Phototransformation of Graphene Oxide on the Removal of Sulfamethazine in a Water Environment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Photochemistry Experiment
2.3. SMZ Degradation
2.4. ROS Generation
2.5. GO Characterization
3. Results and Discussion
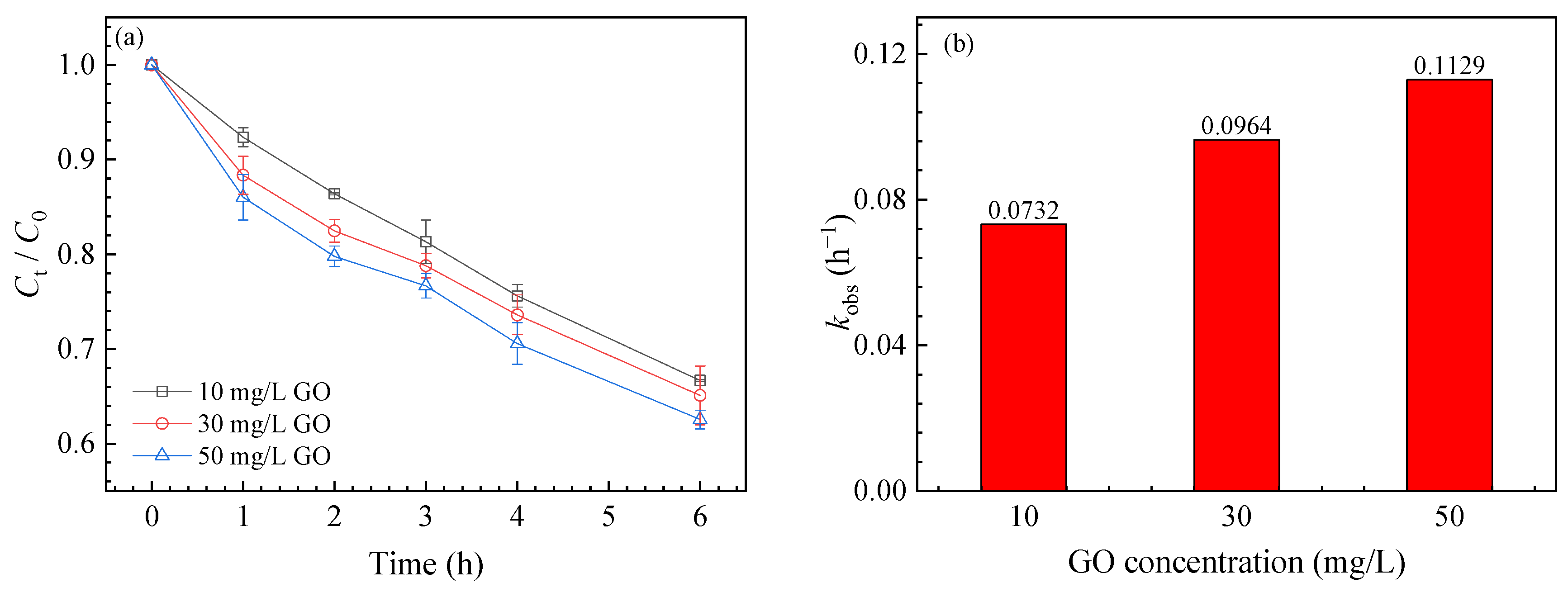
3.1. SMZ Degradation
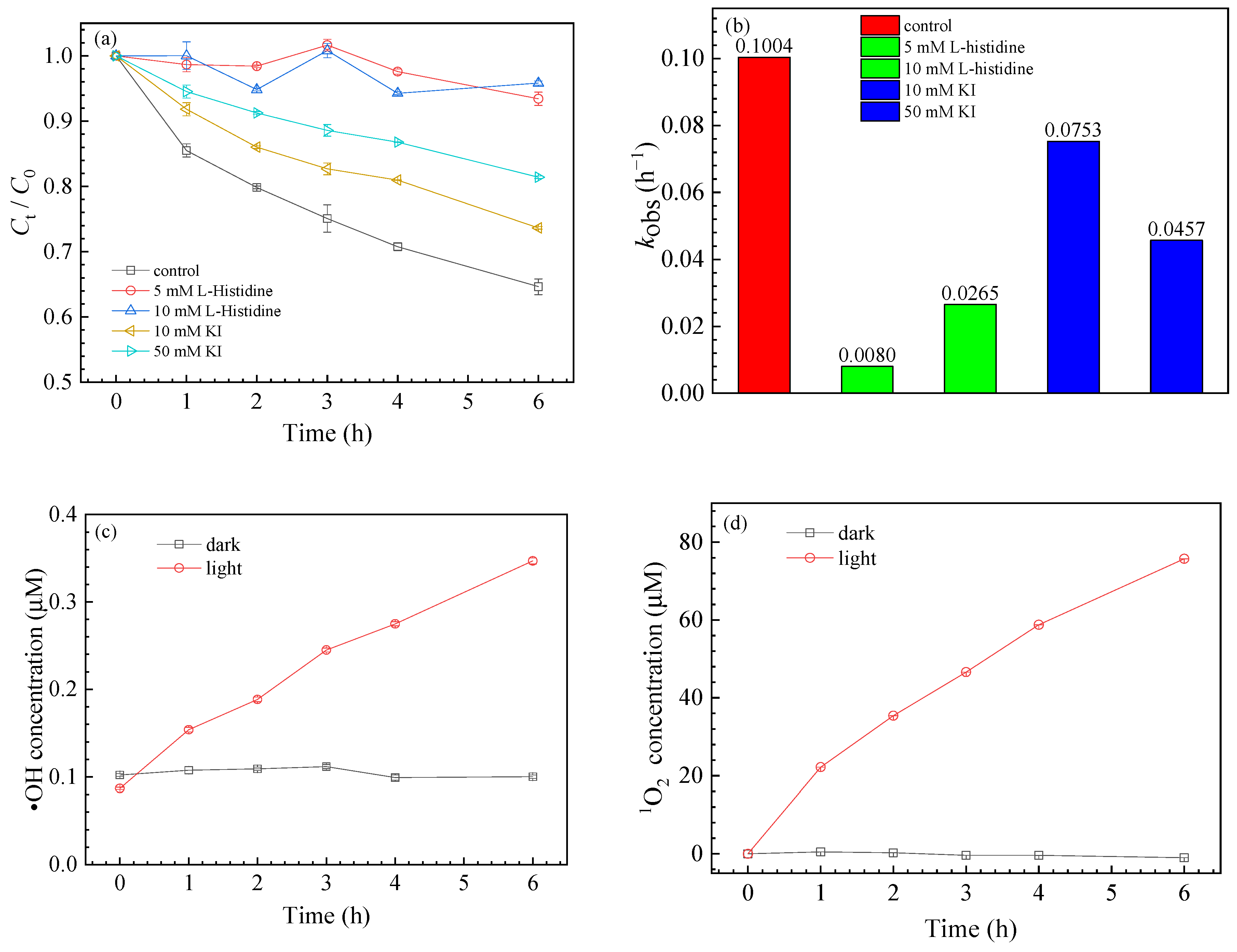
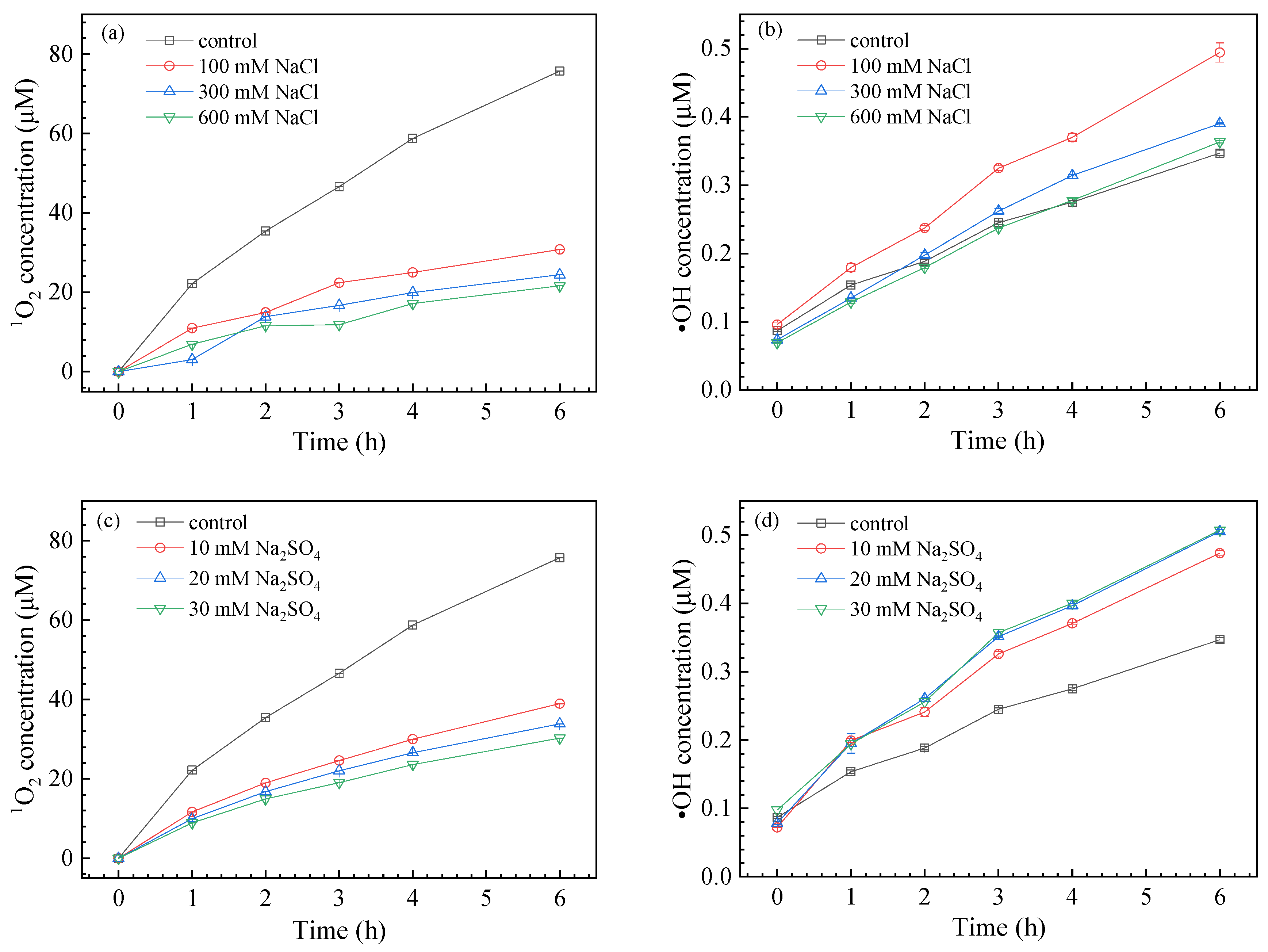
3.2. ROS Generation
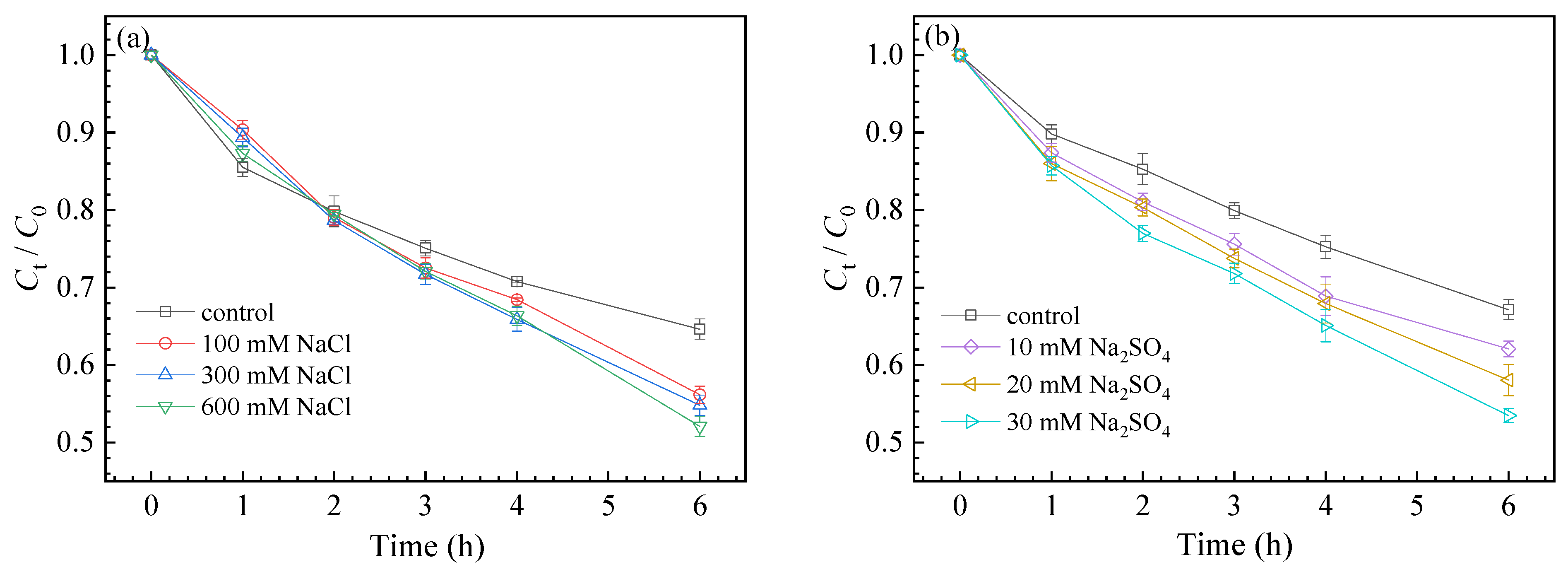
3.3. Effects of Different Conditions on SMZ Degradation
3.3.1. Effect of pH
3.3.2. Effect of Coexisting Anions
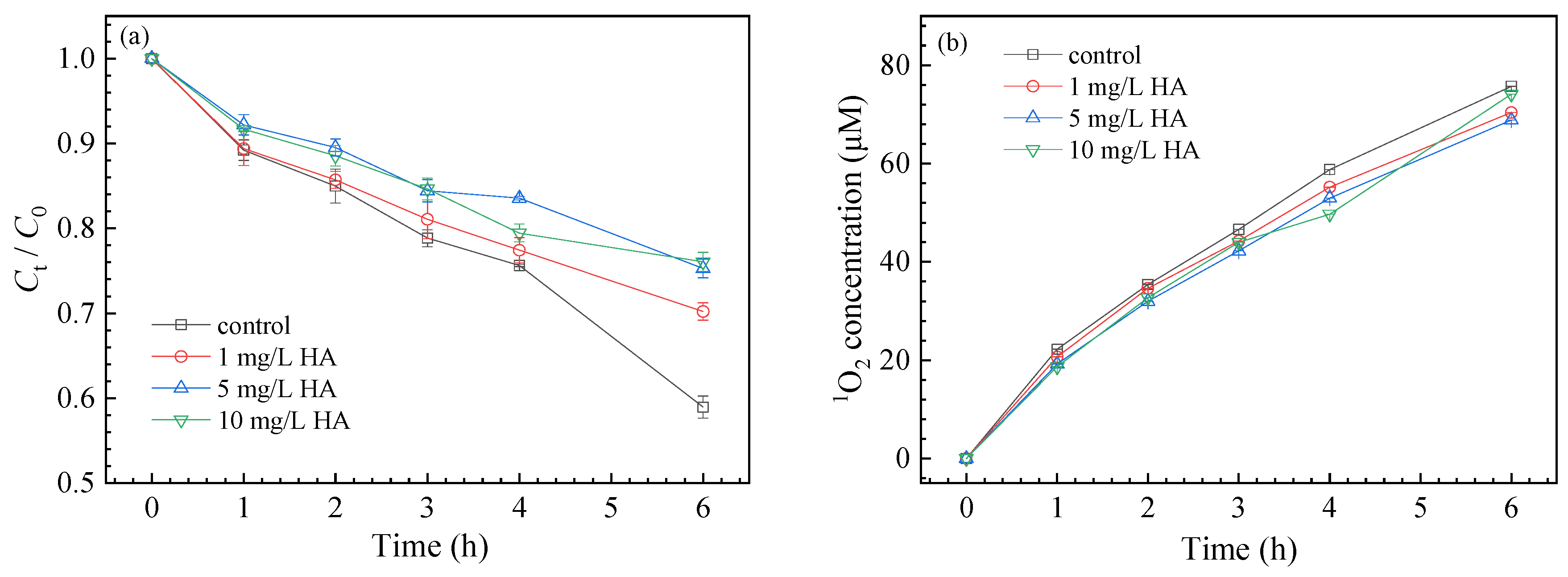
3.3.3. Effect of NOM
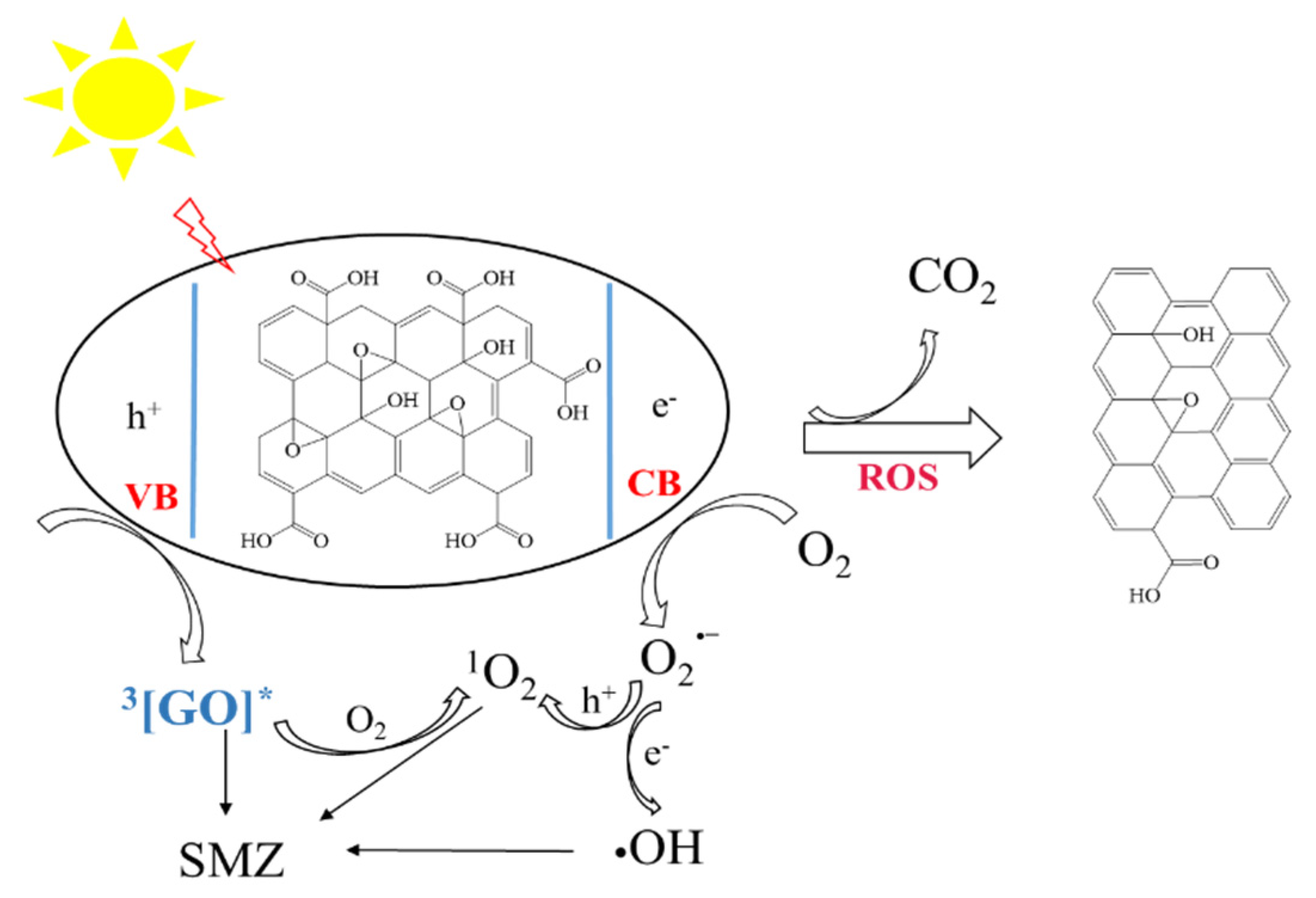
3.4. GO Transformation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef]
- Goodwin, D.G.; Adeleye, A.S.; Sung, L.; Ho, K.T.; Burgess, R.M.; Petersen, E.J. Detection and quantification of graphene-family nanomaterials in the environment. Environ. Sci. Technol. 2018, 52, 4491–4513. [Google Scholar] [CrossRef]
- Guo, Z.L.; Xie, C.J.; Zhang, P.; Zhang, J.Z.; Wang, G.H.; He, X.; Ma, Y.H.; Zhao, B.; Zhang, Z.Y. Toxicity and transformation of graphene oxide and reduced graphene oxide in bacteria biofilm. Sci. Total. Environ. 2017, 580, 1300–1308. [Google Scholar] [CrossRef]
- Zhao, J.; Cao, X.S.; Wang, Z.Y.; Dai, Y.H.; Xing, B.S. Mechanistic understanding toward the toxicity of graphene-family materials to freshwater algae. Water Res. 2017, 111, 18–27. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.L.; Zhou, Q.X.; Zou, W.; Hu, X.G. Molecular mechanisms of developmental toxicity induced by graphene oxide at predicted environmental concentrations. Environ. Sci. Technol. 2017, 51, 7861–7871. [Google Scholar] [CrossRef] [PubMed]
