Abstract
The combination of consumer’s ongoing demand for chia (Salvia hispanica L.) alongside the increased demand for regionally produced food products provided the impetus for this study. Its aim was to test if a regional cultivation of new chia genotypes, which were adapted to day lengths greater than 12 h, is feasible under Central European conditions. Therefore, three early flowering chia genotypes (Sahi Alba 914, W13.1, G8) were cultivated in a randomized block design at two experimental stations in Southwestern Germany (Ihinger Hof, Eckartsweier) over the course of two years (2015, 2016). Mean yields ranged from 100 to 1290 kg ha−1. Mucilage content ranged from 9.5% to 12.2%, while the crude protein content ranged from 17.2% to 25.0%. Crude oil content fell in the range of 30.9–33.7% and the PUFA:SAT ratio ranged from 4.0 to 9.4, whereas the omega6:omega3 ratio varied from 0.27 to 0.5. As chia seed yields surpassed yield levels obtained by their countries of origin and as quality parameters obtained, were in line with the genotypes cultivated in their countries of origin, it can be assumed that a regional chia production in Southwestern Germany offers great potential, being ecologically and economically profitable.
1. Introduction
Chia (Salvia hispanica L.) is a summer annual herbaceous plant belonging to the Lamiaceae family. Evidence suggests the Aztecs first used chia as a food prior to 3500 BC [1] (p. 63). Later, by 1500 to 900 BC, it was grown in the center of Mexico as a cash crop [1] (p. 63). In pre-Columbian times chia was one of the four basic dietary elements of the Central American civilization besides corn, beans and amaranth [1,2]. It was used as a tithe or form of sacrificial offering, among other things [1] (p. 72). Chia nearly passed into extinction due to the destruction and eradication of everything related to pre-Hispanic religion [1] (p. 65).
As worldwide public health awareness and the demand for functional food with multitudinously health benefits has increased [3], chia seeds have gained popularity in recent years due its numerous nutritional characteristics including high concentrations of extractable fatty acids, large quantities of polyunsaturated fatty acids (ω3 and ω6), extraordinary mucilaginous fiber content, vitamins, minerals and antioxidants [2,4,5,6,7]. Further, due to its high adaptability to different pedoclimatic conditions, chia is considered as an alternative crop in terms of food security and climate change [8].
Chia grows naturally in tropical and subtropical environments. Its cultivation is spreading rapidly from its center of origin in Central America to new areas of cultivation such as Africa, Australia, Europe and North America as the market for chia is profitable and favorable [9]. Chia is intolerant to freezing in all development stages [9,10] and has optimum growth temperatures of 16–26 °C [11]. It is considered to be a short-day plant with a threshold of 12–13 h [12,13] and as such, its period of growth and fruiting depends on the latitude where it is grown. Due to the dedication of breeders, new long-day flowering genotypes have been produced, which are able to induce flower formation at 12 h day lengths or more. Unlike regular chia, these genotypes can therefore flower earlier in summer when day length are longer than 12 h, allowing for maturation before frost. This no longer limits chia cultivation to latitudes lower than 25 degrees near the equator, therefore enabling its cultivation to a wider range of environmental conditions [12,14].
In Europe, promising studies have already been conducted in Italy (latitude 40° N) and Greece (latitude 22° N) but as of now, chia has never been cultivated further north from the equator [9,15]. Cultivating chia at a latitude of 48 degrees north, were mean temperatures are lower and day length of 13.86 to 15.12 h are given has never been tested before. Hence, the objective of this study was to examine three early flowering chia genotypes with regard to their growth and yield performance under the given day length and climatic conditions of Southwestern Germany. Additionally, quality parameters like protein and oil content, mucilage content as well as fatty acid profiles were investigated. Based on the results of this research work, suitable chia genotypes for the cultivation in Southwestern Germany should be selected that are in line with the Novel Food-Regulation (EU) 2015/2283 in order to ensure a domestic chia supply [16,17].
2. Materials and Methods
2.1. Site Descriptions
Field trials were conducted in two consecutive growing seasons (2015/2016) at the experimental station Ihinger Hof (IHO) (Upper Neckarland, Lat. N 48°44′40,70′′ Lon. E 8°55′26,36′′) of the University of Hohenheim. An additional field trial was conducted in 2016 at the University’s experimental station in Eckartsweier (EWE) (Upper Rhine Valley, Lat. N 48°31′45,24′′ Lon. E 7°51′12,81′′) in order to determine the influence of different environmental conditions on yield and quality parameters of chia.
Precipitation during the experimental period at IHO in 2015 and 2016 amounted to 246.6 and 354.8 mm respectively and the mean temperature during the experimental period was 15.0 and 15.5 °C respectively. At EWE, the precipitation amounted to 212.4 mm during the experimental period in 2016 and the mean temperature was 18.8 °C (Figure 1). Meteorological data was obtained by the weather stations at IHO and EWE.
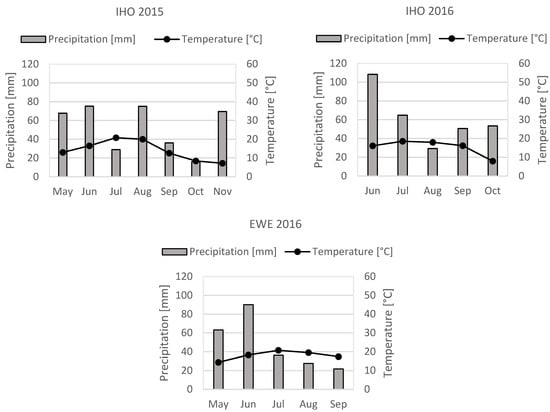
Figure 1.
Precipitation [mm, bars] and mean temperature [°C; ●] during the experimental period at Ihinger Hof in 2015, 2016 and EWE in 2016. Jun, January. Jul, July. Aug, August. Sep, September. Oct, October. Nov, November.
GDD for both locations was calculated using the growing degree-day formula Equation (1) of McMaster and Wilhelm [18], where Tmax is defined as the daily maximum air temperature and Tmin is defined as the daily minimum air temperature, and Tbase as the crop base temperature. The crop base temperature of 10 °C according to Baginsky et al. [2], was subtracted from the daily average air temperature.
If the daily maximum air temperature was less than crop base temperature, ((TMAX + TMIN)/2) < TBASE, then ((TMAX + TMIN)/2) = TBASE
According to the World Reference Base [19] experimental soils at IHO can be characterized as Terra Fusca brown soil and Pelosol brown soil in 2015 and 2016, respectively. The composition of clay, sand and silt contents of the top soils in both years were quite similar. On average sand content was about 10%, silt about 64% and clay about 26%. The pH values differed only marginally from each other (7.1 to 7.45). Prior to sowing, soil mineral nitrogen content (Nmin) from 0 to 90 cm amounted to 9.45 kg ha−1 (Stockacker) and 31.82 kg ha−1 (Dreispitz) in 2015 and 22.96 kg ha−1 (Härdtle) in 2016. A soil mineral nitrogen content of 6.30 kg ha−1 was identified for EWE in 2016 (Table S1).
2.2. Plant Material
At both locations the three genotypes cultivated, produced inflorescences under long day conditions as they were either day length insensitive (Sahi Alba 914) or adapted to day lengths greater than 12 h (G8, W13.1) (Table 1). Genotype G8 was generated by gamma ray-mutagenesis according to Hildebrand et al. [20]. W13.1 derived from a cross between G8 and a white-flowered, white-seeded commercial chia (wild type). Sahi Alba 914 was obtained by individual selection of Sahi Alba 912 for early flowering plants over a period of 6 years in Argentina [14].

Table 1.
Cultivated chia genotypes at IHO (2015, 2016) and EWE (2016).
2.3. Trial Setup
In both years and locations, field trials were carried out as randomized block designs with three replications. Plot size for each cultivar at IHO was 6 m × 5 m in 2015 and 10 m × 3 m in 2016, respectively. At EWE in 2016 plot sizes were 7 m × 1.5 m. Mechanical sowing at IHO took place on 21 May 2015 and 27 May 2016 using a Deppe D82 Sower (Agrar Markt DEPPE GmbH, Rosdorf, Germany). Hand sowing at EWE took place on 11 May 2016. Sowing density amounted to 2.5 kg ha−1 in 2015 with a row spacing of 75 cm, and 1.5 kg ha−1 in 2016 with a row spacing of 50 cm at IHO, while 2.0 kg ha−1 sowing density at 37.5 cm was chosen for EWE. In order to prevent outcrossing during flowering, the two white seeded genotypes W13.1 and Sahi Alba 914 were cultivated spatially separated from G8 in 2015 (different experimental areas), whereas in 2016 a spatial distance was kept on the same experimental area. The different row spacing and sowing densities were accounted for in the statistics later on. In 2015, harrowing at IHO took place one day before sowing, in 2016 it took place 73 days before sowing, and at EWE in 2016 51 and 30 days prior to sowing. Seedbed preparation was conducted at IHO on the same day as sowing in 2015 and eight days prior to sowing in 2016. Seed bed preparation at EWE was done one day before sowing. Nitrogen fertilization (20 kg N ha−1) as calcium ammonium nitrate was applied 23 days after sowing at IHO in 2015 and 15 days after sowing in 2016. In 2016, 31 days after sowing 40 kg N ha−1 were applied as ‘ENTEC® 26’ (EuroChem Agro GmbH, Mannheim, Germany), at EWE. Weed control was performed manually in the plots and mechanically by hoeing around the plots if necessary at both locations in both years.
