Abstract
Solar-to-electricity energy conversion and large scale electricity storage technologies are key to achieve a sustainable development of society. For energy conversion, photoelectrochemical solar cells were proposed as an economic alternative to the conventional Si-based technology. For energy storage, metal-ion batteries are a very promising technology. Titania (TiO2) based anodes are widely used in photoelectrochemical cells and have recently emerged as safe, high-rate anodes for metal-ion batteries. In both applications, titania interacts with electrolyte species: molecules and metal ions. Details of this interaction determine the performance of the electrode in both technologies, but no unified theoretical description exists, e.g., there is no systematic description of the effects of Li, Na insertion into TiO2 on solar cell performance (while it is widely studied in battery research) and no description of effects of surface adsorbents on the performance of battery anodes (while they are widely studied in solar cell research). In fact, there is no systematic description of interactions of electrolyte species with TiO2 of different phases and morphologies. We propose a computation-focused study that will bridge the two fields that have heretofore largely been developing in parallel and will identify improved anode materials for both photoelectrochemical solar cells and metal-ion batteries.
1. Introduction
Solar-to-electricity energy conversion technologies are key to achieve a sustainable development of society. To fully utilize the potential of solar and other intermittent sources of energy (e.g., wind, waves), development of electrical energy storage technologies is necessary [1].
For energy conversion, photoelectrochemical solar cells and, specifically, dye-sensitized solar cells (DSSC) [2,3] and direct injection cells [4], were proposed as an economic alternative to the conventional Si-based technology, as they can avoid the use of high-purity Si and face no resource limitations [5,6]. Dye-sensitized cells are obtained by sensitizing a wide band gap semiconductor anode (usually TiO2) with molecules—purely organic or metal-organic dyes—that effectively absorb solar radiation. Absorption promotes electrons into the dye’s excited state(s), and the electrons are then injected into the conduction band of the oxide and can travel through the external circuit to the counterelectrodes [7], from where they are carried by a redox species in an electrolyte (usually I-/I3- in MeCN [8]) back to the sensitized anode to regenerate the dye (Figure 1). To achieve commercialization, a number of issues need to be resolved, including the stability. Rapid temporal degradation is in fact the most important impediment to a widespread application of this technology. Stability is in particular related to interactions of the anode with electrolyte species; for example, it has been known that dye desorption is influenced by the presence of water [9] and also that phase changes in the anode can occur after a while.
For energy storage, metal ion batteries are the most promising technology. In a metal ion battery, metal ions (usually Li+, Na+, Mg2+, but other metals have also been used [10,11,12,13,14,15,16]) travel in an ion-conducting electrolyte between electrodes with different chemical potentials (Figure 2). During discharge, this potential difference drives the ions from the anode to the cathode, while the electrons travel through the external circuit. During charge, the current direction is reversed by applying external voltage. While Li ion batteries today provide the highest energy density among commercial batteries, to achieve economic large scale storage [1], non-Li batteries, such as Na-ion batteries, are most promising, as Li resources are limited and very unevenly distributed [17,18]. Development of practical batteries passes again through optimization of electrode-electrolyte (including Na+ or Li+) interactions [19,20,21,22,23].
TiO2 is the most widely used and best-performing anode in mesoscopic photoelectrochemical solar cells [2,3,24], due to its band structure and a reasonable conductance of nanostructured TiO2. At the same time, it has been recognized that there is much room for improvement of electronic properties of TiO2 anodes: it is desirable to improve their conductance and to be able to control the band gap, the conduction band edge position and the density of states at the dye-semiconductor interface [25,26], in order to improve the short-circuit current, open circuit voltage and to decrease charge recombination. This can be done by using co-adsorbent molecules [27,28,29] and counterions, such as Li+, Na+, Mg2+ [30,31,32].
At the same time, TiO2 anodes are receiving more and more attention by the battery community [33], as they are able to form lithiated phases with up to one Li atom per formula unit (and even 1.25 predicted recently [34]) with a minimal volume expansion [35,36], and therefore, they have a good theoretical capacity (335 mAh/g). They are safer than graphite [37] and have a notable cycling ability (high Li mobility): Li insertion into TiO2 can be facile (albeit dependent on the initial phase) and accompanied by phase transitions [34,38,39]. On the contrary, insertion of non-Li ions (such as Na+) into TiO2 phases is largely unstudied, even though it is non-Li batteries that hold the greatest promise as large-scale storage devices to be deployed together with intermittent energy sources (wind, solar). Also, much effort is being spent to develop new electrolytes for metal-ion batteries [40,41,42,43,44,45]. However, the effects of key molecular functional groups on the electronic properties of a TiO2 interface are also largely unstudied.
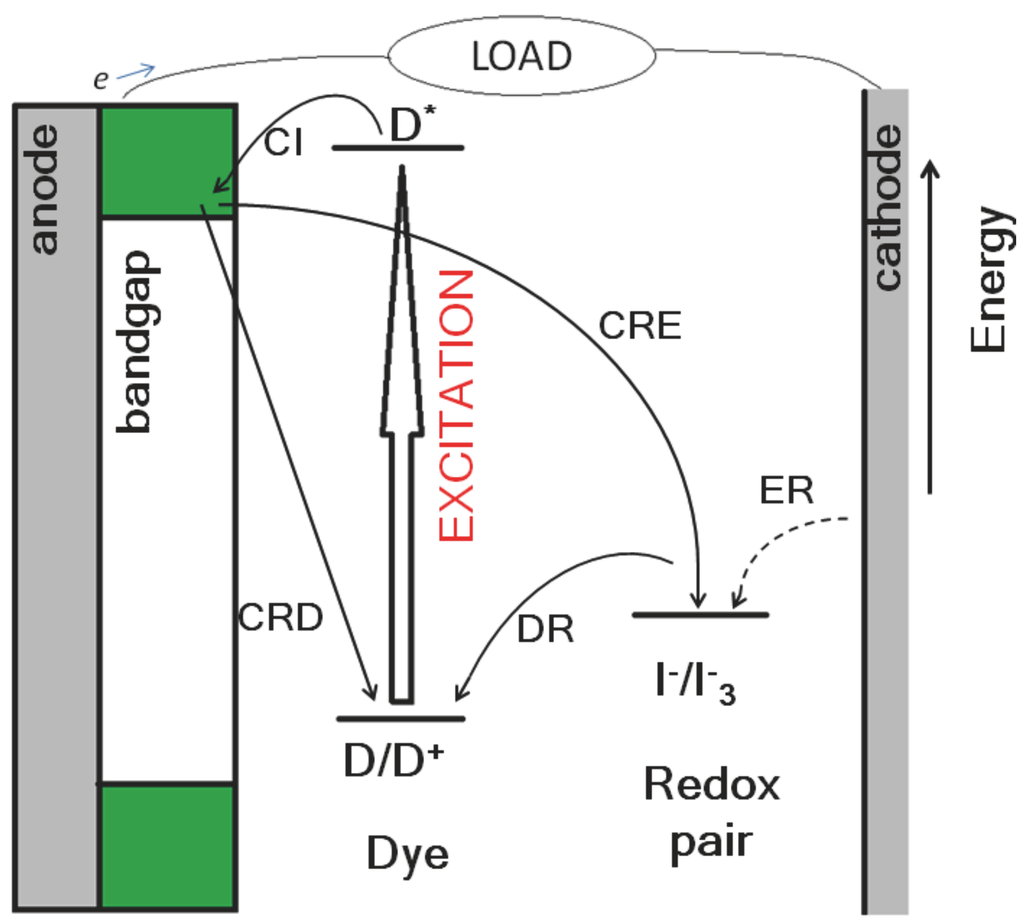
Figure 1.
A typical photoelectrochemical cell, where dye molecules are adsorbed on a semiconductor (TiO2). Photons excite the dye from the ground (D/D+) into an excited state (D*). The excited electron is transferred into the TiO2 conduction band (CI: charge injection) and the oxidized dye is regenerated by electrolyte species (DR: dye regeneration, ER: electrolyte regeneration). The cell’s current, voltage and the unwanted recombination (CRD/CRE: charge recombination to dye/electrolyte) can be controlled by functionalizing TiO2 with co-adsorbent molecules.
We see, therefore, that in both applications, titania interacts with electrolyte species—molecules and metal ions, and details of this interaction determine the performance of the electrode, including its lifetime. In solar cell research, electrolyte additives—atomic and molecular—are routinely used to improve performance. Specifically, Li, Na and Mg ions are used to control the energy of the conduction band edge of titania [30,31,32]. In theoretical models that include the effects of these ions, they are usually considered as surface-adsorbates. On the other hand, from battery research, we now know that insertion of large amounts of metal atoms into TiO2 is possible [16,46,47,48]. It has also been reported that anatase TiO2 anodes in DSSC can undergo a phase transition to the B phase [49]. One can surmise that there should be significant insertion of metal ions into the DSSC anodes. There exist experimental reports of irreversible Li insertion in DSSC anodes [50]. Yet, insertion of Li+, Na+, Mg2+ in DSSC anodes has not been systematically studied and is an example of a separation between two research fields that should inform each other.
