Elemental Composition of Magnetic Nanoparticles in Wildland–Urban Interface Fire Ashes Revealed by Single Particle-Inductively Coupled Plasma-Time-of-Flight-Mass Spectrometer
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Location and Sample Collection
2.2. Magnetic Particles Separation
2.3. Multi-Metal Nanoparticle Composition on Single Particle Basis
2.4. Statistical Analysis
3. Results
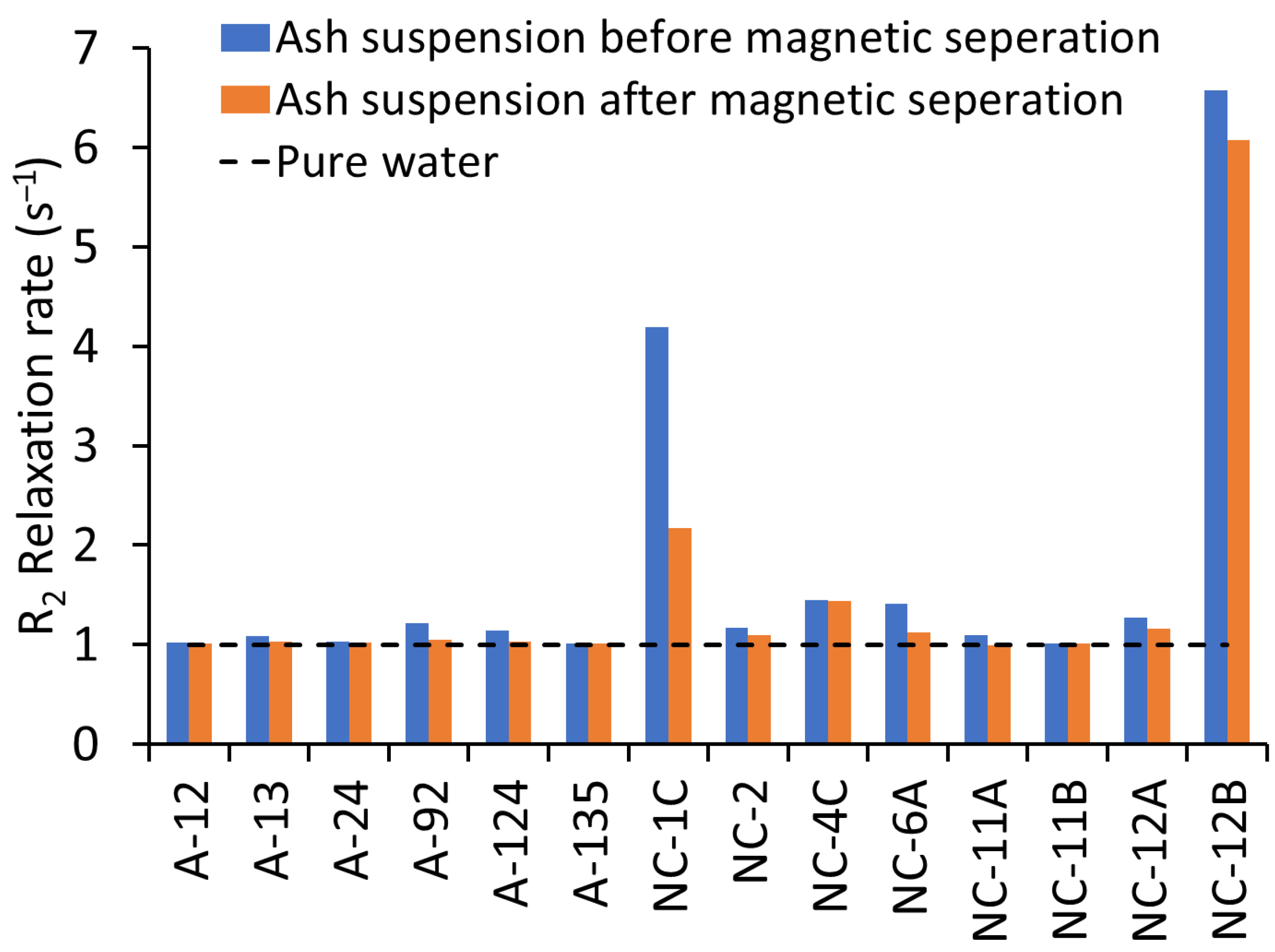
3.1. Efficiency of the Magnetic Separation
3.2. Particle Number Concentration
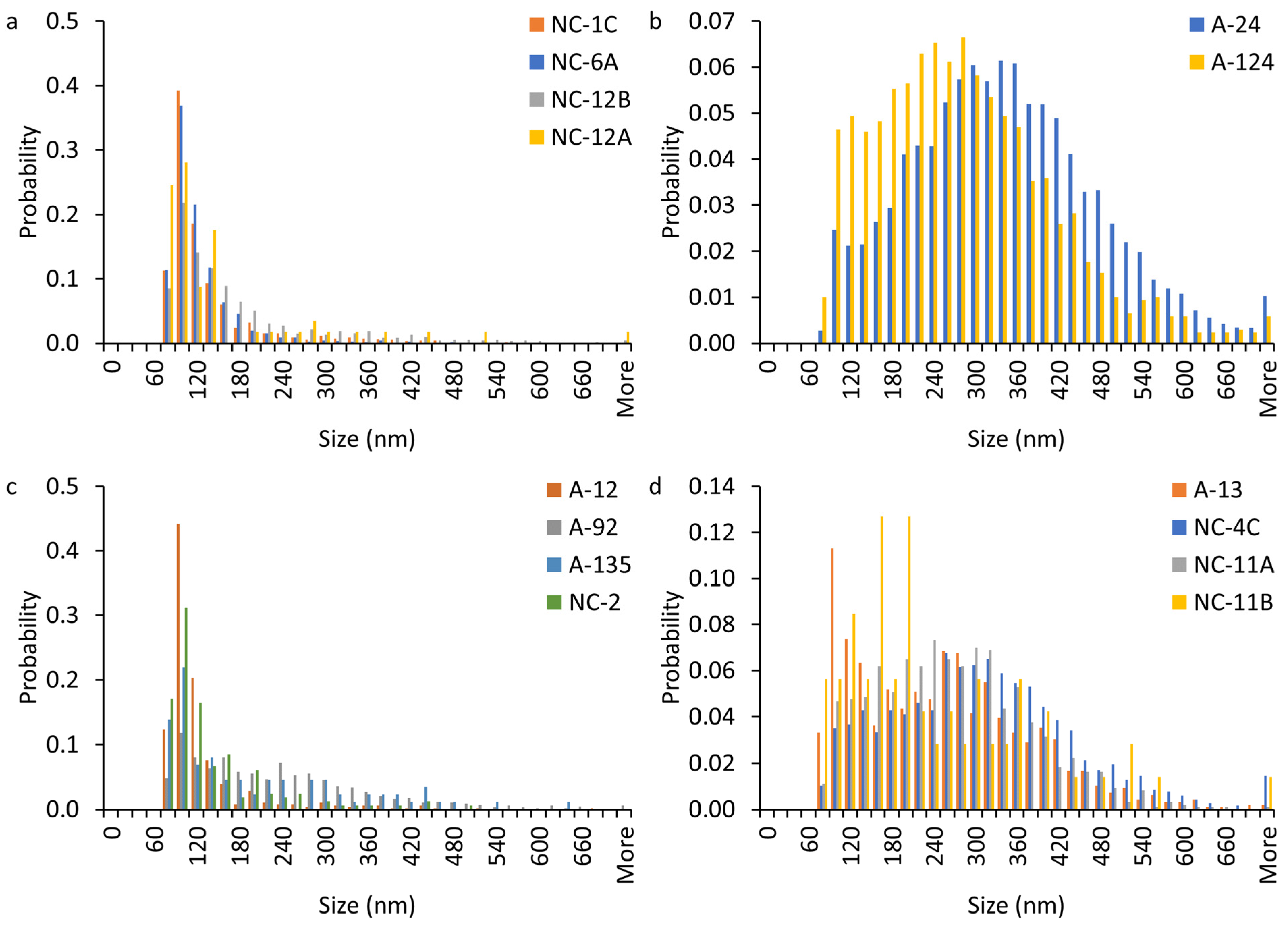
3.3. Particle Size Distribution
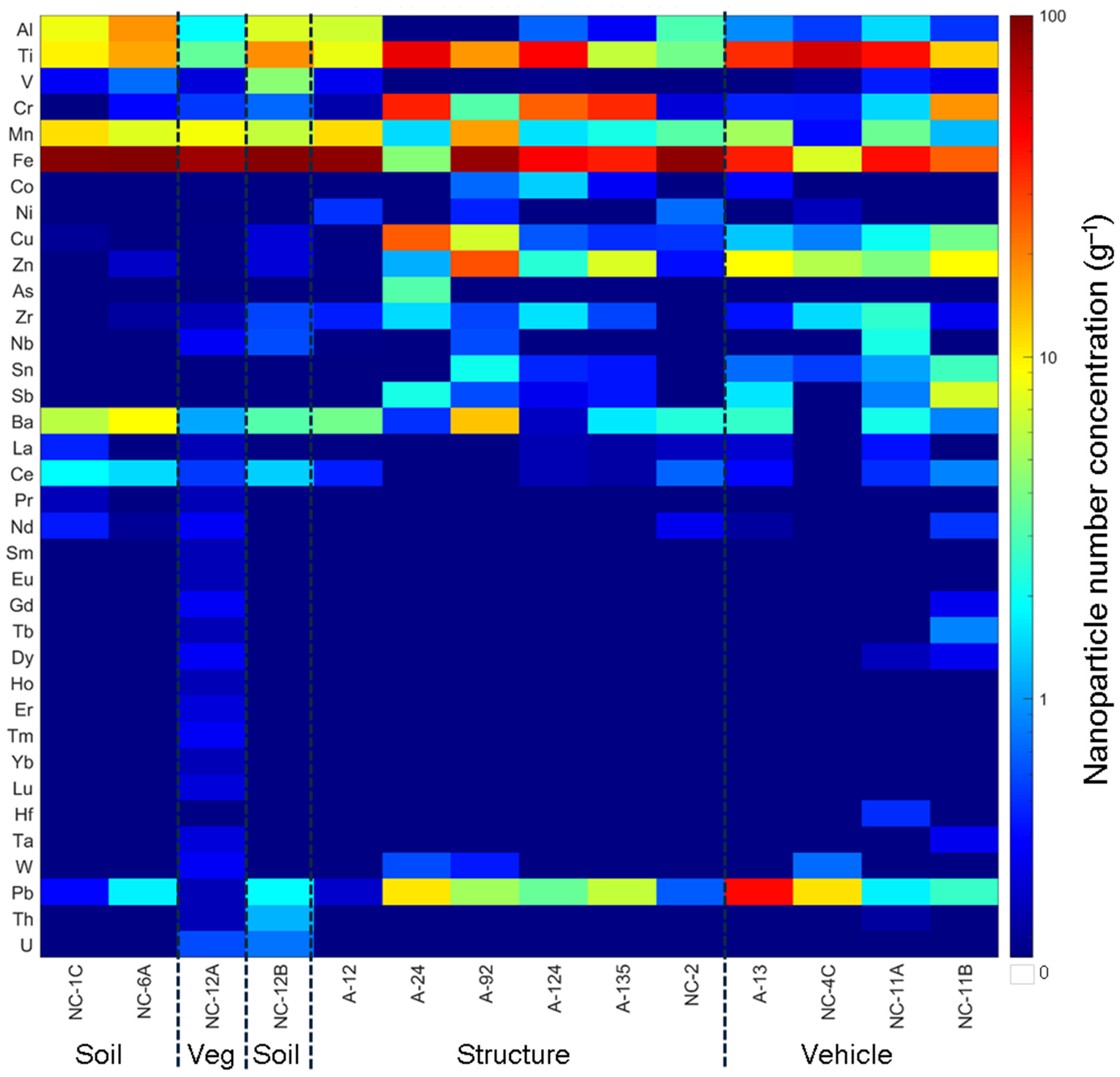
3.4. Particle Composition
3.4.1. smNPs vs. mmNPs
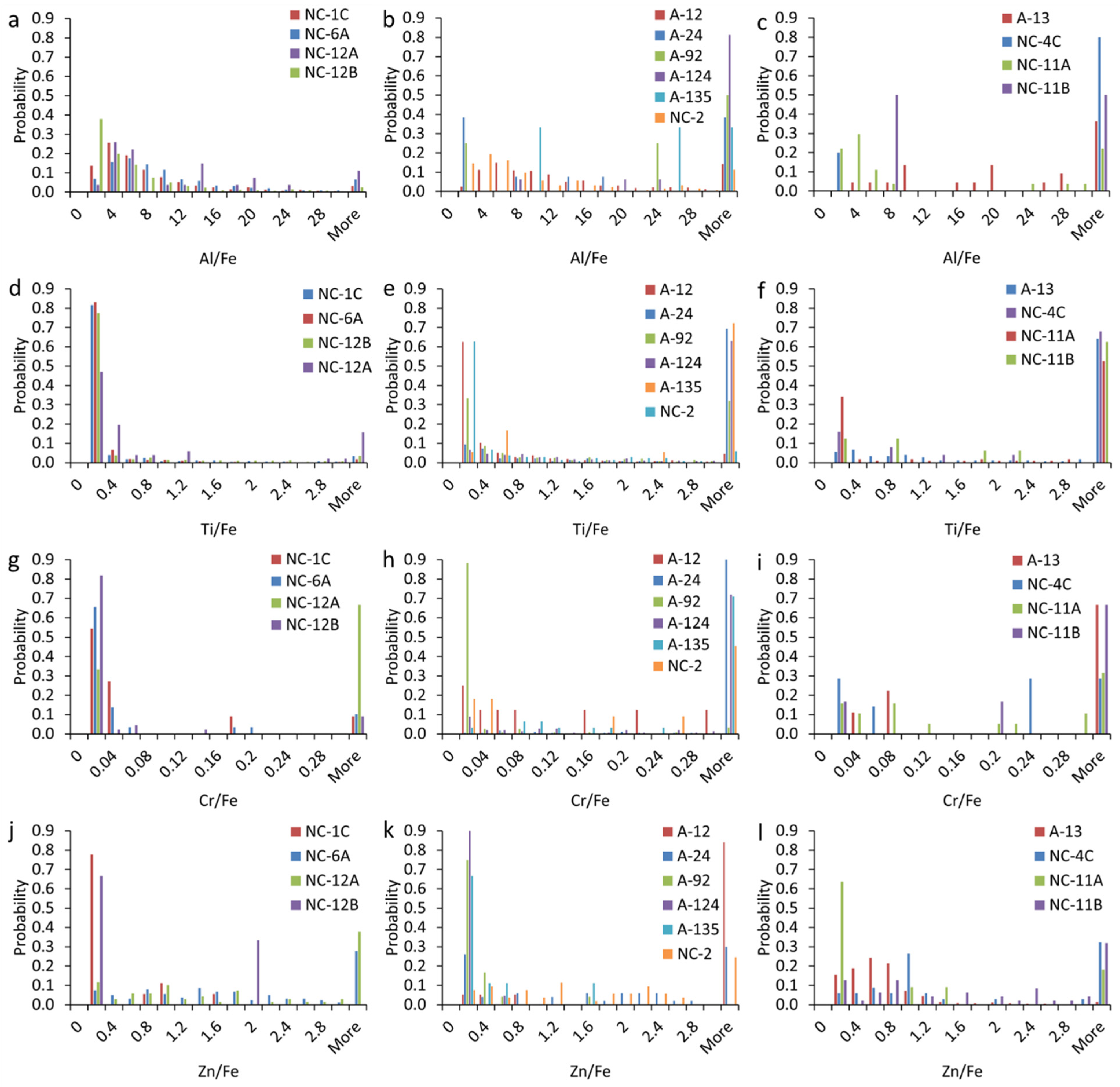
3.4.2. Elemental Ratios in mmNPs
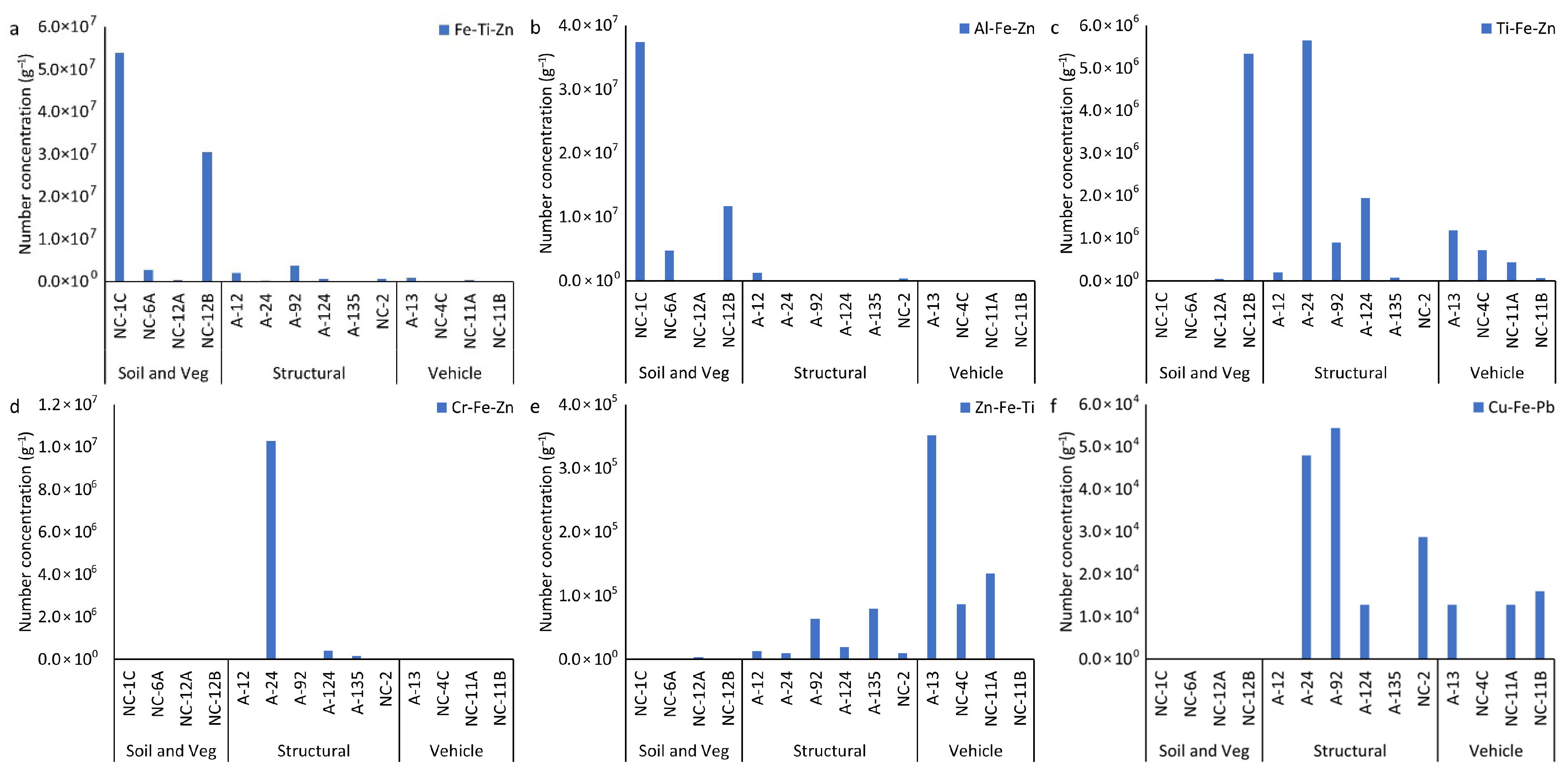
3.4.3. Multi-Element Nanoparticle Clusters
4. Discussion
5. Conclusions and Environmental Implications
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- National Academies of Sciences, Engineering, and Medicine. The Chemistry of Fires at the Wildland-Urban Interface; The National Academies Press: Washington, DC, USA, 2022. [Google Scholar] [CrossRef]
- Bladon, K.D.; Emelko, M.B.; Silins, U.; Stone, M. Wildfire and the future of water supply. Environ. Sci. Technol. 2014, 48, 8936–8943. [Google Scholar] [CrossRef] [PubMed]
- Brito, D.Q.; Passos, C.J.S.; Muniz, D.H.; Oliveira-Filho, E.C. Aquatic ecotoxicity of ashes from Brazilian savanna wildfires. Environ. Sci. Pollut. Res. 2017, 24, 19671–19682. [Google Scholar] [CrossRef]
- Bodi, M.B.; Martin, D.A.; Balfour, V.N.; Santín, C.; Doerr, S.H.; Pereira, P.; Cerdà, A.; Mataix-Solera, J. Wildland fire ash: Production, composition and eco-hydro-geomorphic effects. Earth Sci. Rev. 2014, 130, 103–127. [Google Scholar]
- Wu, Y.; Fang, M.; Lan, L.; Zhang, P.; Rao, K.V.; Bao, Z. Rapid and direct magnetization of goethite ore roasted by biomass fuel. Sep. Purif. Technol. 2012, 94, 34–38. [Google Scholar] [CrossRef]
- Abd Rashid, R.Z.; Salleh, H.M.; Ani, M.H.; Yunus, N.A.; Akiyama, T.; Purwanto, H. Reduction of low grade iron ore pellet using palm kernel shell. Renew. Energy 2014, 63, 617–623. [Google Scholar] [CrossRef]