2.4. Experimental Procedure
2.4.1. Soil Mineral Nitrogen Content
Soil mineral nitrogen content was determined prior to sowing according to Bassler and Hoffman [21]. Soil samples were dried at 100 °C for 24 h in a drying oven. Afterwards the samples were ground with a type SR3 rotor beater mill (Retsch, Haan, Germany) (5 mm sieve). 20 g of soil were weighed in a 250 mL PE bottle with screw cap. The extraction was carried out with 200 mL of 0.025 n CaCl2 solution (extraction ratio 1:10) by shaking for one hour. The nitrate content of the filtrate, which was filtered through a folded filter (15 cm diameter, Type 619 G 1/4) (Macherey-Nagel GmbH & Co. KG, Düren, Germany), was analyzed by using the FIAstar 5000 Analyzer (FOSS Analytical, Hilleroed Denmark).
2.4.2. Phenological and Morphological Data Collection
During the course of the field experiment in 2016, ten plants per plot were recorded and assessed in detail every week. Days until flowering and maturity were monitored, single plant height and final seed weight was measured. Mean length and width of seeds were determined with the seed analyzer MARVIN (GTA Sensorik GmbH, Neubrandenburg, Germany). The number of non-senescent first degree branches at the main stem, number of inflorescences (>5 cm) and seeds produced per single plant were counted and the length of the central axis inflorescence was measured.
2.4.3. Yield Parameters
Ten plants per plot were harvested manually at maturity in order to obtain the mean single plant yield. All inflorescences per single plant were cut off and dried for 24 h at 40 °C. Afterwards the inflorescences were threshed manually and the cleaned seeds were weighed and counted.
Plot harvest took place mechanically with a plot combine Classic (Wintersteiger AG, Ried, Austria). Yield was evaluated on the basis of absolute dry matter (0% grain moisture) and the mean thousand kernel was additionally identified on the basis of the mechanically threshed yield. Thousand seeds per repetition were counted by Contador (Pfeuffer GmbH, Kitzingen, Germany) and weighed in order to determine the mean thousand kernel mass. Additionally, the final number of plants per square meter was ascertained.
2.4.4. Quality Parameters
Mucilage extraction took place according to Muñoz et al. [22]. In order to extract the mucilage, whole chia seeds (5 g) were placed in a 250 mL beaker. Distilled water was added in a ratio of 1:40. At the beginning of soaking and after 2 h of hydration process, the pH was recorded. During extraction, the temperature was maintained at 80 ± 1.5 °C using a temperature controller. The mixtures were stirred by ultrasound bathing and hydrated for 2 h. In deviation to Muñoz et al. [22], extraction took place three times. Afterwards the aqueous suspension was spread on a drying tray and exposed to 50 °C for at least 10 h. By rubbing the dried mucilage over a 0.5 mm mesh screen was separated and the weight was recorded. Mucilage weight was represented by the difference in weight before and after its extraction.
By using the vario MACRO cube CHNS (Elementar Analysensysteme GmbH, Langenselbold, Germany) the total seed N content was determined according to Dumas [23]. The values were multiplied by 5.71 (conversion factors) in order to estimate the crude protein content [24].
Crude oil content was determined by Soxhlet extraction with petroleum benzene as solvent according to the European Commission’s Regulation 152/III H procedure B [25].
Producing fatty acid methyl esters (FAMEs) with methanolic BF3 (BF3·CH3OH) by rapid saponification and esterification led to the determination of the fatty acid profile [26,27,28]. To do so, 20 µL of nonadecylic acid (C19:0) (Merck KGaA, Darmstadt, Germany) (5 mg mL−1 hexane), the internal standard, was pipetted into a screw cap (culture tube) (DURAN GL14) and evaporated for 15 min beneath the fume hood. By adding 0.5 mL methanolic KOH (0.5 M) to a tube with 5 mg crude fat sample at 80 °C for 5 min saponification of the triglycerides took place. Subsequent to the cooling process, 2 mL of methanolic BF3 was added to each sample and heated to 80 °C for 5 min one more time. Samples were put in an ice bath for 10 min to cool before 1 mL of saturated NaCl solution and 2 mL of n-hexane were added. The organic phase, including the FAMEs, which resulted from the above-mentioned procedure, was isolated and stored at −24 °C for the following gas chromatography analysis. In order to calibrate the gas chromatograph, a FAME standard (10 mg/mL hexane, Marine Oil FAME Mix, Restek Corporation, Bellefonte, PA, USA), containing a mixture of fatty acids with even and uneven numbers of C-atoms (nonadecylic acid included), concentrations of 0.25, 0.5 and 1 mg mL−1 was used. A GC appliance (Shimadzu GC-2010 Plus, Shimadzu Corp., Kyōto, Japan) equipped with a flame ionization detector (Shimadzu FID-2010 Plus) was used for FAME analysis. By the use of an autosampler (Shimadzu AOC-201) 1.0 μL of sample solution was injected. Coated with 0.25 μm polyethylene glycol a 30 mm × 0.25 mm inner diameter fused silica capillary column (FAMEWAX, Restek Corporation, Bellefonte, PA, USA) was installed in the GC oven. Helium (purity 99.9%) was used as carrier gas at a constant flow rate of 25.7 mL min−1. Total runtime of the continuous process was 26 min. The temperature program of the GC oven began at 180 °C, heated at 5 °C min−1 to 220 °C (kept for 1 min). Thereafter it was heated at 5 °C min−1 to 240 °C (kept for 8 min). Lastly, it was heated at a rate of 5 °C min−1 to 250 °C (kept for 4 min). Analyses were performed in duplicates. Fatty acid contents were determined using their peak area relative to that of the internal standard.
2.5. Statistical Analysis
Statistical analysis was conducted by using the statistical software R version 3.2.1 (The R Foundation for Statistical Computing, Vienna, Austria). The following linear mixed model was fit to different traits, which were determined by single plant measurements (10 per plot), namely plant height, length of central axis inflorescence, number of branches, number of inflorescences, number of seeds, seed weight, seed length and seed width:
where is the observation of the traits of the l-th plant from the k-th variety, in the j-th block and the i-th location. is the overall intercept, is the effect of the i-th location, . is the effect of the j-th block within the i-th location, is the effect of the k-th variety, is the interaction of location and variety, is the effect of the ijk-th plot and are the errors associated with . , and were considered random effects with mean zero and variances , and .
The following model was used for analysis of the assessed yield and quality traits such as yield, TKM, crude fat and protein content, mucilage content, fatty acids and the corresponding ratios.
where is the observation of the k-th variety in the j-th block of the i-th location and the a-th year, is the common intercept, is the effect of the i-th location, is the effect of the a-th year, is the effect of the k-th variety, is the interaction of year and variety, is the interaction of location and variety, is the effect of the -th block and are the errors associated with . and were considered random effects with mean zero variances , and .
Referring to the different sowing rates and distances given in Section 2.3 it should be pointed out that sowing rate and row distance were integral parts of the site and annual effects.
In order to display means and standard errors for the genotype G8, simplified versions of models (2) and (3) were used which did not contain variety effects. Normality of residuals and homogeneity of variance was tested by the inspection of residual plots. If residual plots indicated any violations the response variable was transformed using logarithm or square root transformation. Variance components were estimated by the REML algorithm of the lmerTest package [29]. Thousand kernel mass revealed variances-heterogeneity, which could not be corrected by transformation, therefore the analysis was proceeded on the untransformed data, knowing that the sensitivity of some tests might be reduced. In order to account for heterogeneous variances between the different combinations of year, location and variety separate error variances were used for each combination of year, location and variety regarding some of the traits. Where heterogeneous residual variances were necessary to improve the model fit the package nlme was used [30]. The fixed effects in the model were tested for significance using F-tests. Denominator degrees of freedom in F-tests were adjusted using the Satterthwaite method [29]. Non-significant results at the α = 5% significance level were removed from the model. The levels of factors found significant were compared with pairwise Tukey-tests and results were presented as letter display. For mean comparisons tools from the package lsmeans were used [31].
3. Results
3.1. Plant Development and Growth
3.1.1. Growth and Development Conditions
Table 2 shows that plants at IHO in 2015 began to flower 74 DAS, having accumulated 545.45 GDD. Until flower induction radiation, mean day length and mean temperature amounted up to 10200.54 Wh/m2, 15.82 h and 17.3 °C in 2015. The mean temperature from flower induction to harvest maturity at IHO in 2015 was 11.9 °C. Harvest maturity was reached 170 days after sowing, which corresponded to 948.3 growing degree-days, respectively.

Table 2.
Accumulated growing degree-days (GDD), vegetation period, radiation, day length and mean temperature until flower induction at Ihinger Hof in 2015, 2016 and EWE in 2016.
