Google Scholar] [CrossRef]
- Ibrahim, E.H.; Sherman, G.; Ward, S.; Fraser, V.J.; Kollef, M.H. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest 2000, 118, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.T.; Gaynes, R.P. Emerging trends in antibiotic use in US hospitals: Quality, quantification and stewardship. Expert Rev. Anti Infect. 2010, 8, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Kollef, M.H.; Sherman, G.; Ward, S.; Fraser, V.J. Inadequate antimicrobial treatment of infections: A risk factor for hospital mortality among critically ill patients. Chest 1999, 115, 462–474. [Google Scholar] [CrossRef]
- Micek, S.T.; Welch, E.C.; Khan, J.; Pervez, M.; Doherty, J.A.; Reichley, R.M.; Kollef, M.H. Empiric combination antibiotic therapy is associated with improved outcome against sepsis due to Gram-negative bacteria: A retrospective analysis. Antimicrob. Agents Chemother. 2010, 54, 1742–1748. [Google Scholar] [CrossRef] [PubMed]
- Ray, W.A.; Murray, K.T.; Hall, K.; Arbogast, P.G.; Stein, C.M. Azithromycin and the Risk of Cardiovascular Death. N. Engl. J. Med. 2012, 366, 1881–1890. [Google Scholar] [CrossRef]
- Ray, W.A.; Murray, K.T.; Meredith, S.; Narasimhulu, S.S.; Hall, K.; Stein, C.M. Oral Erythromycin and the Risk of Sudden Death from Cardiac Causes. N. Engl. J. Med. 2004, 351, 1089–1096. [Google Scholar] [CrossRef]
- Shehab, N.; Patel, P.R.; Srinivasan, A.; Budnitz, D.S. Emergency department visits for antibiotic-associated adverse events. Clin. Infect. Dis. 2008, 47, 735–743. [Google Scholar] [CrossRef]
- Zahar, J.R.; Rioux, C.; Girou, E.; Hulin, A.; Sauve, C.; Bernier-Combes, A.; Brun-Buisson, C.; Lesprit, P. Inappropriate prescribing of aminoglycosides: Risk factors and impact of an antibiotic control team. J. Antimicrob. Chemother. 2006, 58, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Davey, P.; Marwick, C.A.; Scott, C.L.; Charani, E.; McNeil, K.; Brown, E.; Gould, I.M.; Ramsay, C.R.; Michie, S. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst. Rev. 2017, 2, Cd003543. [Google Scholar] [CrossRef]
- Schuts, E.C.; Hulscher, M.; Mouton, J.W.; Verduin, C.M.; Stuart, J.; Overdiek, H.; van der Linden, P.D.; Natsch, S.; Hertogh, C.; Wolfs, T.F.W.; et al. Current evidence on hospital antimicrobial stewardship objectives: A systematic review and meta-analysis. Lancet Infect. Dis. 2016, 16, 847–856. [Google Scholar] [CrossRef]
- de Kraker, M.E.; Stewardson, A.J.; Harbarth, S. Will 10 Million People Die a Year due to Antimicrobial Resistance by 2050? PLoS Med. 2016, 13, e1002184. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; 2016. Available online: https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf (accessed on 20 September 2022).