- Baalousha, M.; Desmau, M.; Singerling, S.; Webster, J.P.; Matiasek, S.; Stern, M.A.; Alpers, C.N. Discovery and potential ramifications of reduced iron-bearing nanomaterials—Magnetite, wüstite, and zero valent iron—In wildland-urban interface fire ashes. Environ. Sci. Nano 2022, 9, 4149. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, B.; Hower, J.; Schindler, M.; Winkler, C.; Brandt, J.; Di Giulio, R.; Ge, J.; Liu, M.; Fu, Y.; et al. Discovery and ramifications of incidental Magnéli phase generation and release from industrial coal-burning. Nat. Commun. 2017, 8, 194. [Google Scholar] [CrossRef]
- McDaniel, D.K.; Ringel-Scaia, V.M.; Morrison, H.A.; Coutermarsh-Ott, S.; Council-Troche, M.; Angle, J.W.; Perry, J.B.; Davis, G.; Leng, W.; Minarchick, V. Pulmonary exposure to Magnéli phase titanium suboxides results in significant macrophage abnormalities and decreased lung function. Front. Immunol. 2019, 10, 2714. [Google Scholar] [CrossRef]
- Alam, M.; Sitter, J.D.; Vannucci, A.K.; Webster, J.P.; Matiasek, S.J.; Alpers, C.N.; Baalousha, M. Environmentally persistent free radicals and other paramagnetic species in wildland-urban interface fire ashes. Chemosphere 2024, 363, 142950. [Google Scholar] [CrossRef] [PubMed]
- Lyu, H.; He, Y.; Tang, J.; Hecker, M.; Liu, Q.; Jones, P.D.; Codling, G.; Giesy, J.P. Effect of pyrolysis temperature on potential toxicity of biochar if applied to the environment. Environ. Pollut. 2016, 218, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Godlewska, P.; Ok, Y.S.; Oleszczuk, P. THE DARK SIDE OF BLACK GOLD: Ecotoxicological aspects of biochar and biochar-amended soils. J. Hazard. Mater. 2021, 403, 123833. [Google Scholar] [CrossRef] [PubMed]
- Buss, W.; Mašek, O.; Graham, M.; Wüst, D. Inherent organic compounds in biochar–Their content, composition and potential toxic effects. J. Environ. Manag. 2015, 156, 150–157. [Google Scholar] [CrossRef]
- Burling, I.R.; Yokelson, R.J.; Griffith, D.W.T.; Johnson, T.J.; Veres, P.; Roberts, J.M.; Warneke, C.; Urbanski, S.P.; Reardon, J.; Weise, D.R.; et al. Laboratory measurements of trace gas emissions from biomass burning of fuel types from the southeastern and southwestern United States. Atmos. Chem. Phys. 2010, 10, 11115–11130. [Google Scholar] [CrossRef]
- Wu, J.; Tou, F.; Yang, Y.; Liu, C.; Hower, J.C.; Baalousha, M.; Wang, G.; Liu, M.; Hochella, M.F. Metal-Containing Nanoparticles in Low-Rank Coal-Derived Fly Ash from China: Characterization and Implications toward Human Lung Toxicity. Environ. Sci. Technol. 2021, 55, 6644–6654. [Google Scholar] [CrossRef]
