The Synthesis of Materials with a Hierarchical Structure Based on Tin Dioxide
Abstract
1. Introduction
2. Materials and Methods
- (1)
- Tin chloride pentahydrate powder SnCl4·5H2O;
- (2)
- Ethyl alcohol C2H5OH (GOST 5962-13);
- (3)
- Concentrated aqueous solution of ammonia NH4OH.
- (1)
- m(SnCl4.5H2O) = 3.9072 g.
- (2)
- V(C2H5OH) = 100 mL.
- (3)
- V(NH4OH) = 0 mL; 0.4 mL; 0.8 mL; 1.6 mL.
Sn(OH)4 → SnO2 + 2H2O
3. Results and Discussion
3.1. Optical Microscopy
3.2. Raman Spectroscopy
3.3. X-Ray Diffraction
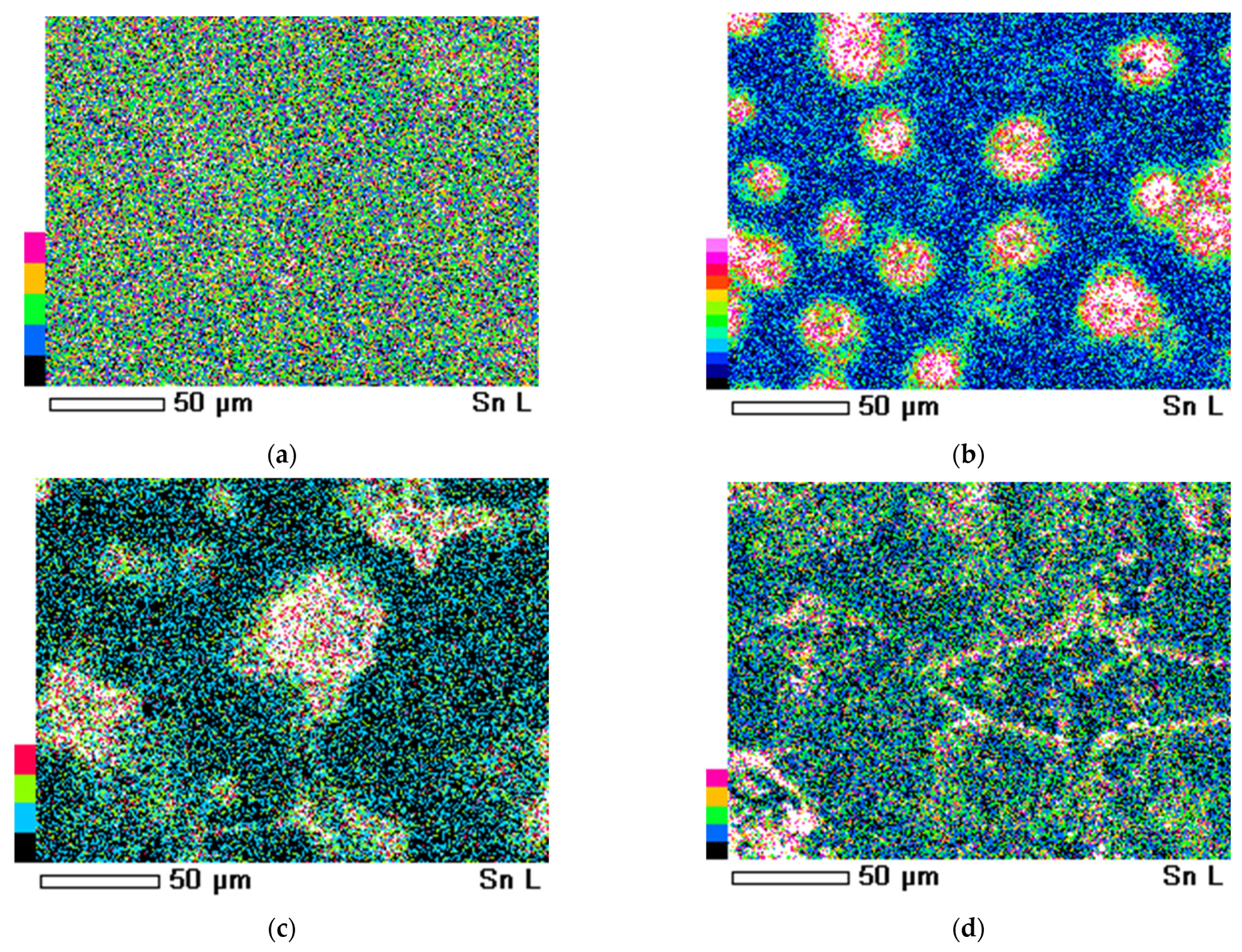
3.4. Scanning Electron Microscopy (SEM)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Murzalinov, D.; Kemelbekova, A.; Seredavina, T.; Spivak, Y.; Serikkanov, A.; Shongalova, A.; Zhantuarov, S.; Moshnikov, V.; Mukhamedshina, D. Self-organization effects of thin ZnO layers on the surface of porous silicon by formation of energetically stable nanostructures. Materials 2023, 16, 838. [Google Scholar] [CrossRef] [PubMed]
- Vargheese, S.; Kumar, R.S.; Kumar, R.T.R.; Shim, J.-J.; Haldorai, Y. Binary metal oxide (MnO2/SnO2) nanostructures supported triazine framework-derived nitrogen-doped carbon composite for symmetric supercapacitor. J. Energy Storage 2023, 68, 107671. [Google Scholar] [CrossRef]