In 2016, beginning of flowering took place 66 (EWE) and 70 (IHO) DAS which corresponded to 500.2 (EWE) and 492 (IHO) GDD. During the growing season of 2016, radiation accumulated to 10,267.59 Wh/m2 (EWE) and 10,155.02 Wh/m2 (IHO) until flower induction. Mean day length until flower induction was 15.83 h (EWE) and 15.81 h (IHO), while the mean temperature until flower induction was 16.8 °C (EWE) and 17.5 °C (IHO). From flower induction to harvest maturity the mean temperature at EWE in 2016 was 20.5 °C whereas the mean temperature at IHO in 2016 was 14.1 °C. Maturity was reached 127 (EWE) and 154 (IHO) days after sowing which corresponded to 1143.9 (EWE) and 910 (IHO) GDD.
3.1.2. Vegetative Growth
In 2016, plant heights of 112.7 cm (Sahi Alba 914), 115.6 cm (W13.1) and 103.8 cm were reached at IHO, whereas at EWE plant heights of 102.3 cm (Sahi Alba 914), 117.5 cm (W13.1) and 104.6 cm (G8) were recorded (Table 3). Significant differences were detected between the two genotypes Sahi Alba 914 and W13.1 (p-value 0.0263).

Table 3.
Mean plant height, number of branches per plant, number of inflorescences per single plant and the length of the central axis inflorescence, of three chia genotypes (G8, Sahi Alba 914, W13.1,) cultivated at two different locations (IHO/EWE) in 2016 (n = 10, α = 0.05, mean ± standard error).
The mean number of branches developed by the genotypes cultivated at IHO were 5.6 (Sahi Alba 914) and 6.9 (W13.1) (Table 3). At EWE, the number of developed branches was 3.5 (Sahi Alba 914), 6.5 (W13.1) and 5.7 (G8), indicating significant differences between the two genotypes Sahi Alba 914 and W13.1 cultivated at EWE (p-value 0.0352).
3.1.3. Generative Growth
At IHO 11.8 (Sahi Alba 914) and 13.3 (W13.1) inflorescences per plant were produced. Genotypes cultivated at EWE produced 6.7 (Sahi Alba 914), 10.2 (W13.1) and 8.3 (G8) inflorescences (Table 3). In general, the number of inflorescences per plant did not vary significantly but it was obvious that G8 produced fewer inflorescences compared to Sahi Alba 914 and W13.1 (Table 3).
The length of the central axis inflorescence was 18.8 cm (W13.1), 19.2 cm (Sahi Alba 914) and 16.8 cm (G8) for the genotypes cultivated at IHO, whereas at EWE it amounted to 16.8 cm (Sahi Alba 914), 19.5 cm (W13.1) and 16.0 cm (G8) (Table 3). No significant difference in length of the central axis inflorescence between Sahi Alba 914 and W13.1 was detected. The interactions between genotypes and locations were not significant.
Genotypes cultivated at IHO produced 4051 (W13.1) and 4289 (Sahi Alba 914) seeds per plant. At EWE seeds per plant amounted to 2073 (Sahi Alba 914), 3519 (W13.1) and 2738 (G8). No significant differences in the amount of seeds produced per plant were recorded for the two locations (Table 4).

Table 4.
Mean number of seeds, seed weight, seed length and seed width per single plant of three chia genotypes (G8, Sahi Alba 914, W13.1) cultivated at two different locations (IHO/EWE) in 2016 (n = 10, α = 0.05, mean ± standard error).
Mean seed weight per plant amounted to 6.3 g (W13.1) and 6.4 g (Sahi Alba 914) for the genotypes cultivated at IHO whereas the seed weights per plant in EWE were considerably lower due to the lower seed numbers, showing weights of 4.5g (W13.1), 2.7 g (Sahi Alba 914) and 3.2 g (G8). Again, no significant difference between genotypes (Sahi Alba 914, W13.1) and locations could be detected (Table 4).
Seed length (Table 4) of genotypes cultivated at IHO was 2.0 mm (Sahi Alba 914), 2.1 mm (W13.1) and 1.9 mm (G8). Differences between Sahi Alba 914 and W13.1 were significant (p-value 0.0008). Samples of the genotypes cultivated at EWE were 1.9 mm (Sahi Alba 914), 2.0 mm (W13.1) and 1.8 mm (G8) long. A significant difference was detected between the two locations regarding Sahi Alba 914 seed length (p-value 0.0126).
Seed width of Sahi Alba 914 (1.3 mm) was significantly lower compared to that of W13.1 (1.4 mm) at both locations (p-value < 0.0001, Table 4). Seeds of G8 cultivated at IHO and EWE were 1.3 mm wide. The above-mentioned data showed no significant genotype-by-location interaction
3.2. Yield Parameters
In 2016 mean plant density per square meter at IHO amounted to 30 (W13.1), 23 (Sahi Alba 914) and 17 plants (G8) (Table 5). At EWE, plant densities amounted to 87 (W13.1), 134 (Sahi Alba 914) and 92 (G8) per square meter in 2016. The study showed that there was a significant difference in plant density between locations (IHO, EWE) and the white seeded genotypes W13.1 and Sahi Alba 914 (p-value 0.0028 and 0.0025, respectively). There was no difference in germination capacity identified. Compared to IHO an increased seedling emergence at EWE was obtained probably due to the fact, that the sandy soil type was less susceptible towards soil surface crust formation resulting in a higher number of plants per square meter [34].

Table 5.
Mean number of plants per square meter, mechanically threshed seed yield [kg ha−1] and corresponding thousand kernel mass (TKM) [g] of three chia genotypes (G8, Sahi Alba 914, W13.1) cultivated at two different locations (IHO/EWE) in 2015 and 2016 (n = 3, α = 0.05, mean ± standard error).
In this regard, it should be mentioned that the different seeding rates and the resulting plant densities were considered in the statistical evaluation.
Table 5 shows that at IHO in 2015 genotype W13.1 generated a significantly higher seed yield (832.7 kg ha−1) compared to Sahi Alba 914 (422.8 kg ha−1) (p-value 0.0154). With 186.3 kg ha−1 G8 obtained a substantially lower yield. In 2016, seed yields at IHO were significantly higher compared to 2015, ranging from 973.4 kg ha−1 (W13.1), 865.3 kg ha−1 (Sahi Alba 914) to 751.7 kg ha−1 (G8) (p-value 0.0003). Yields obtained at EWE in 2016 were significantly higher compared to the yields obtained at IHO in 2016 (p-value 0.0166). Genotype W13.1 generated a yield of 1274.7 kg, Sahi Alba 914 generated a yield of 1161.4 kg ha−1 and G8 obtained a yield of 1286.0 kg ha−1.
The TKM in 2015 at IHO was 1.2 g (Sahi Alba 914), 1.3 g (W13.1) and 1.1 g (G8). In 2016 TKM has increased significantly by 0.1 g each to 1.3 g (Sahi Alba 914), 1.4 g (W13.1) and 1.2 g (G8) (p-value 0.0066). In 2016 TKM at EWE was 1.3 g (Sahi Alba 914), 1.4 g (W13.1) and 1.2 g (G8). Significant differences could be observed between the genotypes Sahi Alba 914 and W13.1 cultivated at IHO (p-value 0.0075, Table 5).
A genotype-by-year interaction was found to be significant (p-value 0.0147) for seed yield (Table 5). No further interactions were found to be significant.
3.3. Quality Parameters
3.3.1. Protein, Mucilage and Oil Content
Genotypes cultivated at IHO in 2015 showed mean crude protein contents of 24.2% (W13.1), 20.6% (Sahi Alba 914) and 23.0% (G8). Protein content was 22.9% (W13.1), 25.0% (Sahi Alba 914) and 21.7% (G8) for genotypes cultivated at IHO in 2016. Genotypes Sahi Alba 914 and W13.1 cultivated at EWE in 2016 showed significantly lower protein contents compared to those cultivated at IHO in 2016, namely 17.8% (W13.1), 18.1% (Sahi Alba 914) and 17.2% (G8) (p-value < 0.0001) (Figure 2). Significant differences between Sahi Alba 914 and W13.1 were detected for IHO in 2016 (p-value < 0.0001).
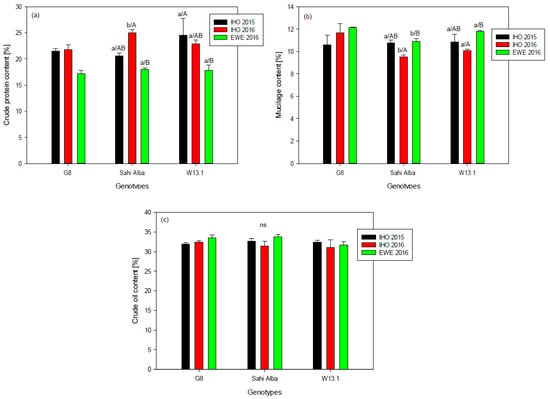
Figure 2.
Mean crude protein (a), mucilage (b) and crude oil (c) content of three chia genotypes (G8, Sahi Alba 914, W13.1,) cultivated at two different locations (IHO/EWE) in 2015 and 2016. Genotype-means within an environment which share a common lower-case letter do not differ significantly at α = 0.05. Environment-means which share a common upper case letter do not differ significantly within a genotype at α = 0.05. (Two way ANOVA, Tukey test, n = 3, means ± standard errors based on model (3)).
