Abstract
Palladium (Pd) nanostructures are highly active non-platinum anodic electrocatalysts in alkaline direct methanol fuel cells (DMFCs), and their electrocatalytic performance relies highly on their morphology and composition. This study reports the preparation, characterizations, and electrocatalytic properties of palladium-copper alloys loaded on the carbon support. XC-72 was used as a support, and hydrazine hydrate served as a reducing agent. PdxCuy/XC-72 nanoalloy catalysts were prepared in a one-step chemical reduction process with different ratios of Pd and Cu. A range of analytical techniques was used to characterize the microstructure and electronic properties of the catalysts, including transmission electron microscopy (TEM), X-ray diffractometry (XRD), X-ray photoelectron spectroscopy (XPS), and inductively coupled plasma emission spectroscopy (ICP-OES). Benefiting from excellent electronic structure, Pd3Cu2/XC-72 achieves higher mass activity enhancement and improves durability for MOR. Considering the simple synthesis, excellent activity, and long-term stability, PdxCuy/XC-72 anodic electrocatalysts will be highly promising in alkaline DMFCs.
1. Introduction
With the rapid development of human society, the demand for energy in all countries is increasing, and the accompanying environmental problems are becoming more serious. It has become an imperative development strategy to develop innovative, sustainable, and environmentally friendly energy conversion technologies and devices. Fortunately, Direct Methanol Fuel Cells (DMFCs), as a new energy conversion device with simple structure, high energy density, fast charging, and environmental advantages, can transform direct chemical energy stored in methanol into electricity, which has attracted much attention in recent years [1,2,3,4,5]. At the same time, methanol is a relatively abundant product of the chemical industry, and the development of DMFCs is also considered to be the key to achieving carbon neutrality [6,7,8,9,10]. However, the anodic catalyst with excellent performance and low cost is a challenge for the commercial use of DMFCs. Therefore, it is essential to design and prepare the anode catalyst of DMFCs scientifically [11].
Pt has been verified to be the best catalyst for DMFCs; however, the active site of Pt is easily poisoned by COads produced in the process of methanol oxidation, and the methanol oxidation activity on the Pt electrode surface consequently decreases. In order to improve the anti-CO poisoning ability of catalysts, the research of DMFCs anode catalysts mainly focused on Pt and PtM (M = Pd [12], Ru [13], Fe [14], Co [15], Ni [16], Cu [17], Sn [18], and Ag [19]). Some studies have shown that there are two mechanisms of bifunctional mechanism and electronic effect in improving the anti-CO poisoning ability of Pt-based alloy catalysts. The bifunctional mechanism holds that methanol takes the adsorption–dehydrogenation processes on the surface of Pt. The second metal or metal oxide which is introduced promotes the dissociation of the electrolyte to form more OHads, which is conducive to faster oxidation removal of COads adsorbed on Pt [20,21]. On the other hand, the second metal reduces the adsorption energy of COads on the Pt surface by affecting the electronic properties of Pt, thus inhibiting the adsorption of COads on the Pt surface. The Pd-based catalyst is more abundant, has higher electrocatalytic activity for small alcohol molecules in alkaline media, and is resistant to CO poisoning. Thus, it can be used as a substitute for Pt-based anode catalysts [22].
Therefore, in order to further improve the electrocatalytic activity and economical utilization of the Pd catalyst, the second metal is usually introduced to form the alloy PdM (M = Cu [23], Co [24], Ag [25], Fe [26], and Sn [27]). Comparing it with a single metal, the bimetallic catalyst exposed to more active sites had higher activity and stability, owing to the shift of the d-band center of Pd, which is caused by the electronic effects and geometric changes of the catalyst. In addition, the support with a large specific surface area can provide a channel for electron transfer, such as carbon black, carbon nanotubes, and graphene, thus maintaining the excellent conductivity and performance of the catalyst.
In recent years, palladium copper alloy nanoparticles have attracted much attention due to their excellent small molecule oxidation properties of alcohols and ability to resist COads poisoning. The reason for this is that the d-band center of Pd shifts after the alloy formation between Cu and Pd, and the addition of copper reduces the binding energy of Pdn+. In contrast, Pd increases the binding energy of Cun+, which weakens the adsorption of COads on the catalyst surface.
Recently, Ye et al. [28] reported a PdCu catalyst with an unique core–shell structure. In an alkaline medium, the catalyst has excellent catalytic performance for methanol oxidation reaction (MOR), and the enhanced activity is rigorously ascribed to the adsorption strength of OHads, which is good for the removal of adsorbed COads and increases the ability of COads to resist COads poisoning. Shih et al. [23] studied porous PdCu NPs for electrocatalytic oxidation of methanol with excellent electrochemical activity, greater stability, and lower cost-effectiveness. The copper content of a catalyst is known to have a significant effect on its morphology and catalytic activity. Additionally, the copper content controls the morphology and affects the catalytic activity toward the MOR in alkaline media. This is an important discovery, because it can help to optimize the performance of catalysts for various applications. Saleem et al. [29] prepared an element segregation phenomenon in two-dimensional (2D) core–shell nanoplates, which showed superior electrocatalytic activity and stability towards the MOR compared to the commercial Pt/C catalyst. Unfortunately, the complex synthesis process impedes its practical application in DMFCs. Therefore, it is urgent to design and synthesize an efficient MOR catalyst using convenient, rapid, and eco-friendly synthetic methods.