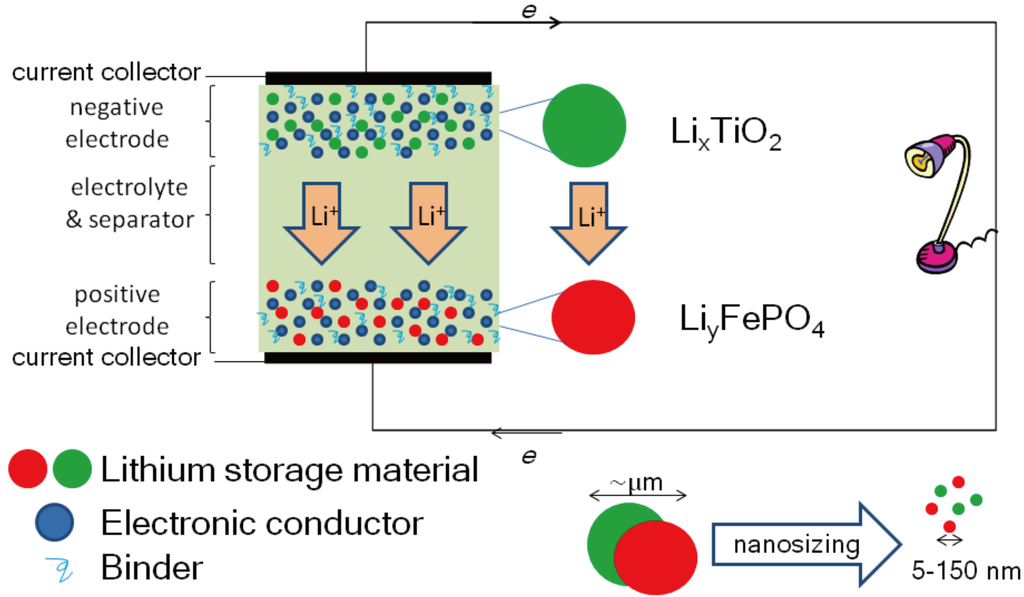
Figure 2.
A typical electrochemical (metal ion) battery. During charging, an external potential is applied; metal ions (Li+, Na+, …) migrate from the cathode and intercalate/insert into the anode. During discharge (shown here), the electrode potential (arising from differences in the chemical potential of Li/Na at the anode and the cathode) induces the reverse process with the electrons travelling through the external circuit to produce electrical work. TiO2 particles have emerged as safe high-rate anodes for electrochemical batteries. What are the effects of nanosizing? What is the dynamics of Na vs. Li insertion, and how to optimize it?
Another evidence of this separation, which we propose to bridge for the benefit of both fields, is in the fact that while DSSC research has paid much attention to the use of co-adsorbate molecules to functionalize TiO2 anodes [28,29,51,52,53], this has not been done for batteries. Co-adsorbed molecules are used to modulate the conduction band edge to enhance electron injection and to prevent recombination (by physically preventing a close approach of the redox species and of the dye oxidation equivalent hole to the oxide surface [27,28,54,55,56,57,58]). They also change adsorption energetics and configuration of the dye [9,54,55,56]. Most theoretical studies of battery anode materials have focused on bulk properties, such as bulk diffusion barriers and rates for metal atoms/ion, voltages and volume expansion [59,60,61,62,63,64]. Interactions of Li with surfaces and nanostructures exposed to vacuum were also considered [65,66,67,68,69]. Interactions with the electrolyte are usually considered in the context of reactions leading to the SEI (solid-electrolyte interface) formation or to an irreversible loss of the active metal (formation of carbonates) [19,20,21,70,71,72,73]. It has, however, been established that surface properties of a battery anode (adsorption energy, insertion barrier) can be more important for storage than its bulk properties [74]. Specifically, the likelihood and the rate of ion insertion are determined, respectively, by the relation between the associated defect formation energy, the metal cohesive energy and the solvation energy and by the insertion barrier through the anode-electrolyte interface. This latter is especially sensitive to other species interacting with the surface [75]. Surface functionalization with molecules could allow for a degree of control over those properties, but remains largely unstudied.
It is therefore important to understand and optimize the interactions of TiO2 with molecules and atomic species for applications in both solar cells and batteries, but no unified description exists, e.g., there is no description of the effects of Li, Na insertion into TiO2 on solar cell performance and no description of the effects of molecular functionalization of the anode surface on battery performance. In fact, there is no systematic description of the interactions of electrolyte species with TiO2 of different phases and morphologies. We propose that such a description be produced based on computational modeling backed by specific experiments. It then will be possible to identify titania nano-morphologies optimal for batter and solar cell performance. Such a study would bridge the two fields that have largely been developing in parallel and would identify improved anode materials for both photoelectrochemical solar cells and metal-ion batteries.
2. The Challenge: A Systematic Study of Functionalized, Alloyed TiO2 Anodes
2.1. Project Scope
We propose to study the dependence of the properties of TiO2 anodes on the molecular structure of co-adsorbents. This will identify structure-property relations, which will guide the experimental design of improved solar cells and batteries. We suggest imidazole derivatives for this study, as this kind of molecules has been used to improve DSSC performance [76,77] and they are known to be compatible with Li battery components [78]. Structural changes of the nanocrystalline TiO2 anode due to the insertion of counterions, such as Li+ or Na+, will then be studied. We hypothesize that there is significant intercalation into the anode of some of the counterions commonly used in photoelectrochemical cell electrolytes and that it could be a determinant of cell (in)stability, which has been preventing commercialization. Such intercalation has been observed in the phases of titania used as prospective anodes for the Li-ion batteries, but is often ignored in the prevailing discourse about and theoretical models of the effect of counterions on the performance of photoelectrochemical cells.
To identify candidate titania anodes for large-scale, non-Li batteries, the insertion of both Na+ and Li+ should be compared. Insertion into TiO2 anodes of different crystal phases and surface cuts/nano-morphologies, which are used in solar cells, as well as those used as prospective battery anodes will be considered.
By considering the effects of molecular additives on metal ion insertion and of inserted metal ions on dye-TiO2 interaction, the study will connect the fields that have largely been developing in parallel, even though dealing with similar elementary processes. This is expected to result in new insight and in the development of improved anodes for both photoelectrochemical solar cells and Li-ion and Na-ion batteries.
2.2. Specific Aims
This proposal aims to answer the following questions:
- 1)
- How do molecular co-adsorbents affect the electronic structure of the dye-semiconductor interface and, consequently, electron injection into TiO2 and recombination of injected electrons with electrolyte species? We need to understand the trends in the effects of molecular co-adsorbents (specifically on dye adsorption configuration and energy and on energy level matching) depending on their molecular structure, including the influence of specific functional groups, such as nitrogen containing heterocycles. This knowledge will help design co-adsorbent-dye combinations enhancing the performance of mesoscopic solar cells.
- 2)
- Do Li or Na counterions insert into and de-insert from the TiO2 anode under the photoelectrochemical cell’s operating conditions? Specifically, does the metal ion concentration in TiO2 affect significantly the electronic structure, conduction, electron injection (short circuit current), diffusion and recombination (open circuit voltage)?
- 3)
- Can the interaction with the metal ion change the phase of the nanostructured anode material? If so, is this a reversible change? This could have a profound effect on the durability of mesoscopic solar cells.
- 4)
- How does this interaction depend on the kind of ion (e.g., Li vs. Na) and the specific polymorph of TiO2? How does it depend on the nanoparticle morphology and the surface indices of the facets approached by the ion? This includes a comparative study of anode structures (phases, facets) used in photoelectrochemical cells and those used as prospective anodes in Li or Na ion batteries. This knowledge will enable the design of anodes with desired insertion (for metal ion batteries), absorption and conduction band (for DSSC) properties.
- 5)
- To what extent is it possible to decouple the design of co-adsorbents and counterions from the design of dyes? There are conflicting reports in the literature about this [32].
With the knowledge derived from studying points 1–5, one can produce structure-property relations, such as trends in the conduction band edge position, conductance, crystal structure and chemical potential depending on adsorbed or inserted electrolyte species. This knowledge is valuable in designing the anode material, as today, it is possible to experimentally control the crystal structure (i.e., anatase vs. rutile) and, to some extent, the exposed surfaces [79].