- Fadeel, B.; Bussy, C.; Merino, S.; Vázquez, E.; Flahaut, E.; Mouchet, F.; Evariste, L.; Gauthier, L.; Koivisto, A.J.; Vogel, U.; et al. Safety assessment of graphene-based materials: Focus on human health and the environment. ACS Nano 2018, 12, 10582–10620. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Müller, M.B.; Gilje, S.; Kaner, R.B.; Wallace, G.G. Processable aqueous dispersions of graphene nanosheets. Nat. Nanotechnol. 2008, 3, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Pavagadhi, S.; Tang, A.L.L.; Sathishkumar, M.; Loh, K.P.; Balasubramanian, R. Removal of microcystin-LR and microcystin-RR by graphene oxide: Adsorption and kinetic experiments. Water Res. 2013, 47, 4621–4629. [Google Scholar] [CrossRef]
- Wei, H.; Yang, W.S.; Xi, Q.; Chen, X. Preparation of Fe3O4@graphene oxide core-shell magnetic particles for use in protein adsorption. Mater. Lett. 2012, 82, 224–226. [Google Scholar] [CrossRef]
- Yang, Z.; Yan, H.; Yang, H.; Li, H.B.; Li, A.M.; Cheng, R.S. Flocculation performance and mechanism of graphene oxide for removal of various contaminants from water. Water Res. 2013, 47, 3037–3046. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, X.; Yin, X.; Sun, H.; Wang, N. Graphene oxide-facilitated transport of Pb2+ and Cd2+ in saturated porous media. Sci. Total Environ. 2018, 631–632, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Dong, S.; Sun, Y.; Gao, B.; Du, W.; Xu, H.; Wu, J. Graphene oxide-facilitated transport of levofloxacin and ciprofloxacin in saturated and unsaturated porous media. J. Hazard. Mater. 