Abstract
Supported Au nanoparticles on TiO2 (1 mol%) are capable of catalyzing the reduction of the carbene-like diazo functionality of α-diazocarbonyl compounds into a methylene group [C=(N2) → CH2] by NH3BH3 or NaBH4 in methanol as solvent. The Au-catalyzed reduction that occurs within a few minutes at room temperature formally requires one hydride equivalent (B-H) and one proton that originates from the protic solvent. This pathway is in contrast to the Pt/CeO2-catalyzed reaction of α-diazocarbonyl compounds with NH3BH3 in methanol, which leads to the corresponding hydrazones instead. Under our stoichiometric Au-catalyzed reaction conditions, the ketone-type carbonyls remain intact, which is in contrast to the uncatalyzed conditions where they are selectively reduced by the boron hydride reagent. It is proposed that the transformation occurs via the formation of chemisorbed carbenes on Au nanoparticles, having proximally activated the boron hydride reagent. This protocol is the first general example of catalytic transfer hydrogenation of the carbene-like α -ketodiazo functionality.
1. Introduction
The reduction of the carbene-like diazo functionality into a methylene group [C=(N2) → CH2] in α-diazocarbonyl compounds is not a well-studied transformation in the past, and there are scattered examples in the literature. Decades ago, the Wolfrom method [1] was reported, which employs hydriodic acid as the reducing agent and then a protocol involving catalytic amounts of diethyl peroxydicarbonate in isopropanol [2]. The interesting transformation of α-diazo-β-hydroxy esters into β-keto esters [3,4] catalyzed by Rh2(OAc)4 could be seen as a relevant example and apparently involves an intramolecular transfer hydrogenation. Bu3SnH has been also used as reducing agent with Cu(acac)2 as catalyst under irradiation [5], as well as a series of early transition metallocenes in the presence of H2O [6]. Finally, the relevant to this concept Pd/C-catalyzed hydrogenation of a specific type of diazo compounds (α-diazo-β-hydroxy esters) [7] appears as a practical reductive method providing β-hydroxy esters in moderate to good yields.
Recently, we presented the first example of Au-catalyzed hydrosilylation of α-diazocarbonyl compounds using recyclable and reusable Au nanoparticles on TiO2 as the active catalytic sites (Scheme 1) [8]. This reaction proceeds in short reaction times in anhydrous 1,2-dichloroethane (DCE) affording α-silyl carbonyl compounds in high yields. It is most likely that this transformation involves not only the activation of the diazo compounds on Au NPs forming chemisorbed Au-carbenes [9] but proximally chemisorbed hydrosilanes as well. The only side product of the hydrosilylation reaction is the replacement of the diazo group by two hydrogen atoms [C=(N2) → CH2]. This side pathway depends on the amount of moisture in the solvent and apparently arises from the chemisorbed H species on the Au nanoparticle. This observation initiated our efforts to develop a new methodology for the reduction of the diazo functionality of α-diazocarbonyl compounds into a methylene group. When we contacted the Au/TiO2-catalyzed hydrosilylation of diazo compound 1a in DCE in the additional presence of H2O (0.5 equivalent), the product selectivity altered, affording the reduced ester 3a in 72% relative yield versus 28% of the anticipated hydrosilylation product 2a. Although the relative yield of 3a can be further increased by increasing the equivalents of water added into the reaction mixture, at the same time, a high excess of hydrosilane should be used, as hydrosilanes react with H2O (a reaction that is also catalyzed by Au/TiO2). Thus, we sought for an alternative and cheaper reducing agent to replace hydrosilane and establish a more practical methodology to achieve this reductive transformation.
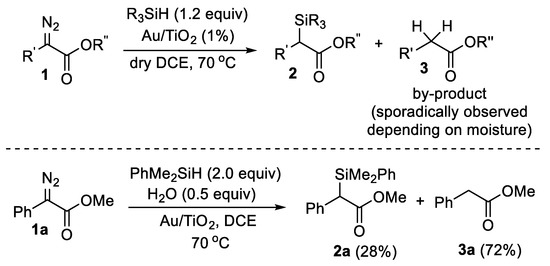
Scheme 1.
Hydrosilylation of α-diazo carbonyl compounds catalyzed by Au/TiO2; reversal of chemoselectivity of the attempted hydrosilylation of diazo compound 1a in the presence of 0.5 equivalents of H2O.
Over the past two decades, supported Au nanoparticles (Au NPs) and other nano Au(0) materials have attracted significant attention due to their surprising ability to catalyze a plethora of organic transformations, despite the metallic character of Au atoms on nanoparticles [10,11,12,13], including transfer hydrogenation reductive processes [14,15]. Given the heterogeneous nature of Au NP-catalyzed processes and that they can be easily recycled and reused on the vast majority of the reported protocols, they can be considered as benign green catalysts.
