Abstract
Recently, the development of nanoparticle pigments has attracted interest in chemical preparation due to their potential functional properties, such as phosphate-based pigments. The present research focuses on the feasibility of synthesising the BaCr2(P2O7)2 pigment under hydrothermal conditions. The effect of the microstructural features of ceramic pigments (the crystalline structure, morphology, and particle size) on their optical properties (colour and reflectance) was also studied. The BaCr2(P2O7)2 compound was prepared in different fluid media, including water and NaOH solutions (0.5–1.0 M), at several reaction temperatures (170–240 °C) and intervals (6–48 h). The single-phase BaCr2(P2O7)2 did not crystallise without by-products (BaCr2O10, BaCr2(PO7)2) in water and the alkaline solutions, even at 240 °C for 48 h; in these fluids, the ionic Cr3+ species oxidised to Cr6+. In contrast, the BaCr2(P2O7)2 single-phase crystallisation was favoured by adding urea as a reductant agent (25.0–300.0 mmol). Monodispersed BaCr2(P2O7)2 fine particles with a mean size of 44.0 nm were synthesised at a low temperature of 170 °C for 6 h with 0.5 M NaOH solution in the presence of 50.0 mmol urea. The phosphate pigment particle grew to approximately 62.0 nm by increasing the treatment temperature to 240 °C. A secondary dissolution–recrystallisation achieved after 24 h triggered a change in the particle morphology coupled with the incrementation of the concentration of NaOH in the solution. The pyrophosphate BaCr2(P2O7)2 pigments prepared in this study belong to the green colour spectral space according to the CIELab coordinates measurement, and exhibit 67.5% high near-infrared (NIR) solar reflectance.
1. Introduction
Extensive attention has been paid to the preparation of inorganic pigments based on anhydrous pyrophosphates, i.e., M2P2O7, CaM3(P2O7)2 and M2(P2O7)2 (M = Co, Ni, among others), since they exhibit interesting physical and chemical functional properties. In addition, these pigments have promising potential in a wide variety of applications in areas including catalysis, magnetic ferroelectrics, optical sensors and luminescence [1,2,3,4,5,6,7,8,9]. In the last decade, especially new ceramic pigments in the pyrophosphate group, with various colours and high chemical stability, have attracted much attention in the chemical industry. According to the crystalline structure, the M site in the phosphate network structure can be occupied by various transition metal cations, producing a variety of solid solutions with good crystalline structural stability. In particular, Zn2P2O7 [10], BaCuP2O7 [11], (Mg/Cu)2P2O7 [12], and Ni2P2O7 [13] have been widely studied. The employment of chromium improves both thermal and chemical stability at elevated temperatures, which hinders the corrosion of oxide pigments by a glassy liquid phase at elevated temperatures, consequently producing satisfactory green optical tonalities in the coated material. Oxide-based nano-pigments containing chromium (III) [14], such as the spinel-structured cobalt chromium (CoCr2O4) [6,15] and cubic garnet known as Victoria green pigment (Ca3Cr2Si3O12) [6], exhibit outstanding stability. Therefore, the pyrophosphate compounds are likely candidates for obtaining a new green hue pigment incorporating Cr3+. Indeed, the first attempt to produce the single-phase green hue BaCr2(P2O7)2 was recently prepared via solid-state reaction; the raw reagent grade BaCO3, Cr2O3 and NH4H2PO4 powders were heated at 1200 °C for 8 h under a reducing atmosphere (H2:N2 = 5:95). In addition to its green colour tonality, the BaCr2(P2O7)2 particles with a mean particle size of 3.0 mm exhibited a high diffuse reflectance response of 85.0% in the near-infrared (NIR) spectrum between 700 and 1800 nm. A detailed crystalline structural analysis led to the establishment of the methodology for developing these oxides. However, a detailed correlation between the microstructural features (morphology and particle size) and the optical features was not found [1].
The hydrothermal processing technique has been effective in the processing of ternary inorganic pigment compounds such as the silicates (BaCuSiO6), which can be crystallised with controlled 3D hierarchical architectures produced by nanostructured primary crystals, followed by spontaneous self-assembly during the growth of the fine pigment aggregates [16]. Our group has recently investigated the synthesis of the analogous silicate pigment Victorian green containing Cr3+ ions under alkaline hydrothermal conditions. The crystallisation of the Ca3Cr2Si3O12 proceeded in highly concentrated NaOH solutions between 2.5–5.0 M at 200 °C for 3 h via a single-step chemical reaction. The garnet-structured particles synthesised by the dissolution of a precursor Ca3Cr2Si3(OH)24 coprecipitated; the solute crystallised simultaneously to form euhedral-shaped nanometric-sized crystals (approximately 10 nm) that self-assembled to form 3D hierarchical popcorn-shaped Ca3Cr2Si3O12 aggregates with sizes in the range of 66.0–156.0 nm [17]. Despite the severe conditions, the oxidation of the precursor Cr3+ ions did not occur in the highly concentrated alkaline hydrothermal medium without reductant reagents and particle-size organic modifiers to assist the crystallisation process. Additionally, the optical diffuse reflectance measurements in the NIR (700–2500 nm) revealed that the hydrothermally prepared Victorian green aggregates exhibit a high response of 95.0%, proving this compound’s potential as a cold pigment [18].
The single-phase BaCr2(P2O7)2 has not been prepared before by a soft chemical processing route, i.e., hydrothermal processing, to the best of our knowledge. Therefore, in this study, we have evaluated the feasibility of obtaining the BaCr2(P2O7)2 crystalline phase via a single-step reaction in alkaline solutions under hydrothermal conditions, and simultaneously, controlling the microstructural aspect (size and morphology) of the particles towards optimising the NIR solar reflectance and the colour chromatic properties of this green pigment. In addition, a systematic analysis devoted to reducing the chemical oxidation potential of the alkaline media was carried out by adding a reductant agent (urea), due to the oxidation reaction of Cr3+ to Cr6+ being the major chemical challenge that might hinder the single-step reaction, to solely produce the target BaCr2(P2O7)2 phase. Hence, the preliminary experiments aimed to produce the pyrophosphate pigment in mildly acidic and concentrated NaOH (0.5–1.0 M) solutions under hydrothermal conditions from an ecological point of view. In addition, the use of urea reactant prevents the oxidation of Cr3+ to Cr6+, hindering the presence of Cr(VI)-containing species as by-products. UV-vis NIR structural analyses of BaCr2(P2O7)2 green pigments were carried out to discuss the differences in the optical pigment properties based on the particle size variation caused by the variations in the experimental parameters (temperature and reaction interval).
2. Materials and Methods
2.1. Materials
The pyrophosphate BaCr2(P2O7)2 particles were prepared using chemical-grade reagents of barium nitrate [Ba(NO3)2] (Sigma-Aldrich, Milwaukee, Wisconsin, USA, 99.0% purity), chromium nitrate nanohydrate [Cr(NO3)3·9H2O] (Sigma-Aldrich, Milwaukee, WI, USA, 99.0% purity) and phosphoric acid [H3PO4] (Sigma-Aldrich, Milwaukee, WI, USA, 85.0% purity), which were used for the precursor gel coprecipitation. The precursor solutions were prepared with deionised water (Hycel, Monterrey, N.L., Mexico) with various concentrations of 0.05 M Ba(NO3)2, 0.1 M Cr(NO3)3 and 0.2 M H3PO4. The NaOH (Sigma-Aldrich, Milwaukee, WI, USA, 99.0% purity) solution (0.5–1.0 M) was used as a hydrothermal solvent to control the solution pH, and urea powder reagent grade [Sigma-Aldrich Milwaukee, WI, USA, 99.0% purity, CO(NH2)2] was a reducing agent to prevent the oxidation of Cr3+ to Cr6+. TiO2 rutile-structured powder (Sigma-Aldrich 99.0% purity, <5.0 μm) was selected as a standard for comparison of the pyrophosphate optical properties characterisation.