- Alshehri, T.; Wang, J.; Singerling, S.A.; Gigault, J.; Webster, J.P.; Matiasek, S.J.; Alpers, C.N.; Baalousha, M. Wildland-urban interface fire ashes as a major source of incidental nanomaterials. J. Hazard. Mater. 2023, 443, 130311. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Alshehri, T.; Wang, J.; Singerling, S.A.; Alpers, C.N.; Baalousha, M. Identification and quantification of Cr, Cu, and As incidental nanomaterials derived from CCA-treated wood in wildland-urban interface fire ashes. J. Hazard. Mater. 2023, 445, 130608. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; González-Maciel, A.; Mukherjee, P.S.; Reynoso-Robles, R.; Pérez-Guillé, B.; Gayosso-Chávez, C.; Torres-Jardón, R.; Cross, J.V.; Ahmed, I.A.M.; Karloukovski, V.V.; et al. Combustion- and friction-derived magnetic air pollution nanoparticles in human hearts. Environ. Res. 2019, 176, 108567. [Google Scholar] [CrossRef] [PubMed]
- Pankhurst, Q.; Hautot, D.; Khan, N.; Dobson, J. Increased Levels of Magnetic Iron Compounds in Alzheimer’s Disease. J. Alzheimer’s Dis. 2008, 13, 49–52. [Google Scholar]
- Hautot, D.; Pankhurst, Q.A.; Khan, N.; Dobson, J. Preliminary evaluation of nanoscale biogenic magnetite in Alzheimer’s disease brain tissue. Proc. R. Soc. Lond. Ser. B 2003, 270, S62–S64. [Google Scholar]
- Till, J.L.; Moskowitz, B.; Poulton, S.W. Magnetic properties of plant ashes and their influence on magnetic signatures of fire in soils. Front. Earth Sci. 2021, 8, 592659. [Google Scholar] [CrossRef]
- Jordanova, N.; Jordanova, D.; Barrón, V. Wildfire severity: Environmental effects revealed by soil magnetic properties. Land Degrad. Dev. 2019, 30, 2226–2242. [Google Scholar] [CrossRef]
- Yamini, V.; Shanmugam, V.; Rameshpathy, M.; Venkatraman, G.; Ramanathan, G.; AL Garalleh, H.; Hashmi, A.; Brindhadevi, K.; Rajeswari, V.D. Environmental effects and interaction of nanoparticles on beneficial soil and aquatic microorganisms. Environ. Res. 2023, 236, 116776. [Google Scholar] [CrossRef]
- Maher, B.A.; Ahmed, I.A.M.; Karloukovski, V.; MacLaren, D.A.; Foulds, P.G.; Allsop, D.; Mann, D.M.A.; Torres-Jardón, R.; Calderon-Garciduenas, L. Magnetite pollution nanoparticles in the human brain. Proc. Natl. Acad. Sci. USA 2016, 113, 10797–10801. [Google Scholar] [PubMed]
- Lu, D.; Luo, Q.; Chen, R.; Zhuansun, Y.; Jiang, J.; Wang, W.; Yang, X.; Zhang, L.; Liu, X.; Li, F.; et al. Chemical multi-fingerprinting of exogenous ultrafine particles in human serum and pleural effusion. Nat. Commun. 2020, 11, 2567. [Google Scholar] [CrossRef]