2. Results
At the outset, we investigated the possible Au/TiO2-catalyzed reduction of the model compound 1a into the desired product 3a, using the readily available boron hydride reagents NH3BH3 and NaBH4. Ammonia borane complex [16,17] as well as sodium borohydride [18] have been used efficiently in the past for the reduction of alkynes or nitro compounds using Au/TiO2 as catalyst. While no reaction occurs in a series of dry aprotic solvents (dichloromethane, diethyl ether, hexane, or benzene), to our pleasure, we found that the treatment of 1a with NH3BH3 (0.4 molar equivalent) or NaBH4 (0.3 molar equivalent) in MeOH for 15 min at room temperature resulted in the quantitative formation of the reduced product 3a (Scheme 2). In the absence of Au/TiO2 or in the presence of the support itself (TiO2, routile, or anatase), no transformation took place even after prolonged reaction times (12 h) at 50 °C, which is indicative that Au NPs are the catalytic sites.
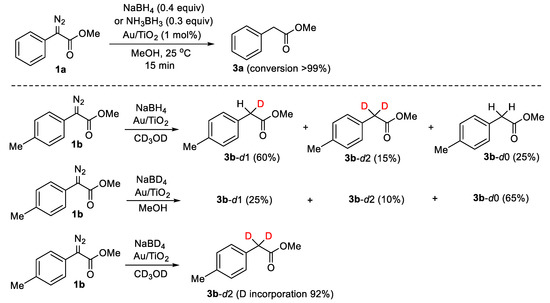
Scheme 2.
Reduction of α-diazocarbonyl compound 1a by boron hydride reagents in methanol catalyzed by Au/TiO2, and D-labeling experiments in the reduction of compound 1b.
The amounts of boron hydride reagents that are necessary to achieve a quantitative reduction indicate that one hydride equivalent from either NH3BH3 or NaBH4 is required; thus, the second hydrogen atom on the product arises from the protic solvent (methanol). To test this hypothesis, we performed the reduction of diazo compound 1b with NaBH4 in methanol-d4 (Scheme 2). Analysis of the reaction mixture revealed the presence of D atoms in the products. The monodeuterated 3b-d1 was formed in 60% relative yield, along with dideuterated 3b-d2 (15%) and non-deuterated 3b-d0 (25%). The incorporation of H atoms from protic solvents in the Au/TiO2-catalyzed semireduction of alkynes using NH3BH3 has been already shown by our group in the past [16]. An analogous H/D scrabbling pattern in products has been reported in the Pd-catalyzed hydrogenation of N-tosylhydrazones in MeOD [19]. However, a different product distribution was seen in the reduction of 1b with NaBD4 (98% D) in methanol-d0 (Scheme 2). Under these conditions, the fully protonated product 3b-d0 was the major one (65% relative yield), along with 3b-d1 (25%) and 3b-d2 (10%). Finally, the reduction of 1b with NaBD4 in methanol-d4 afforded product 3a-d2 with 92% deuterium incorporation. The incomplete deuteration in the latter experiment is attributed to traces of moisture present in the reaction medium.
These observations support our consideration regarding the origins of the two hydrogen atoms: one hydride from NaBH4 or NH3BH3 and one proton from MeOH. In the accompanying mechanistic analysis, we will comment on the differences in the product distribution between 3b-d2, 3b-d1, and 3b-d0, upon replacing NaBH4 with NaBD4 or methanol-d0 with methanol-d4.
The observed result is outstanding, given that the reduction is very fast, occurs at room temperature with low loading of the catalyst, and the amount of the reducing agent is stoichiometric in terms of hydride equivalents. Thus, for instance, one molar equivalent of NH3BH3 can formally achieve the reduction of three molar equivalents of the α-diazocarbonyl compound. The catalyst can be recycled at the end of each reaction by simple filtration and then reused after being washed with dichloromethane and dried in the oven at 90 °C for 2 h. We were able to perform five consecutive catalytic runs with the same batch of catalyst without observing any obvious deterioration in terms of yields. We emphasize herein the unique nature of our catalytic system, as it known [20] that in the analogous treatment of α-diazocarbonyl compounds with three molar equivalents of NH3BH3 under single-atom platinum on ceria catalysis conditions (Pt1/CeO2), the corresponding hydrazones are formed instead, as seen in Scheme 3.
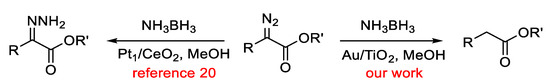
Scheme 3.
The reaction of α-diazocarbonyl compounds with NH3BH3 in methanol under different catalytic conditions.