Mean mucilage content was 10.8% (W13.1/Sahi Alba 914) and 10.6% (G8) for the genotypes cultivated in 2015 at IHO. In 2016, mucilage content was 10.1% (W13.1), 9.5% (Sahi Alba 914) and 11.7% (G8). Mucilage contents of Sahi Alba 914 and W13.1 cultivated at EWE were significantly higher reaching a mucilage content of 11.8% (W13.1), 10.9% (Sahi Alba 914) and 12.2% (G8) (p-value 0.0003, Figure 2). In 2016, Sahi Alba 914 and W13.1 obtained significantly different mucilage contents at both locations (p-value 0.0016, Figure 2).
No statistically significant difference was identified for total crude oil content. Mean contents shown in Figure 2 were 32.4% (W13.1), 32.7% (Sahi Alba 914) and 32.0% (G8) for genotypes cultivated at IHO in 2015. In 2016, total crude oil content was 30.9% (W13.1), 31.3% (Sahi Alba 914) and 32.5% (G8) for the genotypes cultivated at IHO. Genotypes cultivated at EWE showed slightly higher total crude oil contents, 31.7% (W13.1), 33.7% (Sahi Alba 914) and 33.5% (G8) in 2016. No significant interactions between genotypes, locations and year of cultivation were found for the above-mentioned data.
3.3.2. Fatty Acid Contents, Proportions and Ratios
The statistical analysis of the identified single fatty acids showed no significant interaction between genotype × year and genotype × location (Table 6). Location and year significantly influenced the content of the saturated palmitic (p-value 0.0209 and p-value 0.0053, respectively) and stearic (p-value 0.0059 and p-value 0.0004, respectively) fatty acids (Table 6).

Table 6.
Fatty acid composition (% of total fatty acid) of three chia genotypes (G8, Sahi Alba 914, W13.1) cultivated at two different locations (IHO/EWE) in 2015 and 2016 (n = 3, α = 0.05, mean ± standard error).
Mean palmitic acid content of the genotypes cultivated at IHO in 2015 was 6.9% (W13.1), 7.3% (Sahi Alba 914) and 6.6% (G8). Significantly, higher contents could be detected in 2016. Mean palmitic acid content amounted to 8.2% (W13.1/Sahi Alba 914) and 7.5% (G8). Mean palmitic acid contents of the genotypes cultivated at EWE in 2016 were significantly higher, varying between 9.7% (W13.1), 11.8% (Sahi Alba 914) and 11.1% (G8).
Genotypes cultivated at IHO showed mean stearic acid contents between 2.0% (W13.1), 2.1% (Sahi Alba 914) and 2.8% (G8) in 2015. Significantly, higher contents could be identified in 2016 varying from 3.4% (W13.1) to 3.8% (Sahi Alba 914/G8). A significant difference in stearic acid content was detected between the genotypes Sahi Alba 914 and W13.1 (p-value 0.0254) in 2016. Mean stearic acid contents of the genotypes cultivated at EWE in 2016 were significantly higher, ranging from 4.3% (W13.1) to 6.1% (Sahi Alba 914) and 5.9% (G8).
The monounsaturated oleic fatty acid was also significantly influenced by location (p-value 0.0067) and year (p-value 0.0009) whereas the monounsaturated vaccenic fatty acid seemed not to be influenced by location, genotype nor year (p-value > 0.05) (Table 6).
Oleic acid content of the genotypes cultivated at IHO in 2015 ranged from 4.6% (Sahi Alba 914) to 5.3% (W13.1) and 6.2% (G8). In 2016 the oleic acid values showed a significant increase, varying between 6.6% (Sahi Alba 914), 6.7% (W13.1) and 8.2% (G8). The oleic acid content of the genotypes cultivated at EWE in 2016 were significantly higher compared to those cultivated at IHO in 2016, namely 8.1% (W13.1), 9.2% (Sahi Alba 914) and 11.8 % (G8).
Vaccenic acid content obtained by all three genotypes (W13.1, Sahi Alba 914, G8) cultivated at IHO in 2015 was 0.8%. In 2016, genotypes cultivated at IHO showed vaccenic acid contents of 0.8% (Sahi Alba 914, G8) and 0.9% (W13.1) whereas those vaccenic acid contents observed at EWE in 2016 were 1.0% (W13.1, Sahi Alba 914) and 1.1% (G8).
Polyunsaturated linoleic and α-Linolenic fatty acid contents were significantly influenced by year (p-value 0.0010 and p-value 0.0001, respectively). Further, it was shown that the linoleic content was significantly influenced by genotype (p-value 0.0099) whereas the α-Linolenic content was significantly influenced by location (p-value 0.0171) (Table 6).
Linoleic acid contents of the genotypes cultivated at IHO in 2015 were 18.0% (Sahi Alba 914), 19.0% (G8) and 20.2% (W13.1). A significant difference was observed between Sahi Alba 914 and W13.1 in that year. In 2016, the linoleic acid content significantly increased compared to the contents recorded in 2015. Contents of 21.1% (Sahi Alba 914), 21.6% (G8) and 22.0% (W13.1) were obtained for linoleic acid. The genotypes cultivated at EWE in 2016 showed linoleic acid contents of 23.1% (Sahi Alba 914), 23.2% (G8) and 21.9% (W13.1).
Contents of α-Linolenic acid were found to be 64.7% (W13.1, G8) and 67.2% (Sahi Alba 914) for the genotypes cultivated at IHO in 2015. In 2016, genotypes cultivated at IHO showed significantly lower α-Linolenic acid contents (Table 6), varying between 58.1% (G8), 58.8% (W13.1) and 59.6% (Sahi Alba 914). α-Linolenic acid contents of genotypes cultivated at EWE in 2016 were significantly lower, namely 46.7% (G8), 48.7% (Sahi Alba 914) and 54.2% (W13.1).
The statistical analysis of the fatty acid proportions and ratios indicated no significant interaction between genotype × year as well as for genotype × location (Table 7).

Table 7.
Fatty acid proportions and ratios of three chia genotypes (G8, Sahi Alba 914, W13.1,) cultivated at two different locations (IHO/EWE) in 2015 and 2016 (n = 3, α = 0.05, mean ± standard error).
The percentage of saturated fatty acids (SFA) obtained in 2015 by the genotypes cultivated at IHO was 9.4 (Sahi Alba 914), 9.0 (W13.1) and 9.3 (G8). In 2016, the percentage of SFA was significantly higher for those genotypes cultivated at IHO (p-value 0.0006) (Table 7). Genotypes showed 12.0 (Sahi Alba 914), 11.5 (W13.1) and 11.2 (G8) percentage of SFA. Compared to the genotypes cultivated at IHO the percentage of SFA from those of EWE cultivated in 2016 was significantly higher, namely 17.8% (Sahi Alba 914), 14.0% (W13.1) and 17.1% (G8) (p-value 0.0169) (Table 7).
Monounsaturated fatty acid (MUFAs) content was 5.4% (Sahi Alba 914), 6.1% (W13.1) and 7.0% (G8) for the genotypes cultivated at IHO in 2015. There was a significant increase in MUFA’S in 2016 (p-value 0.0008) (Table 7). Genotypes cultivated at IHO showed MUFA contents of 7.3% (Sahi Alba 914), 7.6% (W13.1) and 9.0% (G8). Results for the genotypes cultivated at EWE were also significantly higher compared to those cultivated at IHO in 2016. MUFA contents were 10.2% (Sahi Alba 914), 9.1% (W13.1) and 12.9% (G8) (p-value 0.0083) (Table 7).
Contents of polyunsaturated fatty acids (PUFAs) were 84.9% (W13.1), 85.2% (Sahi Alba 914) and 83.7% (G8) for the genotypes cultivated in 2015 at IHO. A significantly lower content of PUFAs was detected for those genotypes cultivated at IHO in 2016 (p-value 0.0009) (Table 7). In this year PUFA contents were 80.8% (W13.1), 80.7% (Sahi Alba 914) and 79.8% (G8). Significant differences were detected between the two locations IHO and EWE in 2016 (p-value 0.0172) (Table 7). PUFAs were significantly lower for the genotypes cultivated at EWE, showing PUFA contents of 76.2% (W13.1), 72.0% (Sahi Alba 914) and 70.0% (G8).
Genotypes cultivated at IHO in 2015 showed PUFA:SFA ratios of 9.4 (W13.1), 9.1 (Sahi Alba 914) and 9.0 (G8) whereas the PUFA:SFA ratios of the genotypes cultivated at IHO in 2016 were significantly lower (p-value 0.0006) (Table 7). Ratios were 7.0 (W13.1), 6.7 (Sahi Alba 914) and 7.1 (G8). Compared to the ratios of the genotypes cultivated at IHO in 2016 the ratios of the genotypes cultivated at EWE in 2016 were significantly lower with 5.5 (W13.1), 4.0 (Sahi Alba 914) and 4.1 (G8) (p-value 0.0171) (Table 7).