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Dellit, T.H.; Owens, R.C.; McGowan, J.E., Jr.; Gerding, D.N.; Weinstein, R.A.; Burke, J.P.; Huskins, W.C.; Paterson, D.L.; Fishman, N.O.; Carpenter, C.F.; et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin. Infect. Dis. 2007, 44, 159–177. [Google Scholar] [CrossRef]
- MacDougall, C.; Polk, R.E. Antimicrobial stewardship programs in health care systems. Clin. Microbiol. Rev. 2005, 18, 638–656. [Google Scholar] [CrossRef]
- Ohl, C.A.; Dodds Ashley, E.S. Antimicrobial stewardship programs in community hospitals: The evidence base and case studies. Clin. Infect. Dis. 2011, 53 (Suppl. S1), S23–S28, quiz S29–30. [Google Scholar] [CrossRef]
- Barnett, M.L.; Linder, J.A. Antibiotic prescribing to adults with sore throat in the United States, 1997-2010. JAMA Intern. Med. 2014, 174, 138–140. [Google Scholar] [CrossRef]
- Honda, H.; Ohmagari, N.; Tokuda, Y.; Mattar, C.; Warren, D.K. Antimicrobial Stewardship in Inpatient Settings in the Asia Pacific Region: A Systematic Review and Meta-analysis. Clin. Infect. Dis. 2017, 64, S119–S126. [Google Scholar] [CrossRef]
- Hu, Y.; Walley, J.; Chou, R.; Tucker, J.D.; Harwell, J.I.; Wu, X.; Yin, J.; Zou, G.; Wei, X. Interventions to reduce childhood antibiotic prescribing for upper respiratory infections: Systematic review and meta-analysis. J. Epidemiol. Community Health 2016, 70, 1162–1170. [Google Scholar] [CrossRef]
- Karanika, S.; Paudel, S.; Grigoras, C.; Kalbasi, A.; Mylonakis, E. Systematic Review and Meta-analysis of Clinical and Economic Outcomes from the Implementation of Hospital-Based Antimicrobial Stewardship Programs. Antimicrob. Agents Chemother. 2016, 60, 4840–4852. [Google Scholar] [CrossRef]
- Saha, S.K.; Hawes, L.; Mazza, D. Effectiveness of interventions involving pharmacists on antibiotic prescribing by general practitioners: A systematic review and meta-analysis. J. Antimicrob. Chemother. 2019, 74, 1173–1181. [Google Scholar] [CrossRef] [PubMed]
- Thoolen, B.; de Ridder, D.; van Lensvelt-Mulders, G. Patient-oriented interventions to improve antibiotic prescribing practices in respiratory tract infections: A meta-analysis. Health Psychol. Rev. 2012, 6, 92–112. [Google Scholar] [CrossRef]
- Aldeyab, M.A.; Kearney, M.P.; Scott, M.G.; Aldiab, M.A.; Alahmadi, Y.M.; Darwish Elhajji, F.W.; Magee, F.A.; McElnay, J.C. An evaluation of the impact of antibiotic stewardship on reducing the use of high-risk antibiotics and its effect on the incidence of Clostridium difficile infection in hospital settings. J. Antimicrob. Chemother. 2012, 67, 2988–2996. [Google Scholar] [CrossRef] [PubMed]
- Bauerle, W.; O’Laughlin, M.; Evans, H. Improving Antibiotic Stewardship in Acute Appendicitis through Risk-Based Empiric Treatment Selection. Surg. Infect. 2022, 23, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Bornard, L.; Dellamonica, J.; Hyvernat, H.; Girard-Pipau, F.; Molinari, N.; Sotto, A.; Roger, P.M.; Bernardin, G.; Pulcini, C. Impact of an assisted reassessment of antibiotic therapies on the quality of prescriptions in an intensive care unit. Med. Mal. Infect. 2011, 41, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Cappanera, S.; Tiri, B.; Priante, G.; Sensi, E.; Scarcella, M.; Bolli, L.; Costantini, M.; Andreani, P.; Sodo, S.; Martella, L.A.; et al. Educational ICU antimicrobial stewardship model: The daily activities of the AMS team over a 10-month period. Infez. Med. 2019, 27, 251–257. [Google Scholar]
- Carratalà, J.; Garcia-Vidal, C.; Ortega, L.; Fernández-Sabé, N.; Clemente, M.; Albero, G.; López, M.; Castellsagué, X.; Dorca, J.; Verdaguer, R.; et al. Effect of a 3-step critical pathway to reduce duration of intravenous antibiotic therapy and length of stay in community-acquired pneumonia: A randomized controlled trial. Arch. Intern. Med. 2012, 172, 922–928. [Google Scholar] [CrossRef]