Subsequently, we examined the reduction of a series of α-diazocarbonyl compounds. The results are summarized in Figure 1. In all cases, the reaction proceeds smoothly within 15 min, the transformation is quantitative, and the isolated yields are very high, as the products do not essentially require any chromatographic purification in the majority of the cases. Noteworthily, the transformation is highly chemoselective given that the reduction of the generated Au-carbenes is faster than that of the ketone moiety. Thus, the reaction of α-diazo ketones 1j–l with 0.35–0.4 molar equivalents of NH3BH3 or with 0.25–0.30 molar equivalents of NaBH4 solely provided ketones 3j–l, respectively, leaving the ketone moiety intact. In the case of using excess of reducing agent (≈2.5 hydride equivalents), both the diazo and the ketone functionalities are reduced quantitatively to the corresponding alcohols. In the literature, it is well established that in the reduction of α-diazocarbonyl compounds with NaBH4, having an extra ketone carbonyl functionality, the diazo group remains intact [21]. The same holds in their reduction using biocatalytic conditions (ketoreductases, NADPH [22]). Notably, we found that in the absence of Au/TiO2, the reaction of 1l with NH3BH3 or NaBH4 yields a complex mixture of products, and this is quite reasonable given the expected instability of the anticipated α-diazo alcohol. Additionally, while the results shown in Figure 1 were done at 0.2–0.3 mmol scale of the α-diazocarbonyl compounds, we performed the reduction of compound 1a in a 3 mmol scale at 0 °C, producing the methylene product 3a in almost quantitative yield within 15 min, which shows that our protocol is operational for larger-scale experiments.

Figure 1.
Reduction of α-diazocarbonyl compounds by NH3BH3 or NaBH4 catalyzed by Au/TiO2. a With one additional B-H equivalent, the corresponding alcohols of the ketones 3j–l were isolated in >90% yield. b In this case, the corresponding hydrazone (Ε/Ζ = 85/15) was formed in 15% relative yield using NH3BH3 and in 25% with NaBH4. The isolated yield refers to the reaction with NH3BH3.
As noted earlier in Scheme 3, hydrazones are formed exclusively in the Pt1/CeO2-catalyzed reaction of α-diazocarbonyl compounds using three molar equivalents of ammonia borane in MeOH [20]. We emphasize that in case of using an excess of reducing agent in our Au/TiO2-catalyzed transformation, the corresponding hydrazones were seen as minor products. For instance, in the reduction of substrate 1a, we observed the following. Using 0.5–0.6 molar equivalents of NaBH4 (≈100% excess in terms of hydride equivalents), hydrazone 4a [20] was observed in 8% yield relative to product 3a. With 1.2 molar equivalents, 4a is formed in 25% relative yield and with three molar equivalents in 38% (Scheme 4). On the other hand, using excess of NH3BH3, the reaction is more selective, with byproduct 4a being formed in merely 3% relative yield if using 1.0 molar equivalent of ammonia borane and 5% relative yield with 3.0 molar equivalents. The only substrate that exhibited an obviously higher selectivity in terms of hydrazone formation was the α-diazocarbonyl compound 1i. The treatment of 1i with one hydride equivalent from NH3BH3 or NaBH4 resulted in the partial formation of the corresponding hydrazone 4i [23] (E/Z = 85/15, see Supplementary Materials) in 15% and 25% relative yield, respectively. Hydrazone 4a was isolated, and we found that it does not lead to product 3a either upon treatment with 1 mol% Au/TiO2 under conditions identical to our protocol reaction or in the presence of the reductants (NH3BH3 or NaBH4) again in the presence of Au/TiO2 (MeOH, 15 min, 25 °C), and it is recovered intact (Scheme 4). Therefore, it is not a reaction intermediate. The same was observed with hydrazone 4i. We comment on the origins of formation of the hydrazone side products in the accompanying mechanistic discussion and in Scheme 5.
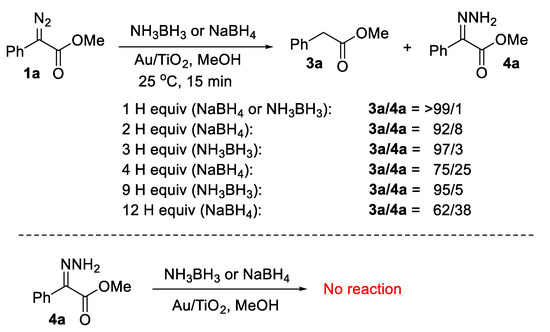
Scheme 4.
Au/TiO2-catalyzed reaction of 1a with excess of NaBH4 or NH3BH3, and a proof that hydrazones are not reaction intermediates.
