Abstract
Control of green mold, caused by Penicillium digitatum, by fungicides raises several problems, such as emergence of resistant pathogens, as well as concerns about the environment and consumers’ health. As potential alternatives, the effects of chitosan on green mold disease and the quality attributes of citrus fruits were investigated. Fruits were wounded then treated with different concentrations of chitosan 24 h before their inoculation with P. digitatum. The results of in vitro experiment demonstrated that the antifungal activity against P. digitatum was improved in concert to the increase of chitosan concentration. In an in vivo study, green mold was significantly reduced by chitosan treatments. In parallel, chitinase and glucanase activities were enhanced in coated fruits. Evidence suggested that effects of chitosan coating on green mold of mandarin fruits might be related to its fungitoxic properties against the pathogen and/or the elicitation of biochemical defense responses in coated fruits. Further, quality attributes including fruit firmness, surface color, juice content, and total soluble solids, were not affected by chitosan during storage. Moreover, the loss of weight was even less pronounced in chitosan-coated fruit.
1. Introduction
With an annual production of over 130 million tons, covering an area of nearly 9 million hectares, citrus fruits are the leading fruit crop in international trade in terms of value. Citrus-based products represent a global market size of many billions of dollars. At present, the Mediterranean basin constitutes one of the most important production areas of citrus, exporting more than half of the world’s citrus fruits [1]. Before reaching the market, harvested fruits are usually stored for fresh consumption. During the postharvest period, fungal disease infection is the leading source of fresh citrus fruit decay [2]. Green mold caused by Penicillium digitatum (Pers.:Fr.) Sacc. is the primary postharvest disease affecting citrus production worldwide in the packing house, during transit and in the market [3,4,5].
The fight against postharvest decays of fruits has been underway for decades but has not yet been won, and presently, control of citrus pathogens is still dependent mainly on the use of chemical fungicides, such as imazalil or thiabendazole [6]. However, in the long run, many of the fungicides widely used to control postharvest decay have short-term effectiveness because of the emergence and proliferation of fungicide-resistant pathogens [3,7]. Likewise, the excessive use of synthetic fungicides is raising consumer concern regarding their adverse effect on the environment, human and animal health [8]. Consequently, current trends in both food industry and consumption are directed towards safer and healthier food production, with no chemical additives, according to principles of sustainability and the need of environmental protection. This has led to an increase in our efforts to discover new natural antimicrobials alternatives, and, in this approach, the impacts of chitosan have been investigated.
Chitosan, a chitin derivative, has been widely identified as a natural antimicrobial agent against many bacteria, fungi and yeasts [9,10]. Although the exact mechanisms of action of chitosan in reducing plant disease are not yet fully understood, different mechanisms have been proposed [10]. Thus, there is growing evidence demonstrating its action through direct toxicity or chelation of nutrients and minerals from pathogens, halting or reducing fungal growth [11]. In addition, chitosan has been stated to elicit diverse host defense responses, offering protection against infection in a variety of host plants against their respective pathogens [11,12,13,14,15].
The overall objectives of this study were to (i) investigate the resistance induced by chitosan to control in vitro and in vivo development of Penicillium digitatum; (ii) evaluate the activity of defense enzymes in citrus fruit induced by chitosan treatment; and (iii) assess quality parameters of chitosan-coated citrus fruit.
2. Materials and Methods
2.1. Fruit Treatments
Mandarin fruits cv. “Ortanique” (Citrus reticulata Blanco) used in this study were harvested from trees in the orchard of the INRA experimental citrus research station (El Menzeh, Morocco), and sorted based on size uniformity and the absence of physical injuries or disease infection. Freshly harvested fruits were surface disinfected by dipping for 2 min in a 10% sodium hypochlorite solution and were rinsed twice with distilled water. After drying for one hour, fruits were randomized into treatment lots then wounded at four equidistant points at the equatorial site. Each wound was 5 mm in diameter and 4 mm in depth. At 24 h before inoculation, fruits were then dipped into chitosan solution for 10 sec, and air-dried for 30 min under a fan to warrant dryness. Fruits dipped in distilled water following the same procedures were used as controls.
2.2. Chitosan
Shrimp shell chitosan was purchased from Sigma Aldrich (France) and ground to a fine powder. Purified chitosan was prepared by dissolving chitosan in 0.25 N acetic acid and the undissolved particles were removed by centrifugation (15 min, 10,000 g). The viscous solution was then neutralized with 2.5 N NaOH to pH 9.8 to precipitate the chitosan. Precipitated chitosan was recovered by filtration, washed extensively with deionized water, and then lyophilized. Chitosan stock solution (10 g·L−1) was prepared by dissolving chitosan in HCl (0.05 N), and the pH solution was adjusted to 5.6 by adding sodium hydroxide 1N. The stock solution was autoclaved and subsequently diluted with sterile distilled water to obtain final chitosan concentrations of 2, 4, 6, and 8 g·L−1.
2.3. Pathogen Inoculum
Highly aggressive isolates of P. digitatum, used in the investigation, were originally isolated from rotted citrus fruit collected from the INRA Citrus orchard. Identification was made based on the morphological criteria of the colony on malt extract agar medium when incubated at 25 ± 1 °C for 7 days. A white mycelium and green conidia were observed. Isolates were grown on potato dextrose agar (PDA) at 25 °C for 7 days. Spores were afterward harvested by flooding the surface of media with sterile distilled water and the plate was agitated gently to dislodge spores. Spores were counted using a hemacytometer, and the spore concentrations from the pathogens were adjusted with sterile distilled water containing 0.05% (v/v) Tween 80 to obtain 105 spores mL−1.
2.4. In Vitro and in Vivo Antifungal Activity of Chitosan
The antifungal properties of chitosan against P. digitatum were determined using PDA plates amended with different concentrations of chitosan. The PDA plates were inoculated with the pathogen using 20 µL of spores suspension. Growth was measured when the control reached the edge of the plate. Growth inhibition was calculated as the percentage of inhibition of radial growth relative to control.
For the in vivo assay, chitosan-coated and control fruits were inoculated with 50 µL of P. digitatum at a concentration of 105 spores mL−1 or 50 µL of Tween 80 solution (0.05% w/v). Fruits were kept at 25°C for 7 days before disease evaluation. For each treatment, four replicates of 10 fruits were used, and results were expressed as the percentage of disease inhibition.
2.5. Anatomical Studies
Rind tissues (1 mm diameter) were immersed in cold fixative solution containing 8% glutaraldehyde and 2% paraformaldehyde in 0.2 M potassium buffer (pH 7.24), vacuum infiltrated for 20 min, and then immersed in fresh fixative solution for 20 h [16]. Samples were subsequently washed with 0.2 M potassium buffer (pH 7.24), post-fixed in 2% osmium tetroxide prepared in the same buffer for 4 h, washed with the buffer, and dehydrated in graded ethanol series. The samples were then washed with acetone series and embedded in araldite (Fluka, France). Semi-thin sections (1 µm) were collected on glass slides and stained with toluidine blue, rinsed in distilled water, air dried, and mounted in Eukitt. The sections were examined under an optical microscope (model no. BH-2; Olympus, Tokyo, Japan).
2.6. Determination of Defense-Related Enzyme Activities: Chitinase and β-1,3-Glucanase
Chitinase and β-1,3-glucanase were assayed from flavedo tissues of mandarin fruits. Flavedo material was peeled from the border of macerated tissue to the healthy zone, immediately dipped in liquid nitrogen, and ground with a mortar and pestle. Enzymes were extracted by dissolving using 100 mg of ground tissues in 5 mL sodium phosphate buffer (0.05 M, pH 6.5) containing 0.5 g of polyvinyl polypyrrolidone (PVPP) for 2 h at 4 °C. The suspension was pelleted by centrifugation at 20,000 g for 30 min at 4 °C. The supernatant phase was collected to determine chitinase and β -1,3-glucanase activities.