- Chowdhury, S.; Sastry, A.; Sureshkumar, S.; Cherian, A.; Sistla, S.; Rajashekar, D. The impact of antimicrobial stewardship programme on regulating the policy adherence and antimicrobial usage in selected intensive care units in a tertiary care center-A prospective interventional study. Indian J. Med. Microbiol. 2020, 38, 362–370. [Google Scholar] [CrossRef]
- Hwang, H.; Kim, B. Impact of an infectious diseases specialist-led antimicrobial stewardship programmes on antibiotic use and antimicrobial resistance in a large Korean hospital. Sci. Rep. 2018, 8, 14757. [Google Scholar] [CrossRef]
- Leo, F.; Bannehr, M.; Valenta, S.; Lippeck, M.; Pachl, S.; Steib-Bauert, M.; Semper, H.; Grohé, C. Impact of a computerized physician order entry (CPOE)-based antibiotic stewardship intervention on the treatment duration for pneumonia and COPD exacerbations. Respir. Med. 2021, 186, 106546. [Google Scholar] [CrossRef]
- Lesprit, P.; Landelle, C.; Brun-Buisson, C. Clinical impact of unsolicited post-prescription antibiotic review in surgical and medical wards: A randomized controlled trial. Clin. Microbiol. Infect. 2013, 19, E91–E97. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rattanaumpawan, P.; Sutha, P.; Thamlikitkul, V. Effectiveness of drug use evaluation and antibiotic authorization on patients’ clinical outcomes, antibiotic consumption, and antibiotic expenditures. Am. J. Infect. Control 2010, 38, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Seidelman, J.L.; Turner, N.A.; Wrenn, R.H.; Sarubbi, C.; Anderson, D.J.; Sexton, D.J.; Moehring, R.W. Impact of Antibiotic Stewardship Rounds in the Intensive Care Setting: A prospective cluster-randomized crossover study. Clin. Infect. Dis. 2021, 74, 1986–1992. [Google Scholar] [CrossRef]
- Trinh, T.D.; Strnad, L.; Damon, L.; Dzundza, J.H.; Graff, L.R.; Griffith, L.M.; Hilts-Horeczko, A.; Olin, R.; Shenoy, S.; Devoe, C.; et al. Reductions in vancomycin and meropenem following the implementation of a febrile neutropenia management algorithm in hospitalized adults: An interrupted time series analysis. Infect. Control Hosp. Epidemiol. 2021, 42, 1090–1097. [Google Scholar] [CrossRef]
- Walsh, T.L.; Bremmer, D.N.; Moffa, M.A.; Chan-Tompkins, N.H.; Murillo, M.A.; Chan, L.; Burkitt, M.J.; Konopka, C.I.; Watson, C.; Trienski, T.L. Effect of Antimicrobial Stewardship Program Guidance on the Management of Uncomplicated Skin and Soft Tissue Infections in Hospitalized Adults. Mayo Clin. Proc. Innov. Qual. Outcomes 2017, 1, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Bishop, P.A.; Isache, C.; McCarter, Y.S.; Smotherman, C.; Gautam, S.; Jankowski, C.A. Clinical impact of a pharmacist-led antimicrobial stewardship initiative evaluating patients with Clostridioides difficile colitis. J. Investig. Med. 2020, 68, 888–892. [Google Scholar] [CrossRef]
- Didiodato, G.; McArthur, L.; Beyene, J.; Smieja, M.; Thabane, L. Evaluating the impact of an antimicrobial stewardship program on the length of stay of immune-competent adult patients admitted to a hospital ward with a diagnosis of community-acquired pneumonia: A quasi-experimental study. Am. J. Infect. Control 2016, 44, e73–e79. [Google Scholar] [CrossRef]
- Doyle, D.; McDonald, G.; Pratt, C.; Rehan, Z.; Benteau, T.; Phillips, J.; Daley, P. Impact of a mobile decision support tool on antimicrobial stewardship indicators in St. John’s, Canada. PLoS ONE 2021, 16, e0252407. [Google Scholar] [CrossRef]
- Du, Y.; Li, J.; Wang, X.; Peng, X.; Wang, X.; He, W.; Li, Y.; Wang, X.; Yang, Q.; Zhang, X. Impact of a Multifaceted Pharmacist-Led Intervention on Antimicrobial Stewardship in a Gastroenterology Ward: A Segmented Regression Analysis. Front. Pharmacol. 2020, 11, 442. [Google Scholar] [CrossRef]