- Könczöl, M.; Ebeling, S.; Goldenberg, E.; Treude, F.; Gminski, R.; Gieré, R.; Grobéty, B.; Rothen-Rutishauser, B.; Merfort, I.; Mersch-Sundermann, V. Cytotoxicity and Genotoxicity of Size-Fractionated Iron Oxide (Magnetite) in A549 Human Lung Epithelial Cells: Role of ROS, JNK, and NF-κB. Chem. Res. Toxicol. 2011, 24, 1460–1475. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Llort, J.; Weis, J.; Perron, M.M.G.; Basart, S.; Li, Z.; Sathyendranath, S.; Jackson, T.; Rodriguez, E.S.; Proemse, B.C.; et al. Widespread phytoplankton blooms triggered by 2019–2020 Australian wildfires. Nature 2021, 597, 370–375. [Google Scholar] [CrossRef]
- Ito, A.; Ye, Y.; Baldo, C.; Shi, Z. Ocean fertilization by pyrogenic aerosol iron. Npj Clim. Atmos. Sci. 2021, 4, 30. [Google Scholar] [CrossRef]
- Moteki, N.; Adachi, K.; Ohata, S.; Yoshida, A.; Harigaya, T.; Koike, M.; Kondo, Y. Anthropogenic iron oxide aerosols enhance atmospheric heating. Nat. Commun. 2017, 8, 15329. [Google Scholar] [CrossRef]
- Matsui, H.; Mahowald, N.M.; Moteki, N.; Hamilton, D.S.; Ohata, S.; Yoshida, A.; Koike, M.; Scanza, R.A.; Flanner, M.G. Anthropogenic combustion iron as a complex climate forcer. Nat. Commun. 2018, 9, 1593. [Google Scholar] [CrossRef]
- Janvrin, W.; Martin, J.; Hancock, D.; Varillas, A.; Downey, A.R.; Pellechia, P.J.; Satme, J.; Won, S.H. Open-source compact time-domain hydrogen (1H) NMR System for Field Deployment. HardwareX 2025, 22, e00651. [Google Scholar] [CrossRef]
- Martin, J.S.; Downey, A.R.J.; Baalousha, M.; Won, S.H. Rapid Measurement of Magnetic Particle Concentrations in Wildland-Urban Interface Fire Ashes and Runoff Using Compact NMR. IEEE Sens. J. 2023, 24, 7355–7363. [Google Scholar] [CrossRef]
- Baalousha, M.; Wang, J.; Erfani, M.; Goharian, E. Elemental fingerprints in natural nanomaterials determined using SP-ICP-TOF-MS and clustering analysis. Sci. Total Environ. 2021, 792, 148426. [Google Scholar] [CrossRef]
- Wang, J.; Nabi, M.D.M.; Erfani, M.; Goharian, E.; Baalousha, M. Identification and quantification of anthropogenic nanomaterials in urban rain and runoff using single particle-inductively coupled plasma-time of flight-mass spectrometry. Environ. Sci. Nano 2022, 9, 714–729. [Google Scholar] [CrossRef]
