Abstract
Semen collection is an important component of conservation and animal husbandry. Semen quality is generally improved using voluntary collection methods, particularly artificial vaginas (AVs). Most commercially available AVs are tube-shaped with few species-specific design augmentations. As genitalia are highly variable across taxa, incorporating species-specific genital morphologies into AV designs may enhance collected semen quality. We compared dolphin semen quality using: (1) silicone bioinspired artificial vaginas (BAVs) that reflect the internal shape of dolphin vaginas, and (2) manual stimulation. Sperm motility and kinematic parameters of five bottlenose dolphins (Tursiops sp.) were assessed using computer-aided sperm analysis (CASA). Sperm collected using BAVs showed non-significant increases in median progressive and rapid motility, and increases in median and mean linear motility, supporting a sexual selection functional hypothesis for the biodiverse vaginal folds unique to whales, dolphins, and porpoises. Sperm concentration decreased with BAV collection, while no consistent trends were detected in volume, pH, velocity, or plasma membrane integrity. Modifications to AVs for other species that incorporate genital morphologies may also optimize collected semen quality for application to artificial insemination.
1. Introduction
Animal breeding programs have become essential to ex situ conservation efforts to offset species extinction [1]. Breeding programs also support the livestock industry, which is projected to produce 100 billion livestock animals globally by 2050 [2]. Assisted reproductive technologies (ART) such as artificial insemination (AI), sperm sexing, and embryo transfer augment the success of animal breeding programs [3]. Artificial insemination can maintain genetic diversity within breeding populations, yet fertilization success rates remain varied within and between species, particularly among non-domesticated species [4]. The success of AI partially depends on the quality of semen. Fertilization success increased when most sperm had rapid progressive motility, normal morphologies, intact acrosomes, and mature genetic material [5]. Semen quality can vary between ejaculates due to a combination of intrinsic and extrinsic factors including genetics, contaminant exposure, and the biochemical composition of the extracellular environment [6]. Methods for preserving high-quality gametes for use in AI have been developed for many domestic and non-domesticated species over recent years [7,8], with most research focused on improving semen quality after ejaculation. There has been limited innovation in techniques to improve semen quality at the time of ejaculate collection.
Breeding programs typically use manual stimulation, artificial vaginas (AVs), or electroejaculation techniques (ejaculation induced by rectal probe electrical stimulation) to obtain semen [8]. Artificial vaginas are often the preferred method of collecting ejaculate from domestic species as AVs yield the highest quality semen and minimize collection risks for animals and handlers compared to manual stimulation and electroejaculation [9,10,11,12,13]. Although AVs have been developed for several mammalian and avian species, most are designed for commercially valuable species (e.g., bovine, equine), with few modifications. Artificial vaginas are usually comprised of a rigid tube lined with rubber that represents the general shape of the vaginal canal [14,15,16]. Most commercially available AV designs have not considered species-specific reproductive anatomical features. The few AVs that incorporate knowledge of mating systems and genital interactions between the sexes have improved ejaculate quality. Examples of such modifications include an external air pump to stimulate the penis [17], adjustments to the AV length for increased tactile stimulation [18], and an imitation cervix for tactile stimulation in species with intra-uterine semen deposition [19,20]. Such modifications to AVs elicited natural male copulatory behaviors and durations, and improved semen quality [17,19,21], suggesting that penile stimulation during semen collection may be a critical factor that has been vastly overlooked in current commercially available AV designs.
Incorporating the physical properties of vaginal tissues into AV designs may be particularly important for species with complex reproductive anatomy. Genital morphologies are highly heterogeneous across species in shape, size, and complexity [22] and can be important in post-copulatory sexual selection (sperm competition, cryptic female choice) [23]. The limited research that explored the importance of genital shape during intromission found that shape contributed to sexual conflict and/or cooperation between the sexes [24,25,26,27]. Female cetaceans (whales, dolphins, and porpoises) are unique among mammals in possessing complex and species-specific vaginal folds, which are protrusions of the vaginal wall into the vaginal lumen (Figure 1A) [28,29]. Vaginal folds may function to exclude saltwater from the vaginal lumen during aquatic copulation [30] and/or allow for female control over paternity by obstructing the penis during intromission [27]. The genitalia of female and male cetaceans exhibit a tight copulatory fit, suggesting genital coevolution between the sexes [27]. When bottlenose dolphins (Turisops sp.) copulate ventrum-to-ventrum, an optimal alignment is produced that allows the penis to contact and bypass the vaginal folds [27], supporting a penile stimulatory functionality of vaginal folds. In this study, a bioinspired artificial vagina (BAV) was developed for bottlenose dolphins that reflects species-specific genital morphology. Bioinspired artificial vaginas were predicted to enhance the quality of collected ejaculate compared to manual stimulation collection techniques.

Figure 1.