Activity of β-1,3-glucanase was determined as described by Yao and Tian [17]. Briefly, the mixture of 50 μL of extracted flavedo enzyme and 50 μL of laminarin (Sigma, USA) 0.4% (w/v) was incubated for 30 min at 37 °C. The reaction was stopped by adding 400 μL of dinitrosalicylic acid (DNS) reagent. The mixture was then heated for 10 min in boiling water. After cooling, reaction solution was appropriately diluted with distilled water and the absorbance was measured at 540 nm. The β-1,3-glucanase activity was defined as the amount of reducing sugar released from laminarin hydrolysis. The β-1,3-glucanase activity unit was defined as the enzyme activity that catalyzes the formation of 1 μmol glucose per minute at 37 °C, and expressed as Glucanases µg−1·min−1·g−1 FW.
Chitinase activity was measured according to the method of Wirth and Wolf [18] with chitin as a substrate. Chitin (Sigma, USA) was dissolved in sodium phosphate buffer (0.05 M, pH 5.2) and shaken at 500 rpm for 30 min. A total of 200 μL of 1% (w/v) colloidal chitin plus 200 μL of enzyme extract solution was shaken at 500 rpm at 37 °C for 1 hour. After stopping the reaction by heating in boiling water, the mixture was centrifuged at 10,000 g for 5 min and the supernatant was collected to determine chitinase activity. The chitinase activity defined as the amount of enzyme required to release 1 μmol of N-Acetyl-d-Glucosamine per minute from chitin hydrolysis under the assay conditions was measured spectrophotometrically at 550 nm and 500 nm using a UV-160 Spectrophotometer (Shimadzu, Japan). The chitinase activity was expressed as chitin hydrolyzed. min−1·g−1 of fresh weight.
The total soluble protein was determined according to the method of Bradford [19] using bovine serum albumin as the standard
2.7. Measurement of Fruit Quality Parameters
Fruit firmness was measured at the end of each storage period using a digital penetrometer (AGROSTA®14ATouchscreen, FR). Each fruit was placed between two flat surfaces and the machine compressed the samples in the equatorial zone until 5% deformation at 5 mm/min, by closing together the upper surface that consists of a probe that ends in a flat area of 8 mm diameter. The machine gave the deformation (mm) after application of a load of 10N to the equatorial region of the fruit. The firmness was reported as peak force and expressed in Newtons as the force required to reach this deformation level. Measurements were taken in 20 mandarins for each treatment and storage time.
Weight loss was monitored 0, 2, 5, 7, 10, 14, and 21 days after chitosan coating. Three replicate of 30 fruits per treatment were used to measure weight loss. The same fruit was weighed at the beginning of the experiment and at the end of each storage period. The results were expressed as the percentage loss of initial weight.
Surface color of the citrus fruit was measured using a Hunter colorimeter (Konica Minolta, model CR-400, Japan). To avoid the effects of heterogeneity in the fruit, measurements were always taken in the same previously marked sample zone in the citrus. L∗ (lightness), a∗ (redness), and b∗ (yellowness) values were recorded. For each fruit, two different sites were measured at equatorial area. Ten fruits were used for each measurement and the measurements were performed in duplicate. The Hunter parameters L*, a* and b* were reported by the colorimeter, obtaining the color index (CI) using the following equation: CI = (1000 x a*)/(L* x b*). The a* parameter indicates the area of variation between red and green spectrum; b* parameter refers to the area of variation between yellow and blue spectrum. L* parameter gives a value of the luminance or brightness of the sample.
To determine juice content, ten representative fruits were weighed and cut into halves before being pressed using a juicer (Santos, France) at 1500 tr/mn. The juice content, expressed as percent juice, is determined by weighing components of the whole fruit and the juice. The % juice = (juice weight/fruit weight) × 100.
Total soluble solids (TSS) content in the juice was determined with a Model PAL-1 digital refractometer (Atago, Tokyo Tech., Tokyo, Japan) and titratable acidity (TA) was measured by titration, with 0.1 N sodium hydroxide to pH 8.1. The TA is expressed as percentage of citric acid anhydride per L of juice by following the AOAC 942.15 method [20]. The maturity index (MI) was calculated as TSS/TA ratio. Total of three juice samples were considered for each treatment/time. Each juice sample corresponded to 15 fruits.
2.8. Statistical Analyses
Each experiment was repeated at least three times, with 24 plants evaluated per treatment, unless indicated otherwise. Antifungal activity test was done using ten petri dishes for each treatment. For chitinase and glucanase activity, the results are expressed as the mean of two separate experiments (in each experiment three different extractions were pooled). For other experiments, results were analyzed statistically through ANOVA. Means for each treatment were separated with a least significant difference (LSD, p < 0.05) multiple comparison test (Fisher’s protected). Bars or means with the same letters represent values that are not significantly different (p < 0.05).
3. Results
3.1. In Vitro and in Vivo Antifungal Effects
In vitro antifungal results showed that P. digitatum growth was reduced on chitosan-supplemented plates relative to fungal growth on chitosan-free plates. This inhibition was chitosan concentration dependent, with a maximum inhibition of 69% at 3.5 g·L−1 (v/v) chitosan (Figure 1). However, the level of fungal growth inhibition never reached 100%, suggesting that fungal growth was not fully controlled by chitosan.
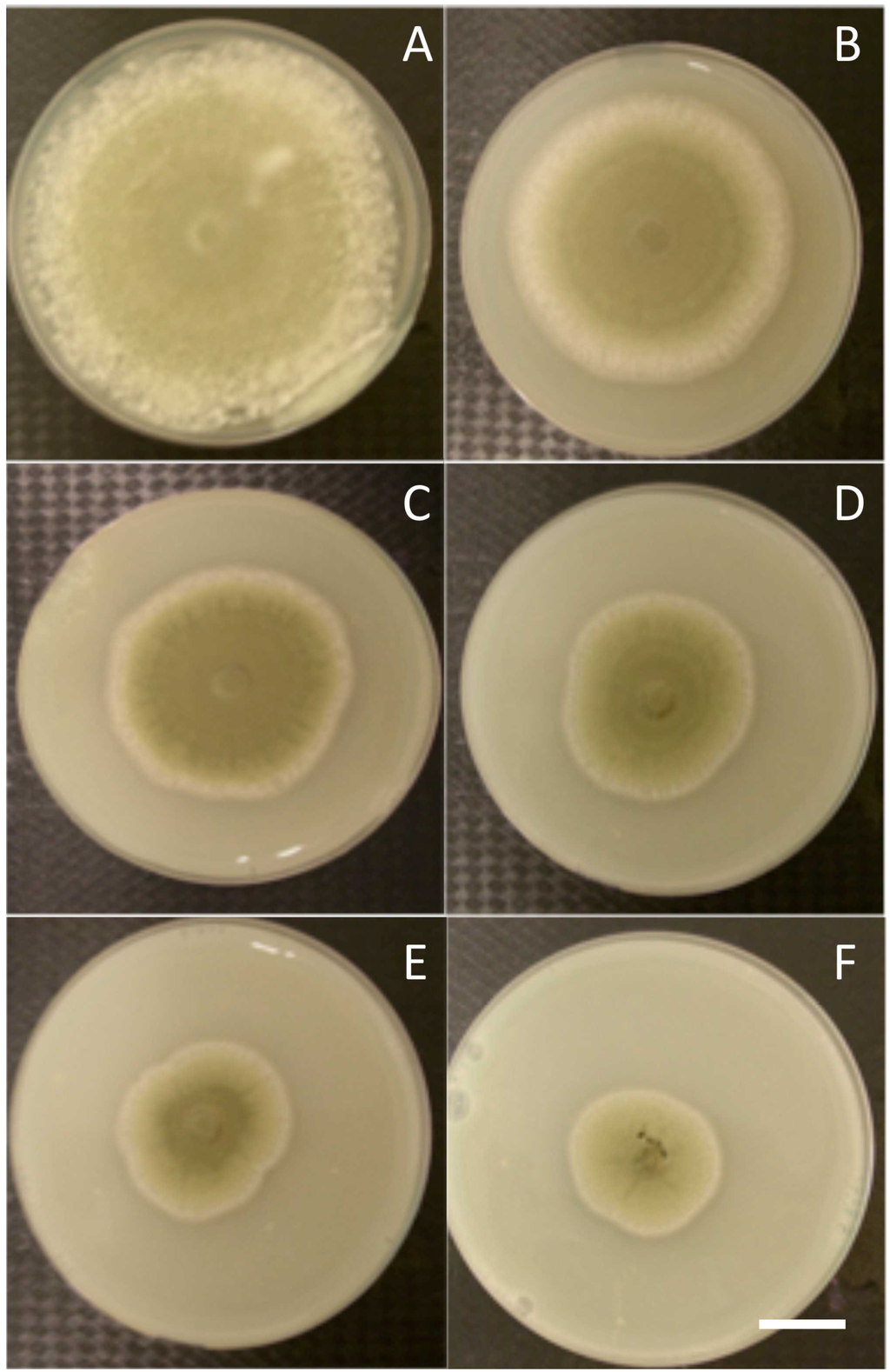
Figure 1.