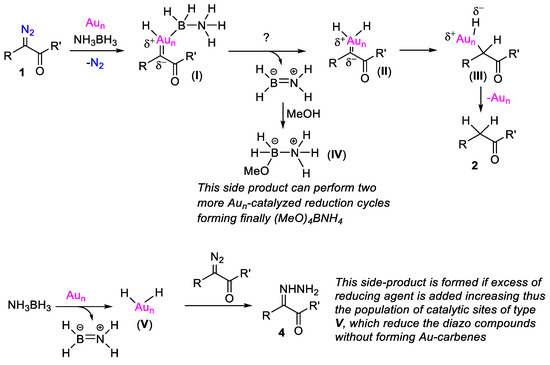
Scheme 5.
Mechanistic considerations in the reduction of α-diazocarbonyl compounds with NH3BH3 catalyzed by Au/TiO2 (the catalytic sites of nanoparticles are indicated as Aun).
The possible mechanistic scenario of the reductive process is presented in Scheme 5. Based on previous studies regarding the Au/TiO2-catalyzed reaction of α-diazocarbonyl compounds with hydrosilanes where Au-carbenes were invoked as intermediates [8], and the Au/TiO2-catalyzed semihydrogenation of alkynes with NH3BH3 [16] where Au-hydrides are the reactive species, we envision the formation of intermediate I, which can be described as a Schrock-type carbene [24] having chemisorbed on the electrophilic Au nanoparticle, which is a molecule of NH3BH3 on the same catalytic site. The elimination of NH2BH2 from I generates Au-dihydride intermediate II, which delivers the two H atoms on the former carbene carbon atom presumably in a stepwise manner (via intermediate III), thus leading to the final reduction product, with its concomitant liberation from the surface of nanoparticle Aun. The NH2BH2 reacts with methanol, providing the methoxy-bearing amine borane adduct IV shown in Scheme 5, which possesses even more reactive hydrides relative to NH3BH3. Amine borane IV reduces a second molecule of α-diazocarbonyl compound through a similar catalytic cycle on Aun, and it is transformed into NH2BH(OMe)2, which finally undergoes a third Aun-catalyzed reduction round. The final fate of ammonia borane is its transformation into NH4B(OMe)4 [25], which is proved by 11B NMR (see Supplementary Materials). Similarly, the final fate of NaBH4 is the formation of the analogous salt NaB(OMe)4, which resonates at about 3 ppm [26] in 11B NMR (see Supplementary Materials). If an excess of reducing agent is added, then the population of Au nanoparticle dihydro sites (intermediate V) increases, and subsequently, these sites can reduce the α-diazocarbonyl compounds through a side pathway that does not require their chemisorption on an Au nanoparticle. This pathway is similar to what was suggested in the Pt1/CeO2-catalyzed reduction of the α-diazocarbonyl compounds with NH3BH3 [20].
The D-labeling experiments presented in Scheme 2 can be rationalized, taking into account the proposed mechanism. Thus, in the reduction of 1b with NaBH4 in methanol-d4, the major product is 3b-d1 having one H from a B-H bond and one D from the O-D functionality of the solvent. The observation in parallel of the disproportionation products 3b-d2 and 3b-d0 implies that boron hydrides are partially exchanged by D atoms from the O-D functionality of the protic solvent under the reaction conditions; likewise, silicon-hydrides do in the Au/TiO2-catalyzed reduction of quinolines [27]. Notably, in analogous metal-catalyzed labeling experiments such as in the semihydrogenation of alkynes employing R3SiH(D) [28] or NaBH4(D4) [25], and MeOH(D) or H2(D2)O as the proton sources, no analogous isotopic scrabbling is observed. The H/D exchange of boron hydrides is very obvious in the reduction of 1b with NaBD4 in CH3OH, where the fully protonated 3b-d0 is the major product, while 3b-d0 and 3b-d2 are the minor ones. The differences in product distribution among these two labeling experiments are rather related to the different degree of Au/TiO2-catalyzed H/D exchange processes of boron-H(D) reagents from the solvent [MeOH or MeOH-d4], arising from intrinsic isotope effects.
3. Experimental Section
3.1. Catalyst
The supported Au nanoparticles on TiO2 having a gold content ≈1 wt%, which were used in our studies, are commercially available from Strem Chemicals Inc. (Newburyport, MA, USA) The average crystallite size of Au nanoparticles is ≈2–3 nm. Prior to use, they were grinded in a mortar, and the resulting purple dust was kept in a dark-colored bottle.