Genital morphologies and bioinspired artificial vagina (BAV) fabrication for bottlenose dolphins (Tursiops sp.). (A) Endocast of the vaginal lumen of a common bottlenose dolphin (T. truncatus). The vaginal fold (indentation into the vaginal lumen) is indicated by the white arrow. (B) Penis of Indo-Pacific bottlenose dolphin (T. aduncus). (C) Three-dimensional print combining the vaginal endocast (left) and penis model (right). The vaginal endocast terminates distally at the cervix (left). The vaginal fold is indicated by the white arrow. (D) Bioinspired dolphin artificial vagina made of Dragon SkinTM silicone with attached silicone handle (yellow). The distal end of the BAV terminates at the cervix with a hole for semen collection (left). The proximal end of the BAV contacts the base of the penis during semen collection as a sleeve (right).
2. Materials and Methods
2.1. Bioinspired Artificial Vagina Development
Vaginal endocasts were created by filling frozen-thawed vaginal lumens of post-mortem, sexually mature, female bottlenose dolphins with Mold Star® 16 FAST silicone; the reproductive tracts were distended with silicone from the vaginal opening through the cervix [27]. Once fully firm, the silicone endocast was removed manually (Figure 1A) and a digital 3D model was created using a hand-held EinScan Pro HD 3D scanner (SHINING 3D Technology Inc., San Francisco, CA, USA). Three dimensional models of dolphin penises were created in Autodesk Fusion 360 (v 2.0.20981) using length and girth measurements provided by aquaria for each male dolphin included in the study. Bioinspired artificial vaginas were custom-sized to eliminate the potential for penis abrasion during this pioneering study. The digitized endocasts (in uniform size, as vaginal sizes are independent of penis sizes) were affixed to the terminal end of 3D penis models. The combined vaginal endocast/penis models were 3D printed with a Phenom Noir 3D printer (Peopoly, Hong Kong) using deft resin (Peopoly, Hong Kong; Figure 1C). Once cured, the 3D prints were coated with Dragon SkinTM (Smooth-On Inc, Macungie, PA, USA), a durable, highly elastic, silicone rubber with a soft, skin-like texture. Dragon SkinTM silicone rubber was mixed 15:1 with THI-VEXTM (Smooth-On Inc., Macungie, PA, USA) to increase the elasticity of the BAV while maintaining sufficient structural integrity to withstand semen collection training and experimental trials. The 3D-printed endocasts were removed, leaving a shell of soft and flexible silicone with an internal morphology identical to the vaginal lumen endocast (Figure 1D). A silicone handle was attached to the dorsal side of the BAV to aid trainers during collection and a hole was cut in the distal end of the BAV to collect the ejaculate (Figure 1D). A sterile plastic bag was attached over the distal end of the BAV during experimentation to collect the entire ejaculate.
2.2. Semen Collection
Semen was collected by the BAV and manual stimulation from one aquarium-reared sexually mature common bottlenose dolphin (Tursiops truncatus) and four Indo-Pacific bottlenose dolphins (Tursiops aduncus). The dolphins had been trained for voluntary semen collection for genetic banking using manual stimulation. The dolphins were trained to invert and hold their ventra above the water surface, allowing trainers to induce erection. Prior to semen collection, the inside of the BAV or the trainer’s gloved hand was lightly coated in a water-based lubricant. When full erection was achieved, the penis tip was directed into the BAV or a sterile plastic bag for semen collection. Males were trained to utilize the BAV approximately daily for 2–3 weeks prior to experimentation trials. The BAV desensitization process was gradual and interspersed with semen collection sessions using manual stimulation to ensure that dolphins maintained voluntary ejaculation.
During the two-week experimentation trials, two semen samples were collected from five dolphins per treatment (BAV and manual stimulation), totaling 20 semen samples. A custom-sized BAV was used for each male based on individual penis length and girth measurements. Bioinspired artificial vaginas were sterilized using chlorhexidine and water between each use. The time of each semen collection was recorded. One semen sample was collected from each dolphin per day with at least one day between collections. Ejaculate quality was evaluated based on semen volume, sperm concentration, sperm motility and kinematic parameters, and sperm plasma membrane integrity. Bottlenose dolphin semen are considered high quality based on volume (range: 3.9–99 mL), sperm concentration (range: 79 × 106–1294 × 106 sperm/mL), percentages of motile (range: 78–90%), progressive (range: 35–87%), and linear moving sperm (range: 43–90%), and percentage of sperm with intact plasma membranes (range: 81–94%) [31,32]. Semen processing, motility and kinematic analyses, and hypoosmotic swelling solution exposures were conducted immediately after sample collection. Aliquots of raw and hypoosmotic swelling solution exposed semen were preserved using 80% ethanol for analyses of concentration and plasma membrane integrity.
2.3. Macroscopic Semen Characteristics and Sperm Concentrations
Semen volume was measured immediately following sample collection using a graduated cylinder. The samples were subsequently transferred to sterile tubes and placed in a dry bath (FisherbrandTM IsotempTM, Fisher Scientific, Waltham, MA, USA) at 37 °C for the duration of motility and kinematic analyses. Semen pH was evaluated using a calibrated hand-held conductivity/pH meter fitted with a Micro pH electrode (STMICRO5, Ohaus Corporation, Parsippany, NJ, USA) and automatic temperature compensation probe (Oakton® Instruments, Cole-Parmer, Vernon Hills, IL, USA). None of the semen samples were urine- contaminated or saltwater-contaminated. Total sperm concentration per semen sample was determined post hoc via a hemacytometer (Hausser Scientific, 0.1 mm depth, Neubauer ruling) using a known dilution of 80% ethanol-fixed sperm [33].