- Yoon, C.-M.; Jekal, S.; Kim, D.-H.; Noh, J.; Kim, J.; Kim, H.-Y.; Kim, C.-G.; Chu, Y.-R.; Oh, W.-C. 3D hierarchically structured tin oxide and iron oxide-embedded carbon nanofiber with outermost polypyrrole layer for high-performance asymmetric supercapacitor. Nanomaterials 2023, 13, 1614. [Google Scholar] [CrossRef]
- Shang, J.; Zhang, T.; Li, X.; Luo, Y.; Feng, D.; Cheng, X. Mn3O4-ZnMn2O4/SnO2 nanocomposite activated peroxymonosulfate for efficient degradation of ciprofloxacin in water. Sep. Purif. Technol. 2023, 311, 123342. [Google Scholar] [CrossRef]
- Gao, M.; Gong, Z.; Li, H.; Zhao, H.; Chen, D.; Wei, Y.; Li, D.; Li, Y.; Yang, L.; Chen, Y. Constructing a multifunctional interlayer toward ultra-high critical current density for garnet-based solid-state lithium batteries. Adv. Funct. Mater. 2023, 33, 2300319. [Google Scholar] [CrossRef]
- Gul, S.; Azam, A.; Imrose, N.; Riaz, S.; Naseem, S. Tin oxide thin films prepared by sol-gel for PV applications. Mater. Today Proc. 2015, 2, 5793–5798. [Google Scholar] [CrossRef]
- Yang, L.; Qin, Z.; Pan, H.; Yun, H.; Min, Y.; Xu, Q. Corrosion protection of 304 stainless steel bipolar plates of PEMFC by coating SnO2 film. Int. J. Electrochem. Sci. 2017, 12, 10946–10957. [Google Scholar] [CrossRef]
- Grushevskaya, E.; Ibraimova, S.; Dmitriyeva, E.; Lebedev, I.; Mit’, K.; Mukhamedshina, D.; Fedosimova, A.; Serikkanov, A.; Temiraliev, A. Sensitivity to ethanol vapour of thin films SnO2 doped with fluorine. Eurasian Chem. Technol. J. 2019, 21, 13–17. [Google Scholar] [CrossRef]
