Abstract
Black TiO2 with abundant oxygen vacancies (OVs)/B-doped graphitic carbon nitride (g-C3N4) Z-scheme heterojunction nanocomposites are successfully prepared by the one-pot strategy. The OVs can improve not only photogenerated carrier separation, but also the sorption and activation of antibiotic compounds (tetracycline hydrochloride, TC). The prepared heterojunction photocatalysts with a narrow bandgap of ∼2.13 eV exhibit excellent photocatalytic activity for the degradation of tetracycline hydrochloride (65%) under visible light irradiation within 30 min, which is several times higher than that of the pristine one. The outstanding photocatalytic property can be ascribed to abundant OVs and B element-dope reducing the bandgap and extending the photo-response to the visible light region, the Z-scheme formation of heterojunctions preventing the recombination of photogenerated electrons and holes, and promoting their effective separation.
1. Introduction
In recent years, the main aspects of environmental problems have been the energy crisis and pollution, with water pollution receiving special attention [1,2,3]. Water pollution is principally caused by heavy-metal ion contaminants and organic pollutants such as hormones, dyes, aromatics, pesticides, and perfluorinated organic compounds (PFOCs). Organic pollutants in wastewater, for example, have high toxicity, carcinogenicity, and refractory degradation, posing a significant threat to human health. As a consequence, it is critical to develop efficient technologies for breaking down organic pollutants from water [4]. Photocatalysis, one of the advanced oxidation methods for producing highly oxidizing free radicals, has been identified as a sustainable and ecologically friendly method for the degradation of pollutants. Photocatalytic oxidation has been acknowledged as a significant and successful candidate for eliminating poisonous and harmful contaminants in aqueous environments [5,6,7,8,9].
Numerous photocatalytic materials with superior band structures, visible light adsorption, charge separation, and transport have been created to date. Due to its huge band gap, the original TiO2—being a mature semiconductor—can only be used to purify wastewater using ultraviolet light, regardless of the fact that TiO2 has much lower biotoxicity than the majority of semiconductors [6,10,11,12]. Fortunately, Chen et al. discovered black TiO2 nanomaterials through a surface hydrogenation strategy, which narrowed the bandgap and extended photo-absorption from ultraviolet to visible light and/or near-infrared [13,14,15,16]. The outstanding solar-driven photocatalytic performance represented a breakthrough for wide-spectrum response TiO2 materials. In recent years, graphitic carbon nitride (g-C3N4), with a narrow band gap, excellent stability, and fast charge transfer, has been considered a potential visible light photocatalyst since the groundbreaking work reported by Wang et al. in 2009 [17,18,19,20]. However, the quick electron-hole recombination, low quantum efficiency, insufficient specific surface area, and other issues continue to restrict the photocatalytic activity of g-C3N4 [21]. Recently, many groups have documented the use of P- or S-doped g-C3N4 to enhance photocatalytic activity [22,23,24]. Wang et al. also discovered that even a small amount of boron doping could significantly increase photocatalytic activity [25].
Here, we proposed a one-pot synthesis of a B-doped g-C3N4/black-TiO2 (BCBT) heterojunction nanocomposite photocatalyst using NaBH4 as a solid reducing agent. This catalyst showed significantly higher photocatalytic degradation activity of high-toxic tetracycline hydrochloride (TC) when exposed to both visible light and simulated sunlight. The superiority of this Z-scheme BCBT heterojunction structure is demonstrated by the remarkable photocatalytic activity. More importantly, the photocatalytic degradation mechanism of the heterojunction is further revealed, which provides guidance for the design of a photocatalyst.
2. Experimental
2.1. Materials
Melamine, potassium borohydride (KBH4), and tetracycline hydrochloride (TC) were purchased from Chinese Medicine Group Chemical Reagent Co., Ltd. (Shanghai, China). Degussa P25 (P25, with 85% anatase and 15% rutile) was purchased from Sigma Aldrich (St. Louis, MO, USA). All chemicals were of analytically pure grade and used without further purification.
2.2. Fabrication of Black TiO2/B-Doped g-C3N4 Heterojunction
Further, 2.5 g Melamine, 2.5 g P25, and 1.0 g KBH4 were ground thoroughly for 15 min. Then, the mixture was calcined in a N2 flow at 520 °C for 3 h under normal pressure conditions with a constant heating rate of 5 °C min−1. The obtained composite was washed with deionized water and ethanol three times, and then dried in an oven at 80 °C overnight. Then, the resulting yellow product of BCBT was collected and ground into powder for further use, as detailed in Scheme 1.

Scheme 1.
Schematic illustration for synthesis of the black TiO2/B-doped g-C3N4 (BCBT).