- Dunn, K.; O’Reilly, A.; Silke, B.; Rogers, T.; Bergin, C.; Dunn, K.; O’Reilly, A.; Silke, B.; Rogers, T.; Bergin, C. Implementing a pharmacist-led sequential antimicrobial therapy strategy: A controlled before-and-after study. Int. J. Clin. Pharm. 2011, 33, 208–214. [Google Scholar] [CrossRef]
- Elligsen, M.; Walker, S.A.; Pinto, R.; Simor, A.; Mubareka, S.; Rachlis, A.; Allen, V.; Daneman, N. Audit and feedback to reduce broad-spectrum antibiotic use among intensive care unit patients: A controlled interrupted time series analysis. Infect. Control Hosp. Epidemiol. 2012, 33, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Foolad, F.; Huang, A.M.; Nguyen, C.T.; Colyer, L.; Lim, M.; Grieger, J.; Li, J.; Revolinski, S.; Mack, M.; Gandhi, T.; et al. A multicentre stewardship initiative to decrease excessive duration of antibiotic therapy for the treatment of community-acquired pneumonia. J. Antimicrob. Chemother. 2018, 73, 1402–1407. [Google Scholar] [CrossRef]
- Fukuda, T.; Watanabe, H.; Ido, S.; Shiragami, M. Contribution of antimicrobial stewardship programs to reduction of antimicrobial therapy costs in community hospital with 429 Beds --before-after comparative two-year trial in Japan. J. Pharm. Policy Pract. 2014, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Golali, E.; Sistanizad, M.; Salamzadeh, J.; Haghighi, M.; Solooki, M. Antibiotic prescribing trends before and after implementation of an audit and feedback program in internal ward of a tertiary hospital in tehran. Iran. J. Pharm. Res. 2019, 18, 2136–2143. [Google Scholar] [CrossRef] [PubMed]
- Heng, S.T.; Wong, J.; Young, B.; Tay, H.L.; Tan, S.H.; Yap, M.Y.; Teng, C.B.; Ang, B.; Lee, T.H.; Tan, H.L.; et al. Effective Antimicrobial StewaRdship StrategIES (ARIES): Cluster randomized trial of computerized decision support system and prospective review and feedback. Open Forum Infect. Dis. 2020, 7, ofaa254. [Google Scholar] [CrossRef]
- Khdour, M.R.; Hallak, H.O.; Aldeyab, M.A.; Nasif, M.A.; Khalili, A.M.; Dallashi, A.A.; Khofash, M.B.; Scott, M.G. Impact of antimicrobial stewardship programme on hospitalized patients at the intensive care unit: A prospective audit and feedback study. Br. J. Clin. Pharmacol. 2018, 84, 708–715. [Google Scholar] [CrossRef]
- Lowe, C.F.; Payne, M.; Puddicombe, D.; Mah, A.; Wong, D.; Kirkwood, A.; Hull, M.W.; Leung, V. Antimicrobial stewardship for hospitalized patients with viral respiratory tract infections. Am. J. Infect. Control 2017, 45, 872–875. [Google Scholar] [CrossRef]
- Magedanz, L.; Silliprandi, E.M.; dos Santos, R.P. Impact of the pharmacist on a multidisciplinary team in an antimicrobial stewardship program: A quasi-experimental study. Int. J. Clin. Pharm. 2012, 34, 290–294. [Google Scholar] [CrossRef]
- Matono, T.; Umeda, Y.; Uchida, M.; Koga, H.; Kanatani, N.; Furuno, Y.; Yamashita, T.; Nakamura, K. Impact of an infectious disease physician-led carbapenem postprescription feedback on prescribing behavior in a Japanese tertiary hospital: A before-after study. J. Infect. Chemother. 2021, 27, 439–444. [Google Scholar] [CrossRef]
- Talpaert, M.J.; Gopal Rao, G.; Cooper, B.S.; Wade, P. Impact of guidelines and enhanced antibiotic stewardship on reducing broad-spectrum antibiotic usage and its effect on incidence of Clostridium difficile infection. J. Antimicrob. Chemother. 2011, 66, 2168–2174. [Google Scholar] [CrossRef]
- Thom, K.A.; Tamma, P.D.; Harris, A.D.; Dzintars, K.; Morgan, D.J.; Li, S.; Pineles, L.; Srinivasan, A.; Avdic, E.; Cosgrove, S.E. Impact of a prescriber-driven antibiotic time-out on antibiotic use in hospitalized patients. Clin. Infect. Dis. 2019, 68, 1581–1584. [Google Scholar] [CrossRef] [PubMed]