- Filippatos, P.-P.; Sharma, R.; Soultati, A.; Kelaidis, N.; Petaroudis, C.; Alivisatou, A.-A.; Drivas, C.; Kennou, S.; Christopoulos, S.-R.G.; Davazoglou, D.; et al. Optimization of the hydrogen response characteristics of halogen-doped SnO2. Sci. Rep. 2023, 13, 2524. [Google Scholar] [CrossRef]
- Kendall, O.; Wainer, P.; Barrow, S.; van Embden, J.; Della Gaspera, E. Fluorine-Doped Tin Oxide Colloidal Nanocrystals. Nanomaterials 2020, 10, 863. [Google Scholar] [CrossRef]
- Zhou, S.; Zhou, H.; Zhang, Y.; Zhu, K.; Zhai, Y.; Wei, D.; Zeng, S. SnO2 Anchored in S and N Co-Doped Carbon as the Anode for Long-Life Lithium-Ion Batteries. Nanomaterials 2022, 12, 700. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Zhao, L.; Liu, R.; Wu, M.; Sun, Y.; Li, L. Electrical properties of bulk and interface layers in Sb doped SnO2 thin films. Ceram. Int. 2019, 45, 2201–2206. [Google Scholar] [CrossRef]
- Padmaja, B.; Dhanapandian, S.; Ashokkumar, K. Hydrothermally derived Mg doped tin oxide nanostructures for photocatalytic and supercapacitor applications. Mater. Sci. Eng. B 2023, 297, 116699. [Google Scholar] [CrossRef]
- Mukhamedshina, D.; Fedosimova, A.; Dmitriyeva, E.; Lebedev, I.; Grushevskaya, E.; Ibraimova, S.; Mit’, K.; Serikkanov, A. Influence of plasma treatment on physical properties of thin SnO2 films obtained from SnCl4 solutions with additions of NH4F and NH4OH. Eurasian Chem. Technol. J. 2019, 21, 57–61. [Google Scholar] [CrossRef]