3. Detailed Proposal
3.1. Detailed Justification
3.1.1. Importance of Developing Functionalized TiO2 Anodes for Mesoscopic Solar Cells
Improved TiO2 anodes are needed to capitalize on recent advances in mesoscopic solar cells. Despite the abundance of Si, Si-based solar cells do suffer from several resource limitations for terawatt-scale deployment, such as the Ag electrode [5]. Other high-efficiency solar cell technologies also suffer from resource limitations [5] and/or require the use of large quantities of highly toxic materials (e.g., CdTe cells) [80].
There are no resource limitations facing organic dye-based photoelectrochemical cells [5], and they can be made with environment-friendly materials. Therefore, there is potential to achieve cost-effective and mass-producible solar cells. The technology was first proposed in 1991 [81], but it is only in the last couple of years that significant advances have been made, which make commercial dye-sensitized cells an achievable goal. Specifically, efficiencies of over 10% have been achieved with organic-based dyes and non-iodine electrolyte and also with a Pt-free cathode [82,83]. In fact, the current record efficiency cell employed both a non-iodine electrolyte, a non-Ru dye, and a co-adsorbent! [24] Further progress is needed to realize high-efficiency cells with long-term stability to make this technology commercial. Theoretical studies are necessary to guide cell designers to efficient chromophore-semiconductor-electrolyte combinations, but understanding of even elementary processes in photoelectrochemical cells is still fragmentary. Such little-understood processes include anode material interactions with electrolyte species.
The effect of electrolyte species on the anode performance is under-studied. Both efficiency and durability depend on the composition of the electrolyte. Various additives were found to change the solar cell’s voltage and the rates of electron injection (desirable) and charge recombination (undesirable) [27,28,29,31]. There is, however, no unified theoretical description of an additive’s effect on cell performance that would ultimately permit rational design of additive-dye pairs. Computational modeling has the ability to make such predictions. To achieve this, structure-property relations need to be derived for additives featuring key functional groups. This is one goal of this proposal.
3.1.2. Ion Insertion into a TiO2 Anode: A Common Yet Little Studied Phenomenon in Photoelectrochemical Solar Cells and Batteries
Another goal of this proposal is to determine the effect of electrolyte species on the structure of the nanocrystalline anode. This includes a study of Li+ and Na+ insertion into TiO2 nanostructures-candidate anodes for metal ion batteries. We also hypothesize that there is significant intercalation into the anode of some of the counterions commonly used in solar cell electrolytes.
For example, the Li+ or Na+ ions are often used to modify the conduction band edge of TiO2 in photoelectrochemical cells [30,31,32]. Now, the interaction of Li+ with TiO2 also governs the performance of anodes of Li ion batteries using the same material, which now attract more and more attention [34,39,84,85,86,87,88,89,90].
It is therefore expected that a significant metal ion insertion into the TiO2 nanostructured anode should also occur under the operating condition of solar cells, but is completely ignored in the prevailing understanding of the effect of counterions on the performance of photoelectrochemical cells. There is anecdotal evidence of the bronze TiO2 formed in an operating cell (Helmut Tributsch, private communication), and irreversible Li update by TiO2 in DSSC was also observed [50]. It was observed previously [49] that sodium uptake by anatase can lead to the destruction of crystal structure and formation of TiO2(B) when the Na/Ti ratio is about 0.2. At the same time, small (7 nm) particles of anatase were shown to maintain their crystal structure up to Li/Ti = 0.21 [36,89]. Clearly, there is ion-type and size dependence of insertion dynamics that must significantly affect the lifetime of both batteries and mesoscopic solar cells (which is the Achilles's heel of this technology). This phenomenon is largely under-studied and is a focus of this proposal.
3.2. Theoretical and Computational Analysis
The proposed project involves a systematic density-functional theory and molecular dynamics analysis of the interaction of electrolyte species (molecules and Li+, Na+ ions) with TiO2 anodes.
3.2.1. The Effect of Molecular Co-Adsorbates on the Electronic Properties of the Anode
First, the dependence of the anode band structure (conduction band minimum, the density of states) on the kind of co-adsorbent needs to be studied to identify structure property relations allowing for a prediction, e.g., of the position of a conduction band minimum from the molecular structure of the adsorbed molecule. This is important for the control of the balance between the injection rate and the open-circuit voltage [28,30,31,32].
Next, the effects of nuclear motions on the electronic properties need to be included. Almost all computational studies of photoelectrochemical cells and all such studies of molecular co-adsorbents were done at the most energetically favored configurations. However, we have shown that dye nuclear motions can cause orders of magnitude changes in the injection and recombination rates [54,55,56,91]. It is therefore expected that the nuclear dynamics of the co-adsorbent will also have a strong effect on electronic properties of the dye-electrolyte-semiconductor interface and, therefore, on solar cell performance. An analysis of the evolution of the electronic structure (e.g., the energy of the conduction band minimum) of a semiconductor-co-adsorbent and semiconductor-dye-co-adsorbent interface under the influence of room-temperature vibrations (including orientational motions of the molecule with respect to the surface) have to be performed (Figure 3).
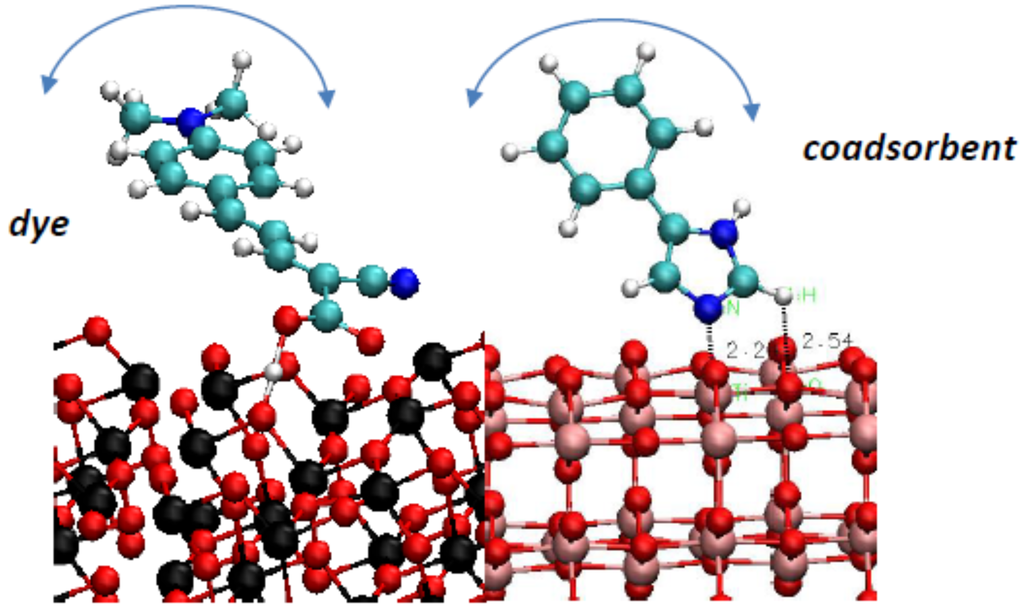
Figure 3.
Modification of the electronic properties of TiO2 surfaces with co-adsorbents is a powerful tool to increase solar cell efficiency. In [54,55,91], we showed that adsorption modes and the nuclear dynamics of a dye adsorbed on TiO2 modify electron injection and recombination rates (left panel: NK1 dye on anatase (101) surface). What is the effect of co-adsorbent molecules’ adsorption mode and dynamics on the electronic properties of TiO2 anodes of different phases, surface cuts and nano-morphologies? What are the synergetic dye/co-adsorbent effects? The proposed study will answer these questions and identify optimal TiO2/co-adsorbent combinations (right panel: a promising 4-phenylimidazole co-adsorbent on the anatase (101) surface).
Nitrogen-containing heterocyclic molecules have been shown to modify the open circuit voltage and the short circuit current depending on the molecular and surface structure [76]. We therefore suggest calculations of a series of imidazole derivatives adsorbed on TiO2 (such as imidazole, 4-phenylimidazole and 2-iso-propenylimidazole). Those will identify adsorption modes and trends in band structure of TiO2, depending on the molecular structure, molecular binding mode, kind of TiO2 surface (e.g., anatase (101) vs. rutile (110)), as well as on the inclusion of nuclear motions. For example, Figure 4 shows selected adsorption modes of three imidazole derivatives, and Figure 5 shows corresponding trends in the adsorption energy and in the energy of the functionalized TiO2 conduction band minimum.