2.3. Characterizations
The structure and phase of materials were determined using a SmartLAb SE X-ray diffractometer (XRD, Rigaku, Tokyo, Japan) with Cu-Kα radiation source at an operating voltage of 40 kV and an operating current of 180 mA. ESCALAB Xi+ X-ray photoelectron spectrometer (XPS, Thermo Fisher, MA, USA) with Al-Kα radiation as the excitation source was used to examine the elements on the surface of the samples. On a Regulus 8220 scanning electron microscope (SEM, Hitachi, Tokyo, Japan) and a JEM-2100 transmission electron microscope (TEM, JEOL, Tokyo, Japan), the microscopic morphologies of the samples were examined. The UV-vis diffuse reflectance spectrum (DRS) was recorded in the range of 200~800 nm using a UV 2600 UV-vis spectrophotometer (Shimadzu, Tokyo, Japan) using BaSO4 as a reference standard. The photoluminescence (PL) spectra of samples were measured using an LS 55 fluorescence spectrometer (Perkin Elmer, MA, USA) with an excitation wavelength of 350 nm.
2.4. Photocatalytic Degradation of Organic Pollutants
Using a Xenon arc lamp (PLS-SXE300+, PerfectLight, Beijing, China) with a cut-off filter (λ > 420 nm) and tetracycline hydrochloride (TC) as a contaminant, the photocatalytic degradation characteristics of BCBT were investigated. Then, 20 mg of the photocatalyst was added to 100 mL of TC solution with an initial concentration of 10 mg L−1. The solution was stirred for 20 min in the dark. The solution (5 mL) was filtered every 20 min. TC residuals were detected using a UV spectrophotometer. Pure distilled water was served as a reference sample.
2.5. Photoelectrochemical Properties
The photocurrent test, electrochemical impedance spectroscopy (EIS), and the Mott–Schottky plots of the samples were performed on a CHI-660E electrochemical workstation (Chenhua, Shanghai, China). To initiate the photoelectrochemical tests, a Xenon arc lamp (300 W, Beijing Aulight) with a cut-off filter (λ > 420 nm) was used as the light source. We started by dissolving 20 mg of material in ethanol. With an art airbrush, the dispersion was then uniformly sprayed on an FTO glass. Finally, the BCBT-coated FTO glass was calcined at 350 °C for 2 h in a N2 environment. The three-electrode electrochemical station included an aqueous Na2SO4 solution as the electrolyte, a platinum plate as the counter electrode, FTO glass as the photoanode, and Ag/AgCl as the reference electrode. To de-aerate the solution, the electrolyte was purged with N2 gas before use.
3. Results and Discussion
As shown in Figure 1a, the strong (200) peak at 32.2° and the (100) peak at 15.9° for the BCN (B-doped C3N4), respectively, belonged to the inter-layer and in-plane crystal facets of g-C3N4 (ICDD 01-078-1691). The diffraction patterns of the as-prepared b-TiO2 were well matched with that of the anatase TiO2 (ICDD 00-004-0477), showing that there was no impurity phase introduced after reduction by NaBH4. All of the diffraction peaks for the BCBT were identical to those for g-C3N4 and b-TiO2. These results indicate that there are no additional impurity peaks, proving that the B-doped g-C3N4/black-TiO2 (BCBT) composite samples were successfully synthesized via the one-pot process. The FT-IR spectra of the BT (black-TiO2), BCN, and BCBT further validated the existence of BCN and BT (Figure 1b). The typical peaks of g-C3N4 can be observed in BCBT at about 3200 cm−1 (C-H) and 1250–1650 cm−1 (C-N), which are consistent with the BCN [26]. Additionally, the characteristic peak of Ti-O is found at 500–1000 cm−1 [14]. All these findings demonstrate the presence of BCN and BT in the BCBT.
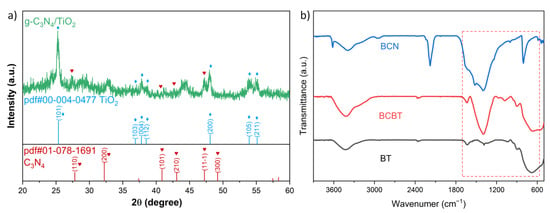
Figure 1.
The XRD patterns of BCBT photocatalysts (TiO2 in blue, C3N4 in red) (a) and FT-IR spectra of all prepared photocatalysts (b).
SEM and TEM were used to characterize the morphology of the obtained samples. In Figure 2a, the thin-layered BCN is associated with the b-TiO2 microspheres, which also demonstrates that b-TiO2 exhibits microspheres with sizes of about 50 nm. Figure 2a shows that the thin-layered BCN is deposited on the surface of b-TiO2 among the BCBT. EDS analysis confirms the existence of Ti, O, B, and C (Figure 2b–e). The TEM image of the BCBT in Figure 2f, which depicts the BCN nanoflakes loaded onto the surfaces of the b-TiO2 nanoparticles, further demonstrates this point and is in line with the findings of the aforementioned SEM studies. These findings demonstrate that b-TiO2 was successfully attached to the BCN surfaces.
