Abstract
Mn-Ce-Zr-O catalysts doped with varying Mn content were prepared and assessed for the catalytic combustion of chlorobenzene (CB). Nanosized MCZ-0.67 catalyst with amorphous phase exhibited a high and stable catalytic activity among the studied catalysts, achieving 90% CB conversion at 226 °C and withstanding stability tests, including time-based stability and the successive influence of various operating conditions. Meanwhile, the MCZ-0.67 catalyst used showed good recyclability by calcination in air. Characterization results suggested that Mn doping played a dominant role in improving the catalytic performance, resulting in larger surface area, better redox properties and greater amounts of surface active oxygen. In addition, the introduction of Zr was also indispensable for maintaining the good catalytic performance of catalysts. Finally, trace amounts of polychlorinated by-products during CB oxidation were monitored and the oxidation process was discussed.
1. Introduction
Chlorinated volatile organic compounds (CVOCs), widely used as solvents, additives and raw materials in industry, are considered serious hazards to human health and the environment owing to their high toxicity, volatility and durability [1,2]. It is therefore of great significance to effectively control the environmental emissions of these compounds. Compared with conventional thermal combustion (operating above 1000 °C), catalytic combustion is a promising heterogeneous catalytic oxidation technology for the elimination of CVOCs due to its much lower energy consumption (operating below 550 °C) and limited secondary pollution (avoiding the production of NOx and dioxins) [3]. However, the development of this technology is dependent on the advent of high-performance catalysts, which are regarded as the crucial enablers in most chemical reaction processes [4].
To date, despite much research on various types of catalysts, including supported noble metals [5,6], transition metal oxides [7,8] and zeolites [9,10] for CVOCs elimination, the occurrence of chlorine poisoning, the mass production of polychlorinated by-products and the low selectivity of HCl remain “bottlenecks” for the practical application of such catalysts. Limited natural resources and their susceptibility to chlorine poisoning restrict the widespread use of noble metals [11] despite their excellent activity and selectivity. Similarly, coking and chlorination reactions readily occur in zeolite catalytic systems [7,12]. Conversely, transition metal oxides possess a better thermal stability, stronger anti-poisoning ability and lower cost, thus arousing widespread interest [13,14]. In recent years, ceria has been used as a good promoter for the activity and structure of transition metal oxides owing to its high oxygen storage capacity (OSC) and relatively easy redox cycle between Ce4+ and Ce3+ [15,16]. Specifically, the redox capability and dispersity of transition metal oxides are modified along with the partial substitution of Ce4+ in the CeO2 lattice with metal ions. The resulting improvements in oxygen vacancy concentration and oxygen mobility contribute to the elimination of adsorbed chlorine species, and thus to the enhancement of catalytic activity and stability for CVOCs elimination [17].
Of the Ce-based transition metal oxides, a CeO2-ZrO2 solid solution, widely applied in the purification of vehicle exhaust [18], the selective catalytic reduction (SCR) of NOx [19] and the catalytic oxidation of VOCs [20], was found to exhibit a high OSC and thermal stability after the doping of a proper amount of ZrO2 into the CeO2 lattice. Gutiérrez-Ortiz et al. [21] prepared a series of CexZr1−xO2 solid solutions for the elimination of chlorinated aliphatic hydrocarbons, and showed that the catalytic performance was dominated by the increased surface acidity and oxygen mobility following Zr doping. However, considerable amounts of Cl2 resulting from the oxidation of HCl (Deacon reaction) were detected during the oxidation of chlorinated reactants. Moreover, due to the relatively low redox ability of ZrO2, the high reaction temperature required for the elimination of CVOCs by CeO2-ZrO2 mixed oxide catalysts was unsatisfactory [22].
MnOx compounds are highly active due to their various labile oxygen species and high efficiency in reaction/oxidation cycles [23] and have been widely used in the catalytic removal of various pollutants, including benzene series [24], chlorinated aromatic hydrocarbons [25], nitrogen oxides [26] and aldehydes [27]. In particular, MnOx-CeO2 solid solution, as an environment-friendly catalyst with high catalytic efficiency, has become the focus of research on multipurpose catalysts [28]. Nevertheless, in the elimination of CVOCs, despite the activity and chlorine resistance of CeO2 having been greatly improved by Mn doping, the achievement of a high and stable catalytic activity still requires higher reaction temperature [29,30]. In addition, trace amounts of polychlorinated by-products resulting from the incomplete oxidation of CVOCs need to be further monitored and assessed.
Considering the respective advantages of MnOx and CeO2-ZrO2 catalysts, Mn-Ce-Zr-O ternary catalyst seems to have promising prospects in catalysis. Shen et al. [19] prepared a series of Mn/Ce-Zr-based catalysts for the SCR of NO. Results showed that the molar ratio of Ce to Zr had a significant influence on the catalytic activity, and MnOx/Ce0.5Zr0.5O2 catalyst possessed the best NO conversion. However, the influence of Mn doping on the catalytic performance of CeO2-ZrO2 catalysts has seldom been systematically studied, especially for the elimination of CVOCs. Herein, Mn-Ce-Zr-O catalysts doped with a varying Mn content were synthesized and used for the elimination of CB, which is a typical CVOCs model widely used in the evaluation of catalysts [31,32]. The physicochemical properties of the prepared catalysts were systematically assessed by various material characterization techniques. The successive influence of CB concentration, space velocity and water vapor on CB destruction was also assessed in the stability study. Moreover, trace amounts of organic by-products during CB oxidation were monitored and the oxidation process was discussed.
2. Materials and Methods
2.1. Catalyst Preparation
Mn-Ce-Zr-O catalysts were synthesized by the sol-gel method as follows: stoichiometric amounts of Mn(NO3)2 (SCRC, 50 wt% solution), Ce(NO3)3·6H2O (SCRC, 99%), ZrO(NO3)2·5H2O (Aladdin, 99.5%) and citric acid (SCRC, 99.5%, citric acid/(Mn + Ce + Zr) = 0.3, molar ratio) were dissolved in deionized water and slowly heated to 80 °C, maintaining this temperature with continuous vigorous stirring until gelation occurred. The obtained gel was dried overnight at 110 °C and then calcined in a muffle furnace at 200 °C for 2 h and further at 550 °C for 5 h. The synthesized catalysts were denoted as MCZ-x (x = 0, 0.33, 0.50, 0.67 and 0.80), where x is the molar ratio of Mn/(Mn + Ce + Zr) and the molar ratio of Ce/Zr was maintained as 1. As a reference, a Mn0.67Ce0.33 catalyst (denoted as MC-0.67) was also prepared by the same method described above. Before use, all synthesized catalysts were finally made into 40–60 mesh particles through pressing, crushing and sieving.
2.2. Catalyst Characterization
Powder X-ray diffraction (XRD) patterns were recorded on a D8-ADVANCE diffractometer (Bruker, Karlsruhe, Germany) equipped with Cu Kα radiation source (λ = 0.15418 nm). The 2θ was measured from 20° to 80° at a scanning rate of 0.04 ° min−1.
N2 adsorption-desorption experiments were carried out on a Tristar II 3020 instrument (Micromeritics, Atlanta, USA) at −196 °C. Prior to measurements, catalysts were degassed at 200 °C under vacuum for 2 h. The Brunauer–Emmett–Teller (BET) and Barrett–Joyner–Halenda (BJH) methods were employed for the calculation of specific surface area and pore-size distribution, respectively.
H2-temperature programmed reduction (H2-TPR) tests were measured under a mixed stream of H2 (10 vol%) and Ar (90 vol%), and 100 mg of the catalyst was tested in the range of 50–800 °C with a heating ramp of 10 °C min−1. During the tests, a thermal conductivity detector (TCD) was employed to monitor the consumed H2.
X-ray photoelectron spectroscopy (XPS) measurements were carried out on a PHI 5000 VersaProbe instrument (Ulvac-Phi, Chigasaki, Japan) equipped with Al Kα (hv = 1486.6 eV) radiation source. Binding energies of the studied atoms were calibrated by 284.6 eV of C 1s. The obtained spectra were deconvoluted by peak fitting using the Shirley background and Gaussian–Lorentzian function provided by the XPSPeak software.