2018, 348, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Song, Y.; Zhang, H.; Lu, T.; Chen, W.; Li, W.; Qi, W.; Qi, Z. Insights into the mutual promotion effect of graphene oxide nanoparticles and tetracycline on their transport in saturated porous media. Environ. Pollut. 2021, 268, 115730. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Lin, Y.; Guo, X.; Li, S.; Cui, J.; Ping, H.; Zhang, J.; Zhong, R.; Du, L.; Han, C.; et al. Co-transport of graphene oxide and titanium dioxide nanoparticles in saturated quartz sand: Influences of solution pH and metal ions. Environ. Pollut. 2019, 251, 723–730. [Google Scholar] [CrossRef]
- Gao, M.; Yang, Y.; Song, Z. Effects of graphene oxide on cadmium uptake and photosynthesis performance in wheat seedlings. Ecotoxicol. Environ. Saf. 2019, 173, 165–173. [Google Scholar] [CrossRef]
- Li, M.; Zhu, J.; Wu, Q.; Wang, Q. The combined adverse effects of cis-bifenthrin and graphene oxide on lipid homeostasis in Xenopus laevis. J. Hazard. Mater. 2021, 407, 124876. [Google Scholar] [CrossRef]
- Cao, X.; Ma, C.; Chen, F.; Luo, X.; Musante, C.; White, J.C.; Zhao, X.; Wang, Z.; Xing, B. New insight into the mechanism of graphene oxide-enhanced phytotoxicity of arsenic species. J. Hazard. Mater. 2021, 410, 124959. [Google Scholar] [CrossRef]
- Bai, H.; Jiang, W.; Kotchey, G.P.; Saidi, W.A.; Bythell, B.J.; Jarvis, J.M.; Marshall, A.G.; Robinson, R.A.; Star, A. Insight into the mechanism of graphene oxide degradation via the photo-Fenton reaction. J. Phys. Chem. C 2014, 118, 10519–10529. [Google Scholar] [CrossRef]
- Gao, Y.; Ren, X.M.; Zhang, X.D.; Chen, C.L. Environmental fate and risk of ultraviolet- and visible-light-transformed graphene oxide: A comparative study. Environ. Pollut. 2019, 251, 821–829. [Google Scholar] [CrossRef]