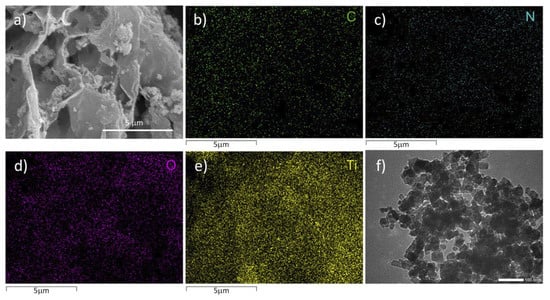
Figure 2.
SEM (a) and TEM (f) images of BCBT; (b–e) EDX elemental mappings of C, N, O, and Ti.
We examined the change of surface chemical bonding of BCBT induced by NaBH4 treatment with XPS. The XPS survey spectrum reveals the presence of Ti, B, C, N, and O elements (Figure 3a). A tiny change for Ti 2p can be observed in Figure 3b. This may imply that oxygen-bound electrons bound to titanium and oxygen ions turn in oxygen vacancies, which serve as electron traps [27]. Figure 3c displays the high-resolution B 1s peaks of BCN and BCBT. The BCN peak at 191.7 eV represents the typical B-N bond [28,29]. The BCBT peak at 190.4 eV, with a lower binding energy than BCN, shows that some boron atoms are less electropositive than BCN. These demonstrate the efficient charge transfer in the BCBT between b-TiO2 and BCN [30]. A peak near 532.5 eV in the O 1 s (Figure 3d) can be ascribed to adsorbed water, which is consistent with a robust interaction between O vacancy sites and water vapor. This peak area clearly grew during the NaBH4 reduction process, which is consistent with the electron transfer to the nearby oxygen vacancies, as shown in the Ti 2p spectrum [31,32,33,34].
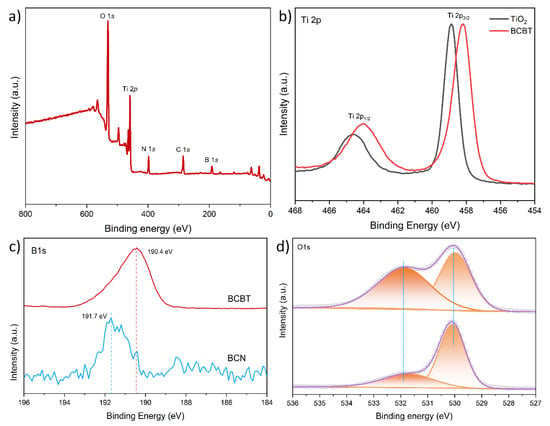
Figure 3.
The XPS spectra of BT, BCB, and BCBT: (a) survey spectrum of BCT; (b) Ti 2p and (d) O 1 s of BT and BCBT; and (c) B 1 s spectra of BCN and BCBT.
The light absorption ability is one of the crucial factors in determining photocatalytic performance. The light absorption properties of the as-prepared samples were characterized by the UV-vis diffuse reflectance spectra (UV-vis DRS). The absorption edges of BT and BCN, as seen in Figure 4a, are at wavelengths of around 400 and 460 nm, respectively, while the two photocatalysts all broaden the range of visible light absorption following NaBH4 reduction. One-pot solid synthesis further enhances the light-harvesting abilities of BCBT, which is attributed to the effective charge transfer between the BCN nanoflakes and BT nanoparticles. An additional broad absorption peak with a wavelength of roughly 400~800 nm is observed in the BCBT hybridized photocatalyst. The O vacancies and doped B elements both promote the activation of BCBT’s e−-h+ couples when exposed to visible light, increasing BCBT’s sensitivity to light. Figure 4b displays the Kubelka–Munk conversion curves for BT, BCN, and BCBT. Band gaps for BT, BCN, and BCBT are estimated to be ~1.98 eV, 2.32 eV, and 2.13 eV, respectively. According to these results, the BCBT, which has a narrower intrinsic bandgap than that of the BCN, is more active in regions of visible light. As a result, it explains why the subsequent photocatalytic activity was improved. The substantial absorption in the visible light range of BCBT is caused by the existence of oxygen vacancies and doped B elements [33,35]. Combining the characterization findings, it can be concluded that the addition of O vacancy sites and doped B elements increases the catalyst’s ability to absorb visible light, which is obviously conducive to the photocatalytic performance of defective BCBT.
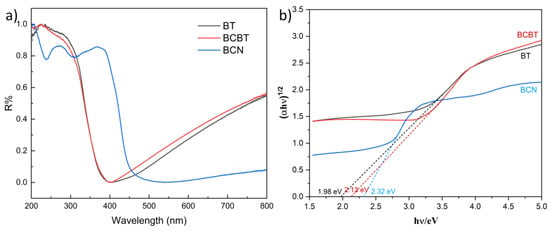
Figure 4.