Effect of chitosan on the radial growth of P. digitatum. A: Control PDA plates, B–F: PDA plates supplemented with chitosan at different concentrations (v/v; B: 1.5, C: 2, D: 2.5, E: 3, F: 3.5 g·L−1). Fungal growth decreased as chitosan concentration increased. Bar = 1 cm.
In vivo analysis revealed that when uncoated fruits were infected with the pathogen, hyphae of P. digitatum invaded rapidly puncture injuries within 4 d, and then mycelia colonized extensively healthy tissue surrounding the injury site (Figure 2A). However, growth of P. digitatum was significantly (p < 0.05) moderated in chitosan-coated fruit dependent on chitosan concentration, with a decrease of colonized area by 95% at 6 g·L−1 (Figure 2D); then the fungus growth was completely halted starting from 8 g·L−1 (Figure 2E,F).
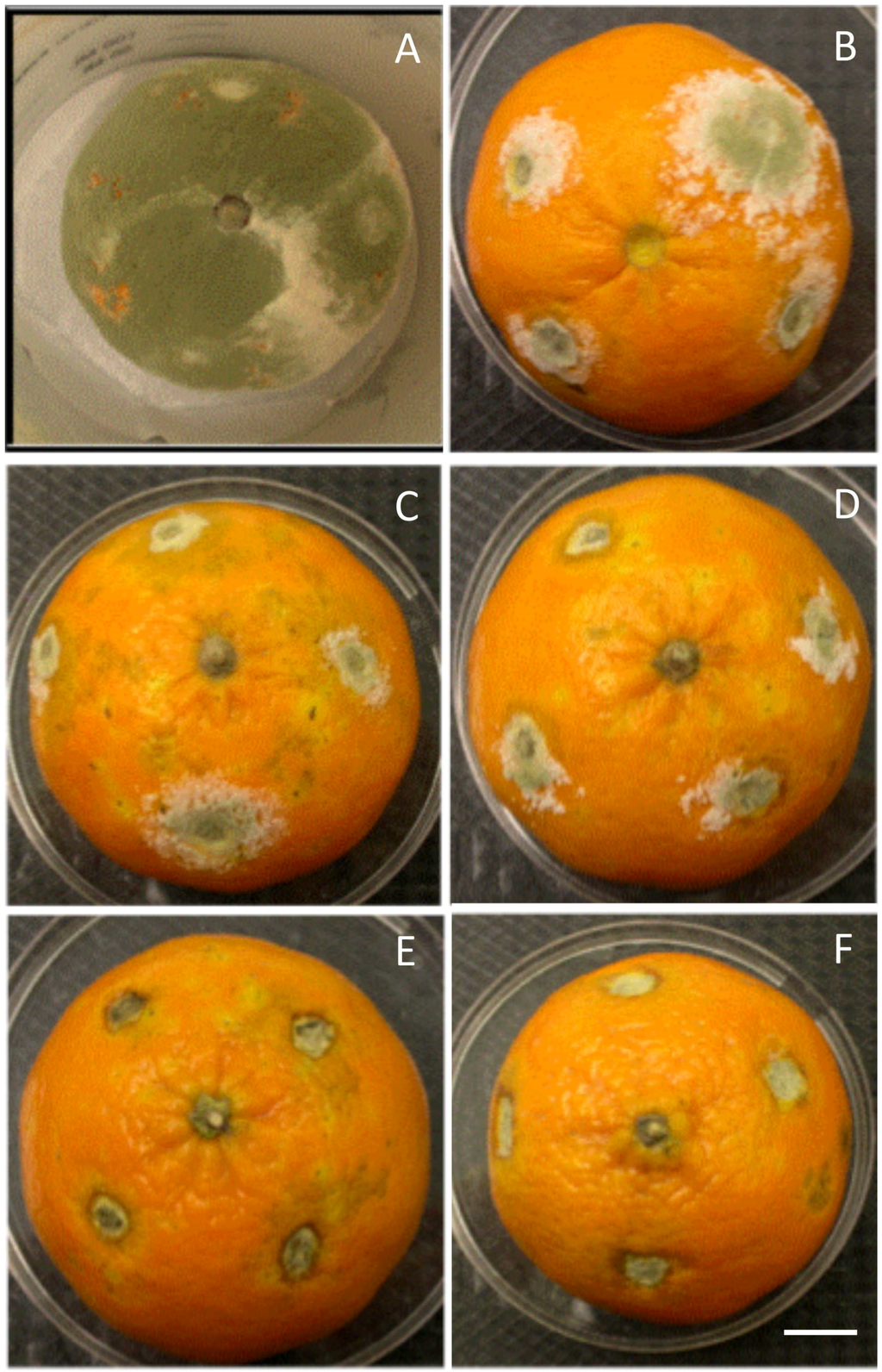
Figure 2.
In vivo phytopathogenicity assay of P. digitatum on Citrus fruits inoculated with different doses of chitosan (A: Control, B: 2, C: 4, D: 6, E: 8, F: 10 g·L−1). The fungus growth on fruit surface was significantly affected by the increase of chitosan concentration, bar = 1 cm.
3.2. Anatomical Studies
Coated fruits exhibited a normal structure with a slight thickening of epidermal cells layers (Figure 3A). On the other hand, when infected with pathogen, non-coated fruit showed an invasion of fungal mycelia after 4 days through physically injured epidermal and subepidermal cells of the chitosan exocarp (Figure 3D–G) at the top of the Citrus fruit. Penetration of injured cells tissues by P. digitatum led to complete cell disorganization (Figure 3D–G). The fungus caused obvious swelling and dissolution of host cell walls in advance of hyphal penetration (Figure 3F,G). Colonization of injured tissue by P. digitatum was essentially complete at 4 to 5 days after the application of pathogen. In puncture injuries, the structures of cells were preserved in chitosan-coated fruits indicating that distribution of the fungal mycelium was halted in these tissues (Figure 3H).