3.2. Reactants
The majority of α-diazocarbonyl compounds used in this study were available from previous studies in our lab [8]. The rest of them were prepared by α-diazotization of the corresponding carbonyl compounds with 4-acetamidobenzenesulfonyl azide in the presence of DBU as base [29]. Copies of the 1H and 13C NMR spectra of the α-diazocarbonyl compounds can be found in the Supplementary Materials.
3.3. Catalytic Reactions
Typical procedure of the title reaction: In a vial are placed 0.3 mmol of the α-diazocarbonyl compound, 0.09 mmol (3.5 mg) of NaBH4 (0.3 equiv) or 0.12 mmol (3.7 mg) of NH3BH3 (0.4 equiv), and 70 mg of Au/TiO2 (1.0 mol% in Au, as the catalytic system contains 1% Au w/w) in 0.5 mL of methanol. The heterogeneous mixture is stirred for 15 min at room temperature and within that period occurs complete consumption of the diazo compound (the supernatant reaction mixture turns to colorless). Then, the slurry is filtered with the aid of 2 mL of dichloromethane or any other volatile solvent such as diethyl ether under a low pressure through a short pad of silica gel, and the filtrate is evaporated. No chromatographic purification of the products is required in most of the cases, as the reactions are quantitative and yield only one product.
3.4. Characterization of Products
Methyl 2-phenylacetate (3a). 1H NMR (500 MHz, CDCl3): 7.35–7.27 (m, 5H), 3.70 (s, 3H), 3.64 (s, 2H); 13C NMR (125 MHz, CDCl3): 172.0, 133.9, 129.2, 128.6, 127.1, 52.0, 41.2.
Methyl 2-(p-tolyl)acetate (3b). 1H NMR (500 MHz, CDCl3): 7.18 (d, J = 8.0 Hz, 2H), 7.13 (d, J = 8.0 Hz, 2H), 3.68 (s, 3H), 3.59 (s, 2H), 3.33 (s, 3H); 13C NMR (125 MHz, CDCl3): 172.2, 136.7, 130.9, 129.2, 129.0, 51.9, 40.7, 21.0.
Methyl 2-(4-methoxyphenyl)acetate (3c). 1H NMR (500 MHz, CDCl3): 7.20 (d, J = 8.5 Hz, 2H), 6.86 (d, J = 8.5 Hz, 2H), 3.79 (s, 3H), 3.69 (s, 3H), 3.57 (s, 2H); 13C NMR (125 MHz, CDCl3): 172.3, 158.6, 130.2, 126.0, 113.9, 55.1, 51.9, 40.2.
Methyl 2-(3-methoxyphenyl)acetate (3d). 1H NMR (500 MHz, CDCl3): 7.24 (t, J = 8.0 Hz, 1H), 6.88–6.81 (m, 3H), 3.80 (s, 3H), 3.70 (s, 3H), 3.61 (s, 2H); 13C NMR (125 MHz, CDCl3): 171.8, 159.6, 135.3, 129.5, 121.5, 114.8, 112.5, 55.1, 52.0, 41.1.
Methyl 2-(4-chlorophenyl)acetate (3e). 1H NMR (500 MHz, CDCl3): 7.29 (d, J = 8.5 Hz, 2H), 7.21 (d, J = 8.5 Hz, 2H), 3.69 (s, 3H), 3.60 (s, 2H); 13C NMR (125 MHz, CDCl3): 171.6, 133.1, 132.3, 130.6, 128.7, 52.1, 40.4.
Methyl 2-(4-fluorophenyl)acetate (3f). 1H NMR (500 MHz, CDCl3): 7.26–7.23 (m, 2H), 7.01 (t, J = 8.5 Hz, 2H), 3.70 (s, 3H), 3.60 (s, 2H); 13C NMR (125 MHz, CDCl3): 171.9, 161.9 (d, JC-F = 243.5 Hz), 130.8 (d, JC-F = 8.0 Hz), 129.6 (d, JC-F = 3.5 Hz), 115.4 (d, JC-F = 21.5 Hz), 52.1, 40.2.
Allyl 2-phenylacetate (3g). 1H NMR (500 MHz, CDCl3): 7.35–7.28 (m, 5H), 5.95–5.87 (m, 1H), 5.28 (qd, J1 = 17.5 Hz, J2 = 1.5 Hz, 1H), 5.22 (qd, J1 = 10.5 Hz, J2 = 1.5 Hz, 1H), 4.61 (td, J1 = 6.0 Hz, J2 = 1.5 Hz, 2H), 3.66 (s, 2H); 13C NMR (125 MHz, CDCl3): 171.2, 133.9, 132.0, 129.2, 128.5, 127.1, 118.2, 65.4, 41.3.
