Phase-Field Simulation on the Effect of Second-Phase Particles on Abnormal Growth of Goss Grains in Fe-3%Si Steels
Abstract
1. Introduction
2. Materials, Model, and Parameters
2.1. Phase Model with Second-Phase Particles
2.2. Model Parameters Setting and Initial Condition
3. Result and Analysis
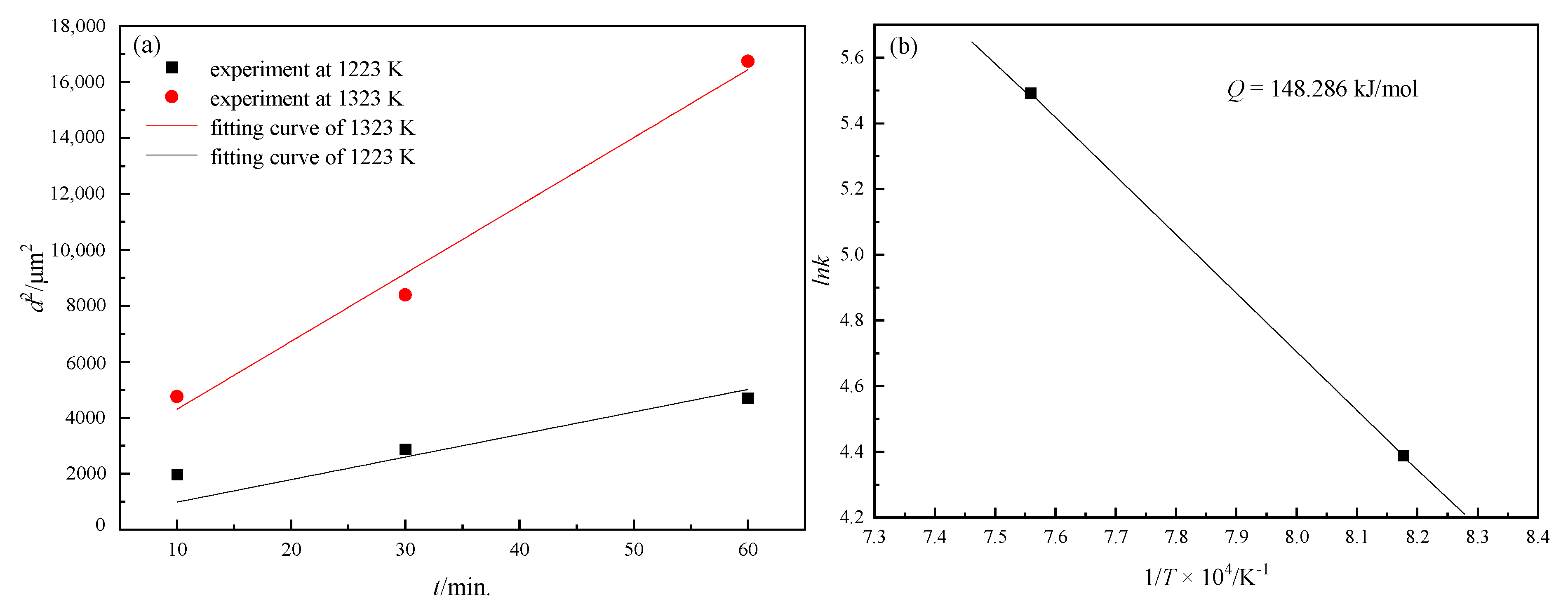
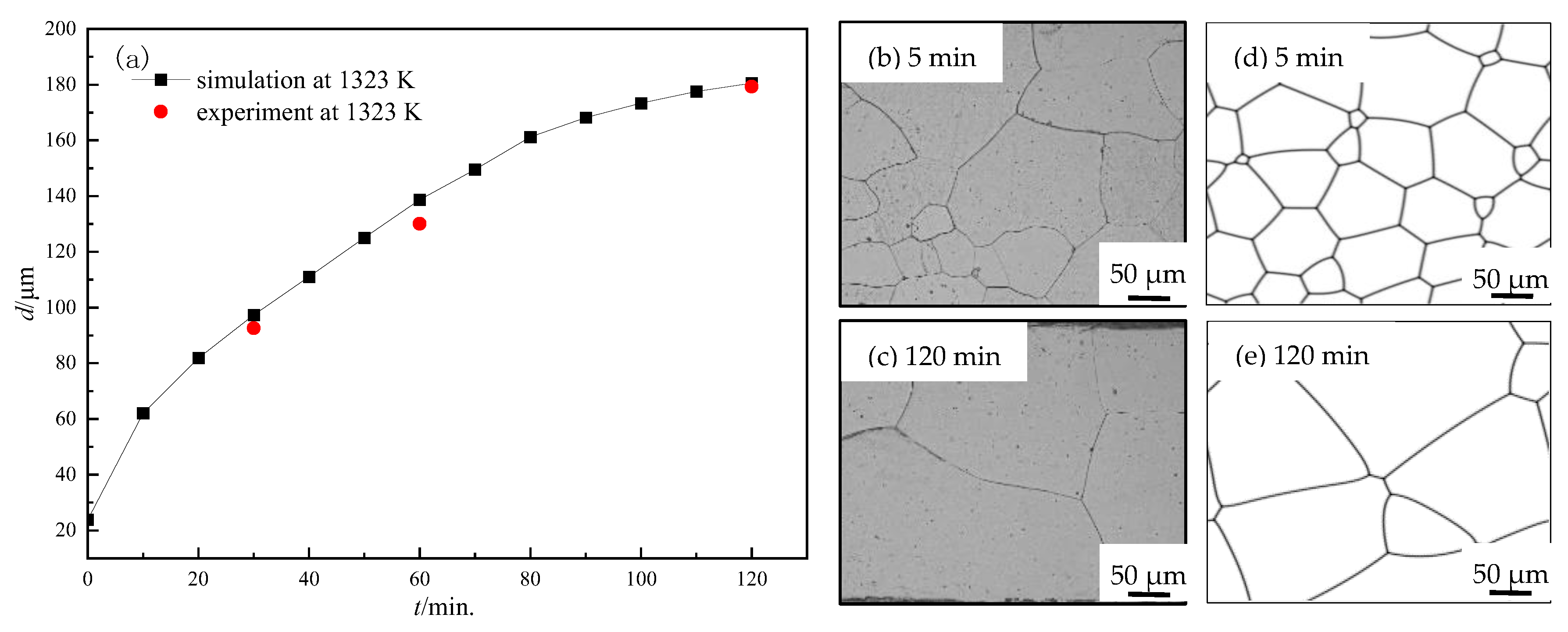
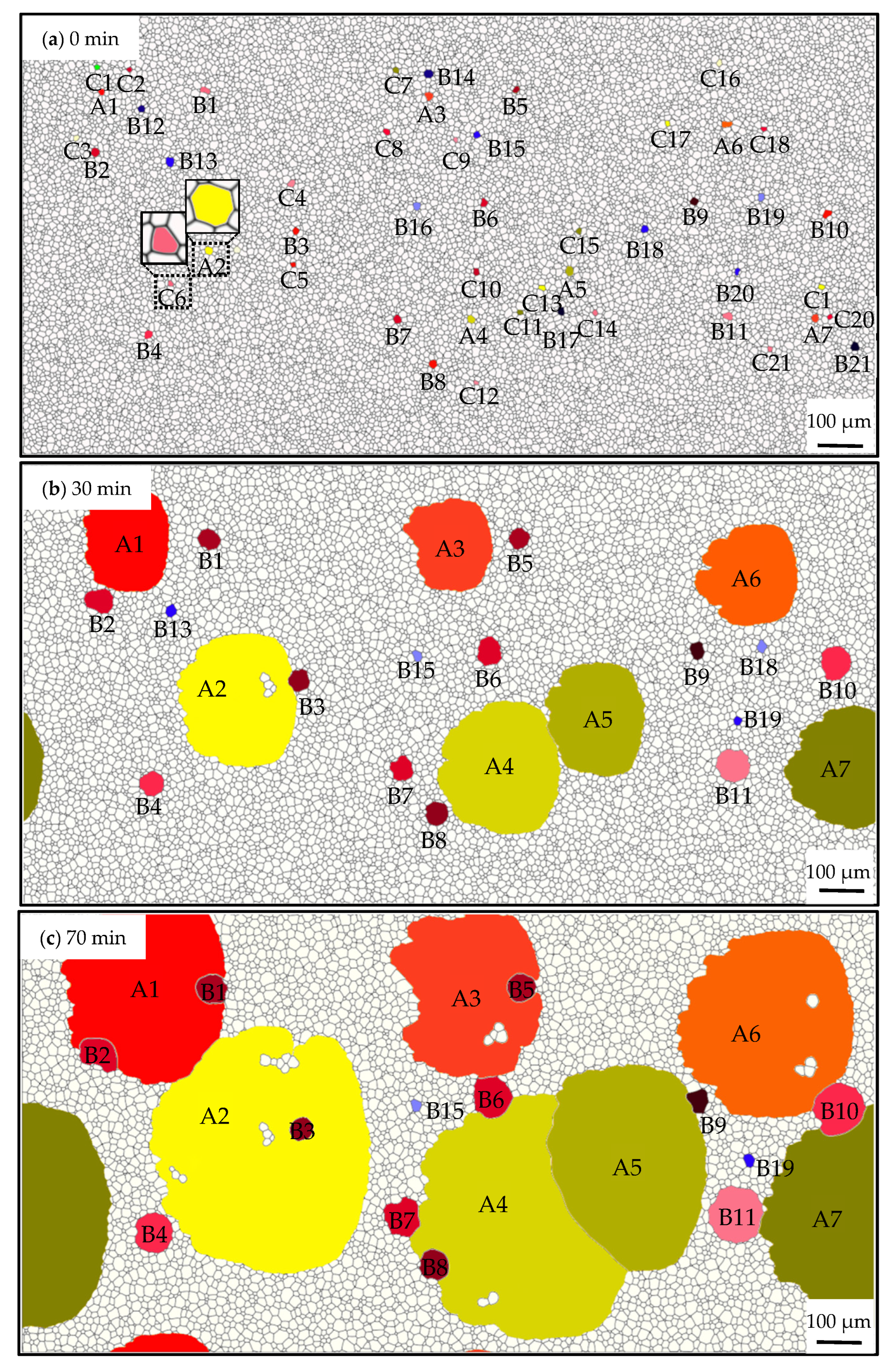
3.1. Grain Growth in Non-Oriented Fe-3%Si Steels at Different Temperatures
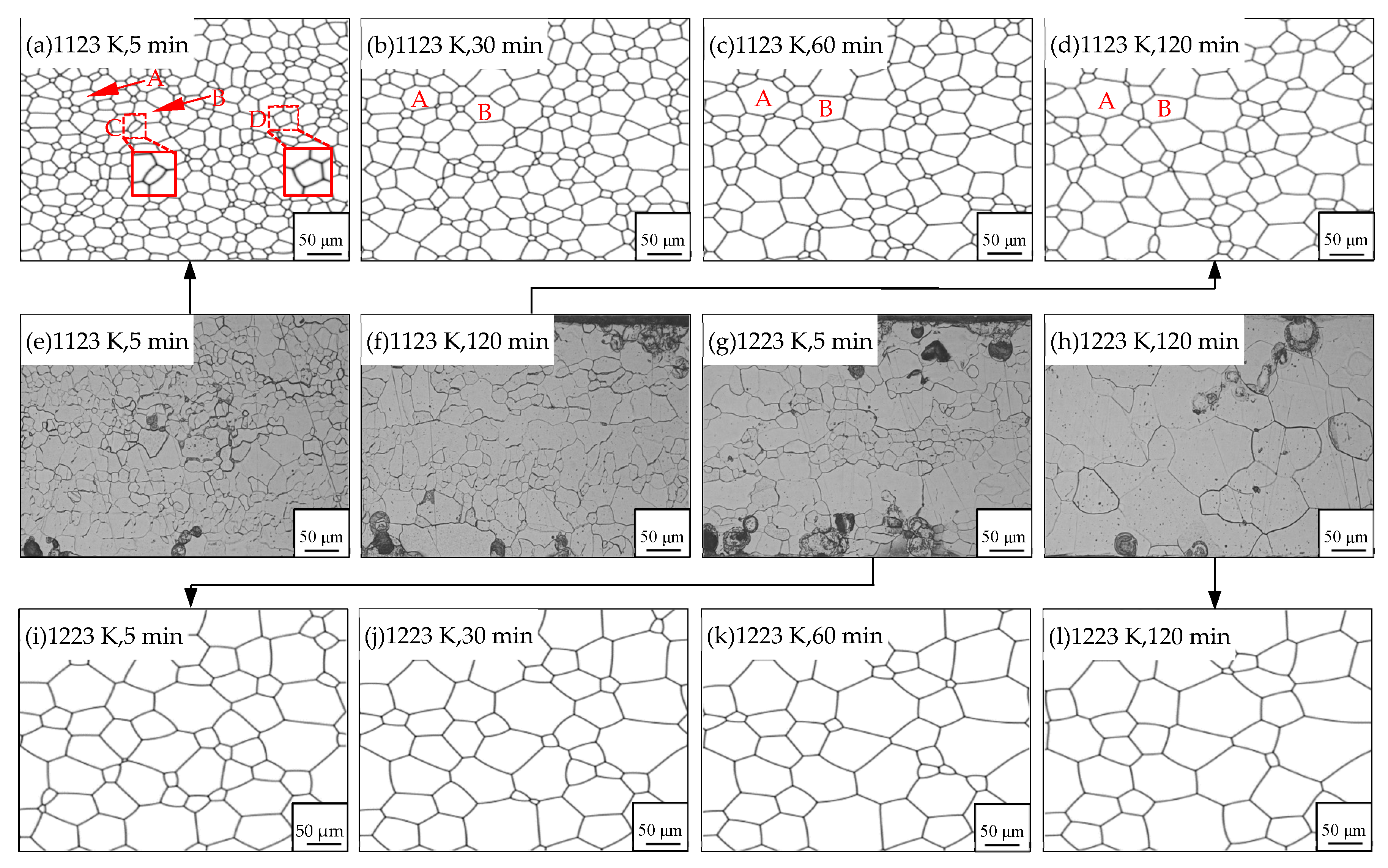
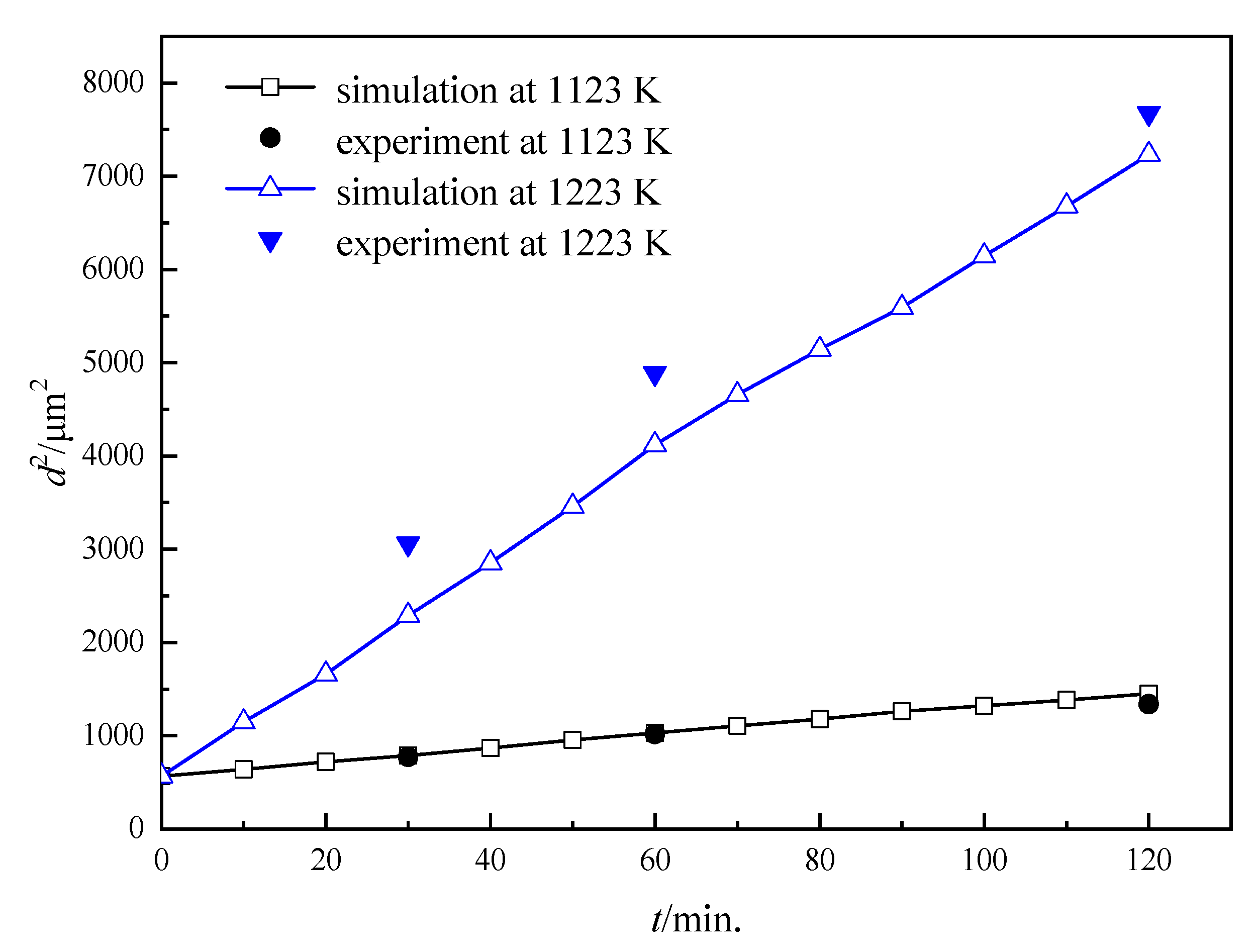
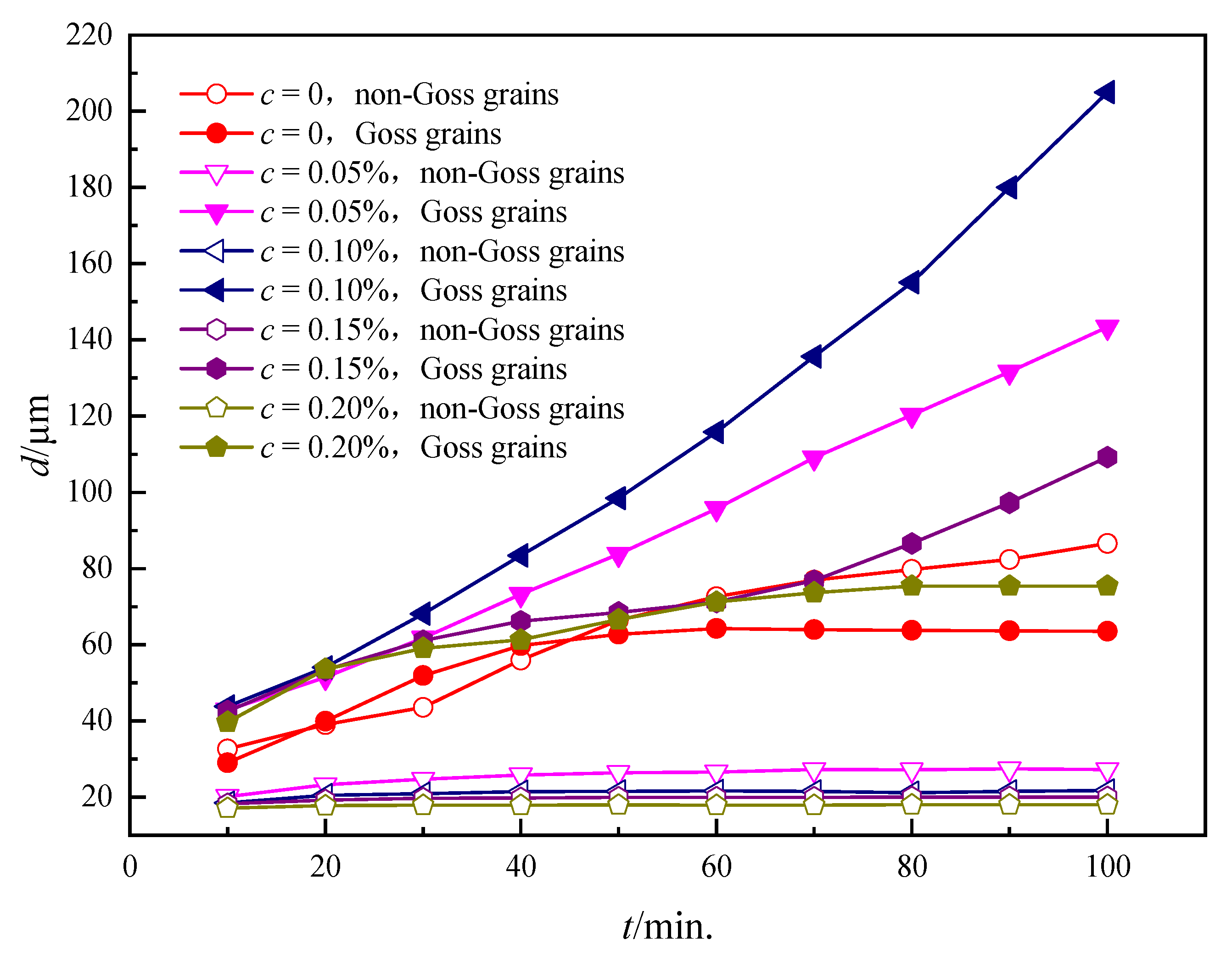
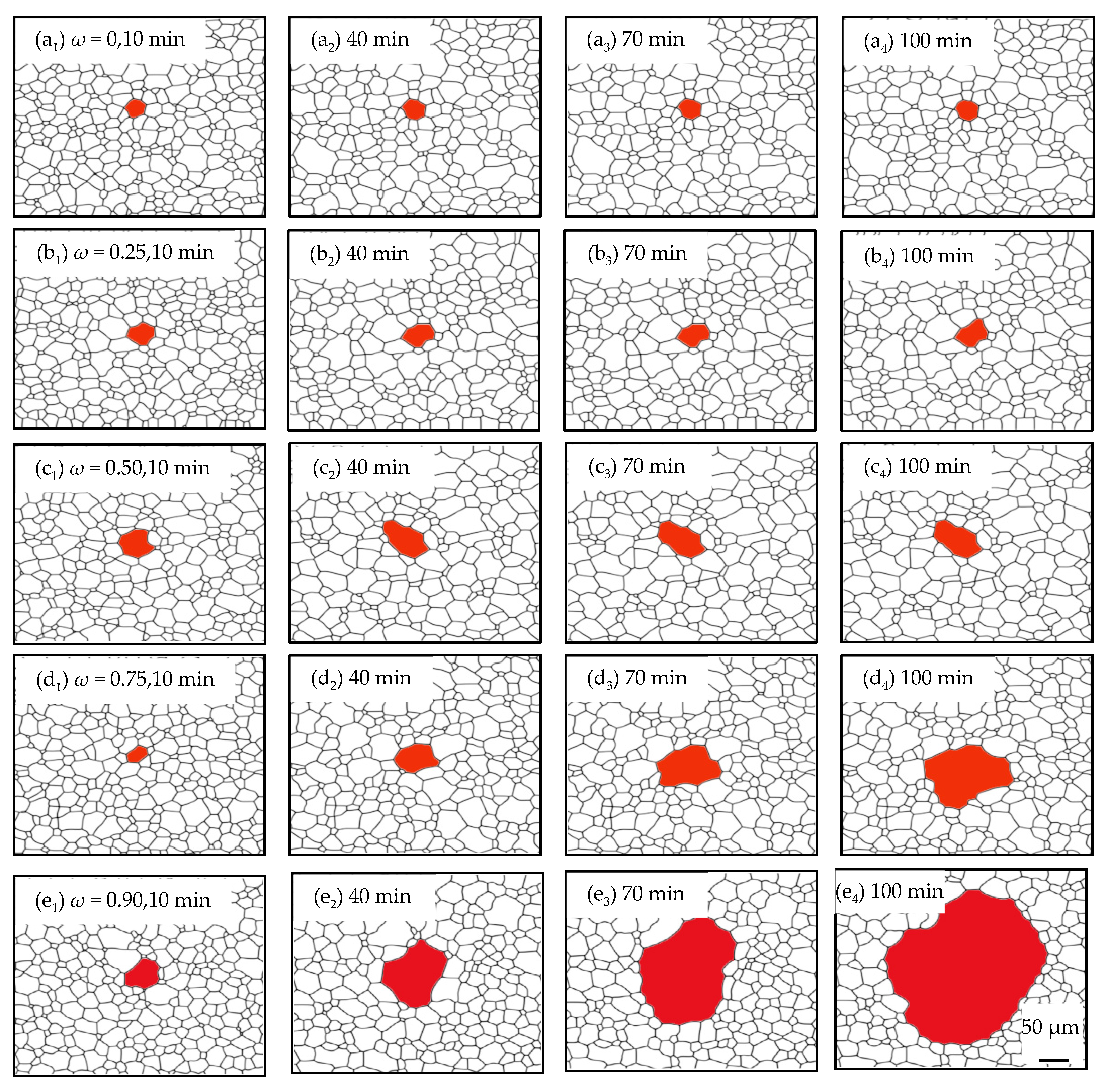
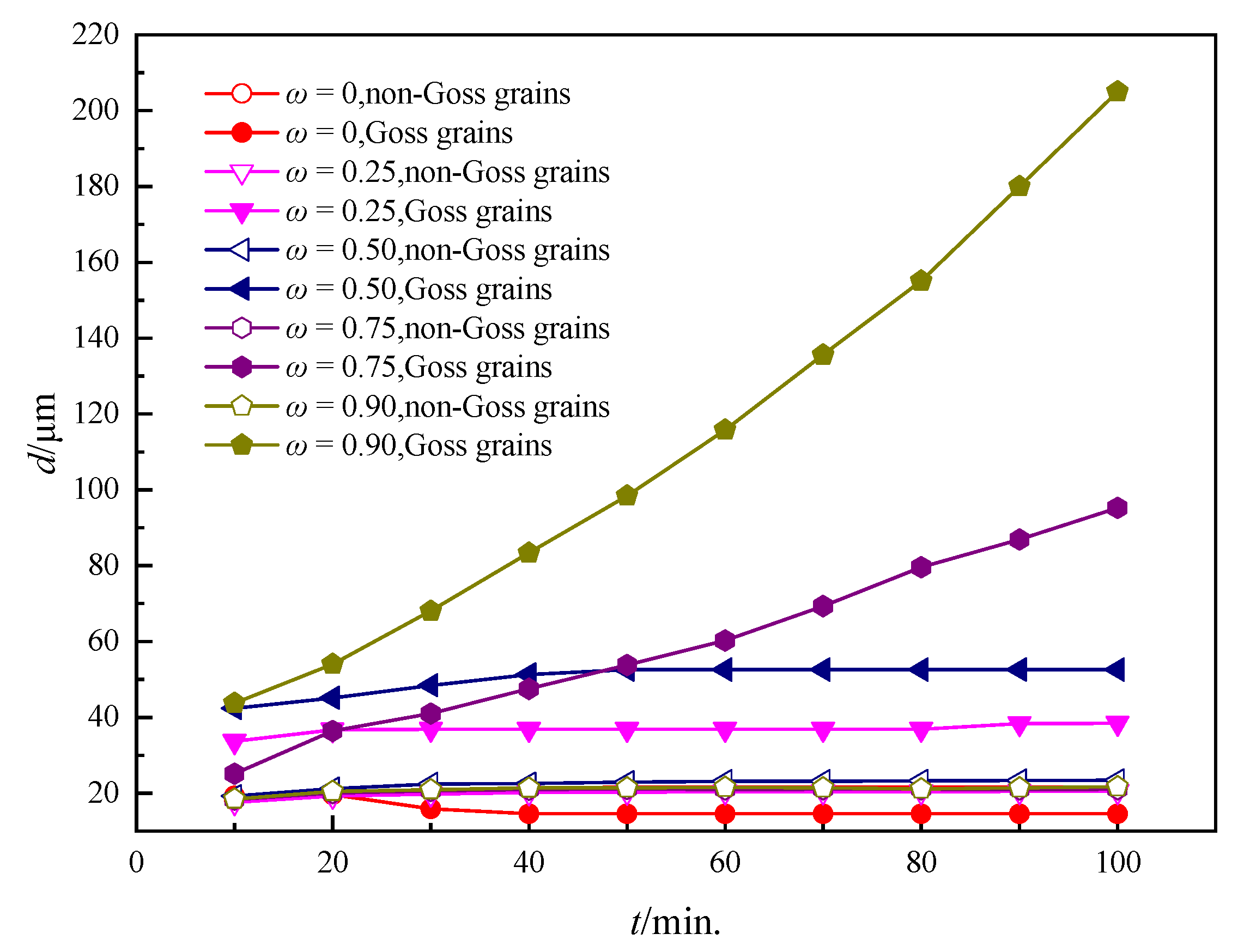
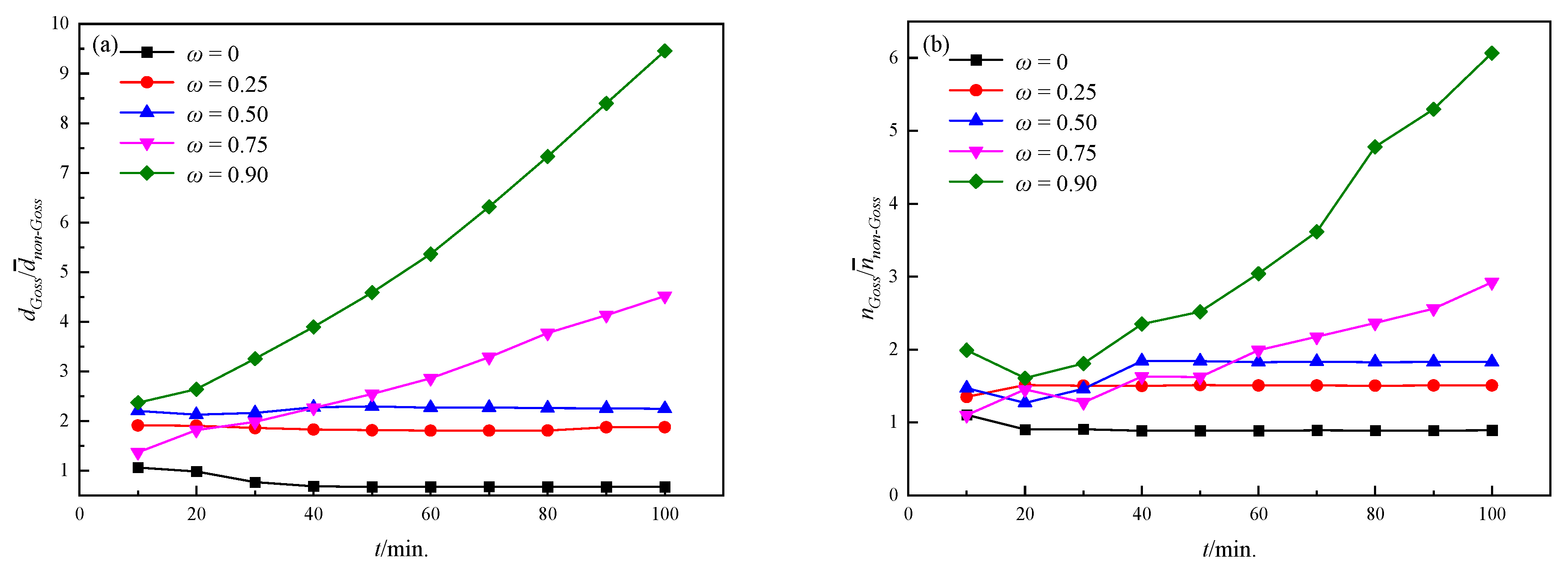
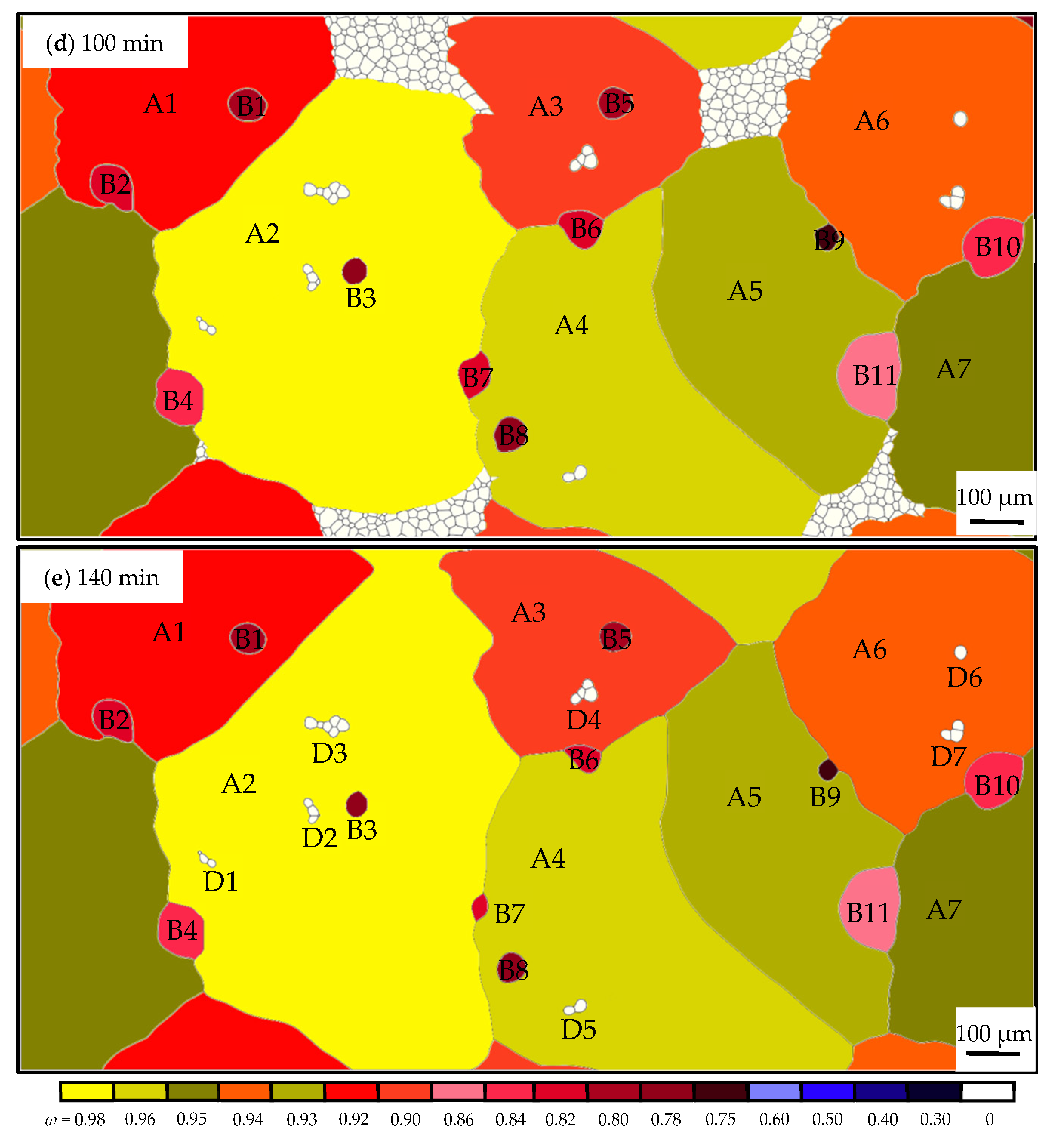
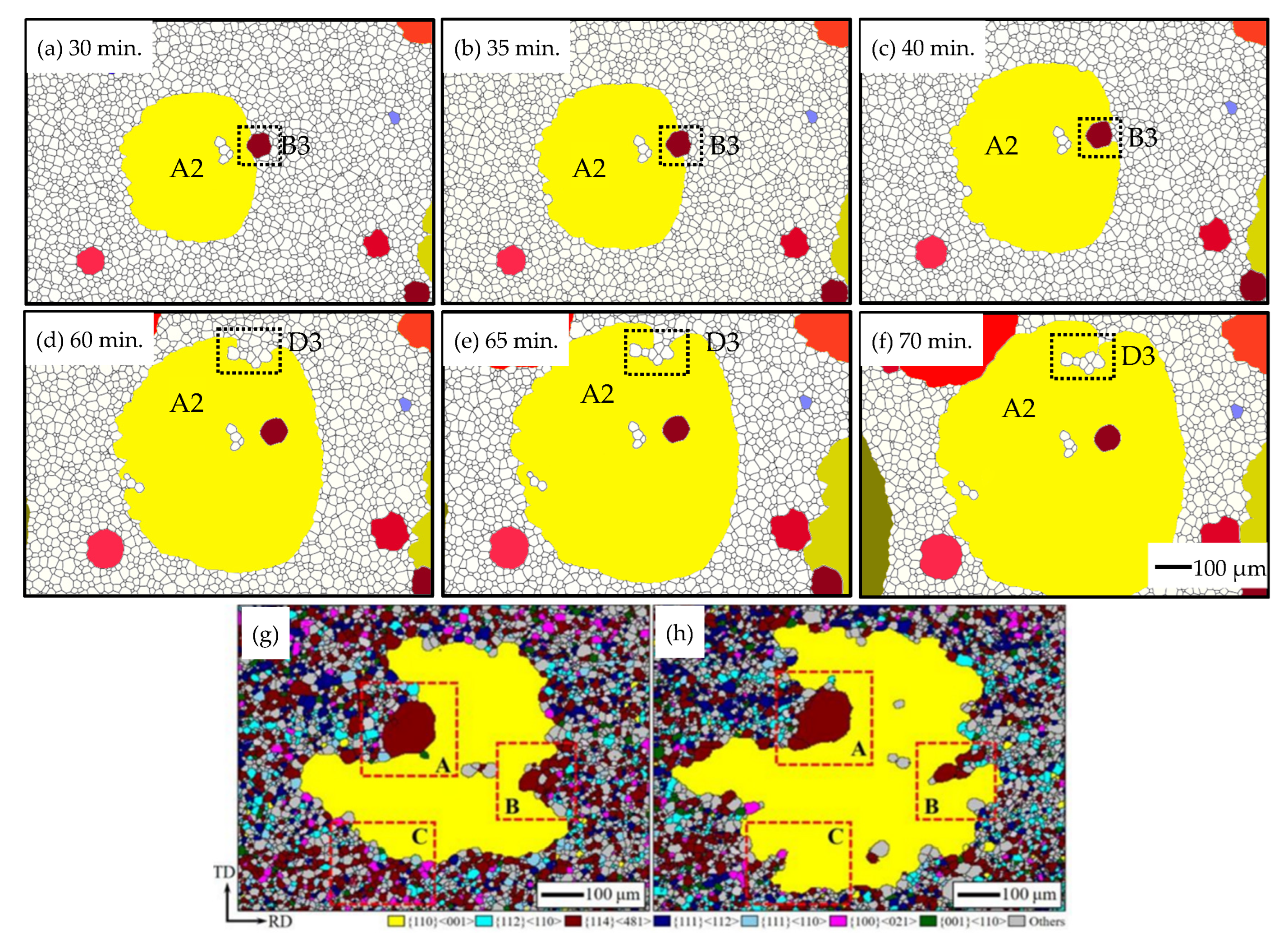
3.2. Influence of the Particles on the Grain Growth Process
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gao, Q.; Wang, X.H.; Li, J.; Gong, J.; Li, B. Effect of aluminum on secondary recrystallization texture and magnetic properties of grain-oriented silicon steel. J. Iron Steel Res. Int. 2021, 28, 479–487. [Google Scholar] [CrossRef]
- May, J.E.; Turnbull, D. Secondary recrystallization in silicon iron. Trans. Metall. Soc. AIME 1958, 212, 769–781. [Google Scholar]
- Inokuti, Y.; Maeda, C.; Ito, Y. Computer color mapping of configuration of Goss grains after transverse cold rolling in grain oriented silicon steel. Trans. Iron Steel Inst. Jpn. 2006, 27, 302–311. [Google Scholar] [CrossRef]