(a) UV-vis diffuse reflectance spectra of the as-prepared BT, BCN, and BCBT. (b) Relationship of (ahν) 1/2 vs. E (ev).
The research results of photocatalytic activity of several samples for TC degradation are shown in Figure 5a. The reaction conditions are given in Section 2.2. Figure 5a displays the photocatalytic degradation rate of BCN at 27% after 30 min. The photocatalytic activity of BCBT was improved greatly, and degradation efficiency was up to 65% within 30 min. These may be due to the fact that the addition of OVs increases the light absorption range and creates a BCN/BT heterojunction that encourages photogenerated charge separation (PL and EIS spectra). By developing a kinetic model of the reaction, the kinetic behavior of the photocatalytic degradation reaction may be investigated further below.
when kC≪1, it can be simplified to pseudo-first-order dynamics.
where k stands for the pseudo-first-order kinetic constant. The kinetic constants for each molecule are shown in Figure 5b. The simplified pseudo-first-order kinetic formula of L-H demonstrates a remarkable linear relationship between the residual concentration of TC in various samples. The first-order kinetic constants for the fitted kinetic curves of BCN and BCBT in Figure 5b are ~0.0065 and 0.0271 min−1, respectively. The kinetic constants of BCBT are 4.17 times higher than that of BCN, indicating that BCBT’s photocatalytic activity greatly increased. Consequently, the BCBT photocatalyst has potential use in wastewater treatment due to its high efficiency, stability, and applicability of antibiotic photodegradation. Additionally, several photocatalysts for the photodegradation of TC published recently are presented in Table 1 and contrasted with the results in this work. The BCBT produced in this work showed superior photodegradation activity with a shorter reaction time when exposed to visible light irradiation when compared to other photocatalysts. This further demonstrates the capability of B-doped g-C3N4/black-TiO2 heterojunction photocatalysts for the photocatalytic degradation of TC.
−dC/dt = kC/(1 + kC)
−dC/dt = kC
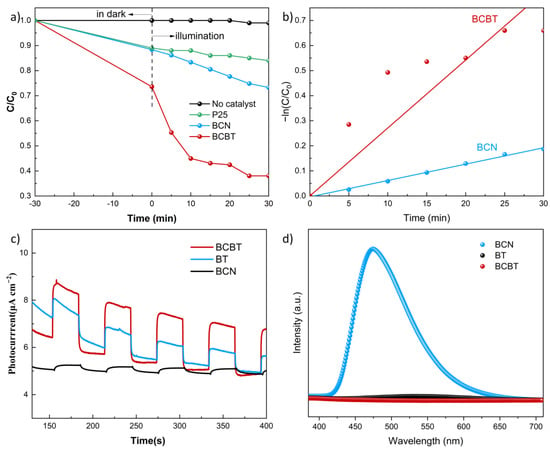
Figure 5.
Photocatalytic degradation efficiencies on the degradation of TC under simulated solar light irradiation (a), and kinetic linear simulation curves (b), photocurrent curves (c), and PL spectra (d) of BT, BCN, and BCBT, respectively.

Table 1.
The comparison of photocatalytic degradation activities of different photocatalysts for TC.
Transient photocurrent responses, which can be utilized to assess charge-transfer properties and photocatalyst stability, were studied using chronoamperometry. As shown in Figure 5c, BCBT has a higher photocurrent density and electron-hole separation efficiency than BT and BCN due to the presence of O vacancy and the heterojunction formation. Additionally, all composite photocurrent responses for both samples are continuous, demonstrating high stability. Figure 5d depicts the PL spectra of BT, BCN, and BCBT. The results reveal that BCBT has the lowest PL response when compared to the other samples, demonstrating that the photogenerated electron-hole pairs efficiently separate after one-pot solid reduction. Charge separation and transfer can be effectively enhanced by decreasing the recombination rate of the photogenerated carriers, directly boosting photocatalytic performance. Electrochemical impedance spectroscopy (EIS) was also used to analyze the migration of the charge carriers. Evaluating the kinetics at the interface requires a knowledge of the as-synthesis electron transfer resistance, which has been expressed as the diameter of the Nyquist circles. The BCBT sample has the median Nyquist circle diameter in contrast to the BT and BCN samples, as shown in Figure S1. Clearly, combining BCN with BT-rich O vacancies promotes the separation of photogenerated charge carriers.
Figure 6a shows the degradation and recovery rates after three cycles of using the BCBT photocatalyst. After three cycles, the 30 min photocatalytic degradation efficiency and recovery rate are still 58.2%, which implies that the sample has high stability, implying the potential applications in fields of environment.