Based on the above views, in this work, we synthesized PdCu nanoalloys on Vulcan XC-72 support by means of the liquid reduction method, which can synthesize many alloy catalysts in a short time under mild conditions, and meets the requirements of green chemistry. In addition, we synthesized a series of PdCu nanoalloys with different atomic ratios to study the influences of Cu addition on the electronic environment and the lattice strain of the catalysts.
2. Materials and Methods
2.1. Materials and Reagents
Palladium chloride PdCl2 (>99.9%); ethylene glycol (>99.8%), hydrazine hydrate (>85%), XC-72, concentrated hydrochloric acid (36~38%), copper nitrate solution (>99%), and all other reagents were of analytical grade and purchased from MACKLIN (Shanghai, China) without further purification.
2.2. Preparation of Catalysts
The supported PdxCuy/XC-72 (x + y = 5) nanoalloy catalysts with different compositions were prepared by one-step chemical reduction, with hydrazine hydrate as a reducing agent and controlling the total metal loading at 30%. Typically, taking Pd1Cu1/XC-72 as an example, 50.0 mg XC-72 was accurately weighed and dispersed in 200 mL ethylene glycol for 30 min by ultrasound, and 2 mL hydrazine hydrate was added to the mixture under stirring. Then, 11.41 mL of H2 PdCl4 (1 mg/mL) and 8.011 mg Cu(NO3)2·3H2O mixed precursor solution was added to it by dropping, and the mixture was reduced by stirring for two hours, followed by filtration, washing, and drying at 70 °C overnight to obtain Pd1Cu1/XC-72.
2.3. Physical Property Characterization
In order to conduct an X-ray diffraction (XRD, Bruker D8 Advance, Germany) analysis, a Bruker D8 Advance, equipped with Cu target Kα radiation as the X-ray source (λ = 0.15404 nm), was employed. Transmission electron microscopy (TEM, FEI Talos F200x, USA) and high-resolution transmission electron microscopy (HRTEM, FEI Talos F200x, USA) were also performed on a JEOL 2100F TEM with an accelerating voltage of 200 kV. Inductively coupled plasma-atomic emission spectroscopy (ICP-OES, Agilent 5110(OES), USA) was a powerful analytical tool that could measure a wide range of elements in a sample. In this study, ICP-OES was used to measure the concentration of elements in the sample. This information was then used to understand the chemical composition of the sample. To this end, a certain amount of the sample was digested by microwave with aqua regia at 200 °C for 1 h. This process was repeated five times until it was clear and transparent, to ensure that the solids were completely digested. Then, the as-obtained solution was volume-constant at 25 mL and diluted 100 times. Moreover, in order to obtain the electronic structure information of the catalyst surface, the PdxCuy/XC-72 was analyzed by X-ray photoelectron spectroscopy (XPS, Thermo Scientific K-Alpha, America). The as-synthesized materials were evaluated for electrochemical characterization using cyclic voltammetry (CV) and chronoamperometry (CA).
2.4. Electrochemical and Physical Characterization
The electrochemical performance of electrocatalysts were evaluated by workstation (CHI 660E) of a three-electrode system. The platinum electrode was the counter electrode, the calomel saturated electrode (SEC) was the reference electrode, and the glassy carbon electrode (GCE, 4.0 mm in diameter, 0.1256 cm [2]) coated with catalyst was the working electrode. The working electrode was prepared as follows: 2.0 mg of catalyst was accurately weighed, and 450 μL absolute ethanol, 50 μL 5% Nafion membrane solution, and 500 μL ultrapure water were successively added to the catalyst. It was then dispersed by ultrasound for 0.5 h to form a uniform inked mixture. Next, 5 μL was removed and dropped onto the surface of the glassy carbon electrode, which was allowed to dry naturally before testing. Cyclic voltammetry (CV) was used to test the activity of electrocatalytic methanol oxidation and the electrochemical active area (ECSA) in an alkaline environment. The MOR activity test electrolyte was 1 M KOH + 1.0 M CH3OH solution saturated with N2, and the ECSA test was placed into 1 M KOH solution saturated with N2. The scanning speed was 50 mV/s, and the potential range was −0.9–0.3 V (vs. SCE). In this work, a chronoamperometry experiment was used to evaluate the stability of the catalyst. The specific operating conditions were as follows: a chronoamperometry test was performed at −0.2 V constant potential, and rapid scanning was performed at 0.1 V/s scanning speed. All above tests were performed at room temperature.
3. Results
3.1. Physical Characterization of PdxCuy/XC-72
Figure 1 indicates the XRD profile of the as-synthesized PdxCuy/XC-72. The XRD profile reveals that the PdCu NPs are well crystallized, as observed from the three prominent diffraction peaks. The three main diffraction peaks appear at 2θ = 40.6°, 46.7°, and 68.2° index to the (111), (200), and (220) crystal planes of face-centered cubic (fcc) Pd NPs. The Pd (111) shift peak is shown in Figure 1. After Cu incorporation, the diffraction peak at 40.6°, corresponding to the Pd (111) plane, slightly shifted to the higher values (namely, 40.61°, 40.26°, 40.28°, 40.20°, 40.00°). This shift may be due to the Pd lattice contraction, owing to the smaller radius of Cu (0.128 nm) compared to that of Pd (0.137 nm), indicating the formation of successful PdCu alloy NPs [30].