O2-temperature programmed desorption (O2-TPD) tests were carried out on an Auto Chem II 2920 instrument (Micromeritics, Atlanta, USA), and 100 mg of the catalyst was tested in the range of 50–700 °C under a He stream at a heating ramp of 10 °C min−1. During the tests, a TCD detector was employed to monitor the desorbed O2. Prior to measurements, catalysts were treated at 300 °C for 2 h under a He stream and then at 50 °C for 2 h under a mixed stream of O2 (5 vol%) and He (95 vol%).
Scanning electron microscopy (SEM) images were taken on a Hitachi S4800 apparatus.
Transmission electron microscopy (TEM) and scanning transmission electron microscopy (STEM) images were taken at a voltage of 200 kV on a JEM-2100F microscope (JEOL, Akishima, Japan) equipped with an energy dispersive X-ray spectrometer (EDX). Powder catalysts were previously treated by dispersing them in ethanol and then depositing on carbon-coated copper grid.
2.3. Catalytic Performance Evaluation
Activity tests were carried out on a conventional fixed-bed microreactor. In each test, 200 mg of the catalyst was packed in the middle of the quartz tube (4 mm i.d.). N2 was used to transport the CB vapor by bubbling the CB solution maintained at 40 °C. The reaction gas, consisting of 1000 ppm CB and 21 vol% O2, was obtained by mixing three gas streams of CB vapor, O2 and N2 (balance) in a mixer. All gas streams were accurately monitored by mass flowmeters and the gas hourly space velocity (GHSV) was set at a typical value of 20,000 h−1. A thermocouple was employed to control the reaction temperature in the range of 100–300 °C. At given temperatures, the exhaust gases were detected on-line using a gas chromatograph (FULI 9790) equipped with two flame ionization detectors (FID), one of which was used for the analysis of CB quantitatively and the other for the measurement of CO2 and CO by means of a methane-converting furnace (Ni catalyst). The concentrations of HCl and Cl2 were determined by bubbling the off-gas through a 0.0125 mol L−1 NaOH solution for 30 min. Ion chromatograph (Thermo Scientific ICS-900) was used to measure the concentration of Cl− and chemical titration with ferrous ammonium sulphate (FAS) using N,N-diethyl-p-phenylenediamine (DPD) as an indicator was employed to determine the concentration of Cl2 in the bubbled solution.
The stability experiments of the prepared catalysts were performed at 300 °C under the same evaluation conditions described above. The water vapor environment was obtained by bubbling the deionized water with a N2 stream.
Assessment of the CB decomposition products was performed by using hexane to absorb the various gaseous organics in the off-gas for 30 min. After concentration, the hexane was analyzed by a gas chromatography-mass spectrometer (GC-MS, Agilent 7890B-5977A) using a weak polar DB-5MS capillary column.
3. Results and Discussion
3.1. X-ray Diffraction (XRD) Characterization and N2 Adsorption-Desorption Analysis
XRD patterns of MCZ-x and MC-0.67 catalysts showed that the crystallinity of the prepared catalysts was significantly affected by Mn content (Figure 1). For the MCZ-x (x = 0, 0.33, 0.50) and MC-0.67 catalysts, only diffraction peaks corresponding to the cubic fluorite-like structure of CeO2 were observed, with the lattice parameters (Table 1) being smaller than 5.41 Å of pure CeO2 (JCPDS #43-1002). Indeed, the ionic radii of Mnn+ (Mn2+ = 0.083 nm, Mn3+ = 0.065 nm, Mn4+ = 0.053 nm) and Zr4+ (0.084 nm) are both smaller than that of Ce4+ (0.097 nm) [29,33]. Thus, the shrinkage of CeO2 unit cell indicates that parts of Ce4+ ions in CeO2 lattice were replaced by Mnn+ and Zr4+ ions to form a solid solution. Remaining Mn- and Zr-related oxides may be uniformly distributed on the surface of CeO2 in a poor crystalline state. At the 2θ range from 24° to 40°, only a weak and widened peak was observed for the MCZ-0.67 catalyst, indicating that it exists in an amorphous state. With the further increase of Mn content, weak diffraction peaks indexed to Mn2O3 (JCPDS #24-0508) were observed for the MCZ-0.80 catalyst, showing that the Mn content has exceeded the maximum that the CeO2 lattice can hold and therefore Mn2O3 phase is formed during calcination.
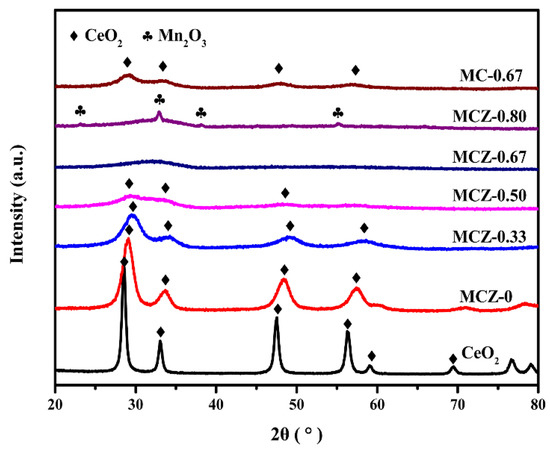
Figure 1.
X-ray diffraction (XRD) patterns of MCZ-x (x = 0, 0.33, 0.50, 0.67, 0.80) and MC-0.67 catalysts.

Table 1.
XRD, N2 adsorption-desorption and H2-temperature programmed reduction (H2-TPR) characterization data.
As is known, a lower intensity diffraction peak indicates a reduction in crystallinity and reveals the generation of more lattice defects and smaller crystallite sizes [29,34]. Thus, from Figure 1, it can be inferred that the CeO2 lattice is constantly distorted with increasing Mn doping, resulting in a decrease in crystallite size (Table 1). Similarly, the specific surface area and pore volume increase, reaching a maximum when x = 0.67. This observation may be attributed to the production of smaller nanoparticles with an amorphous phase. Further, the addition of Zr promotes the transformation of the MC-0.67 catalyst from a solid solution to an amorphous material and leads to a doubling of the specific surface area. This significant increase may be related to the larger solubility of Zr (maximum of 75 at%) in the CeO2 lattice than that of Mn (maximum of 20 at%) [35], as improved solubility can lead to the formation of more lattice defects, which is beneficial for the increase of specific surface area.
With regards to N2 adsorption-desorption and pore size distribution, all catalysts prepared by the sol-gel method showed a typical irreversible type IV isotherm (Figure 2A), with the MCZ-0 and MCZ-0.33 catalysts displaying a hysteresis loop of type H3 and the remaining four catalysts displaying a hysteresis loop of type H2. Furthermore, the sizes of the formed pores were within the range of 2 to 10 nm (Figure 2B and Table 1), suggesting the presence of a mesoporous structure.
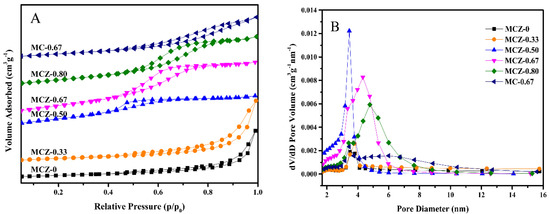
Figure 2.
N2 adsorption-desorption isotherms (A) and pore size distributions (B) of MCZ-x (x = 0, 0.33, 0.50, 0.67, 0.80) and MC-0.67 catalysts.
3.2. H2-Temperature Programmed Reduction (H2-TPR) Characterization
The influence of Mn doping on the redox properties of the catalysts was studied using H2-TPR (Figure 3). For the MCZ-0 catalyst, just one reduction peak appeared at 555 °C, ascribed to the reduction of Ce4+ on the catalyst surface [20] due to the difficulty of Zr4+ reduction at temperatures below 900 °C under this test condition [19]. After Mn doping, two overlapped reduction peaks at 351 °C and 478 °C were observed from the MCZ-0.33 catalyst, ascribed to the two-step reduction of MnO2/Mn2O3 leading to the sequential formation of Mn3O4 and MnO [36]. Similarly, with the increase of Mn content, the reduction peaks of Mn oxides moved towards lower temperatures, with the lowest reduction temperature of 321 °C being observed for the MCZ-0.67 catalyst. This was attributed to the generation of more lattice defects following Mn doping, which is conducive to the mobility of oxygen species and thus makes the catalyst more prone to reduction.