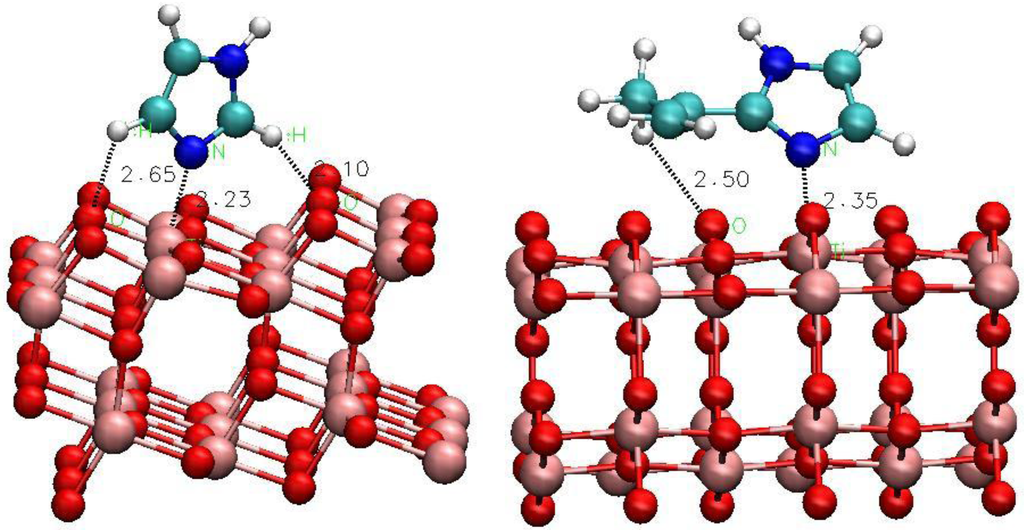
Figure 4.
Selected adsorption configurations of (left to right) imidazole, 4-phenyl-imidazole and 2-iso-propenyl-imidazole on the anatase (101) surface of TiO2.
This should be followed with similar calculations including both co-adsorbent molecules and selected organic dyes into the model (candidate dyes are aminophenyl acid [54], indoline and carbazole dyes [92]). While interactions between Ru dyes and some co-adsorbents have been theoretically analyzed [93], there are no systematic studies of organic dyes co-adsorbed with additives on a TiO2 surface. The dependence of the electronic properties of the interface on both dye and co-adsorbent molecular structures and any synergetic effects will thus be identified. We want to understand to what extent the design of co-adsorbents can be decoupled from the design of dyes, as this is an open and important design question [31,32,94].
3.2.2. The Effect of Metal Ion Insertion on the Electronic Properties of the Anode and of Co-Adsorbents on Insertion
Previous studies of Li diffusion into other anode materials [69,95] identified the dependence of lithiated structure and diffusion dynamics on surface indices. The uptake of Li and Na is therefore expected to depend on the phase and on the surface cut of TiO2. Alloy structures with different metal concentrations and their electronic structure can be determined using DFT for selected phases of TiO2 (such as anatase, rutile, brookite, (B)) with and without the presence of co-adsorbent molecules (such as imidazole derivatives). We want to understand what alloys and compounds are formed and how much the band structure, and possibly, the crystal structure, of the original anode change, due to the intake of the ions of different metals [90] and, also, in the presence of molecular species at the surface.
Furthermore, an analysis of barriers to diffusion and molecular dynamics simulations of lithiation/sodiation proceeding through different facets (surface indices) of these phases should be performed. We want to understand how facile insertion/de-insertion of each kind of ion (Li, Na) is and, therefore, how significant this phenomenon is in typical photoelectrochemical cells and what phases and surfaces of TiO2 are expected to be good anodes for metal ion batteries, especially non-Li batteries (such as Na-ion batteries). Energetics (which determines the battery voltage), barriers to diffusion (which determines rate capability), volume expansion and crystal structure stability (which determine cyclability and anode lifetime) will then be studied, and the molecular dynamics of metal ion uptake by different TiO2 nanomorphologies will be performed.
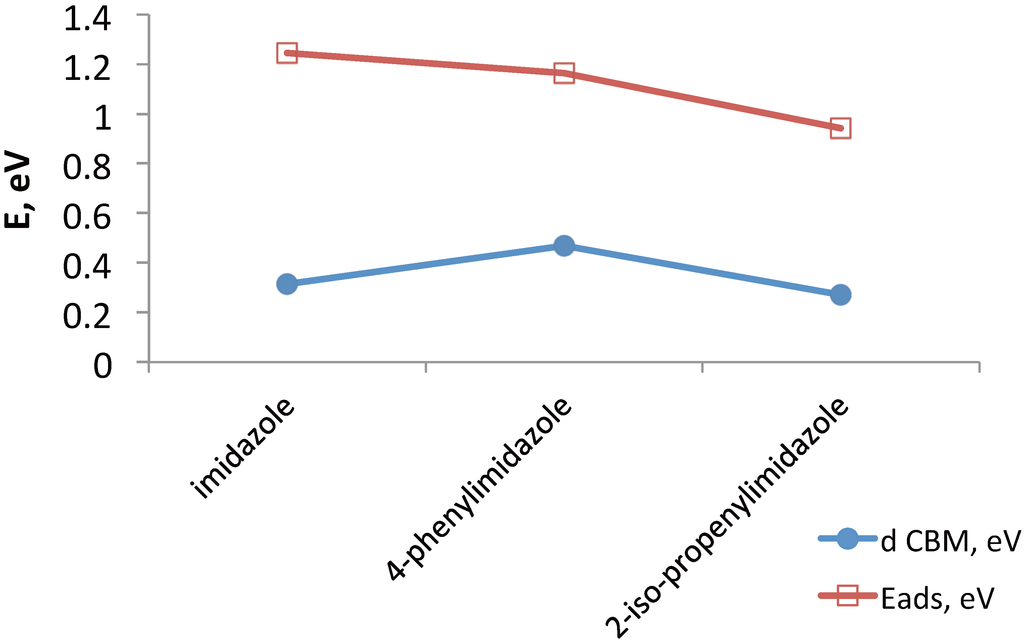
Figure 5.
The co-adsorbent’s adsorption energy (Eads) and the change in the energy of the functionalized TiO2 conduction band minimum (d CBM) for three imidazole derivatives adsorbed on the anatase (101) surface of TiO2, as shown in Figure 4.
Conversely, the effect of adsorbed molecules on Li+ and Na+ insertion in battery anodes should be studied. There is mounting evidence that electrolyte molecules influence metal ion insertion dynamics in batteries, e.g., solvent adsorption and decomposition was found to influence the barrier of Li+ insertion into Si [75], and changing the solvent composition was found to enable Na+ and Mg2+ insertion into a Prussian blue analog [96]. This suggests the use of molecular co-adsorbents to influence insertion. Therefore, a systematic study on Li+ and Na+ insertion into the TiO2 of a series of molecular co-adsorbents, like those shown above, as well as electrolyte species (ethylene or propylene carbonate [97]) should be performed.
3.3. Interaction between Theory/Computation and Experiment
This computation-focused proposal can be implemented in close collaboration with experimental labs. The following measurements are proposed and can be done in most solar cell and battery labs:
- -
- Investigation of electrochemical insertion of Li- and Na-ions into TiO2- anatase, rutile and bronze phases (standard battery characterization measurements of capacity-voltage curves at different rates) upon anode exposure to electrolytes with different Li/Na salt concentrations.
- -
- Estimation of metal ion content based on the storage capacity obtained.
- -
- Estimation of diffusion coefficients of Li and Na-ions in these structures, using GITT (galvanostatic intermittent titration technique), CV (cyclic voltammetry) and impedance studies.
- -
- Evaluation of electronic conductivity on prelithiated TiO2 films.
- -
- Experiments on photoelectrochemical cells with chemically pre-lithiated TiO2 anodes to investigate their performance.
- -
- Measurements of the effect on electron injection and recombination rates in dye-sensitized cells of different co-adsorbent-dyes pairs (using, for example, spectroscopy and impedance techniques [98]). This, together with calculations, will be used to build structure-property relations. These relations will facilitate computational screening of co-adsorbents used to achieve a desired effect on the electronic structure of the dye-semiconductor interface, to facilitate injection and inhibit recombination.
- -
- Measurements of I-V curves under different illumination intensities, transient photocurrent and photovoltage and AC impedance of solar cells utilizing different co-adsorbent molecules.
3.4. Expected Outcomes
The main expected outcome is computational discovery of TiO2-based materials (crystal phases, nano-sized objects of specific size and shape, surface cuts and molecular functionalizations), which best perform specific functions as anodes in batteries (high capacity and high rate metal ion insertion and de-insertion) and mesoscopic solar cells (control of conduction band edge, improved electron injection and inhibited recombination).
The proposed study will produce systematized knowledge of the interaction of molecular co-adsorbents and metal ions with TiO2 anodes of different structures. This will include trends of electronic properties of TiO2 anodes used in mesoscopic solar cells as a function of co-adsorbate and dye molecular structure. For atomic ions interacting with TiO2 anodes used in metal ion batteries and in mesoscopic solar cells, the effects of different TiO2 morphologies and different kinds of ions will be understood. Trends in key properties as a function of the initial crystal phase, surface cut, etc., will emerge. It is the knowledge of these trends that is important for the rational design of functional materials. Specifically, the resulting systematic knowledge of the properties of sodium-TiO2 co-adsorbate complexes or alloys is non-existent today. The knowledge produced in this study will therefore help in the design of better anodes for both solar cells and batteries.