- Zhang, X.F.; Shao, X.; Liu, S. Dual fluorescence of graphene oxide: A time-resolved study. J. Phys. Chem. A 2012, 116, 7308–7313. [Google Scholar] [CrossRef]
- Hou, W.C.; Chowdhury, I.; Goodwin, D.G.; Henderson, W.M.; Fairbrother, D.H.; Bouchard, D.; Zepp, R.G. Photochemical transformation of graphene oxide in sunlight. Environ. Sci. Technol. 2015, 49, 3435–3443. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Ning, F.; Cao, X.; Yao, H.; Wang, Z.; Xing, B. Photo-transformation of graphene oxide in the presence of co-existing metal ions regulated its toxicity to freshwater algae. Water Res. 2020, 176, 115735. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.F.; Wang, S.C.; Zhu, Z.L.; Wang, S.G.; Liu, F.F.; Liu, G.Z. Effects of oxidation degree on photo-transformation and the resulting toxicity of graphene oxide in aqueous environment. Environ. Pollut. 2019, 249, 1106–1114. [Google Scholar] [CrossRef] [PubMed]
- Katsnelson, M.I. Graphene: Carbon in two dimensions. Mater. Today 2007, 10, 20–27. [Google Scholar] [CrossRef]
- Meyer, J.C.; Geim, A.K.; Katsnelson, M.I.; Novoselov, K.S.; Booth, T.J.; Roth, S. The structure of suspended graphene sheets. Nature 2007, 446, 60–63. [Google Scholar] [CrossRef]
- Cao, X.; Ma, C.; Zhao, J.; Guo, H.; Dai, Y.; Wang, Z.; Xing, B. Graphene oxide mediated reduction of silver ions to silver nanoparticles under environmentally relevant conditions: Kinetics and mechanisms. Sci. Total Environ. 2019, 679, 270–278. [Google Scholar] [CrossRef]
- Cao, X.; Ma, C.; Zhao, J.; Musante, C.; White, J.C.; Wang, Z.; Xing, B. Interaction of graphene oxide with co-existing arsenite and arsenate: Adsorption, transformation and combined toxicity. Environ. Int. 2019, 131, 104992. [Google Scholar] [CrossRef]