- Tompakova, N.M.; Dmitriyeva, E.A.; Lebedev, I.A.; Serikkanov, A.S.; Grushevskaya, E.A.; Fedosimova, A.I. Influence of hydrogen plasma on SnO2 thin films. Mater. Today Proc. 2020, 25, 83–87. [Google Scholar] [CrossRef]
- Nomura, K. Magnetic properties and oxygen defects of dilute metal doped tin oxide based semiconductor. Croat. Chem. Acta. 2015, 88, 579–590. [Google Scholar] [CrossRef]
- Kwoka, M.; Lyson-Sypien, B.; Kulis, A.; Zappa, D.; Comini, E. Surface Properties of SnO2 Nanowires Deposited on Si Substrate Covered by Au Catalyst Studies by XPS, TDS and SEM. Nanomaterials 2018, 8, 738. [Google Scholar] [CrossRef]
- Habte, A.G.; Hone, F.G.; Dejene, F.B. Effect of solution pH on structural, optical and morphological properties of SnO2 nanoparticles. Phys. B Condens. Matter 2019, 580, 411832. [Google Scholar] [CrossRef]
- Murzalinov, D.; Dmitriyeva, E.; Lebedev, I.; Bondar, E.A.; Fedosimova, A.I.; Kemelbekova, A. The effect of pH solution in the sol–gel process on the structure and properties of thin SnO2 films. Processes 2022, 10, 1116. [Google Scholar] [CrossRef]
- Ullah, E.; Shah, M.Z.U.; Ahmad, S.A.; Sajjad, M.; Khan, S.; Alzahrani, F.M.; Yahya, A.E.; Eldin, S.M.; Akkinepally, B.; Shah, A.; et al. Hydrothermal assisted synthesis of hierarchical SnO2 micro flowers with CdO nanoparticles based membrane for energy storage applications. Chemosphere 2023, 321, 138004. [Google Scholar] [CrossRef]