(1R,2S,5R)-2-Isopropyl-5-methylcyclohexyl 2-phenylacetate (3h). 1H NMR (500 MHz, CDCl3): 7.34–7.24 (m, 5H), 4.71–4.66 (m, 1H), 3.61 (d, J = 16.0 Hz, 1H), 3.60 (d, J = 16.0 Hz, 1H), 1.99–1.96 (m, 1H), (1.77–1.71, m, 1H), 1.70–1.63 (m, 2H), 1.51–1.43 (m, 1H), 1.39–1.33 (m, 1H), 1.08–0.97 (m, 2H), 0.90 (d, J = 7.0 Hz, 3H), 0.84 (d, J = 7.0 Hz, 3H), 0.79 (d, J = 7.0 Hz, 3H); 13C NMR (125 MHz, CDCl3): 171.1, 134.3, 129.1, 128.4, 126.9, 74.6, 47.0, 41.8, 40.7, 34.2, 31.3, 26.1, 23.4, 22.0, 20.7, 16.2.
Ethyl 3-phenylpropanoate (3i). 1H NMR (500 MHz, CDCl3): 7.30–7.19 (m, 5H), 4.13 (q, J = 7.0 Hz, 2H), 2.96 (t, J = 7.5 Hz, 2H), 2.62 (t, J = 7.5 Hz, 2H), 1.24 (t, J = 7.0 Hz, 3H); 13C NMR (125 MHz, CDCl3): 172.9, 140.6, 128.5, 128.3, 126.2 60.4, 35.9, 31.0, 14.2.
1-Phenylpropan-2-one (3j). 1H NMR (500 MHz, CDCl3): 7.34 (t, J = 7.5 Hz, 2H), 7.28 (t, J = 7.5 Hz, 1H), 7.21 (d, J = 7.5 Hz, 2H), 3.70 (s, 2H), 2.16 (s, 3H); 13C NMR (125 MHz, CDCl3): 206.4, 134.2, 129.4, 128.8, 127.1, 51.0, 29.3.
1-(4-Chlorophenyl)propan-2-one (3k). 1H NMR (500 MHz, CDCl3): 7.30 (t, J = 8.5 Hz, 2H), 7.12 (t, J = 8.5 Hz, 2H), 3.67 (s, 2H), 2.16 (s, 3H); 13C NMR (125 MHz, CDCl3): 205.6, 133.0, 132.5, 130.7, 128.8, 50.0, 29.4.
Propiophenone (3l). 1H NMR (500 MHz, CDCl3): 7.97 (d, J = 8.5 Hz, 2H), 7.55 (t, J = 7.5 Hz, 1H), 7.46 (d, J = 7.5 Hz, 2H), 3.02 (q, J = 7.5 Hz, 2H), 1.24 (t, J = 7.5 Hz, 3H); 13C NMR (125 MHz, CDCl3): 200.8, 136.9, 132.9, 128.5, 128.0, 31.8, 8.2.
Methyl (E)-2-hydrazono-2-phenylacetate (4a). 1H NMR (500 MHz, CDCl3): 7.51–7.29 (m, 5H), 7.55 (t, J = 7.5 Hz, 1H), 6.28 (br s, 2H), 3.84 (s, 3H); 13C NMR (125 MHz, CDCl3): 164.8, 137.3, 129.4, 129.4, 129.2, 128.8, 52.4.
Ethyl 2-hydrazono-3-phenylpropanoate (4i). 1H NMR (500 MHz, CDCl3), E-isomer (major): 7.32–7.20 (m, 5H), 6.04 (br s, 2H), 4.33 (q, J = 7.0 Hz, 2H), 3,90 (s, 2H), 1.37 (t, J = 7.0 Hz, 3H). Z-isomer (minor): 8.16 (br s, 2H), 7.32–7.20 (m, 5H), 4.14 (q, J = 7.0 Hz, 2H), 3,69 (s, 2H), 1.22 (t, J = 7.0 Hz, 3H); 13C NMR (125 MHz, CDCl3), E-isomer (major): 165.2, 138.1, 134.8, 129.0, 128.0, 126.9, 61.5, 30.5, 14.3. Z-isomer (minor): 162.7, 139.2, 130.4, 128.8, 128.1, 126.1, 60.2, 39.5, 14.0.
Copies of the 1H and 13C NMR spectra of all products can be found in the Supplementary Materials.
4. Conclusions
In summary, we presented a novel and useful transfer hydrogenation methodology for the reduction of the carbene moiety of α-diazocarbonyl compounds into a methylene group catalyzed by recyclable and reusable supported Au nanoparticles on TiO2. The reductant can be readily available NH3BH3 or NaBH4. The characteristic of the reduction protocol is that one stoichiometric hydride equivalent from the boron hydride reagent and one proton that arises from the protic solvent (methanol) are required. In case of using an excess of reducing agent, a side catalytic pathway operates that reduces the α-diazocarbonyl compounds into the corresponding hydrazones. This work exemplifies the unique catalytic behavior of Au nanoparticles relative to other supported metallic noble metals such as Pt [20], where the reductive pathway of the formation of hydrazones occurs exclusively.