- Li, W.H.; Shi, Y.L.; Gao, L.H.; Liu, J.M.; Cai, Y.Q. Occurrence of antibiotics in water, sediments, aquatic plants, and animals from Baiyangdian Lake in North China. Chemosphere 2012, 89, 1307–1315. [Google Scholar] [CrossRef]
- Zhang, Q.Q.; Ying, G.G.; Pan, C.G.; Liu, Y.S.; Zhao, J.L. Comprehensive evaluation of antibiotics emission and fate in the river basins of China: Source analysis, multimedia modeling, and linkage to bacterial resistance. Environ. Sci. Technol. 2015, 49, 6772–6782. [Google Scholar] [CrossRef]
- Ma, Y.; Li, M.; Wu, M.; Li, Z.; Liu, X. Occurrences and regional distributions of 20 antibiotics in water bodies during groundwater recharge. Sci. Total Environ. 2015, 518–519, 498–506. [Google Scholar] [CrossRef]
- Zhang, Q.; Jia, A.; Wan, Y.; Liu, H.; Wang, K.; Peng, H.; Dong, Z.; Hu, J. Occurrences of three classes of antibiotics in a natural river basin: Association with antibiotic-resistant Escherichia coli. Environ. Sci. Technol. 2014, 48, 14317–14325. [Google Scholar] [CrossRef]
- Gao, Y.; Li, Y.; Zhang, L.; Huang, H.; Hu, J.J.; Shah, S.M.; Su, X.G. Adsorption and removal of tetracycline antibiotics from aqueous solution by graphene oxide. J. Colloid. Interface Sci. 2012, 368, 540–546. [Google Scholar] [CrossRef]
- Latch, D.E.; Stender, B.L.; Packer, J.L.; Arnold, W.A.; McNeill, K. Photochemical fate of pharmaceuticals in the environment: Cimetidine and ranitidine. Environ. Sci. Technol. 2003, 37, 3342–3350. [Google Scholar] [CrossRef]