ω6:ω3 ratios of genotypes cultivated at IHO in 2015 were 0.31 (W13.1), 0.27 (Sahi Alba 914) and 0.29 (G8). Significantly higher ω6:ω3 ratios were obtained in 2016 showing ratios of 0.37 (W13.1), 0.35 (Sahi Alba 914) and 0.37 (G8) (p-value < 0.0001) (Table 7). Significantly higher ω6:ω3 ratios were obtained by the genotypes cultivated at EWE in 2016, 0.41 (W13.1) 0.48 (Sahi Alba 914) and 0.50 (G8), respectively (p-value 0.0018) (Table 7). This study generally indicated that the fatty acid proportions and ratios were strongly influenced by location and year rather than by genotype.
4. Discussion
Being a frost sensitive short day plant, requiring day length < 12 h to induce flower formation, the cultivation of chia is traditionally limited to latitudes up to about 25 degrees [35]. In temperate regions of the northern hemisphere chia only starts to produce flower buds in short days of late September/October, which are killed by first frost shortly thereafter [35]. According to Grimes et al. [35] the photoperiodic sensitivity can thus be seen as a bottleneck for the expansion of chia cultivation towards more northern and southern latitudes. By overcoming this barrier, chia cultivation could be introduced to a wider range of environmental conditions outside of its Mesoamerican origin [12,14].
4.1. Plant Development
4.1.1. Impact of Day Length and Temperature
In this study, the cultivation of three chia genotypes either day length insensitive (Sahi Alba 914) or adapted to day lengths greater than 12 h (G8, W13.1) were tested under the given climatic and day length conditions of Southwestern Germany. To the best of our knowledge, it was the first attempt to grow these genotypes at latitudes > 48° N.
Jamboonsri et al. [12] was able to proof the existence of new chia genotypes, which were able to induce flower formation in greenhouse (15.9 h) and field (14 h 41 min) experiments exceeding the known threshold. With flower induction occurring 66 to 74 days after sowing by mid-July (EWE, 2016) and early August (IHO, 2015 & 2016) at a mean day length of 15.81 to 15.83 h (Table 2), this study verified the findings of Jamboonsri et al. [12] indicating that these genotypes can cope with the given day length conditions in Southwestern Germany. With 66 (EWE, 2016) to 70 (IHO, 2016) and 74 (IHO, 2015) days until flower initiation, findings of this study were centered between the data reported by Baginsky et al. [2], indicating that flower initiation occurred 40 to 60 (coastal dessert/dessert climate) and 80 to 110 (Mediterranean climate) days after sowing at a latitude of 18°–33° S.
In order to induce flowering, Baginsky et al. [2] stated that chia plants have to accumulate 600 to 700 growing degree-days, if day length is not sufficiently long enough. As within this study, lesser growing degree-days were accumulated until flower induction (Table 2), the identified threshold of 15.8 h day length for flower induction seemed to be adequate for the three cultivated genotypes. It is therefore suggested that an adequate adoption to day length is of higher importance for the development of the tested genotypes than the accumulated growing degree days. Growing degree-days observed in this study (Table 2) fell amongst the data obtained by Baginsky et al. [2] which reported 1549.1 to 1638.8 (coastal dessert and dessert climate) and 610.3 (Mediterranean climate) accumulated growing degree-days, respectively.
Temperature until flower induction was higher (0.7 °C) at EWE in 2016 compared to IHO (2016), which led to an accelerated plant development accompanied with an earlier beginning of flowering. Referring to temperature Ayerza and Coats [11] stated that chia’s minimum and maximum growth temperature is 11 °C and 36 °C with an optimum range between 16 °C and 26 °C, hence thermal requirements could be fulfilled within this study (Table 2).
As the mean day length until flowering, did not vary between the two locations in 2016 (Table 2), the early flower induction at EWE is therefore most likely attributed to temperature. In general, it can be stated that all genotypes at both locations completed their phenological development and were able to reach maturity. Nevertheless, the climatic conditions of the upper Rhine Valley can be considered favorable in terms of the limited period of time (frost-free), in which the cultivation of chia under Southwestern German conditions is feasible.
In this regard, it should be mentioned that sowing date is crucial as it affects the above-mentioned findings (day length, GDD, temperature) to a significant extent. Sowing date determines the duration of the crop cycle and therefore its ability to reach maturity depending on the variations in environmental temperature and day length to which it is exposed during cultivation [10]. Chosen sowing dates in May seem to match with the given requirements of chia and can therefore be recommended for chia cultivation in Southwestern Germany. Earlier sowing dates might increase the risk of frost damages to the emerging chia plants, as the region of Southwestern Germany is often exposed to a cooler weather period and the last spring frost often occurs at the end of April/beginning of May (ice saints).
4.2. Vegetative Growth
Identified plant heights of 102.3 to 117.5 cm were in line with studies of Sorondo [14] who measured plant heights of Sahi Alba 914 cultivated in Argentina between 106 and 121 cm (Table 3). In a study by Nyamweha et al. [36] carried out in Uganda (latitude ~ 0° N) common chia cultivars reached plant heights of 110.6 cm while a study in Ghana (latitude ~ 10° N) documented heights of 92.9 to 101.1 cm [37]. Chia plant heights decrease with shorter day length according to Baginsky et al. [2]. At latitudes of zero to ten (Uganda/Ghana) shorter plants were developed compared to those grown at the higher latitude (48° N) presented in this study (Table 3) [36,37]. This pattern is reported also for other short day plants such as soybean and Amaranthus spp. and could be observed for the chia genotypes, which were adopted to day length greater > 12 h (W13.1, G8), but in consequence not for the day length insensitive genotype (Sahi Alba 914) (Table 3) [38,39].
The mean number of branches identified by the present study was below the values reported so far in literature (Table 3). Souza & Chaves [40] reported 7.9 to 9.3 branches under greenhouse conditions (latitude 7° S, mean temp. 23–34°C) while Yeboah et al. [37] indicated 21.2 to 26.8 branches per plant under field conditions (latitude 6° N, mean temp. 24–28 °C). As the number of branches per plant is directly influenced by row spacing and seeding density, the given variability between the different studies indicated a high plasticity of chia regarding the number of branches, wherefore the chosen density has to be matched to the specific environmental conditions at each growing site [41].
4.3. Yield Parameters and Yield
Under Chilean conditions, Baginsky et al. [2] showed that black and white seeded genotypes produced lesser inflorescences in the Mediterranean climate (6.3, 6.1) compared to the coastal dessert (11.2, 10.8) and dessert climate (8.7, 10.0) conditions. With increased day length the number of inflorescences produced per single plant decreased according to Baginsky et al. [2]. This contradicts the findings of the present study as the number of inflorescences produced by the genotypes cultivated in this study (Table 3) was in line with results of Karim et al. [42] who determined 4.67 to 13.67 inflorescences per single plant in a field trial conducted in Bangladesh (latitude ~ 25° N). However, as the number of inflorescences as well as the number of branches per plant might also be influenced by agronomic parameters like sowing density, row spacing etc., differences in yield parameters might rather be related to complex interactions between agronomic and environmental parameters instead of being related to a single parameter.
Mean length of the central axis inflorescences produced by the three genotypes cultivated at IHO and EWE in 2016 were between 16.0 to 19.5 cm. Sorondo [14] referred to the mean length of the central axis inflorescence of 22.4 cm for Sahi Alba 914, which could not be reached. Baginsky et al. [2] reached central axis inflorescences length of 13.6 to 34.1 cm, depending on sowing date and location. In this regard, they observed that delayed sowing dates (shorter day length) tend to increase inflorescence length in all locations and that the length of the central axis inflorescences was in general negatively and significantly associated with day length at the beginning of flowering. This would explain why reported lengths of inflorescences in literature could not be reached in our study, but might have been rather compensated by a higher number of inflorescences per plant.
The amount of seeds produced per single plant has not yet been reported in the existing literature. The study conducted at IHO and EWE produced 2073 to 4289 seeds per single plant as shown in Table 4. Regarding the different sowing rates and row distances it should again be mentioned that they were integral parts of the site and annual effects and that the resulting plant densities are hence to be disregarded. Referring to this, the higher seed numbers per single plant at IHO indicate that due to its high plasticity, chia is able to compensate lower plant numbers with higher seed numbers per single plant [41], finally leading to satisfactory yields under the given climatic conditions in Southwestern Germany. However, it has to be stated that the right choice of harvesting time and harvest intensity (manually/mechanically) is of crucial importance in regard to potentially achievable yields, as chia is highly susceptible to seed shattering [43]. Amongst others, it was reported that suboptimal combine operations and harsh weather conditions between manual and mechanical harvest, could led to yield losses of up to 37% [44], which both would explain the observed differences between manually and mechanically harvested yields (Table 4 and Table 5).
When speaking of yield, thousand kernel mass is another important yield determining trait. Ixtaina et al. [4] showed average thousand kernel masses of dark and white chia seeds of 1.323 g and 1.301 g, respectively. Sorondo [14] stated thousand kernel masses between 1.1 and 1.4 g for Sahi Alba 914. Amato et al. [45] reported first biometric data on chia seeds produced in Europe showing thousand kernel masses of 1.26 to 1.37 g. Those values are in line with the findings of this study (Table 5). Indicating that genotype and year influenced thousand kernel mass significantly. Therefore, more studies would be beneficial in order to identify the specific influencing factors on chia’s thousand kernel mass.