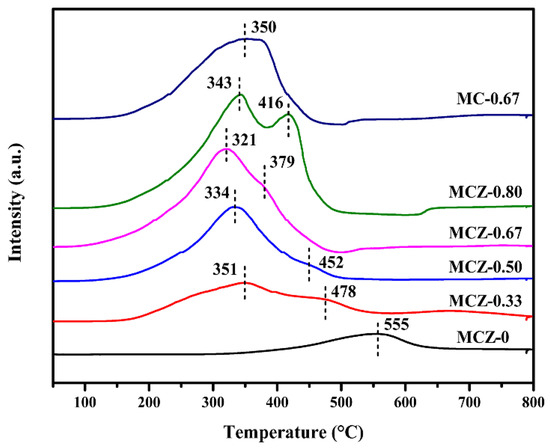
Figure 3.
H2-TPR profiles of MCZ-x (x = 0, 0.33, 0.50, 0.67, 0.80) and MC-0.67 catalysts.
Additionally, the introduction of Mn significantly increased the total H2 consumption, from 0.834 mmol g−1 for the MCZ-0 catalyst to 6.151 mmol g−1 for the MCZ-0.80 catalyst (Table 1), indicating that Mn oxides play a crucial role in the reducibility of MCZ-x catalysts. Nevertheless, despite an equal Mn content, the total H2 consumption of MC-0.67 catalyst (4.334 mmol g−1) was smaller than that of MCZ-0.67 catalyst (5.081 mmol g−1), suggesting that the introduction of Zr contributes to the generation of more reactive oxygen. It is generally acknowledged that a lower reduction temperature shows better redox properties and that greater H2 consumption improves the catalytic oxidation activity. Therefore, it can be predicted that MCZ-x catalysts with a high Mn content should have a good catalytic performance in CB elimination.
3.3. X-ray Photoelectron Spectroscopy (XPS) Characterization
The surface characteristics of the synthesized catalysts were analyzed using XPS measurements (Figure 4 and Table 2). The Ce 3d XPS spectra were fitted and deconvolved into 10 peaks (Figure 4A), wherein those labeled as v, v′′, v′′′, u, u′′ and u′′′ were ascribed to Ce4+ and those labeled as v′, v0, u′ and u0 were ascribed to Ce3+ [37]. These peaks provided evidence of the coexistence of Ce4+ and Ce3+ on the catalysts surface. With the increase of Mn content, the percentage of Ce3+/(Ce3+ + Ce4+) increased firstly and then decreased, reaching a maximum value of 34.3% for the MCZ-0.67 catalyst. In contrast, the Ce3+ concentration on the MC-0.67 catalyst surface was 31.3%; therefore, the introduction of Zr promoted the transformation of Ce4+ to Ce3+. As previously reported, the presence of Ce3+ ions indicates the generation of oxygen vacancy and creates a charge imbalance [38]; therefore, for maintaining electrical neutrality within the catalyst, more active oxygen species (i.e., O2−, O22−, O−) will be generated on the catalyst surface. Thus, it can be inferred that a catalyst with a higher percentage of Ce3+/(Ce3+ + Ce4+) could promote the rapid elimination of CB effectively because of the greater number of active oxygen species.
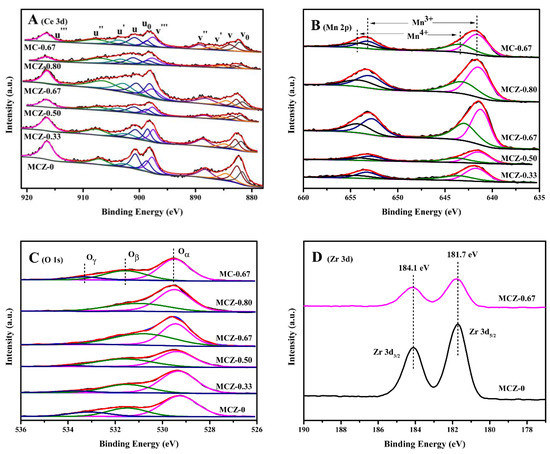
Figure 4.
X-ray photoelectron spectroscopy (XPS) spectra of Ce 3d (A); Mn 2p (B); O 1s (C) and Zr 3d (D) of MCZ-x (x = 0, 0.33, 0.50, 0.67, 0.80) and MC-0.67 catalysts.

Table 2.
XPS results of MCZ-x (x = 0, 0.33, 0.50, 0.67, 0.80) and MC-0.67 catalysts.
The measured Mn 2p XPS spectra showed four peaks fitted from the spin-orbit doublet of Mn 2p3/2 and Mn 2p1/2 at approximately 641, 653 eV and 643, 654 eV (Figure 4B), ascribed to Mn3+ and Mn4+ species on catalyst surface, respectively [39]. The actual molar ratio of Mn/(Mn + Ce + Zr) measured by XPS was close to the theoretical value (Table 2), indicating that Mn oxides are uniformly distributed on the surface and bulk of the prepared catalysts. It is noteworthy that the increase of Mn content is accompanied by the simultaneous increase in Mn4+/(Mn3+ + Mn4+) and Ce3+/(Ce3+ + Ce4+) percentages, except for MCZ-0.80 catalyst, indicating that the doping of Mn could promote the electron transfer from Mn3+ to Ce4+ on the catalyst surface. As previously reported, more Mn4+ ions could offer more sufficient active sites during the CB oxidation, since the oxidation reaction usually takes place through the redox cycles between high and low valence cations [40]. For the MCZ-0.80 catalyst, the lower percentage of Mn4+/(Mn3+ + Mn4+) may be attributed to the formation of more Mn2O3 phase during the calcination process, as evidenced by XRD characterization.
The O 1s XPS spectra obtained were fitted into three peaks (Figure 4C), with those at approximately 529.3–529.5 eV being ascribed to lattice oxygen (O2−, labeled as Oα), and those at approximately 530.8–531.6 eV and above 533.0 eV being ascribed to surface chemisorbed oxygen (O2− and O−, labeled as Oβ) and hydroxyl species and/or chemisorbed water species (labeled as Oγ), respectively [41]. It can be observed that the percentage of Oβ/(Oα + Oβ + Oγ) experiences a similar variation tendency to that of Ce3+ concentration as a result of Mn doping (Table 2), confirming the analysis of surface active oxygen species in Ce 3d XPS characterization. As is known, surface chemisorbed oxygen is vital for most catalytic oxidation reactions [33,42]. Therefore, compared with the other studied catalysts herein, the high Oβ concentration on the MCZ-0.67 catalyst surface could be responsible for the enhancement in catalytic performance for CB elimination. Likewise, the introduction of Zr could likely promote the generation of more chemisorbed oxygen on the MCZ-0.67 catalyst surface compared to that on the MC-0.67 catalyst surface (Table 2), in agreement with TPR characterization.
The Zr 3d XPS spectra of MCZ-0 and MCZ-0.67 catalysts exhibited two peaks at 181.7 eV and 184.1 eV (Figure 4D), ascribed to Zr 3d5/2 and Zr 3d3/2, respectively [43], confirming the solo presence of Zr4+ ions in the prepared catalysts [42]. As can be observed, for MCZ-0 and MCZ-0.67 catalysts, the binding energy position of Zr is highly similar, indicating that the chemical state of Zr is stable and hardly affected by the introduction of Mn.
3.4. O2-Temperature Programmed Desorption (O2-TPD) Characterization
The distribution of oxygen species was also explored by O2-TPD for the MCZ-0, MCZ-0.67 and MC-0.67 catalysts (Figure 5). The desorption peak below 200 °C was attributed to physically adsorbed oxygen molecule (O2), while the desorption peaks within 200–500 °C were ascribed to chemisorbed oxygen species (O2− and O−, where O2− is easier to desorb than O−). The desorption peak observed above 500 °C was attributed to surface lattice oxygen (O2−) [41].
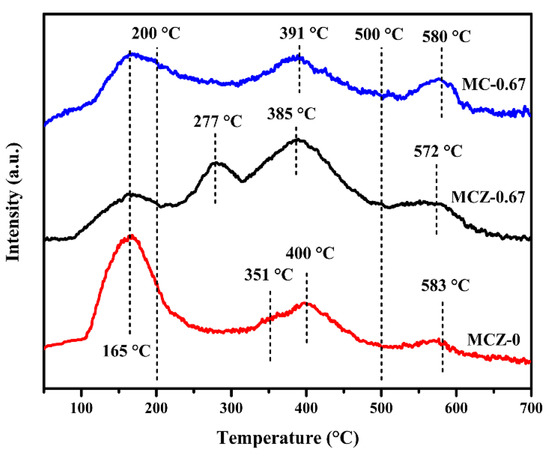
Figure 5.