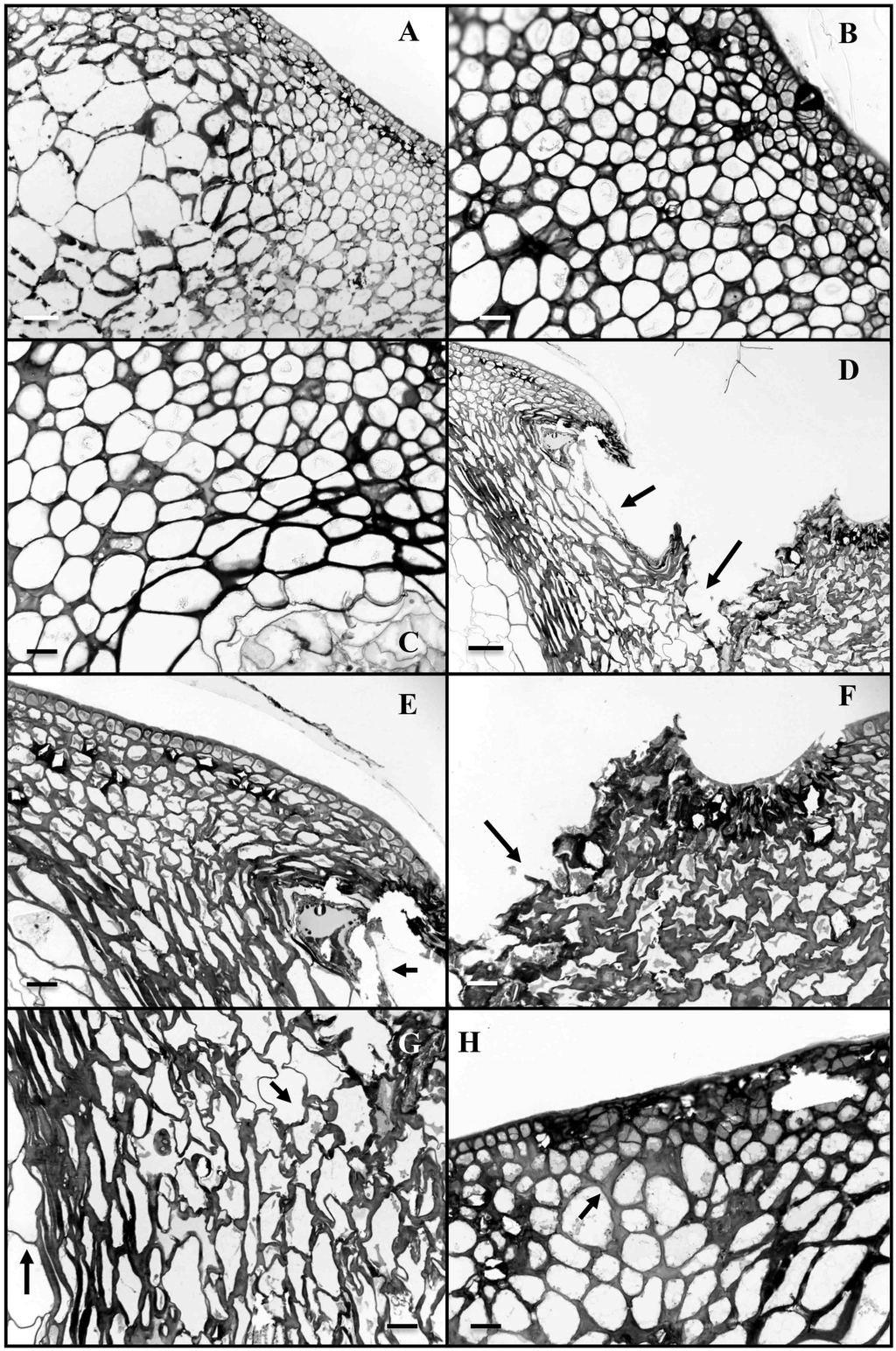
Figure 3.
Light micrographs of samples from Citrus peel tissues infected by Penicillium digitatum. A: cross-section of control fruit, B,C: cross-section of Citrus fruit treated with chitosan (6 g·L−1); D–G: cross-section of fruit inoculated Penicillium digitatum (Note disruption of tissue integrity in cells, arrows) H: cross-section of fruit pre-treated with chitosan and infected by Penicillium digitatum. Fungal growth is mainly restricted to the epidermis. Restriction of fungal growth correlates with establishment of discrete structural changes mainly characterized by an increased thickness of the host cell wall. Scale bar = 20 μm.
3.3. Biochemical Defense Responses
Compared to control, chitosan-coated fruit exhibited a significant increase of chitinase activity (Figure 4A). Meanwhile, in P. digitatum-infected fruit, the chitinase activity was induced in Citrus fruit tissues with an increase of 100% compared to non-infected fruit. Further, the level of chitinase activity was highest in chitosan-coated fruits that were infected by the pathogen.
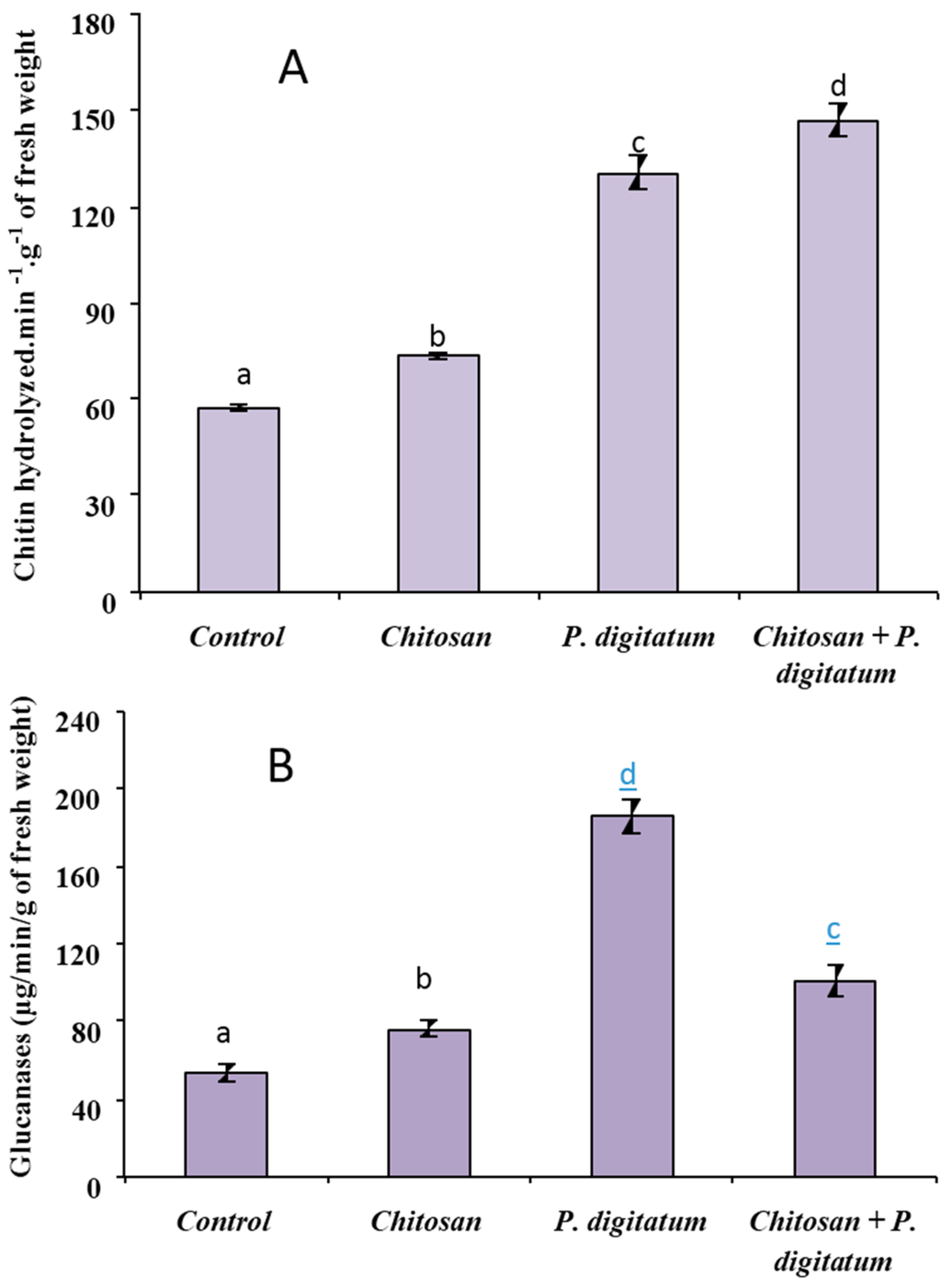
Figure 4.