As reported in Table 5, seed yields of the white seeded genotypes Sahi Alba 914 and W13.1 obtained in this study (422.8–1286.0 kg ha−1) are in line with the yields obtained by Baginsky et al. [2] and Sosa et al. [46]. Seed yields observed at EWE were significantly higher compared to those obtained at IHO in 2016. In this regard, it should again be mentioned that the different sowing rates and row distances were integral parts of the site and annual effects and could therefore be disregarded as well as the resulting plant densities. The combination of higher temperature and growing degree day accumulation along with higher radiation (Table 2), which represents the energy source for photosynthesis during the whole crop cycle, very likely influenced seed yield at EWE in 2016 positively. As temperature in general seemed to be more constant at EWE a higher metabolic activity during grain filling and maturation compared to IHO might have occurred. This may have contributed to a possible superior rate of matured seeds, leading to an overall higher yield potential at EWE [2,9].
A field experiment conducted in Southern Italy, at ~40 ° N produced seed yields between 126.5 and 490.4 kg (total dry mass) and increased significantly with plant density according to Bochicchio et al. [9]. This demonstrates the enormous potential of chia cultivation outside its center of origin. Relatively low seed yields are often the result of phenology (late flowering), temperature conditions (peaks of low temperatures, winter kill) and lodging which affected the seed yield negatively [9].
Dependent on the environment and cultivation conditions seed yields of chia seem to vary greatly. In Argentina for example, Sosa et al. [46] reported yields from 150 to 1200 kg ha−1 whereas seed yields of about 2600 kg ha−1 were obtained under Ghanaian conditions [37]. Another trial conducted by Baginsky et al. [2] resulted in significantly varying seed yields between the Chilean dessert/coastal dessert climate (950–2900 kg ha−1) and the Chilean Mediterranean climate (70–130 kg ha−1). Sowing date is highly relevant as it determines the development period of the crop given the fluctuations in environmental temperature and day length, which according to Ayerza [47,48] are mainly responsible for yield potential and seed quality. Even though the genotypes cultivated in this study were adapted to temperate regions, the cultivation period in Southwestern Germany is highly restricted due to low temperatures and frost occurrences in the first and last quarter of the year, which unfortunately strictly limits chia cultivation from April/May until October/November. In temperate regions, late sowing dates would kill the plants before they reached maturity.
4.4. Quality Parameters
4.4.1. Protein, Mucilage and Oil Content
Chia seeds cultivated at IHO contained between 20.6 and 25% of crude protein which is in line or rather higher compared to findings of Amato et al. [45] and Silva et al. [49]. Ayerza and Coats [50] showed that the protein content tends to decrease as the temperature increases. When looking at the protein contents of genotypes cultivated at EWE (Figure 2), where mean temperature from flower induction to harvest maturity was 6.4 degrees higher this tendency could be verified and a negative correlation between seed yield and protein content as for most plants was observed. In order to realize a higher protein content at EWE, which holds a high yield potential, the level of nitrogen fertilization should therefore be raised.
Mucilage (gel, which surrounds the seeds when hydrated) yields are reported to vary between 5 to 15.1% [24,51,52,53]. With mean mucilage yield values between 9.51 and 12.15% results of this study can be assessed as quite high and therefore favorable (Figure 2). It has been reported to be essentially composed of complex high molecular weight polysaccharides [9,54,55] and to have exceptional physical properties on the basis of the high soluble to insoluble fiber ratio [54]. In recent years, scientists have been researching chia seeds mucilage and its possible areas of application showing promising functional properties from a technological and physiological perspective [56].
Total crude oil contents varied from 30.9 to 33.7% and were in line with the findings of Ixtaina et al. [52], Silveira Coelho & de las Mercedes Salas-Mellado [53], Amato et al. [45], Baginsky et al. [2] and Silva et al. [49] where oil yields ranged from 20.3% to 34.4%. According to Ixtaina et al. [52] and Silva et al. [49] seed oil yield was crucially affected by extraction technique and solvent used, regarding n-hexane as the solvent yielding the highest oil yields. It was additionally stated that the seed oil yield generally depends on factors, such as climatic conditions and the region of provenance [5,50]. Ayerza [5] was able to demonstrate that oil contents of a single chia line varied from 25.93% to 33.50% in five different ecosystems. As oil contents were not influenced by genotype, location or year in our study, farmers might profit from stable oil contents while meeting the given EU requirements of the Novel Food guideline [57].
4.4.2. Fatty Acid Contents, Proportions and Ratios
The determined fatty acid profiles were in line with the given profiles in literature [49,52]. Within this study it could be shown that the fatty acid composition was influenced by year > location > genotype in descending order. Overall, temperature affects the determined oil quality [58]. Cool climatic conditions are supposed to postpone the maturity of the seeds and thus offer a longer period for oil and fatty acid synthesis leading to an increase in the level of unsaturated fatty acids [5,59]. Taking the mean temperature from flower induction to harvest maturity of both locations in 2016 into account, the location IHO was cooler than EWE, resulting in higher amounts of PUFAs. In addition, mean temperature at IHO in 2015 during grain filling was lower than in 2016 leading to an even higher amount of PUFAs in 2015 at the same location (Table 2).
The reduction of inflammatory conditions, tumor growth, angiogenesis and metastasis and the lower risk of cardiovascular disease and mortality are just some of the health benefits associated with ω-3 PUFAs [60,61,62,63,64,65,66]. It is known from the literature that the main components of chia oil are PUFA’s with α-linolenic acid (ω3) as the predominant one and linoleic acid (ω6) as the second most common PUFA [45]. The fatty acids of the genotypes cultivated in our study can be classified in the following frequency of occurrence: α-linolenic acid (C18:3) > linoleic acid (C18:2) > palmitic acid (C16:0) > oleic acid (C18:1) > stearic acid (C18:0) > vaccenic acid (C18:1) [49,52]. In comparison to marine α-linolenic acid (ω3) sources, like algae or fish in the family Clupeidae, chia contains significantly less saturated fatty acids and a much higher α-linolenic acid content, while being odor- and tasteless which constitutes an advantage particularly in regard to enriching foods [1].
Humans require the essential polyunsaturated fatty acids ω6 and ω3 from food intake as they lack the endogenous enzymes for ω3 desaturation and therefore cannot be synthesized by the human body [1,67,68]. Modern agriculture has led to a disproportionately high level of ω6 PUFAs parallel to an extreme reduction of ω3 PUFAs in western diets, leading to an unbalanced and unhealthy ω6:ω3 ratio of between 10:1 and 20:1, compared to the 1:1 ratio during the Paleolithic period [68,69,70]. Balancing the ω6:ω3 ratio plays an important role in the prevention and management of obesity and coronary heart diseases [1,71]. With ω6:ω3 ratios between 2.7:1 and 5:1 as shown in Table 7, chia oil intake could contribute to a more balanced ω6:ω3 uptake therefore.
5. Conclusions
The three chia genotypes cultivated surpassed the yields obtained by their countries of origin and are thus an attractive alternative to local farmers in Southwestern Germany. Oil, protein and mucilage content are in line with current literature and the Novel Food-Regulation (EU) 2015/2283. Location, year and genotype choices appeared to be of equal importance for a successful chia production whereas the fatty acid profile seemed directly impacted by the factors as follows: year > location > genotype. Underlined by the results of this study, Southwestern Germany could be seen as a seminal opportunity to expand the cultivation area of chia to latitudes up to 48° N. Nevertheless, additional studies should be conducted in order to establish an optimized cultivation system for chia under the given conditions of Southwestern Germany.
Supplementary Materials
The following are available online at http://www.mdpi.com/2077-0472/8/10/154/s1, Table S1: Soil characteristics of the experimental site at IHO in 2015 and 2016.
Author Contributions
Conceptualization, S.J.G. and S.G.-H.; Formal analysis, S.J.G. and F.C.; Funding acquisition, S.G.-H.; Investigation, S.J.G. and V.H.; Supervision, S.G.-H.; Validation, S.J.G. and F.C.; Visualization, S.J.G.; Writing–original draft, S.J.G.; Writing–review & editing, S.J.G., T.D.P., V.H., F.C. and S.G.-H.
Funding
This research was funded by the German Federal Ministry for Economic Affairs and Energy within the Central Innovation Program for SMEs (16KN050524).