Supplementary Materials
Copies of 1H and 13C NMR of reactants and products are available online at https://www.mdpi.com/2079-4991/11/1/248/s1.
Author Contributions
M.K. performed all the experiments and prepared the manuscript. M.S. supervised this work and reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The research work was supported by the Hellenic Foundation for Research and Innovation (HFRI) and the General Secretariat for Research and Technology (GSRT), under the HFRI PhD Fellowship grant (GA No. 31449).
Data Availability Statement
Data is contained within the article or Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References and Note
- Wolfrom, M.L.; Brown, R.L. The action of diazomethane upon acyclic sugar derivatives. V. Halogen derivatives. J. Am. Chem. Soc. 1943, 65, 1516–1521. [Google Scholar] [CrossRef]
- Horner, L.; Schwarz, H. Die durch radicale ausgelgste “reductive eliminierung von diazostickstoff” aus diazocarbonyl und diazosulfonylvebbindungen. Tetrahedron Lett. 1966, 30, 3579–3583. [Google Scholar] [CrossRef]
- Pellicciari, R.; Fringuelli, R.; Ceccherelli, P.; Sisani, E. β-Keto esters from the rhodium(II) acetate catalysed conversion of α-diazo-β-hydroxy esters. J. Chem. Soc. Chem. Commun. 1979, 959–960. [Google Scholar] [CrossRef]
- Ye, T.; McKewey, M.A. Synthesis of chiral N-protected α-amino-β-diketones from α-diazoketones derived from natural amino acids. Tetrahedron 1992, 37, 8007–8022. [Google Scholar] [CrossRef]
- Tan, Z.; Qu, Z.; Chen, B.; Wang, J. Diazo decomposition in the presence of tributyltin hydride. Reduction of α-diazo carbonyl compounds. Tetrahedron 2000, 56, 7457–7461. [Google Scholar] [CrossRef]
- Schobert, R.; Hohlein, U. Reduction and isomerization of oxiranes and α-diazoketones by various early transition metallocenes. Synlett 1990, 8, 465–466. [Google Scholar] [CrossRef]
- Pellicciari, R.; Natalini, B.; Cecchetti, S.; Fringuelli, R. Reduction of α-diazo-β-hydroxy esters to β-hydroxy esters: Application in one of two convergent syntheses of a (22S)-22-hydroxy bile acid from fish bile and its (22R)-epimer. J. Chem. Soc. Perkin Trans. I 1985, 493–497. [Google Scholar] [CrossRef]
- Kidonakis, M.; Stratakis, M. Au nanoparticle-catalyzed insertion of carbenes from α-diazocarbonyl compounds into hydrosilanes. Org. Lett. 2018, 20, 4086–4089. [Google Scholar] [CrossRef]
- Oliver-Meseguer, J.; Boronat, M.; Vidal-Moya, A.; Concepcion, P.; Rivero-Crespo, M.A.; Leyva-Perez, A.; Corma, A. Generation and reactivity of electron-rich carbenes on the surface of catalytic gold nanoparticles. J. Am. Chem. Soc. 2018, 140, 3215–3218. [Google Scholar] [CrossRef]
- Stratakis, M.; Garcia, H. Catalysis by supported gold nanoparticles: Beyond aerobic oxidative processes. Chem. Rev. 2012, 112, 4469–4506. [Google Scholar] [CrossRef]
- Stratakis, M.; Lykakis, I.N. Nanogold(0)-catalyzed addition of heteroelement σ linkages to functional groups. Synthesis 2019, 51, 2435–2454. [Google Scholar] [CrossRef]
- Takale, B.S.; Bao, M.; Yamamoto, Y. Gold nanoparticle (AuNPs) and gold nanopore (AuNPore) catalysts in organic synthesis. Org. Biomol. Chem. 2014, 12, 2005–2027. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Terada, M.; Bao, M.; Yamamoto, Y. Catalytic performance of nanoporous metal skeleton catalysts for molecular transformations. ChemSusChem 2019, 12, 2936–2954. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Ge, L.; Yuan, H.; Liu, Y.; Gui, Y.; Zhang, B.; Zhou, L.; Fang, S. Heterogeneous gold catalysts for selective hydrogenation: From nanoparticles to atomically precise nanoclusters. Nanoscale 2019, 11, 11429–11436. [Google Scholar] [CrossRef]
