Kinetic and Surface Study of Single-Walled Aluminosilicate Nanotubes and Their Precursors
Abstract
:1. Introduction
2. Experimental Section
3. Results and Discussion
3.1. Structural Characterisation
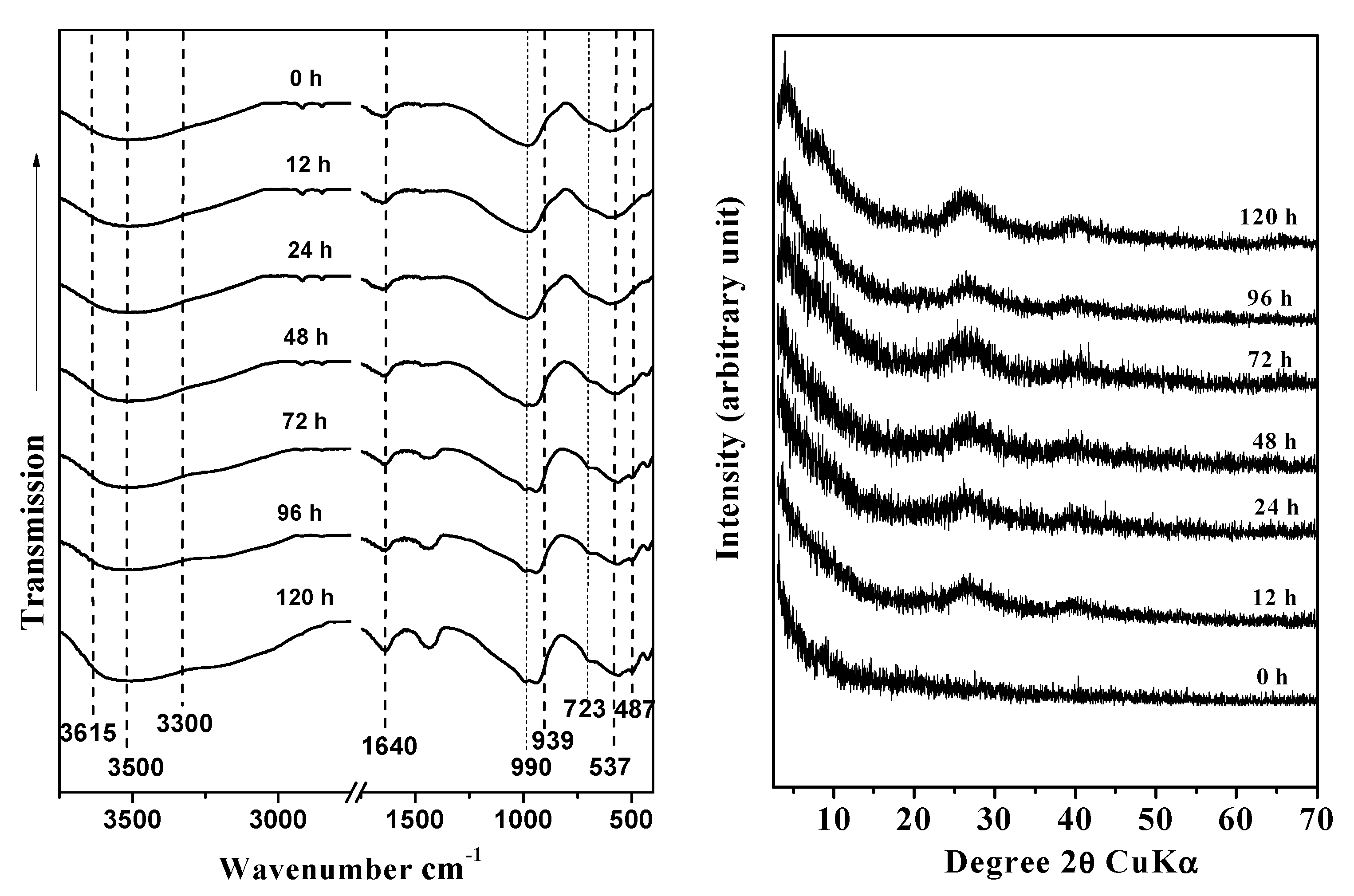
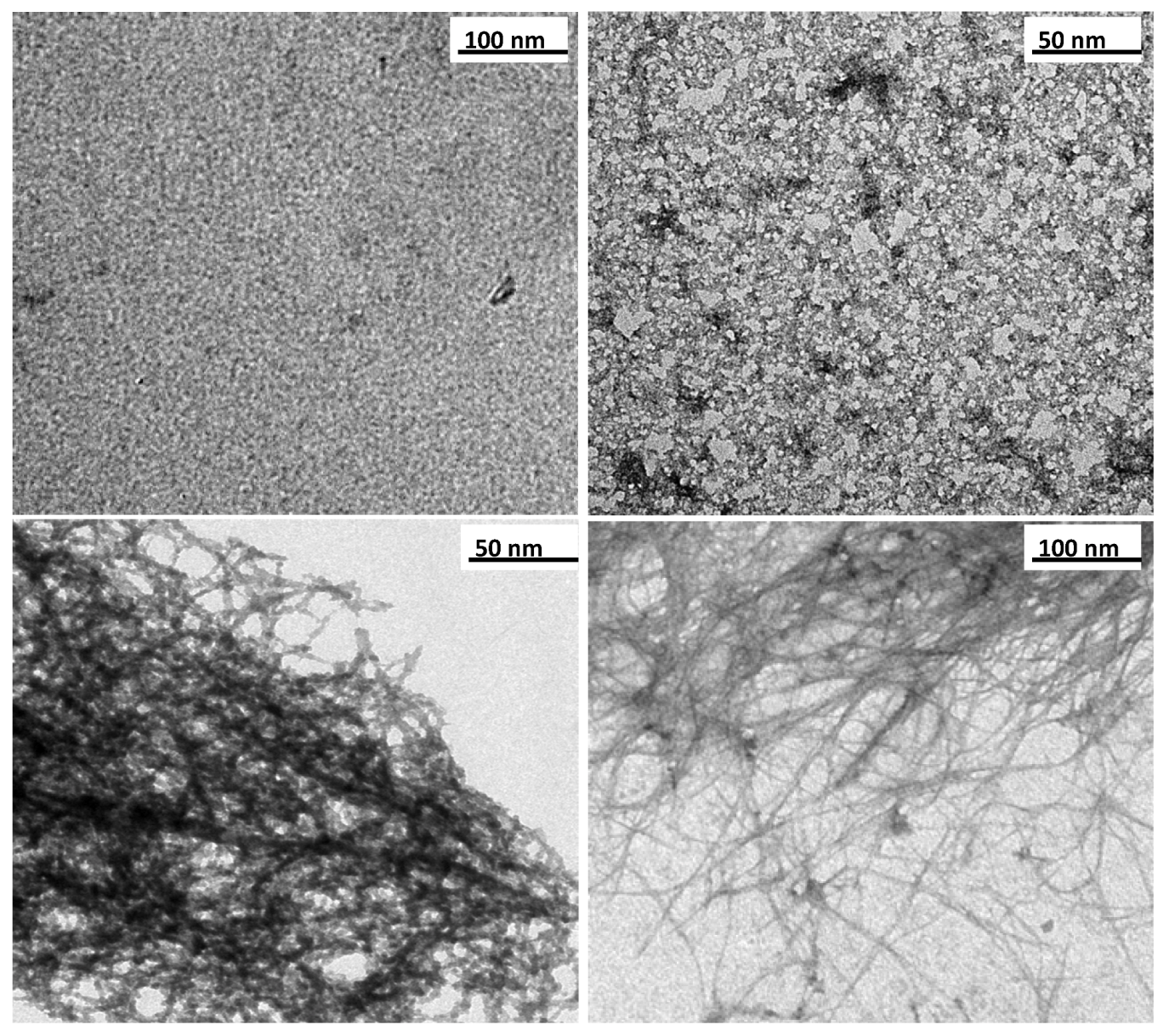
| Time (h) | Surface area a (m2/g) | Pore volume b (cm3/g) | Microporous volume c (cm3/g) | Inner diameter (nm) | Outer diameter (nm) |
|---|---|---|---|---|---|
| 0 | 170 | 0.10 | 0.04 | --- | --- |
| 12 | 181 | 0.13 | 0.03 | --- | --- |
| 24 | 197 | 0.15 | 0.03 | --- | --- |
| 72 | 214 | 0.20 | 0.02 | 1.0 | 2.3 |
| 96 | 253 | 0.23 | 0.02 | 1.0 | 2.5 |
| 120 | 303 | 0.25 | 0.02 | 1.0 | 2.5 |
3.2. Surface Characterization
| Time (h) | IEP | ∆IEP/∆t | pHrx | ∆pHrx/∆t |
|---|---|---|---|---|
| 0 | 6.6 a | 0 | 4.49 a | 0 |
| 12 | 7.1 b | 4.2 | 4.26 b | 1.90 |
| 24 | 7.8 c | 5.8 | 3.85 c | 3.40 |
| 48 | 8.5 d | 2.9 | 3.61 d | 1.80 |
| 72 | 10.0 e | 6.3 | 2.78 e | 3.50 |
| 96 | 10.3 ef | 1.3 | 2.72 f | 0.03 |
| 120 | 10.6 fg | 1.3 | 2.70 fg | 0.01 |
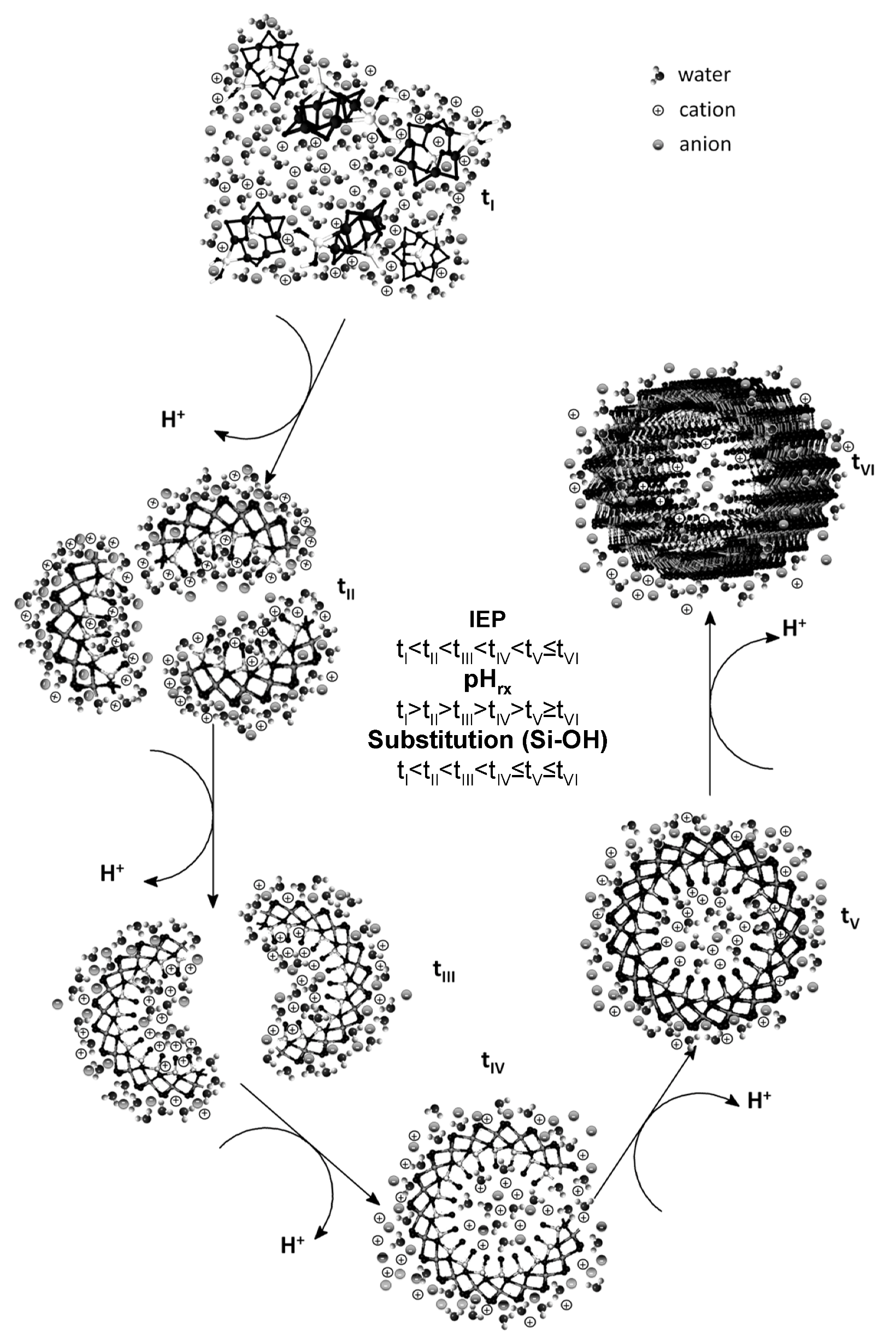
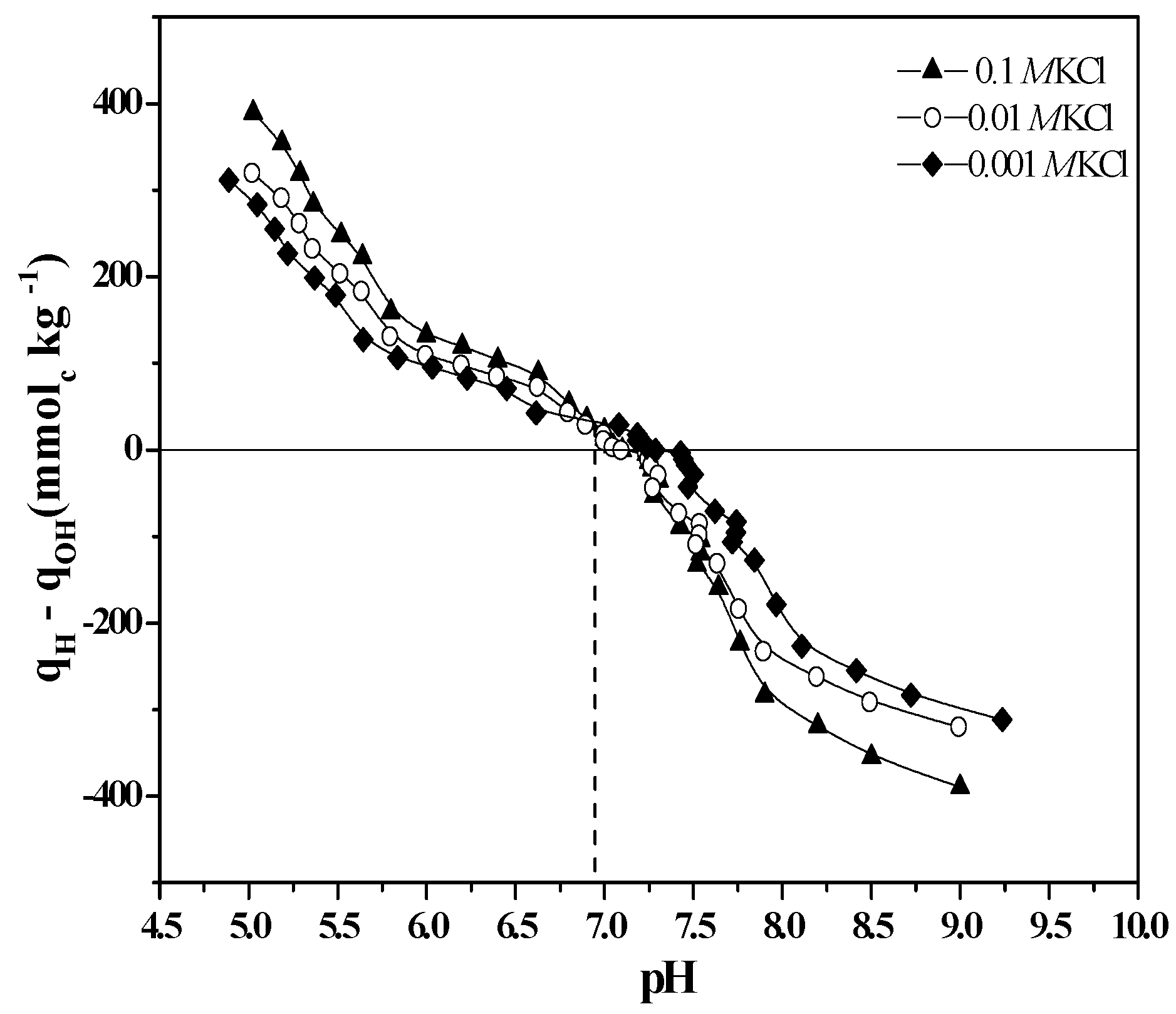


3.3. Surface Evidence of the Formation of Imogolite
4. Conclusions
Acknowledgments
References
- Bursill, L.A.; Peng, J.L.; Bourgeois, L.N. Imogolite: An aluminosilicate nanotube material. Philog. Mag. A 2000, 80, 105–117. [Google Scholar]