- Mehrabi, K.; Kaegi, R.; Günther, D.; Gundlach-Graham, A. Emerging investigator series: Automated single-nanoparticle quantification and classification: A holistic study of particles into and out of wastewater treatment plants in Switzerland. Environ. Sci. Nano 2021, 8, 1211. [Google Scholar] [CrossRef]
- Pace, H.E.; Rogers, N.J.; Jarolimek, C.; Coleman, V.A.; Higgins, C.P.; Ranville, J.F. Determining transport efficiency for the purpose of counting and sizing nanoparticles via single particle inductively coupled plasma mass spectrometry. Anal. Chem. 2011, 83, 9361–9369. [Google Scholar] [CrossRef]
- Alam, M.; Singerling, S.A.; Erfani, M.; Alpers, C.N.; Baalousha, M. Comparative study of the elemental composition of metal(loid)-bearing nanomaterials in wildland-urban interface fire ashes using icp-TOF-MS. Environ. Sci. Nano 2025, 12, 3217–3230. [Google Scholar] [CrossRef]
- Tada-Oikawa, S.; Ichihara, G.; Fukatsu, H.; Shimanuki, Y.; Tanaka, N.; Watanabe, E.; Suzuki, Y.; Murakami, M.; Izuoka, K.; Chang, J.; et al. Titanium Dioxide Particle Type and Concentration Influence the Inflammatory Response in Caco-2 Cells. Int. J. Mol. Sci. 2016, 17, 576. [Google Scholar] [CrossRef]
- Reimann, C.; Koller, F.; Frengstad, B.; Kashulina, G.; Niskavaara, H.; Englmaier, P. Comparison of the element composition in several plant species and their substrate from a 1500000-km2 area in Northern Europe. Sci. Total Environ. 2001, 278, 87–112. [Google Scholar] [CrossRef]
- Hosseini, S.; Li, Q.; Cocker, D.; Weise, D.; Miller, A.; Shrivastava, M.; Miller, J.W.; Mahalingam, S.; Princevac, M.; Jung, H. Particle size distributions from laboratory-scale biomass fires using fast response instruments. Atmos. Chem. Phys. 2010, 10, 8065–8076. [Google Scholar] [CrossRef]
- Lighty, J.S.; Veranth, J.M.; Sarofim, A.F. Combustion aerosols: Factors governing their size and composition and implications to human health. J. Air Waste Manag. Assoc. 2000, 50, 1565–1618. [Google Scholar] [CrossRef] [PubMed]
- Sunaryono, S.; Taufiq, A.; Mashuri, M.; Pratapa, S.; Zainuri, M.; Triwikantoro, T.; Darminto, D. Various magnetic properties of magnetite nanoparticles synthesized from iron-sands by coprecipitation method at room temperature. Mater. Sci. Forum 2015, 827, 229–234. [Google Scholar] [CrossRef]
- Tombach, B.; Reimer, P. Soluble paramagnetic chelates and stabilized colloidal particle solutions of iron oxides as contrast agents for magnetic resonance imaging. Curr. Med. Chem. 2005, 12, 2795–2804. [Google Scholar] [CrossRef]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2003. [Google Scholar]