O2-temperature programmed desorption (O2-TPD) patterns of MCZ-0, MCZ-0.67 and MC-0.67 catalysts.
For the MCZ-0 catalyst specifically, four peaks were observed at 165 °C, 351 °C, 400 °C and 583 °C, corresponding to the desorption of O2, O2−, O− and O2−, respectively. The MCZ-0.67 catalyst presented similar desorption peaks, yet with a much greater peak intensity of chemisorbed oxygen species, indicating that the addition of Mn contributes to the adsorption of active oxygen species on the catalyst surface. This is in good agreement with the O 1s XPS characterization, and likely also correlated to the large BET and mesoporous structure of the MCZ-0.67 catalyst, which are conducive to the adsorption and activation of gas-phase oxygen on the catalyst surface. The desorption peaks of the MCZ-0.67 catalyst showed a shift towards lower temperatures as compared with the MCZ-0 catalyst. As is known, a larger peak intensity and lower desorption temperature correspond to improved catalytic activity [44], which perhaps forecast the better catalytic performance of the MCZ-0.67 catalyst for CB elimination. Finally, the MC-0.67 catalyst exhibited three distinct desorption peaks; it is likely that the O2− desorption peak may have been covered by adjacent peaks. As compared with the MCZ-0.67 catalyst, it is obvious that the addition of Zr contributes to the improvement of catalytic performance by providing more active oxygen species.
3.5. Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM) Characterization
The correlation between the catalytic performance and morphological characteristics of the promising MCZ-0.67 catalyst was studied using SEM and TEM. Figure 6A,B showed that the MCZ-0.67 catalyst presented sponge-like surface with a large degree of porosity, in agreement with its large BET. Closer observation using TEM (Figure 6C,D) showed that this catalyst was composed of well-distributed nanoparticles with a size of 6–10 nm, and their accumulation led to the formation of mesopores with a size of 3–9 nm. This morphology could contribute to the good catalytic performance to some extent by providing a large quantity of adsorption sites [45]. It is reported that nanoparticles showed promising applications for catalytic reactions due to their high surface-to-volume ratio [46]. The selected area electron diffraction (SAED) pattern exhibited in the inset further confirmed that the prepared MCZ-0.67 catalyst is amorphous since no typical diffraction rings or spot patterns were observed [47], in agreement with the results of the XRD characterization. The bright squares in Figure 6E,F indicated that the concentration and distribution of Mn species in MCZ-0.67 catalyst were better than those in MCZ-0.33 catalyst. As reported, the content and distribution of active Mn species can greatly influence the performance of the catalyst [48].
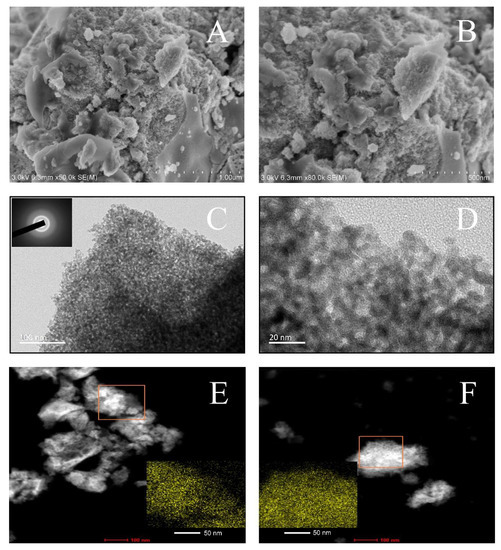
Figure 6.
Scanning electron microscope (SEM) (A,B) and transmission electron microscope (TEM) (C,D) images of MCZ-0.67 catalyst, the inset is the pattern of the selected area electron diffraction (SAED). Scanning transmission electron microscope (STEM) images of MCZ-0.33 (E) and MCZ-0.67 (F) catalysts, the insets are Mn-element mappings.
3.6. Catalytic Activity for Chlorobenzene (CB) Combustion
The catalytic activity of the prepared catalysts during CB combustion was assessed (Figure 7 and Table 3). The MCZ-0 catalyst exhibited the lowest catalytic activity, with deactivation occurring after 250 °C. Following Mn doping, the catalytic activity and anti-poisoning ability were significantly improved. Specifically, MCZ-0.33 (Rs = 0.598 μmol m−2 h−1) exhibited an increased reaction rate compared to that of MCZ-0 (Rs = 0.468 μmol m−2 h−1) at 130 °C. This increase in reaction rate continued until x = 0.67 (Rs = 0.716 μmol m−2 h−1), after which a reversal was observed. Thus, the most abundant active sites may be generated on the MCZ-0.67 catalyst surface, as evidenced from Figure 6 that more active Mn species were evenly distributed in the catalyst. Therefore, the optimal Mn content for CB elimination was defined at 0.67.
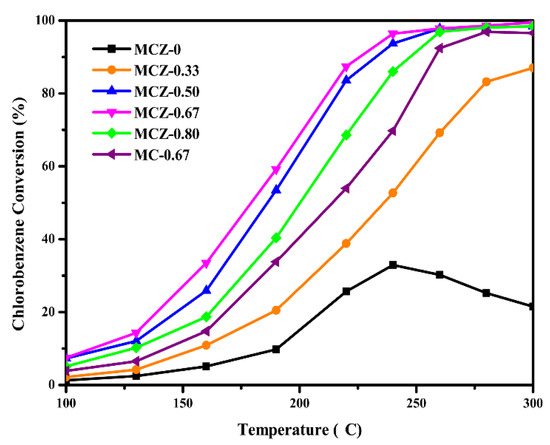
Figure 7.
The catalytic activity of MCZ-x (x = 0, 0.33, 0.50, 0.67, 0.80) and MC-0.67 catalysts for chlorobenzene (CB) combustion; gas composition: 1000 ppm CB, 21% O2; gas hourly space velocity (GHSV) = 20,000 h−1.

Table 3.
Results of activity tests on CB combustion over the prepared catalysts.
The MCZ-0.67 catalyst achieved 90% CB conversion at approximately 226 °C (Table 3), which is lower than most transition metal oxide catalysts reported in previous literature (Table 4). Furthermore, this temperature was approximately 32 °C lower compared with the T90 of the MC-0.67 catalyst, likely due to the changes in physicochemical properties of the MC-0.67 catalyst following Zr doping, including the increase in BET surface area and active oxygen species. Additionally, the measurement of CO2 and CO concentrations in the off-gas (Table 3) showed that the prepared MCZ-x catalysts, except for the MCZ-0 catalyst, presented a greater than 98% CO2 selectivity, indicating that these catalysts possess a good deep catalytic oxidation of CB.

Table 4.
Main data of literature reports on CB elimination over transition metal oxide catalysts.
3.7. Catalyst Stability
Stability is another important consideration in the evaluation of catalytic performance, especially for the removal of CVOCs. Generally, dissociated chlorine species can occupy the catalyst active sites through adsorption or metal chloride formation, resulting in a decline in activity and stability, commonly known as chlorine poisoning [1]. Additionally, due to the strong electronegativity, chlorine deposited on the catalyst surface will lead to an electron shift towards the chlorine species [29], inhibiting the generation of active oxygen species. Accordingly, the timely removal of chlorine species from the catalyst surface is crucial for maintaining a stable catalytic activity.
The variation in catalytic activity over time at 300 °C for 30 h was assessed herein (Figure 8). The activity of the prepared catalysts decreased to varying degrees within the initial 2.5 h likely due to the rapid deposition of chlorine species on the active sites. The activity of the MCZ-0 catalyst was largely exhausted at the end of the stability test, mainly due to its poor redox properties (revealed in TPR characterization). With the introduction of Mn, the increased BET surface area and surface active oxygen could significantly improve the catalyst chlorine resistance by providing more active sites and accelerating the removal of deposited chlorine. It is worth noting that the MCZ-0.67 catalyst maintained a high and stable catalytic activity (>95%) and showed the best resistance to chlorine poisoning throughout the stability test. In contrast, the MC-0.67 catalyst showed a significant decrease in catalytic activity, from 96% to 52%, after 30 h of continuous reaction. Therefore, the addition of Zr led to an improvement in catalytic stability.