- Hwang, N.M.; Lee, B.J.; Han, C.H. Texture evolution by grain growth under a system of anisotropic grain boundary energy. Scripta Mater. 1997, 37, 1761–1767. [Google Scholar] [CrossRef]
- Chen, N.; Zaefferer, S.; Lahn, L.; Günther, K.; Raabe, D. Effects of topology on abnormal grain growth in silicon steel. Acta Mater. 2003, 51, 1755–1765. [Google Scholar] [CrossRef]
- Lücke, K.; Brandt, R.; Abbruzzese, G. Normal and abnormal grain growth as transient phenomena. Interface Sci. 1998, 6, 67–76. [Google Scholar] [CrossRef]
- Thompson, C.V.; Frost, H.J.; Spaepen, F. The relative rates of secondary and normal grain growth. Acta Metall. 1987, 35, 887–890. [Google Scholar] [CrossRef]
- Harase, J.; Shimizu, R.; Takahashi, N. Coincidence grain boundary and (100) [001] secondary recrystallization in Fe-3% Si. Acta Metall. Mater. 1990, 38, 1849–1856. [Google Scholar] [CrossRef]
- Shimizu, R.; Harase, J. Coincidence grain boundary and texture evolution in Fe-3%Si. Acta Metall. 1989, 37, 1241–1249. [Google Scholar] [CrossRef]
- Hayakawa, Y.; Szpunar, J.A.; Palumbo, G.; Lin, P. The role of grain boundary character distribution in Goss texture development in electrical steels. J. Magn. Magn. Mater. 1996, 160, 143–144. [Google Scholar] [CrossRef]
- Cui, F.E.; Yang, P.; Mao, W.M. Behaviors of different inhibitors during secondary recrystallization of a grain-orientated silicon steel. Int. J. Min. Met. Mater. 2011, 18, 314–319. [Google Scholar] [CrossRef]