- Li, Y.J.; Liu, X.L.; Zhang, B.J.; Zhao, Q.; Ning, P.; Tian, S.L. Aquatic photochemistry of sulfamethazine: Multivariate effects of main water constituents and mechanisms. Environ. Sci. Process. Impacts 2018, 20, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.F.; Zhao, J.; Wang, S.G.; Xing, B.S. Adsorption of sulfonamides on reduced graphene oxides as affected by pH and dissolved organic matter. Environ. Pollut. 2016, 210, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.F.; Zhao, J.; Wang, S.G.; Du, P.; Xing, B.S. Effects of solution chemistry on adsorption of selected pharmaceuticals and personal care products (PPCPs) by graphenes and carbon nanotubes. Environ. Sci. Technol. 2014, 48, 13197–13206. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xu, X.; Li, Y.W.; Su, R.D.; Li, Q.; Zhou, W.Z.; Gao, B.Y.; Yue, Q.Y. One-step synthesis of "nuclear-shell" structure iron-carbon nanocomposite as a persulfate activator for bisphenol A degradation. Chem. Eng. J. 2020, 382, 12. [Google Scholar] [CrossRef]
- Niu, X.Z.; Busetti, F.; Langsa, M.; Croue, J.P. Roles of singlet oxygen and dissolved organic matter in self-sensitized photo-oxidation of antibiotic norfloxacin under sunlight irradiation. Water Res. 2016, 106, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, W.; Niu, J.F.; Chen, Y.S. Mechanism of photogenerated reactive oxygen species and correlation with the antibacterial properties of engineered metal-oxide nanoparticles. ACS Nano 2012, 6, 5164–5173. [Google Scholar] [CrossRef]
- Qu, X.L.; Alvarez, P.J.J.; Li, Q.L. Photochemical transformation of carboxylated multiwalled carbon nanotubes: Role of reactive oxygen species. Environ. Sci. Technol. 2013, 47, 14080–14088. [Google Scholar] [CrossRef]