2.4. Motility and Kinematic Analyses
An aliquot of semen was centrifuged for 10 min at 2000× g to separate seminal plasma from sperm. The seminal plasma without sperm was maintained at 37 °C until it was used to dilute semen to appropriate concentrations for motility and kinematic analyses. Seminal plasma was used instead of phosphate-buffered saline or semen extender to prevent altering natural sperm parameters (e.g., pH, salinity) [34]. Immediately after dilution in seminal plasma, 3 μL diluted semen was pipetted into a four-chambered, 20 μm deep counting slide (CV 1020-4cz, CellVision Technologies, Heerhugowaard, The Netherlands), which was pre-warmed and maintained at 37 °C using a microscope-stage slide warmer (HTM-MiniTherm®, Hamilton Thorne, Beverly, MA, USA). Sperm movement parameters were objectively assessed using a computer-aided sperm analysis (CASA) system (Microptic Sperm Class Analyzer®, v6.6.16; Microptic S.L., Barcelona, Spain). CASA analyses of motility and kinematics were conducted in phase contrast at 100× total magnification using a camera (Basler Ace acA1300-200uc; Basler Inc., Exton, PA, USA) attached to a microscope (Nikon Eclipse E200 LED MV R; Nikon Corp., Tokyo, Japan). Analyses were conducted at 50 fps with default SCA® mammalian settings (immotile cells = 0 μm/s, slow-medium = 5 μm/s, rapid > 25 μm/s, progressive > 80% STR) and a cell size range of 2–60 μm2. Motility parameters included total motile, immotile, progressive, non-progressive, rapid-speed, medium-speed, and slow-speed spermatozoa. Kinematic parameters included curvilinear velocity (VCL), straight-line velocity (VSL), average pathway velocity (VAP), straightness of movement (STR), linearity (LIN), amount of wobble (WOB), amplitude of lateral head displacement (ALH), and beat cross-frequency (BCF) of spermatozoa. All motility and kinematic analyses were completed within 1 h of semen collection to reduce the effects of time on parameters [35]. Files were assessed to remove non-intact sperm, cellular debris identified as sperm, and sperm with motility improperly categorized from the analyses. After cleaning the data, four samples had <200 sperm suitable for analysis. The number of sperm analyzed ranged from 126–356 per sample using at least five randomly selected visual fields.
2.5. Plasma Membrane Integrity Analysis
To assess the structural and functional integrity of sperm plasma membranes, a hypoosmotic swelling (HOS) test was applied [36]. Semen was diluted 1:10 in HOS solution (50% 1.5 mM sodium citrate, 50% 1.5 mM fructose) and incubated at least 30 min at 37 °C [36]. After incubation, the sample was fixed 1:1 with 80% ethanol. Tail abnormalities in fully intact sperm were manually assessed in wet mounts under brightfield illuminations of 600× magnification. Sperm with swollen tails possessed an intact plasma membrane [36]. A minimum of 200 randomly selected sperm were analyzed per semen sample.
2.6. Data Analyses
Data were analyzed and visualized using R statistical software (v4.2.3; [37]) and Microsoft Excel (v16.86). A linear mixed-effects model was used to analyze volume, pH, concentration, motility (percentages of total motile, immotile, progressive, non-progressive, rapid speed, medium speed, slow speed) and kinematic parameters (VCL, VSL, VAP, STR, LIN, WOB, ALH, BCF) using the nlme package (v3.1.164; [38]). Normality was verified using the Shapiro–Wilks test. Homogeneity in the variances was verified using the Brown Forsythe test. Outliers with a difference > 100 units from the mean were excluded from models. When data could not be transformed to a normal distribution, the robustlmm package was applied to the models [39]. Binomial plasma membrane integrity was assessed with a generalized linear mixed-effects model using the lme4 package (v1.1.35.5; [40]). The DHARMa package (v 0.4.6; [41]) was used to verify absence of overdispersion, normal distributions of random effects, and residual diagnostics. Collection technique (BAV or manual stimulation) was the fixed independent variable and individual was the random effect for all models.
3. Results
3.1. Macroscopic Semen Characteristics and Sperm Concentrations
The mean (±SD) semen volume collected with BAVs was 20.5 mL ± 17.07 (range: 1.8–46 mL, n = 10 ejaculates) compared to 20.12 mL ± 14.18 (range: 1.1–36 mL, n = 10 ejaculates) with manual stimulation (Figure 2A). Semen volume was not significantly different between collection methods (F = 0.12, p = 0.73, n = 20 samples; Table 1). The mean (±SD) semen pH for BAV collection was 7.68 ± 0.37 (range: 6.93–8.16, n = 10 ejaculates) and 7.55 ± 0.27 (range: 7.25–8.00, n = 7 ejaculates) for manual stimulation collection (Figure 2B). Semen pH was not significantly different between collection methods (F = 0.59, p = 0.46, n = 17 samples; Table 1). The mean (±SD) sperm concentration for BAV collection was 66.07 × 106 sperm/mL ± 103.71 × 106 (range: 1 × 106–335 × 106 sperm/mL, n = 6 samples) and 333.68 × 106 sperm/mL ± 787.56 × 106 (range: 6 × 106–1941 × 106 sperm/mL, n = 9 samples) for manual stimulation collection (Figure 2C). While a decreased trend in sperm concentration was observed with BAV collections, the concentration of sperm cells was not significantly different between collection methods (F = 2.42, p = 0.16, n = 15 samples; Table 1).