The effect of chitosan on the induction of chitinase (A) and glucanase (B) activities in the rind of Citrus fruits. The results are expressed as the mean of three separate experiments (in each experiment three different extractions were pooled). Means indicated with different letters are significantly different (p < 0.05). Data are means of three independent experiments with standard error.
As for chitinase, glucanase activity was affected by all treatments. As shown in Figure 4B, chitosan coating significantly (p < 0.05) enhanced glucanase activity. Moreover, the activity was boosted more than three-fold in fruits infected by P. digitatum. In the meantime, in fruit coated with chitosan before being inoculated with the pathogen, glucanase activity was higher than control or chitosan-coated fruit, but significantly lower than for P. digitatum-treated fruits.
3.4. Effects on Quality Parameters of Citrus Fruits
There was no effect of chitosan treatment between the chitosan-coated and control fruit on fruit firmness (Figure 5). However, when inoculated with P. digitatum, firmness decreased in both coated and uncoated fruits, but was more significantly affected in uncoated fruits. Furthermore, results revealed a clear evolution of the color index during storage, regardless of chitosan treatment (Figure 6A–C), indicating that the coating has a very low impact on the color of the fruit skin. The acidity values of the fruits also showed a significant decrease throughout storage time, regardless of coating (Figure 6E). The soluble solids fluctuated during the storage period without clear treatment-dependent tendency (Figure 6F). This could be explained by a better contribution of the natural variability of the sample than that of the treatment or storage time. In both treatments, there is a growing trend in the index of fruit maturity. However, there was no difference in this parameter between the treated and untreated fruit (Figure 6G). Both storage time and coating were found to have a significant effect (p < 0.05) on sample weight loss. The weight loss increased over the storage time and tended to be lower for chitosan-coated fruits than the uncoated (Figure 6H).
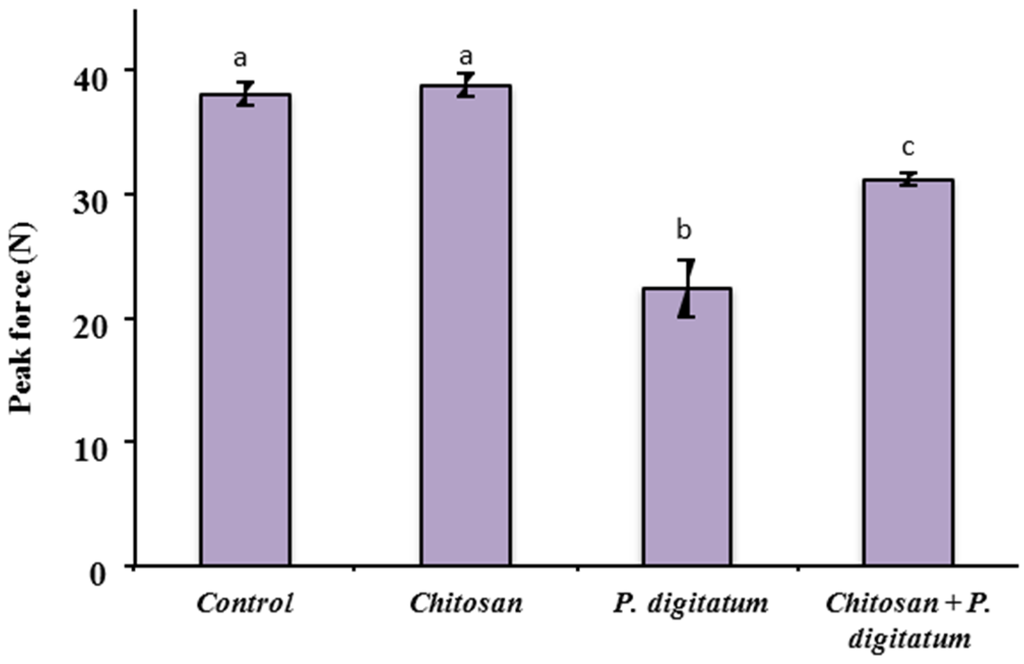
Figure 5.
The effect of chitosan on firmness of fresh citrus fruits inoculated by P. digitatum. Vertical bars indicate standard error. The fruit firmness was affected by the presence of pathogen, but less when they were previously coated with chitosan. Means indicated with different letters are significantly different (p < 0.05). Data are means of three independent experiments with standard errors.
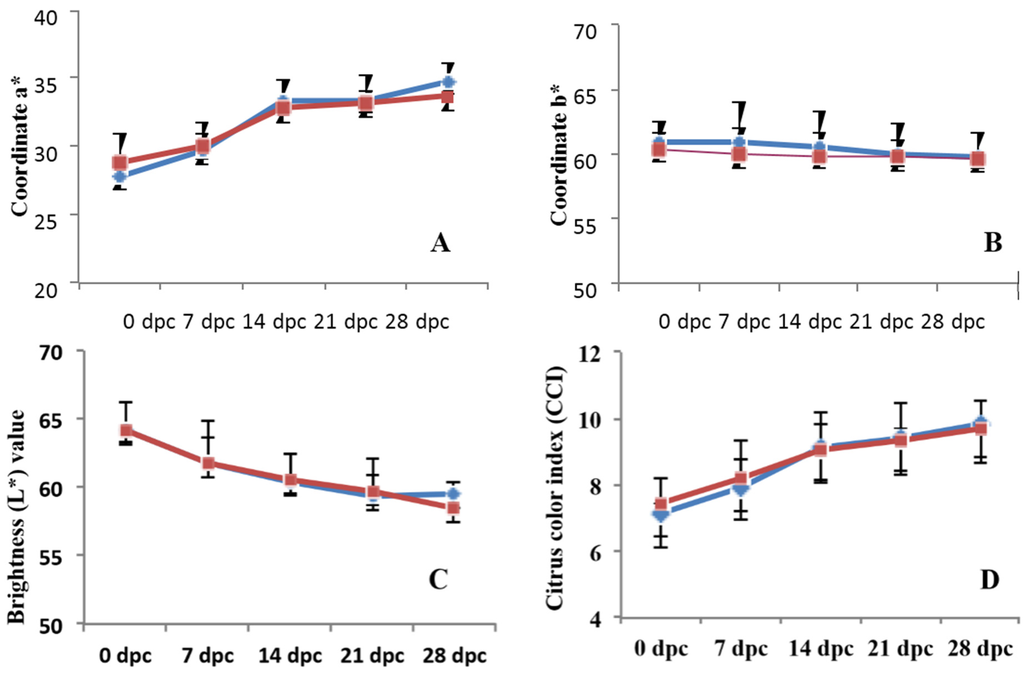
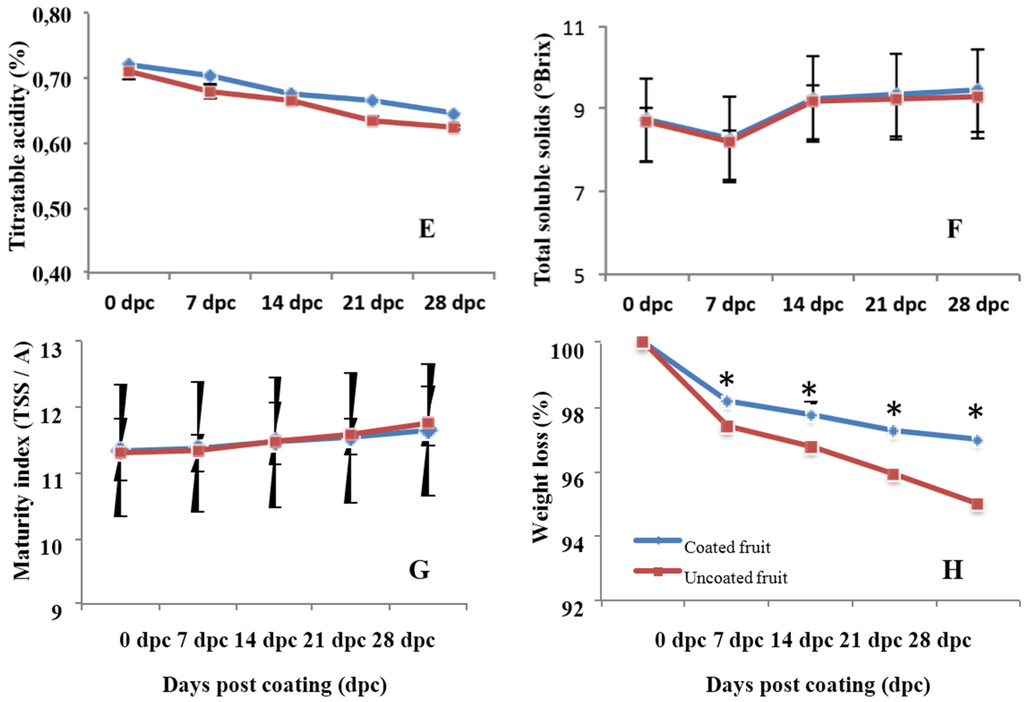
Figure 6.
