Abstract
Millets are small-seeded cereals belonging to the family Poaceae. They are considered to be climate-resilient and future nutritional food cereals for humans. Millets are resistant to biotic and abiotic stressors compared to other major cereals and thrive in low-quality soils with little maintenance and less rainfall. The importance of millets is still not well known to many people due to the lack of popularity and cultivation in semi-arid tropics of Asia and Africa. The United Nations has declared 2023 as the International Year of Millets (IYM 2023) to promote millet cultivation and popularize their health benefits globally. A few years ago, the application of molecular biology was in its infancy in millets due to the unavailability of genome sequences. Genome sequences are available for most of the millets on NCBI and Phytozome databases. In this review, we discuss the details of genome sequences for millets, candidate genes identified from the native genome of millets. The current status of quantitative trait loci and genome-wide association studies in millets are also discussed. The utilization of millet genome sequences in functional genomics research and translating the information for crop improvement will help millet and non-millet cereals survive harsh environments in the future. Such efforts will help strengthen food security and reduce malnutrition worldwide in 2050.
1. Introduction
Millets are a group of small-seeded cereal crops grown largely on marginal dry lands or with limited access to irrigation. There are several millet species cultivated worldwide, such as sorghum (Sorghum bicolor), pearl millet (Cenchrus americanus), finger millet (Eleusine coracona), foxtail millet (Setaria italica), proso millet (Panicum miliaceum), little millet (Panicum sumatrense), kodo millet (Paspalum scrobiculatum), barnyard millet (Echinochloa esculenta), brown top millet (Urochloa ramosa), tef (Eragrostis tef), fonio millet (Digitaria exilis), job’s tear (Coix lacryma-jobi), guinea millet (Brachiaria deflexa), raishan (Digitaria cruciate), etc. Millets are generally called gluten-free and are highly nutritious and contain higher protein, minerals, vitamins, and fiber compared to major cereal crops, rice (Oryza sativa), wheat (Triticum aestivum), and maize (Zea mays) [1]. Millets can grow with less water than most other major cereals and are well suited to drought-like conditions [2]. Millets’ production traditionally does not depend on artificial fertilizers, and most are unaffected by storage pests [3]. In addition, millets have natural biodiversity that makes them suitable for cultivation in various agro-climatic conditions [4]. They have a short lifecycle and can be grown between main cropping seasons and enrich the soil with their micronutrients [5]. The affordability of millets also makes it the “poor man’s food grain” [6]. The world is now looking at millets for their enormous potential. In addition to the cultivation advantages of millets, they are highly nutritious and climate-resilient. Because of this, mainstream society is beginning to understand and appreciate the long-term benefits of millets. Millets are a nutritional powerhouse, and they are now referred to as “Nutri-Cereals” [7]. They are rich in protein, fiber, key vitamins, and mineral sources. About 80% of millets have long been an important part of a nourishing diet [8]. Millets are high in fiber, which aids digestion and prevents constipation [9]. They are also gluten-free and suitable for celiac patients [10]. They contain antioxidants, which help to protect our cells from free radicals [11]. Calcium is necessary for bone health, blood vessel and muscular contractions, and proper nerve function. Finger millet contains more calcium (162–487 mg/100 g) than milk (124 mg/100 mL) and other cereals (10 mg/100 g of rice, 21.40/100 g of maize, 33 mg/100 g of wheat and 21.40 mg/100 g of sorghum), which supports boosting the calcium level in the human body [12]. The highest iron content (>11 mg/100 g) is found in pearl millet and has the potential to treat anemia [13]. It is also high in zinc (>3 mg/100 g) and folic acid, making it ideal for pregnant women [14]. Pearl millet contains twice (9–21 g/100 g) as much protein as milk (3.4 g/100 mL) [15]. Kodo millet has three times the fiber (10.2 g/100 mg) of wheat (2.5 gm/100 mg) and maize (2.0 g/100 g) and ten times the fiber of rice (1 gm/100 g) [1]. Hence, millets are considered the world’s next superfood. Millets have the potential to help achieve many sustainable development goals (SDGs) (Figure 1) [3].
