Nanostructured ZnFe2O4: An Exotic Energy Material
Abstract
1. Introduction
2. Material Properties of ZnFe2O4
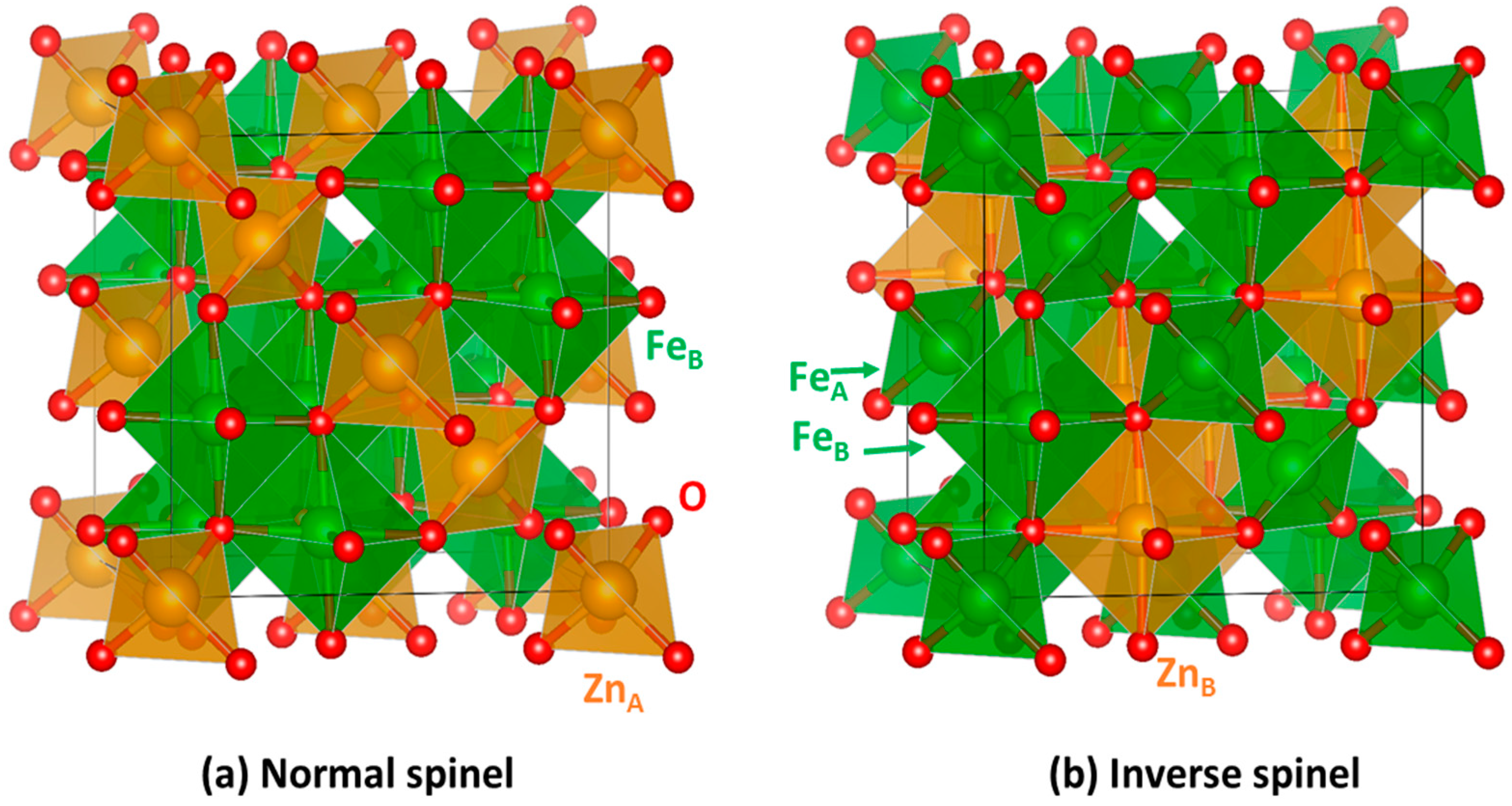
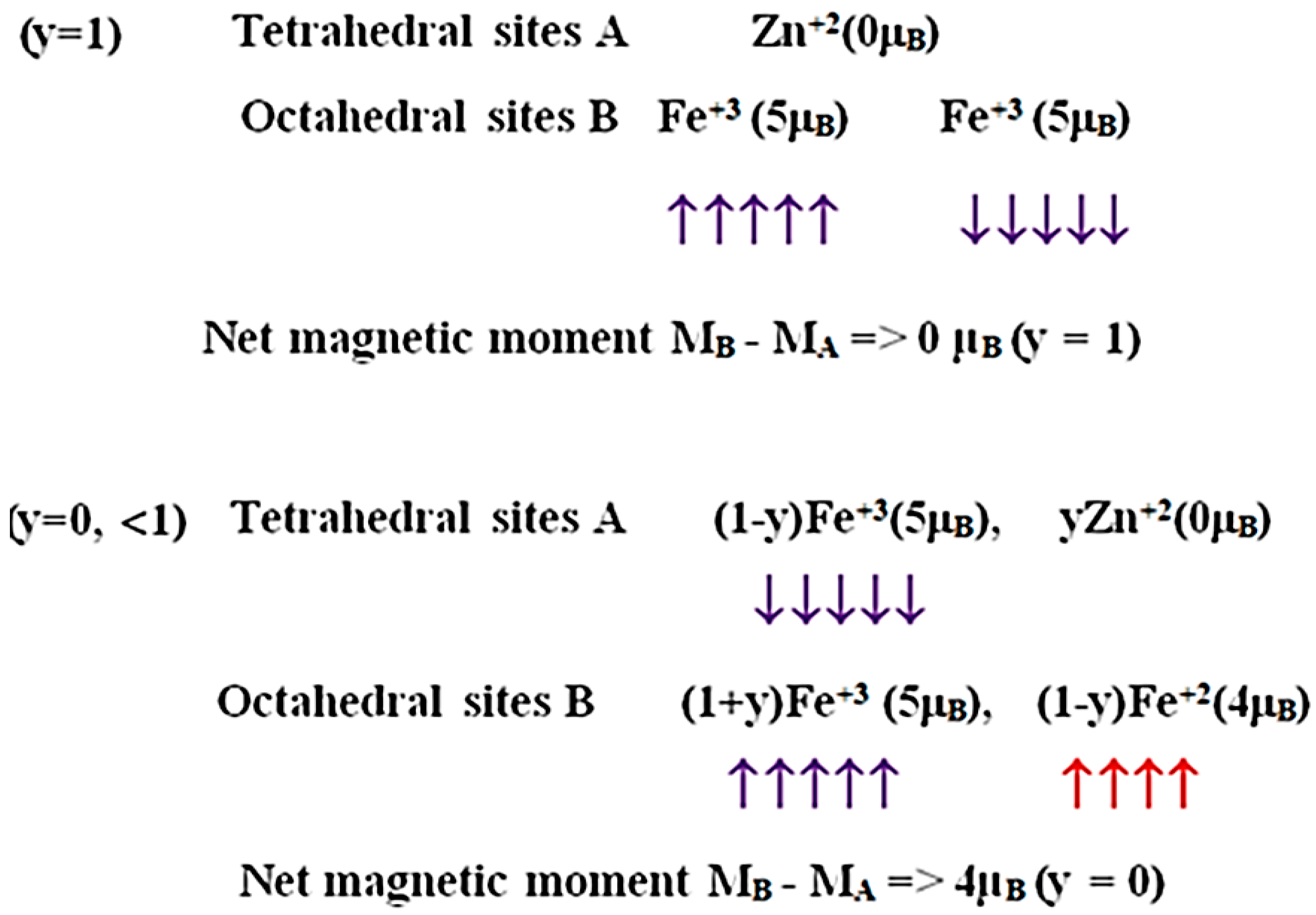
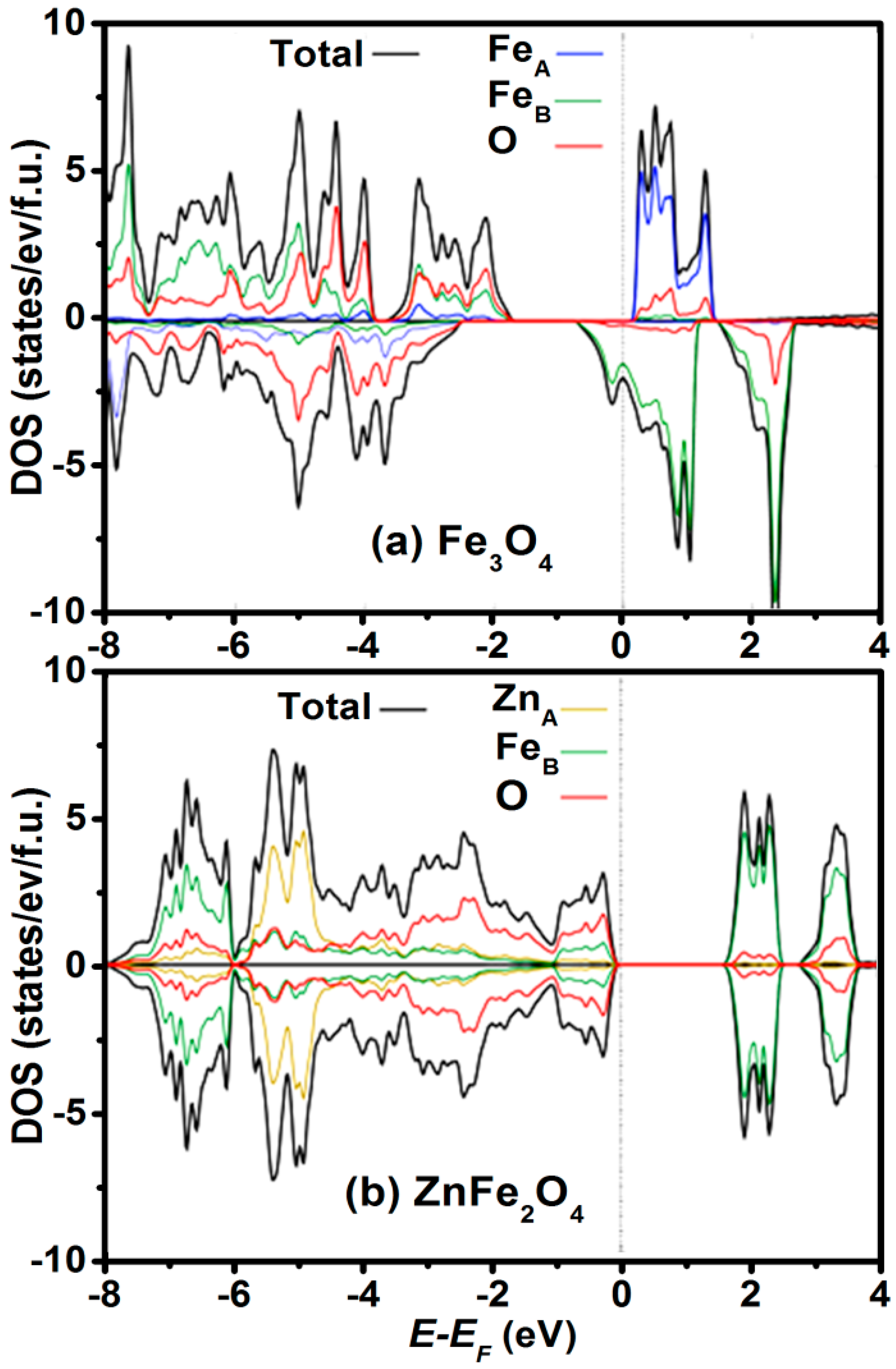
2.1. Bulk Crystalline and Spin Structure
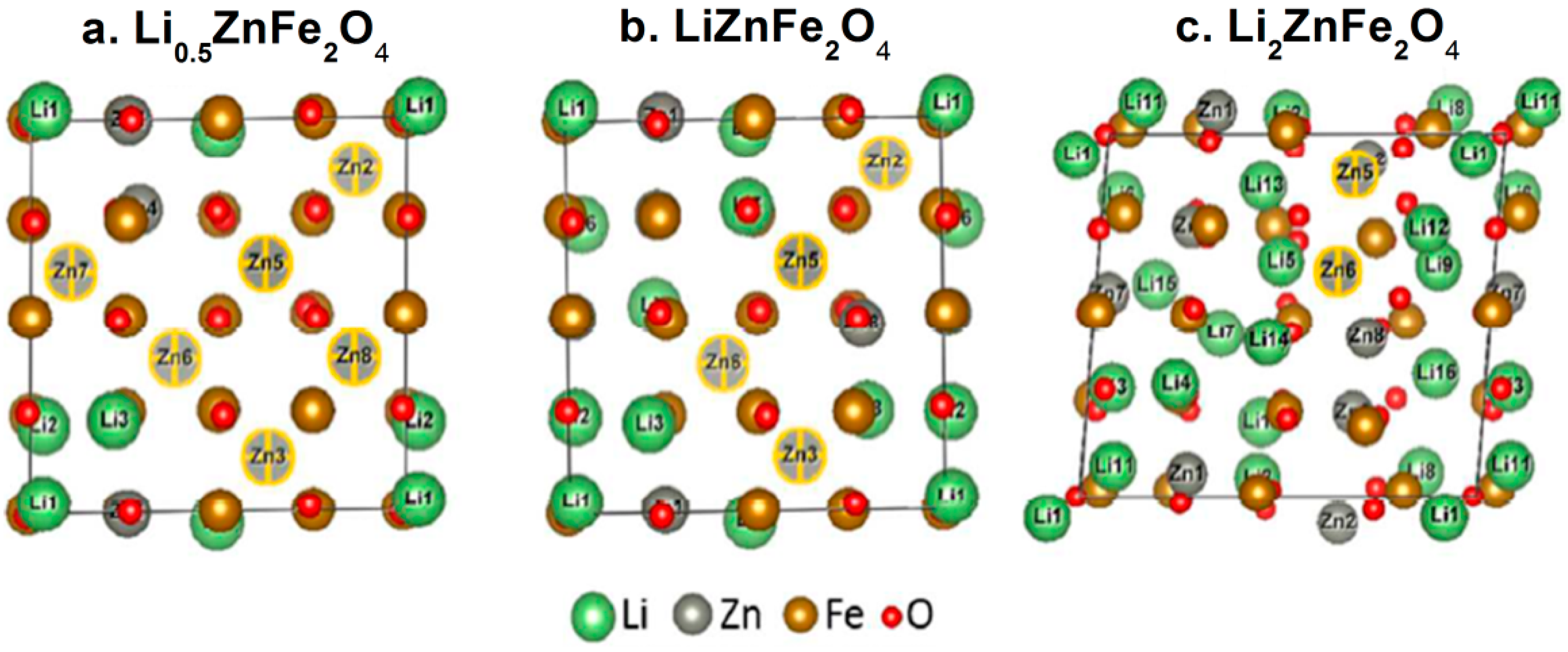
2.2. Cation Engineering in Nano Regime
3. Various ZnFe2O4 Nanostructure Morphologies
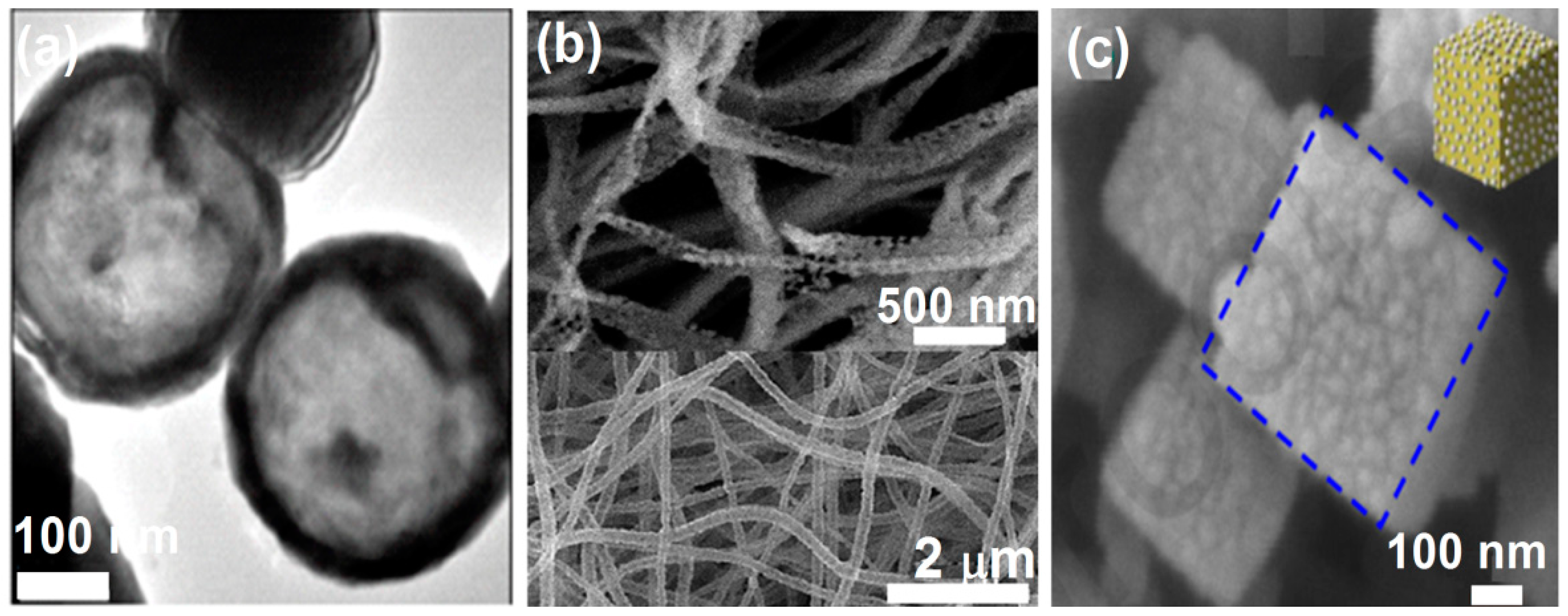
3.1. Nanoparticles (1 nm < Particle Size < 100 nm)
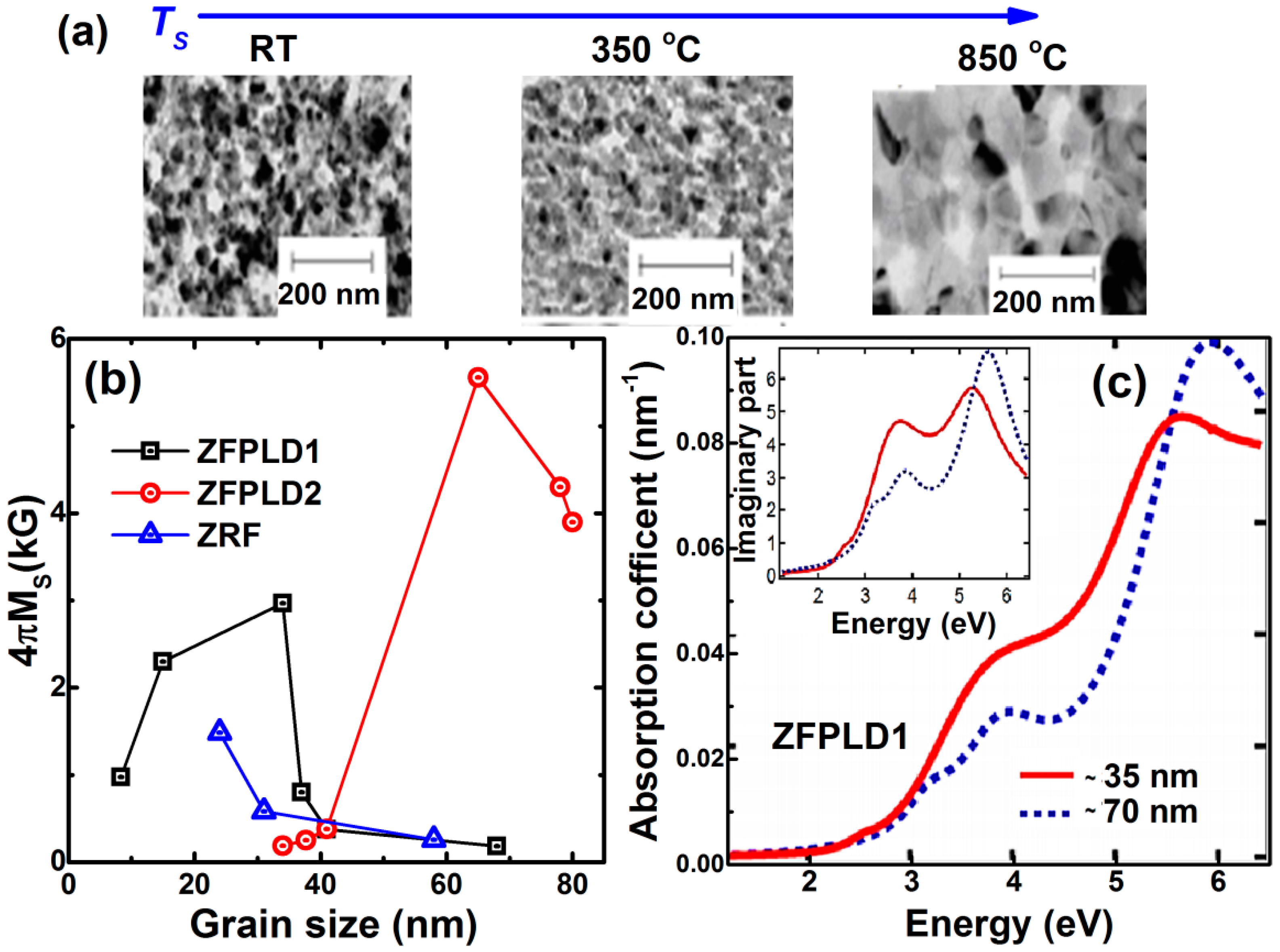
3.2. Nanocrystalline Thin Films (1 nm < Grain Size < 100 nm)