- Moshnikov, V.; Kononova (Grachova), I.; Kuznezov, V.; Maximov, A.; Nalimova, S.; Ponomareva, A. Hierarchical nanostructured semiconductor porous materials for gas sensors. J. Non-Cryst. Solids 2010, 356, 2020–2025. [Google Scholar] [CrossRef]
- Lee, J.H. Gas sensors using hierarchical and hollow oxide nanostructures: Overview. Sens. Actuators B Chem. 2009, 140, 319–336. [Google Scholar] [CrossRef]
- Sun, M.H.; Huang, S.Z.; Chen, L.H.; Li, Y.; Yang, X.Y.; Yuan, Z.Y.; Su, B.L. Applications of hierarchically structured porous materials from energy storage and conversion, catalysis, photocatalysis, adsorption, separation, and sensing to biomedicine. Chem. Soc. Rev. 2016, 45, 3479–3563. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-P.; Ren, T.-Z.; Ma, T.-Y.; Yuan, Z.-Y. Hierarchical Structures from Inorganic Nanocrystal Self-Assembly for Photoenergy Utilization. Int. J. Photoenergy 2014, 498540, 15. [Google Scholar] [CrossRef]
- Lei, C.; Pi, M.; Jiang, C.; Cheng, B.; Yu, J. Synthesis of hierarchical porous zinc oxide (ZnO) microspheres with highly efficient adsorption of Congo red. J. Colloid Interface Sci. 2017, 490, 242–251. [Google Scholar] [CrossRef]