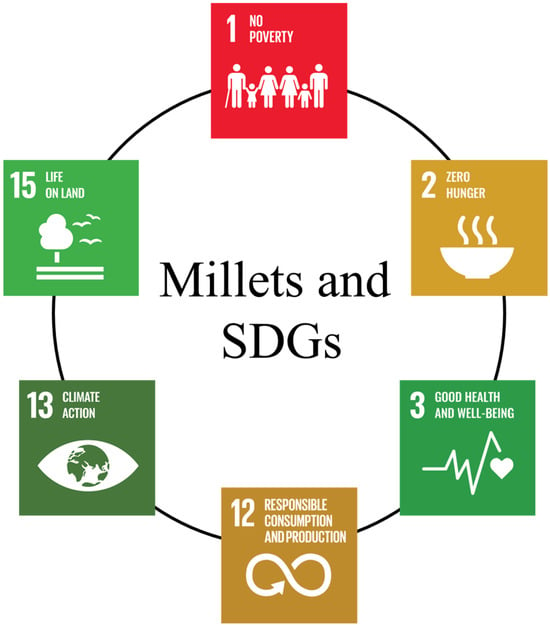
Figure 1.
Role of millets in achieving the United Nations Sustainable Development Goals.
Understanding the need to promote millets’ diversity, nutritional, and ecological benefits, the United Nations has declared the year 2023 as the International Year of Millets (IYM 2023), following a proposal by the Government of India. The main intention of the IYM 2023 is to increase millet production and consumption with four strategies to improve millets’ cultivation: (1) promoting sustainable production, (2) enhanced nutrition, (3) wider acceptance, and (4) increased consumption. However, millets’ research is still in its infancy with limited genomic resources. Some research laboratories have released draft/annotated genome sequences for some millets. In this review, we have discussed the availability of genome sequences for major (sorghum and pearl millet) and seven minor millets (finger millet, barnyard millet, foxtail millet, proso millet, teff, fonio, and job’s tear) and the way forward to utilize millets’ genome sequences and genomics resources for crop improvement. We have discussed genome-wide association studies (GWASs) in millets and summarized the identified candidate genes and the current status of millets’ molecular breeding. Finally, the role of millet genome sequences to achieve food availability in 2050 is discussed. This review will raise awareness for millet researchers to utilize millets’ genomes for their future experiments through various molecular tools. Identifying and characterizing more candidate genes from millets will help improve millet and non-millet cereals for biotic/abiotic stresses and nutritional traits.
2. Nutritional Profile and Health Benefits of Millets
Millets are nutritionally excellent because their grains are rich in proteins, minerals, flavonoids, polyphenols, and vitamins; therefore, they may offer multiple health benefits. About 80% of millet grains have long been an important part of the nutritious diet. Many research/review articles have already discussed the nutritional importance and health benefits of millets [16,17,18]. Millets are now considered “God’s own cereal” due to their rich nutritional profile. Consuming millets in our daily diet raises the levels of proteins (especially adiponectin) that help protect against cardiovascular diseases [19]. Millets also contain a higher amount of vitamin B3/niacin, which helps lower certain risk factors of heart diseases such as high cholesterol and triglycerides and is effective in lowering oxidative stress [20]. Millet grains contain the lowest carbohydrate content compared to other cereals (especially rice) and so are highly recommended for people with type 2 diabetes [21]. Oxidative stress can cause various chronic diseases (neurodegenerative disorders, arthritis, and diabetes) [22]. A high-fat diet is also a risk factor for the development of dementia because it increases oxidative stress in the brain [23]. Millets are a good source of antioxidants, which can help support the body’s ability to fight oxidative stress, a factor in illness and aging [24]. Hence, consuming millets could decrease the risk of chronic diseases [24]. Millets are rich in phytochemicals (polyphenols, lignans, phytosterols, phyto-oestrogens, phytocyanins) that help protect people from age-related degenerative diseases like diabetes, cancer, etc. [25]. Each millet has some unique nutritional properties that help improve human health. For example, a sufficient amount of calcium is essential for bone health, blood vessel and muscular contractions, and to ensure proper nerve function [12]. Finger millet has a higher calcium content than all other millets, cereals, and milk [17] and hence is one of the best grain sources to improve/maintain proper calcium levels in humans [26]. Proso millet contains high lecithin which supports the neural health system [27]. Kodo millet contains a high amount of potassium (>120 mg/100 g), which helps reduce abdominal cramps during the menstrual cycle [28,29]. Including pearl millet in our daily diet is an effective way to prevent iron deficiency anemia as its grains are rich in iron [13]. Overall, the consumption of millets reduces the risk of heart disease, protects from diabetes, improves the digestive system, lowers the risk of cancer, detoxifies the body, increases immunity in respiratory health, increases energy levels and improves muscular and neural systems, and is protective against several degenerative diseases.