Figure 2.
Box and whisker plots of collection method effect on semen mean and median macroscopic characteristics: (A) semen volume (mL), (B) semen pH, and (C) sperm concentration (×106 sperm/mL) of five bottlenose dolphins (Tursiops sp.). n = 20 samples (2/treatment/individual) for semen volume. n = 10 for bioinspired artificial vagina and n = 7 for manual stimulation for semen pH. n = 9 for bioinspired artificial vagina and n = 6 for manual stimulation for sperm concentration. The “×” in each box represents the mean while the horizontal line represents the median. Outliers in sperm concentration indicate concentrations > 0.75 quantile threshold.

Table 1.
Linear mixed-effects model F and p values. Individual was the random effect in all models. n = 10 samples for volume and all motility and kinematic parameter models. n = 10 for bioinspired artificial vagina and n = 7 for manual stimulation for semen pH. n = 8 for bioinspired artificial vagina and n = 5 for manual stimulation for sperm concentration after exclusion of outliers. Kinematic parameter abbreviations: VCL = curvilinear velocity, VSL = straight-line velocity, VAP = average pathway velocity, STR = straightness of movement, LIN = linearity index, WOB = amount of wobble, ALH = amplitude of lateral head displacement, BCF = beat cross-frequency.
3.2. Motility and Kinematics
There were no statistically significant differences in motility parameters attributable to semen collection technique (Table 1). However, percentages of motile, progressive, and rapid sperm had higher medians and lower means when collected by BAV compared to manual stimulation (Figure 3; Table 2). The plots indicate that the sperm collected with the BAVs dichotomously performed well or poorly for these three parameters, without an intermediate spread of data as found with manual stimulation samples. Percentages of immotile and slow speed sperm had higher means but lower medians when collected by BAV (Figure 3; Table 2). No notable trends occurred in means or medians of the percentages of non-progressive and medium-speed sperm between collection techniques (Figure 3; Table 2).

Figure 3.
Box and whisker plots of collection method effect on sperm mean and median motility: (A) motile (%), (B) immotile (%), (C) progressive (%), (D) non-progressive (%), (E) rapid speed (%), (F) medium speed (%), and (G) slow speed (%) of five bottlenose dolphins (Tursiops sp.). n = 20 samples (2/treatment/individual). The “×” in each box represents the mean while the horizontal line represents the median.

Table 2.
Motility and kinematic parameter means, standard deviations, and medians. n = 10 samples for each treatment. Means or medians ≥ 4% difference between bioinspired artificial vagina and manual stimulation are in bold.
Sperm kinematic parameters were not significantly affected by semen collection technique (Table 1), although parameters associated with straightness of movement had higher means and medians when semen were collected with BAVs (straightness of movement, linearity index, amount of wobble; Figure 4; Table 2). No notable trends between collection methods were observed for the means or medians of curvilinear velocity, straight-line velocity, average pathway velocity, amplitude of lateral head displacement, and beat cross-frequency (Figure 4; Table 2).

Figure 4.
Box and whisker plots of collection method effect on sperm mean and median kinematics: (A) curvilinear velocity (μm/s), (B) straight-line velocity (μm/s), (C) average pathway velocity (μm/s), (D) straightness of movement, (E) linearity index (%), (F) amount of wobble (%), (G) amplitude of lateral head displacement (μm), and (H) beat cross-frequency (Hz) of five bottlenose dolphins (Tursiops sp.). n = 20 samples (2/treatment/individual). The “×” in each box represents the mean while the horizontal line represents the median.
3.3. Plasma Membrane Integrity
When semen was collected with BAVs, the mean (±SD) percentage of sperm with intact plasma membranes was 74.9% ± 6.92 (range: 60–85%, n = 1800 sperm; Figure 5). The mean (±SD) percentage of sperm with intact plasma membranes collected by manual stimulation was 74.5% ± 17.41 (range: 46–89%, n = 1000 sperm; Figure 5). The percentage and median of sperm cells with intact plasma membranes was not significantly different between collection methods (F = 12.08, p = 0.999, n = 2800 sperm; Table 1).
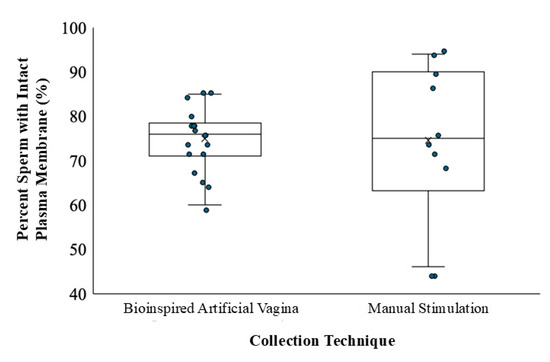
Figure 5.