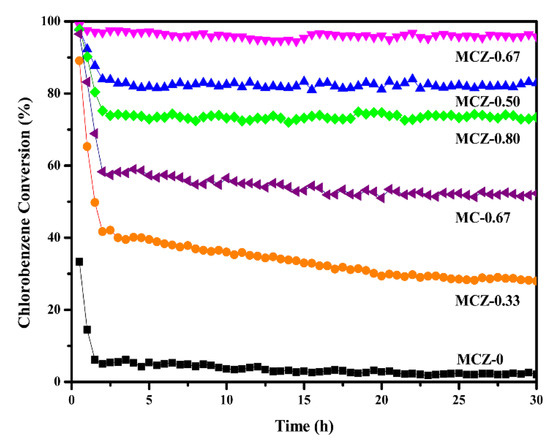
Figure 8.
Stability tests of MCZ-x (x = 0, 0.33, 0.50, 0.67, 0.80) and MC-0.67 catalysts for CB elimination during 30 h of continuous reaction at 300 °C, gas composition: 1000 ppm CB, 21% O2; GHSV = 20,000 h−1.
Chlorine resistance was assessed according to the Cl 2p XPS spectra of the used MCZ-0, MCZ-0.67 and MC-0.67 catalysts after the stability tests (Figure 9). The two overlapping peaks appearing at approximately 198.1 eV and 199.6 eV were attributed to the Cl 2p3/2 and Cl 2p1/2 core levels, respectively [54]. Their presence confirmed the occurrence of chlorine deposition on the catalyst surface. Specifically, according to the estimation from the peak area of Cl 2p3/2, the surface chlorine content of the used MCZ-0, MCZ-0.67 and MC-0.67 catalysts was 5.9 at%, 4.0 at% and 5.2 at%, respectively. Obviously, the MCZ-0.67 catalyst had the best chlorine resistance, with Zr addition effectively inhibiting chlorine poisoning.
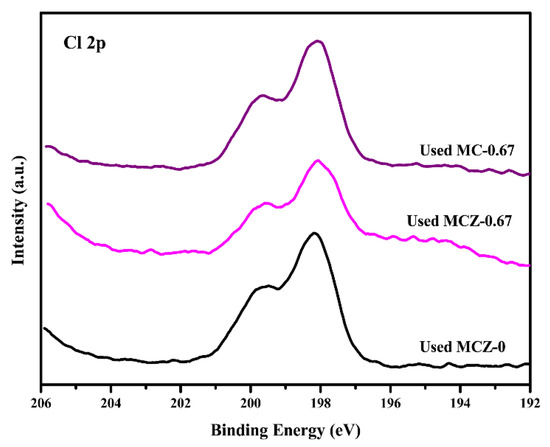
Figure 9.
Cl 2p XPS spectra of the used MCZ-0, MCZ-0.67 and MC-0.67 catalysts.
Apart from time-based stability, the practical application process subjects catalysts to complicated and variable operating conditions. Therefore, the successive influence of CB concentration, space velocity and water vapor on CB elimination and HCl selectivity ([HCl]/([HCl] + [Cl2])) were studied for the MCZ-0.67 catalyst at 300 °C (Figure 10). Both higher CB concentration (2500 ppm) and space velocity (40,000 h−1) led to a serious decrease in activity (lower than 60%), while lower space velocity (10,000 h−1) resulted in an almost complete elimination of CB. The influence of CB concentration indicated that the catalytic efficiency would be overloaded when handling a large amount of reactants, and the influence of lower space velocity was attributed to a more sufficient oxidation caused by the longer residence time of reactants through the catalyst bed. Meanwhile, Deacon reaction (the generation of Cl2) was similarly influenced, as can be seen from the higher HCl selectivity at greater space velocity. Under water vapor condition (2.3 vol%), the conversion of CB dropped to 72%, which is attributed to the competitive adsorption of CB and H2O on the surface active sites [55]. In contrast, the selectivity of HCl was as high as 99%, showing that water can effectively inhibit the generation of Cl2. According to previous report, the flowing water vapor can wash the catalyst surface and promote the formation of HCl by the dissociated chlorine species [56]. Thus, as shown above, despite undergoing various operating conditions, CB conversion was maintained at approximately 90%, further confirming the good catalytic performance of the MCZ-0.67 catalyst.
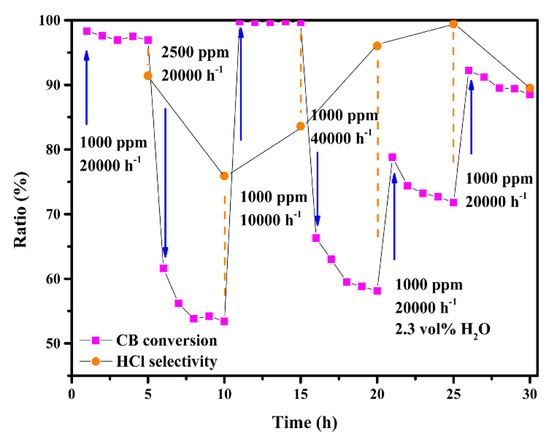
Figure 10.
The successive influence of CB concentration, space velocity and water vapor on CB conversion and HCl selectivity over the MCZ-0.67 catalyst at 300 °C.
3.8. Regeneration of Catalyst
Catalytic combustion is a heterogeneous catalytic oxidation process, varying from homogeneous catalysis, catalysts used in the tests can be easily separated from the reaction system [57]. Thus, it is of great significance to explore the reuse of catalysts (Figure 11). After the fourth reaction run, MCZ-0.67 catalyst suffers a decrease in catalytic efficiency from 98% (fresh catalyst) to approximately 80% at 300 °C. Regeneration was carried out by calcining the above used catalyst at 350 °C for 12 h in air and nitrogen atmosphere respectively. As shown in Figure 11, after treatment in air, the activity of MCZ-0.67 catalyst recovered to approximately 95%, while that of the catalyst treated in nitrogen remained almost unchanged. This phenomenon indicates that the presence of oxygen contributes to the regeneration of the catalyst and the used MCZ-0.67 catalyst can be recycled by calcination in air. The re-enhancement of catalytic activity may be attributed to the reaction of oxygen species with chlorine species at high temperature, which exposes the active sites occupied by chlorine species again [11].
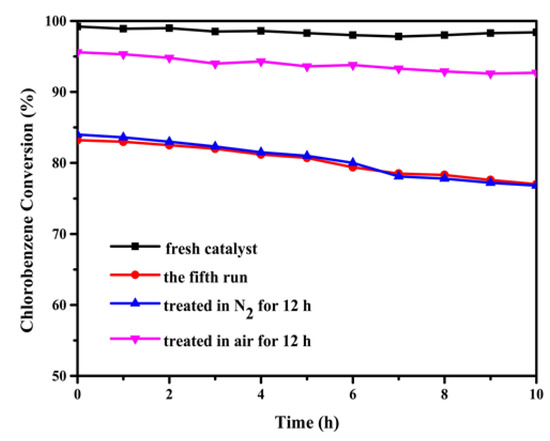
Figure 11.
The catalytic efficiency of the MCZ-0.67 catalyst treated under different conditions; activity test condition: 300 °C for 10 h; gas composition: 1000 ppm CB, 21% O2; GHSV = 20,000 h−1.
During the above 10 h of continuous test, all the off-gas was bubbled through a 5% HNO3 solution. After concentration, inductively coupled plasma–atomic emission spectroscopy (ICP–AES, Thermo Scientific 6300) was used to measure the metal elements in the solution. Results showed that the corresponding Mn, Ce and Zr elements were not detected (detection limit is 0.01 ppm), indicating that the prepared MCZ-0.67 catalyst was stable in composition, and no metal elements were leached into the final effluents under this reaction media.