- Fang, F.; Zhang, Y.X.; Lu, X.; Wang, Y.; Cao, G.M.; Yuan, G.; Xu, Y.B.; Wang, G.D.; Misra, R.D.K. Inhibitor induced secondary recrystallization in thin-gauge grain oriented silicon steel with high permeability. Mater. Des. 2016, 105, 398–403. [Google Scholar] [CrossRef]
- Yang, F.Y.; He, C.X.; Meng, L.; Ma, G.; Chen, X.; Mao, W.M. Effect of annealing atmosphere on secondary recrystallization in thin-gauge grain-oriented silicon steel: Evolution of inhibitors. J. Magn. Magn. Mater. 2017, 439, 403–410. [Google Scholar] [CrossRef]
- Kim, D.U.; Cha, P.R.; Kim, S.G.; Kim, W.T.; Cho, J.H.; Han, H.N.; Lee, H.J.; Kim, J.Y. Effect of micro-elasticity on grain growth and texture evolution: A phase field grain growth simulation. Comput. Mater. Sci. 2012, 56, 58–68. [Google Scholar] [CrossRef]
- Tonks, M.; Millett, P. Phase field simulations of elastic deformation-driven grain growth in 2D copper polycrystals. Mater. Sci. Eng. A 2011, 528, 4086–4091. [Google Scholar] [CrossRef]
- Yadav, A.K.; Nelson, C.T.; Hsu, S.L.; Hong, Z.; Clarkson, J.D.; Schlepütz, C.; Damodaran, A.R.; Shafer, P.; Arenholz, E.; Dedon, L.R. Corrigendum: Observation of polar vortices in oxide superlattices. Nature 2016, 534, 138. [Google Scholar] [CrossRef] [PubMed]
- Tsakmakis, A.; Vormwald, M. Thermodynamics and analysis of predicted responses of a phase field model for ductile fracture. Materials 2021, 14, 5842. [Google Scholar] [CrossRef]