- Cradwick, P.D.G.; Farmer, V.C.; Russell, J.D.; Masson, C.R.; Wada, K.; Yoshinaga, N. Imogolite—A hydrated aluminium silicate of tubular structure. Nat. Phys. Sci. 1972, 240, 187–189. [Google Scholar] [CrossRef]
- Farmer, V.C.; Fraser, A.R.; Tait, J.M. Synthesis of imogolite: A tubular aluminium silicate polymer. J. Chem. Soc. Chem. Commun. 1977, 13, 462–463. [Google Scholar] [CrossRef]
- Denaix, L.; Lamy, I.; Bottero, J.Y. Structure and affinity towards Cd2+, Cu2+, Pb2+ of synthetic colloidal amorphous aluminosilicates and their precursors. Colloid Surf. 1999, 158, 315–325. [Google Scholar] [CrossRef]
- Farmer, V.C.; Adams, M.J.; Fraser, A.R.; Palmieri, F. Synthetic imogolite—Properties, synthesis, and possible applications. Clay Miner. 1983, 18, 459–472. [Google Scholar]
- Geraldo, D.A.; Arancibia-Miranda, N.; Villagra, N.A.; Mora, G.C.; Arratia-Perez, R. Synthesis of CdTe QDs/single-walled aluminosilicate nanotubes hybrid compound and their antimicrobial activity on bacteria. J. Nanopart. Res. 2012, 14, 1286–1293. [Google Scholar]
- Imamura, S.; Hayashi, Y.; Kajiwara, K.; Hoshino, H.; Kaito, C. Imogolite—A possible new type of shape-selective catalyst. Ind. Eng. Chem. Res. 1993, 32, 600–603. [Google Scholar] [CrossRef]
- Qi, X.; Yoon, H.; Lee, S.H.; Yoon, J.; Kim, S.J. Surface-modified imogolite by 3-APS-OsO4 complex: Synthesis, characterization and its application in the dihydroxylation of olefins. J. Ind. Eng. Chem. 2008, 14, 136–141. [Google Scholar] [CrossRef]