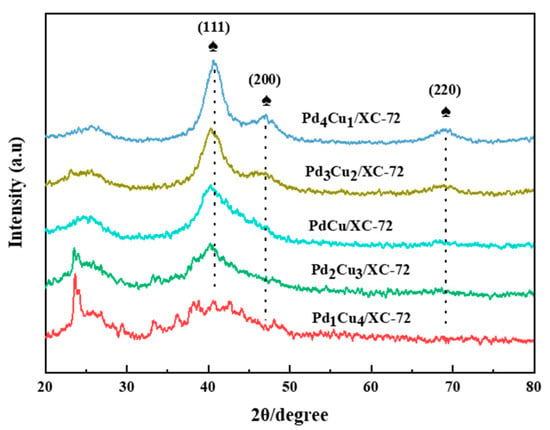
Figure 1.
XRD pattern of the electrocatalysts.
The dispersion of several catalysts with different PdCu ratios is reflected in the TEM images of PdxCuy/XC-72 in Figure 2. It can be seen that the catalysts formed with our synthesized are well-dispersed, with a uniform size distribution, and the particle size of the formed catalysts gradually rises with the increase in the Pd ratio.
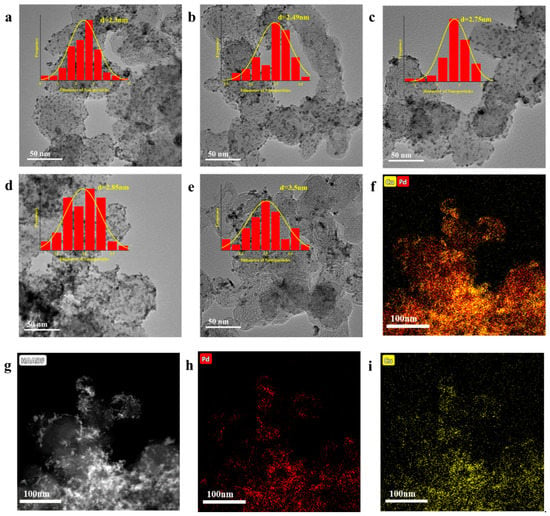
Figure 2.
TEM Micrographs of PdxCuy/XC-72 (a) Pd1Cu4/XC-72 (2.3 ± 1 nm), (b) Pd2Cu3/XC-72 (2.49 ± 1 nm), (c) PdCu/XC-72 (2.75 ± 1 nm), (d) Pd3Cu2/XC-72 (2.85 ± 2 nm), and (e) Pd4Cu1/XC-72 (3.5 ± 1 nm). (f–i) EDX element maps of Pd3Cu2/XC-72.
Figure 3 shows the HRTEM profile of the Pd3Cu2/XC-72. It can be seen that the metal is dispersed more evenly on the carbon support, and in the Figure 3c, the lattice fringes of the Pd(111) plane, Pd (200) plane, and Cu (111) plane can be clearly seen, which further verifies the formation of its alloy [31].
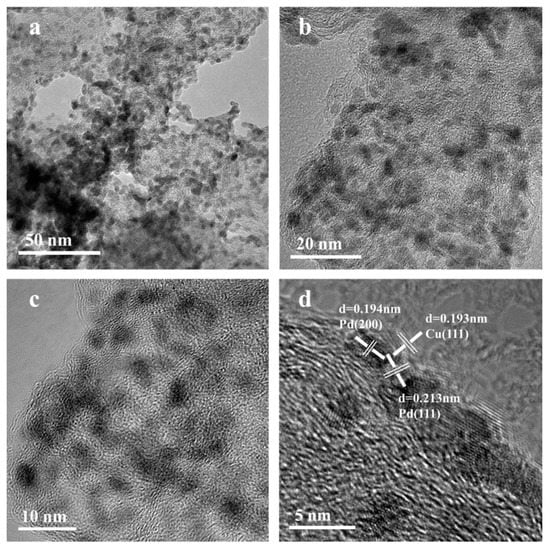
Figure 3.
HRTEM image of Pd3Cu2/XC-72 (a) 50 nm, (b) 20 nm, (c) 10 nm, (d) 5 nm.
Moreover, we used the microwave digestion technique combined with inductively coupled plasma optical emission spectrometry (ICP-OES) analysis. Table 1 shows that the Pd metal content of Pd1Cu4/XC-72, Pd2Cu3/XC-72, PdCu/XC-72, Pd3Cu2/XC-72, and Pd4Cu1/XC-72 is 7.6, 12.4, 18.2, 19.7, and 25.8 wt%, respectively. In addition, the Cu metal content of Pd1Cu4/XC-72, Pd2Cu3/XC-72, PdCu/XC-72, Pd3Cu2/XC-72, and Pd4Cu1/XC-72 is 15.4, 11.1, 10.2, 6.9, and 3.5 wt%, respectively. This generally agrees with the ratio of PdCu alloy in our experimental design.