- Chen, C.; Ma, W.; Zhao, J. Semiconductor-mediated photodegradation of pollutants under visible-light irradiation. Chem. Soc. Rev. 2010, 39, 4206–4219. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A.Y. Generation and detection of reactive oxygen species in photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef]
- Chen, W.; Chen, X.; Yu, H. Photochemical degradation of sulfamethazine in aqueous solution. J. Agro-Environ. Sci. 2016, 35, 346–352. [Google Scholar]
- Zhao, Y.C.; Jafvert, C.T. Environmental photochemistry of single layered graphene oxide in water. Environ. Sci. Nano 2015, 2, 136–142. [Google Scholar] [CrossRef]
- Khodja, A.A.; Sehili, T.; Pilichowski, J.F.; Boule, P. Photocatalytic degradation of 2-phenylphenol on TiO2 and ZnO in aqueous suspensions. J. Photochem. Photobiol. A 2001, 141, 231–239. [Google Scholar] [CrossRef]
- Li, M.R.; Liu, F.F.; Wang, S.C.; Cheng, X.; Zhang, H.; Huang, T.Y.; Liu, G.Z. Phototransformation of zinc oxide nanoparticles and coexisting pollutant: Role of reactive oxygen species. Sci. Total Environ. 2020, 728, 12. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Hao, R.; Xu, Z.; He, X.; Adeleye, A.S.; Li, Y. Removal of graphene oxide nanomaterials from aqueous media via coagulation: Effects of water chemistry and natural organic matter. Chemosphere 2017, 168, 1051–1057. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, J.; Shang, E.X.; Xia, X.H.; Niu, J.F.; Crittenden, J. Effects of chloride ions on dissolution, ROS generation, and toxicity of silver nanoparticles under UV irradiation. Environ. Sci. Technol. 2018, 52, 4842–4849. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, I.; Duch, M.C.; Mansukhani, N.D.; Hersam, M.C.; Bouchard, D. Colloidal properties and stability of graphene oxide nanomaterials in the aquatic environment. Environ. Sci. Technol. 2013, 47, 6288–6296. [Google Scholar] [CrossRef]