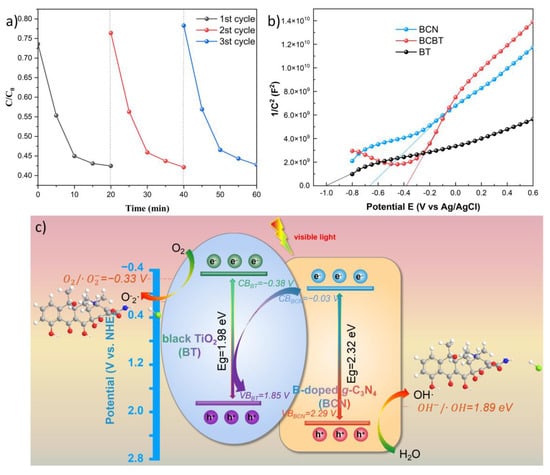
Figure 6.
Recycling experiments (a), Mott–Schottky plots (b) of BCBT, and the presented mechanism (c) for photocatalytic TC elimination by BCBT photocatalyst.
The Mott–Schottky (MS) plots for BT, BCN, and BCBT demonstrated that they were typical n-type semiconductors with relatively positive slopes, as shown in Figure 6b. Calculated from x-intercepts of the linear region, the flat-band potentials of BT, BCN, and BCBT were shown to be −1.01 V, 0.66 V, and 0.38 V vs. SCE. As a result, BT and BCN had conduction band potentials (ECB) of −0.38 V and −0.03 V vs. NHE, respectively. Comparing the ECB of BCBT composite to those of BT and BCN, it appears that there was a significant positive movement. The conduction band potential was believed to have shifted positively as a result of the electrical interactions between BT and BCN, leading to a low conduction band position and a higher observable absorption power for the BCBT composite.
The proposed photocatalytic mechanism over the BCBT photocatalyst is shown in Figure 6c. Both the BCN and BT produced photoinduced carriers when exposed to visible light. The photoinduced electrons were then transported from the CB of the B-doped g-C3N4 to the VB of the black TiO2 to create Z-scheme photocatalysts [42,43,44]. In addition, the photogenerated electrons produced by the BT reduced oxygen to form O2 − (E0 (O2/O2·−) = −0.33 eV) [45]. The photogenerated holes produced by the VB of BCN were sufficiently positive to cause the oxidation of OH− to OH (E0(OH−/·OH = +1.89 eV) [46]. Then, the TC interacted with RSs (reactive species: O2–, ·OH, and h+) to promote the degradation process. In addition, the absorption of visible light increases when in situ black TiO2 is combined with B-doped g-C3N4. The Ovs level and the introduction of B components considerably increase the BCBT photocatalysts’ ability to absorb light, giving them exceptional photo-absorption properties.
4. Conclusions
In conclusion, the successful synthesis of the Z-scheme black TiO2/B-doped g-C3N4 heterojunction photocatalyst and evaluation of the photocatalytic processes were accomplished. Black TiO2/B-doped g-C3N4 had a higher photocatalytic activity than black TiO2 and B-doped g-C3N4. The TC removal ratio for BCBT reached up to 65% within 30 min, which was much higher than that for pure BT and BCN. The abundance of OVs and B-doped elements in BCBT was largely responsible for its outstanding photocatalytic activity. These led to effective photogenerated carrier separation and enough visible light absorption, which improved BCBT’s photocatalytic efficiency.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nano13030518/s1, Figure S1: EIS plot of BT, BCN and BCBT, respectively.
Author Contributions
Methodology, Y.W., Y.J. and Y.Y.; software, K.X.; formal analysis, K.X.; investigation, K.X.; resources, L.F.; data curation, Y.W. and Y.J.; writing—original draft preparation, Y.W.; supervision, L.F. and H.J.; funding acquisition, Y.W. All authors have read and agreed to the published version of the manuscript.