Table 1.
ICP-OES results for PdxCuy/XC-72 catalysts.
The composites were further examined by XPS in order to learn more about the surface electronic state of PdxCuy/XC-72 (Figure 4). To ascertain the valence state of Pd in the hybrids, the XPS spectra of Pd 3d were also gathered. Metallic Pd0 is responsible for the Pd 3d5/2 peak at 335.2 and the 3d3/2 peak at 340.5 eV, and also the Pd 3d5/2 peak at 342.4 eV and 3d5/2 peak at 337.2 eV assigned to Pd2+. It can be seen that the majority of the metal precursors are successfully reduced and loaded onto the support by comparing the quantities of Pd0 and Pd2+. According to the Cu 2p spectra, the PdxCuy/XC-72 peaks at 952.56 and 932.7 eV, respectively, correspond to Cu0 2p1/2 and 2p3/2; the peaks at 953.7 and 933.6 eV, respectively, correspond to Cu2+ 2p1/2 and 2p3/2. The peaks at 944.8 and 941.9 eV, correspond to Cu2+ 2p3/2 satellite peaks. The overall intensity of the Pd peaks decreases with the addition of Cu, indicating less Pd exposure in the bimetallic.
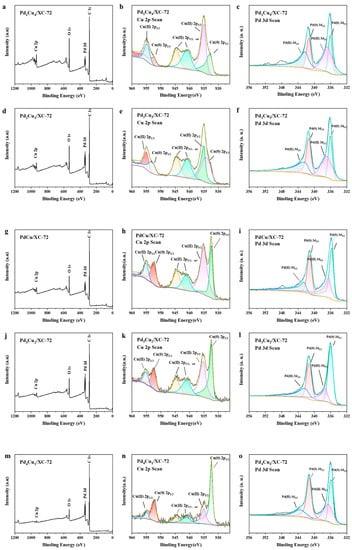
Figure 4.
XPS data showing the wide spectra of (a–c) Pd1Cu4/XC-72 wide spectra, Cu 2p, and Pd 3d; (d–f) Pd2Cu3/XC-72 wide spectra, Cu 2p, and Pd 3d; (g–i) PdCu/XC-72 wide spectra, Cu 2p, and Pd 3d; (j–l) Pd3Cu2/XC-72 wide spectra, Cu 2p, and Pd 3d; (m–o) Pd4Cu1/XC-72 wide spectra, Cu 2p, and Pd 3d.
Area ratios for various PdxCuy/XC-72 catalysts are shown in Figure 5. Since Pdn+ to Pd0 and Cun+ to Cu0 are the alloys formed during reduction, other metals will change to create a relatively stable state during the alloy formation process. At this point, low-valence Cu 2p electrons will be transferred to Pd, reducing the positive state of Pdn+ while increasing the valence state of low-valence Cu, resulting in a gradual rise in the concentration of Cun+.
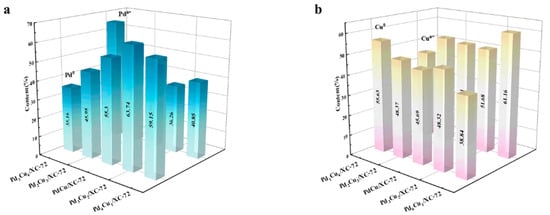
Figure 5.
(a) The corresponding Pd0 and Pdn+ contents of Pd1Cu4/XC-72, Pd2Cu3/XC-72, PdCu/XC-72, Pd3Cu2/XC-72, and Pd4Cu1/XC-72, as well as (b) the corresponding Cu0 and Cun+ contents of Pd1Cu4/XC-72, Pd2Cu3/XC-72, PdCu/XC-72, Pd3Cu2/XC-72, and Pd4Cu1/XC-72.
However, Pd3Cu2/XC-72 has the highest value of Pd0 and Cun+ content. There is also a change in the binding energy of a fraction of the Pd, represented by the broadening of the peaks. The electronic structure of the surface Pd atoms is modified by alloying with the Cu atoms as the Cu content increases and the binding energy shifts to a higher value. These results further confirm that the alloy of PdxCuy nanoparticles supported on XC-72 has been successfully synthesized by the chemical reduction method. In addition, the presence of Cu2+ may be caused by the surface oxidation or chemisorption of environmental oxygen during the synthesis process, as indicated by the shake-up satellite (sat.) peaks at the high binding energy side of the Cu 2p3/2 and Cu 2p1/2. The XPS spectra of the other catalysts are shown in Figure 4. The characteristic peaks for each catalyst are the same as the PdxCuy/XC-72 XPS results.