Box and whisker plot of collection method effect on percentage of sperm with intact plasma membranes of five bottlenose dolphins (Tursiops sp.). n = 2800 sperm. The “×” in each box represents the mean while the horizontal line represents the median.
4. Discussion
A novel bioinspired artificial vagina was successfully developed and implemented for semen collection from bottlenose dolphins in the present study, representing the first AV developed for any cetacean species incorporating unique genital morphologies. Sperm motility and kinematic parameters fell within the upper range of parameters previously described for high-quality bottlenose dolphin semen [31,32]. Although the BAV did not statistically significantly affect sperm quality compared to manual stimulation collection, patterns of improved sperm motility and kinematic parameters suggest that further exploration of BAVs as semen collection devices is warranted. Ejaculate collected with BAVs produced dichotomous percentages of progressive and rapid-speed sperm, with higher median values for these parameters compared to manual stimulation collection. The observed trend of BAVs improving dolphin sperm motility is consistent with patterns in camels (Camelus dromedarius) in which AVs reflecting reproductive morphology improved sperm motility compared to commercially available AVs and electroejaculation [42]. Mean and median values for sperm kinematic parameters related to linearity of movement improved non-significantly when semen were collected with BAVs. As linear movement patterns are necessary in the female reproductive tract to reach the ovum, improved linearity of sperm movement (straightness, linearity, amount of wobble) is associated with augmented semen quality and fertility in boars (Sus scrofa domesticus) [43].
In contrast to previous research on bulls (Bos taurus) and sheep (Ovis aries) that compared AV and electroejaculation semen collection techniques [44,45,46], bottlenose dolphin sperm concentration decreased non-significantly when collected by BAV compared to manual stimulation. Electroejaculation stimulates the prostate gland and simultaneously increases the volume of seminal fluid in semen [45,47]. Increased seminal fluid composition of semen is also associated with increased total semen volume and pH [44,45,48]. Despite differences in semen volume between AV and electroejaculation methods in llamas (Lama glama), no differences in total sperm number were observed between collection methods in the present study [49]. As semen concentration, volume, and pH did not significantly differ in bottlenose dolphins between collection methods, the BAV likely does not affect the relative ratio of sperm-to-seminal fluid in semen. Interpretation of the observed decrease in sperm concentration with BAV collection was impeded by agglutination in fixed sperm samples from both collection techniques, resulting in limited sample sizes. Future analyses of semen samples collected by BAV could explore alternative fixation protocols to increase samples sizes for concentration analysis. Examination of the proteomic profiles of semen samples collected with the BAV could provide insights into how the semen constituents are affected by the collection method.
Bottlenose dolphin sperm plasma membrane integrity was lower using both collection techniques in the present study compared to another published study [32]. No trends were detected in bottlenose dolphin sperm plasma membrane integrity based on collection technique, consistent with a study on sheep [46], but inconsistent with other studies on sheep and camels [42,50]. AV component materials such as rubber and latex had detrimental effects on sperm plasma membrane integrity in horses (Equus caballus) and cows [51,52,53]. As silicone AV liners did not harm camel semen [54], future studies should continue to examine the effects of silicone on sperm properties across species for widespread implementation in commercial AV models.
The effects of variable AV designs on collected semen quality have identified conflicting results [14,19], highlighting the complex nature of assessing factors that affect collected semen quality and the need for more research exploring BAV implementation. While semen samples were collected once daily for analysis, ejaculations outside of voluntary semen collection sessions likely occurred as dolphin socio-sexual behaviors are frequently observed in aquaria [55]. Ejaculations occurring outside of experimental trials may have affected results as semen quality decreases with successive sampling [56,57]. The limited sample size for common bottlenose dolphins (one individual) compared to Indo-Pacific bottlenose dolphins (four individuals) precluded comparisons of semen parameters across species. Individual variation in sperm parameters between T. aduncus and T. truncatus may be impacted by variations in training techniques used between facilities rather than differences in reproductive biology as vaginal and penile morphology are conserved within the Tursiops genus (D.N.O. pers. observ.). A longer desensitization/training period may improve the consistency of sample collection for all dolphins and increase sample size, generating more pronounced patterns. Future studies could explore how social/environmental factors affect sperm quality, such as the presence of nearby conspecifics, shifts in social groupings/hierarchies, trainers involved in semen collection, variability in training methods, and ambient noise from weather/construction.
Bioinspired artificial vaginas constructed using soft silicone that reflect species-specific genital morphologies present a promising avenue for future research in advancing semen collection techniques. Developing BAVs for species with commercially available AVs may further illuminate patterns in collected semen quality. Future BAV prototypes could explore a single-size BAV with manipulatable internal widths based on penis girth as applied to livestock AVs using an internal bladder [16,17,19]. The observed trends of improved sperm motility and linearity of movement and a lack of negative effects on overall dolphin semen quality suggest continued research on BAVs as tools could support breeding program success. Breeding programs are increasingly important to support species conservation and fertilization success rates [4]. Applying custom-designed BAVs that incorporate genital morphological features may augment ejaculate collection for artificial insemination application in a range of species.