With the above, anode-electrolyte species combinations with the best expected performance can be identified. The knowledge produced will therefore enable identification of promising candidate materials for both solar cells and batteries before extensive and costly experimentation is begun. The resulting cross-fertilization of the two fields will be beneficial for the development of better anode materials, through enhanced knowledge sharing, awareness and collaboration.
4. Conclusions
We proposed a computation-focused and experiment-supported study that would bridge the gap between the researches done so far in photoelectrochemical cells and battery communities and help design high-performance titania anodes for use in dye-sensitized cells and Li and Na batteries. The proposal’s novelty and competitive advantage over studies done in these two separate communities is due to the following salient points.
4.1. A Systematic Theoretical Study to Derive Structure-Property Relations
Today, there does not exist a systematic understanding of the effect of electrolyte species on electronic structure and dynamics (insertion, electron injection and recombination) in photoelectrochemical cells and batteries. The complexity of nanoscale TiO2 materials and the difficulty observing them in situ call for a theoretical study proposed here. A computer model will have full control of the factors mentioned above (surface cut, crystal structure and size, etc.). For the first time, structure-property relations as a function of key molecular building blocks or atomic species interacting with TiO2 would be built. The trends of key electronic properties of the anode with the co-adsorbent molecular size and type of functional groups, with counterion concentration, etc., will permit rational design of electrolyte-anode interfaces with improved performance.
4.2. Inclusion of Effects Due to Nuclear Dynamics
We are the first who identified a large difference in electron injection and recombination rates in photoelectrochemical cells between their static estimates and averages over nuclear motions [54,55,56,91]. We were able to do that without performing the extremely costly joint electron-nuclei dynamics [99]. We expect a strong nuclear dynamics effect to be also present in the case of a TiO2 anode interacting with electrolyte species, such as co-adsorbent molecules. One of the advantages of our approach is the inclusion of molecular dynamics. Molecular dynamics should also be used to study the atomic counterion insertion into the anode.
4.3. Considering the Effect of Counterion Intercalation on the Anode
We propose a new focus on the effects due to intercalation of electrolyte species into TiO2 anodes. To the best of our knowledge, this brings for the first time under one roof the studies of Li and Na insertion into TiO2 in photoelectrochemical solar cells and in electrochemical batteries. The phenomenon is similar in both applications, and this study will help in the design of both types of devices. The effects due to ion insertion are usually ignored in the analysis of electrochemical solar cells; therefore, this study will significantly expand the knowledge about the performance of TiO2 anodes, specifically, of the evolution of their electronic properties when exposed to the electrolyte, which could degrade the performance. Remember that durability is the most important problem preventing a wide use of the photoelectrochemical cell technology. There is currently only fragmentary understanding of the effect of counterion insertion into TiO2 on solar cell performance. There exist experimental studies of only Li insertion into different phases and morphologies of TiO2 [100,101,102], including anatase [35,36,39,84,103,104,105,106] and rutile [38,107], commonly used for solar cell anodes. These studies have been focused on applications in Li ion batteries. Theoretical studies of the insertion of Li into TiO2 are scarce [34,87,107] and of Na, non-existent. Na+ is a commonly used counterion in photoelectrochemical cells [31,32] that could also form an alloy with TiO2 and possibly cause a phase transition and, in any case, a change in the electronic properties of the anode and in cell performance [49]. A study of sodiation is also necessary to understand if TiO2 is a good potential anode for sodium batteries—a promising large-scale storage technology [15]. We propose, for the first time, a comparative and systematic (i.e., for different counterions, surfaces, etc.) computational study of Li and Na insertion into TiO2 phases and facets that have been traditionally used in both dye-sensitized cells and batteries.
4.4. Impact of Nanosizing
Most previous computational studies of the interaction of co-adsorbent molecules or atoms/ions with TiO2 were done for bulk or for extended low-index surfaces [53,76,107]. It is known that nanosizing significantly changes the electronic properties of TiO2 anodes (including suppression of first order phase transitions and a large irreversible loss of Li capacity upon the first cycle) [38,84,90,107]. We propose to include modeling nano-sized materials via nanowires, nanoparticles or high-index surfaces, narrowing the gap between theory and experiment.
4.5. A Theoretical Study Tailored to Practice
The proposed theoretical studies can be conducted in close collaboration with experimental groups to focus on the computational analysis of systems, which are practically feasible. The proposed experimental measurements are doable in most solar cell and battery labs. It will be possible to implement, in practice, anode-electrolyte combinations, which will be identified by calculations as promising, as the analysis is done on materials already widely used in practice (e.g., titania nanoparticles or imidazole derivatives).
Acknowledgments
This work was supported by the AcRF Tier 1 grant R-265-000-430-133 by the Ministry of Education of Singapore and also by the Funding Program for World-Leading Innovative R&D on Science and Technology (FIRST) "Development of Organic Photovoltaics toward a Low-Carbon Society", Cabinet Office, Japan.
The authors also thank Dr. Kentaro Kawata of Merck Japan, Dr. Palani Balaya of the Department of Mechanical Engineering, National University of Singapore, Prof. Hiroshi Segawa of the Research Center for Advances Science and Technology, University of Tokyo, and Prof. Koichi Yamashita of the Department of Chemical System Engineering, University of Tokyo, for fruitful discussions.
References and Notes
- Barbour, E.; Wilson, I.A.G.; Bryden, I.G.; McGregor, P.G.; Mulheran, P.A.; Hall, P.J. Towards an objective method to compare energy storage technologies: Development and validation of a model to determine the upper boundary of revenue available from electrical price arbitrage. Energy Environ. Sci. 2012, 5, 5425–5436. [Google Scholar] [CrossRef]
- Hagfeldt, A.; Boschloo, G.; Sun, L.; Kloo, L.; Pettersson, H. Dye-sensitized solar cells. Chem. Rev. 2010, 110, 6595–6663. [Google Scholar] [CrossRef]
- Wei, D. Dye sensitized solar cells. Int. J. Mol. Sci. 2010, 11, 1103–1113. [Google Scholar] [CrossRef]
- Manzhos, S.; Jono, R.; Yamashita, K.; Fujisawa, J.-I.; Nagata, M.; Segawa, H. Study of interfacial charge transfer bands and electron recombination in the surface complexes of TCNE, TCNQ, and TCNAQ with TiO2. J. Phys. Chem. C 2011, 115, 21487–21493. [Google Scholar]
- Tao, C.S.; Jiang, J.; Tao, M. Natural resource limitations to terawatt-scale solar cells. Solar Energy Mater. Solar Cells 2011, 95, 3176–3180. [Google Scholar] [CrossRef]
- Branker, K.; Pathak, M.J.J.; Pearce, J.M. A review of solar photovoltaic levelized cost of electricity. Renew. Sust. Energ. Rev. 2011, 15, 4470–4482. [Google Scholar] [CrossRef]