Effect of chitosan on the evolution of different physio-chemical parameters during storage of Citrus fruit. If noted, asterisks (*) show significant difference (p < 0.05) between coated and uncoated fruits. Data are means of three independent experiments’ standard errors.
4. Discussion
Chitosan has a double impact on host-pathogen interactions through its antifungal activity and its ability to induce plant defense mechanisms [21]. Coating fruit and vegetables with chitosan has some positive advantages for the long-term storage of foods. Previous findings have reported that applying a chitosan coating to fruits including strawberry, bell pepper, cucumber, pear, peach and litchi, controlled postharvest diseases [9,21,22,23]. In the present work, the faculty and mode of action of chitosan to inhibit the development of green mold caused by P. digitatum in citrus fruits in addition to its impact on fruit quality parameters were monitored.
4.1. Chitosan as Antifungal
The radial growth of P. digitatum on PDA plates decreased as chitosan concentration increased, thereby corroborating the literature, which indicates that the level of inhibition of fungi is highly correlated with chitosan concentration. When applied at a rate of 1 g/L, chitosan inhibits the in vitro growth of a several fungi and oomycetes. Thus, the radial growth of Alternaria alternata, Aspergillus niger, Botrytis cinerea, Colletrotichum gloeosporioides, Penicillium, Rhizopus stolonifer, and Sclerotinia sclerotiorum, decreased as chitosan concentration increased [9,11,24,25,26,27].
In addition to its inhibitory impact on fungal growth, several studies have reported that chitosan is able to also induce obvious morphological and structural disorganization in parallel to molecular changes of the fungal cells [9,22,24]. Chen et al. [26] showed that the mycelium and conidia of A. alternata were affected at the structural level when chitosan was applied. One of the reasons for the antimicrobial proprieties of chitosan is its positively charged amino group. The latter interacts with negatively charged microbial cell membranes, leading to the leakage of proteinaceous and other intracellular constituents of the pathogens [11,13]. Additionally, chitosan may enter into fungal cells inhibiting adsorption of essential nutrients, and therefore to an inhibition or slowing of mRNA and protein synthesis [23,28].
4.2. Chitosan and Postharvest Fungal Disease
Resistance of plants to disease might be systemically improved by earlier infections with pathogens or by prior elicitors’ treatments [29]. Various reports have indicated that chitosan efficiently controls postharvest rots during storage, delays the onset of infection and slows down the infection progress. In this study, a significant delay in the rate of fungal decay was observed when fruits were previously coated with chitosan confirming earlier findings indicating that chitosan coatings are effective against blue and green postharvest citrus decay in citrus fruit [30,31,32]. This result appeared to be linked to the antifungal activity of chitosan previously reported against several postharvest fungi including A. alternate, Fusarium, Rhizopus, and B. cinerea [26,33,34,35].
Analysis of sections from treated tissues revealed that chitosan prevented the disintegration of cell structure. These results are in line with earlier observations reporting disorganized hyphae associated with inhibition of fungal growth as consequence of a sequence of morphological and structural modifications induced by chitosan [24,36]. Chitosan has been shown to trigger resistance locally at the site of contact in carrot foliage [13], therefore partially explaining why, in our study, P. digitatum fails to progress around the site of infection. Because of its biopolymer properties, chitosan might also form physical barriers around the sites of pathogen attack, blocking them from spreading to healthy tissues.
4.3. Biochemical Defense as Response to Chitosan Application
Enhancing the natural defense capabilities of fruits through induction of resistance is one of the alternative strategies that have been explored to attenuate the chemical fungicide use during postharvest handling and storage. In this respect, the elicitor impact of chitosan is well known, through the induction of a variety of plant responses both locally near the attack sites and systemically to alert healthy parts of the plant [9]. Plants may also employ an arsenal of inducible defenses as retaliation to the pathogen assault in order to slow spread of the disease [37]. Some of these defenses include early signaling events as well as the accumulation of defense-related proteins. Among pathogenesis-related (PR) proteins, chitinase and glucanase with potential antifungal activity are induced in plants in response to pathogen attack and frequently associated with necrotic reactions [38]. Besides its ability to attack the fungal cell wall directly, chitinase may also contribute indirectly to induce defense-related responses in plant cells through the release of non-specific elicitors [39]. Glucanase acts as a mechanical barrier to obstruct the fungal invasion inside the plant tissues and also protects them against fungal phytotoxic substances. Moreover, the accumulated glucanase may hydrolyze β-1,4-glucan, which is the major component of fungal cell wall [25].
Several PR proteins, including chitinase and glucanase, were induced by chitosan in orange, raspberries and strawberry as compared with the uncoated controls [15] Evidence that glucanase and chitinase may be responsible for limiting fungal development have been reported in cucumbers [40], dragon fruit [25], strawberries and raspberries [41], and citrus fruit [3,32], by inducing systemic resistance. In line with these findings, in this study, we report significant increase of chitinase and glucanase activities of the chitosan-treated fruit or that inoculated with P. digitatum as compared with the control fruit. It seems conceivable to hypothesize that the activation of a combined group of defense responses is required to prevent P. digitatum infection. By inducing and hastening chitinase and glucanase activities, chitosan may delay the reactivation of latent infections, which naturally occurs when resistance of tissue declines [42]. However, Fajardo et al. [43] did not report induced-PR proteins in the flavedo of oranges treated with different biological derived elicitors before being inoculated with P. digitatum.
4.4. Effect of Chitosan on the Postharvest Quality
Firmness was less affected by the presence of P. digitatum when fruits were coated previously with chitosan. The reported delay of firmness decline may be associated with the histo-cytological observation where fungal growth was halted in chitosan-coated fruits. In agreement with our finding, several examples indicate that the loss of firmness of the chitosan-coated fruit—including papayas, citrus, strawberries, peaches, raspberries, tomatoes, and others—was delayed during postharvest storage [9,26,31,35]. Furthermore, the lower weight loss observed on chitosan-coated fruits correlates with a higher firmness, confirming reports of Rodov et al. [44] who indicate that firmness of fruit depends primarily on weight loss rate. Since chitosan is able to form an edible film when applied to the surface of fruit, it is clear that it will be able to confer an effective physical barrier to moisture loss, delaying dehydration and fruit shriveling. Additionally, postharvest water loss may also alter metabolism before symptoms become apparent [45]. Hence, coating with chitosan may prolong storage life, delay the drop in sensory quality, and control the decay of the coated fruit.
Divergent reports were listed in the literature regarding the impact of chitosan on the color. While a deeper green color than control was detected on cucumber and bell peppers [9], our results revealed that the color was not affected with chitosan treatment. In accordance with our results, Baldwin et al. [46] and Obenland et al. [47] have found that chitosan did not affect physicochemical characteristics of fruits during postharvest storage. In this study, application of chitosan did not affect physicochemical characteristics of fruits during postharvest storage. In contrast, another study has reported a decline in SSC and TA losses in chitosan-coated fruits, which was associated with a decrease in weight loss and respiration rate [48].