Author Contributions
Conceptualization, D.N.O. and J.R.C.; methodology, D.N.O., J.R.C., and J.R.; formal analysis, J.R.; resources, D.N.O. and G.J.S.-C.; data curation, J.R. and G.J.S.-C.; writing—original draft preparation, J.R.; writing—review and editing, D.N.O., J.R.C., and G.J.S.-C.; visualization, J.R.; supervision, D.N.O.; funding acquisition, D.N.O. and J.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Science Foundation [grant #2216595 awarded to D.N.O.]; Texas A&M University–Corpus Christi’s Office of Research and Innovation [Equipment and Infrastructure Grant and Texas Comprehensive Research Fund awarded to D.N.O.]; the Society for Integrative and Comparative Biology [Fellowship of Graduate Student Travel awarded to J.R.], and the Mount Holyoke College Alumnae Association [Bardwell Fellowship awarded to J.R.].
Institutional Review Board Statement
Data were collected and analyzed under permits from Texas A&M University, Corpus Christi’s Institutional Animal Care and Use Committee (IACUC-2023-0008), Texas A&M University, Corpus Christi’s Institutional Biosafety Committee (IBC-2023-0017), and consent from collaborating aquaria. Post-mortem dolphins were collected under a National Oceanic and Atmospheric Administration National Marine Fisheries Services parts authorization letter to DNO.
Data Availability Statement
The data and code from this project are available upon request to the corresponding author.
Acknowledgments
We thank Diane Kelly and Patricia Brennan for their feedback on the BAV fabrication. We thank the dolphin care and training teams at Dolphin Discovery Akumal, Dolphin Discovery Cancun, Dolphin Discovery Maroma, and Dolphin Discovery Puerto Aventuras, particularly Alejandra, Alicia, Calli, Cony, Esmerelda, Luisa, Mario, and Nayeli for training the dolphins and conducting the coordination and collection of semen samples. We thank the dolphin husbandry teams at SeaWorld Orlando, particularly Gisele Montano, and The Dolphin Company, particularly Sarah Gordon and Caitlin Rose, for testing the BAV prototypes. We thank Rosario Alvarado for assistance with data collection. We thank Hussain Abdulla, Christopher Hollenbeck and three anonymous reviewers for providing helpful feedback on the manuscript.
Conflicts of Interest
Author G.J.S.C. was employed by The Dolphin Company. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| AI | artificial insemination |
| ART | assisted reproductive technologies |
| AV | artificial vagina |
| BAV | bioinspired artificial vagina |
| CASA | computer-aided sperm analysis |
References
- Andermann, T.; Faurby, S.; Turvey, S.T.; Antonelli, A.; Silvestro, D. The past and future human impact on mammalian diversity. Sci. Adv. 2020, 6, eabb2313. [Google Scholar] [CrossRef] [PubMed]
- Yitbarek, M.B. Livestock and livestock product trends by 2050: Review. Int. J. Anim. Res. 2019, 4, 30. [Google Scholar]
- Herrick, J.R. Assisted reproductive technologies for endangered species conservation: Developing sophisticated protocols with limited access to animals with unique reproductive mechanisms. Biol. Reprod. 2019, 100, 1158–1170. [Google Scholar] [CrossRef] [PubMed]
- Comizzoli, P.; Holt, W.V. Breakthroughs and new horizons in reproductive biology of rare and endangered animal species. Biol. Reprod. 2019, 101, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Snook, R.R. Sperm in competition: Not playing by the numbers. Trends Ecol. Evol. 2005, 20, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Holt, W.V.; Van Look, K.J.W. Concepts in sperm heterogeneity, sperm selection and sperm competition as biological foundations for laboratory tests of semen quality. Reproduction 2004, 127, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Dziekońska, A.; Partyka, A. Current status and advances in semen preservation. Animals 2023, 13, 123. [Google Scholar] [CrossRef] [PubMed]
- Prieto, M.T.; Sanchez-Calabuig, M.J.; Hildebrandt, T.B.; Santiago-Moreno, J.; Saragusty, J. Sperm cryopreservation in wild animals. Eur. J. Wildl. Res. 2014, 60, 851–864. [Google Scholar] [CrossRef]
- Aral, F.; Aral, S. Comparison of semen collection methods in Merino rams. Turk. J. Vet. Anim. Sci. 2004, 28, 47–53. [Google Scholar]
- Gvaryahu, G.; Robinzon, B.; Meltzer, A.; Perek, M.; Snapir, N. An improved method for obtaining semen from Muscovy drakes and some of its quantitative and qualitative characteristics. Poult. Sci. 1984, 63, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Rabadán, P.; Ramón, M.; García-Álvarez, O.; Maroto-Morales, A.; del Olmo, E.; Pérez-Guzmán, M.D.; Bisbal, A.; Fernández-Santos, M.R.; Garde, J.J.; Soler, A.J. Effect of semen collection method (artificial vagina vs. electroejaculation), extender and centrifugation on post-thaw sperm quality of Blanca-Celtibérica buck ejaculates. Anim. Reprod. Sci. 2012, 132, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Matthews, N.; Bester, N.; Schwalbach, L.M.J. A comparison of ram semen collected by artificial vagina and electro-ejaculation. S. Afr. J. Anim. Sci. 2003, 4, 28–30. [Google Scholar]