- Graetzel, M. Photoelectrochemical cells. Nature 2001, 414, 338–344. [Google Scholar] [CrossRef]
- Boschloo, G.; Hagfeldt, A. Characteristics of the iodide/triiodide redox mediator in dye-sensitized solar cells. Acc. Chem. Res. 2009, 42, 1819–1826. [Google Scholar] [CrossRef]
- De Angelis, F.; Fantacci, S. Simulating dye-sensitized TiO2 Heterointerfaces in explicit solvent: Absorption spectra, energy levels, and dye desorption. J. Phys. Chem. Lett. 2011, 2, 813–817. [Google Scholar] [CrossRef]
- Aurbach, D.; Lu, Z.; Schechter, A.; Gofer, Y.; Gizbar, H.; Turgeman, R.; Cohen, Y.; Moshkovich, M.; Levi, E. Prototype systems for rechargeable magnesium batteries. Nature 2000, 407, 724–727. [Google Scholar] [CrossRef]
- Tran, T.T.; Obrovac, M.N. Alloy negative electrodes for high energy density metal-ion cells. J. Electrochem. Soc. 2011, 158, A1411–A1416. [Google Scholar] [CrossRef]
- Chevrier, V.L.; Ceder, G. Challenges for Na-ion negative rlectrodes. J. Electrochem. Soc. 2011, 158, A1011–A1014. [Google Scholar] [CrossRef]
- Kim, S.W.; Seo, D.H.; Ma, X.H.; Ceder, G.; Kang, K. Electrode materials for rechargeable sodium-ion batteries: Potential alternatives to current lithium-ion batteries. Adv. Energy Mater. 2012, 2, 710–721. [Google Scholar] [CrossRef]
- Hayashi, M.; Arai, H.; Ohtsuka, H.; Sakurai, Y. Electrochemical characteristics of calcium in organic electrolyte solutions and vanadium oxides as calcium hosts. J. Power Sources 2003, 119–121, 617–620. [Google Scholar]
- Palomares, V.; Serras, P.; Villaluenga, I.; Hueso, K.B.; Gonzalez, J.C.; Rojo, T. Na-ion batteries, recent advances and present challenges to become low cost energy storage systems. Energy Environ. Sci. 2012, 5, 5884–5901. [Google Scholar] [CrossRef]
- Liu, S.; Hu, J.J.; Yan, N.F.; Pan, G.L.; Li, G.R.; Gao, X.P. Aluminum storage behavior of anatase TiO2 nanotube arrays in aqueous solution for aluminum ion batteries. Energy Environ. Sci. 2012, 5, 9743–9746. [Google Scholar] [CrossRef]
- Shukla, A.K.; Kumar, T.P. Lithium economy: Will it get the electric traction? J. Phys. Chem. Lett. 2013, 4, 551–555. [Google Scholar] [CrossRef]
- Tarascon, J.-M. Is lithium the new gold? Nat. Chem. 2010, 2, 510. [Google Scholar] [CrossRef]
- Xu, K.; von Cresce, A. Li+-solvation/desolvation dictates interphasial processes on graphitic anode in Li ion cells. J. Mater. Res. 2012, 27, 2327–2341. [Google Scholar] [CrossRef]
- Leung, K. First-principles modeling of the initial stages of organic solvent decomposition on LixMn2O4(100) surfaces. J. Phys. Chem. C 2012, 116, 9852–9861. [Google Scholar] [CrossRef]
- Leung, K. Electronic structure modeling of electrochemical reactions at electrode/electrolyte interfaces in lithium ion batteries. J. Phys. Chem. C 2013, 117, 1539–1547. [Google Scholar] [CrossRef]
- Vatamanu, J.; Borodin, O.; Smith, G.D. Molecular dynamics simulation studies of the structure of a mixed carbonate/LiPF6 electrolyte near graphite surface as a function of electrode potential. J. Phys. Chem. C 2012, 116, 1114–1121. [Google Scholar] [CrossRef]
- Muldoon, J.; Bucur, C.B.; Oliver, A.G.; Sugimoto, T.; Matsui, M.; Kim, H.S.; Allred, G.D.; Zajicek, J.; Kotani, Y. Electrolyte roadblocks to a magnesium rechargeable battery. Energy Environ. Sci. 2012, 5, 5941–5950. [Google Scholar] [CrossRef]
- Yella, A.; Lee, H.W.; Tsao, H.N.; Yi, C.; Chandiran, A.K.; Nazeeruddin, M.K.; Diau, E.W.G.; Yeh, C.Y.; Zakeeruddin, S.M.; Grätzel, M. Porphyrin-sensitized solar cells with cobalt (II/III)-based electrolyte exceed 12% efficiency. Science 2011, 334, 629–634. [Google Scholar] [CrossRef]
- Auvinen, S.; Alatalo, M.; Haario, H.; Jalava, J.-P.; Lamminmäki, R.-J. Size and shape dependence of the electronic and spectral properties in TiO2 nanoparticles. J. Phys. Chem. C 2011, 115, 8484–8493. [Google Scholar]
- Spadavecchia, F.; Cappelletti, G.; Ardizzone, S.; Ceotto, M.; Falciola, L. Electronic structure of pure and N-doped TiO2 nanocrystals by electrochemical experiments and first principles calculations. J. Phys. Chem. C 2011, 115, 6381–6391. [Google Scholar] [CrossRef]
- Han, L.; Islam, A.; Chen, H.; Malapaka, C.; Chiranjeevi, B.; Zhang, S.; Yang, X.; Yanagida, M. High-efficiency dye-sensitized solar cell with a novel co-adsorbent. Energy Environ. Sci. 2012, 5, 6057–6060. [Google Scholar] [CrossRef]
- Lon, H.; Zhou, D.; Zhang, M.; Peng, C.; Uchida, S.; Wang, P. Probing dye-correlated interplay of energetics and kinetics in mesoscopic titania solar cells with 4-tert-butylpyridine. J. Phys. Chem. C 2011, 115, 14408–14414. [Google Scholar] [CrossRef]
- Ren, X.; Feng, Q.; Zhou, G.; Huang, C.-H.; Wang, Z.-S. Effect of cations in coadsorbate on charge recombination and conduction band edge movement in dye-sensitized solar cells. J. Phys. Chem. C 2010, 114, 7190–7195. [Google Scholar] [CrossRef]
- Koops, S.E.; O’Regan, B.C.; Barnes, P.R.; Durrant, J.R. Parameters influencing the efficiency of electron injection in dye-sensitized solar cells. J. Am. Chem. Soc. 2009, 131, 4808–4818. [Google Scholar]
- Wang, H.; Peter, L.M. Influence of electrolyte cations on electron transport and electron transfer in dye-sensitized solar cells. J. Phys. Chem. C 2012, 116, 10468–10475. [Google Scholar] [CrossRef]
- Shi, Y.S.; Wang, Y.H.; Zhang, M.; Dong, X.D. Influences of cation charge density on the photovoltaic performance of dye-sensitized solar cells: Lithium, sodium, potassium, and dimethylimidazolium. Phys. Chem. Chem. Phys. 2011, 13, 14590–14597. [Google Scholar] [CrossRef]
- Saravanan, K.; Ananthanarayanan, K.; Balaya, P. Mesoporous TiO2 with high packing density for superior lithium storage. Energy Environ. Sci. 2010, 3, 939–948. [Google Scholar] [CrossRef]
- Dalton, A.S.; Belak, A.A.; van der Ven, A. Thermodynamics of Lithium in TiO2(B) from first principles. Chem. Mater. 2012, 24, 1568–1574. [Google Scholar] [CrossRef]
- Kavan, L.; Kalbáč, M.; Zukalová, M.; Exnar, I.; Lorenzen, V.; Nesper, R.; Graetzel, M. Lithium storage in nanostructured TiO2 made by hydrothermal growth. Chem. Mater. 2004, 16, 477–485. [Google Scholar] [CrossRef]
- Wagemaker, M.; Borghols, W.J.H.; Mulder, F.M. Large impact of particle size on insertion reactions. A case for anatase LixTiO2. J. Am. Chem. Soc. 2007, 129, 4323–4327. [Google Scholar] [CrossRef]
- Yang, Z.; Choi, D.; Kerisit, S.; Rosso, K.M.; Wang, D.; Zhang, J.; Graff, G.; Liu, J. Nanostructures and lithium electrochemical reactivity of lithium titanites and titanium oxides: A review. J. Power Sources 2009, 192, 588–598. [Google Scholar] [CrossRef]
- Hu, Y.S.; Kienle, L.; Guo, Y.G.; Maier, J. High lithium electroactivity of nanometer-sized rutile TiO2. Adv. Mater. 2006, 18, 1421–1426. [Google Scholar] [CrossRef]
- Shin, J.-Y.; Joo, J.H.; Samuelis, D.; Maier, J. Oxygen-deficient TiO2-δ nanoparticles via hydrogen reduction for high rate capability lithium batteries. Chem. Mater. 2012, 24, 543–551. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Kim, Y. Challenges for rechargeable Li batteries. Chem. Mater. 2010, 22, 587–603. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Z.; Redfern, P.C.; Curtiss, L.A.; Amine, K. Molecular engineering towards safer lithium-ion batteries: A highly stable and compatible redox shuttle for overcharge protection. Energy Environ. Sci. 2012, 5, 8204–8207. [Google Scholar] [CrossRef]
- Nakayama, M.; Kotobuki, M.; Munakata, H.; Nogami, M.; Kanamura, K. First-principles density functional calculation of electrochemical stability of fast Li ion conducting garnet-type oxides. Phys. Chem. Chem. Phys. 2012, 14, 10008–10014. [Google Scholar] [CrossRef]