3. Germplasm Resources of Millets
Germplasm resources are an essential strategic resource for continued progress in crop improvement for global food security and nutrition. Many millet researchers have already discussed the genetic resource of millets in various review articles [30,31]. The recent report on a global millets’ conservation strategy indicates that more than 479,000 germplasms of sorghum and millets are conserved globally [32]. Millet germplasms are majorly conserved in Asian and African countries such as India, China, Japan, Kenya, Ethiopia, Uganda, and Zambia. It is noteworthy that developed countries such as the US, Canada, France, Russia, and Italy also conserve millet germplasms [31]. The International Crops Research Institute for the Semi-Arid Tropics (ICRISAT), Hyderabad, Telangana, India, conserves the largest collection of millets (about 80,000 accessions of eight millets) which includes sorghum (42,869 accessions), pearl millet (25,537 accessions), finger millet (7513 accessions), foxtail millet (1542 accessions), barnyard millet (749 accessions), kodo millet (665 accessions), little millet (473 accessions), and proso millet (849 accessions) (https://genebank.icrisat.org/ accessed on 6 March 2024). The ICARI-National Bureau of Plant Genetic Resources Institute (ICAR-NBPGRI), New Delhi, India, conserves over 58,000 accessions of millet including sorghum (25,669), pearl millet (8699), finger millet (11,667), foxtail millet (4685), proso millet (1055), barnyard millet (2010), kodo millet (2404), little millet (2226), and brown top millet (44). (http://genebank.nbpgr.ernet.in/SeedBank/Default.aspx; accessed on 6 March 2024). The United States Department of Agriculture (USDA-ARS) gene bank conserved five millets’ germplasms (such as 1452 finger millet, 1314 pearl millet, 300 kodo millet, 212 little millet, and 719 proso millet) (https://www.ars.usda.gov/southeast-area/griffin-ga/pgrcu/; accessed on 6 March 2024). Over the past 15 years, the conservation of millets’ germplasm resources has become an important part of national and international research programs. However, the number of germplasms available for small millets particularly little millet, kodo millet, barnyard millet, brown top millet, and other minor millets is very low [31], because researchers have not yet prioritized those millets and most traditional genotypes have already disappeared due to the dominance of other cash crops. Hence, the collection and conservation of existing small millets are crucial before we lose them forever, which may help to support millets’ improvement globally.
4. Evolution of Millets
Millets are believed to be the oldest ancient domesticated crop. There is evidence that millets were cultivated in Asia and Africa over 5000 to 10,000 years ago [33]. Archaeobotanical evidence has proved that foxtail millet and proso millet were first domesticated in China ~10,500 calendar years before the present (cal. BP) [34]. Pearl millet has its origin in Africa, and the earliest evidence of its cultivation is reported from northeast Mali (4500 cal. BP) [35,36]. Kodo millet originated in India, and its domestication took place about 3000 years ago [28,37]. Little millet was domesticated in several sites across India around 6400 cal. BP [38]. Hence, millets are not new to the world; they have always been a part of our staple foods since ancient times. Due to the ability of millets to grow on marginal lands with low external inputs such as water, fertilizer, pesticides, etc., farmers are trying to revive its cultivation and bring it back to the market.
5. Ploidy Level of Millets
The genome size, ploidy level, and chromosome number are of great importance for studying the evolution of millets and the development of the breeding program [39]. In addition, the knowledge of a plant’s genome size and ploidy status can provide clues about the mechanisms responsible for decreases or increases in the genomic content along the evolutionary pathway [39]. The genome size and ploidy levels varied extensively among the millets. The ploidy level and chromosome number of each millet are provided in Table 1. Among the millets, pearl millet, kodo millet, and finger millet have the largest genome sizes. The genome size for little millet has not yet been estimated. Foxtail millet is a member of the subfamily Panicoideae and the tribe Paniceae, with chromosome numbers of 2n = 2x = 18 [40,41]. It is recognized as a diploid but is closely related to many tetraploid and higher ploidy level species. Finger millet is an allotetraploid (2n = 4x = 36) that belongs to the Chloridoideae subfamily [42,43,44]. Barnyard millet is a hexaploid (2n = 6x = 54), and it belongs to the subfamily Panicoideae [45]. Three millets such as proso millet, kodo millet, and little millet are tetraploid, and their chromosome range is 36–40 [46,47] (Table 1).