- Nadaf, S.M.; Ramesh, V.; Mech, M.; Khan, M.H.; Ahmed, F.A.; Ponraj, P.; Mitra, A. Comparative ejaculatory response, fresh and frozen semen quality and fertility to artificial vagina vs electroejaculation method of semen collection in mithun (Bos frontalis) bulls. Andrologia 2022, 53, e14330. [Google Scholar] [CrossRef] [PubMed]
- Cholakkal, I.K.; Koroth, A.; Sharifi, S.A. Observations on semen collection and suitability of different modifications of artificial vagina for dromedary camels (Camelus dromedarius). J. Camel Pract. Res. 2016, 23, 169–174. [Google Scholar] [CrossRef]
- Dascanio, J.J. Missouri artificial vagina. In Equine Reproductive Procedures; Dascanio, J., McCue, P., Eds.; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2014; pp. 329–331. [Google Scholar]
- Love, C.C. Semen collection techniques. Vet. Clin. N. Am. Equine Pract. 1992, 8, 111–128. [Google Scholar] [CrossRef] [PubMed]
- Harrop, A.E. A new type of canine artificial vagina. Br. Vet. J. 1954, 110, 194–196. [Google Scholar] [CrossRef]
- Seidel, G.E.; Foote, R.H. Influence of semen collection interval and tactile stimuli on semen quality and sperm output in bulls. J. Dairy Sci. 1969, 52, 1074–1079. [Google Scholar] [CrossRef] [PubMed]
- Bravo, P.W.; Flores, U.; Garnica, J.; Ordoñez, C. Collection of semen and artificial insemination of alpacas. Theriogenology 1997, 47, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Skidmore, J.A.; Morton, K.M.; Billah, M. Artificial insemination in dromedary camels. Anim. Reprod. Sci. 2013, 136, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Romano, J.E.; Christians, C.J. Sperm loss using different artificial vaginas in rams. Small Rumin. Res. 2009, 83, 85–87. [Google Scholar] [CrossRef]
- Brennan, P.L.R. Studying genital coevolution to understand intermittent organ morphology. Integr. Comp. Biol. 2016, 56, 669–681. [Google Scholar] [CrossRef] [PubMed]
- Eberhard, W.G. Sexual Selection and Animal Genitalia; Harvard University Press: Cambridge, UK, 1985. [Google Scholar]
- Brennan, P.L.; Prum, R.O.; McCracken, K.G.; Sorenson, M.D.; Wilson, R.E.; Birkhead, T.R. Coevolution of male and female genital morphology in waterfowl. PLoS ONE 2007, 2, e418. [Google Scholar] [CrossRef] [PubMed]
- Friesen, C.R.; Uhrig, E.J.; Squire, M.K.; Mason, R.T.; Brennan, P.L.R. Sexual conflict over mating in red-sided garter snakes (Thamnophis sirtalis) as indicated by experimental manipulation of genitalia. Proc. R. Soc. B 2014, 281, 20132694. [Google Scholar] [CrossRef] [PubMed]
- Hedrick, B.P.; Antalek-Schrag, P.; Conith, A.J.; Natanson, L.J.; Brennan, P.L.R. Variability and asymmetry in the shape of the spiny dogfish vagina revealed by 2D and 3D geometric morphometrics. J. Zool. 2019, 308, 16–27. [Google Scholar] [CrossRef]
- Orbach, D.N.; Kelly, D.A.; Solano, M.; Brennan, P.L.R. Genital interactions during simulated copulation among marine mammals. Proc. R. Soc. B 2017, 284, 20171265. [Google Scholar] [CrossRef] [PubMed]
- Orbach, D.N.; Marshall, C.D.; Mesnick, S.L.; Würsig, B. Patterns of cetacean vaginal folds yield insights into functionality. PLoS ONE 2017, 12, e0175037. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, J.P. Breeding bottlenose dolphins in captivity. In The Bottlenose Dolphin; Leatherwood, S., Reeves, R.R., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 435–446. [Google Scholar]
- Robeck, T.R.; Curry, B.F.; McBain, J.F.; Kraemer, D.C. Reproductive biology of the bottlenose dolphin (Tursiops truncatus) and the potential application of advanced reproductive technologies. J. Zoo. Wildl. Med. 1994, 25, 321–336. [Google Scholar]
- van der Horst, G.; Medger, K.; Steckler, D.; Luther, I.; Bartels, P. Bottlenose dolphin (Tursiops truncatus) sperm revisited: Motility, morphology and ultrastructure of fresh sperm of consecutive ejaculates. Anim. Reprod. Sci. 2018, 195, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Yuen, Q.W.H.; Brook, F.M.; Kinoshita, R.E.; Ying, M.T.C. Semen collection and ejaculate characteristics in the Indo-Pacific bottlenose dolphin (Tursiops aduncus). J. Androl. 2009, 30, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Dinnel, P.A.; Link, J.M.; Stober, Q.J. Improved methodology for a sea urchin sperm cell bioassay for marine waters. Arch. Environ. Contam. Toxicol. 1987, 16, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Rich, J.; Cowart, J.R.; Montano, G.; Sánchez-Contreras, G.J.; Orbach, D.N. Salinity and pH affect common bottlenose dolphin (Tursiops truncatus) sperm viability. Anim. Reprod. Sci. 2025, 277, 107838. [Google Scholar] [CrossRef] [PubMed]