- Jalem, R.; Yamamoto, Y.; Shiiba, H.; Nakayama, M.; Munakata, H.; Kasuga, T.; Kanamura, K. Concerted migration mechanism in the Li ion dynamics of garnet-type Li7La3Zr2O12. Chem. Mater. 2013, 25, 425–430. [Google Scholar] [CrossRef]
- Yamagata, M.; Matsui, Y.; Sugimoto, T.; Kikuta, M.; Higashizaki, T.; Kono, M.; Ishikawa, M. High-performance graphite negative electrode in a bis(fluorosulfonyl)imide-based ionic liquid. J. Power Sources 2013, 227, 60–64. [Google Scholar] [CrossRef]
- Ryan, K.R.; Trahey, L.; Ingram, B.J.; Burrell, A.K. Limited stability of ether-based solvents in lithium–oxygen batteries. J. Phys. Chem. C 2012, 116, 19724–19728. [Google Scholar] [CrossRef]
- Fehse, M.; Fischer, F.; Tessier, C.; Stievano, L.; Monconduit, L. Tailoring of phase composition and morphology of TiO2-based electrode materials for lithium-ion batteries. J. Power Sources 2013, 231, 23–28. [Google Scholar] [CrossRef]
- Yu, J.; Sushko, M.L.; Kerisit, S.; Rosso, K.M.; Liu, J. Kinetic Monte Carlo study of ambipolar lithium ion and electron-polaron diffusion into nanostructured TiO2. J. Phys. Chem. Lett. 2012, 3, 2076–2081. [Google Scholar] [CrossRef]
- Ren, Y.; Zhang, J.; Liu, Y.; Li, H.; Wei, H.; Li, B.; Wang, X. Synthesis and superior anode performances of TiO2-carbon-rGO composites in lithium-ion batteries. Appl. Mater. Interfaces 2012, 4, 4776–4780. [Google Scholar] [CrossRef]
- Nishizawa, H.; Aoki, Y. The crystallization of anatase and the conversion to bronze-type TiO2 under hydrothermal conditions. J. Solid. State Chem. 1985, 56, 158–165. [Google Scholar] [CrossRef]
- Kopidakis, N.; Benkstein, K.D.; van de Lagemaat, J.; Frank, A.J. Transport-limited recombination of photocarriers in dye-sensitized nanocrystalline TiO2 solar cells. J. Phys. Chem. B 2003, 107, 11307–11315. [Google Scholar]
- Sun, Z.; Zhang, R.-K.; Xie, H.-H.; Wang, H.; Liang, M.; Xue, S. Nonideal charge recombination and conduction band edge shifts in dye-sensitized solar cells based on adsorbent doped poly(ethylene oxide) electrolytes. J. Phys. Chem. C 2013, 117, 4364–4373. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Kim, J.Y.; Lee, D.-K.; Kim, B.; Kim, H.; Koe, M.J. Importance of 4-tert-butylpyridine in electrolyte for dye-sensitized solar cells employing SnO2 electrode. J. Phys. Chem. C 2012, 116, 22759–22766. [Google Scholar] [CrossRef]
- Asaduzzaman, A.M.; Schreckenbach, G. Computational studies on the interactions among redox couples, additives and TiO2: Implications for dye-sensitized solar cells. Phys. Chem. Chem. Phys. 2010, 12, 14609–14618. [Google Scholar] [CrossRef]
- Manzhos, S.; Segawa, H.; Yamashita, K. The effect of ligand substitution and water co-adsorption on the adsorption dynamics and energy level matching of amino-phenyl acid dyes on TiO2. Phys. Chem. Chem. Phys. 2012, 14, 1749–1755. [Google Scholar] [CrossRef]
- Manzhos, S.; Segawa, H.; Yamashita, K. Effect of nuclear vibrations, temperature, and orientation on injection and recombination conditions in amino-phenyl acid dyes on TiO2. Proc. SPIE 2012, 8438, 843814. [Google Scholar]
- Manzhos, S.; Segawa, H.; Yamashita, K. Effect of nuclear vibrations, temperature, co-adsorbed water, and dye orientation on light absorption, charge injection and recombination conditions in organic dyes on TiO2. Phys. Chem. Chem. Phys. 2013, 15, 1141–1147. [Google Scholar] [CrossRef]
- Haque, S.A.; Handa, S.; Peter, K.; Palomares, E.; Thelakkat, M.; Durrant, J.R. Supermolecular control of charge transfer in dye-sensitized nanocrystalline TiO2 films: Towards a quantitative structure–function relationship. Angew. Chem. Int. Ed. 2005, 44, 5740–5744. [Google Scholar] [CrossRef]
- Clifford, J.N.; Palomares, E.; Nazeeruddin, M.K.; Grätzel, M.; Nelson, J.; Li, X.; Long, N.J.; Durrant, J.R. Molecular control of recombination dynamics in dye-sensitized nanocrystalline TiO2 films: Free energy vs distance dependence. J. Am. Chem. Soc. 2004, 126, 5225–5233. [Google Scholar] [CrossRef]
- Ceder, G.; Hautier, G.; Jain, A.; Ong, S.P. Recharging lithium battery research with first-principles methods. MRS Bull. 2011, 36, 185–191. [Google Scholar]
- Malyi, O.I.; Tan, T.L.; Manzhos, S. A comparative computational study of structures, diffusion, and dopant interactions between Li and Na insertion into Si. Appl. Phys. Express 2013, 6, 027301. [Google Scholar] [CrossRef]
- Malyi, O.I.; Tan, T.L.; Manzhos, S. In search of high performance anode materials for Mg batteries: Computational studies of Mg in Ge, Si, and Sn. J. Power Sources 2013, 233, 341–345. [Google Scholar] [CrossRef]
- Wan, W.H.; Zhang, Q.F.; Cui, Y.; Wang, E.G. First principles study of lithium insertion in bulk silicon. J. Phys. Condens. Matter 2010, 22, 415501–415509. [Google Scholar] [CrossRef]
- Cui, Z.; Gao, F.; Cui, Z.; Qu, J. A second nearest neighbor embedded atom method interatomic potential for Li-Si alloys. J. Power Sources 2012, 207, 150–159. [Google Scholar] [CrossRef]
- Miao, L.; Wu, J.; Jiang, J.; Liang, P. First-principles study on the synergetic mechanism of SnO2 and graphene as a lithium ion battery anode. J. Phys. Chem. C 2013, 117, 23–27. [Google Scholar] [CrossRef]
- Kaghazchi, P. Theoretical studies of Li incorporation into Si(111). J. Phys. Condens. Matter 2013, 25, 095008. [Google Scholar] [CrossRef]
- Kaghazchi, P. Theoretical studies of lithium incorporation into α-Sn(100). J. Chem. Phys. 2013, 138, 054706. [Google Scholar] [CrossRef]
- Peng, B.; Cheng, F.; Tao, Z.; Chen, J. Lithium transport at silicon thin film: Barrier for high-rate capability anode. J. Chem. Phys. 2010, 133, 034701. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, W.; Wan, W.; Cui, Y.; Wang, E. Lithium insertion in silicon nanowires: An ab initio study. Nano Lett. 2010, 10, 3243–3249. [Google Scholar] [CrossRef]
- Jung, S.C.; Han, Y.-K. Facet-dependent lithium intercalation into Si crystals: Si(100) vs. Si(111). Phys. Chem. Chem. Phys. 2011, 13, 21282–21287. [Google Scholar] [CrossRef]
- Leung, K.; Qi, Y.; Zavadil, K.R.; Jung, Y.S.; Dillon, A.C.; Cavanagh, A.S.; Lee, S.-H.; George, S.M. Using atomic layer deposition to hinder solvent decomposition in lithium ion batteries: First-principles modeling and experimental studies. J. Am. Chem. Soc. 2011, 133, 14741–14754. [Google Scholar]
- Shao, N.; Sun, X.-G.; Dai, S.; Jiang, D. Oxidation potentials of functionalized sulfone solvents for high-voltage Li-ion batteries: A computational study. J. Phys. Chem. B 2012, 116, 3235–3238. [Google Scholar] [CrossRef]
- Kim, S.-P.; van Duin, A.C.T.; Shenoy, V.B. Effect of electrolytes on the structure and evolution of the solid electrolyte interphase (SEI) in Li-ion batteries: A molecular dynamics study. J. Power Sources 2011, 196, 8590–8597. [Google Scholar] [CrossRef]
- Ganesh, P.; Kent, P.R.C.; Jiang, D. Solid–electrolyte interphase formation and electrolyte reduction at Li-ion battery graphite anodes: Insights from first-principles molecular dynamics. J. Phys. Chem. C 2012, 116, 24476–24481. [Google Scholar] [CrossRef]
- Malyi, O.I.; Tan, T.L.; Manzhos, S. A computational study of the insertion of Li, Na, and Mg atoms into Si(111) nanosheets. Nano Energy 2013. [Google Scholar] [CrossRef]
- Carvalho, A.; Rayson, M.J.; Briddon, P.R.; Manzhos, S. Effect of the adsorption of ethylene carbonate on Si surfaces on the Li intercalation behavior. (Submitted).
- Kusama, H.; Orita, H.; Sugihara, H. TiO2 band shift by nitrogen-containing heterocycles in dye-sensitized solar cells: A periodic density functional study. Langmuir 2008, 24, 4411–4419. [Google Scholar] [CrossRef]
- Kawata, K.; Goto, T.; Yoshizaki, H. Additives for dye-sensitized solar cells. February 2013. Available online: http://www.sumobrain.com/patents/WO2013026563.html (accessed on 18 June 2013).