Funding
The financial support from the Heilongjiang Provincial Natural Science Foundation of China (LH2020E127)”, “Fundamental Research Funds in Heilongjiang Provincial Universities (135309347)”, “Key research and development guidance projects in Heilongjiang Province (GZ20210034)”, “Opening Foundation of Heilongjiang Provincial Key Laboratory of Polymeric Composition materials (CLKFKT2021B3)”, “Undergraduate Training Programs for Innovation and Entrepreneurship of Qiqihar University (YJSCX2021039)” and Key Laboratory of Functional Inorganic Material Chemistry (Heilongjiang University), Ministry of Education.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw/processed data required to reproduce these findings cannot be shared at this time due to technical or time limitations.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liu, X.; Iocozzia, J.; Wang, Y.; Cui, X.; Chen, Y.; Zhao, S.; Li, Z.; Lin, Z. Noble Metal–Metal Oxide Nanohybrids with Tailored Nanostructures for Efficient Solar Energy Conversion, Photocatalysis and Environmental Remediation. Energy Environ. Sci. 2017, 10, 402–434. [Google Scholar] [CrossRef]
- Meng, N.; Ren, J.; Liu, Y.; Huang, Y.; Petit, T.; Zhang, B. Engineering Oxygen-Containing and Amino Groups into Two-Dimensional Atomically-Thin Porous Polymeric Carbon Nitrogen for Enhanced Photocatalytic Hydrogen Production. Energy Environ. Sci. 2018, 11, 566–571. [Google Scholar] [CrossRef]
- Rahman, M.Z.; Kwong, C.W.; Davey, K.; Qiao, S.Z. 2D Phosphorene as a Water Splitting Photocatalyst: Fundamentals to Applications. Energy Environ. Sci. 2016, 9, 709–728. [Google Scholar] [CrossRef]
- Wu, C.; Xing, Z.; Yang, S.; Li, Z.; Zhou, W. Nanoreactors for Photocatalysis. Coordin. Chem. Rev. 2023, 477, 214939. [Google Scholar] [CrossRef]
- Fang, B.; Xing, Z.; Sun, D.; Li, Z.; Zhou, W. Hollow Semiconductor Photocatalysts for Solar Energy Conversion. Adv. Powder Mater. 2022, 1, 100021. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Yu, C.; Zhou, W.; Liu, H.; Liu, Y.; Dionysiou, D.D. Design and Fabrication of Microsphere Photocatalysts for Environmental Purification and Energy Conversion. Chem. Eng. J. 2016, 287, 117–129. [Google Scholar] [CrossRef]
- Pi, Y.; Li, X.; Xia, Q.; Wu, J.; Li, Y.; Xiao, J.; Li, Z. Adsorptive and Photocatalytic Removal of Persistent Organic Pollutants (POPs) in Water by Metal-Organic Frameworks (MOFs). Chem. Eng. J. 2018, 337, 351–371. [Google Scholar] [CrossRef]
- Fang, B.; Xing, Z.; Kong, W.; Li, Z.; Zhou, W. Electron Spin Polarization-Mediated Charge Separation in Pd/CoP@CoNiP Superstructures toward Optimized Photocatalytic Performance. Nano Energy 2022, 101, 107616. [Google Scholar] [CrossRef]
- Zhou, W.; Sun, F.; Pan, K.; Tian, G.; Jiang, B.; Ren, Z.; Tian, C.; Fu, H. Well-Ordered Large-Pore Mesoporous Anatase TiO2 with Remarkably High Thermal Stability and Improved Crystallinity: Preparation, Characterization, and Photocatalytic Performance. Adv. Funct. Mater. 2011, 21, 1922–1930. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Ghiaci, M.; Kulinich, S.A.; Wunderlich, W.; Ghaziaskar, H.S.; Koupaei, A.J. Ethyl Benzene Oxidation under Aerobic Conditions Using Cobalt Oxide Imbedded in Nitrogen-Doped Carbon Fiber Felt Wrapped by Spiral TiO2-SiO2. Appl. Catal. A-Gen. 2022, 630, 118456. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Ghiaci, M.; Kulinich, S.A.; Wunderlich, W.; Monjezi, B.H.; Ghorbani, Y.; Ghaziaskar, H.S.; Javaheri Koupaei, A. Au-Pd Nanoparticles Enfolded in Coil-like TiO2 Immobilized on Carbon Fibers Felt as Recyclable Nanocatalyst for Benzene Oxidation under Mild Conditions. Appl. Surf. Sci. 2020, 506, 144644. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Yu, P.Y.; Mao, S.S. Increasing Solar Absorption for Photocatalysis with Black Hydrogenated Titanium Dioxide Nanocrystals. Science 2011, 331, 746–750. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Huang, F. Black Titanium Dioxide (TiO2) Nanomaterials. Chem. Soc. Rev. 2015, 44, 1861–1885. [Google Scholar] [CrossRef]