Acknowledgments
The authors would like to thank Augustin Sorondo for providing the day length insensitive Sahi Alba 914 seeds and the University of Kentucky (USA) which generously provided the mutant genotypes G8 and W13.1 within the framework of an agreement between the University of Hohenheim and the University of Kentucky.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- Ayerza, R.; Coates, W. Chia: Rediscovering a Forgotten Crop of the Aztecs; University of Arizona Press: Tucson, AZ, USA, 2005; ISBN 978-0-8165-2488-4. [Google Scholar]
- Baginsky, C.; Arenas, J.; Escobar, H.; Garrido, M.; Valero, N.; Tello, D.; Pizarro, L.; Valenzuela, A.; Morale, L.; Silva, H. Growth and yield of chia (Salvia hispanica L.) in the Mediterranean and desert climates of Chile. Chil. J. Agric. Res. 2016, 76, 255–264. [Google Scholar] [CrossRef]
- Mohd Ali, N.; Yeap, S.K.; Ho, W.Y.; Beh, B.K.; Tan, S.W.; Tan, S.G. The promising future of chia, Salvia hispanica L. J. Biomed. Biotechnol. 2012, 2012, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ixtaina, V.Y.; Nolasco, S.M.; Tomás, M.C. Physical properties of chia (Salvia hispanica L.) seeds. Ind. Crops Prod. 2008, 28, 286–293. [Google Scholar] [CrossRef]
- Ayerza, R. The seed’s protein and oil content, fatty acid composition, and growing cycle length of a single genotype of chia (Salvia hispanica L.) as affected by environmental factors. J. Oleo Sci. 2009, 58, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, L.A.; Cobos, A.; Diaz, O.; Aguilera, J.M. Chia Seed (Salvia hispanica): An Ancient Grain and a New Functional Food. Food Rev. Int. 2013, 29. [Google Scholar] [CrossRef]
- Segura-Campos, M.R.; Ciau-Solís, N.; Rosado-Rubio, G.; Chel-Guerrero, L.; Betancur-Ancona, D. Physicochemical characterization of chia (Salvia hispanica) seed oil from Yucatán, México. Agric. Sci. 2014, 5, 220–226. [Google Scholar] [CrossRef]
- Herman, S.; Marco, G.; Cecilia, B.; Alfonso, V.; Luis, M.; Cristián, V.; Sebastián, P.; Sebastián, A. Effect of water availability on growth, water use efficiency and omega 3 (ALA) content in two phenotypes of chia (Salvia hispanica L.) established in the arid Mediterranean zone of Chile. Agric. Water Manag. 2016, 173, 67–75. [Google Scholar] [CrossRef]
- Bochicchio, R.; Phillips, T.D.; Lovelli, S.; Labella, R.; Galgano, F.; Marisco, A.D.; Perniola, M.; Amato, M. Innovative Crop Productions for Healthy Food: The Case Chia (Salvia hispanica L.). In The Sustainability of Agro-Food and Natural Resource Systems in the Mediterranean Basin; Vastola, A., Ed.; Springer International Publishing: Basel, Switzerland, 2015; pp. 29–45. [Google Scholar]
- Zavalía, R.L.; Alcocer, M.G.; Fuentes, F.J.; Rodríguez, A.W.; Morandini, M.; Devani, M.R. Desarrollo del cultivo de chía en Tucumán, Republica Agentina. EEAOC-Av. Agroindustrial 2010, 32, 27–30. [Google Scholar]
- Ayerza, R.; Coates, W. Influence of environment on growing period and yield, protein, oil and α-linolenic content of three chia (Salvia hispanica L.) selections. Ind. Crops Prod. 2009, 30, 321–324. [Google Scholar] [CrossRef]
- Jamboonsri, W.; Phillips, T.D.; Geneve, R.L.; Cahill, J.P.; Hildebrand, D.F. Extending the range of an ancient crop, Salvia hispanica L.—A new ω3 source. Genet. Resour. Crop Evol. 2012, 59, 171–178. [Google Scholar] [CrossRef]
- Busilacchi, H.; Quiroga, M.; Bueno, M.; Di Sapio, O.; Flores, V.; Severin, C. Evaluación de Salvia hispanica L. cultivada em el Sur de Santa Fe (Republica Argentina). Cultiv. Trop. 2013, 34, 55–59. [Google Scholar]
- Sorondo, A. Chia (Salvia hispanica L.) Variety Sahi Alba 914. U.S. Patent 9,686,926, 27 June 2014. [Google Scholar]
- Karkanis, A.C.; Kontopoulou, C.K.; Lykas, C.; Kakabouki, I.; Petropoulos, S.A.; Bilalis, D. Efficacy and selectivity of pre- and post-emergence herbicides in chia (Salvia hispanica L.) under mediterranean semi-arid conditions. Not. Bot. Horti Agrobot. Cluj-Napoca 2018, 46, 183–189. [Google Scholar] [CrossRef]
- National Sustainable Agriculture Coalition NSAC’s Blog—New $22 million Investment in Organic Farming Research. Available online: http://sustainableagriculture.net/blog/fy12_orei_org_award/ (accessed on 15 January 2018).
- European Commission. Regulation (EU) 2015/2283 on novel foods, amending Regulation (EU) No 1169/2011 of the European Parliament and of the Council and repealing Regulation (EC) No 258/97 of the European Parliament and of the Council and Commission Regulation (EC) No 1852/2001. Off. J. Eur. Union 2015, 327, 1–22. [Google Scholar]
- Mcmaster, G.S.; Wilhelm, W.W. Growing degree-days: One equation, two interpretations. Agric. For. Meteorol. 1997, 87, 291–300. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2006; FAO: Rome, Italy, 2006. [Google Scholar]
- Hildebrand, D.; Jamboonsri, W.; Phillips, T. Early Flowering Chía and uses thereof. U.S. Patent 8,586,831, 19 November 2013. [Google Scholar]
- Bassler, R.; Hoffmann, G. Bestimmung von mineralischem, (Nitrat-) Stickstoff in Bodenprofilen, Nmin-Labormethode. In VDLUFA Methodenbuch Band I, 4th ed.; Deller, B., Ed.; VDLUFA-Verlag: Darmstadt, Germany, 1997; ISBN 978-3-941273-21-4. [Google Scholar]
- Muñoz, L.A.; Cobos, A.; Diaz, O.; Aguilera, J.M. Chia seeds: Microstructure, mucilage extraction and hydration. J. Food Eng. 2012, 108, 216–224. [Google Scholar] [CrossRef]
- Dumas, A. Stickstoffbestimmung nach Dumas. In Die Praxis des org. Chemikers, 41st ed.; Garschagen, H., Ed.; Walter de Gruyter: Nürnberg, Germany, 1962. [Google Scholar]
- AOAC Micro-Kjeldahl Method. In Official Methods of Analysis; Cunniff, P., AOAC International, Eds.; Association of Official Analytical Chemists: Arlington VA, USA, 1995. [Google Scholar]
- European Commission Regulation (EC) No 152/III H procedure B laying down the methods of sampling and analysis for the official control of feed. Off. J. Eur. Union 2009, 54, 1–130.
- Metcalfe, L.D.; Schmitz, A.A.; Pelka, J.R. Rapid preparation of fatty acid esters from lipids for gas chromatographic Analysis. Anal. Chem. 1966, 38, 514–515. [Google Scholar] [CrossRef]
- DGF. Fettsäuremethylester. In Einheitsmethoden zur Untersuchung von Fetten, Fettprodukten, Tensiden und verwandten Stoffen, 2nd ed.; Deutsche Gesellschaft für Fettwissenschaft e.V, Ed.; Wissenschaftliche Verlagsgesellschaft mbH: Stuttgart, Germany, 2018; ISBN 978-3-8047-3024-3. [Google Scholar]
- Thurnhofer, S.; Vetter, W. A gas chromatography/electron ionization-mass spectrometry-selected ion monitoring method for determining the fatty acid pattern in food after formation of fatty acid methyl esters. J. Agric. Food Chem. 2005, 53, 8896–8903. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82. [Google Scholar] [CrossRef]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.R.C.T. nlme: Linear and Nonlinear Mixed Effects Models 2018. Available online: https://cran.r-project.org/web/packages/nlme/nlme.pdf (accessed on 15 June 2018).
- Lenth, R.V. Least-Squares Means: The R Package lsmeans. J. Stat. Softw. 2016, 69. [Google Scholar] [CrossRef]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration—Guidelines for Computing Crop Water Requirements; FAO: Rome, Italy, 1998; Volume 56. [Google Scholar]
- ESRL Solar Calculation Details. Available online: https://www.esrl.noaa.gov/gmd/grad/solcalc/calcdetails.html (accessed on 15 January 2018).
- Anzooman, M.; Christopher, J.; Mumford, M.; Dang, Y.; Menzies, N.; Peter, K. Selection for rapid germination and emergence may improve wheat seedling establishment in the presence of soil surface crusts. Plant Soil 2018, 1–13. [Google Scholar] [CrossRef]
- Grimes, S.; Phillips, T.; Claupein, W.; Graeff-Hönninger, S. Chia^4: Is a new tetraploid chia (Salvia hispanica L.) genotype able to revolutionize European agriculture? In The Food System Approach: New Challenges for Education, Research and Industry; ISEKI Food Association: Stuttgart, Germany, 2018; ISBN 978-3-900932-57-2. [Google Scholar]
- Nyamweha, R.B.; Akoraebirungi, B.; Geoffrey, C.; Kakyo, T. Chia (Salvia hispanica) to Different Plant Densities and the Ecological Conditions of Kabarole District. Available online: https://www.researchgate.net/publication/311087315_Response_of_Chia_Salvia_hispanica_to_different_plant_densities_and_the_ecological_conditions_of_Kabarole_district/citations (accessed on 16 May 2018).