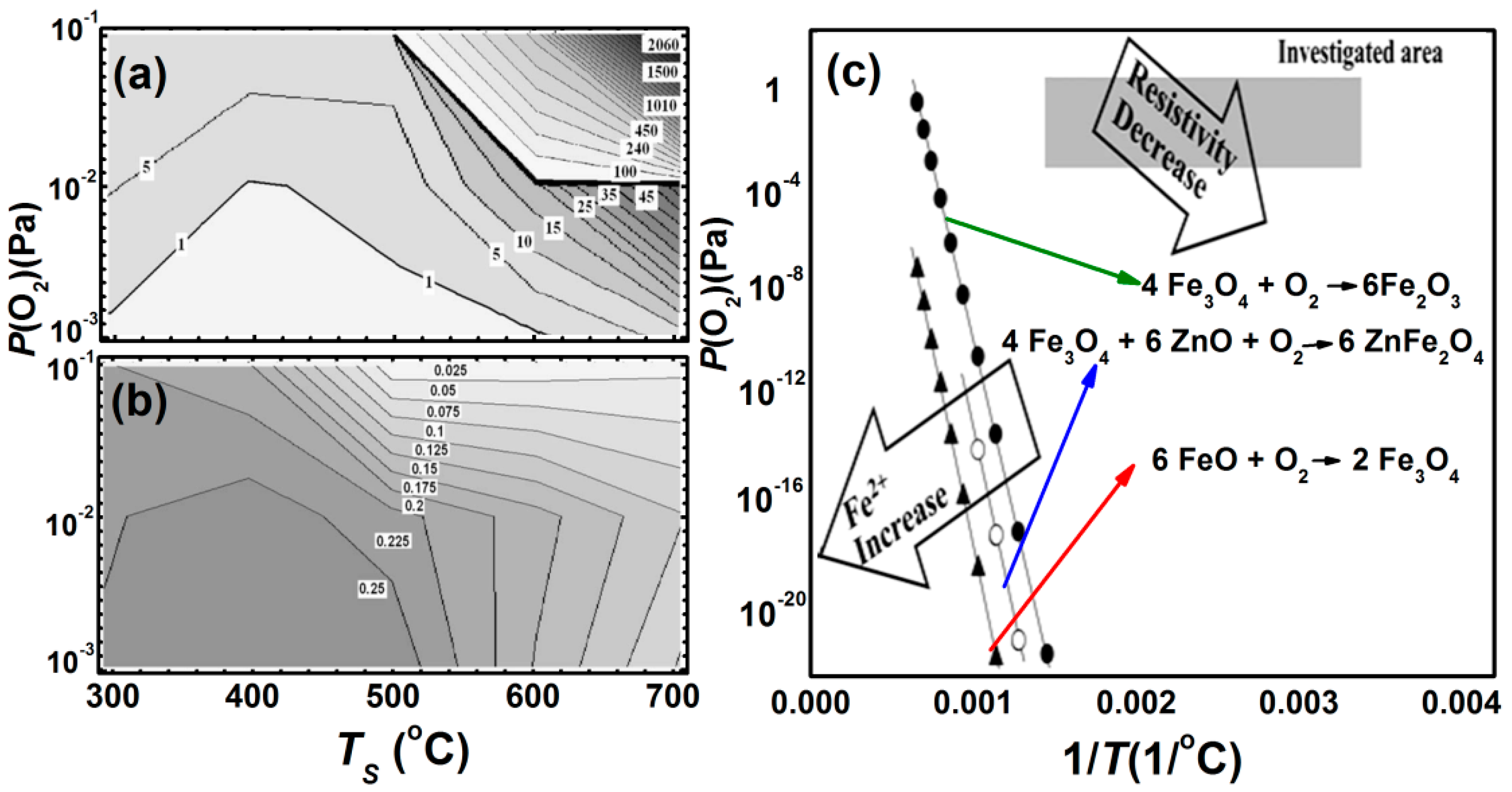
3.3. Epitaxial Films (1 nm < Nano-Thick < 200 nm)
3.4. Other Nanostructured ZnFe2O4 Geometries
4. Applications
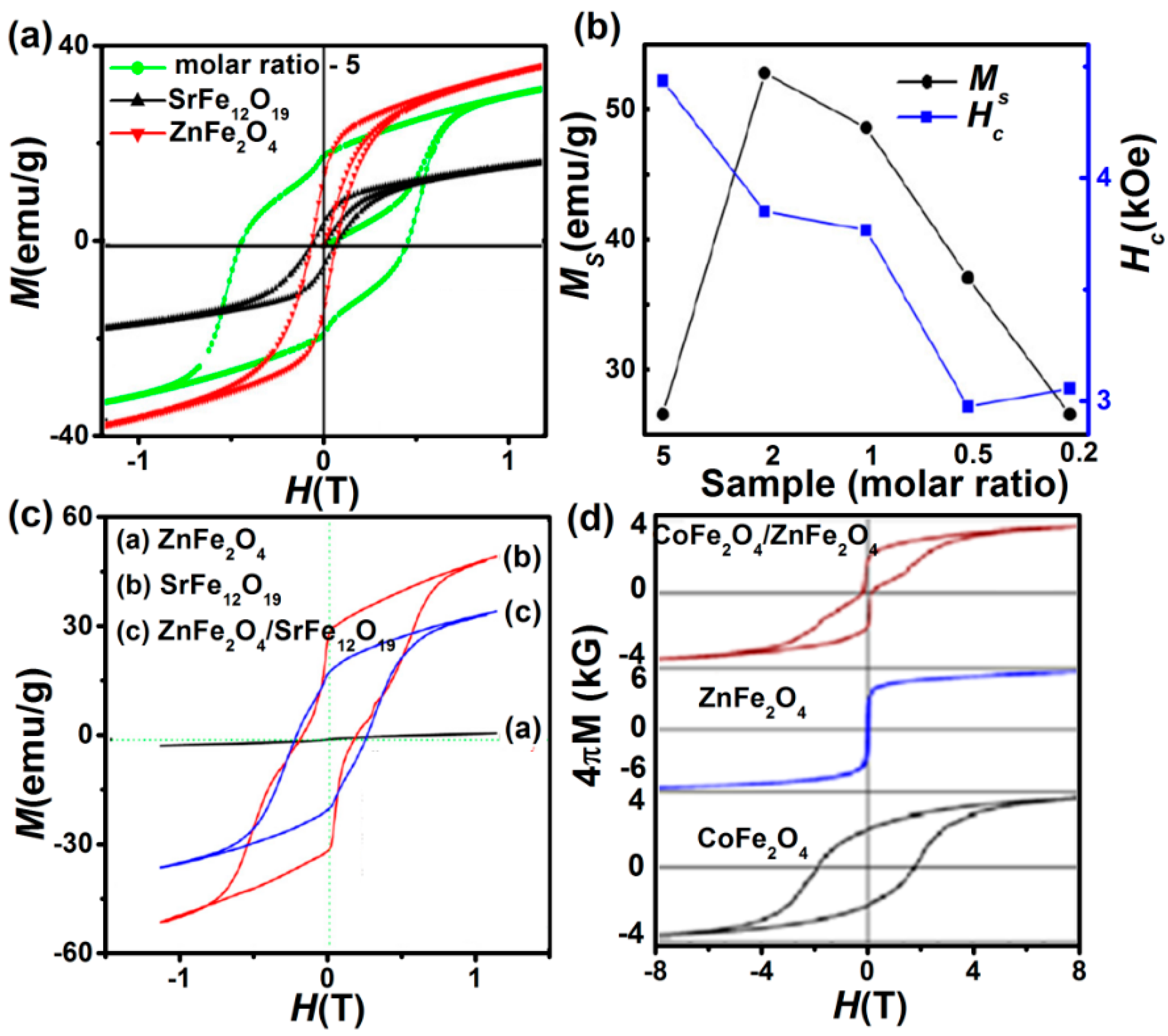
4.1. Exchange Coupling
4.1.1. Exchange Spring System (Soft + Hard Ferrite)
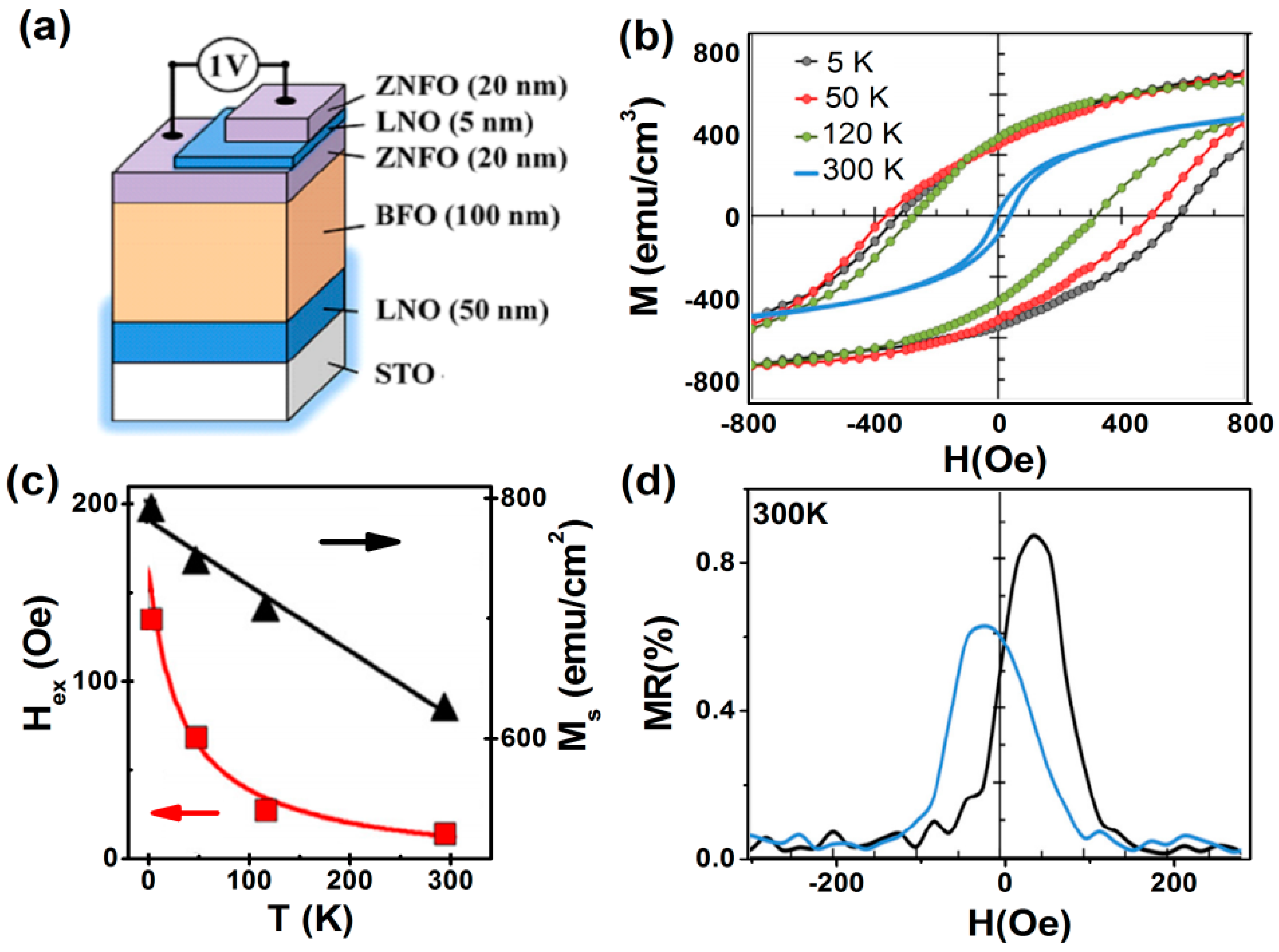
4.1.2. Exchange Bias (AFM/FM Interfaces)
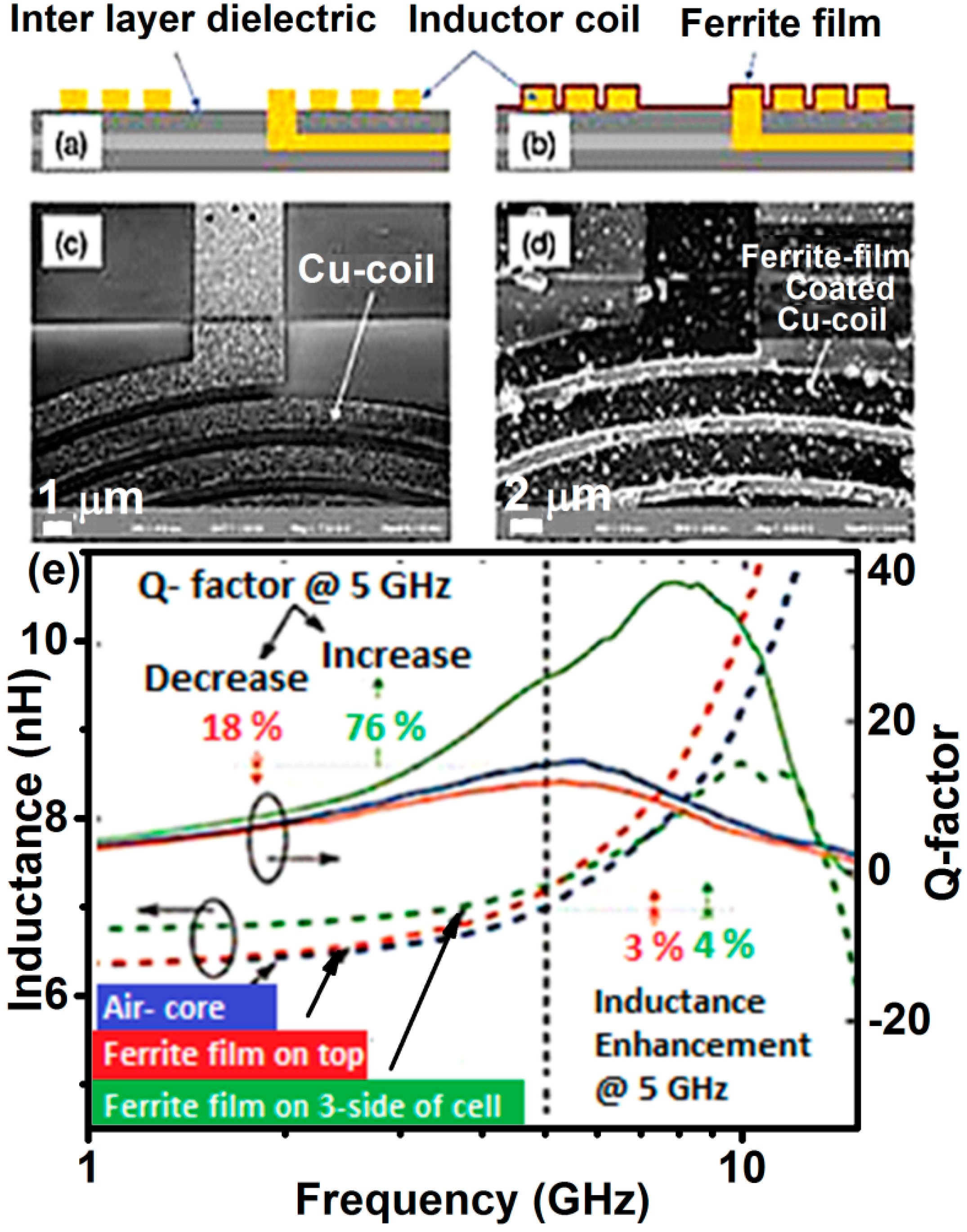
4.2. High-Frequency Applications
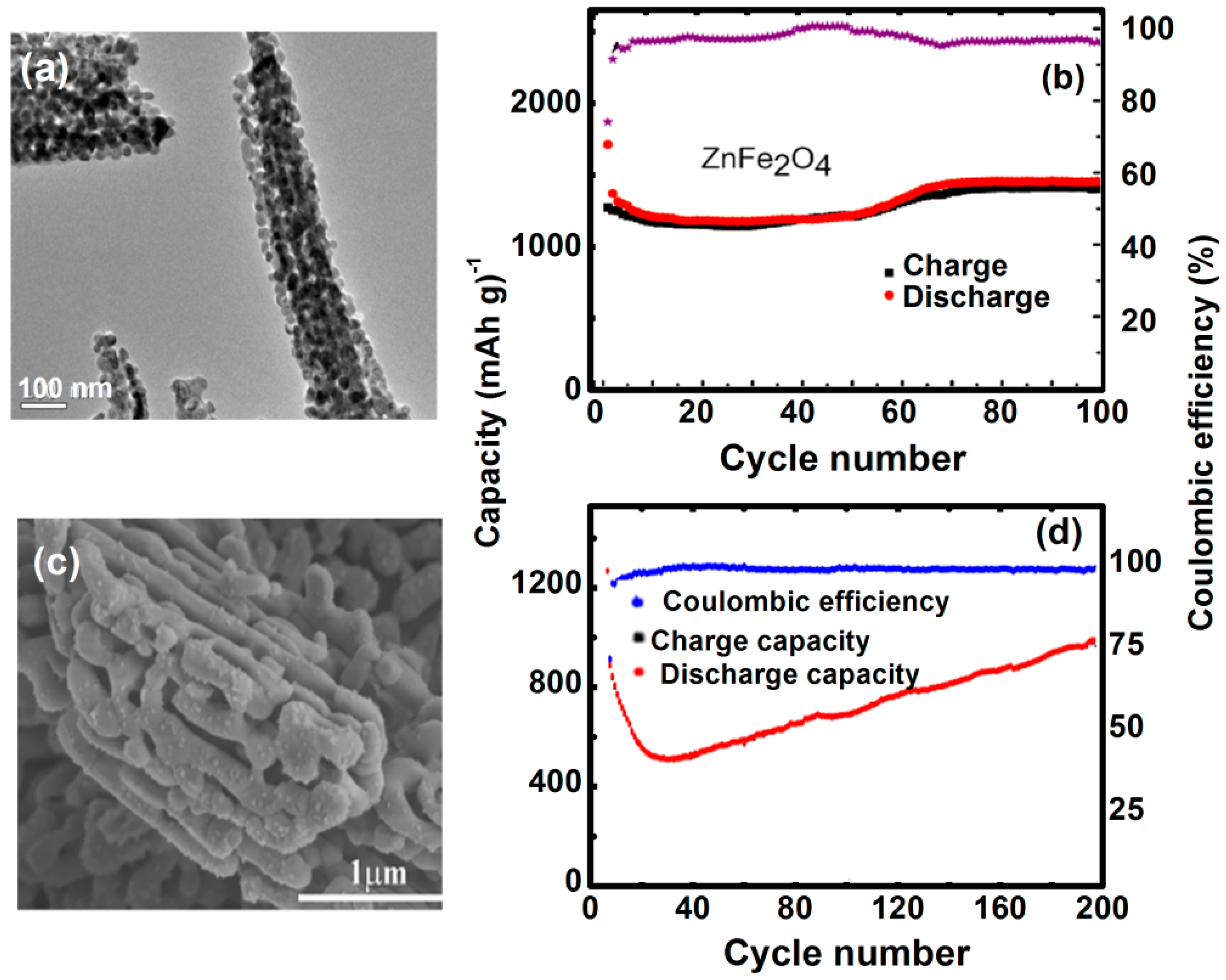
4.3. Lithium-Ion Batteries
| Morphology | Reversible Capacity mAh g−1 | Cycle | Current Rate mA g−1 | Ref. |