2.2. Hydrothermal Treatments
Reaction mother liquor for the hydrothermal treatments was prepared by mixing equal volumes of the precursor solutions, with a Ba:Cr:P ratio of 7.5:7.5:7.5 mL, which corresponds to the pyrophosphate stoichiometric cationic Ba:Cr:P molar ratio of 1:2:4, adding Ba, Cr and P-containing solutions into the Teflon chamber bottom; and pouring 12.5 mL of water or NaOH solution. In a different set of experiments, urea powder [CO(NH2)2, 25 to 300 mmol/dm3] was placed at the bottom of the autoclave before adding any solution. It is noted that no precipitation occurred from the mixture liquor when water was mixed in, whilst a milky green colloid spontaneously coprecipitated on the addition of the NaOH solution. All experiments were carried out at a constant mother liquor solution volume of 35 mL, corresponding to a 50% autoclave filling ratio. The preliminary hydrothermal treatments were carried out at different reaction intervals (6–48 h) and various temperatures (170–240 °C) to investigate the chemical equilibrium that produces solely BaCr2(P2O7)2. After each treatment, the reaction products were vigorously washed three times with hot water (80 °C) to clean off the remaining mother liquor; the resultant powders were then dried overnight in an oven at 70 °C.
2.3. Characterisation
Powder X-ray diffraction (XRD): The analyses were conducted in an X-ray diffraction apparatus (Empyrean, Malvern Panalytical, Almelo, The Netherlands). Each sample was scanned at the following conditions: 40 kV and 20 mA, using Cu-Ka radiation (λ = 1.5418 Å). The XRD patterns were recorded in the 2θ range of 10–90° at a constant scanning speed of 10°/min with a step of 0.02°. Rietveld refinement analysis was carried out using the TOPAS 4.2 (Bruker AXS: Karlsruhe, Germany, 2009) software. The space group and the atomic spatial positions (Wyckoff number and coordinates) of the BaCr2(P2O7)2 crystalline structure were determined based on the card MP-1192170, German crystallographic database [19], shown in Table 1. Details of the parameters considered in the refining algorithm and the secondary phase crystallographic information (Table S1) are described in Section S1 of the Supplementary Supporting Information File (hereafter referred to as SSIF).

Table 1.
Atomic coordinates and Wyckoff positions of the BaCr2(P2O7)2 with triclinic structure (MP card no. 1192170, space group P–1, 2) were used to carry out the Rietveld refinements by TOPAS 4.2 software.
X-ray photoelectron spectroscopy (XPS): Compositional analyses of selected pigment powder samples prepared with and without urea were conducted by XPS. All the analyses were conducted by selecting the carbonaceous C 1s peak (284.6 eV) as a standard calibrating binding energy. The spectra were recorded on a Thermo Fisher Scientific Model K-Alpha, Waltham, MA, USA.
Fourier transform infrared spectroscopy (FT-IR): Additional structural details associated with atomic bond differences in the BaCr2(P2O7)2 and the presence of water molecules were investigated by FT–IR analyses, which were conducted using JASCO 4000 equipment (Hachioji, Tokyo, Japan). Pelletised samples consisting of 5 mg pigment powder and 200 mg KBr were used for the analyses. The powder samples were dried overnight at 60 °C before pellet preparation.
Morphology and microstructural observations: The microstructural aspects of the pyrophosphate BaCr2(P2O7)2 particles were observed using a field emission scanning electron microscope (FE-SEM JEOL JSM–7100F, Tokyo, Japan). The microscope was operated at 10 kV and 69 mA. The statistical analysis of particle size and its distribution was calculated using SEM images, considering 80 particles. Crystalline structural details of pyrophosphate particles were revealed by high-resolution transmission electron microscopy (HR–TEM, Philips Talos F200X, Seoul, Republic of Korea) operated at 200 kV.
Differential scanning calorimetry (DSC). The thermal stability of BaCr2(P2O7)2 powders was evaluated via thermogravimetry (TG) and differential scanning calorimetry analysis (Mettler Toledo 3+ DTA/DSC, Los Angeles, CA, USA). The sample was heated from 30 to 900 °C at a constant heating rate of 10 °C/min in an air atmosphere.
Optical properties. The colour CIELab* and reflectance spectra of pyrophosphate BaCr2(P2O7)2 powder were measured in a UV-Visible–NIR spectrometer (Jasco V-770, Hachioji, Tokyo, Japan) equipped with an integrating sphere device. Baseline calibration was carried out with BaSO4 fine powder as the reference, and the spectrometer colour space parameters were measured according to the standard CIELab* colourimetry method. Additional details on the measurement of band-gap, the chromatic parameters and the solar reflectance of the pigments are described in detail in Section SF1 in the SSIF.
3. Results and Discussion
The one-pot hydrothermal processing treatment was employed as a new chemical route for preparing synthetic BaCr2(P2O7)2 pigment particles, which have been prepared solely by the conventional solid-state reaction at high temperatures (1200 °C). Hence, the appropriate reaction conditions were investigated in terms of the solution pH to trigger the crystallisation of only the single-phase BaCr2(P2O7)2, taking into consideration the prevention of the oxidation of the precursor Cr3+ to Cr6+ by addition of a reductant agent (urea, CO(NH2)2) under hydrothermal conditions [20]. Table 2 summarises the relevant reaction conditions of selected experiments and the structural information on the reaction products calculated by Rietveld Analysis. Further details of all the experiments conducted in this work are given in Table S1 of the SSIF. The condition of the mother liquor strongly depends on the particle crystallisation under the hydrothermal treatment. In the case of water (pH 6.2), the formation of an amorphous powder is triggered due to the low chemical stability of the mother liquor under hydrothermal conditions. A different reaction pattern was observed in the case of the alkaline solutions, but two different crystalline phases, including the BaCr2(P2O7)2 pigment, were obtained in these experiments, as discussed in the next section.

Table 2.
Summary of the experiments proposed to investigate the preparation of BaCr2(P2O7)2 powders under hydrothermal conditions employing various solutions at different temperatures and reaction times.
3.1. Chemical Stability of the Pyrophosphate BaCr2(P2O7)2 Single-Phase Under Acidic and Alkaline Hydrothermal Conditions
Figure 1 shows the typical X-ray diffraction patterns of reaction products hydrothermally obtained at mildly acidic (pH = 6.17) and mild alkaline (pH = 12.5–13.59) conditions. In mildly acidic conditions, a green powder formed in the mother liquor irrespective of the treatment temperature; which exhibited no trace of crystalline phases, instead showing the amorphous phase (Figure 1a). Although precipitation proceeds in the mildly acidic media with counter NO3− anions, we surmise that crystallisation could not be triggered because the solute supersaturation stage to produce the metastable state required for embryo crystallisation is not reached in the fluid.
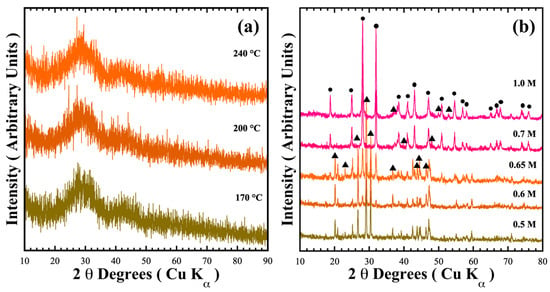
Figure 1.
XRD patterns of the reaction products obtained under hydrothermal conditions for 48 h with (a) water varying the temperature, and (b) at 240 °C with different NaOH solution concentrations. Crystalline phases: (●) BaCr2(P2O7)2 and (▲) BaHCr2PO10.
On the other hand, the treatment conducted in NaOH alkaline media provoked a different reaction pathway to form two major crystalline phases (Figure 1b). The peaks of the crystalline phases obtained in the 0.5 M NaOH solution were indexed with those of the pyrophosphate compounds BaCr2(P2O7)2 (●, MP Card No. 1192170) and BaHCr2PO10 (▲, COD card no. 96-901-6408). The content of each phase indexed on each XRD powder pattern was determined by a computer algorithm, which comprises two subroutines containing the crystallographic phase (spatial coordinates and Wykoff values) shown in Table 1 and Table S1 in the SSIF. Figure S2a,b show examples of the refinement calculation. The accuracy of the phase content computation is supported by the low values of the GOF χ2 parameter, which is associated with the algorithm’s refinement goodness. The ratio of single-phase BaCr2(P2O7)2 increased with the gradual augmentation of the NaOH concentration; the maximum yield of BaCr2(P2O7)2 was 75.9 ± 0.8 wt.% in the 0.65 M NaOH solution. In the 1.0 M NaOH solution, the target pyrophosphate pigment amount remained almost constant (74.0 ± 1.8 wt.%). These results indicate that the precursor coprecipitated a hydrous gel identified by Equation (1) (BaCr2P4(OH)28∙xH2Ogel) rapidly reacted in the alkaline media during the hydrothermal treatment. The chemical equilibrium that triggers the crystallisation of pyrophosphate compounds should be Equation (2), through the dimerisation of phosphate into pyrophosphate achieved in the pH alkaline range of 12.61–13.61 [21]. However, the coexistence of NO3− in the mother liquor could oxidise Cr3+ into Cr6+, resulting in the prevention of the complete crystallisation of the precursor hydrated gel BaCr2P4(OH)28∙xH2O to BaCr2(P2O7)2 pigment. To overcome this issue, addition of a reductant agent, namely urea, to prevent chromium oxidation, was used to prepare the sole formation of BaCr2(P2O7)2 [20].