- Li, Z.; Li, H.; Wang, S.; Yang, F.; Zhou, W. Mesoporous Black TiO2/MoS2/Cu2S Hierarchical Tandem Heterojunctions toward Optimized Photothermal-Photocatalytic Fuel Production. Chem. Eng. J. 2022, 427, 131830. [Google Scholar] [CrossRef]
- Li, Z.; Wang, S.; Wu, J.; Zhou, W. Recent Progress in Defective TiO2 Photocatalysts for Energy and Environmental Applications. Renew. Sustain. Energy Rev. 2022, 156, 111980. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A Metal-Free Polymeric Photocatalyst for Hydrogen Production from Water under Visible Light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef]
- Ong, W.-J.; Tan, L.-L.; Ng, Y.H.; Yong, S.-T.; Chai, S.-P. Graphitic Carbon Nitride (g-C3N4)-Based Photocatalysts for Artificial Photosynthesis and Environmental Remediation: Are We a Step Closer To Achieving Sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef]
- Fu, J.; Yu, J.; Jiang, C.; Cheng, B. G-C3N4-Based Heterostructured Photocatalysts. Adv. Energy Mater. 2018, 8, 1701503. [Google Scholar] [CrossRef]
- Mamba, G.; Mishra, A.K. Graphitic Carbon Nitride (g-C3N4) Nanocomposites: A New and Exciting Generation of Visible Light Driven Photocatalysts for Environmental Pollution Remediation. Appl. Catal. B-Environ. 2016, 198, 347–377. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Ghiaci, M.; Kulinich, S.A.; Wunderlich, W.; Farrokhpour, H.; Saraji, M.; Shahvar, A. Au-Pd@g-C3N4 as an Efficient Photocatalyst for Visible-Light Oxidation of Benzene to Phenol: Experimental and Mechanistic Study. J. Phys. Chem. C 2018, 122, 27477–27485. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J.; Jaroniec, M. Preparation and Enhanced Visible-Light Photocatalytic H2-Production Activity of Graphene/C3N4 Composites. J. Phys. Chem. C 2011, 115, 7355–7363. [Google Scholar] [CrossRef]
- Zhang, Y.; Mori, T.; Ye, J.; Antonietti, M. Phosphorus-Doped Carbon Nitride Solid: Enhanced Electrical Conductivity and Photocurrent Generation. J. Am. Chem. Soc. 2010, 132, 6294. [Google Scholar] [CrossRef]
- Liu, G.; Niu, P.; Sun, C.; Smith, S.C.; Chen, Z.; Lu, G.Q.; Cheng, H.-M. Unique Electronic Structure Induced High Photoreactivity of Sulfur-Doped Graphitic C3N4. J. Am. Chem. Soc. 2010, 132, 11642–11648. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yang, L.; Wang, Y.; Li, L.; Chen, S. High Yield Synthesis of Homogeneous Boron Doping C3N4 Nanocrystals with Enhanced Photocatalytic Property. Appl. Surf. Sci. 2019, 489, 631–638. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, J.; Li, C.; Ji, W.; Yang, M.; Huang, H.; Liu, Y.; Kang, Z. Tunable Ternary (N, P, B)-Doped Porous Nanocarbons and Their Catalytic Properties for Oxygen Reduction Reaction. ACS Appl. Mater. Interfaces 2014, 6, 22297–22304. [Google Scholar] [CrossRef] [PubMed]
- Kang, Q.; Cao, J.; Zhang, Y.; Liu, L.; Xu, H.; Ye, J. Reduced TiO2 Nanotube Arrays for Photoelectrochemical Water Splitting. J. Mater. Chem. A 2013, 1, 5766. [Google Scholar] [CrossRef]
- Kawaguchi, M.; Kawashima, T.; Nakajima, T. Syntheses and Structures of New Graphite-like Materials of Composition BCN(H) and BC3N(H). Chem. Mater. 1996, 8, 1197–1201. [Google Scholar] [CrossRef]
- Song, L.; Ci, L.; Lu, H.; Sorokin, P.B.; Jin, C.; Ni, J.; Kvashnin, A.G.; Kvashnin, D.G.; Lou, J.; Yakobson, B.I.; et al. Large Scale Growth and Characterization of Atomic Hexagonal Boron Nitride Layers. Nano Lett. 2010, 10, 3209–3215. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, Y.; Dong, C.-L.; Huang, Y.-C.; Chen, J.; Xue, F.; Shen, S.; Guo, L. Boron-Doped Nitrogen-Deficient Carbon Nitride-Based Z-Scheme Heterostructures for Photocatalytic Overall Water Splitting. Nat. Energy 2021, 6, 388–397. [Google Scholar] [CrossRef]
- Yu, H.; Chen, F.; Li, X.; Huang, H.; Zhang, Q.; Su, S.; Wang, K.; Mao, E.; Mei, B.; Mul, G.; et al. Synergy of Ferroelectric Polarization and Oxygen Vacancy to Promote CO2 Photoreduction. Nat. Commun 2021, 12, 4594. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, H.; Sun, B.; Qiao, P.; Ren, L.; Tian, G.; Jiang, B.; Pan, K.; Zhou, W. Surface Oxygen Vacancy Defect-Promoted Electron-Hole Separation for Porous Defective ZnO Hexagonal Plates and Enhanced Solar-Driven Photocatalytic Performance. Chem. Eng. J. 2020, 379, 122295. [Google Scholar] [CrossRef]