|---|---|---|---|---|
| Thin film | 434 | 100 | 10 | [78] |
| Nanoparticles | 841 | 50 | 60 | [79] |
| Nanofibers | 733 | 30 | 60 | [80] |
| Nano-octahedrons | 910 | 80 | 60 | [81] |
| Nanorod | 900 | 50 | 100 | [82] |
| Cubic nanoparticles | 367 | 50 | 60 | [83] |
| Hollow spheres | 900 | 50 | 65 | [70] |
| Hollow microspheres | 1200 | 120 | 100 | [84] |
| Hollow nanospheres | 1101 | 120 | 200 | [85] |
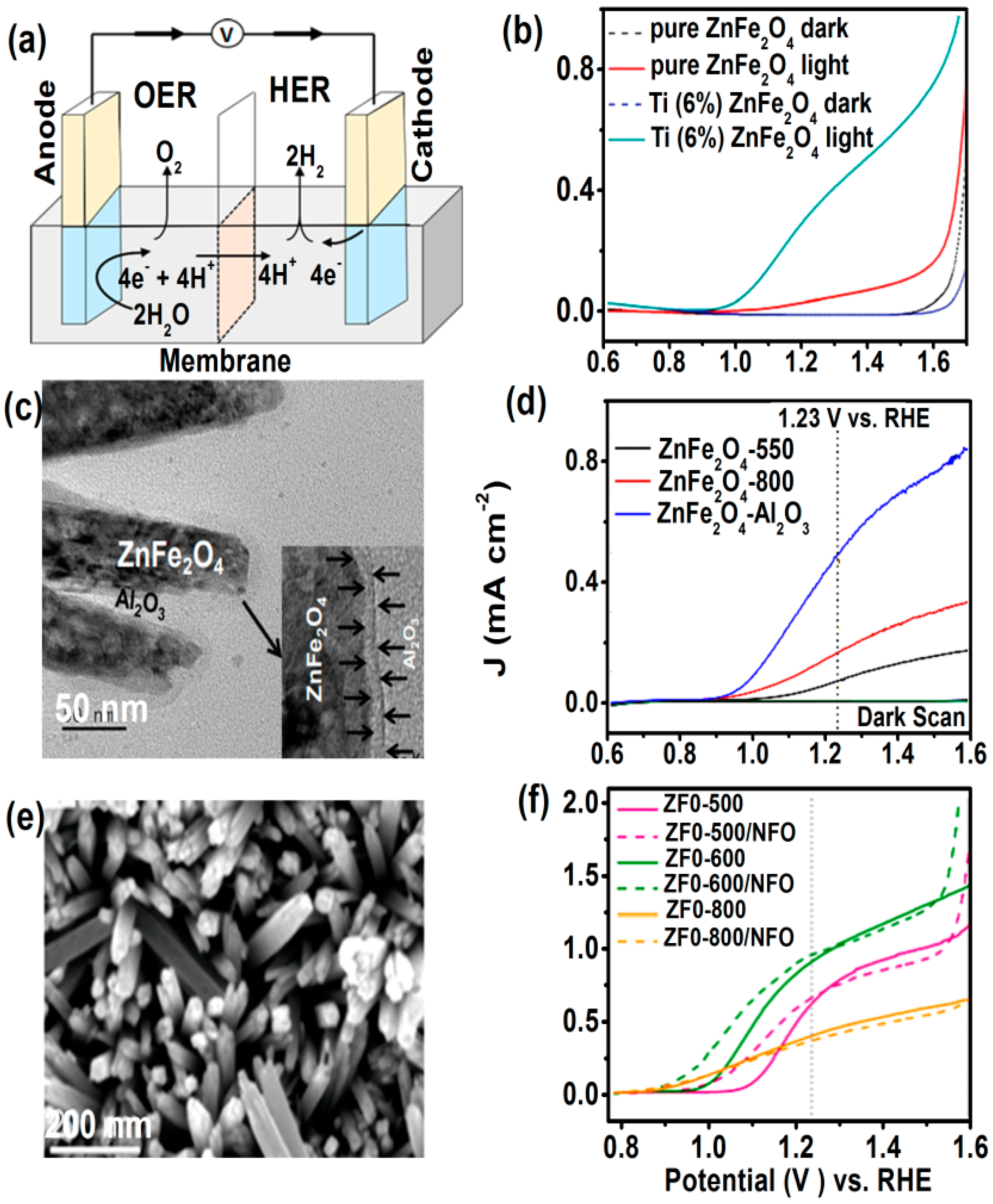
4.4. Photoelectrochemical (PEC) Water Splitting
4.5. Electrochemical Supercapacitors
5. Conclusions
- Different spintronics devices, possibly with low-energy operation cost, can be constructed by using an inverted stoichiometric ZnFe2O4 thin film as ferrimagnetic layer in magnetic tunnel junctions, as a barrier layer in spin filtering devices, oxygen-deficient ZnyFe3−yO4−δ thin film as a conducting layer could be used in homo-epitaxial devices, provided with a fine control of the stoichiometry during the growth.
- Inverted ZnFe2O4 thin layer with low microwave loss can be a potential material for high-frequency applications, such as 5G mobile communication.
- Inverted ZnFe2O4 nanostructures are emerging photoanode material for photoelectrochemical solar fuel productions. Cation disorder in ZnFe2O4 facilitates photogenerated charge separation and increased charge carrier transport.
- ZnFe2O4 used as an electrode in a Li-ion battery demonstrated large charge/discharge capacity and cycle stability. Highly porous surface and wide voids in ZnFe2O4 nanostructures play a critical role in enhancing electrochemical reactions. The suitable cathode and stable electrolyte materials are the prerequisite to form ZnFe2O4-based Li-ion battery considering high working voltage of electrode.