The new series of preparation were conducted specifically at 240 °C for 48 h in a 0.7 M NaOH alkaline solution in the presence of designated concentrations of urea (25.0–300.0 mmol/dm3) under hydrothermal conditions to form green powders. Although the pigment synthesis proceeded at mild alkaline conditions of pH 9.46–10.66, the polyatomic anions (HCO3−) generated from urea decomposition decreased the pH of the mother liquor, which is likely to promote a reductive environment in mildly alkaline media.
Figure 2 shows the typical XRD pattern of the green powders obtained via the standard conditions mentioned above, with variation of the urea concentration. In the presence of 25 mmol/dm3 of urea, two phases of BaCr2(P2O7)2 and BaHCr2PO10 formed simultaneously. At the same time, the single-phase BaCr2(P2O7)2, for which the peaks in the XRD pattern were indexed with those in the MP Card No. 1192170, was formed in the presence of 50 mmol/dm3 urea, which is appropriate for completion of the precursor gel dissolution. Subsequently, solvent supersaturation with the solute triggers BaCr2(P2O7)2 embryonic nucleation and continuous growth, as suggested by the single-step chemical reaction given in Equation (3). The addition of 100 mmol/dm3 urea contents resulted in the formation of two phases: the primary BaCr2(P2O7)2 and amorphous species (Figure 2). The amount of the primary BaCr2(P2O7)2 phase gradually decreased with increasing amounts of urea (see Table 2). In the presence of >150 mmol/dm3 urea, the amorphous powder was formed during treatments conducted for 48 h. Interestingly, this reaction pathway is analogous to that observed under mildly acidic conditions. Urea saturation decreased the solvent pH from 12.88 to 9.7 (see Table 2), leading to limited pyrophosphate crystallisation because the solute supersaturation condition for triggering crystallisation did not occur at lower pH. Hence, the BaCr2(P2O7)2 particles crystallisation occurred preferentially at a mild pH range of 10.16 to 10.52 by a single-step reaction involving a dissolution–crystallisation mechanism [18]. The urea reductant agent controls the reaction media pH and avoids chromium (III) oxidation. Under these conditions, the precursor gel dehydration and dissolution occurred in the alkaline buffer medium. Consequently, solute supersaturation was reached in the solvent media, triggering the BaCr2(P2O7)2 compound crystallisation without byproducts, according to Equation (3).
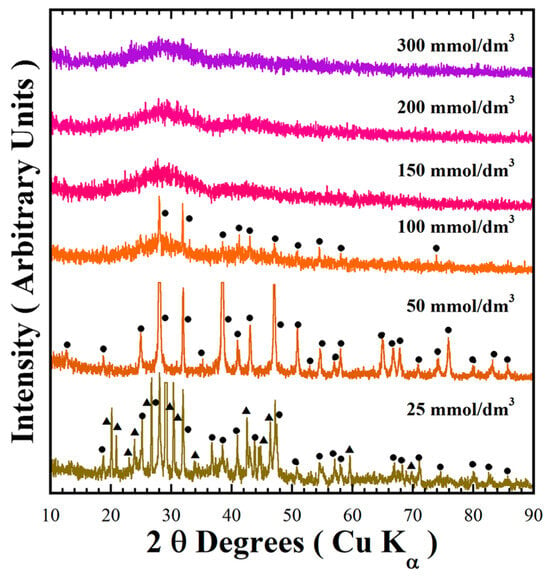
Figure 2.
XRD patterns of the reaction products obtained at 240 °C for 48 h using a 0.7 M NaOH solution with different urea contents. Crystalline phases (●) BaCr2(P2O7)2 and (▲) BaHCr2PO10.
3.2. Tailoring the BaCr2(P2O7)2 Pigment Synthesis Assisted by Urea Under Hydrothermal Conditions
Figure 3 shows XRD patterns of the powder products prepared in various conditions of mother liquors containing designated concentrations of NaOH (0.5–1.0 M) in the presence of 50 mmol/dm3 urea at different reaction temperatures (170–240 °C) for various reaction times (6–48 h) to check the stability of the pyrophosphate compounds. The major peak of the triclinic structured BaCr2(P2O7)2 with Miller index (001), observed at a 2θ angle of 12.50°, is too small in intensity [19]. This peak intensity did not increase with increasing NaOH concentration, while a gradual increase in intensity of the peak at a 2θ angle of 38.22° (as seen in Figure 3a) implied the preferential crystal growth of the BaCr2(P2O7)2 particles along the [112] crystallographic direction. The BaCr2(P2O7)2 single-phase crystallisation proceeds in mildly basic media; the urea decomposed above 120 °C to keep the Cr3+ species stable (Equation (3)).
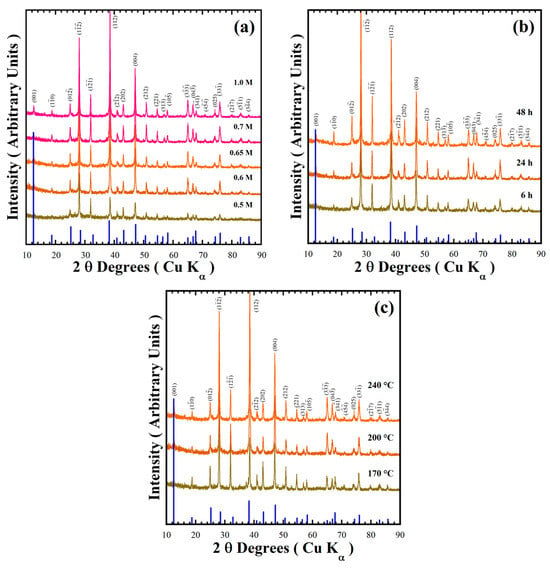
Figure 3.
X-ray diffraction patterns of BaCr2(P2O7)2 powders prepared with a concentration of 50 mmol/dm3 of urea, at 240 °C for 24 h varying (a) the NaOH solution concentration; (b) with a 0.7 M NaOH solution for different reaction times; and (c) for 24 h with a 0.7 M NaOH solution at various reaction temperatures. The blue solid lines correspond to the BaCr2(P2O7)2 single XRD pattern.
All diffraction patterns of the powders prepared in a 0.7 M NaOH solution at 240 °C for different reaction times were indexed with those of the BaCr2(P2O7)2 compound (Figure 3b). The reaction time did not remarkably affect the stability of the pyrophosphate pigment, indicating a short reaction time of 6 h is enough to obtain the single phase BaCr2(P2O7)2 crystal. Below a reaction interval of 24 h, the particles preferentially grew parallel to the crystallographic plane with Miller indexes (112) at 2θ = 38.22°. However, the particles coarsened above 24 h reaction time, and the peak at 2θ = 28.1°, parallel to the Miller index plane (1), which belongs to the {112} plane family, for the powder prepared for 48 h, increased remarkably in intensity (Figure S1 in the SSIF). A dissolution–recrystallisation reaction should occur, resulting in the change in particle coarsening associated with the increase in diffraction peaks intensities due to local volume molar changes of the solvent under hydrothermal conditions. The single-phase BaCr2(P2O7)2 compound was obtained even at 170 °C for 24 h (Figure 3c). Finally, the single-phase BaCr2(P2O7)2 powder was prepared in the NaOH solution at a sharp pH range of 10.16–10.53 in presence of 50.0 mmol/dm3 under hydrothermal conditions at 170–200 °C for less than 6 h.