- van den Bergh, D.; Messina, A.P.; Goff, D.A.; van Jaarsveld, A.; Coetzee, R.; de Wet, Y.; Bronkhorst, E.; Brink, A.; Mendelson, M.; Richards, G.A.; et al. A pharmacist-led prospective antibiotic stewardship intervention improves compliance to community-acquired pneumonia guidelines in 39 public and private hospitals across South Africa. Int. J. Antimicrob. Agents 2020, 56, 106189. [Google Scholar] [CrossRef] [PubMed]
- Yeo, C.L.; Chan, D.S.; Earnest, A.; Wu, T.S.; Yeoh, S.F.; Lim, R.; Jureen, R.; Fisher, D.; Hsu, L.Y. Prospective audit and feedback on antibiotic prescription in an adult hematology-oncology unit in Singapore. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Sadeq, A.A.; Shamseddine, J.M.; Babiker, Z.O.E.; Nsutebu, E.F.; Moukarzel, M.B.; Conway, B.R.; Hasan, S.S.; Conlon-Bingham, G.M.; Aldeyab, M.A. Impact of Multidisciplinary Team Escalating Approach on Antibiotic Stewardship in the United Arab Emirates. Antibiotics 2021, 10, 1289. [Google Scholar] [CrossRef]
- Durante, J.; McBride, J.; Miklo, L.; Killeen, M.; Creech, C. Implementation of an Educational Intervention to Reduce Inappropriate Antibiotic Use in Upper Respiratory Infections. J. Dr. Nurs. Pract. 2017, 10, 45–49. [Google Scholar] [CrossRef]
- Gonzales, R.; Anderer, T.; McCulloch, C.E.; Maselli, J.H.; Bloom, F.J., Jr.; Graf, T.R.; Stahl, M.; Yefko, M.; Molecavage, J.; Metlay, J.P. A cluster randomized trial of decision support strategies for reducing antibiotic use in acute bronchitis. JAMA Intern. Med. 2013, 173, 267–273. [Google Scholar] [CrossRef]
- Légaré, F.; Labrecque, M.; Cauchon, M.; Castel, J.; Turcotte, S.; Grimshaw, J. Training family physicians in shared decision-making to reduce the overuse of antibiotics in acute respiratory infections: A cluster randomized trial. CMAJ 2012, 184, E726–E734. [Google Scholar] [CrossRef]
- Linder, J.A.; Schnipper, J.L.; Tsurikova, R.; Yu, D.T.; Volk, L.A.; Melnikas, A.J.; Palchuk, M.B.; Olsha-Yehiav, M.; Middleton, B. Electronic health record feedback to improve antibiotic prescribing for acute respiratory infections. Am. J. Manag. Care 2010, 16, e311–e319. [Google Scholar]
- Little, P.; Moore, M.V.; Turner, S.; Rumsby, K.; Warner, G.; Lowes, J.A.; Smith, H.; Hawke, C.; Leydon, G.; Arscott, A.; et al. Effectiveness of five different approaches in management of urinary tract infection: Randomised controlled trial. BMJ (Online) 2010, 340, 405. [Google Scholar] [CrossRef]
- Manns, B.; Laupland, K.; Tonelli, M.; Gao, S.; Hemmelgarn, B. Evaluating the impact of a novel restricted reimbursement policy for quinolone antibiotics: A time series analysis. BMC Health Serv. Res. 2012, 12, 290. [Google Scholar] [CrossRef]
- Wasylyshyn, A.I.; Kaye, K.S.; Chen, J.; Haddad, H.; Nagel, J.; Petrie, J.G.; Gandhi, T.N.; Petty, L.A. Improving antibiotic use for sinusitis and upper respiratory tract infections: A virtual-visit antibiotic stewardship initiative. Infect. Control Hosp. Epidemiol. 2022, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Worrall, G.; Kettle, A.; Graham, W.; Hutchinson, J. Postdated versus usual delayed antibiotic prescriptions in primary care: Reduction in antibiotic use for acute respiratory infections? Can. Fam. Physician 2010, 56, 1032–1036. [Google Scholar] [PubMed]
- Burns, K.W.; Johnson, K.M.; Pham, S.N.; Egwuatu, N.E.; Dumkow, L.E. Implementing outpatient antimicrobial stewardship in a primary care office through ambulatory care pharmacist-led audit and feedback. J. Am. Pharm. Assoc. 2020, 60, e246–e251. [Google Scholar] [CrossRef] [PubMed]