- Mitsudome, T.; Kaneda, K. Gold nanoparticle catalysts for selective hydrogenations. Green Chem. 2013, 15, 2636–2654. [Google Scholar] [CrossRef]
- Vasilikogiannaki, E.; Titilas, I.; Vassilikogiannakis, G.; Stratakis, M. cis-Semihydrogenation of alkynes with amine borane complexes catalyzed by gold nanoparticles under mild conditions. Chem. Commun. 2015, 51, 2384–2387. [Google Scholar] [CrossRef]
- Vasilikogiannaki, E.; Gryparis, C.; Kotzabasaki, V.; Lykakis, I.N.; Stratakis, M. Activation of ammonia-borane complex by gold nanoparticles: Facile reduction of nitroarenes into anilines and nitroalkanes into hydroxylamines. Adv. Synth. Catal. 2013, 355, 907–911. [Google Scholar] [CrossRef]
- Fountoulaki, S.; Daikopoulou, V.; Gkizis, P.L.; Tamiolakis, I.; Armatas, G.S.; Lykakis, I.N. Mechanistic studies of the reduction of nitroarenes by NaBH4 or hydrosilanes catalyzed by supported gold nanoparticles. ACS Catal. 2014, 4, 3504–3511. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, Z.; Liu, Y.; Zhang, Y.; Wang, J. N-Tosylhydrazine-mediated deoxygenative hydrogenation of aldehydes and ketones catalyzed by Pd/C. Tetrahedron 2013, 69, 6083–6087. [Google Scholar] [CrossRef]
- Liu, C.; Chen, Z.; Yan, H.; Xi, S.; Yam, K.M.; Gao, J.; Du, Y.; Li, J.; Zhao, X.; Xie, K.; et al. Expedient synthesis of E-hydrazone esters and 1H-indazole scaffolds through heterogeneous single-atom platinum catalysis. Sci. Adv. 2019, 5, eaay1537. [Google Scholar] [CrossRef]
- Gonzalez-Granda, S.; Costin, T.A.; Sa, M.M.; Gotor-Fernandez, V. Stereoselective bioreduction of α-diazo-β-keto esters. Molecules 2020, 25, 931. [Google Scholar] [CrossRef] [PubMed]
- Mittmann, E.; Hu, Y.; Peschke, T.; Rabe, K.S.; Niemeyer, C.M.; Brase, S. Chemoenzymatic synthesis of O-containing heterocycles from α-diazo esters. ChemCatChem 2019, 11, 5519–5523. [Google Scholar] [CrossRef]
- Yasui, E.; Wada, M.; Nagumo, S.; Takamura, N. A novel method for the synthesis of 3,4-disubstituted pyrrole-2,5-dicarboxylates from hydrazones derived from α-diazo esters. Tetrahedron 2013, 69, 4325–4330. [Google Scholar] [CrossRef]
- In contrast to the Fischer metal-carbenes where the carbon atom that is bonded to the metal is electrophilic, in Schrock-type carbenes the carbon atom is nucleophilic. Chemisorbed α-diazocarbonyl compounds on Au nanoparticles are Schrock-type carbenes (see references 8 and 9).
- Ramachandran, P.V.; Gagare, P.D. Preparation of ammonia borane in high yield and purity, methanolysis, and regeneration. Inorg. Chem. 2007, 46, 7810–7817. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Shi, X.; Qiao, X.; Wu, Z.; Bai, G. Ligand-free nickel-catalyzed semihydrogenation of alkynes with sodium borohydride: A highly efficient and selective process for cis-alkenes under ambient conditions. Chem. Commun. 2017, 53, 5372–5375. [Google Scholar] [CrossRef] [PubMed]
- Louka, A.; Gryparis, C.; Stratakis, M. Reduction of quinolines to 1,2,3,4-tetrahydroquinolines with hydrosilane/ethanol catalyzed by TiO2-supported gold nanoparticles under solvent free conditions. Arkivoc 2015, 2015, 38–51. [Google Scholar] [CrossRef]
- Yan, M.; Jin, T.; Ishikawa, Y.; Minato, T.; Fujita, T.; Chen, L.-Y.; Bao, M.; Asao, N.; Chen, M.-W.; Yamamoto, Y. Nanoporous gold catalyst for highly selective semihydrogenation of alkynes: Remarkable effect of amine additives. J. Am. Chem. Soc. 2012, 134, 17536–17542. [Google Scholar] [CrossRef]
- Keipour, H.; Jalba, A.; Delage-Laurin, L.; Ollevier, T. Copper-catalyzed carbenoid insertion reactions of α-diazoesters and α-diazoketones into Si−H and S−H Bonds. J. Org. Chem. 2017, 82, 3000–3010. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).