Table 1.
Details on ploidy level and genome size of millets based on available reports.
6. Genome Sequences of Millets
The field of millet genomics has grown rapidly in the past 10 years. Millet researchers have developed genome sequences for major and minor millets except two minor millets (little millet and kodo millet). Notably, five millet genome sequences were assembled at the chromosome level (Figure 2). In addition, the quality of sorghum, foxtail millet, and finger millet genome assemblies is much improved now compared to what it was a few years ago (https://phytozome-next.jgi.doe.gov/; accessed on 8 March 2024). The genomic resources of millets will enable millet researchers to identify candidate genes related to biotic/abiotic stresses and nutritional and other agronomically important traits and develop various DNA markers to conduct genetic mapping studies, which will increase millet production in the near future. In this section, we have listed out the availability of draft and annotated genomes of major and minor millets.
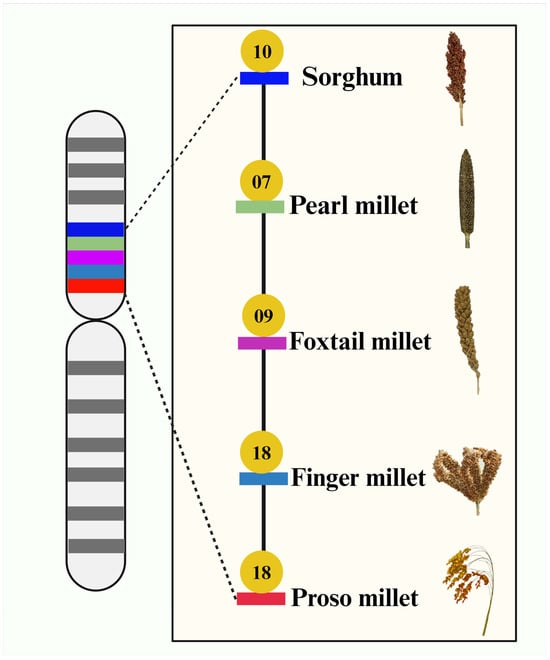
Figure 2.
Genome assembly of millets at chromosome level. Sorghum, pearl millet, finger millet, foxtail millet, and proso millet genomes were assembled at chromosome level. Number of chromosomes in assembled genomes is indicated by golden circle for each millet.
6.1. Major Millets
6.1.1. Sorghum
The first sorghum genome was released in 2009 at the chromosome level using the BTx623 genotype [48]. The assembled genome size was ~730 Mb with 229 scaffolds and 10 chromosomes. From the genome, they have identified more than 70,000 simple sequence repeat (SSR) markers and >34,000 annotated genes. Likewise, around 35,490 genes were identified from the genome sequence of the Rio genotype (Table 2). A total of 21 genome assemblies are currently available at NCBI, and many high-quality improved annotated genome sequences are available at the Phytozome database. The current version 5.1 is available in the Phytozome database (https://phytozome-next.jgi.doe.gov/info/Sbicolor_v5_1; accessed on 8 March 2024). After releasing the genome sequences of sorghum, several studies related to GWASs, transcriptomes, functional genomics, and molecular breeding have been extensively conducted. Sorghum has entered an exciting and fruitful era due to the abundant availability of genetic, genomic, and breeding resources for millet researchers. Hence, the genomic resources of sorghum can serve as a reference genome for all other millet species.

Table 2.
Summary of available millet genome assemblies.