- Dai, R.; Yang, S.; Zhang, T.; Zhong, J.; Chen, L.; Deng, C.; Zhang, L. High-throughput screening of optimal process parameters for PVD TiN coatings with best properties through a combination of 3-d quantitative phase-field simulation and hierarchical multi-objective optimization strategy. Front. Mater. 2022, 9, 924294. [Google Scholar] [CrossRef]
- Han, G.; Han, Z.; Luo, A.A.; Liu, B. Three-dimensional phase-field simulation and experimental validation of β-Mg17Al12 phase precipitation in Mg-Al-based alloys. Metall. Mater. Trans. A 2014, 46, 948–962. [Google Scholar] [CrossRef]
- Tomic, Z.; Jukic, K.; Jarak, T.; Fabijanic, T.A.; Tonkovic, Z. Phase-field modeling of fused silica cone-crack vickers Indentation. Nanomaterials 2022, 12, 2356. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, H.; Xu, H.; Xu, D.; Yang, R. Phase-field study on the formation of α variant clusters induced by elastoplastic stress field around the void in titanium alloys. Comput. Mater. Sci. 2022, 215, 111781. [Google Scholar] [CrossRef]
- Kim, H.K.; Kim, S.G.; Dong, W.P.; Steinbach, I.; Lee, B.J. Phase-field modeling for 3D grain growth based on a grain boundary energy database. Model. Simul. Mat. Sci. Eng. 2014, 22, 034004. [Google Scholar] [CrossRef]
- Krill, C.E.; Chen, L.Q. Computer simulation of 3-D grain growth using a phase-field model. Acta Mater. 2002, 50, 3057–3073. [Google Scholar] [CrossRef]
- Song, Y.H.; Wang, M.T.; Ni, J.; Jin, J.F.; Zong, Y.P. Effect of grain boundary energy anisotropy on grain growth in ZK60 alloy using a 3D phase-field modeling. Chin. Phys. B 2020, 29, 604–615. [Google Scholar] [CrossRef]