- Choi, B.J.; Kim, S.H.; Jeon, Y.T.; Moon, J.Y.; Lee, G.H.; Chang, Y.; Park, J. Magnetic properties of γ-Fe2O3 nanoparticles. Int. J. Mod. Phys. B 2006, 20, 4422–4425. [Google Scholar] [CrossRef]
- Zulfiqar; Rahman, M.U.; Usman, M.; Hasanain, S.K.; Rahman, Z.U.; Ullah, A.; Kim, I.W. Static magnetic properties of Maghemite nanoparticles. J. Korean Phys. Soc. 2014, 65, 1925–1929. [Google Scholar] [CrossRef]
- Tannous, C.; Gieraltowski, J. Magnetic Properties: From Traditional to Spintronic. In Springer Handbook of Electronic and Photonic Materials, Springer Handbooks; Kasap, S., Capper, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar] [CrossRef]
- Gutfleisch, O.; Willard, M.A.; Brück, E.; Chen, C.H.; Sankar, S.G.; Liu, J.P. Magnetic Materials and Devices for the 21st Century: Stronger, Lighter, and More Energy Efficient. Adv. Mater. 2011, 23, 821–842. [Google Scholar] [CrossRef]
- Si, P.Z.; Wang, H.X.; Jiang, W.; Lee, J.G.; Choi, C.J.; Söhnel, T. Structure and magnetic properties of manganese oxide nanoparticles prepared by arc sublimation. Mod. Phys. Lett. B 2010, 24, 3025–3032. [Google Scholar] [CrossRef]
- Ahmad, T.; Ramanujachary, K.; Lofland, S.E.; Ganguli, A.K. Nanorods of manganese oxalate: A single source precursor to different manganese oxide nanoparticles (MnO, Mn2O3, Mn3O4). J. Mater. Chem. 2004, 14, 3406–3410. [Google Scholar] [CrossRef]
- Bañobre-López, M.; Vázquez-Vázquez, C.; Rivas, J.; López-Quintela, M.A. Magnetic properties of chromium (III) oxide nanoparticles. Nanotechnology 2003, 14, 318–322. [Google Scholar] [CrossRef]
- Rinaldi-Montes, N.; Gorria, P.; Fuertes, A.B.; Martínez-Blanco, D.; Amghouz, Z.; Puente-Orench, I.; Olivi, L.; Herrero-Martín, J.; Fernandez-Garcia, M.P.; Alonso, J.; et al. Entangled core/shell magnetic structure driven by surface magnetic symmetry-breaking in Cr2O3 nanoparticles. J. Mater. Chem. C 2022, 10, 1798–1807. [Google Scholar] [CrossRef]
- Chamberland, B.L. The chemical and physical properties of CrO2 and tetravalent chromium oxide derivatives. Crit. Rev. Solid State Mater. Sci. 1997, 7, 1–31. [Google Scholar] [CrossRef]
- Sutto, E.T. Magnetite fine particle and nanoparticle environmental contamination from industrial uses of coal. Environ. Pollut. 2018, 243 Pt A, 528–533. [Google Scholar] [CrossRef]