3.9. Product Analysis
In order to better elucidate the CB oxidation process, the CB decomposition products in the off-gas were collected and analyzed. Based on the calculation of carbon balance and chlorine balance of reactants, the carbon distribution and chlorine distribution of converted CB at different conversion rates (i.e., 20%, 50%, 90% and 100% CB conversion, the corresponding temperature was at approximately 145, 185, 235 and 300 °C, respectively) over the MCZ-0.67 catalyst were obtained (Figure 12). The carbon in the decomposed CB mainly existed in the form of CO2, with CO2 selectivity increasing with the increase in catalytic activity (Figure 12A). When CB was completely oxidized, the selectivity of carbon to carbon oxides (CO2 and CO) exceeded 99.5%, of which the CO content was less than 1%. However, the selectivity of chlorine to HCl in converted CB was low at 20% and 50% CB conversion (Figure 12B), likely related to the easier adsorption of chlorine species on the catalyst surface at lower temperatures [12]. Furthermore, Cl2 was detected at 90% CB conversion, indicating the occurrence of the Deacon reaction. Indeed, it has been previously reported that the higher temperature and modified Mn species are both favorable for the production of Cl2 [49]. Cl balance gives the calculation result of 84% at 100% CB conversion, combined with the high carbon oxides selectivity at this time, the occurrence of chlorine deposition on catalyst surface can be confirmed, which may be related to the formation of metal chlorides or metal oxychlorides (MClx or MOClx).
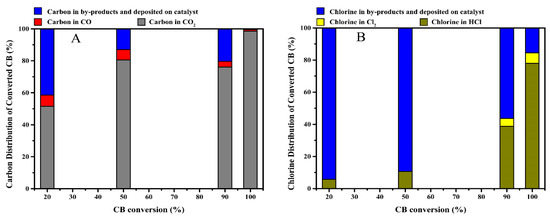
Figure 12.
Carbon distribution (A) and chlorine distribution (B) of converted CB at different conversion rates over the MCZ-0.67 catalyst.
The production of polychlorinated by-products is inevitable during the oxidation of chlorinated aromatic hydrocarbons due to the strong reactivity of chlorine species. Thus, an evaluation of the CB decomposition products is required. Herein, the GC-MS method was employed for the detection of probable trace products (Figure 13), showing that various polychlorinated by-products were produced at 90% CB conversion, including dichlorobenzene (C6H4Cl2, ortho—6 ppm, meta—2 ppm, para—16 ppm), tetrachloroethylene (C2Cl4, 15 ppm), trichloroethylene (C2HCl3, 7 ppm), dichloromethane (CH2Cl2, 3 ppm) and trace amounts of trichlorobenzene (C6H3Cl3, <1 ppm) and hexachlorobutadiene (C4Cl6, <1 ppm). Their presence directly confirms the occurrence of chlorination reactions during CB oxidation and clearly depicts the break-down process of the C–C bond from a hexatomic ring to a short chain. It has been previously reported that the formation of these polychlorinated by-products is initiated from the electrophilic substitution of chlorine species [55], and according to the chlorine distribution during CB oxidation (Figure 12B), it can be reasonably speculated that the chlorine species dissociated from the catalyst surface and the Cl2 resulting from the Deacon reaction are responsible for the substitution reaction. In addition, it is well acknowledged that the destruction of CB molecule begins with dechlorination reaction due to the lower dissociation energy of the C–Cl bond than that of C–H bond [49], and the resulting intermediate benzene is then further ring-opened and oxidized by active oxygen species. Herein, among the products of CB decomposition, benzene, the incomplete oxidation product of CB, was not detected, while it is the only organic by-product during the oxidation of CB over Mn0.8Ce0.2O2 catalyst [55]. This phenomenon may be ascribed to the competitive adsorption for the same active sites between polychlorobenzene and benzene; herein, benzene seems to be preferentially oxidized over the MCZ-0.67 catalyst. This result may be correlated to the higher electron cloud density of benzene than that of polychlorobenzene, thus facilitating the formation of π-complex in the adsorption process [52]. As the reaction temperature increases, these small amounts of polychlorinated by-products can be further oxidized under the strong oxidation ability of active oxygen species, as evidenced from the observations in Figure 13, wherein the corresponding peaks are almost invisible at 100% CB conversion. Based on the above analyses, a possible reaction path of CB oxidation over the MCZ-0.67 catalyst was proposed and presented in Scheme 1.
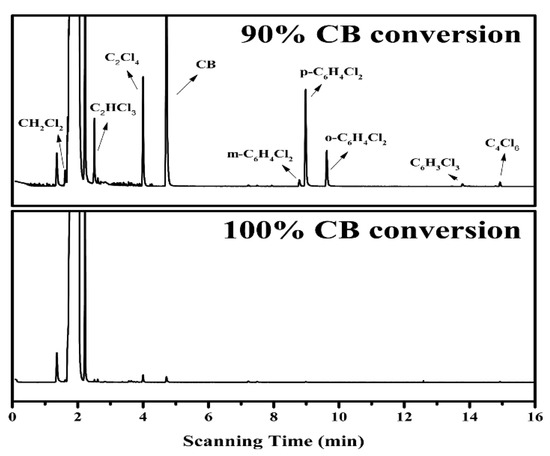
Figure 13.
The gas chromatography-mass spectrometer (GC-MS) chromatograms of organic by-products in off-gas at different CB conversion rates over the MCZ-0.67 catalyst.
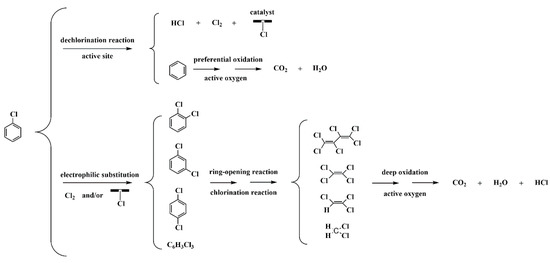
Scheme 1.
Proposed reaction path of CB oxidation over the MCZ-0.67 catalyst.
4. Conclusions
Mn-Ce-Zr-O catalysts doped with a varying Mn concentration were prepared and assessed for the catalytic combustion of CB. Nanosized MCZ-0.67 catalyst with amorphous phase was found to exhibit superior catalytic activity and chlorine resistance among the studied catalysts, and presented excellent performance in stability tests, including time-based stability and the successive influence of the various operating conditions. At the same time, the used MCZ-0.67 catalyst can be recycled by calcination in air at 350 °C for 12 h. Characterization results showed that the doping of Mn played a dominant role in improving the catalytic performance by increasing the BET surface area, improving the redox properties and increasing the percentage of surface active oxygen. Product analysis showed that trace amounts of polychlorinated by-products did occur during CB oxidation, attributed to the chlorination reactions caused by the dissociated chlorine species and the Cl2 generated. At 300 °C, these by-products can be completely oxidized and a high CO2 selectivity (>99%) was obtained under the strong oxidation ability of the MCZ-0.67 catalyst. Therefore, the MCZ-0.67 catalyst prepared herein shows promise for applications to eliminate CVOCs. In addition, although ZrO2 is inactive, the changes in physical structure and chemical properties of catalysts resulting from Zr introduction are essential for maintaining their high and stable catalytic activity.
Author Contributions
Y.X. and Z.L. designed the experiments; L.Z., X.L. and L.Y. performed the experiments; P.Y. contributed to the characterization analysis; L.Z. wrote the manuscript; P.W. and X.J. revised the manuscript. All authors read and approved the manuscript.