- Liu, Y.; Militzer, M.; Perez, M. Phase field modelling of abnormal grain growth. Materials 2019, 12, 4048. [Google Scholar] [CrossRef] [PubMed]
- Moelans, N.; Blanpain, B.; Wollants, P. A phase field model for the simulation of grain growth in materials containing finely dispersed incoherent second-phase particles. Acta Mater. 2005, 53, 1771–1781. [Google Scholar] [CrossRef]
- Chang, K.; Kwon, J.; Rhee, C.K. Role of second-phase particle morphology on 3D grain growth: A phase-field approach. Comput. Mater. Sci. 2016, 124, 438–443. [Google Scholar] [CrossRef]
- Chang, K.; Moelans, N. Phase-field simulations of the interaction between a grain boundary and an evolving second-phase particle. Philos. Mag. Lett. 2015, 95, 202–210. [Google Scholar] [CrossRef]
- Chang, K.; Chen, L.Q. Quantitative evaluation of particle pinning force on a grain boundary using the phase-field method. Model. Simul. Mat. Sci. Eng. 2012, 20, 055004. [Google Scholar] [CrossRef]
- Chang, K.; Feng, W.; Chen, L.Q. Effect of second-phase particle morphology on grain growth kinetics. Acta Mater. 2009, 57, 5229–5236. [Google Scholar] [CrossRef]
- He, R.; Wang, M.T.; Zhang, X.G.; Zong, Y.P. Influence of second-phase particles on grain growth in AZ31 magnesium alloy during equal channel angular pressing by phase field simulation. Model. Simul. Mat. Sci. Eng. 2016, 24, 055017. [Google Scholar] [CrossRef]
- Mallick, A. Effect of second phase mobile particles on polycrystalline grain growth: A phase-field approach. Comput. Mater. Sci. 2013, 67, 27–34. [Google Scholar] [CrossRef]