3.2. Electrochemical Tests for MOR (Three-Electrode Cell)
As is summarized in Figure 6, the electrochemical performance of the five electrocatalysts is shown. Cyclic voltammetry (CV) was performed on each of the Pd1Cu4/XC-72, Pd2Cu3/XC-72, PdCu/XC-72, Pd3Cu2/XC-72, and Pd4Cu1/XC-72 electrodes in N2-saturated 1.0 M KOH and 1.0 M CH3OH. It can be seen that there are obvious differences in electrocatalytic activities with different amounts of Cu. The results depict that Pd3Cu2/XC-72, the maximum value, was obtained (1719 mA·mg−1 Pd). Therefore, combined with XPS (Figure 4) and XRD (Figure 1) data, we can conclude that Pd3Cu2/XC-72 exhibits excellent catalytic performance due to its excellent electronic structure. In addition, it was found that the performance of the catalyst synthesized in this work was much higher than that of the catalysts synthesized in other works (Table 2).
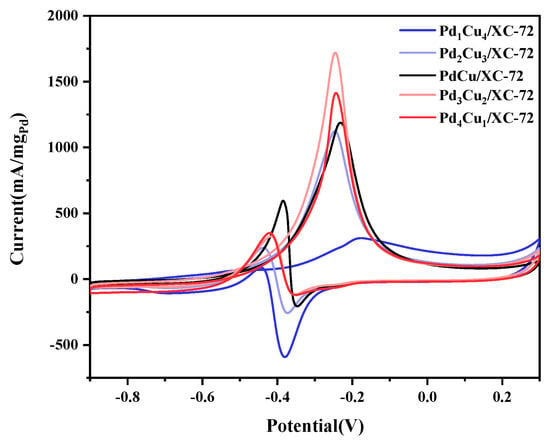
Figure 6.
CVs in 1.0 M KOH + 1.0 M CH3OH-purged N2 at 50 mVs−1.

Table 2.
Performance comparison of different palladium-based catalysts.
Generally, the ratio between the peak current in the forward (If) and the backward scan (Ib) is the measure for the tolerance of the catalyst against CO poisoning, with a higher If:Ib indicating higher tolerance. In our experiment, Pd3Cu2/XC-72 displays a higher If:Ib, which reflects its higher CO tolerance (Table 3). In other words, If:Ib is positively correlated with the area ratio of Pd0 and Cun+, which proves that higher Pd0 and Cun+ in the catalyst results in higher anti-CO performance.

Table 3.
Comparison of CO resistance of different catalysts.
Figure 7 summarizes the electrochemical performance of five electrocatalysts. Cyclic voltammetry(CV) is performed on each of the Pd1Cu4/XC-72, Pd2Cu3/XC-72, PdCu/XC-72, Pd3Cu2/XC-72 and Pd4Cu1/XC-72 electrode in N2-saturated 1.0 M KOH. The PdO reduction peaks of the catalysts containing Cu occur at earlier potentials, as seen in Figure 6. This means less energy was needed to reduce PdO in the presence of Cu. This is probably the attraction of Pd to metal oxides like CuO, which provides more electronics to the Pd and PdO active sites. In other words, it is more difficult to reduce PdO oxides as CuO withdraws electrons from Pd.
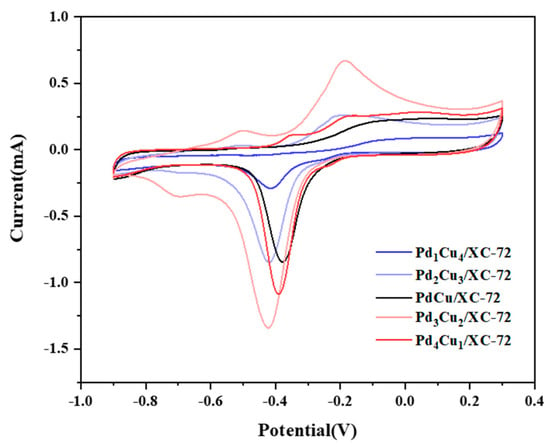
Figure 7.
CVs in 1.0 M KOH + CH3OH-purged N2 at 50 mVs−1.
The electrochemical active surface area (ECSA) is also an important index for evaluating the activity of electrocatalysts. In this work, the ESCA of different ratios of PdxCuy/XC-72 is estimated by integrating the Pd reduction peaks in order to find the Coulombic charge of each catalyst, and then following the proposed method of Rand and Wood (Equation (1))
where Q is the Coulombic charge (0.0536, 0.1316, 0.1173, 0.216562, and 0.1444 mC for Pd1Cu4/XC-72, Pd2Cu3/XC-72, PdCu/XC-72, Pd3Cu2/XC-72, and Pd4Cu1/XC-72, respectively), S is the Coulombic constant for a monolayer of Pd (0.424 mC cm−2), and L is the electrocatalyst loading (∼x μg (Pd)). The calculated ECSA Pd values decrease as follows: Pd3Cu2/XC-72 (2.384 m2 gPd−1) > Pd2Cu3/XC-72 (1.961 m2 gPd−1) > PdCu/XC-72 (1.47 m2 gPd−1) > Pd1Cu4/XC-72 (1.428 m2 gPd−1) > Pd4Cu1/XC-72 (1.305 m2 gPd−1). These results reveal that Cu acts as a promoter to boost the electrochemical activity of Pd.