6.1.2. Pearl Millet
Pearl millet is a cross-pollinated crop with a genome size of ~1.7 Gb. Its genome was partially assembled and published in 2017 using the inbred Tift 23D2B1-P1-P5 genotype of pearl millet [49]. They used whole-genome shotgun and bacterial artificial chromosome (BAC) sequencing techniques to assemble the genome for the Tift 23D2B1-P1-P5 genotype. After stringent filtering and correction steps, 1.49 Tb of sequence data was assembled into 1.58 Gb of contigs and 1.82 Gb of scaffolds. Finally, they generated a genome sequence around 1.76 Gb for the Tift 23D2B1-P1-P5 genotype, indicating that ~90% of the pearl millet genome was assembled. A total of 1.22 Gb repeat elements was predicted from the Tift 23D2B1-P1-P5 genome assembly which indicates that 77.2% of the assembled genome is repetitive. From the genome, a total of 27,893 genes were annotated, and 10,686 genes were unannotated. They also identified transfer RNA (tRNA) (909), ribosomal RNA (rRNA) (235), messenger RNA (mRNA) (183), and small nuclear RNA (snRNA) (752) genes from the genome. Apart from this, 88,256 SSRs were also identified in the pearl millet genome using a microsatellite program, which can be used for future genetics and breeding applications. Based on their resequencing data, they also predicted 29,542,173 single-nucleotide polymorphisms (SNPs) by PMiGAP lines. Among these, 450,000 high-quality SNPs were identified based on principal component analysis which would be helpful for advancing molecular breeding studies on pearl millet. Apart from this genome assembly, a total of nine partial genome assemblies were developed by Sichuan Agricultural University using various cultivars (Table 2). They also generated a partial genome assembly for pearl millet at the chromosome level, and the generated genome size is between 1.9 and 2.0 Gb. There is no doubt the available draft genome of pearl millet will provide a resource for the millet research community to understand trait variation and accelerate the genetic improvement of pearl millet.
6.2. Minor Millets
6.2.1. Foxtail Millet
Among the minor millets, the genome sequence was first released for foxtail millet. It is a diploid cereal with a comparatively smaller genome (~515 Mb) than other minor millets. Two institutes (US Department of Energy Joint Genome Institute, Berkeley, CA, USA and Beijing Genomics Institute, Beijing, China) have released completely annotated genome sequences for two different foxtail millet cultivars [40,41]. Zhang et al. (2012) developed a draft genome (~423 Mb) for the Zhang gu genotype of foxtail millet [41]. The scaffold N50 was 1.0 Mb, and 90% of the scaffolds were 380 Mb. They predicted 542,322 SNPs from the annotated genome of foxtail millet. The Zhang gu genome comprised >46% of transposable elements (both retroelements and DNA transposons), indicating that the genome contains many repetitive genes. They identified 38,801 genes by integrated annotation pipeline methods, and the average length of annotated genes was 2522 bp. In addition, they also identified 1367 pseudogenes in the genome. Several noncoding RNA genes were also detected from the Zhang gu genome assembly. The second high-quality reference genome sequence for foxtail millet was generated for the Yugu1 cultivar [40]. The Yugu1 assembly contains 405.7 Mb of the sequence in nine chromosomes and an additional 4.2 Mb in 327 scaffolds that are unanchored by the genetic map, with an estimated genome coverage of ~80%. Like the Zhang gu genome, the Yugu1 genome also composed of 40% of transposable elements. The Yugu1 genome contained 24,000 to 29,000 protein-encoding genes. Genome sequences for both cultivars are available in the NCBI database (Table 2). Apart from this, a completely annotated genome sequence of foxtail millet (version 2.2) is also currently available at the Phytozome database. The version 2.2 assemblies were constructed by Program to Assemble Spliced Alignments (PASA) from ~1.28 million foxtail millet expressed sequence tag (EST) reads sequenced at the Joint Genome Institute (JGI) against the 8.3X. Each locus of the current version of the foxtail millet genome was determined by BLASTX alignments of proteins from sorghum, rice, Arabidopsis thaliana, and grapevine (Vitis vinifera) genomes. Homology-based predictors FGENESH+ and GenomeScan were used to predict models of each gene. Therefore, foxtail millet could serve as an ideal surrogate genome for the future research and development of switchgrass and related biofuel crops.