- Suzuki, M.; Inukai, K.; Maeda, M. Synthesis of imogolite from inorganic solution influence of solution concentration on forming precursor for the synthesis of large quantities of imogolite. J. Vac. Soc. Jpn. 2005, 49, 29–33. [Google Scholar] [CrossRef]
- Levard, C.; Masion, A.; Rose, J.; Doelsch, E.; Borschneck, D.; Dominici, C.; Ziarelli, F.; Bottero, J.Y. Synthesis of imogolite fibers from decimolar concentration at low temperature and ambient pressure: A promising route for inexpensive nanotubes. J. Am. Chem. Soc. 2009, 131, 17080–17081. [Google Scholar]
- Levard, C.; Rose, J.; Thill, A.; Masion, A.; Doelsch, E.; Maillet, P.; Spalla, O.; Olivi, L.; Cognigni, A.; Ziarelli, F.; et al. Formation and growth mechanisms of imogolite-like aluminogermanate nanotubes. Chem. Mater. 2010, 22, 2466–2473. [Google Scholar]
- Yang, H.X.; Wang, C.; Su, Z.H. Growth mechanism of synthetic imogolite nanotubes. Chem. Mater. 2008, 20, 4484–4488. [Google Scholar]
- Mukherjee, S.; Kim, K.; Nair, S. Short, highly ordered, single-walled mixed-oxide nanotubes assemble from amorphous nanoparticles. J. Am. Chem. Soc. 2007, 129, 6820–6826. [Google Scholar] [CrossRef]
- Mukherjee, S.; Bartlow, V.A.; Nair, S. Phenomenology of the growth of single-walled aluminosilicate and aluminogermanate nanotubes of precise dimensions. Chem. Mater. 2005, 17, 4900–4909. [Google Scholar] [CrossRef]
- Barrett, S.M.; Budd, P.M.; Price, C. The synthesis and characterization of imogolite. Eur. Polym. J. 1991, 7, 609–612. [Google Scholar] [CrossRef]