ECSA = Q/SL
Chronoamperometry (CA) curves are recorded in 1.0 M CH3OH + 1.0 M KOH solution under a constant potential of −0.2 V, as shown in Figure 8. The high initial current density is caused by double-layer charging and abundant active sites for methanol activation. The current density dropped quickly within the first dozens of seconds due to the adsorption of CO produced from the MOR on the catalytic surface.
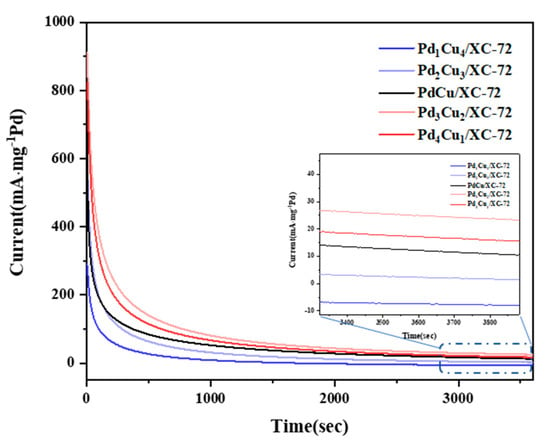
Figure 8.
CAs in 1.0 M KOH + 1.0 M CH3OH−purged N2 at 50 mVs−1.
After 500 s of testing, Pd3Cu2/XC-72 still had the highest ultimate current density (122.3 mA mg−1 Pd), further confirming that the Pd3Cu2/XC-72 nanocatalyst has the best electrocatalytic activity and long-term electrochemical durability for MOR. The synthesized Pd3Cu2/XC-72 nanoparticles have the advantages of large specific surface area, high alloying, strong electronic interactions between Pd and Cu atoms, a clear surface, and many active sites, which can significantly improve the electrocatalytic performance of MOR.
4. Conclusions
In summary, we studied a highly efficient palladium copper alloy catalyst with a coordination structure which was successfully synthesized by the liquid phase reduction method. During the synthesis, the ratios between Pd and Cu determined the alloying degree. The particular electronic characteristics endowed Pd3Cu2/XC-72 with large ECSA, fast mass transfer, and high self-stability, which contributed to the high electrocatalytic activity and excellent stability of Pd3Cu2/XC-72 for MOR in alkaline media. Additionally, Pd3Cu2/XC-72 exhibited enhanced MOR activity compared to Pd/C, indicating that the PdCu interface improved the MOR activity of Pd nanostructures. Experimental results demonstrated that the synergistic effect between Pd and Cu accelerated the oxidation of COads, which are also responsible for MOR activity/stability enhancement of Pd3Cu2/XC-72. This study provides new ideas for the design of highly active Pd electrocatalysts, and demonstrates the great potential of Pd3Cu2/XC-72 as a DMFCs anode electrocatalyst.
Author Contributions
Conceptualization, G.L. and S.W.; methodology, S.W. and P.G.; software, H.L. and X.Z.; validation, G.L., H.L. and S.W.; formal analysis, P.G.; investigation, S.W.; resources, H.L.; data curation, H.L. and D.J.; writing—original draft preparation, S.W. and H.L.; writing—review and editing, S.W. and H.L.; visualization, S.W. and Y.L.; supervision, G.L.; project administration, H.L.; funding acquisition, H.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Basic Research Innovation Group Project of Gansu Province, grant number NO. 22JR5RA219.
Data Availability Statement
The data presented in this study are available upon request from the first author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fu, X.; Wan, C.; Huang, Y.; Duan, X. Noble Metal Based Electrocatalysts for Alcohol Oxidation Reactions in Alkaline Media. Adv. Funct. Mater. 2022, 32, 2106401. [Google Scholar] [CrossRef]
- Bianchini, C.; Shen, P.K. Palladium-Based Electrocatalysts for Alcohol Oxidation in Half Cells and in Direct Alcohol Fuel Cells. Chem. Rev. 2009, 109, 4183–4206. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Zhang, X.; Sun, H.; Wang, S.; Sun, G. Recent Advances in Multi-Scale Design and Construction of Materials for Direct Methanol Fuel Cells. Nano Energy 2019, 65, 104048. [Google Scholar] [CrossRef]
- Tong, Y.Y.; Gu, C.D.; Zhang, J.L.; Huang, M.L.; Tang, H.; Wang, X.L.; Tu, J.P. Three-Dimensional Astrocyte-Network Ni–P–O Compound with Superior Electrocatalytic Activity and Stability for Methanol Oxidation in Alkaline Environments. J. Mater. Chem. A 2015, 3, 4669–4678. [Google Scholar] [CrossRef]
- de Sá, M.H.; Pinto, A.M.F.R.; Oliveira, V.B. Passive Direct Methanol Fuel Cells as a Sustainable Alternative to Batteries in Hearing Aid Devices—An Overview. Int. J. Hydrogen Energy 2022, 47, 16552–16567. [Google Scholar] [CrossRef]