The XRD results indicate that the oxidation reaction of Cr3+ to Cr6+ partially occurs in the hydrothermal fluid without addition of the urea agent. XPS analyses of the powders prepared at standard reaction conditions at 240 °C for 48 h in 0.7 M NaOH, in the absence and in the presence of urea as a reductant agent, were conducted to determine the effect of urea addition in terms of limitation of the oxidation reaction of Cr3+ to Cr6+ in the hydrothermal system. The typical XPS spectra of the Cr 2p3/2 and O 1s core levels are shown in Figure 4. The deconvolution of the Cr 2p3/2 spectrum of the powders synthesised in the absence of urea showed that this peak with a FHWM of 2.754 eV is constituted by two Cr oxidation states that correspond to the signals at energy binding levels of 577.0 eV and 578.7 eV, which correspond to Cr3+ and Cr6+ species (Figure 4a), respectively [22,23]. In contrast, the deconvolution of the core level Cr 2p3/2 spectrum for the powders prepared in the solution containing 50.0 mmol urea only proceeded by the binding energy at 577.0 eV, which corresponds to the Cr3+ cation (Figure 4b), because the FHWM (2.686 eV) of the deconvoluted peak matched the same position in the XPS experimental peak. The linearity of the residual line calculated by subtracting the deconvoluted spectrum from the experimental one supports that adding urea can maintain the valence of Cr3+ in alkaline aqueous media. Furthermore, the analyses of the O 1s core-level of the samples prepared without (Figure 4c) exhibited three different peaks at the binding energies of 530.7 eV, 531.6 eV and 532.9 eV, corresponding to the oxygen coordinated to Cr3+, the O-P-O oxygen bridging in the pyrophosphate group, and to Cr6+-O, respectively, while the spectrum of the sample prepared in the presence of urea only comprises two binding energy peaks at 530.7 eV and 531.6 eV of the oxygen binding O-P-O in the P2O7 units and the oxygen binding to Cr3+ species, respectively (Figure 4d). These results are quantitatively consistent based on the peak areas and the residual line shapes portrayed in Figure 4c,d.
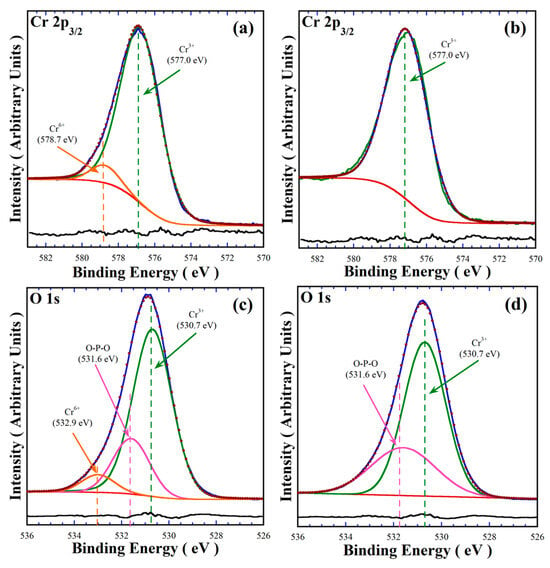
Figure 4.
XPS spectra of pigment powders prepared (a,c) in the absence of urea, and (b,d) with the addition of 50 mmol/dm3 of urea. The green dashed line corresponds to the Cr3+ oxidation state, the orange line represents the Cr6+ species, and the pink line indicates the O-P-O bridging group associated with the pyrophosphate structure.
3.3. Structural Features of the BaCr2(P2O7)2 Powders Prepared Under Hydrothermal Conditions
An additional feature revealed from the XRD patterns of all BaCr2(P2O7)2 powders exhibited a systematic diffraction peak displacement regardless of the peak positions on the single-phase pattern corresponding to the triclinic-structured pyrophosphate (MP Card No. 1192170). Therefore, the detailed Rietveld refinements revealed differences in the samples’ unit cell parameters and the lattice strain factor (Table 2). The goodness-of-fit refinement values (GOF, χ2) and the continuous residual line computed from the experimental and calculated patterns indicate that the detailed structure was solved, showing the high accuracy of the refinement algorithm (Figure 5). The average BaCr2(P2O7)2 cell lattice parameters, together with standard deviation computed by the refinements, are “a0” = 5.4465 Å ± 0.0176 Å, “b0” = 7.5562 Å ± 0.0174 Å and “c0” = 7.6975 Å ± 0.0091 Å (see Table 2). These values are nearly similar to the original lattice parameters of BaCr2(P2O7)2 with triclinic structure, namely a0 = 5.4490 Å, b0 = 7.5698 Å and c0 = 7.6927 Å. These structural variations are likely due to partially incorporating the anionic OH− species, which can provoke the slight displacement observed on the XRD pattern of samples prepared via urea-assisted synthesis [20,24]. The reaction pathway of BaCr2(P2O7)2 under hydrothermal conditions is analogous to that of uvarovite silicate-based pigments (Ca3Cr2Si3O12) in highly concentrated alkaline hydrothermal media (NaOH 2.5–5.0 M) [18]. Therefore, the H4O4 anion units may be incorporated into the O2 positions at polyhedral P2O74− units; thereby, the systematic variation on both unit cell lattice parameters and the lattice strain is caused by the partial incorporation of OH−.
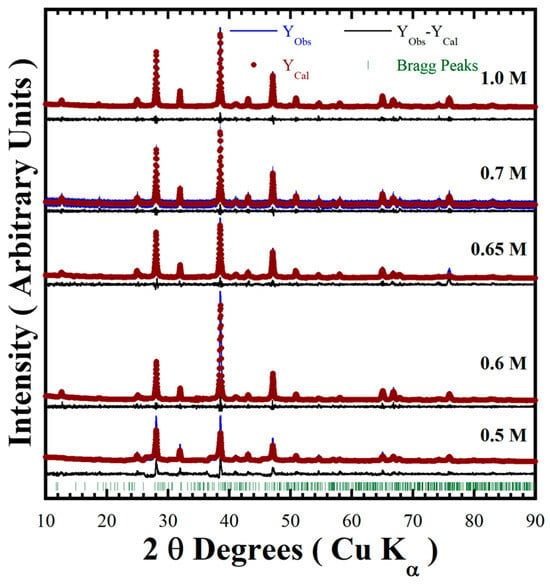
Figure 5.
Rietveld refinement plot of BaCr2(P2O7)2 powders prepared with 50 mmol/dm3 of urea at 170 °C for 6 h, varying the NaOH concentration. The Bragg lines correspond to the triclinic structured pyrophosphate (MP card no. 1192170) [19].
The BaCr2(P2O7)2 crystalline structural features were also investigated by FT–IR spectroscopy in the wavelength range of 400–4000 cm−1, to reveal aspects of the chemical bonding of the pigments. Figure 6 shows typical FT–IR spectra of samples prepared at 240 °C for 24 h in different concentrations of NaOH solutions in the presence of 50 mmol/dm3 of urea. The presence of the polyhedral P2O74− anion is associated with the vibrations of the P–O–P and P–O bonds, respectively [1]. The band due to the P–O bond in the single PO3− tetrahedra is larger than that due to the P–O–P bridge associated with polyhedral P2O74−; it is important to emphasise that this behaviour is caused by the preferential crystal growth of the particles that proceeds in the triclinic structure (112) plane, thus, a preferential aligning of the PO3− units in these specific positions markedly promotes a large transmission peak at 985 cm−1. The symmetric and asymmetric stretching bands of P2O74− and P–O bonds are typically observed at 1407 cm−1 and 985 cm−1, respectively. Moreover, the symmetric and asymmetric bridge stretching modes for the P–O–P bonds constituting the polyhedral P2O74− units were revealed at 920 cm−1 and 798 cm−1, respectively [25,26]. These analyses demonstrate the existence of the paired PO3− tetrahedral in the triclinic BaCr2(P2O7)2 powders prepared under hydrothermal conditions assisted by urea addition.