- Vedantam, S.; Mallick, A. Phase-field theory of grain growth in the presence of mobile second-phase particles. Acta Mater. 2010, 58, 272–281. [Google Scholar] [CrossRef]
- Song, Y.H.; Wang, M.T.; Zong, Y.P.; He, R.; Jin, J.F. Grain refinement by second phase particles under applied stress in ZK60 Mg alloy with Y through phase field simulation. Materials 2018, 11, 1903. [Google Scholar] [CrossRef] [PubMed]
- Han, C.H.; Kwon, S.J. Effect of nitrogen on the size and distribution of AlN precipitates in 3% silicon steel. Scripta Mater. 1996, 34, 543–549. [Google Scholar] [CrossRef]
- Ueshima, N.; Maeda, T.; Oikawa, K. Effect of Cu addition on precipitation and growth behavior of MnS in silicon steel sheets. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2017, 48, 3843–3851. [Google Scholar] [CrossRef]
- Ginzburg, V.L.; Landau, L.D. On the Theory of Superconductivity. In Collected Papers of L.D. Landau; Pergamon Press: Oxford, UK, 1965. [Google Scholar]
- Fang, F.; Yang, J.; Zhang, Y.X.; Wang, Y.; Zhang, X.M.; Yuan, G.; Misra, R.D.K.; Wang, G.D. Microstructure and magnetic properties of ultra-thin grain-oriented silicon steel: Conventional process versus strip casting. J. Magn. Magn. Mater. 2021, 535, 168087. [Google Scholar] [CrossRef]
- Liu, H.T.; Liu, Z.Y.; Li, C.G.; Cao, G.M.; Wang, G.D. Solidification structure and crystallographic texture of strip casting 3 wt.% Si non-oriented silicon steel. Mater. Charact. 2011, 62, 463–468. [Google Scholar] [CrossRef]