- Yeboah, S.; Owusu Danquah, E.; Lamptey, J.N.L.; Mochiah, M.B.; Lamptey, S.; Oteng-Darko, P.; Adama, I.; Appiah-Kubi, Z.; Agyeman, K. Influence of Planting Methods and Density on Performance of Chia (Salvia hispanica) and its Suitability as an Oilseed Plant. Agric. Sci. 2014, 2, 14–26. [Google Scholar] [CrossRef]
- Allen, L.H.; Zhang, L.; Boote, K.J.; Hauser, B.A. Elevated temperature intensity, timing, and duration of exposure affect soybean internode elongation, mainstem node number, and pod number per plant. Crop J. 2018, 6, 148–161. [Google Scholar] [CrossRef]
- Stetter, M.G.; Zeitler, L.; Steinhaus, A.; Kroener, K.; Biljecki, M.; Schmid, K.J. Crossing Methods and Cultivation Conditions for Rapid Production of Segregating Populations in Three Grain Amaranth Species. Front. Plant Sci. 2016, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Souza, R.S.; Chaves, L.H.G. Initial growth of chia (Salvia hispanica L.) submitted to nitrogen, phosphorus and potassium fertilization. Aust. J. Crop Sci. 2017, 11, 610–615. [Google Scholar] [CrossRef]
- Sosa-Baldivia, A.; Ruiz Ibarra, G. Inadequate Nitrogen Fertilization: Main Cause of the Low Seed Yield on the Chia Crop (Salvia Hispanica L.). Biomed. J. Sci. Tech. Res. 2018, 2, 6–7. [Google Scholar] [CrossRef]
- Karim, M.M.; Ashrafuzzaman, M.; Hossain, M.A. Effect of planting time on the growth and yield of chia (Salvia hispanica L.). Asian J. Med. Biol. Res. 2016, 1, 502–507. [Google Scholar] [CrossRef]
- Boisclair, J.; Richard, G.; Boislard, T.; Phillips, T.; Leblanc, M.; Grenier, M.; Belzile, L.; Thibault, C. Chia: A New Crop for Organic Production in Québec, Results from 2015 Preliminary Trial. In Sharing Research that Grows Organic; IRDA: Montreal-Longueuil, QC, Canada, 2016. [Google Scholar]
- Coates, W.; Ayerza, R. Commercial production of chia in Northwestern Argentina. J. Am. Oil Chem. Soc. 1998, 75, 1417–1420. [Google Scholar] [CrossRef]
- Amato, M.; Caruso, M.C.; Guzzo, F.; Galgano, F.; Commisso, M.; Bochicchio, R.; Labella, R.; Favati, F. Nutritional quality of seeds and leaf metabolites of Chia (Salvia hispanica L.) from Southern Italy. Eur. Food Res. Technol. 2015, 241, 615–625. [Google Scholar] [CrossRef]
- Sosa, A.; Ruiz, G.; Jat, R.; Gerardo, G.; West, H.; Sharma, M.; Liu, X.; Robles de laTorre, R.R. Chia Crop (Salvia hispanica L.): Its History and Importance as a Source of Polyunsaturated Fatty Acids Omega-3 Around the World: A Review. J. Crop Res. Fertil. 2016, 1, 1–9. [Google Scholar] [CrossRef]
- Ayerza, R. Effects of seed color and growing locations on fatty acid content and composition of two chia (Salvia hispanica L.) genotypes. J. Am. Oil Chem. Soc. 2010, 87, 1161–1165. [Google Scholar] [CrossRef]
- Ayerza, R. The seed’s oil content and fatty acid composition of chia (Salvia hispánica L.) var. Iztac 1, grown under six tropical ecosystems conditions. Interciencia 2011, 36, 620–624. [Google Scholar]
- Silva, C.; Garcia, V.A.S.; Zanette, C.M. Chia (Salvia hispanica L.) oil extraction using different organic solvents: Oil yield, fatty acids profile and technological analysis of defatted meal. Int. Food Res. J. 2016, 23, 998–1004. [Google Scholar]
- Ayerza, R.; Coates, W. Protein content, oil content and fatty acid profiles as potential criteria to determine the origin of commercially grown chia (Salvia hispanica L.). Ind. Crops Prod. 2011, 34, 1366–1371. [Google Scholar] [CrossRef]
- Ayerza, R.; Coates, W. Omega-3 enriched eggs: The influence of dietary α-linolenic fatty acid source on egg production and composition. Can. J. Anim. Sci. 2001, 81, 355–362. [Google Scholar] [CrossRef]
- Ixtaina, V.Y.; Martínez, M.L.; Spotorno, V.; Mateo, C.M.; Maestri, D.M.; Diehl, B.W.K.; Nolasco, S.M.; Tomás, M.C. Characterization of chia seed oils obtained by pressing and solvent extraction. J. Food Compos. Anal. 2011, 24, 166–174. [Google Scholar] [CrossRef]
- Silveira Coelho, M.; de las Mercedes Salas-Mellado, M. Chemical Characterization of Chia (Salvia hispanica L.) for Use in Food Products. J. Food Nutr. Res. 2014, 2, 263–269. [Google Scholar] [CrossRef]
- De la Paz Salgado-Cruz, M.; Georgina, C.D.; Jorge, C.P.; Reynold, R.F.R.; Juan, V.M.M.; Díaz-Ramíreza, M. Chia (Salvia hispanica L.) seed mucilage release characterisation. A microstructural and image analysis study. Ind. Crops Prod. 2013, 51, 453–462. [Google Scholar] [CrossRef]
- Campos, B.E.; Dias Ruivo, T.; da Silva Scapim, M.R.; Madrona, G.S.; de C. Bergamasco, R. Optimization of the mucilage extraction process from chia seeds and application in ice cream as a stabilizer and emulsifier. LWT-Food Sci. Technol. 2016, 65, 874–883. [Google Scholar] [CrossRef]
- Capitani, M.I.; Ixtaina, V.Y.; Nolasco, S.M.; Tomás, M.C. Microstructure, chemical composition and mucilage exudation of chia (Salvia hispanica L.) nutlets from Argentina. J. Sci. Food Agric. 2013, 93, 3856–3862. [Google Scholar] [CrossRef] [PubMed]
- European Commission: Commission implementing decision authorizing the placing on the market of chia oil (Salvia hispanica) as a novel food ingredient under Regulation (EC) No 258/97 of the European Parliament and of the Council. Off. J. Eur. Union 2014, 353, 15–16.
- Flagella, Z.; Rotunno, T.; Tarantino, E.; Di Caterina, R.; de Caro, A. Changes in seed yield and oil fatty acid composition of high oleic sunflower (Helianthus annuus L.) hybrids in relation to the sowing date and the water regime. Eur. J. Agron. 2002, 17, 221–230. [Google Scholar] [CrossRef]
- Mirshekari, M.; Amiri, R.; Nezhad, H.I.; A Sadat Noori, S.; Zandvakili, O.R. Effects of Planting Date and Water Deficit on Quantitative and Qualitative Traits of Flax Seed. Am.-Eurasian J. Agric. Environ. Sci. 2012, 12, 901–913. [Google Scholar] [CrossRef]
- Calder, P.C. n-3 Fatty acids and cardiovascular disease: Evidence explained and mechanisms explored. Clin. Sci. 2004, 107, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Very long chain omega-3 (n-3) fatty acids and human health. Eur. J. Lipid Sci. Technol. 2014, 116, 1280–1300. [Google Scholar] [CrossRef]
- Calder, P.C. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2015, 1851, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.S. The omega-3 index: From biomarker to risk marker to risk factor. Curr. Atheroscler. Rep. 2009, 11, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Marik, P.E.; Varon, J. Omega-3 dietary supplements and the risk of cardiovascular events: A systematic review. Clin. Cardiol. 2009, 32, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Wu, J.H.Y. Omega-3 fatty acids and cardiovascular disease: Effects on risk factors, molecular pathways, and clinical events. J. Am. Coll. Cardiol. 2011, 58, 2047–2067. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Panigrahy, D.; Mahakian, L.M.; Yang, J.; Liu, J.-Y.; Stephen Lee, K.S.; Wettersten, H.I.; Ulu, A.; Hu, X.; Tam, S.; et al. Epoxy metabolites of docosahexaenoic acid (DHA) inhibit angiogenesis, tumor growth, and metastasis. Proc. Natl. Acad. Sci. USA 2013, 110, 6530–6535. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. Evolutionary aspects of diet and essential fatty acids. World Rev. Nutr. Diet. 2001, 88, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.X. The Importance of Omega–6/ Omega–3 Fatty Acid Ratio in Cell Function—The Gene Transfer of Omega–3 Fatty Acid Desaturase. In Omega–6/Omega–3 Essential Fatty Acid Ratio: The Scientific Evidence. World Review of Nutrition and Dietetics; Simopoulos, A.P., Cleland, L.G., Eds.; Karger: Basel, Switzerland, 2003; Volume 92, pp. 23–36. [Google Scholar]
- Eaton, S.B.; Eaton Iii, S.B.; Sinclair, A.J.; Cordain, L.; Mann, N.J. Dietary Intake of Long-Chain Polyunsaturated Fatty Acids during the Paleolithic. World Rev. Nutr Diet. Basel 1998, 83, 12–23. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The Importance of the Omega-6/Omega-3 Fatty Acid Ratio in Cardiovascular Disease and Other Chronic Diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. An increase in the Omega-6/Omega-3 fatty acid ratio increases the risk for obesity. Nutrients 2016, 8, 128. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).