- Scheers, J.; Johansson, P.; Szczeciński, P.; Wieczorek, W.; Armand, M.; Jacobsson, P. Benzimidazole and imidazole lithium salts for battery electrolytes. J. Power Sources 2010, 195, 6081–6087. [Google Scholar] [CrossRef]
- Ichimura, A.S.; Mack, B.M.; Usmani, S.M.; Mars, D.G. Direct synthesis of anatase films with ~100% (001) facets and [001] preferred orientation. Chem. Mater. 2012, 24, 2324–2329. [Google Scholar] [CrossRef]
- Werner, J.H.; Zapf-Gottwick, R.; Koch, M.; Fischer, K. Toxic substances in photovoltaic modules. In Proceedings of the 21st International Photovoltaic Science and Engineering Conference, Fukuoka, Japan, 28 November–2 December 2011.
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Zeng, W.; Cao, Y.; Bai, Y.; Wang, Y.; Shi, Y.; Zhang, M.; Wang, F.; Pan, C.; Wang, P. Efficient dye-sensitized solar cells with an organic photosensitizer featuring orderly conjugated ethylenedioxythiophene and dithienosilole blocks. Chem. Mater. 2010, 22, 1915–1925. [Google Scholar] [CrossRef]
- Yum, J.-H.; Baranoff, E.; Kessler, F.; Moehl, T.; Ahmad, S.; Bessho, T.; Marchioro, A.; Ghadiri, E.; Moser, J.-E.; Yi, C.; et al. A cobalt complex redox shuttle for dye-sensitized solar cells with high open-circuit potentials. Nat. Commun. 2012, 3, 631–638. [Google Scholar] [CrossRef]
- Shin, J.-Y.; Samuelis, D.; Maier, J. Sustained lithium-storage performance of hierarchical, nanoporous anatase TiO2 at high rates: Emphasis on interfacial storage phenomena. Adv. Funct. Mater. 2011, 21, 3464–3472. [Google Scholar] [CrossRef]
- Armstrong, A.R.; Arrouvel, C.; Gentili, V.; Parker, S.C.; Islam, M.S.; Bruce, P.G. Lithium coordination sites in LixTiO2(B): A structural and computational study. Chem. Mater. 2010, 22, 6426–6432. [Google Scholar] [CrossRef]
- Shen, L.; Zhang, X.; Li, H.; Yuan, C.; Cao, G. Design and tailoring of a three-dimensional TiO2-graphene-carbon nanotube nanocomposite for fast lithium storage. J. Phys. Chem. Lett. 2011, 2, 3096–3101. [Google Scholar] [CrossRef]
- Kang, J.; Wei, S.H.; Zhu, K.; Kim, Y.H. First-principles theory of electrochemical capacitance of nanostructured materials: Dipole-assisted subsurface intercalation of lithium in pseudocapacitive TiO2 anatase nanosheets. J. Phys. Chem. C 2011, 115, 4909–4915. [Google Scholar] [CrossRef]
- Zakharova, G.C.; Jähne, C.; Popa, A.; Täschner, Ch.; Gemming, Th.; Leonhardt, A.; Büchner, B.; Klingeler, R. Anatase nanotubes as an electrode material for lithium-ion batteries. J. Phys. Chem. C 2012, 116, 8714–8720. [Google Scholar] [CrossRef]
- Zhu, K.; Wang, Q.; Kim, J.-H.; Pesaran, A.A.; Frank, J.A. Pseudocapacitive lithium-ion storage in oriented anatase TiO2 nanotube arrays. J. Phys. Chem. C 2012, 116, 11895–11899. [Google Scholar] [CrossRef]
- Wagemaker, M.; Mulder, F.M. Properties and promises of nanosized insertion materials for Li-ion batteries. Acc. Chem. Res. 2012. [Google Scholar] [CrossRef]
- Manzhos, S.; Segawa, H.; Yamashita, K. Effect of isotopic substitution on elementary processes in dye-sensitized solar cells: Deuterated amino-phenyl acid dyes on TiO2. Computation 2013, 1, 1–15. [Google Scholar] [CrossRef]
- Miyasaka, T. Toward printable sensitized mesoscopic solar cells: Light-harvesting management with thin TiO2 films. J. Phys. Chem. Lett. 2011, 2, 262–269. [Google Scholar] [CrossRef]
- Kusama, H.; Sugihara, H.; Sayama, K. Effect of cations on the interactions of Ru dye and iodides in dye-sensitized solar cells: A density functional theory study. J. Phys. Chem. C 2011, 115, 2544–2552. [Google Scholar] [CrossRef]
- Li, R.; Liu, J.; Cai, N.; Zhang, M.; Wang, P. Synchronously reduced surface states, charge recombination, and light absorption length for high-performance organic dye-sensitized solar cells. J. Phys. Chem. B 2010, 114, 4461–4464. [Google Scholar] [CrossRef]
- Yang, H.; Huang, S.; Huang, X.; Fan, F.F.; Liang, W.T.; Liu, X.H.; Chen, L.Q.; Huang, J.Y.; Li, J.; Zhu, T.; et al. Orientation-dependent interfacial mobility governs the anisotropic swelling in lithiated silicon nanowires. Nano Lett. 2012, 12, 1953–1958. [Google Scholar] [CrossRef]
- Mizuno, Y.; Okubo, M.; Hosono, E.; Kudo, T.; Zhou, H.; Oh-ishi, K. Suppressed activation energy for interfacial charge transfer of a Prussian blue analog thin film electrode with hydrated ions (Li+, Na+, and Mg2+). J. Phys. Chem. C 2013, 117, 10877–10882. [Google Scholar] [CrossRef]
- Tatsumi, H.; Sasahara, A.; Tomitori, M. Adsorption of propylene carbonate molecules on a TiO2(110) surface. J. Phys. Chem. C 2013, 117, 10410–10416. [Google Scholar] [CrossRef]
- Listorti, A.; Creager, C.; Sommeling, P.; Kroon, J.; Palomares, E.; Fornelli, A.; Breen, B.; Barnes, P.R.F.; Durrant, J.R.; Law, C.; et al. The mechanism behind the beneficial effect of light soaking on injection efficiency and photocurrent in dye sensitized solar cells. Energy Environ. Sci. 2011, 4, 3494–3501. [Google Scholar] [CrossRef]
- Duncan, W.R.; Prezhdo, O.V. Theoretical studies of photoinduced electron transfer in dye-sensitized TiO2. Annu. Rev. Phys. Chem. 2007, 58, 143–184. [Google Scholar] [CrossRef]
- Hibino, M.; Abe, K.; Mochizuki, M.; Miyayama, M. Amorphous titanium dioxide electrode for high-rate discharge and charge. J. Power Sources 2004, 126, 139–143. [Google Scholar] [CrossRef]
- Armstrong, A.R.; Armstrong, G.; Canales, J.; Bruce, P.G. TiO2-B nanowires as negative electrodes for rechargeable lithium batteries. J. Power Sources 2005, 146, 501–506. [Google Scholar] [CrossRef]
- Wang, Q.; Wen, Z.H.; Li, J.H. Solvent-controlled synthesis and electrochemical lithium storage of one-dimensional TiO2 nanostructures. Inorg. Chem. 2006, 45, 6944–6949. [Google Scholar] [CrossRef]
- Kavan, L.; Rathousky, J.; Gratzell, M.; Shklover, V.; Zukal, A. Surfactant-templated TiO2 (anatase): Characteristic features of lithium insertion electrochemistry in organized nanostructures. J. Phys. Chem. B 2000, 104, 12012–12020. [Google Scholar] [CrossRef]
- Wagemaker, M.; Lutzenkirchen-Hecht, D.; van Well, A.A.; Frahm, R. Atomic and electronic bulk versus surface structure: Lithium intercalation in anatase TiO2. J. Phys. Chem. B 2004, 108, 12456–12464. [Google Scholar] [CrossRef]
- Li, J.R.; Tang, Z.L.; Zhang, Z.T. Preparation and novel lithium intercalation properties of titanium oxide nanotubes. Electrochem. Solid-State Lett. 2005, 8, A316–A319. [Google Scholar] [CrossRef]
- Subramanian, V.; Karki, A.; Gnanasekar, K.I.; Eddy, F.P.; Rambabu, B. Nanocrystalline TiO2 (anatase) for Li-ion batteries. J. Power Sources 2006, 159, 186–192. [Google Scholar] [CrossRef]
- Borghols, W.J.H.; Wagemaker, M.; Lafont, U.; Keller, E.M.; Mulder, F.M. Impact of nanosizing on lithiated rutile TiO2. Chem. Mater. 2008, 20, 2949–2955. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).