- Bi, W.; Zhu, J.; Zheng, B.; Liu, S.; Zhang, L. Synthesis of Pd-Doped SnO2 and Flower-like Hierarchical Structures for Efficient and Rapid Detection of Ethanolamine. Molecules 2024, 29, 3650. [Google Scholar] [CrossRef]
- Xiong, C.; Xiong, Y.; Zhu, H.; Zhang, Y. Investigation of Raman spectrum for nano-SnO2. Sci. China 1997, 40, 1222–1227. [Google Scholar] [CrossRef]
- Lebedev, I.; Dmitriyeva, E.; Bondar, E.; Ibraimova, S.; Fedosimova, A.; Temiraliev, A. Signal-to-noise ratio enhancement by accumulation of signal and noise along the spectrum. Fluct. Noise Lett. 2021, 21, 2250016. [Google Scholar] [CrossRef]
- Maheswari, S.; Karunakaran, M.; Chandrasekar, L.B.; Kasirajan, K. Ammonia sensors on the base of gadolinium doped tin oxide thin films and its characterization: Effect of doping concentration. Physica B Condens. Matter 2021, 602, 412477. [Google Scholar] [CrossRef]
- Moshnikov, V.A.; Tairov, U.M.; Hamova, T.V.; Shilova, O.A. Sol-Gel Technology of Micro- and Nanocomposites; Lan: Saint Petersburg, Russia, 2013; 95p. [Google Scholar]
- Shilova, O.A. Fractals, morphogenesis and triply periodic minimal surfaces in sol-gel-derived thin films. J. Sol-Gel Sci. Technol. 2020, 95, 599–608. [Google Scholar] [CrossRef]
- Stauffer, D.; Aharony, A.; Mandelbrot, B.B. Self-similarity and covered neighborhoods of fractals: A random walk test. Phys. A Stat. Mech. Its Appl. 1993, 196, 1–5. [Google Scholar] [CrossRef]