- Zhou, Y.; Xie, Z.; Jiang, J.; Wang, J.; Song, X.; He, Q.; Ding, W.; Wei, Z. Author Correction: Lattice-Confined Ru Clusters with High CO Tolerance and Activity for the Hydrogen Oxidation Reaction. Nat. Catal. 2021, 4, 341. [Google Scholar] [CrossRef]
- Wan, X.; Liu, X.; Li, Y.; Yu, R.; Zheng, L.; Yan, W.; Wang, H.; Xu, M.; Shui, J. Fe–N–C Electrocatalyst with Dense Active Sites and Efficient Mass Transport for High-Performance Proton Exchange Membrane Fuel Cells. Nat. Catal. 2019, 2, 259–268. [Google Scholar] [CrossRef]
- Anson, C.W.; Stahl, S.S. Mediated Fuel Cells: Soluble Redox Mediators and Their Applications to Electrochemical Reduction of O2 and Oxidation of H2, Alcohols, Biomass, and Complex Fuels. Chem. Rev. 2020, 120, 3749–3786. [Google Scholar] [CrossRef]
- Luo, M.; Zhao, Z.; Zhang, Y.; Sun, Y.; Xing, Y.; Lv, F.; Yang, Y.; Zhang, X.; Hwang, S.; Qin, Y.; et al. PdMo Bimetallene for Oxygen Reduction Catalysis. Nature 2019, 574, 81–85. [Google Scholar] [CrossRef]
- Zhu, J.; Xia, L.; Yu, R.; Lu, R.; Li, J.; He, R.; Wu, Y.; Zhang, W.; Hong, X.; Chen, W.; et al. Ultrahigh Stable Methanol Oxidation Enabled by a High Hydroxyl Concentration on Pt Clusters/MXene Interfaces. J. Am. Chem. Soc. 2022, 144, 15529–15538. [Google Scholar] [CrossRef]
- Zuo, Y.; Sheng, W.; Tao, W.; Li, Z. Direct Methanol Fuel Cells System–A Review of Dual-Role Electrocatalysts for Oxygen Reduction and Methanol Oxidation. J. Mater. Sci. Technol. 2022, 114, 29–41. [Google Scholar] [CrossRef]
- Ali, A.; Shen, P.K. Recent Advances in Graphene-Based Platinum and Palladium Electrocatalysts for the Methanol Oxidation Reaction. J. Mater. Chem. A 2019, 7, 22189–22217. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, L.; Liao, S.; Zeng, J. Pt∧Ru/C Catalysts Synthesized by a Two-Stage Polyol Reduction Process for Methanol Oxidation Reaction. J. Power Sources 2011, 196, 10570–10575. [Google Scholar] [CrossRef]
- Lee, E.; Kim, S.; Jang, J.-H.; Park, H.-U.; Matin, M.A.; Kim, Y.-T.; Kwon, Y.-U. Effects of Particle Proximity and Composition of Pt–M (M = Mn, Fe, Co) Nanoparticles on Electrocatalysis in Methanol Oxidation Reaction. J. Power Sources 2015, 294, 75–81. [Google Scholar] [CrossRef]
- Li, Y.; Li, H.; Li, G.; Wang, D.; Wang, S.; Zhao, X. Low-Temperature N-Anchored Ordered Pt3Co Intermetallic Nanoparticles as Electrocatalysts for Methanol Oxidation Reaction. Nanoscale 2022, 14, 14199–14211. [Google Scholar] [CrossRef] [PubMed]
- Fan, A.; Qin, C.; Zhao, R.; Sun, H.; Sun, H.; Dai, X.; Ye, J.-Y.; Sun, S.-G.; Lu, Y.; Zhang, X. Phosphorus-Doping-Tuned PtNi Concave Nanocubes with High-Index Facets for Enhanced Methanol Oxidation Reaction. Nano Res. 2022, 15, 6961–6968. [Google Scholar] [CrossRef]
- Chen, G.; Yang, X.; Xie, Z.; Zhao, F.; Zhou, Z.; Yuan, Q. Hollow PtCu Octahedral Nanoalloys: Efficient Bifunctional Electrocatalysts towards Oxygen Reduction Reaction and Methanol Oxidation Reaction by Regulating near-Surface Composition. J. Colloid Interface Sci. 2020, 562, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Lei, Z.; Zeng, T.; Wang, L.; Cheng, N.; Tan, Y.; Mu, S. Structurally Ordered PtSn Intermetallic Nanoparticles Supported on ATO for Efficient Methanol Oxidation Reaction. Nanoscale 2019, 11, 19895–19902. [Google Scholar] [CrossRef]
- Qiao, M.; Wu, H.; Meng, F.; Zhuang, Z.; Wang, J. Defect-Rich, Highly Porous PtAg Nanoflowers with Superior Anti-Poisoning Ability for Efficient Methanol Oxidation Reaction. Small 2022, 18, 2106643. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xia, T.; Wang, S.; Yang, G.; Dong, B.; Wang, C.; Ma, Q.; Sun, Y.; Wang, R. Oriented-Assembly of Hollow FePt Nanochains with Tunable Catalytic and Magnetic Properties. Nanoscale 2016, 8, 11432–11440. [Google Scholar] [CrossRef]