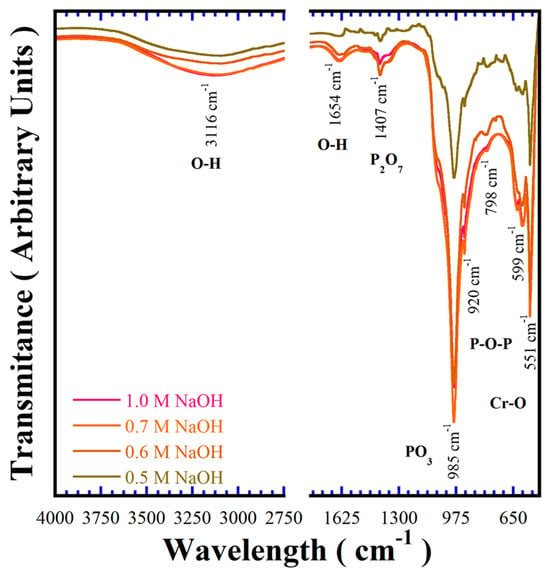
Figure 6.
Fourier transform infrared (FT–IR) spectrum of BaCr2(P2O7)2 pigment powders crystallised at 240 °C for 24 h with 50 mmol/dm3 of urea using different concentrations of NaOH solution.
Additionally, bands within the 400 and 650 cm−1 range correspond to Cr–O bond vibrations [27]. The signal detected in the range between 2650–3700 cm−1 is attributed to water molecules adsorbed on the surface of the BaCr2(P2O7)2, which is commonly promoted by the buffer solution being saturated in OH− ions. The gradual increase in the alkalinity of the solution caused a slight increase in the content of the OH− molecules in the structure of pyrophosphate pigment, which is ascribed to the lattice parameter variations [18]. The polyhedral P2O74− units occur as the principal constituent of the pyrophosphate crystalline structure, along with the OH− molecules structurally adsorbed. The content of OH− in the BaCr2(P2O7) powders was determined by TG-DSC thermal analysis (Section SF5 in SSIF), and the maximum OH− amount in the oxygen positions at the polyhedral P2O74− units was 1.4 molar%. Thus, the compound’s chemical formula is BaCr2(P2O6.9OH0.1)2.
3.4. Microstructural Analysis of BaCr2(P2O7)2 Pigments Produced Under Hydrothermal Conditions
Figure 7 shows the FE–SEM micrographs of the reaction products obtained in different concentrations of NaOH solutions (0.5 M, 0.7 M and 1.0 M) in the presence of 50 mmol/dm3 of urea at 240 °C for 24 h. Generally, the primary mesocrystals of the powders prepared in 0.5 M NaOH solution have a pseudo-spherical morphology (Figure 7a) and an average particle size of 54.7 nm ± 1.2 nm. The BaCr2(P2O7)2 particles prepared in <0.7 M NaOH solution did not change remarkably in their morphology regardless of NaOH concentrations (Figure 7b). The particles are seemingly monodispersed, showing a sharp monomodal distribution with an average size of 69.9 nm ± 1.4 nm. The increase in the OH− ions concentration in the hydrothermal medium provoked a slight particle growth of the BaCr2(P2O7)2 primary mesocrystals. Interestingly, a particle agglomeration occurred in the >1.0 M NaOH solution, resulting in irregular-shaped agglomerates with a size of approximately 110 nm. This sample exhibited a bimodal size distribution: a reduced number of small particles with pseudocubic-shaped morphology had an average particle size of 75.0 ± 9.4 nm (Figure 7c). The differences in the BaCr2(P2O7)2 pigment particles’ morphology are likely provoked by the precursor gel’s rapid dissolution, leading to the agglomeration of primary mesocrystals due to accelerated particle growth. The small particles may crystallise from the remaining solute, supersaturating the NaOH solution without particle coarsening due to the fast decrease in the hydrothermal medium’s temperature during the last step of the treatment.
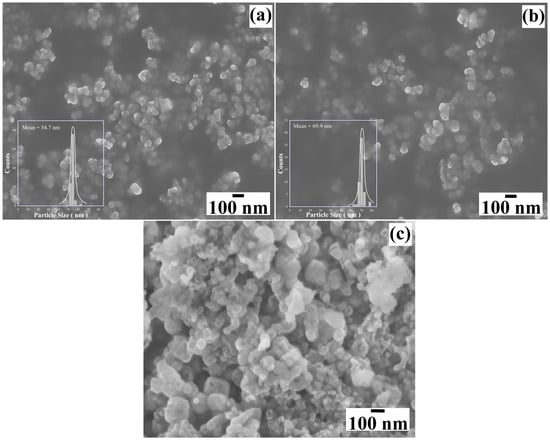
Figure 7.
FE–SEM micrographs of the BaCr2(P2O7)2 particles produced with 50 mmol/dm3 of urea at different NaOH concentrations of (a) 0.5 M, (b) 0.7 M, and (c) 1.0 M. The treatments were carried out at 240 °C for 24 h.
Figure 8 shows the micrographs of the BaCr2(P2O7)2 powders prepared for different reaction times. The particles produced in short reaction times (<24 h) did not show marked differences in their quasi-spherical-like morphology (Figure 8a,b), while the ones produced in longer reaction times (>48 h) had a different morphology as thin platelet-shaped particles with an average length size of 309.26 nm ± 12.9 nm (Figure 8c). Such platelet-shaped particles could be recrystallised from the solute-saturated solution generated from the dissolution of the quasi-spherical mesocrystals produced during early and intermediate reaction steps (before 24 h). This particular behaviour is caused by the low chemical stability of the BaCr2(P2O7)2 particles and the variation in the chemical composition of the solution due to the partial dilution caused by the precursor gel’s dehydration reaction, which occurred before the gel dissolution–crystallisation process. Furthermore, the morphological differences in the particle size and distribution can change in their colour CIELab coordinates and NIR reflectance behaviour [18], as discussed in Section 3.5.
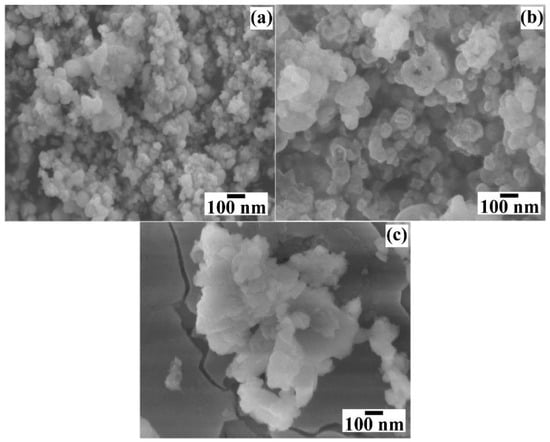
Figure 8.
FE–SEM micrographs of BaCr2(P2O7)2 powders produced with 50 mmol/dm3 of urea and 0.7 M of NaOH solution at 240 °C for different reaction times of (a) 6, (b) 24, and (c) 48 h.
Further details of the BaCr2(P2O7)2 particle morphology were investigated by HR-TEM (Figure 9). Even though the pigment powders had a homogeneous quasi-spherical morphology according to FE-SEM, irrespective of the concentration of NaOH in the preparation (Figure 7a,b), the particles obtained in the 0.5 M NaOH solution consisted of euhedral fine crystals and quasi-spherical mesocrystals according to the TEM images (Figure 9a,b). The quasi-spherical aggregates could be produced by partially dissolving the finest euhedral particles with the solute recrystallised almost in the same place. The remaining euhedral crystals bonded with each other to form a 3D architecture with some tiny pores, as depicted in the inset micrograph of the pseudo-spherical mesocrystal in Figure 9a. Irregular nodular-shaped particle recrystallisation was triggered in the highly concentrated 1.0 M NaOH solution, producing the irregular nodular mesocrystals as seen in Figure 9c. The highly OH-concentrated solvent accelerates the euhedral pigment particles’ dissolution rate, resulting in spontaneous solute recrystallisation which leads to random formation of bulky irregular nodular-shaped mesocrystals. As can be seen from the HR-TEM images (Figure 9b,d), the recrystallised mesocrystals exhibited a high crystallinity, as depicted by the well-defined atomic stacking ordering in both samples prepared in the NaOH solution with different concentrations. Interestingly, the lattice fringe interplanar spacing is similar in both samples, averaging 3.123 Å, which was indexed with the plane with Miller indexes (112) corresponding to the triclinic structure of BaCr2(P2O7)2 [19]. The images indicate that the mesocrystals’ recrystallisation occurred preferentially along the {112} crystallographic family plane, which corresponds to the peak at a 2θ angle of 38.22° in the XRD patterns in Figure 3. Therefore, the pseudo-spherical pyrophosphate mesocrystals should be formed by a bulky 3D hierarchical self-assembly that proceeds along a specific direction, which is analogous to the process for producing other inorganic cold pigments prepared under hydrothermal conditions [16,18].