- Martínez-López, F.; Cabrerizo-Vílchez, M.A.; Hidalgo-Álvarez, R. On the self-similarity of fractal colloidal aggregates in two dimensions. J. Phys. A Math. Gen. 2001, 34, 7393. [Google Scholar] [CrossRef]
- Fedosimova, A.I.; Dmitrieva, E.A.; Lebedev, I.A.; Temiraliev, A.T.; Temiraliev, T.; Abishev, M.E.; Baitimbetova, B.A.; Ryabikin, Y.A.; Serikkanov, A.S. Modeling the process of formation of fractal structures in thin films. J. Phys. Conf. Ser. 2018, 1141, 012004. [Google Scholar] [CrossRef]
- Zöttl, A.; Stark, H. Modeling Active Colloids: From Active Brownian Particles to Hydrodynamic and Chemical Fields. Annu. Rev. Condens. Matter Phys. 2023, 14, 109–127. [Google Scholar] [CrossRef]
- Nekrasov, V.M.; Polshchitsin, A.A.; Yurkin, M.A.; Yakovleva, G.E.; Maltsev, V.P.; Chernyshev, A.V. Chernyshev Brownian aggregation rate of colloid particles with several active sites. J. Chem. Phys. 2014, 141, 064309. [Google Scholar] [CrossRef]
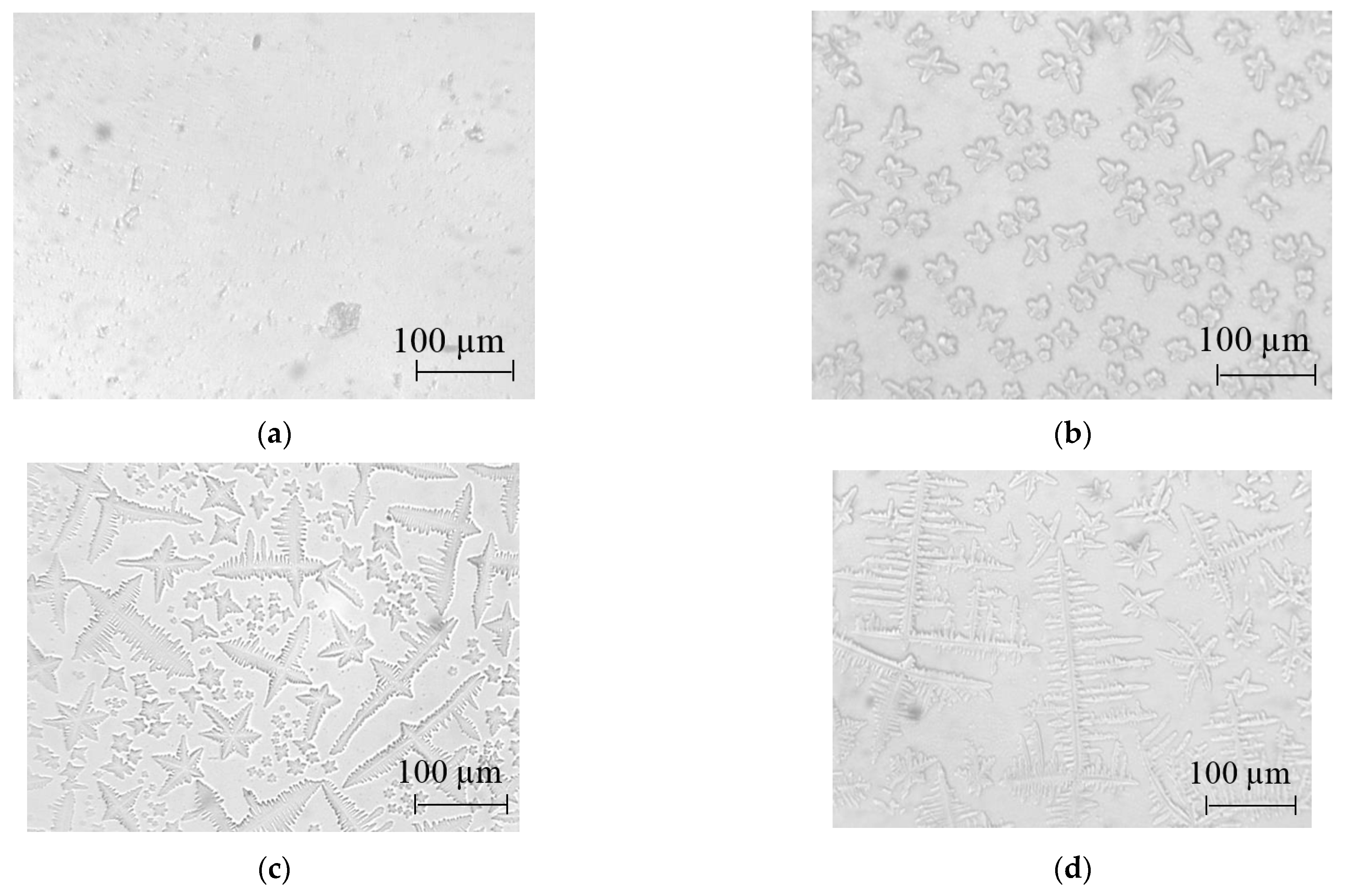
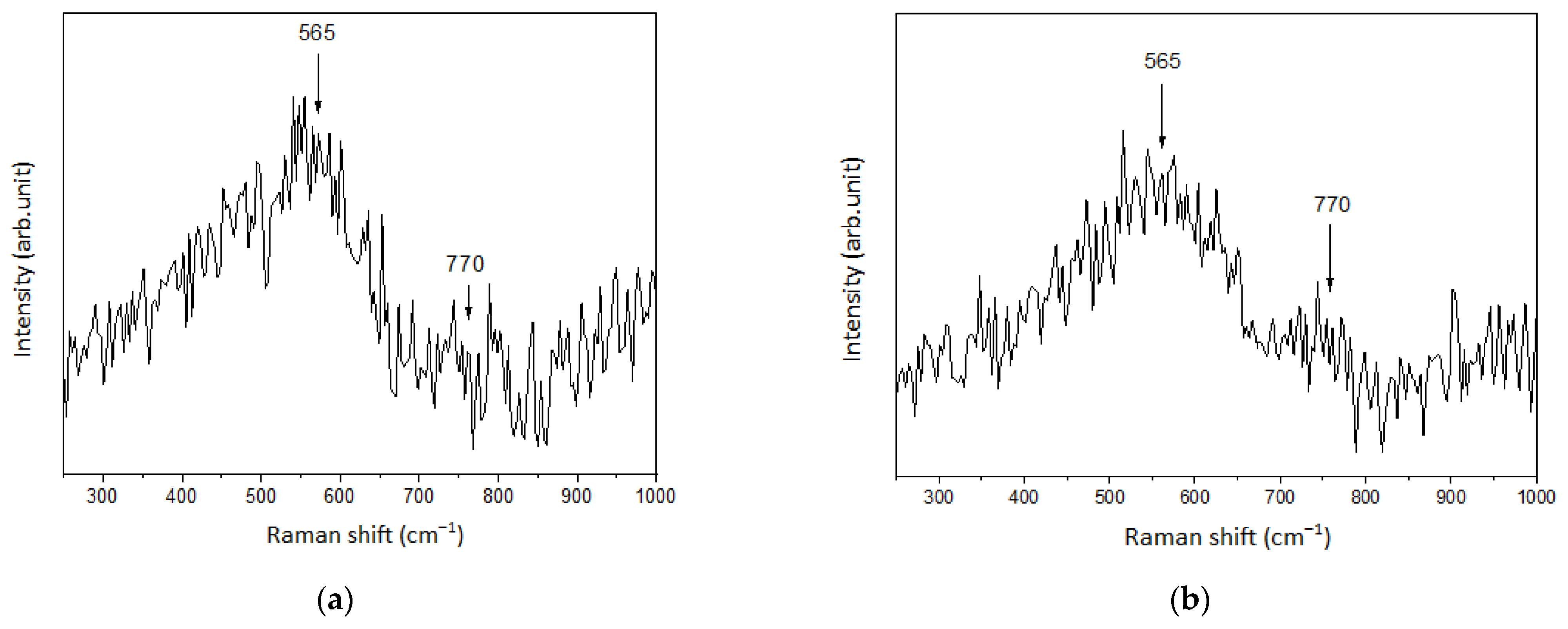
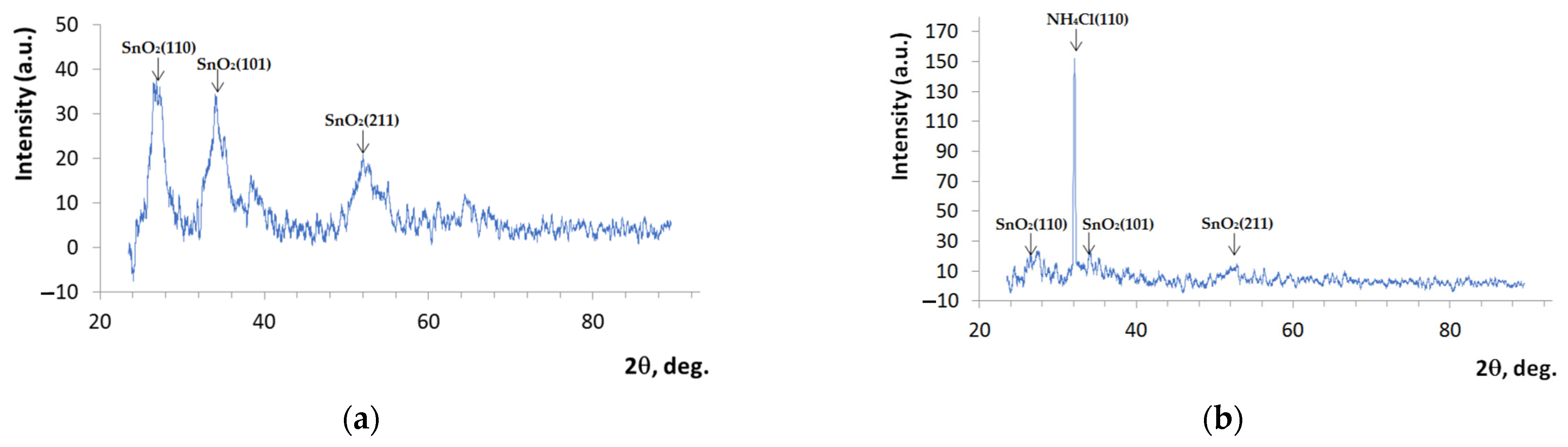
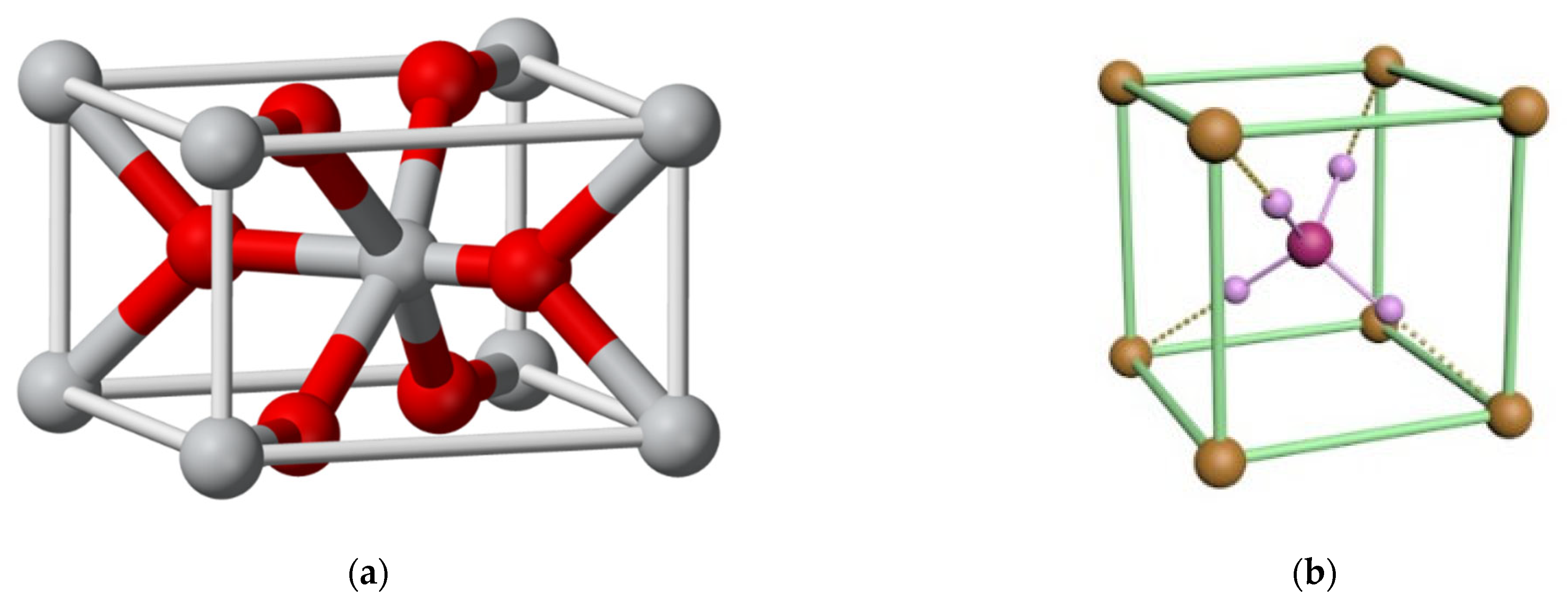
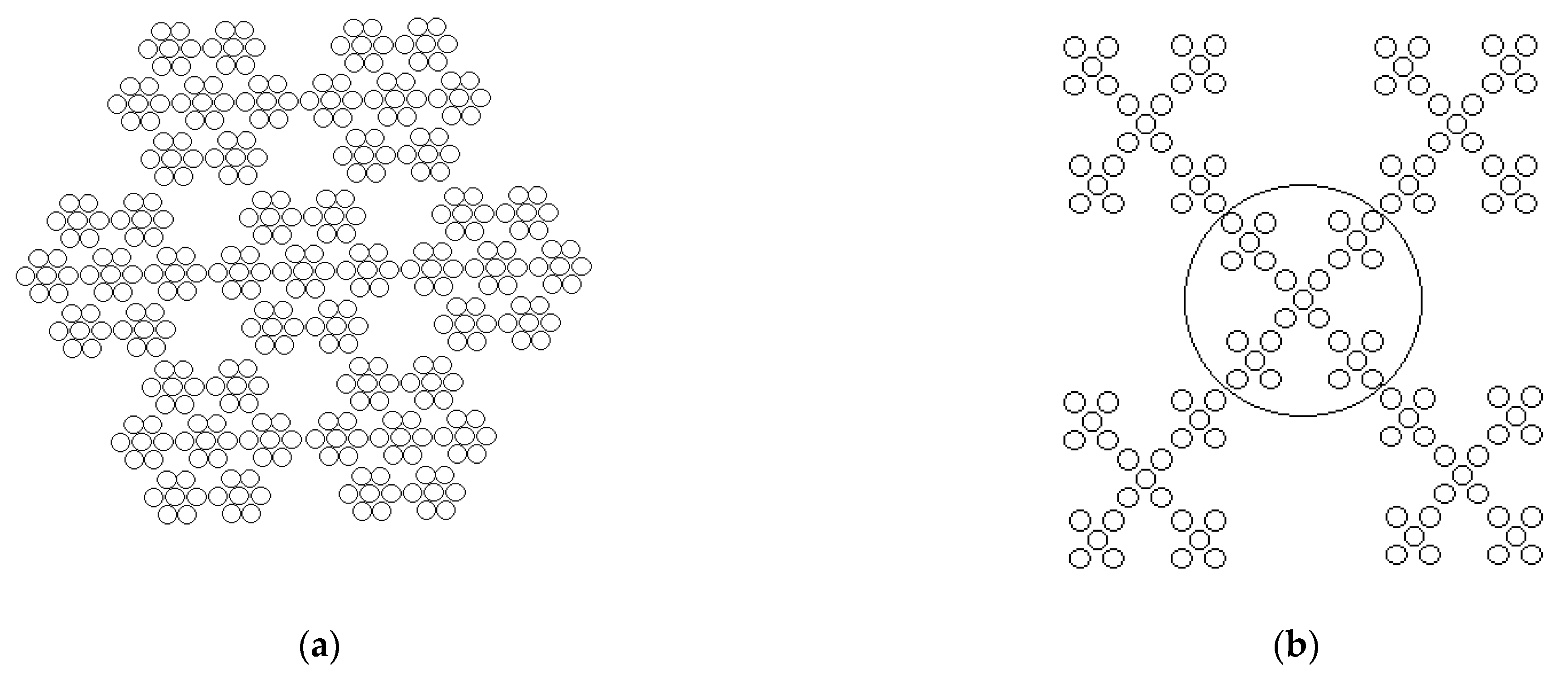
| V(NH4OH), mL | pH of Film-Forming Systems | Tin Ions in 100 mL (in Moles) | Ammonium Ions in 100 mL (in Moles) | Ammonium/Tin Ratio |
|---|---|---|---|---|
| 0 | 1.40 | 0.011 | 0 | 0 |
| 0.4 | 1.44 | 0.011 | 0.005 | 0.455 |