- Jolivet, J.P.; Chanéac, C.; Chiche, D.; Cassaignon, S.; Durupthy, O.; Hernandez, J. Basic concepts of the crystallization from aqueous solutions: The example of aluminum oxy(hydroxi)des and aluminosilicates. C. R. Geosci. 2011, 343, 113–122. [Google Scholar] [CrossRef]
- Wada, S.-I. Imogolite synthesis at 25 °C. Clays Clay Miner. 1987, 5, 379–384. [Google Scholar]
- Maillet, P.; Levard, C.; Spalla, O.; Masion, A.; Rose, J.; Thill, A. Growth kinetic of single and double-walled aluminogermanate imogolite-like nanotubes: An experimental and modeling approach. Phys. Chem. Chem. Phys. 2011, 13, 2682–2689. [Google Scholar]
- Yucelen, G.I.; Choudhury, R.; Vyalikh, A.; Scheler, U.; Beckham, H.W.; Nair, S. Formation and growth mechanisms of imogolite-like aluminogermanate nanotubes. J. Am. Chem. Soc. 2011, 133, 5397–5412. [Google Scholar]
- Thill, A.; Maillet, P.; Guiose, B.; Spalla, O.; Belloni, L.; Chaurand, P.; Auffan, M.; Olivi, L.; Rose, J. Physico-chemical control over the single- or double-wall structure of aluminogermanate imogolite-like nanotubes. J. Am. Chem. Soc. 2012, 134, 3780–3786. [Google Scholar]
- Gil-Llambías, F.J.; Escudey-Castro, A.M. Use of zero point charge measurements in determining apparent surface coverage of molybdena in MoO3/Al2O3 catalysts. J. Chem. Soc. Chem. Commun. 1982, 9, 478–479. [Google Scholar]
- Escudey, M.; Galindo, G.; Ervin, J. Effect of iron oxide dissolution treatment on the isoelectric point of allophanic soils. Clays Clay Miner. 1986, 34, 108–110. [Google Scholar]