- Yang, D.; Xu, Y.; Pan, K.; Yu, C.; Wu, J.; Li, M.; Yang, F.; Qu, Y.; Zhou, W. Engineering Surface Oxygen Vacancy of Mesoporous CeO2 Nanosheets Assembled Microspheres for Boosting Solar-Driven Photocatalytic Performance. Chin. Chem. Lett. 2022, 33, 378–384. [Google Scholar] [CrossRef]
- Zhou, W.; Li, W.; Wang, J.-Q.; Qu, Y.; Yang, Y.; Xie, Y.; Zhang, K.; Wang, L.; Fu, H.; Zhao, D. Ordered Mesoporous Black TiO2 as Highly Efficient Hydrogen Evolution Photocatalyst. J. Am. Chem. Soc. 2014, 136, 9280–9283. [Google Scholar] [CrossRef]
- Sinhamahapatra, A.; Jeon, J.-P.; Yu, J.-S. A New Approach to Prepare Highly Active and Stable Black Titania for Visible Light-Assisted Hydrogen Production. Energy Environ. Sci. 2015, 8, 3539–3544. [Google Scholar] [CrossRef]
- Lu, Z.; Zeng, L.; Song, W.; Qin, Z.; Zeng, D.; Xie, C. In Situ Synthesis of C-TiO2/g-C3N4 Heterojunction Nanocomposite as Highly Visible Light Active Photocatalyst Originated from Effective Interfacial Charge Transfer. Appl. Catal. B-Environ. 2017, 202, 489–499. [Google Scholar] [CrossRef]
- Kumar Ray, S.; Dhakal, D.; Gyawali, G.; Joshi, B.; Raj Koirala, A.; Wohn Lee, S. Transformation of Tetracycline in Water during Degradation by Visible Light Driven Ag Nanoparticles Decorated α-NiMoO4 Nanorods: Mechanism and Pathways. Chem. Eng. J. 2019, 373, 259–274. [Google Scholar] [CrossRef]
- Song, W.; Zhao, H.; Ye, J.; Kang, M.; Miao, S.; Li, Z. Pseudocapacitive Na+ Insertion in Ti–O–C Channels of TiO2 –C Nanofibers with High Rate and Ultrastable Performance. ACS Appl. Mater. Interfaces 2019, 11, 17416–17424. [Google Scholar] [CrossRef]
- Bao, S.; Liang, H.; Li, C.; Bai, J. The Synthesis and Enhanced Photocatalytic Activity of Heterostructure BiOCl/TiO2 Nanofibers Composite for Tetracycline Degradation in Visible Light. J. Disper. Sci. Technol. 2021, 42, 2000–2013. [Google Scholar] [CrossRef]
- Huang, X.; Guo, F.; Li, M.; Ren, H.; Shi, Y.; Chen, L. Hydrothermal Synthesis of ZnSnO3 Nanoparticles Decorated on G-C3N4 Nanosheets for Accelerated Photocatalytic Degradation of Tetracycline under the Visible-Light Irradiation. Sep. Purif. Technol. 2020, 230, 115854. [Google Scholar] [CrossRef]
- Bao, S.; Liu, H.; Liang, H.; Li, C.; Bai, J. Electrospinned Silk-Ribbon-like Carbon-Doped TiO2 Ultrathin Nanosheets for Enhanced Visible-Light Photocatalytic Activity. Colloid. Surface A 2021, 616, 126289. [Google Scholar] [CrossRef]
- Geng, R.; Yin, J.; Zhou, J.; Jiao, T.; Feng, Y.; Zhang, L.; Chen, Y.; Bai, Z.; Peng, Q. In Situ Construction of Ag/TiO2/g-C3N4 Heterojunction Nanocomposite Based on Hierarchical Co-Assembly with Sustainable Hydrogen Evolution. Nanomaterials 2020, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, M.; Wu, C.; Gao, J.; Li, M.; Xing, Z.; Li, Z.; Zhou, W. Hollow Nanoboxes Cu2-xS@ZnIn2S4 Core-Shell S-Scheme Heterojunction with Broad-Spectrum Response and Enhanced Photothermal-Photocatalytic Performance. Small 2022, 18, 2202544. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Zhou, W.; Li, H.; Ren, L.; Qiao, P.; Li, W.; Fu, H. Synthesis of Particulate Hierarchical Tandem Heterojunctions toward Optimized Photocatalytic Hydrogen Production. Adv. Mater. 2018, 30, 1804282. [Google Scholar] [CrossRef]
- Chen, P.; Wang, F.; Chen, Z.-F.; Zhang, Q.; Su, Y.; Shen, L.; Yao, K.; Liu, Y.; Cai, Z.; Lv, W.; et al. Study on the Photocatalytic Mechanism and Detoxicity of Gemfibrozil by a Sunlight-Driven TiO2/Carbon Dots Photocatalyst: The Significant Roles of Reactive Oxygen Species. Appl. Catal. B-Environ. 2017, 204, 250–259. [Google Scholar] [CrossRef]
- Huang, H.; He, Y.; Li, X.; Li, M.; Zeng, C.; Dong, F.; Du, X.; Zhang, T.; Zhang, Y. Bi2O2(OH)(NO3) as a Desirable [Bi2O2]2+ Layered Photocatalyst: Strong Intrinsic Polarity, Rational Band Structure and {001} Active Facets Co-Beneficial for Robust Photooxidation Capability. J. Mater. Chem. A 2015, 3, 24547–24556. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).