- Oudin, A.; Segersson, D.; Adolfsson, R.; Forsberg, B. Association between air pollution from residential wood burning and dementia incidence in a longitudinal study in Northern Sweden. PLoS ONE 2018, 13, e0198283. [Google Scholar] [CrossRef]
- Schuller, A.; Montrose, L. Influence of Woodsmoke Exposure on Molecular Mechanisms Underlying Alzheimer’s Disease: Existing Literature and Gaps in Our Understanding. Epigenetics Insights 2020, 13, 2516865720954873. [Google Scholar] [CrossRef]
- Maher, B.A. Airborne magnetite-and iron-rich pollution nanoparticles: Potential neurotoxicants and environmental risk factors for neurodegenerative disease, including Alzheimer’s disease. J. Alzheimer’s Dis. 2019, 71, 361–375. [Google Scholar] [CrossRef]
- Nowak-Jary, J.; Machnicka, B. Comprehensive Analysis of the Potential Toxicity of Magnetic Iron Oxide Nanoparticles for Medical Applications: Cellular Mechanisms and Systemic Effects. Int. J. Mol. Sci. 2024, 25, 12013. [Google Scholar] [CrossRef]
- Luo, X.; Ren, B.; Hursthouse, A.S.; Jiang, F.; Deng, R.J. Potentially toxic elements (PTEs) in crops, soil, and water near Xiangtan manganese mine, China: Potential risk to health in the foodchain. Environ. Geochem Health 2020, 42, 1965–1976. [Google Scholar] [CrossRef]
- Su, C. Environmental implications and applications of engineered nanoscale magnetite and its hybrid nanocomposites: A review of recent literature. J. Hazard. Mater. 2017, 322, 48–84. [Google Scholar] [CrossRef] [PubMed]
- Neal, A.P.; Guilarte, T.R. Mechanisms of lead and manganese neurotoxicity. Toxicol. Res. 2013, 2, 99–114. [Google Scholar] [CrossRef]
- Iavicoli, I.; Fontana, L.; Bergamaschi, A. The Effects of Metals as Endocrine Disruptors. J. Toxicol. Environ. Health Part B 2009, 12, 206–223. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.; Huh, M.; Ryu, B.; Do, J.; Jin, S.U.; Moon, M.J.; Jung, J.C.; Chang, Y.; Kim, E.; Chi, S.G.; et al. The effect of static magnetic fields on the aggregation and cytotoxicity of magnetic nanoparticles. Biomaterials 2011, 32, 9401–9414. [Google Scholar] [CrossRef] [PubMed]
- DeBano, L.F.; Neary, D.G.; Ffolliott, P.F. Fire Effects on Ecosystems; John Wiley & Sons: Hoboken, NJ, USA, 1998. [Google Scholar]
- Gunawan, C.; Sirimanoonphan, A.; Teoh, W.Y.; Marquis, C.P.; Amal, R. Submicron and nano formulations of titanium dioxide and zinc oxide stimulate unique cellular toxicological responses in the green microalga Chlamydomonas reinhardtii. J. Hazard. Mater. 2013, 260, 984–992. [Google Scholar] [CrossRef] [PubMed]