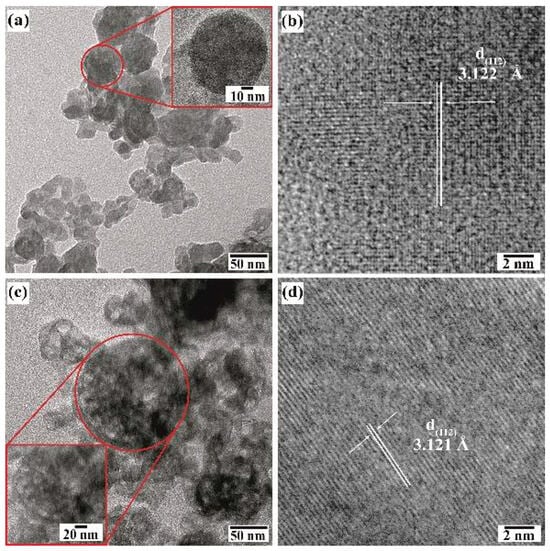
Figure 9.
TEM (a,c) and HR-TEM (b,d) micrographs of BaCr2(P2O7)2 particles prepared with urea at 240 °C for 24 h at different NaOH concentrations of (a,b) 0.5 M and (c,d) 1.0 M. The inset micrograph corresponds to the area in the red circle.
3.5. BaCr2(P2O7)2 Pigments Colour and NIR Solar Reflectance Analyses
The colour of the hydrothermally prepared BaCr2(P2O7)2 pigments was determined by CIELab* measurements using the UV–Vis spectroscopy colourimetry technique. The chroma value was calculated using the mathematical expression [28]. Table 3 summarises the chromatic coordinates L*a*b* and the chroma values determined for various pigments prepared under different experimental conditions. The RGB colour coordinates, obtained by converting the L*a*b* values and the pigment colour tonality associated with their RGB coordinates, are also given in Table 3. In general, the colour of the powder pigment falls within the standard CIELab coordinates of the pyrophosphate BaCr2(P2O7)2 green pigment. However, the hue is brighter than the sample prepared by solid-state reaction at high temperature (L = 72.5, a* = −20.5, b* = 14.7 and Cab* = 25.22) [1].

Table 3.
BaCr2(P2O7)2 pigments particle size, CIELab* values, chroma, RGB coordinates, and colour hue; prepared with 50 mmol/dm3 of urea at different temperatures, reaction intervals and NaOH media concentrations.
On the other hand, the single-phase pigment’s b* coordinate exhibited negative values, which caused a bright green hue in most of the samples prepared with urea under alkaline hydrothermal conditions. The pigment’s chroma (Cab*) is consistent with the CIELab coordinates, with only a variation within the range of 8.89 to 15.50. According to these results, the change in green colour tonality determined on the powders prepared under different treatment conditions strongly depends on the inherent microstructural parameters, such as particle size and morphology. Indeed, the increase in the OH− ions in the alkaline solution provoked the growth of BaCr2(P2O7)2, the pseudo-spherical-shaped particles during the first step. Subsequently, mesocrystals produced by recrystallisation during the hydrothermal processing led to a slight particle size increase of approximately 25.5 nm. Even the slight dimensional change from the tiny increase in the particle size physically increased the brightness of BaCr2(P2O7)2 powders, resulting in low chroma values related to the pigment’s dull and muted colour tonality. This behaviour is analogous to the phenomenon inherent in powder pulverisation, where the particle size reduction enhances an optimum interaction between the surface area and the sunlight, resulting in a light and bright colour tonality. Furthermore, the particle morphology is less dependent on the pigments’ colour intensity and colour saturation, even if the particle size of pseudo-spherical (Cab* = 9.80) intrinsic morphologies is considerably different from that of plate-like (Cab* = 15.80) ones, resulting in vibrant green tonalities in the recrystallised BaCr2(P2O7)2 plate-like particles.
Near infrared (NIR) radiation comprises 52% of the total solar light spectrum, which gives rise to the urban heat island phenomenon. Thus, several research groups have developed cold pigments to decrease electric consumption during air conditioning and suppress the heat generated by the absorption of NIR radiation in solids [29,30]. The optical properties in the NIR, regarding the band gap and solar reflectance (% R*), were investigated. The Kubelka–Munk function provided the BaCr2(P2O7)2 pigment bandgap values given in Table 3; the average pigment band gap is 3.12 ± 0.03 eV (the plots are shown in Figure S7 in SSIF), while the solar reflectance showed a marked difference between 55.41 and 67.46%. The maximum solar reflectance of the BaCr2(P2O7)2 pigment is 10.0% lower than that of the Rutile TiO2 standard (79.01%, <5 mm in size), considered as an adequate cold pigment. According to the microstructural differences in Table 3 and Figure 8 and Figure S5 in SSIF, the optical properties depend on the particle size and morphology, rather than the free carrier density mechanism that is argued to increase the reflectivity of an inorganic pigment [31,32,33]. Indeed, the larger-sized BaCr2(P2O7)2 powders provided larger specular reflectance behaviour from 300 nm to 2500 nm, as shown in Figure 10.
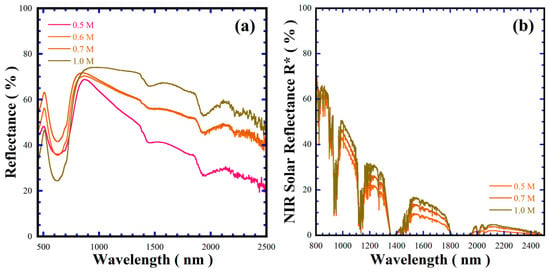
Figure 10.
(a) Visible-NIR spectral reflectance and (b) solar reflectance spectra of BaCr2(P2O7)2 powders prepared with the assistance of urea addition at 240 °C for 48 h, varying the NaOH solution concentration.
On the other hand, the broad absorption peak observed at a wavelength of approximately 522 nm for all the samples falls within the reflectance range of other inorganic green pigments reported elsewhere [34]. As shown in Figure 10a, the peak increased slightly in intensity with increasing alkaline concentration in the hydrothermal medium. This variation might be associated with differences in particle size, which influence the optical properties of the pigment, particularly the a* and b* chromaticity coordinates, resulting in the variation of the pigment’s colour hue property. In addition, the differences in the BaCr2(P2O7)2 pigment colour CIELab* coordinates with those of other green pigments containing the Cr3+ chromophore are discussed in Section SF6 in the SSIF.
On the other hand, Figure 10 shows the NIR reflectance spectrum and the NIR solar reflectance for the BaCr2(P2O7)2 pigments with different particle size and morphology produced in various concentrations of NaOH solution in the presence of 50 mmol/dm3 urea at 240 °C for 48 h, in the range of 300–2500 nm calculated according to ASTM G173-03 [35]. The control of particle size and morphology of the BaCr2(P2O7)2 pigments could optimise their reflectance, based on the results in Figure 10 and Table 3. In general, the pigment with pseudo-spherical-shaped morphology exhibited the lowest spectral reflectance within 800–2500 nm as well as the lowest reflectance within 800–1500 nm, averaging a NIR solar reflectance of 60.0% as revealed at 800 nm (Figure 10a,b). The change in the particle morphology to agglomerated mesocrystals results in a decrease in the spectral reflectance to a minimum of 40% at 2500 nm; these results indicate that the reflectance decrease is irrespective of the particle size rather than the particle morphology. Indeed, the variation in the pigment’s reflectance is analogous to that on the samples with a small particle size (44.3–75.07 nm), Figure S8 in the SSFI. Interestingly, the decrease in reflectance of mesocrystal powder is similar to that of the TiO2 powder in the wavelength range of 800–1700 nm; the reflectance difference is approximately 7.5%. The plate-like shaped BaCr2(P2O7)2 powder improved in reflectance by nearly 20% in the NIR spectra (800–2500 nm), as shown in Figure 10a, and the maximum spectral solar reflectance (67.46%). Solar spectral reflectance can be related to the particle surface aspect: flatter and more faceted particle surfaces provoke an optimum reflection of NIR spectrum photons. Moreover, the NIR solar reflectance capability of the hydrothermally prepared BaCr2(P2O7)2 pigment is 10% lower than that of the one prepared via solid-state reaction [1]. Since the reported functional cold pigment inorganic oxides exhibited bulk solar reflectance with R* values of above 35.0%, such as the spinel-structured ZnCr2O4, CoCr2O4 and CoCrAlO4 prepared by sol-gel calcination [28,29,30,31,32,33,34]. The solar reflectance values of these pigments are summarised in Table S2 in the SSIF. The differences in the green pigments’ solar reflectance are discussed in Section SF6 of the SSIF. According to this evaluation, we surmise that the BaCr2(P2O7)2 pigment hydrothermally prepared here has great potential for cold pigment applications based on the spectral reflectance and the NIR solar reflectance behaviour in Figure 10a,b, and the NIR solar reflectance in Table S2 in SSIF.