- Various ZnFe2O4-based heterostructures and nanocomposites with high conducting property can boost cycle stability and energy density for high-performance supercapacitors.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dusastre, V.; Martiradonna, L. Materials for sustainable energy. Nat. Mater. 2017, 16, 15. [Google Scholar] [CrossRef]
- Dalapati, G.K.; Chua, C.S.; Kushwaha, A.; Liew, S.L.; Suresh, V.; Chi, D. All earth abundant materials for low cost solar-driven hydrogen production. Mater. Lett. 2016, 183, 183–186. [Google Scholar] [CrossRef]
- Jang, J.-W.; Du, C.; Ye, Y.; Lin, Y.; Yao, X.; Thorne, J.; Liu, E.; McMahon, G.; Zhu, J.; Javey, A.; et al. Enabling unassisted solar water splitting by iron oxide and silicon. Nat. Commun. 2015, 6, 7447–7452. [Google Scholar]
- Abdel Maksoud, M.I.A.; Fahim, R.A.; Shalan, A.E.; Abd Elkodous, M.; Olojede, S.O.; Osman, A.I.; Farrell, C.; Al-Muhtaseb, A.H.; Awed, A.S.; Ashour, A.H.; et al. Advanced materials and technologies for supercapacitors used in energy conversion and storage: A review. Environ. Chem. Lett. 2021, 19, 375–439. [Google Scholar]
- Wong, H.; Iwai, H. The road to miniaturization. Phys. World 2005, 18, 40–44. [Google Scholar] [CrossRef]
- Lorenz, M.; Rao, M.S.R.; Venkatesan, T.; Fortunato, E.; Barquinha, P.; Branquinho, R.; Salgueiro, D.; Martins, R.; Carlos, E.; Liu, A.; et al. The 2016 oxide electronic materials and oxide interfaces roadmap. J. Phys. D Appl. Phys. 2016, 49, 433001. [Google Scholar] [CrossRef]
- Coll, M.; Fontcuberta, J.; Althammer, M.; Bibes, M.; Boschker, H.; Calleja, A.; Cheng, G.; Cuoco, M.; Dittmann, R.; Dkhil, B.; et al. Towards oxide electronics: A roadmap. Appl. Surf. Sci. 2019, 482, 1–93. [Google Scholar]
- Giustino, F.; Lee, J.H.; Trier, F.; Bibes, M.; Winter, S.M.; Valentí, R.; Son, Y.-W.; Taillefer, L.; Heil, C.; Figueroa, A.I.; et al. The 2021 quantum materials roadmap. J. Phys. Mater. 2020, 3, 042006. [Google Scholar] [CrossRef]
- Fritsch, D. Electronic and optical properties of spinel zinc ferrite: Ab initio hybrid functional calculations. J. Phys. Condens. Matter 2018, 30, 095502. [Google Scholar] [CrossRef]
- Heda, N.L.; Panwar, K.; Kumar, K.; Ahuja, B.L. Performance of hybrid functional in linear combination of atomic orbitals scheme in predicting electronic response in spinel ferrites ZnFe2O4 and CdFe2O4. J. Mater. Sci. 2020, 55, 3912–3925. [Google Scholar] [CrossRef]
- Ulpe, A.C.; Bauerfeind, K.C.; Bredow, T. Influence of spin state and cation distribution on stability and electronic properties of ternary transition-metal oxides. ACS Omega 2019, 4, 4138–4146. [Google Scholar] [CrossRef] [PubMed]
- Ulpe, A.C.; Bredow, T. GW-BSE calculations of electronic band gap and optical spectrum of ZnFe2O4: Effect of cation distribution and spin configuration. Chem. Phys. Chem. 2020, 21, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Harris, V.G. Modern Microwave Ferrites. IEEE Trans. Magn. 2012, 48, 1075–1104. [Google Scholar] [CrossRef]
- Lišková-Jakubisová, E.; Višňovský, Š.; Široký, P.; Hrabovský, D.; Pištora, J.; Sahoo, S.C.; Prasad, S.; Venkataramani, N.; Bohra, M.; Krishnan, R. Nanocrystalline zinc ferrite films studied by magneto-optical spectroscopy. J. Appl. Phys. 2015, 117, 17B726. [Google Scholar] [CrossRef]
- Zviagin, V.; Kumar, Y.; Lorite, I.; Esquinazi, P.; Grundmann, M.; Schmidt-Grund, R. Ellipsometric investigation of ZnFe2O4 thin films in relation to magnetic properties. Appl. Phys. Lett. 2016, 108, 131901. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, H.E.; Kim, J.H.; Lee, J.S. Ferrites: Emerging light absorbers for solar water splitting. J. Mater. Chem. A 2020, 8, 9447–9482. [Google Scholar] [CrossRef]
- Srivastava, C.M.; Shringi, S.N.; Srivastava, R.G.; Nanadikar, N.G. Magnetic ordering and domain-wall relaxation in zinc-ferrous ferrites. Phys. Rev. B 1976, 14, 2032–2040. [Google Scholar] [CrossRef]
- Bohra, M.; Prasad, S.; Venkataramani, N.; Sahoo, S.C.; Kumar, N.; Krishnan, R. Low temperature magnetization studies of nanocrystalline Zn-ferrite thin films. IEEE Trans. Magn. 2013, 49, 4249–4252. [Google Scholar] [CrossRef]
- Nakashima, S.; Fujita, K.; Tanaka, K.; Hirao, K. High magnetization and the high-temperature superparamagnetic transition with intercluster interaction in disordered zinc ferrite thin film. J. Phys. Condens. Matter. 2005, 17, 137–149. [Google Scholar] [CrossRef]
- Jedrecy, N.; Hebert, C.; Perriere, J.; Nistor, M.; Millon, E. Magnetic and magnetotransport properties of ZnxFe3−xO4−y thin films. J. Appl. Phys. 2014, 116, 213903. [Google Scholar] [CrossRef]
- Chinnasamy, C.N.; Narayanasamy, A.; Ponpandian, N.; Chattopadhyay, K.; Guérault, H.; Greneche, J.-M. Magnetic properties of nanostructured ferrimagnetic zinc ferrite. J. Phys. Condens. Matter. 2000, 12, 7795–7805. [Google Scholar] [CrossRef]
- Hufnagel, A.G.; Peters, K.; Müller, A.; Scheu, C.; Fattakhova-Rohlfing, D.; Bein, T. Zinc ferrite photoanode nanomorphologies with favorable kinetics for water-splitting. Adv. Funct. Mater. 2016, 26, 4435–4443. [Google Scholar] [CrossRef]
- Vadiyar, M.M.; Bhise, S.C.; Patil, S.K.; Patil, S.A.; Pawar, D.K.; Ghule, A.V.; Patil, P.S.; Kolekar, S.S. Mechanochemical growth of a porous ZnFe2O4 nano-flake thin film as an electrode for supercapacitor application. RSC Adv. 2015, 5, 45935–45942. [Google Scholar] [CrossRef]
- Ding, Y.; Yang, Y.; Shao, H. High capacity ZnFe2O4 anode material for lithium ion batteries. Electrochim. Acta 2011, 56, 9433–9438. [Google Scholar] [CrossRef]
- Saraf, M.; Natarajan, K.; Gupta, A.K.; Kumar, P.; Rajak, R.; Mobin, S.M. Electrochemical energy storage properties of solvothermally driven ZnFe2O4 microspheres. Mater. Res. Express 2019, 6, 095534. [Google Scholar] [CrossRef]
- Kamazawa, K.; Tsunoda, Y.; Kadowaki, H.; Kohn, K. Magnetic neutron scattering measurements on a single crystal of frustrated ZnFe2O4. Phys. Rev. B 2003, 68, 024412. [Google Scholar] [CrossRef]
- Bohra, M.; Agarwal, N.; Singh, V. A short review on verwey transition in nanostructured Fe3O4 materials. J. Nanomater. 2019, 2019, 1–18. [Google Scholar] [CrossRef]
- Ferrari, S.; Kumar, R.S.; Grinblat, F.; Aphesteguy, J.C.; Saccone, F.D.; Errandonea, D. In-situ high-pressure x-ray diffraction study of zinc ferrite nanoparticles. Solid State Sci. 2016, 56, 68–72. [Google Scholar] [CrossRef]
- Goodenough, J.B. Magnetism and the Chemical Bond; Interscience: New York, NY, USA, 1963. [Google Scholar]
- Kanamori, J. Superexchange interaction and symmetry properties of electron orbitals. J. Phys. Chem. Solids 1959, 10, 87–98. [Google Scholar] [CrossRef]
- Srivastava, M.; Alla, S.K.; Meena, S.S.; Gupta, N.; Mandal, R.K.; Prasad, N.K. ZnxFe3−xO4 (0.01 ≤ x ≤ 0.8) nanoparticles for controlled magnetic hyperthermia application. New J. Chem. 2018, 42, 7144–7153. [Google Scholar] [CrossRef]
- Tian, Q.; Wang, Q.; Xie, Q.; Jiangong, L. Aqueous solution preparation, structure, and magnetic properties of nano-granular ZnxFe3−xO4 ferrite films. Nanoscale Res. Lett. 2010, 5, 1518–1523. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metal–amorphous-semiconductor transition in germanium. Phys. Rev. B 1994, 49, 14251–14269. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Csonka, G.I.; Perdew, J.P.; Ruzsinszky, A.; Philipsen, P.H.T.; Lebègue, S.; Paier, J.; Vydrov, O.A.; Ángyán, J.G. Assessing the performance of recent density functionals for bulk solids. Phys. Rev. B 2009, 79, 155107. [Google Scholar] [CrossRef]
- Dudarev, S.L.; Botton, G.A.; Savrasov, S.Y.; Humphreys, C.J.; Sutton, A.P. Electron-energy-loss spectra and the structural stability of nickel oxide: An LSDA+U study. Phys. Rev. B 1998, 57, 1505–1509. [Google Scholar] [CrossRef]
- Bohra, M.; Arras, R.; Bobo, J.-F.; Singh, V.; Kumar, N.; Chou, H. Multiple spintronic functionalities into single zinc-ferrous ferrite thin films. J. Alloys Compd. 2021. submitted for publication. [Google Scholar]