- Vedantam, S.; Patnaik, B.S.V. Efficient numerical algorithm for multiphase field simulations. Phys. Rev. E 2006, 73, 016703. [Google Scholar] [CrossRef] [PubMed]
- Moelans, N.; Blanpain, B.; Wollants, P. Quantitative analysis of grain boundary properties in a generalized phase field model for grain growth in anisotropic systems. Phys. Rev. B 2008, 78, 024113. [Google Scholar] [CrossRef]
- Mullins, W.W. Two-dimensional motion of idealized grain boundaries. J. Appl. Phys. 1956, 27, 900–904. [Google Scholar] [CrossRef]
- Feng, Y.L.; Guo, J.; Li, J.; Ning, J.L. Effect of Nb on solution and precipitation of inhibitors in grain-oriented silicon steel. J. Magn. Magn. Mater. 2017, 426, 89–94. [Google Scholar] [CrossRef]
- Oh, J.H.; Cho, S.H.; Jonas, J.J. AlN precipitation in dual-phase 3% Si electrical steels. ISIJ Int. 2007, 41, 484–491. [Google Scholar] [CrossRef][Green Version]
- Wang, Y.; Zhu, C.Y.; Li, G.Q.; Liu, Y.L.; Liu, Y. Influence of Nb content on precipitation, grain microstructure, texture and magnetic properties of grain-oriented silicon steel. Materials 2020, 13, 5581. [Google Scholar] [CrossRef]
- Smith, C.S. Grains, phases, and interphases: An interpretation of microstructure. Trans. Metall. Soc. AIME 1948, 175, 15–51. [Google Scholar] [CrossRef][Green Version]
- He, Z.Z. Electrical Steel, 1st ed.; Metallurgical Industry Press: Beijing, China, 2012; p. 118. [Google Scholar]