- Panagiotou, G.D.; Petsi, T.; Bourikas, K.; Kordulis, C.; Lycourghiotis, A. The interfacial chemistry of the impregnation step involved in the preparation of Tungsten(VI) supported titania catalysts. J. Catal. 2009, 262, 266–279. [Google Scholar] [CrossRef]
- Díaz de León, J.N.; Picquarta, M.; Villarroel, M.; Vrinat, M.; Gil Llambias, F.J.; Murrieta, F.; de los Reyes, J.A. Effect of gallium as an additive in hydrodesulfurization WS2/γ-Al2O3 catalysts. J. Mol. Catal. A 2010, 323, 1–6. [Google Scholar] [CrossRef]
- Taffarel, S.R.; Rubio, J. On the removal of Mn2+ ions by adsorption onto natural and activated Chilean zeolites. Miner. Eng. 2010, 23, 771–779. [Google Scholar] [CrossRef]
- Cáceres, L.; Escudey, M.; Fuentes, E.; Báez, M.E. Modeling the sorption kinetic of metsulfuron-methyl on Andisols and Ultisols volcanic ash-derived soils: Kinetics parameters and solute transport mechanisms. J. Hazard. Mater. 2010, 179, 795. [Google Scholar] [CrossRef]
- Sposito, G. The operational definition of the zero point of charge in soils. Soil Sci. Soc. Am. J. 1981, 45, 292–297. [Google Scholar] [CrossRef]
- Vayssieres, L. On the effect of nanoparticle size on water-oxide interfacial chemistry. J. Phys. Chem. 2009, 113, 4733–4736. [Google Scholar]
- Qafoku, N. Terrestrial nanoparticles and their controls on soil-/geo-processes and reactions. Adv. Agron. 2010, 107, 33–91. [Google Scholar] [CrossRef]