- Desai, M.; Prasad, S.; Venkataramani, N.; Samajdar, I.; Nigam, A.K.; Krishnan, R. Annealing induced structural change in sputter deposited copper ferrite thin films and its impact on magnetic properties. J. Appl. Phys. 2002, 91, 2220–2227. [Google Scholar] [CrossRef]
- Bohra, M.; Prasad, S.; Kumar, N.; Misra, D.S.; Sahoo, S.C.; Venkataramani, N. Large room temperature magnetization in nanocrystalline zinc ferrite thin films. Appl. Phys. Lett. 2006, 88, 262506. [Google Scholar] [CrossRef]
- Cobos, M.A.; Presa, P.D.L.; Llorente, I.; Alonso, J.M.; García-Escorial, A.; Marína, P.; Hernando, A.; Jiménez, J.A. Magnetic phase diagram of nanostructured zinc ferrite as a function of inversion degree δ. J. Phys. Chem. C 2019, 123, 17472–17482. [Google Scholar] [CrossRef]
- Yao, C.; Zeng, Q.; Goya, G.F.; Torres, T.; Liu, J.; Wu, H.; Ge, M.; Zeng, Y.; Wang, Y.; Jiang, J.Z. ZnFe2O4 nanocrystals: Synthesis and magnetic properties. J. Phys. Chem. C 2007, 111, 12274–12278. [Google Scholar] [CrossRef]
- Granone, L.I.; Ulpe, A.C.; Robben, L.; Klimke, S.; Jahns, M.; Renz, F.; Gesing, T.M.; Bredow, T.; Dillert, R.; Bahnemann, D.W. Effect of the degree of inversion on optical properties of spinel ZnFe2O4. Phys. Chem. Chem. Phys. 2018, 20, 28267–28278. [Google Scholar] [CrossRef]
- Zviagin, V.; Sturm, C.; Esquinazi, P.D.; Grundmann, M.; Schmidt-Grund, R. Control of magnetic properties in spinel ZnFe2O4 thin films through intrinsic defect manipulation. J. Appl. Phys. 2020, 128, 165702. [Google Scholar] [CrossRef]
- Marcu, A.; Yanagida, T.; Nagashima, K.; Tanaka, H.; Kawai, T. Transport properties of ZnFe2O4−δ thin films. J. Appl. Phys. 2007, 102, 023713. [Google Scholar] [CrossRef]
- Rivero, M.; Campo, A.D.; Mayoral, Á.; Mazario, E.; Sánchez-Marcos, J.; Muñoz-Bonilla, A. Synthesis and structural characterization of ZnxFe3−xO4 ferrite nanoparticles obtained by an electrochemical method. RSC Adv. 2016, 6, 40067–40076. [Google Scholar] [CrossRef]
- Venkateshvaran, D.; Althammer, M.; Nielsen, A.; Geprägs, S.; Ramachandra Rao, M.S.; Sebastian, T.; Goennenwein, B.; Opel, M.; Gross, R. Epitaxial ZnxFe3−xO4 thin films: A spintronic material with tunable electrical and magnetic properties. Phys. Rev. B 2009, 79, 134405. [Google Scholar] [CrossRef]
- Sahu, B.N.; Venkataramani, N.; Prasad, S.; Krishnan, R. Effect of thickness on magnetic and microwave properties of RF-sputtered Zn-ferrite thin films. AIP Adv. 2017, 7, 056102. [Google Scholar]
- Hwang, H.; Shin, H.; Lee, W.-J. Effects of calcination temperature for rate capability of triple-shelled ZnFe2O4 hollow microspheres for lithium ion battery anodes. Sci. Rep. 2017, 7, 46378. [Google Scholar] [CrossRef]
- Takaobushi, J.; Tanaka, H.; Kawai, T.; Ueda, S.; Kim, J.-J.; Kobata, M.; Ikenaga, E.; Yabashi, M.; Kobayashi, K.; Nishino, Y.; et al. Fe3−xZnxO4 thin film as tunable high Curie temperature ferromagnetic semiconductor. Appl. Phys. Lett. 2006, 89, 242507. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Tanaka, H.; Kawai, T. The control of cluster-glass transition temperature in spinel-type ZnFe2O4−δ thin film. Jpn. J. Appl. Phys. 2001, 40, L545–L547. [Google Scholar] [CrossRef]
- Lorenz, M.; Brandt, M.; Mexner, K.; Brachwitz, K.; Ziese, M.; Esquinazi, P.; Hochmuth, H.; Grundmann, M. Ferrimagnetic ZnFe2O4 thin films on SrTiO3 single crystals with highly tunable electrical conductivity. Phys. Status Solidi RRL 2011, 5, 438–440. [Google Scholar] [CrossRef]
- Saha, P.; Rakshit, R.; Alam, M.; Mandal, K. Magnetic and electronic properties of Zn doped Fe3O4 hollow nanospheres. Phys. Rev. Appl. 2019, 11, 024059–024069. [Google Scholar] [CrossRef]
- Bohra, M.; Prasad, S.; Venkataramani, N.; Kumar, N.; Sahoo, S.C.; Krishnan, R. Narrow ferromagnetic resonance line width polycrystalline Zn-ferrite thin films. IEEE Trans. Magn. 2011, 47, 345–348. [Google Scholar] [CrossRef]
- Sai, R.; Endo, Y.; Shimada, Y.; Yamaguchi, M.; Shivashankar, S.A. Oriented nanometric aggregates of partially inverted zinc ferrite: One-step processing and tunable high-frequency magnetic properties. J. Appl. Phys. 2015, 117, 17E511. [Google Scholar]
- Sai, R.; Shivashankar, S.A.; Yamaguchi, M.; Bhat, N. Magnetic nanoferrites for RF CMOS: Enabling 5G and beyond. Electrochem. Soc. Interface 2017, 26, 71–76. [Google Scholar]
- Sai, R.; Vinoy, K.J.; Bhat, N.; Shivashankar, S.A. CMOS-compatible and scalable deposition of nanocrystalline zinc ferrite thin film to improve inductance density of integrated RF inductor. IEEE Trans. Magn. 2013, 49, 4323–4326. [Google Scholar]
- Ameer, S.; Gul, I.H.; Mahmood, N.; Mujahid, M. Semiconductor-to-metallic flipping in a ZnFe2O4–graphene based smart nano-system: Temperature/microwave magneto-dielectric spectroscopy. Mater. Charact. 2015, 99, 254–265. [Google Scholar] [CrossRef]
- Shi, R.; Zhang, Y.; Wang, X.; Ma, Q.; Zhang, A.; Yang, P. Electrospun ZnFe2O4 nanotubes and nanobelts: Morphology evolution, formation mechanism and Fenton-like photocatalytic activities. Mater. Chem. Phys. 2018, 207, 114–122. [Google Scholar]
- Wang, K.; Zhan, S.; Sun, H.; Zhang, D.; Wang, J. Hollow porous core–shell ZnFe2O4/AgCl nanocubes coated with EDTA and Ag nanoparticles for enhanced photocatalytic performances of visible-light-driven. Chem. Eng. J. 2020, 400, 125908. [Google Scholar]
- Sahoo, S.C.; Venkataramani, N.; Prasad, S.; Bohra, M.; Krishnan, R. Magnetic Properties of Nanocrystalline CoFe2O4/ZnFe2O4 Bilayers. J. Supercond. Nov. Magn. 2012, 25, 2653–2657. [Google Scholar] [CrossRef]
- Wu, Q.; Yu, Z.; Wu, Y.; Gao, Z.; Xie, H. The magnetic and photocatalytic properties of nanocomposites SrFe12O19 /ZnFe2O4. J. Magn. Magn. Mater. 2018, 465, 1–8. [Google Scholar]
- Taiping, X.; Longjun, X.; Chenglun, L.; Yuan, W. Magnetic composite ZnFe2O4/SrFe12O19: Preparation, characterization, and photocatalytic activity under visible light. Appl. Surf. Sci. 2013, 273, 684–691. [Google Scholar]
- Bohra, M.; Singh, V.; Sowwan, M.; Bobo, J.-F.; Chung, C.-J.; Clemens, B. Influence of packaging on the surface oxidation and magnetic properties of cobalt nanocrystals. J. Phys. D Appl. Phys. 2014, 47, 305002. [Google Scholar] [CrossRef]
- Lin, W.-J.; Chang, W.-C.; Qi, X. Exchange bias and magneto-resistance in an all-oxide spin valve with multi-ferroic BiFeO3 as the pinning layer. Acta Mater. 2013, 61, 7444–7453. [Google Scholar] [CrossRef]
- Gao, X.; Wang, J.; Zhang, D.; Adair, K.; Feng, K.; Sun, N.; Zheng, H.; Shao, H.; Zhong, J.; Ma, Y. Carbon coated bimetallic sulfide nanodots/carbon nanorod heterostructure enabling long-life lithium-ion batteries. J. Mater. Chem. 2017, 5, 25625–25631. [Google Scholar] [CrossRef]
- Hou, L.; Lian, L.; Zhang, L.; Pang, G.; Yuan, C.; Zhang, X. Self-sacrifice template fabrication of hierarchical mesoporous Bi-component-active ZnO/ZnFe2O4 sub-microcubes as superior anode towards high-performance lithium-ion battery. Adv. Funct. Mater. 2015, 25, 238–246. [Google Scholar] [CrossRef]
- Yu, S.-H.; Lee, S.H.; Lee, D.J.; Sung, Y.-E.; Hyeon, T. Conversion reaction-based oxide nanomaterials for lithium ion battery anodes. Small 2016, 12, 2146–2172. [Google Scholar] [CrossRef]
- Yuan, C.; Wu, H.B.; Xie, Y.; Lou, X.W. Mixed transition-metal oxides: Design, synthesis, and energy-related applications. Angew. Chem. Int. Ed. Engl. 2014, 53, 1488–1504. [Google Scholar]
- Lu, G.; Qiu, S.; Liu, J.; Wang, X.; He, C.; Bai, Y.J. Enhanced electrochemical performance of Zn-doped Fe3O4 with carbon coating. Electrochim. Acta 2014, 117, 230–238. [Google Scholar] [CrossRef]
- Guo, X.; Lu, X.; Fang, X.; Mao, Y.; Wang, Z.; Chen, L.; Xu, X.; Yang, H.; Liu, Y. Lithium storage in hollow spherical ZnFe2O4 as anode materials for lithium ion batteries. Electrochem. Commun. 2010, 12, 847–850. [Google Scholar] [CrossRef]
- Bresser, D.; Paillard, E.; Kloepsch, R.; Krueger, S.; Fiedler, M.; Schmitz, R.; Baither, D.; Winter, M.; Passerini, S. Carbon coated ZnFe2O4 nanoparticles for advanced lithium-ion anodes. Adv. Energy Mater. 2013, 3, 513–523. [Google Scholar] [CrossRef]