- Birosca, S.; Nadoum, A.; Hawezy, D.; Robinson, F.; Kockelmann, W. Mechanistic approach of Goss abnormal grain growth in electrical steel: Theory and argument. Acta Mater. 2019, 185, 370–381. [Google Scholar] [CrossRef]
- Xu, Z.Y.; Sha, Y.H.; He, Z.H.; Zhang, F.; Liu, W.; Zhang, H.B.; Zuo, L. Complete Goss secondary recrystallization by control of the grain size and texture of primary recrystallization in grain-oriented silicon steel. Materials 2021, 14, 5383. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Xu, Y.; Hu, J.; Fang, F.; Jin, J.; Jia, T.; Peng, Q. Phase-Field Simulation on the Effect of Second-Phase Particles on Abnormal Growth of Goss Grains in Fe-3%Si Steels. Nanomaterials 2022, 12, 4148. https://doi.org/10.3390/nano12234148
Wang M, Xu Y, Hu J, Fang F, Jin J, Jia T, Peng Q. Phase-Field Simulation on the Effect of Second-Phase Particles on Abnormal Growth of Goss Grains in Fe-3%Si Steels. Nanomaterials. 2022; 12(23):4148. https://doi.org/10.3390/nano12234148
Chicago/Turabian StyleWang, Mingtao, Yongkai Xu, Jinlong Hu, Feng Fang, Jianfeng Jin, Tao Jia, and Qing Peng. 2022. "Phase-Field Simulation on the Effect of Second-Phase Particles on Abnormal Growth of Goss Grains in Fe-3%Si Steels" Nanomaterials 12, no. 23: 4148. https://doi.org/10.3390/nano12234148
APA StyleWang, M., Xu, Y., Hu, J., Fang, F., Jin, J., Jia, T., & Peng, Q. (2022). Phase-Field Simulation on the Effect of Second-Phase Particles on Abnormal Growth of Goss Grains in Fe-3%Si Steels. Nanomaterials, 12(23), 4148. https://doi.org/10.3390/nano12234148