- inciweb. Incident Information System. North Complex. United States Department of Agriculture. Burned-Area Report: North Complex; United States Department of Agriculture FOREST SERVICE, 2020. 2022. Available online: https://inciweb.nwcg.gov/incident/6997/ (accessed on 29 July 2025).
- CALFire. LNU Lightning Complex (Includes Hennessey, Gamble, 15-10, Spanish, Markley, 13-4, 11-16, Walbridge) Incident. 2022. Available online: https://www.fire.ca.gov/incidents/2020/8/17/lnu-lightning-complex-includes-hennessey-gamble-15-10-spanish-markley-13-4-11-16-walbridge/ (accessed on 29 July 2025).
- Gundlach-Graham, A.; Hendriks, L.; Mehrabi, K.; Günther, D. Monte Carlo Simulation of Low-Count Signals in Time-of-Flight Mass Spectrometry and Its Application to Single-Particle Detection. Anal. Chem. 2018, 90, 11847–11855. [Google Scholar] [CrossRef] [PubMed]
- Rousseeuw, P.J. Silhouettes: A graphical aid to the interpretation and validation of cluster analysis. J. Comput.-Tional Appl. Math. 1987, 20, 53–65. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alam, M.; Downey, A.R.J.; Cai, B.; Baalousha, M. Elemental Composition of Magnetic Nanoparticles in Wildland–Urban Interface Fire Ashes Revealed by Single Particle-Inductively Coupled Plasma-Time-of-Flight-Mass Spectrometer. Nanomaterials 2025, 15, 1420. https://doi.org/10.3390/nano15181420
Alam M, Downey ARJ, Cai B, Baalousha M. Elemental Composition of Magnetic Nanoparticles in Wildland–Urban Interface Fire Ashes Revealed by Single Particle-Inductively Coupled Plasma-Time-of-Flight-Mass Spectrometer. Nanomaterials. 2025; 15(18):1420. https://doi.org/10.3390/nano15181420
Chicago/Turabian StyleAlam, Mahbub, Austin R. J. Downey, Bo Cai, and Mohammed Baalousha. 2025. "Elemental Composition of Magnetic Nanoparticles in Wildland–Urban Interface Fire Ashes Revealed by Single Particle-Inductively Coupled Plasma-Time-of-Flight-Mass Spectrometer" Nanomaterials 15, no. 18: 1420. https://doi.org/10.3390/nano15181420
APA StyleAlam, M., Downey, A. R. J., Cai, B., & Baalousha, M. (2025). Elemental Composition of Magnetic Nanoparticles in Wildland–Urban Interface Fire Ashes Revealed by Single Particle-Inductively Coupled Plasma-Time-of-Flight-Mass Spectrometer. Nanomaterials, 15(18), 1420. https://doi.org/10.3390/nano15181420