- Teh, P.F.; Pramana, S.S.; Kim, C.; Chen, C.M.; Chuang, C.H.; Sharma, Y.; Cabana, J.; Madhavi, S. Electrochemical reactivity with lithium of spinel-type ZnFe2–yCryO4 (0≤ y ≤ 2). J. Phys. Chem. C 2013, 117, 24213–24223. [Google Scholar]
- Bourrioux, S. Laser-Pyrolysed ZnFe2O4 Anode for Lithium-Ion Batteries: Understanding of the Lithium Storage Mechanisms. Ph.D. Thesis, Université Grenoble Alpes and NTU Singapore, Singapore, 2018. [Google Scholar]
- Zhang, Y.; Pelliccione, C.J.; Brady, A.B.; Guo, H.; Smith, P.F.; Liu, P.; Marschilok, A.C.; Takeuchi, K.J.; Takeuchi, E.S. Probing the Li insertion mechanism of ZnFe2O4 in Li-ion batteries: A combined X-ray diffraction, extended X-ray absorption fine structure, and density functional theory study. Chem. Mater. 2017, 29, 4282–4292. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, Y.; Marschilok, A.C.; Takeuchi, K.J.; Takeuchi, E.S.; Liu, P. A first principles study of spinel ZnFe2O4 for electrode materials in lithium-ion batteries. Phys. Chem. Chem. Phys. 2017, 19, 26322–26329. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liang, Z.; Xiong, W.L. Metal oxide hollow nanostructures for lithium ion batteries. Adv. Mater. 2012, 24, 1903–1911. [Google Scholar]
- Hou, X.; Wang, X.; Yao, L.; Hu, S.; Wu, Y.; Liu, X. Facile synthesis of ZnFe2O4 with inflorescence spicate architecture as anode materials for lithium-ion batteries with outstanding performance. New J. Chem. 2015, 39, 1943–1952. [Google Scholar] [CrossRef]
- Nuli, Y.N.; Chu, Y.Q.; Qin, Q.Z. Nanocrystalline ZnFe2O4 and Ag-doped ZnFe2O4 films used as new anode materials for Li-ion batteries. J. Electrochem. Soc. 2003, 151, A1077–A1083. [Google Scholar] [CrossRef]
- Sharma, Y.; Sharma, N.; Rao, G.V.S.; Chowdari, B.V.R. Li-storage and cyclability of urea combustion derived ZnFe2O4 as anode for li-ion batteries. Electrochim. Acta 2008, 53, 2380–2385. [Google Scholar] [CrossRef]
- Pei, F.T.; Sharma, Y.; Pramana, S.S.; Srinivasan, M. Nanoweb anodes composed of one-dimensional, high aspect ratio, size tunable electrospun ZnFe2O4 nanofibers for lithium ion batteries. J. Mater. Chem. 2011, 21, 14999–15008. [Google Scholar]
- Xing, Z.; Ju, Z.; Yang, J. One-step hydrothermal synthesis of ZnFe2O4 nano-octahedrons as a high capacity anode material for Li-ion batteries. Nano Res. 2012, 5, 477–485. [Google Scholar] [CrossRef]
- Zhong, X.B.; Yang, Z.Z.; Wang, H.Y.; Lu, L.; Jin, B.; Zha, M.; Jiang, Q.C. A novel approach to facilely synthesize mesoporous ZnFe2O4 nanorods for lithium ion batteries. J. Power Source 2016, 306, 718–723. [Google Scholar] [CrossRef]
- Zhong, X.B.; Jin, B.; Yang, Z.Z.; Wang, C.; Wang, H.Y. Facile shape design and fabrication of ZnFe2O4 as an anode material for Li-ion batteries. RSC Adv. 2014, 4, 55173–55178. [Google Scholar] [CrossRef]
- Fang, Z.; Zhang, L.; Qi, H.; Yue, H.; Zhang, T.; Zhao, X.; Chen, G.; Wei, Y.; Wang, C.; Zhang, D. Nanosheet assembled hollow ZnFe2O4 microsphere as anode for lithium-ion batteries. J. Alloy. Compd. 2018, 762, 480–487. [Google Scholar] [CrossRef]
- Yu, M.; Huang, Y.; Wang, K.; Han, X.; Wang, M.; Zhu, Y.; Liu, L. Complete hollow ZnFe2O4 nanospheres with huge internal space synthesized by a simple solvothermal method as anode for lithium ion batteries. Appl. Surf. Sci. 2018, 462, 955–962. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Y.; Zhu, H.; Wang, X. Hierarchical bead chain ZnFe2O4-PEDOT composites with enhanced Li-ion storage properties as anode materials for lithium-ion batteries. Appl. Surf. Sci. 2020, 529, 147078. [Google Scholar] [CrossRef]
- Roger, I.; Shipman, M.A.; Symes, M.D. Earth-abundant catalysts for electrochemical and photoelectrochemical water splitting. Nat. Rev. Chem. 2017, 1, 1–13. [Google Scholar]
- Polo, A.; Lhermitte, C.R.; Dozzi, M.V.; Selli, E.; Sivula, K. Hydrogenation of ZnFe2O4 flat films: Effects of the pre-annealing temperature on the photoanodes efficiency for water oxidation. Surfaces 2020, 3, 9. [Google Scholar] [CrossRef]
- Kim, J.H.; Jang, Y.J.; Choi, S.H.; Lee, B.J.; Kim, J.H.; Park, Y.B.; Nam, C.M.; Kim, H.G.; Lee, J.S. A multitude of modifications strategy of ZnFe2O4 nanorod photoanodes for enhanced photoelectrochemical water splitting activity. J. Mater. Chem. A 2018, 6, 12693–12700. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, J.H.; Jang, J.W.; Kim, J.Y.; Choi, S.H.; Magesh, G.; Lee, J.; Lee, J.S. Awakening solar water-splitting activity of ZnFe2O4 nanorods by hybrid microwave annealing. Adv. Energy Mater. 2014, 5, 1401933. [Google Scholar] [CrossRef]
- Lan, Y.; Liu, Z.; Guo, Z.; Ruan, M.; Li, X. A promising p-type Co-ZnFe2O4 nanorod film as a photocathode for photoelectrochemical water splitting. Chem. Commun. 2020, 56, 5279–5282. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, N.; Wang, X.; Qian, Q.; Zhang, S.; Li, Z.; Zou, Z. A facile spray pyrolysis method to prepare Ti-doped ZnFe2O4 for boosting photoelectrochemical water splitting. J. Mater. Chem. A 2017, 5, 7571–7577. [Google Scholar] [CrossRef]
- Sahu, T.K.; Shah, A.K.; Gogoi, G.; Patra, A.S.; Ansari, M.S.; Qureshi, M. Effect of surface overlayer in enhancing the photoelectrochemical water oxidation of in-situ grown one dimensional spinel zinc ferrite nanorods directly onto the substrate. Chem. Commun. 2018, 54, 10483–10486. [Google Scholar] [CrossRef]
- Guijarro, N.; Bornoz, P.; Prévot, M.; Yu, X.; Zhu, X.; Johnson, M.; Jeanbourquin, X.; Formal, F.L.; Sivula, K. Evaluating spinel ferrites MFe2O4 (M = Cu, Mg, Zn) as photoanodes for solar water oxidation: Prospects and limitations. Sustain. Energy Fuels 2018, 2, 103–117. [Google Scholar] [CrossRef]
- Zhu, X.; Guijarro, N.; Liu, Y.; Schouwink, P.; Wells, R.A.; Formal, F.L.; Sun, S.; Gao, C.; Sivula, K. Spinel structural disorder influences solar-water-splitting performance of ZnFe2O4 nanorod photoanodes. Adv. Mater. 2018, 30, 1801612. [Google Scholar] [CrossRef]
- Zhang, D.; Li, W.; Ye, R.; Guo, X.; Wang, S.; Wang, X.; Xiang, Q. A facile strategy for ZnFe2O4 coating preparing by electrophoretic deposition and its supercapacitor performances. J. Mater. Sci. Mater. Electron. 2018, 29, 5454–5458. [Google Scholar] [CrossRef]
- Israr, M.; Iqbal, J.; Arshad, A.; Aisida, S.O.; Ahmad, I. A unique ZnFe2O4/graphene nanoplatelets nanocomposite for electrochemical energy storage and efficient visible light driven catalysis for the degradation of organic noxious in wastewater. J. Phys. Chem. Solids 2020, 140, 109333. [Google Scholar] [CrossRef]
- Javed, M.S.; Jiang, Z.; Yang, Q.; Wang, X.; Han, X.; Zhang, C.; Gu, X.; Hu, C. Exploring Li-ion hopping behaviour in zinc ferrite and promoting performance for flexible solid-state supercapacitor. Electrochem. Acta 2019, 295, 558–568. [Google Scholar] [CrossRef]
- Vadiyar, M.M.; Kolekar, S.S.; Chang, J.Y.; Ye, Z.; Ghule, A.V. Anchoring ultrafine ZnFe2O4/C nanoparticles on 3D ZnFe2O4 nanoflakes for boosting cycle stability and energy density of flexible asymmetric supercapacitor. ACS Appl. Mater. Interfaces 2017, 9, 26016–26028. [Google Scholar] [CrossRef] [PubMed]

 x = 0.104;
x = 0.104;  x = 0.134;
x = 0.134;  x = 0.159;
x = 0.159;  x = 0.203).
x = 0.203).
 x = 0.104;
x = 0.104;  x = 0.134;
x = 0.134;  x = 0.159;
x = 0.159;  x = 0.203).
x = 0.203).


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bohra, M.; Alman, V.; Arras, R. Nanostructured ZnFe2O4: An Exotic Energy Material. Nanomaterials 2021, 11, 1286. https://doi.org/10.3390/nano11051286
Bohra M, Alman V, Arras R. Nanostructured ZnFe2O4: An Exotic Energy Material. Nanomaterials. 2021; 11(5):1286. https://doi.org/10.3390/nano11051286
Chicago/Turabian StyleBohra, Murtaza, Vidya Alman, and Rémi Arras. 2021. "Nanostructured ZnFe2O4: An Exotic Energy Material" Nanomaterials 11, no. 5: 1286. https://doi.org/10.3390/nano11051286
APA StyleBohra, M., Alman, V., & Arras, R. (2021). Nanostructured ZnFe2O4: An Exotic Energy Material. Nanomaterials, 11(5), 1286. https://doi.org/10.3390/nano11051286