4. Conclusions
The pyrophosphate pigment BaCr2(P2O7)2 has been successfully prepared under alkaline hydrothermal conditions in the presence of urea, used as a reducing agent. The formation of single–phase BaCr2(P2O7)2 with triclinic structure was triggered via a single-step reaction of the precursor gel achieved by dissolution–crystallisation at a pH range of 9.46–10.53. The polyatomic HCO3− ions from urea decomposition provided a buffered, mildly alkaline reaction environment, leading to the crystallisation of single-phase BaCr2(P2O7)2 pigment without byproducts at a low temperature, 170 °C, for a short reaction time of 6 h. Fine pyrophosphate particles with a size averaging 55.0 ± 10.0 nm and a quasi-spherical morphology were preferentially crystallised by the initial single-step reaction at temperatures below 240 °C for 24 h. The BaCr2(P2O7)2 powders underwent a secondary dissolution–recrystallisation process favoured by the NaOH solution concentration increase (0.5–1.0 M). Under these conditions, a marked morphology variation occurred in the BaCr2(P2O7)2 recrystallised particles, namely quasi-spherical (133.8 nm), nodular-like mesocrystals (221.7–309.3 nm) and plate-like shapes (342.5 nm). The differences in the morphology of BaCr2(P2O7)2 pigments improved the NRI spectral reflectance (57.7–67.5%) and NIR solar reflectance (55.4–67.5%). Plate-like shaped particles exhibited the optimum NIR reflectance, due to their flat faceted surfaces rather than their particle size. Therefore, the solar reflectance capability of BaCr2(P2O7)2 provides great potential for eco–friendly applications such as the preparation of thermally reflective coatings, synergistically contributing to the sustainable development of pigments to mitigate the urban heat island phenomenon.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nano15130982/s1, Figure S1: X-ray diffraction patterns of BaCr2(P2O7)2 powders prepared under hydrothermal conditions with a concentration of 50 mmol/dm3 of urea and 0.7 M NaOH at 240 °C for different reaction times; Figure S2: DSC and TGA curves of the precursor gel and pyrophosphate powders (BaCr2(P2O7)2) prepared under hydrothermal conditions at 240 °C for 24 h with 50 mmol/dm3 of urea, varying the NaOH solution concentration; Figure S3: XPS survey spectrum of the hydrothermally prepared green pigment powders (a) without urea and (b) with urea; Figure S4: Core-level Cr 2p and O 1s high-resolution XPS spectrum of the hydrothermally prepared green pigment powders; Figure S5: FE-SEM micrographs of the BaCr2(P2O7)2 pigments produced under hydrothermal conditions with 50 mmol/dm3 of urea at 240 °C for 48 h in NaOH solutions with concentrations of a) 0.5 M, b) 0.6 M, and c) 1.0 M; Figure S6. Differential scanning calorimetry and thermogravimetry curves of the precursor gel and pyrophosphate powders (BaCr2(P2O7)2) prepared at 240 °C for 24 h with 50 mmol/dm3 urea, varying the NaOH solution concentration; Figure S7: Kubelka–Munk curves of BaCr2(P2O7)2 pigments prepared under hydrothermal conditions with 50 mmol/dm3 at 240 °C for 48 h, varying concentration of NaOH solution; Figure S8: UV-vis NIR spectral reflectance curves and b) NIR solar reflectance spectra of BaCr2(P2O7)2 pyrophosphate pigments prepared under hydrothermal conditions at 240 °C for 24 h by varying the NaOH concentration and Rutile (TiO2), Table S1: CIF file of the secondary crystalline phase determined in the XRD patterns of the powders prepared in hydrothermal conditions.
Author Contributions
D.E.C.-R.: conceptualisation, design and research work; Z.M.-V.: chemical and crystalline structural characterisation and data analysis; I.J.-R.: colour and reflectance characterisation; T.U.: sample holder donation for UV-vis NIR characterisation, manuscript editing, research stay assistance at Kochi University to conduct the hydrothermal experiments; J.C.R.-A.: funding, research supervision, data analysis and manuscript preparation; J.L.-C.: manuscript preparation and discussions. All authors have read and agreed to the published version of the manuscript.
Funding
The Centre for Research and Advanced Studies of the NPI C-3000-2023 federal research budget supported a part of this research.
Data Availability Statement
Data will be made available upon request.
Acknowledgments
J.C.R.-A., Z.M.-V. and J.L.-C. are indebted to the support of SECIHTI-SNII. D.E.C.-R is indebted to the PhD scholarship from SECIHTI. Thanks to T. Matsuzaki and S. Yanagimoto at Marine Core Research Institute, Kochi University, Japan, for their assistance with the FE-Scanning Electron Microscopy observations, and to Maria del Socorro Garcia Guillermo for her commitment to performing the X-ray photoelectron spectroscopy conducted at Cinvestav Saltillo Campus.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this paper.