- Hu, J.; Kannangara, G.S.K.; Wilson, M.A.; Reddy, N. The fused silicate route to protoimogolite and imogolite. J. Non-Cryst. Solids. 2004, 347, 224–230. [Google Scholar] [CrossRef]
- Wilson, M.A.; Lee, G.S.H.; Taylor, R.C. Tetrahedral rehydration during imogolite formation. J. Non-Cryst. Solids. 2001, 296, 172–181. [Google Scholar] [CrossRef]
- McBride, M.B.; Farmer, V.C.; Russell, J.D.; Tait, J.M.; Goodman, B. Iron substitution in aluminosilicate sols synthesized at low pH. Clay Miner. 1984, 19, 1–8. [Google Scholar]
- Gil, B.; Zones, S.I.; Hwang, S.-J.; Bejblová, M.; Čejka, J. Acidic properties of SSZ-33 and SSZ-35 novel zeolites: A complex infrared and MAS NMR study. J. Phys. Chem. C 2008, 112, 2997–3007. [Google Scholar]
- Harsh, J.B.; Traina, S.J.; Boyle, J.; Yang, Y. Adsorption of cations on imogolite and their effect on surface charge characteristics. Clays Clay Miner. 1992, 40, 700–706. [Google Scholar]
- Farmer, V.C.; Smith, B.F.L.; Tait, J.M. Stability free-energy and heat of formation of imogolite. Clay Miner. 1979, 14, 103–107. [Google Scholar]
- Bonelli, B.; Bottero, I.; Ballarini, N.; Passeri, S.; Cavani, F.; Garrone, G. IR spectroscopic and catalytic characterization of the acidity of imogolite-based systems. J. Catal. 2009, 264, 15–30. [Google Scholar]
- Gustafsson, J.P. The surface chemistry of imogolite. Clays Clay Miner. 2001, 49, 73–80. [Google Scholar]