Generally, at the end of the postharvest storage, titratable acidity was stated to increase on the chitosan-coated commodity (strawberries, tomatoes, and peaches), but in other crops such as mangoes and longan, the acidity was slowly reduced, correlating this decline with loss of eating quality [49,50,51]. In our study, titratable acidity declined significantly during the storage period in both uncoated and coated fruit. However, the decline was less significant in chitosan-coated fruits, thereby supporting the idea that chitosan may delay fruit senescence as reported by Gol et al. [52] who report that the decline of acidity during storage is linked to the progress of fruit senescence.
During postharvest storage, TSS of chitosan-treated fruits diverged depending on the commodity: lower content was reported in mangoes and bananas, whereas higher values were reported on treated peaches. However, as observed in our study, other reports showed that TSS of chitosan-dipped papayas and zucchinis were not affected by chitosan treatment [51,53].
5. Conclusions
The present study showed that chitosan, as a natural substance, inhibits the growth of P. digitatum on mandarins in vitro and in vivo. Chitosan also sensitizes the fruit to respond more rapidly to a pathogen attack by elaborating defensive mechanisms. Further, quality attributes were not affected during the storage period. The maintenance of quality and the extension of shelf life of chitosan-coated fruits suggest that the application of chitosan should be considered for use during commercial storage and marketing. Evidence suggests that chitosan may be promising as a natural fungicide to partly substitute synthetic fungicides to extend postharvest shelf life and, to some extent, control decay of mandarins.
Acknowledgments
The authors are grateful to the France-Morocco Bilateral Cooperation for its financial support in the case of PRAD project N° 04-03.
Author Contributions
These authors contributed equally to this work.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Faostat, 2012. FAO Statistical Division. Available online: http://faostat3.fao.org/home/index.html (accessed on 19 December 2012).
- González-Candelas, L.; Alamar, S.; Sánchez-Torres, P.; Zacarías, L.; Marcos, J.F. A transcriptomic approach highlights induction of secondary metabolism in citrus fruit in response to Penicillium digitatum infection. BMC Plant Biol. 2010, 10, 194–211. [Google Scholar] [CrossRef] [PubMed]
- Ballester, A.R.; Izquierdo, A.; Lafuente, M.T.; González-Candelas, L. Biochemical and molecular characterization of induced resistance against Penicillium digitatum in citrus fruit. Postharvest Biol. Technol. 2010, 56, 31–38. [Google Scholar] [CrossRef]
- Cunningham, N.M.; Taverner, P.D. Efficacy of integrated postharvest treatments against mixed innoculations of Penicillium digitatum and Geotrichumcitri-aurantii in ‘leng’ navel oranges (Citrus sinensis). N. Zeal. J. Crop Hort. 2007, 35, 187–192. [Google Scholar] [CrossRef]
- Droby, S.; Eick, A.; Macarisin, D.; Cohen, L.; Rafael, G.; Stange, R.; McColum, G.; Dudai, N.; Nasser, A.; Wisniewski, M.; et al. Role of citrus volatiles in host recognition, germination and growth of Penicillium digitatum and Penicillium italicum. Postharvest Biol. Technol. 2008, 49, 386–396. [Google Scholar] [CrossRef]
- Smilanick, J.L.; Brown, G.E.; Eckert, J.W. Postharvest citrus diseases and their control. In Fresh Citrus Fruits, 2nd ed.; Wardowski, W.F., Miller, W.M., Hall, D.J., Grierson, W., Eds.; Florida Science Source, Inc.: Longboat Key, FL, USA, 2006; pp. 339–396. [Google Scholar]
- Montesinos-Herrero, C.; Smilanick, J.L.; Tebbets, J.S.; Walse, S.; Palou, L. Control of citrus postharvest decay by ammonia gas fumigation and its influence on the efficacy of the fungicide imazalil. Postharvest Biol. Technol. 2011, 59, 85–93. [Google Scholar] [CrossRef]
- Basta, N.T.; Ryan, J.A.; Chaney, R.L. Trace element chemistry in residual-treated soil: Key concepts and metal bioavailability. J. Environ. Qual. 2005, 34, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Bautista-Baños, S.; Hernández-Lauzardo, A.N.; Velázquez-del Valle, M.G.; Hernández-López, M.; Ait Barka, E.; Bosquez-Molina, E.; Wilson, C.L. Chitosan as a potential natural compound to control pre and postharvest diseases of horticultural commodities. Crop Prot. 2006, 25, 108–118. [Google Scholar] [CrossRef]
- Rabea, E.I.; Badawy, M.E.; Stevens, C.V.; Smagghe, G.; Sterbault, W. Chitosan as antimicrobial agent: Application and mode of action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tian, S.P.; Meng, X.; Xu, Y. Effects of chitosan on control of postharvest diseases and physiological responses of tomato fruit. Postharvest Biol. Technol. 2007, 44, 300–306. [Google Scholar] [CrossRef]
- El Hadrami, A.; El Hadrami, I.; Daayf, F. Suppression of induced plant defense responses byfungal pathogens. In Molecular Plant-Microbe Interaction; Bouarab, K., Brisson, N., Daayf, F., Eds.; CABI: Wallingford, UK, 2009; pp. 231–268. [Google Scholar]
- Jayaraj, J.; Rahman, M.; Wan, A.; Punja, Z.K. Enhanced resistance to foliar fungal pathogens in carrot by application of elicitors. Ann. Appl. Biol. 2009, 155, 71–80. [Google Scholar] [CrossRef]
- Nandeeshkumar, P.; Sudisha, J.; Ramachandra, K.K.; Prakash, H.S.; Niranjana, S.R.; Shekar, S.H. Chitosan induced resistance to downy mildew in sunflower caused by Plasmopara halstedii. Physiol. Mol. Plant Path. 2008, 72, 188–194. [Google Scholar] [CrossRef]
- Romanazzi, G.; Lichter, A.; Gabler, F.M.; Smilanick, J. Natural and safe alternatives to conventional methods to control postharvest gray mold of table grapes. Postharvest Biol. Technol. 2012, 63, 141–147. [Google Scholar] [CrossRef]
- Ait Barka, E.; Nowak, J.; Christophe, C. Enhancement of chilling resistance of inoculated grapevine plantlets with a plant growth-promoting rhizobacterium, Burkholderia phytofirmans strain PsJN. Appl. Environ. Microbiol. 2006, 72, 7246–7252. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Tian, S. Effects of pre- and post-harvest application of salicylic acid or methyl jasmonate on inducing disease resistance of sweet cherry fruit in storage. Postharvest Biol. Technol. 2005, 35, 253–262. [Google Scholar] [CrossRef]
- Wirth, S.A.; Wolf, G.A. Dye-labelled substrates for the assay and detection of chitinase and lysozyme activity. J. Microbiol. Meth. 1990, 11, 197–205. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 17th ed.; Association of Analytical Chemists International: Washington, DC, USA, 1995. [Google Scholar]
- Romanazzi, G.; Murolo, S.; Feliziani, E. Effects of an innovative strategy to contain grapevine Bois noir: Field treatment with resistance inducers. Phytopathology 2013, 103, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Zhao, X.; Du, Y. Oligochitosan: A plant diseases vaccine—A review. Carbohydr. Polym. 2010, 82, 1–8. [Google Scholar] [CrossRef]
- Zhang, H.; Li, R.; Liu, W. Effects of chitin and its derivative chitosan on postharvest decay of fruits: A review. Int. Mol. Sci. 2011, 12, 917–934. [Google Scholar] [CrossRef] [PubMed]