References
- Tao, Z.; Zhang, W.; Huang, Y.; Wei, D.; Jin, S.H. A novel pyrophosphate BaCr2(P2O7)2 as green pigment with high NIR solar reflectance and durable chemical stability. Solid State Sci. 2014, 34, 78–84. [Google Scholar] [CrossRef]
- Béjaoui, A.; Horchani–Naifer, K.; Hahhi, M.; Férid, M. Crystal structure, physical properties and bond valence analysis of NaLuP2O7. Solid State Sci. 2014, 31, 46–53. [Google Scholar] [CrossRef]
- Handizi, A.; Boukhari, A.; Holt, E.M.; Aride, J.; Flandrois, S. Synthesis, structural characterization and magnetism of the solid solution: Copper nickel pyrophosphate. Mat. Res. Bull. 1993, 28, 1241–1247. [Google Scholar] [CrossRef]
- Rousse, G.; Rodríguez–Carvajal, J.; Wurm, C.; Masquelier, C. A neutron diffraction study of the antiferromagnetic diphosphate LiFeP2O7. Solid State Sci. 2002, 4, 973–978. [Google Scholar] [CrossRef]
- Wilson, S.T.; Lok, B.M.; Messina, C.A.; Cannan, T.R.; Flanigen, E.M. Aluminophosphate molecular sieves: A new class of microporous crystalline inorganic solids. J. Am. Chem. Soc. 1982, 104, 1146–1147. [Google Scholar] [CrossRef]
- Al–Thabaiti, S.A. Synthesis and characterization of a new cobalt polymeric spinels. Commun. Fac. Sci. Univ. Ank. 2003, 49, 5–14. [Google Scholar]
- Kwang–Hwa, L.; Pei–Fen, S.; Teng–Ming, C. AM3(P2O7)2 (A = alkaline–earth metals; M = Fe, Co, Ni): Diphosphates containing infinite chains of edge–sharing MO6 octahedra. Inorg. Chem. 1993, 32, 4373–4377. [Google Scholar]
- Llusar, M.; García, A.; Gargori, C.; Galindo, R.; Badenes, J.A.; Monrós, G. Synthesis of diphosphate Mn2-xMgxP2O7 solid solutions with thortveitite structure: New pink ceramic dyes for the colouration of ceramic glazes. J. Eur. Ceram. Soc. 2012, 32, 765–776. [Google Scholar] [CrossRef]
- Battle, P.D.; Gibb, T.C.; Nixon, S. The magnetic properties of the synthetic langbeinite KBaCr2(PO4)3. J. Solid State Chem. 1988, 75, 21–29. [Google Scholar] [CrossRef]
- Jarboui, A.; Rhaeim, A.B.; Hlel, F.; Guidara, K.; Gargouri, M. NMR study and electrical properties investigation of Zn2P2O7. Ionics 2010, 16, 67–73. [Google Scholar] [CrossRef]
- Mahajan, A.V.; Nath, R.; Büttgen, N.; Kegler, C.; Loidl, A.; Bobroff, J. 31P NMR study of the spin S = ½ quasi–1D Heisenberg antiferromagnet BaCuP2O7. Phys. B Condens. Matter 2006, 378–380, 1148–1149. [Google Scholar] [CrossRef]
- Boukhari, A.; Moquine, A.; Flandrois, S. Synthesis and characterization of new copper (II) mixed diphosphates (M, Cu)2P2O7 with M = Mg, Ca, Sr and Ba. J. Solid State Chem. 1990, 87, 251–256. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, C.; Li, X.; Wu, D.; Li, J.; Huo, P.; Wang, H. Chemical precipitation synthesis of porous Ni2P2O7 nanowires for supercapacitor. J. Alloys Compd. 2019, 790, 36–41. [Google Scholar] [CrossRef]
- Sangeetha, S.; Basha, R.; Sreeram, K.J.; Sangilimuthu, S.N.; Unni–Nair, B. Functional pigments from chromium (III) oxide nanoparticles. Dyes Pigm. 2012, 94, 548–552. [Google Scholar] [CrossRef]
- Barba, A.; Gazulla, M.F.; Gómez, M.P.; Orduña, M. Characterisation of cobalt–containing ceramic pigments by WD–XRF and XRD. X–Ray Spectrom. 2006, 35, 383–389. [Google Scholar] [CrossRef]
- Corona–Martínez, D.A.; Rendón–Angeles, J.C.; Gonzalez, L.A.; Matamoros–Veloza, Z.; Yanagisawa, K.; Tamayo, A.; Alonso, J.R. Controllable synthesis of BaCuSi2O6 fine particles via a one–pot hydrothermal reaction with enhanced violet colour hue. Adv. Powder Technol. 2019, 30, 1473–1483. [Google Scholar] [CrossRef]
- Della, V.P.; Junkes, J.A.; Rambo, C.R.; Hotza, D. Synthesis of the ceramic pigment Victoria green (Ca3Cr2Si3O12) from CaCO3, Cr2O3 and SiO2. Quim. Nova. 2008, 31, 1004–1007. [Google Scholar] [CrossRef]
- Rendón–Angeles, J.C.; Matamoros–Veloza, Z.; Rodríguez–Galicia, J.L.; Seong, G.; Yanagisawa, K.; Tamayo, A.; Rubio, J.; Anaya–Chavira, L.A. One–pot hydrothermal synthesis of Victoria green (Ca3Cr2Si3O12) nanoparticles in alkaline fluids and its colour hue characterization. Nanomaterials. 2021, 11, 521. [Google Scholar] [CrossRef]
- Zoubitzky, L.; Coudert, F.X. CrystalNets.jl: Data retrieved from the materials project for BaCr2(P2O7)2 (mp–1192170) from database version v2025.02.12. post1. Available online: https://next-gen.materialsproject.org/materials/mp-1192170#related_materials (accessed on 15 June 2025).
- Vasquez–Elizondo, L.J.; Rendón–Angeles, J.C.; Matamoros–Veloza, Z.; López–Cuevas, J.; Yanagisawa, K. Urea decomposition enhancing the hydrothermal synthesis of lithium iron phosphate powders: Effect of the lithium precursor. Adv. Powder Technol. 2017, 28, 1593–1602. [Google Scholar] [CrossRef]
- Ridlington, J.W.; Buttler, L.G. Yeast inorganic pyrophosphatase. I. Binding of pyrophosphate, metal ion, and metal ion–pyrophosphate complexes. J. Biol. Chem. 1972, 22, 7303–7307. [Google Scholar] [CrossRef]
- Bandara, P.C.; Peña-Bahamonde, J.; Rodrigues, D.F. Redox mechanisms of conversion of Cr(VI) to Cr(III) by graphene oxide-polymer composite. Sci. Rep. 2020, 10, 9237. [Google Scholar] [CrossRef] [PubMed]
- Gazzoli, D.; Occhiuzzi, M.; Cimino, A.; Minelli, G.; Valigi, M. Chromium oxidation states and XPS analysis of the chromia/zirconia system. Surf. Interface Anal. 1992, 18, 315–322. [Google Scholar] [CrossRef]
- Khalaf, M.M.; El–Lateef, H.M.A.; Mohamed, I.M.A. Novel electrocatalysts for ethylene glycol oxidation based on functionalized phosphates of bimetals Mn/Ni: Morphology, crystallinity, and electrocatalytic performance. Surf. Interfaces 2023, 38, 102850. [Google Scholar] [CrossRef]
- Fang, Y.; Chen, Z.; Shi, Y.; Zhang, W.; Dong, X. Li1.52Na4.48Cd8(PO4)2(P2O7)4: A new mixed–anion diphosphate containing four types of dimensions groups. Inorg. Chem. Commun. 2017, 86, 253–257. [Google Scholar] [CrossRef]
- Navarro, R.; Hernanz, A. Profile análisis of PO3–symmetric stretching infrared band. J. Mol. Struct. 1988, 175, 335–338. [Google Scholar] [CrossRef]
- Subba–Rao, G.V.; Rao, C.N.R. Infrared and electronic spectra of rare earth perovskites: Ortho–chromites, manganites and ferrites. Appl. Spectrosc. 1970, 24, 436–445. [Google Scholar]
- Dolić, S.D.; Jovanović, D.J.; Štrbac, D.; Đačanin–Far, L. Improved coloristic properties and high NIR reflectance of environment–friendly yellow pigments based on bismuth vanadate. Ceram. Int. 2018, 15, 22731–22737. [Google Scholar]
- Gao, Q.; Wu, X.; Zhu, R. Antifouling energy–efficient coatings based on BiOClxBr1–x microflowers: NIR reflective property and superhydrophobicity. Constr. Build Mater. 2020, 257, 119569. [Google Scholar] [CrossRef]
- Rosati, A.; Fedel, M.; Rossi, S. NIR reflective pigments for cool roof applications: A comprehensive review. J. Clean. Prod. 2021, 313, 127826. [Google Scholar] [CrossRef]
- Bai, M.; Li, W.; Zhu, H.; Guo, W.; Wang, S.; Wang, Y. Enhanced NIR reflectance black pigments based on CuCo2–xTixO4: Synthesis and properties. J. Solid State Chem. 2025, 341, 125056. [Google Scholar] [CrossRef]
- Wang, J.; Han, A.; Ye, M.; Chen, C. Thermal insulation performance of novel orange fabric coated with Fe3+ -doped La2Zr2O7 NIR solar reflectivity pigment. Sol. Energy 2022, 244, 218–226. [Google Scholar] [CrossRef]
- Oka, R.; Masui, T. Synthesis and characterization of black pigments based on calcium manganese oxides for high near–infrared (NIR) reflectance. RSC. Adv. 2016, 6, 90952–90957. [Google Scholar] [CrossRef]
- Yu, J.; Jiang, F.; Liu, J.; Wang, T.; Zhang, X.; Zhang, X.; Zhang, Q.; Zhang, R.; Wu, Q.; Hu, Q.; et al. Coloring and near-infrared reflection performance of low temperature synthesized novel (Cr, V)-ZrSiO4 jewel green pigments. Ceram. Int. 2023, 49, 38602–38613. [Google Scholar] [CrossRef]
- ASTM G173-03; Standard Tables for Reference Solar Spectral Irradiances: Direct Normal and Hemispherical on 37° Tilted Surface. ASTM: West Conshohocken, PA, USA, 2012. Available online: https://www.astm.org/Standards/G173.htm (accessed on 15 June 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).