- Arancibia-Miranda, N.; Escudey, M.; Molina, M.; García-González, M.T. Use of isoelectric point and pH to evaluate the synthesis of a nanotubular aluminosilicate. J. Non-Cryst. Solids. 2011, 357, 1750–1756. [Google Scholar] [CrossRef]
- Yucelen, G.I.; Choudhury, R.P.; Leisen, J.; Nair, S.; Beckham, H.W. Defect structures in aluminosilicate single-walled nanotubes: A solid-state nuclear magnetic resonance investigation. J. Phys. Chem. C 2012, 116, 17149–17157. [Google Scholar]
- Tsuchida, H.; Ooi, S.; Nakaishi, K.; Adachi, Y. Effects of pH and ionic strength on electrokinetic properties of imogolite. Colloid Surf. 2005, 265, 131–134. [Google Scholar] [CrossRef]
- Huittinen, N.; Rabung, T.; Lutzenkirchen, J.; Mitchell, S.C.; Bickmore, B.R.; Lehto, J.; Geckeis, H. Sorption of Cm(III) and Gd(III) onto gibbsite, α-Al(OH)3: A batch and TRLFS study. J. Colloid Interface Sci. 2009, 332, 158–164. [Google Scholar] [CrossRef]
- Singh, B.P.; Menchavez, R.; Takai, C.; Fuji, M.; Takahashi, M. Stability of dispersions of colloidal alumina particles in aqueous suspensions. J. Colloid Interface Sci. 2005, 291, 181–186. [Google Scholar] [CrossRef]
- Steefel, C.I.; van Cappellen, P.; Nagy, K.L.; Lasaga, A.C. Modeling water-rock interaction in the surficial environment: The role of precursors, nucleation and Ostwald ripening. Chem. Geol. 1990, 84, 322–325. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W. Sol–Gel Science. In The Physics and Chemistry of Sol–Gel Processing; Harcourt Brace Jovanovich: Boston, MA, USA, 1989. [Google Scholar]