| 0.8 | 1.46 | 0.011 | 0.01 | 0.909 |
| 1.6 | 1.49 | 0.011 | 0.02 | 1.818 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bondar, E.; Dmitriyeva, E.; Lebedev, I.; Fedosimova, A.; Shongalova, A.; Ibraimova, S.; Kemelbekova, A.; Issayeva, U.; Rakymetov, B.; Nurbaev, B. The Synthesis of Materials with a Hierarchical Structure Based on Tin Dioxide. Nanomaterials 2024, 14, 1813. https://doi.org/10.3390/nano14221813
Bondar E, Dmitriyeva E, Lebedev I, Fedosimova A, Shongalova A, Ibraimova S, Kemelbekova A, Issayeva U, Rakymetov B, Nurbaev B. The Synthesis of Materials with a Hierarchical Structure Based on Tin Dioxide. Nanomaterials. 2024; 14(22):1813. https://doi.org/10.3390/nano14221813
Chicago/Turabian StyleBondar, Ekaterina, Elena Dmitriyeva, Igor Lebedev, Anastasiya Fedosimova, Aigul Shongalova, Sayora Ibraimova, Ainagul Kemelbekova, Ulzhalgas Issayeva, Bagdat Rakymetov, and Bedelbek Nurbaev. 2024. "The Synthesis of Materials with a Hierarchical Structure Based on Tin Dioxide" Nanomaterials 14, no. 22: 1813. https://doi.org/10.3390/nano14221813
APA StyleBondar, E., Dmitriyeva, E., Lebedev, I., Fedosimova, A., Shongalova, A., Ibraimova, S., Kemelbekova, A., Issayeva, U., Rakymetov, B., & Nurbaev, B. (2024). The Synthesis of Materials with a Hierarchical Structure Based on Tin Dioxide. Nanomaterials, 14(22), 1813. https://doi.org/10.3390/nano14221813