Funding
This research was funded by the National Key Research and Development Project of China during the 13th Five-Year Plan Period (2017YFB0602500), the Natural Science Foundation of China (21373034), and the University Science Research Project of Jiangsu Province (16KJA610002).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yang, S.; Zhao, H.; Dong, F.; Zha, F.; Tang, Z. Highly efficient catalytic combustion of o-dichlorobenzene over three-dimensional ordered mesoporous cerium manganese bimetallic oxides: A new concept of chlorine removal mechanism. Mol. Catal. 2019, 463, 119–129. [Google Scholar] [CrossRef]
- Huang, B.; Lei, C.; Wei, C.; Zeng, G. Chlorinated volatile organic compounds (Cl-VOCs) in environment—Sources, potential human health impacts, and current remediation technologies. Environ. Int. 2014, 71, 118–138. [Google Scholar] [CrossRef] [PubMed]
- Maghsoodi, S.; Towfighi, J.; Khodadadi, A.; Mortazavi, Y. The effects of excess manganese in nano-size lanthanum manganite perovskite on enhancement of trichloroethylene oxidation activity. Chem. Eng. J. 2013, 215–216, 827–837. [Google Scholar] [CrossRef]
- Shokouhimehr, M.; Hong, K.; Lee, T.; Moon, C.; Hong, S.; Zhang, K.; Suh, J.; Choi, K.; Varma, R.; Jang, H. Magnetically retrievable nanocomposite adorned with Pd nanocatalysts: Efficient reduction of nitroaromatics in aqueous media. Green Chem. 2018, 20, 3809–3817. [Google Scholar] [CrossRef]
- Cao, S.; Fei, X.; Wen, Y.; Sun, Z.; Wang, H.; Wu, Z. Bimodal mesoporous TiO2 supported Pt, Pd and Ru catalysts and their catalytic performance and deactivation mechanism for catalytic combustion of Dichloromethane (CH2Cl2). Appl. Catal. A Gen. 2018, 550, 20–27. [Google Scholar] [CrossRef]
- Huang, H.; Dai, Q.; Wang, X. Morphology effect of Ru/CeO2 catalysts for the catalytic combustion of chlorobenzene. Appl. Catal. B Environ. 2014, 158–159, 96–105. [Google Scholar] [CrossRef]
- Yang, P.; Shi, Z.; Yang, S.; Zhou, R. High catalytic performances of CeO2–CrOx catalysts for chlorinated VOCs elimination. Chem. Eng. Sci. 2015, 126, 361–369. [Google Scholar] [CrossRef]
- Zhang, Z.; Xia, H.; Dai, Q.; Wang, X. Dichloromethane oxidation over FexZr1-x oxide catalysts. Appl. Catal. A Gen. 2018, 557, 108–118. [Google Scholar] [CrossRef]
- Romero-Sáez, M.; Divakar, D.; Aranzabal, A.; González-Velasco, J.R.; González-Marcos, J.A. Catalytic oxidation of trichloroethylene over Fe-ZSM-5: Influence of the preparation method on the iron species and the catalytic behavior. Appl. Catal. B Environ. 2016, 180, 210–218. [Google Scholar] [CrossRef]
- Su, J.; Liu, Y.; Yao, W.; Wu, Z. Catalytic Combustion of Dichloromethane over HZSM-5-Supported Typical Transition Metal (Cr, Fe, and Cu) Oxide Catalysts: A Stability Study. J. Phys. Chem. C 2016, 120, 18046–18054. [Google Scholar] [CrossRef]
- Xingyi, W.; Qian, K.; Dao, L. Catalytic combustion of chlorobenzene over MnOx–CeO2 mixed oxide catalysts. Appl. Catal. B Environ. 2009, 86, 166–175. [Google Scholar] [CrossRef]
- Kan, J.; Deng, L.; Li, B.; Huang, Q.; Zhu, S.; Shen, S.; Chen, Y. Performance of co-doped Mn-Ce catalysts supported on cordierite for low concentration chlorobenzene oxidation. Appl. Catal. A Gen. 2017, 530, 21–29. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, C.; Wang, L.; Li, G.; Song, L.; Li, G.; Tang, S.; Li, X. Promotional effect of HZSM-5 on the catalytic oxidation of toluene over MnOx/HZSM-5 catalysts. Catal. Sci. Technol. 2016, 6, 4260–4270. [Google Scholar] [CrossRef]
- Jodłowski, P.; Jędrzejczyk, R.; Chlebda, D.; Dziedzicka, A.; Kuterasiński, Ł.; Gancarczyk, A.; Sitarz, M. Non-Noble Metal Oxide Catalysts for Methane Catalytic Combustion: Sonochemical Synthesis and Characterisation. Nanomaterials 2017, 7, 174. [Google Scholar] [CrossRef]
- Dai, Y.; Wang, X.; Dai, Q.; Li, D. Effect of Ce and La on the structure and activity of MnOx catalyst in catalytic combustion of chlorobenzene. Appl. Catal. B Environ. 2012, 111–112, 141–149. [Google Scholar] [CrossRef]
- Yang, P.; Li, J.; Zuo, S. Promoting oxidative activity and stability of CeO2 addition on the MnOx modified kaolin-based catalysts for catalytic combustion of benzene. Chem. Eng. Sci. 2017, 162, 218–226. [Google Scholar] [CrossRef]
- He, C.; Xu, B.; Shi, J.; Qiao, N.; Hao, Z.; Zhao, J. Catalytic destruction of chlorobenzene over mesoporous ACeOx (A=Co, Cu, Fe, Mn, or Zr) composites prepared by inorganic metal precursor spontaneous precipitation. Fuel Process. Technol. 2015, 130, 179–187. [Google Scholar] [CrossRef]
- Zhao, B.; Li, G.; Ge, C.; Wang, Q.; Zhou, R. Preparation of Ce0.67Zr0.33O2 mixed oxides as supports of improved Pd-only three-way catalysts. Appl. Catal. B Environ. 2010, 96, 338–349. [Google Scholar] [CrossRef]
- Shen, B.; Wang, Y.; Wang, F.; Liu, T. The effect of Ce–Zr on NH3-SCR activity over MnOx(0.6)/Ce0.5Zr0.5O2 at low temperature. Chem. Eng. J. 2014, 236, 171–180. [Google Scholar] [CrossRef]
- Azalim, S.; Brahmi, R.; Agunaou, M.; Beaurain, A.; Giraudon, J.M.; Lamonier, J.F. Washcoating of cordierite honeycomb with Ce–Zr–Mn mixed oxides for VOC catalytic oxidation. Chem. Eng. J. 2013, 223, 536–546. [Google Scholar] [CrossRef]
- Gutiérrez-Ortiz, J.I.; de Rivas, B.; López-Fonseca, R.; González-Velasco, J.R. Combustion of aliphatic C2 chlorohydrocarbons over ceria–zirconia mixed oxides catalysts. Appl. Catal. A Gen. 2004, 269, 147–155. [Google Scholar] [CrossRef]
- De Rivas, B.; López-Fonseca, R.; González-Velasco, J.R.; Gutiérrez-Ortiz, J.I. On the mechanism of the catalytic destruction of 1,2-dichloroethane over Ce/Zr mixed oxide catalysts. J. Mol. Catal. A Chem. 2007, 278, 181–188. [Google Scholar] [CrossRef]
- Zhao, C.; Hao, Q.; Zhang, Q.; Yan, N.; Liu, J.; Dou, B.; Bin, F. Catalytic self-sustained combustion of toluene and reaction pathway over CuxMn1-xCe0.75Zr0.25/TiO2 catalysts. Appl. Catal. A Gen. 2019, 569, 66–74. [Google Scholar] [CrossRef]
- Deng, L.; Ding, Y.; Duan, B.; Chen, Y.; Li, P.; Zhu, S.; Shen, S. Catalytic deep combustion characteristics of benzene over cobalt doped Mn-Ce solid solution catalysts at lower temperatures. Mol. Catal. 2018, 446, 72–80. [Google Scholar] [CrossRef]
- Zhao, H.; Han, W.; Dong, F.; Tang, Z. Highly-efficient catalytic combustion performance of 1,2-dichlorobenzene over mesoporous TiO2–SiO2 supported CeMn oxides: The effect of acid sites and redox sites. J. Ind. Eng. Chem. 2018, 64, 194–205. [Google Scholar] [CrossRef]
- Leng, X.; Zhang, Z.; Li, Y.; Zhang, T.; Ma, S.; Yuan, F.; Niu, X.; Zhu, Y. Excellent low temperature NH3-SCR activity over MnaCe0.3TiOx (a = 0.1–0.3) oxides: Influence of Mn addition. Fuel Process. Technol. 2018, 181, 33–43. [Google Scholar] [CrossRef]