- Choi, P.W.; Benzer, J.A.; Coon, J.; Egwuatu, N.E.; Dumkow, L.E. Impact of pharmacist-led selective audit and feedback on outpatient antibiotic prescribing for UTIs and SSTIs. Am. J. Health-Syst. Pharm. 2021, 78, S62–S69. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Urrusuno, R.; Meseguer Barros, C.M.; Benavente Cantalejo, R.S.; Hevia, E.; Martino, C.S.; Aldasoro, A.I.; Mora, J.L.; Navas, A.L.; de la Pisa, B.P. Successful improvement of antibiotic prescribing at Primary Care in Andalusia following the implementation of an antimicrobial guide through multifaceted interventions: An interrupted time-series analysis. PLoS ONE 2020, 15, e0233062. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, T.C.; Irwin, A.; Coombs, L.; Dealleaume, L.; Ross, S.E.; Rozwadowski, J.; Webster, B.; Dickinson, L.M.; Sabel, A.L.; Mackenzie, T.D.; et al. Effects of clinical pathways for common outpatient infections on antibiotic prescribing. Am. J. Med. 2013, 126, 327–335.e312. [Google Scholar] [CrossRef] [PubMed]
- March-López, P.; Madridejos, R.; Tomas, R.; Boix, L.; Arcenillas, P.; Gómez, L.; Padilla, E.; Xercavins, M.; Martinez, L.; Riera, M.; et al. Impact of a Multifaceted Antimicrobial Stewardship Intervention in a Primary Health Care Area: A Quasi-Experimental Study. Front. Pharmacol. 2020, 11, 398. [Google Scholar] [CrossRef]
- May, L.; Nguyen, M.H.; Trajano, R.; Tancredi, D.; Aliyev, E.R.; Mooso, B.; Anderson, C.; Ondak, S.; Yang, N.; Cohen, S.; et al. A multifaceted intervention improves antibiotic stewardship for skin and soft tissues infections. Am. J. Emerg. Med. 2021, 46, 374–381. [Google Scholar] [CrossRef]
- Slekovec, C.; Leroy, J.; Vernaz-Hegi, N.; Faller, J.P.; Sekri, D.; Hoen, B.; Talon, D.; Bertrand, X. Impact of a region wide antimicrobial stewardship guideline on urinary tract infection prescription patterns. Int. J. Clin. Pharm. 2012, 34, 325–329. [Google Scholar] [CrossRef]
- Vinnard, C.; Linkin, D.R.; Localio, A.R.; Leonard, C.E.; Teal, V.L.; Fishman, N.O.; Hennessy, S. Effectiveness of interventions in reducing antibiotic use for upper respiratory infections in ambulatory care practices. Popul. Health Manag. 2013, 16, 22–27. [Google Scholar] [CrossRef]
- Aldeyab, M.A.; López-Lozano, J.M.; Gould, I.M. Global antibiotics use and resistance. In Global Pharmaceutical Policy; Babar, Z.U.D., Ed.; Palgrave Macmillan: Singapore, 2020; pp. 331–344. ISBN 978-981-15-2723-4. [Google Scholar]
- Jirjees, F.J.; Al-Obaidi, H.J.; Sartaj, M.; ConlonBingham, G.; Farren, D.; Scott, M.G.; Gould, I.M.; López-Lozano, J.M.; Aldeyab, M.A. Antibiotic Use and Resistance in Hospitals: Time-Series Analysis Strategy for Determining and Prioritising Interventions. Hosp. Pharm. Eur. 2020, 95, 13–19. Available online: https://hospitalpharmacyeurope.com/news/reviews-research/antibiotic-use-and-resistance-in-hospitals-time-series-analysis-strategy-for-determining-and-prioritising-interventions/ (accessed on 20 September 2022).
- Raban, M.Z.; Gasparini, C.; Li, L.; Baysari, M.T.; Westbrook, J.I. Effectiveness of interventions targeting antibiotic use in long-term aged care facilities: A systematic review and meta-analysis. BMJ Open 2020, 10, e028494. [Google Scholar] [CrossRef] [PubMed]
- Laka, M.; Milazzo, A.; Merlin, T. Can evidence-based decision support tools transform antibiotic management? A systematic review and meta-analyses. J. Antimicrob. Chemother. 2020, 75, 1099–1111. [Google Scholar] [CrossRef]
- Lee, C.F.; Cowling, B.J.; Feng, S.; Aso, H.; Wu, P.; Fukuda, K.; Seto, W.H. Impact of antibiotic stewardship programmes in Asia: A systematic review and meta-analysis. J. Antimicrob. Chemother. 2018, 73, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, R.K.; Gillani, S.W.; Saeed, M.W.; Vippadapu, P.; Alzaabi, M.J.M.A. Impact of pharmacist-led services on antimicrobial stewardship programs: A meta-analysis on clinical outcomes. J. Pharm. Health Serv. Res. 2021, 12, 615–625. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).