- Fan, H.; Cheng, M.; Wang, Z.; Wang, R. Layer-Controlled Pt-Ni Porous Nanobowls with Enhanced Electrocatalytic Performance. Nano Res. 2017, 10, 187–198. [Google Scholar] [CrossRef]
- Zhou, M.; Guo, J.; Fang, J. Nanoscale Design of Pd-Based Electrocatalysts for Oxygen Reduction Reaction Enhancement in Alkaline Media. Small Struct. 2022, 3, 2100188. [Google Scholar] [CrossRef]
- Shih, Z.-Y.; Wang, C.-W.; Xu, G.; Chang, H.-T. Porous Palladium Copper Nanoparticles for the Electrocatalytic Oxidation of Methanol in Direct Methanol Fuel Cells. J. Mater. Chem. A 2013, 1, 4773. [Google Scholar] [CrossRef]
- Lei, H.; Zhang, Q. In Situ Electrochemical Redox Tuning of Pd-Co Hybrid Electrocatalysts for High-Performance Methanol Oxidation: Strong Metal-Support Interaction. J. Colloid Interface Sci. 2021, 588, 476–484. [Google Scholar] [CrossRef]
- Lao, X.; Sun, T.; Zhang, X.; Pang, M.; Fu, A.; Guo, P. Controllable Lattice Expansion of Monodisperse Face-Centered Cubic Pd–Ag Nanoparticles for C1 and C2 Alcohol Oxidation: The Role of Core–Sheath Lattice Mismatch. ACS Sustain. Chem. Eng. 2022, 10, 6843–6852. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, S.; Sun, X.; Sun, S. Synthesis of Ultrathin FePtPd Nanowires and Their Use as Catalysts for Methanol Oxidation Reaction. J. Am. Chem. Soc. 2011, 133, 15354–15357. [Google Scholar] [CrossRef]
- Chen, S.; Liu, N.; Zhong, J.; Yang, R.; Yan, B.; Gan, L.; Yu, P.; Gui, X.; Yang, H.; Yu, D.; et al. Engineering Support and Distribution of Palladium and Tin on MXene with the Modulation D-Band Center for CO-resilient Methanol Oxidation. Angew. Chem. Int. Ed. 2022, 61, e202209693. [Google Scholar] [CrossRef] [PubMed]
- Ye, N.; Bai, Y.; Jiang, Z.; Fang, T. Design the PdCu/TaN C Electrocatalyst with Core-Shell Structure Having High Efficiency for Methanol and Formic Acid Oxidation Reactions. Electrochim. Acta 2021, 383, 138365. [Google Scholar] [CrossRef]
- Saleem, F.; Zhang, Z.; Cui, X.; Gong, Y.; Chen, B.; Lai, Z.; Yun, Q.; Gu, L.; Zhang, H. Elemental Segregation in Multimetallic Core–Shell Nanoplates. J. Am. Chem. Soc. 2019, 141, 14496–14500. [Google Scholar] [CrossRef]
- Hu, S.; Munoz, F.; Noborikawa, J.; Haan, J.; Scudiero, L.; Ha, S. Carbon Supported Pd-Based Bimetallic and Trimetallic Catalyst for Formic Acid Electrochemical Oxidation. Appl. Catal. B Environ. 2016, 180, 758–765. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, B.; Dong, S.; Wang, C.; Feng, A.; Fan, X.; Li, Y. Reduced Graphene Oxide Supported Pd-Cu-Co Trimetallic Catalyst: Synthesis, Characterization and Methanol Electrooxidation Properties. J. Energy Chem. 2019, 29, 72–78. [Google Scholar] [CrossRef]
- Tan, Q.; Shu, C.; Abbott, J.; Zhao, Q.; Liu, L.; Qu, T.; Chen, Y.; Zhu, H.; Liu, Y.; Wu, G. Highly Dispersed Pd-CeO 2 Nanoparticles Supported on N-Doped Core–Shell Structured Mesoporous Carbon for Methanol Oxidation in Alkaline Media. ACS Catal. 2019, 9, 6362–6371. [Google Scholar] [CrossRef]
- Jin, L.; Xu, H.; Chen, C.; Shang, H.; Wang, Y.; Wang, C.; Du, Y. Three-Dimensional PdCuM (M = Ru, Rh, Ir) Trimetallic Alloy Nanosheets for Enhancing Methanol Oxidation Electrocatalysis. ACS Appl. Mater. Interfaces 2019, 11, 42123–42130. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Li, F.; Huang, H.; Yin, S.; Chen, P.; Jin, P.; Chen, Y. Porous Pd-PdO Nanotubes for Methanol Electrooxidation. Adv. Funct. Mater. 2020, 30, 2000534. [Google Scholar] [CrossRef]
- Luo, X.; Liu, C.; Wang, X.; Shao, Q.; Pi, Y.; Zhu, T.; Li, Y.; Huang, X. Spin Regulation on 2D Pd–Fe–Pt Nanomeshes Promotes Fuel Electrooxidations. Nano Lett. 2020, 20, 1967–1973. [Google Scholar] [CrossRef]
- Xue, J.; Hu, Z.; Li, H.; Zhang, Y.; Liu, C.; Li, M.; Yang, Q.; Hu, S. Pd-Sn Alloy Nanoparticles for Electrocatalytic Methanol Oxidation: Phase Evolution from Solid Solution to Intermetallic Compounds. Nano Res. 2022, 15, 8819–8825. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).