- Zhu, B.; Li, X.; Sun, P.; Liu, J.; Ma, X.; Zhu, X.; Zhu, A. A novel process of ozone catalytic oxidation for low concentration formaldehyde removal. Chin. J. Catal. 2017, 38, 1759–1769. [Google Scholar] [CrossRef]
- Arena, F. Multipurpose composite MnCeOx catalysts for environmental applications. Catal. Sci. Technol. 2014, 4, 189–1898. [Google Scholar] [CrossRef]
- Yu, D.; Xingyi, W.; Dao, L.; Qiguang, D. Catalytic combustion of chlorobenzene over Mn-Ce-La-O mixed oxide catalysts. J. Hazard. Mater. 2011, 188, 132–139. [Google Scholar] [CrossRef]
- Tang, A.; Hu, L.; Yang, X.; Jia, Y.; Zhang, Y. Promoting effect of the addition of Ce and Fe on manganese oxide catalyst for 1,2-dichlorobenzene catalytic combustion. Catal. Commun. 2016, 82, 41–45. [Google Scholar] [CrossRef]
- He, F.; Luo, J.; Liu, S. Novel metal loaded KIT-6 catalysts and their applications in the catalytic combustion of chlorobenzene. Chem. Eng. J. 2016, 294, 362–370. [Google Scholar] [CrossRef]
- Li, J.; Zhao, P.; Liu, S. SnOx–MnOx–TiO2 catalysts with high resistance to chlorine poisoning for low-temperature chlorobenzene oxidation. Appl. Catal. A Gen. 2014, 482, 363–369. [Google Scholar] [CrossRef]
- Venkataswamy, P.; Rao, K.N.; Jampaiah, D.; Reddy, B.M. Nanostructured manganese doped ceria solid solutions for CO oxidation at lower temperatures. Appl. Catal. B Environ. 2015, 162, 122–132. [Google Scholar] [CrossRef]
- Chen, H.; Sayari, A.; Adnot, A.; Larachi, F. Composition–activity effects of Mn–Ce–O composites on phenol catalytic wet oxidation. Appl. Catal. B Environ. 2001, 32, 195–204. [Google Scholar] [CrossRef]
- Wu, X.; Liang, Q.; Weng, D. Effect of Manganese Doping on Oxygen Storage Capacity of Ceria-Zirconia Mixed Oxides. J. Rare Earths 2006, 24, 549–553. [Google Scholar] [CrossRef]
- Trawczyński, J.; Bielak, B.; Miśta, W. Oxidation of ethanol over supported manganese catalysts—Effect of the carrier. Appl. Catal. B Environ. 2005, 55, 277–285. [Google Scholar] [CrossRef]
- Zou, Z.; Meng, M.; Zha, Y. Surfactant-Assisted Synthesis, Characterizations, and Catalytic Oxidation Mechanisms of the Mesoporous MnOx−CeO2 and Pd/MnOx−CeO2 Catalysts Used for CO and C3H8 Oxidation. J. Phys. Chem. C 2009, 114, 468–477. [Google Scholar] [CrossRef]
- Li, H.; Wu, C.; Li, Y.; Zhang, J. CeO2–TiO2 Catalysts for Catalytic Oxidation of Elemental Mercury in Low-Rank Coal Combustion Flue Gas. Environ. Sci. Technol. 2011, 45, 7394–7400. [Google Scholar] [CrossRef]
- Tang, X.; Li, Y.; Huang, X.; Xu, Y.; Zhu, H.; Wang, J.; Shen, W. MnOx–CeO2 mixed oxide catalysts for complete oxidation of formaldehyde: Effect of preparation method and calcination temperature. Appl. Catal. B Environ. 2006, 62, 265–273. [Google Scholar] [CrossRef]
- Bai, B.; Li, J.; Hao, J. 1D-MnO2, 2D-MnO2 and 3D-MnO2 for low-temperature oxidation of ethanol. Appl. Catal. B Environ. 2015, 164, 241–250. [Google Scholar] [CrossRef]
- Deng, W.; Dai, Q.; Lao, Y.; Shi, B.; Wang, X. Low temperature catalytic combustion of 1,2-dichlorobenzene over CeO2–TiO2 mixed oxide catalysts. Appl. Catal. B Environ. 2016, 181, 848–861. [Google Scholar] [CrossRef]
- Jampaiah, D.; Ippolito, S.J.; Sabri, Y.M.; Tardio, J.; Selvakannan, P.R.; Nafady, A.; Reddy, B.M.; Bhargava, S.K. Ceria–zirconia modified MnOx catalysts for gaseous elemental mercury oxidation and adsorption. Catal. Sci. Technol. 2016, 6, 1792–1803. [Google Scholar] [CrossRef]
- Cao, F.; Xiang, J.; Su, S.; Wang, P.; Sun, L.; Hu, S.; Lei, S. The activity and characterization of MnOx–CeO2–ZrO2/γ-Al2O3 catalysts for low temperature selective catalytic reduction of NO with NH3. Chem. Eng. J. 2014, 243, 347–354. [Google Scholar] [CrossRef]
- Ma, C.Y.; Mu, Z.; Li, J.J.; Jin, Y.G.; Cheng, J.; Lu, G.Q.; Hao, Z.P.; Qiao, S.Z. Mesoporous Co3O4 and Au/Co3O4 Catalysts for Low-Temperature Oxidation of Trace Ethylene. J. Am. Chem. Soc. 2010, 132, 2608–2613. [Google Scholar] [CrossRef] [PubMed]
- Njagi, E.C.; Genuino, H.C.; King’ondu, C.K.; Dharmarathna, S.; Suib, S.L. Catalytic oxidation of ethylene at low temperatures using porous copper manganese oxides. Appl. Catal. A Gen. 2012, 421–422, 154–160. [Google Scholar] [CrossRef]
- Shokouhimehr, M.; Shin, K.; Lee, J.; Hackett, M.; Jun, S.; Oh, M.; Jang, J.; Hyeon, T. Magnetically recyclable core-shell nanocatalysts for efficient heterogeneous oxidation of alcohols. J. Mater. Chem. A 2014, 2, 7593–7599. [Google Scholar] [CrossRef]
- Njagi, E.C.; Chen, C.; Genuino, H.; Galindo, H.; Huang, H.; Suib, S.L. Total oxidation of CO at ambient temperature using copper manganese oxide catalysts prepared by a redox method. Appl. Catal. B Environ. 2010, 99, 103–110. [Google Scholar] [CrossRef]
- Chen, J.; Chen, X.; Chen, X.; Xu, W.; Xu, Z.; Jia, H.; Chen, J. Homogeneous introduction of CeOy into MnOx-based catalyst for oxidation of aromatic VOCs. Appl. Catal. B Environ. 2018, 224, 825–835. [Google Scholar] [CrossRef]
- Wang, X.; Ran, L.; Dai, Y.; Lu, Y.; Dai, Q. Removal of Cl adsorbed on Mn–Ce–La solid solution catalysts during CVOC combustion. J. Coll. Interf. Sci. 2014, 426, 324–332. [Google Scholar] [CrossRef]
- He, C.; Yu, Y.; Shi, J.; Shen, Q.; Chen, J.; Liu, H. Mesostructured Cu–Mn–Ce–O composites with homogeneous bulk composition for chlorobenzene removal: Catalytic performance and microactivation course. Mater. Chem. Phys. 2015, 157, 87–100. [Google Scholar] [CrossRef]
- Lu, Y.; Dai, Q.; Wang, X. Catalytic combustion of chlorobenzene on modified LaMnO3 catalysts. Catal. Commun. 2014, 54, 114–117. [Google Scholar] [CrossRef]
- Huang, H.; Gu, Y.; Zhao, J.; Wang, X. Catalytic combustion of chlorobenzene over VOx/CeO2 catalysts. J. Catal. 2015, 326, 54–68. [Google Scholar] [CrossRef]
- Wu, M.; Wang, X.; Dai, Q.; Li, D. Catalytic combustion of chlorobenzene over Mn–Ce/Al2O3 catalyst promoted by Mg. Catal. Commun. 2010, 11, 1022–1025. [Google Scholar] [CrossRef]
- Mao, D.; He, F.; Zhao, P.; Liu, S. Enhancement of resistance to chlorine poisoning of Sn-modified MnCeLa catalysts for chlorobenzene oxidation at low temperature. RSC Adv. 2015, 5, 10040–10047. [Google Scholar] [CrossRef]
- Sun, P.; Wang, W.; Dai, X.; Weng, X.; Wu, Z. Mechanism study on catalytic oxidation of chlorobenzene over MnxCe1-xO2/H-ZSM5 catalysts under dry and humid conditions. Appl. Catal. B Environ. 2016, 198, 389–397. [Google Scholar] [CrossRef]
- Hetrick, C.E.; Patcas, F.; Amiridis, M.D. Effect of water on the oxidation of dichlorobenzene over V2O5/TiO2 catalysts. Appl. Catal. B Environ. 2011, 101, 622–628. [Google Scholar] [CrossRef]
- Shokouhimehr, M.; Kim, T.; Jun, S.; Shin, K.; Jang, Y.; Kim, B.; Kim, J.; Hyeon, T. Magnetically separable carbon nanocomposite catalysts for efficient nitroarene reduction and Suzuki reactions. Appl. Catal. A Gen. 2014, 476, 133–139. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).