6.2.2. Finger Millet
Two draft assemblies and one completely annotated genome assembly are currently available for finger millet. In 2017, the first draft genome assembly was released for an Indian cultivar (ML-365) using Illumina and sequencing by oligonucleotide ligation and detection (SOLiD) sequencing technologies [42]. Around 45 Gb of paired-end and 21 Gb of mate-pair data by Illumina and SOLiD sequencing technologies were generated and further used to assemble the ML-365 genome, followed by gap closure which gave a consensus genome size of 1196 Mb, representing 82.31% of the estimated finger millet genome. About 85,243 genes (78,647 non-transposable elements related and 6596 transposable elements related genes) were predicted based on the de novo method of gene prediction using Augustus. Of the 85,243 genes, 52,541 genes contained the Pfam domain, and these genes were distributed in 3254 gene families. Also, most of the identified genes were involved in ATP binding activities and zinc ion and nucleic acid binding. Furthermore, 2866 drought-responsive genes, 1766 disease-resistance genes, 330 calcium transport and accumulation-related genes, and 146 C4 photosynthetic pathways were captured in the genome. Apart from this, a total of 114,083 SSRs were detected from which di- (66,805), tri- (40,578), tetra- (2179), penta- (3010), and hexa- (1511) repeats were identified. The identified SSR markers from this genome assembly can be further used in diverse studies, linkage map construction, association mapping, the quantitative trait loci (QTL) mapping of agronomically important traits, and marker-assisted breeding programs. This is the first breakthrough report on the finger millet genome. In the next year, Hatakeyama et al. (2018) generated draft genome sequences for another Indian cultivar (PR-202) using Illumina MiSeq [43]. They estimated a genome around 1.5 Gb by the flow cytometry method. The total number of scaffolds was 1897 with an N50 length > 2.6 Mb. The N50 of PR-202 was higher than ML-365 which may allow for an RNAseq analysis of each homeolog separately and resequencing analyses. Overall, 62,348 genes were predicted from the PR-202 genome assembly. Among these, 57,066 genes were annotated and submitted to NCBI. In addition, a total of 1440 universal single-copy genes were identified. Of these, 606 genes were found to be single-copy genes, and 783 genes were duplicate genes.
The complete annotated genome sequence (version 1.0 assembly) was recently generated by Prof. Devos’ group of University of Georgia, USA for the Kenyan finger millet cultivar (KNE 796) using mapping, error correction, and a de novo assembly tool (MECAT) assembler [44]. The assembled size of the KNE 796 genome is 1129.7 Mb. Overall, the assembled KNE 796 genome comprised 1058 scaffolds, with a contig N50 of 12.1 Mb, and the average length of scaffolds was 2275 bp. The assembled genome contained 18 chromosomes and 1.11 billion bases (Gb). About 48,836 high-confidence and 24,176 low-confidence genes were distributed across the annotated finger millet genome. The annotated genome is a great opening to identify and validate the genes and understand the genetic basis of finger millet. The annotated genome will help mine and characterize calcium and other nutrients’ transporter and regulatory genes for further research (for example, identifying genes involved in grain nutrient filling). The development of finger millet against blast and other diseases by conventional breeding methods is hampered by limited genetic variability. Hence, generating blast disease-resistant finger millet using antifungal protein-encoding genes would be useful. The annotated genome sequence of finger millet could pave the way to identify genes associated with blast and other diseases. Undoubtedly, the annotated genome sequence of finger millet will be a great resource to accelerate food security and nutrient fortification in less developed countries of Asia and Africa.
6.2.3. Proso Millet
The normal genome size of proso millet is ~900 Mb. It is an allotetraploid cereal with a chromosome number of 2n = 4x = 36. A total of 35 draft genome assemblies for proso millet have been developed and submitted to NCBI (Table 2). Of these, 34 partial genome assemblies were developed by the Chinese Academy of Sciences, and the single genome assembly was developed by Shanghai Center for Plant Stress Biology. Interestingly, all the partial genome sequences were assembled at the chromosome level (Table 2). Unfortunately, no candidate genes and markers were predicted for 34 genome assemblies developed by the Chinese Academy of Sciences. However, the partial genome assembly developed by Shanghai Center for Plant Stress Biology predicted many candidate genes and markers from their genome [46]. The assembled partial genome of the Pm_0390 genotype was 923 Mb. They predicted 221,787 SNPs and 112,158 SSR markers from the Pm_0390 genotype’s partial genome. All the identified markers were composed of di- and tri-nucleotide motifs with an average length of ~22 bp. This resource can serve for developing SSR- and SNP-based genetic markers for proso millet. They also predicted 55,930 protein-coding genes, 339 micro RNAs (miRNAs), 1420 tRNAs, 1640 rRNAs, and 2302 snRNAs from the genome. Compared to the other millets, proso millet has a higher number of partial genome assemblies. All currently available partial genome sequences are valuable resources for small millet breeders and will provide a foundation for studying the exceptional stress tolerance.