- Ait Barka, E.; Eullaffroy, P.; Clément, C.; Vernet, G. Chitosan improves development, and protects Vitis vinifera L. against Botrytis cinerea. Plant Cell Rep. 2004, 22, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Zahid, N.; Manickam, S.; Siddiqui, Y.; Alderson, P.G.; Maqbool, M. Induction of lignin and pathogenesis related proteins in dragon fruit plants in response to submicron chitosan dispersions. Crop Prot. 2014, 63, 83–88. [Google Scholar] [CrossRef]
- Chen, J.; Zou, X.; Liu, Q.; Wang, F.; Feng, W.; Wan, N. Combination effect of chitosan and methyl jasmonate on controlling Alternaria alternata and enhancing activity of cherry tomato fruit defense mechanisms. Crop Protection 2014, 56, 31–36. [Google Scholar] [CrossRef]
- Heng, Y.; Yan, L.; Zhang, H.Y.; Wang, W.X.; Lu, H.; Grevsen, K.; Zhao, X.; Du, Y. Chitosan oligosaccharides-triggered innate immunity contributes to oilseed rape resistance against Sclerotinia sclerotiorum. Int. J. Plant Sci. 2013, 174, 722–732. [Google Scholar]
- Avadi, M.R.; Jalali, A.; Sadeghi, A.M.; Shamimi, K.; Bayati, K.H.; Nahid, E.; Dehpour, A.R.; RafieeTehrani, M. Diethyl methyl chitosan as an intestinal paracellular enhancer: Ex vivo and in vivo studies. Int. J. Pharm. 2005, 293, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Boller, T.; Felix, G. A renaissance of elicitors: Perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Ann. Rev. Plant Biol. 2009, 60, 379–406. [Google Scholar] [CrossRef] [PubMed]
- Chien, P.J.; Chou, C.C. Antifungal activity of chitosan and its application to control postharvest quality and fungal rotting of Tankan citrus fruit (Citrus tankan Hayata). J. Sci. Food Agric. 2006, 86, 1964–1969. [Google Scholar] [CrossRef]
- Chien, P.J.; Sheu, F.; Lin, H.R. Coating citrus (Murcott tangor) fruit with low molecular weight chitosan increases postharvest quality and shelf life. Food Chem. 2007, 100, 1160–1164. [Google Scholar] [CrossRef]
- Long, L.T.; Tien, N.T.T.; Trang, N.H.; Ha, T.T.T.; Hieu, N.M. Study on Antifungal Ability of Water Soluble Chitosan against Green Mould Infection in Harvested Oranges. J. Agric. Sci. 2014, 6, 205. [Google Scholar] [CrossRef]
- Li, Y.C.; Sun, X.J.; Bi, Y.; Ge, Y.H.; Wang, Y. Antifungal activity of chitosan on Fusarium sulphureum in relation to dry rot of potato tuber. Agric. Sci. China 2009, 8, 597–604. [Google Scholar] [CrossRef]
- Perdones, A.; Sánchez-González, L.; Chiralt, A.; Vargas, M. Effect of chitosan–lemon essential oil coatings on storage-keeping quality of strawberry. Postharvest Biol. Technol. 2012, 70, 32–41. [Google Scholar] [CrossRef]
- Ramos-García, M.; Bosquez-Molina, E.; Hernández-Romano, J.; Zavala-Padilla, G.; Terrés-Rojas, E.; Alia-Tejacal, I.; Barrera-Necha, L.; Hernández-López, M.; Bautista-Baños, S. Use of chitosanbased edible coatings in combination with other natural compounds, to control Rhizopus stolonifer and Escherichia coli DH5α in fresh tomatoes. Crop Prot. 2012, 38, 1–6. [Google Scholar] [CrossRef]
- Benhamou, N.; Kloepper, J.W.; Tuzun., S. Induction of resistance against Fusarium wilt of tomato by combination of chitosan with an endophytic bacterial strain: Ultrastructure and cytochemistry of the host response. Planta 1998, 204, 153–168. [Google Scholar] [CrossRef]
- Szandala, E.S.; Backhouse, D. Effect of sporulation of Botrytis cinerea by antagonists applied after infection. Australas. Plant Pathol. 2001, 30, 165–170. [Google Scholar] [CrossRef]
- Van Loon, L.C.; Bakker, P.A.H.M. Signalling in Rhizobacteria-Plant Interactions. In Root ecology (Ecological Studies); De Kroon, J., Visser, E.J.W., Eds.; Springer Verlag: Berlin, Gremany, 2004; Vol. 168, pp. 287–330. [Google Scholar]
- Shibuya, N.; Minami, E. Oligosaccharide signalling for defence responses in plant. Physiol. Mol. Plant Pathol. 2001, 59, 223–233. [Google Scholar] [CrossRef]
- Zhang, D.; Quantick, P.C. Antifungal effects of chitosan coating on fresh strawberries and raspberries during storage. J. Hort. Sci. Biotechnol. 1998, 73, 763–767. [Google Scholar] [CrossRef]
- Ji, C.; Kuc, J. Purification and characterization of an acidic beta-1,3-glucanase from cucumber and its relationship to systemic disease resistance induced by Colletotrichum lagenarium and tobacco necrosis virus. Mol. Plant Microbe Interact. 1995, 8, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Barkai-Golan, R. Postharvest Diseases of Fruits and Vegetables: Development and Control; Elsevier: Philadelphia, PA, USA, 2001. [Google Scholar]
- Fajardo, J.E.; McCollum, T.G.; McDonald, R.E.; Mayer., R.T. Differential induction of proteins in orange flavedo by biologically based elicitors and challenged by Penicillium digitatum Sacc. Biol. Control 1998, 13, 143–151. [Google Scholar] [CrossRef]
- Rodov, V.; Agar, T.; Peretz, J.; Nafussi, B.; Kim, J.J.; Ben-Yehoshua, S. Effect of combined application of heat treatments and plastic packaging on keeping quality of ‘Oroblanco’ fruit (Citrus grandis L. x C. paradisi Macf.). Postharvest Biol. Technol. 2000, 20, 287–294. [Google Scholar] [CrossRef]
- Burdon, J.N.; Dori, S.; Lomaniec, E.; Marinansky, R.; Pesis, E. The post-harvest ripening of water stressed banana fruits. J. Hortic. Sci. 1994, 69, 799–804. [Google Scholar] [CrossRef]
- Baldwin, E.A. Edible coatings for fresh fruits and vegetables, past, present, and future. In Edible Coatings and Films to Improve Food Quality; Krochta, J.M., Baldwin, E.A., Nisperos-Carriedo, M.O., Eds.; Technomic Publishing Co.: Lancaster, PA, USA, 1994; pp. 25–64. [Google Scholar]
- Obenland, D.; Collin, S.; Sievert, J.; Fjeld, K.; Doctor, J.; Arpaia, M.L. Commercial packing and storage of navel oranges alters aroma volatiles and reduces flavor quality. Postharvest Biol. Technol. 2008, 47, 159–167. [Google Scholar] [CrossRef]
- Togrul, H.; Arslan, N. Carboxymethyl cellulose from sugar beet pulp cellulose as a hydrophilic polymer in coating of mandarin. J. Food Eng. 2004, 62, 271–279. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, Y. Effects of chitosan coating on postharvest life and quality of longan fruit. Food Chem. 2001, 73, 139–143. [Google Scholar] [CrossRef]
- Li, H.; Yu, T. Effect of chitosan on incidence of brown rot, quality and physiological attributes of postharvest peach fruit. J. Sci. Food Agric. 2000, 81, 269–274. [Google Scholar] [CrossRef]
- Srinivasa, P.C.; Baskaran, R.; Armes, M.N.; Harish Prashanth, K.V.; Tharanathan, R.N. Storage studies of mango packed using biodegradable chitosan film. Eur. Food Res. Technol. 2002, 215, 504–508. [Google Scholar]
- Gol, N.B.; Patel, P.R.; Ramana Rao, T.V. Improvement of quality and shelf-life of strawberries with edible coatings enriched with chitosan. Postharvest Biol. Technol. 2013, 85, 185–195. [Google Scholar] [CrossRef]
- Bautista-Baños, S.; Hernández-López, M.; Bosquez-Molina, E. Growth inhibition of selected fungi by chitosan and plant extracts. Mexican J. Phytopathol. 2004, 22, 178–186. [Google Scholar]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).