- Yucelen, G.I.; Kang, D.-Y.; Guerrero-Ferreira, R.C.; Wright, E.R.; Beckham, H.W.; Nair, S. Shaping single-walled metal oxide nanotubes from precursors of controlled curvature. Nano Lett. 2012, 12, 827–832. [Google Scholar] [CrossRef]
- Kuc, A.; Heine, T. Shielding nanowires and nanotubes with imogolite: A route to nanocables. Adv. Mater. 2009, 21, 4353–4356. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Arancibia-Miranda, N.; Escudey, M.; Molina, M.; García-González, M.T. Kinetic and Surface Study of Single-Walled Aluminosilicate Nanotubes and Their Precursors. Nanomaterials 2013, 3, 126-140. https://doi.org/10.3390/nano3010126
Arancibia-Miranda N, Escudey M, Molina M, García-González MT. Kinetic and Surface Study of Single-Walled Aluminosilicate Nanotubes and Their Precursors. Nanomaterials. 2013; 3(1):126-140. https://doi.org/10.3390/nano3010126
Chicago/Turabian StyleArancibia-Miranda, Nicolás, Mauricio Escudey, Mauricio Molina, and María Teresa García-González. 2013. "Kinetic and Surface Study of Single-Walled Aluminosilicate Nanotubes and Their Precursors" Nanomaterials 3, no. 1: 126-140. https://doi.org/10.3390/nano3010126
APA StyleArancibia-Miranda, N., Escudey, M., Molina, M., & García-González, M. T. (2013). Kinetic and Surface Study of Single-Walled Aluminosilicate Nanotubes and Their Precursors. Nanomaterials, 3(1), 126-140. https://doi.org/10.3390/nano3010126