- Chomsrimek, N.; Choktanasiri, W.; Wongkularb, A.; O-Prasertsawat, P. Effect of time between ejaculation and analysis on sperm motility. Thai J. Obstet. Gynaecol. 2008, 16, 109–114. [Google Scholar]
- Ramu, S.; Jeyendran, R.S. The hypo-osmotic swelling test for evaluation of sperm membrane integrity. In Spermatogenesis: Methods in Molecular Biology; Carrell, D., Aston, K., Eds.; Humana Press: Totowa, NJ, USA, 2013; pp. 21–25. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Pinheiro, J.; Bates, D. ; R Core Team. nlme: Linear and Nonlinear Mixed Effects Models, R Package, version 3; R Core Team: Vienna, Austria, 2024; pp. 1–166. [Google Scholar]
- Koller, M. robustlmm: An R package for robust estimation of linear mixed-effects models. J. State Softw. 2016, 75, 1–24. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. State Softw. 2015, 67, 1–48. [Google Scholar]
- Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models. R Package, version 4; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Mansour, N. A novel, patented method for semen collection in dromedary camel (Camelus dromedarius). Reprod. Dom. Anim. 2023, 58, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Fernández-López, P.; Garriga, J.; Casas, I.; Yeste, M.; Bartumeus, F. Predicting fertility from sperm motility landscapes. Commun. Biol. 2022, 5, 1027. [Google Scholar] [CrossRef] [PubMed]
- Brogliatti, G.; Garcia Migliaro, F.; Cavia, R.; Larraburu, G.; Albrecht, A. CASA parameters of fresh bull semen collected by artificial vagina or electroejaculation in Argentina. Reprod. Fertil. Dev. 2005, 17, 156. [Google Scholar] [CrossRef]
- Clarke, R.H.; Hewetson, R.W.; Thompson, B.J. Comparison of the fertility of bovine semen collected by artificial vagina and electro-ejaculation from bulls with low libido. Aust. Vet. J. 1973, 49, 240–241. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Rabadán, P.; Soler, A.J.; Ramón, M.; García-Álvarez, O.; Maroto-Morales, A.; Iniesta-Cuerda, M.; Fernández-Santos, M.R.; Montoro, V.; Pérez-Guzmán, M.D.; Garde, J.J. Influence of semen collection method on sperm cryoresistance in small ruminants. Anim. Reprod. Sci. 2016, 167, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Abraham, M.C.; de Verdier, K.; Bage, R.; Morrell, J.M. Semen collection methods in alpacas. Vet. Rec. 2017, 180, 613–614. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, L.C.; Masken, J.F.; Hopwood, M.L. Fractionation of the bovine ejaculate. J. Dairy Sci. 1964, 47, 823–825. [Google Scholar] [CrossRef]
- Giuliano, S.; Director, A.; Gambarotta, M.; Trasorras, V.; Miragaya, M. Collection method, season and individual variation on seminal characteristics in the llama (Lama glama). Anim. Reprod. Sci. 2008, 104, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Ledesma, A.; Manes, J.; Ríos, G.; Aller, J.; Cesari, A.; Alberio, R.; Hozbor, F. Effect of seminal plasma on post-thaw quality and functionality of Corriedale ram sperm obtained by electroejaculation and artificial vagina. Reprod. Dom. Anim. 2015, 50, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Colborn, D.R.; Merilan, C.P.; Loch, W.E. Temperature and pH effects on rubber toxicity for equine spermatozoa. J. Equine Vet. Sci. 1990, 10, 343–347. [Google Scholar] [CrossRef]
- Flick, D.L.; Merilan, C.P. Toxicity of artificial vagina liners for bovine spermatozoa. Theriogenology 1988, 29, 1207–1213. [Google Scholar] [CrossRef]
- Merilan, C.P.; Loch, W.E. The effect of artificial vagina liners on livability of stallion spermatozoa. J. Equine Vet. Sci. 1987, 7, 226–228. [Google Scholar] [CrossRef]
- Monaco, D.; Fatnassi, M.; Lamia, D.; Padalino, B.; Seddik, M.-M.; Khorchani, T.; Hammadi, M.; Lacalandra, G.M. Mating behaviour and semen parameters in dromedary camel bulls (Camelus dromedarius): A comparison between two types of artificial vagina. Emir. J. Food Agric. 2018, 30, 326–334. [Google Scholar] [CrossRef]
- Serres, A.; Hao, Y.; Wang, D. Socio-sexual interactions in captive finless porpoises and bottlenose dolphins. Mar. Mammal Sci. 2022, 38, 812–821. [Google Scholar] [CrossRef]
- Carlsen, E.; Peterson, J.H.; Andersson, A.-M.; Skakkebaek, N.E. Effects of ejaculatory frequency and season on variations in semen quality. Fertil. Steril. 2004, 82, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Ollero, M.; Muniño-Blanco, T.; López-Pérez, M.J.; Cebrián-Pérez, J.A. Viability of ram spermatozoa in relation to the abstinence period and successive ejaculations. Int. J. Androl. 1996, 19, 287–292. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).