- Budarz, J.F.; Turolla, A.; Piasecki, A.F.; Bottero, J.Y.; Antonelli, M.; Wiesner, M.R. Influence of aqueous inorganic anions on the reactivity of nanoparticles in TiO2 photocatalysis. Langmuir 2017, 33, 2770–2779. [Google Scholar] [CrossRef]
- Chen, X.R.; Fang, G.D.; Liu, C.; Dionysiou, D.D.; Wang, X.L.; Zhu, C.Y.; Wang, Y.J.; Gao, J.; Zhou, D.M. Cotransformation of carbon dots and contaminant under light in aqueous solutions: A mechanistic study. Environ. Sci. Technol. 2019, 53, 6235–6244. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, F.-F.; Li, M.-R.; Wang, S.-C.; Zhang, Y.-X.; Liu, G.-Z.; Fan, J.-L. Phototransformation of Graphene Oxide on the Removal of Sulfamethazine in a Water Environment. Nanomaterials 2021, 11, 2134. https://doi.org/10.3390/nano11082134
Liu F-F, Li M-R, Wang S-C, Zhang Y-X, Liu G-Z, Fan J-L. Phototransformation of Graphene Oxide on the Removal of Sulfamethazine in a Water Environment. Nanomaterials. 2021; 11(8):2134. https://doi.org/10.3390/nano11082134
Chicago/Turabian StyleLiu, Fei-Fei, Meng-Ru Li, Su-Chun Wang, Yu-Xue Zhang, Guang-Zhou Liu, and Jin-Lin Fan. 2021. "Phototransformation of Graphene Oxide on the Removal of Sulfamethazine in a Water Environment" Nanomaterials 11, no. 8: 2134. https://doi.org/10.3390/nano11082134
APA StyleLiu, F.-F., Li, M.-R., Wang, S.-C., Zhang, Y.-X., Liu, G.-Z., & Fan, J.-L. (2021). Phototransformation of Graphene Oxide on the Removal of Sulfamethazine in a Water Environment. Nanomaterials, 11(8), 2134. https://doi.org/10.3390/nano11082134