6.2.4. Barnyard Millet
A species-specific genome sequence for barnyard millet has yet to be developed; however, the partial genome has been developed for barnyard grass related to barnyard millet. The draft genome for barnyard grass was developed by Zhejiang University, China [45]. They developed two partial genome sequences for barnyard grass at the scaffold level. Around 108,771 protein-coding genes were predicted from the partial genome. In addition to protein-coding genes, 785 miRNAs, 2306 tRNAs, 1890 rRNAs, and 3378 snRNAs were identified in the barnyard grass genome. They predicted several gene families associated with detoxification such as cytochrome P450 monooxygenase genes (917), glutathione S-transferase genes (277), and many differentially expressed genes (4945) from the draft genome of barnyard grass. Apart from this, 4945 differentially expressed genes (2534 up-regulated and 2411 down-regulated) were identified. Two partial genome assemblies are currently available for barnyard grass at NCBI (Table 2). This genome sequence provides new insights into the adaptive molecular mechanisms for barnyard millet survival.
6.2.5. Other Minor Millets
Genome sequences are now available for some other minor millets. For example, the draft genome sequence for tef was released in 2014 by the University of Bern, Switzerland [51]. The draft genome of tef is currently available at the NCBI (Accession: GCA_000970635.1) and Ensembl Plants (https://plants.ensembl.org/Eragrostis_tef/Info/Index; accessed on 8 March 2024) databases. The estimated draft genome of tef is about 672 Mb, which clearly indicates that the tef genome is almost 87% sequenced. A total of 49,600 SSR markers and 38,000 transcripts were found in the draft genome of tef. The draft genome sequence of tef is more reliable for developing molecular markers and identifying candidate genes related to abiotic stress tolerance. In addition, the tef draft genome will pave the way for conducting GWASs and GBS for tef improvement.
The genomic resources for fonio millet were released in 2020 at the chromosome level using the CM05836 cultivar [52]. From the fonio genome, they predicted 59,844 protein-coding genes with an average length of 2.5 kb. In addition, they also identified 11,046,501 high-quality SNPs, and they were all evenly distributed across the 18 chromosomes. In the same study, two genes, such as grain size 5 (GS5) and shattering 1 (Sh1), were identified to play an important role in regulating the grain size of fonio and conserving the seed shattering of fonio, respectively. The draft genome of job’s tear was developed for the Korean cultivar (Johyun) by de novo assembly [53,54]. Around 3362 scaffolds were generated, with a total length of 1.28 Gb [53]. A total of 3988 differentially expressed genes (1470 up-regulated and 2518 down-regulated) related to seeds and other tissues were identified in the draft genome of job’s tear. In addition, a total of 317 transcription factors (including basic region/leucine zipper moti (bZIP), MYB, NAC, basic helix–loop–helix (bHLH), and ethylene response factor (ERF)) were identified as related to seed development. All identified genes and transcription factors will support the development of high-quality seeds of job’s tear and other millets through molecular breeding and other biotechnological approaches. Apart from this, 76 genes (including 57 cupin superfamily genes, 18 coixin genes, and one glutelin gene) associated with seed storage proteins and 13 genes involved in the biosynthesis of benzoxazinoids were also identified from the job’s draft genome. The chloroplast genome sequence is now available for little millet [50]. The developed chloroplast genome sequence length was 139,384 bp, and the genome contained 91 protein-coding genes, 4 rRNA genes, and 30 tRNA genes. This chloroplast genome of little millet may provide valuable information for this cereal, which will help to initiate further molecular experiments for improving this crop. The sequencing of millet genomes made it possible to assess intraspecific polymorphism, identify key genes influencing the formation of significant features, and develop molecular markers of economically valuable traits, and this has become the basis for the genomics-assisted breeding for crop improvement. An adequate review of the molecular breeding and functional genomics of sorghum has already been published. Hence, hereafter, this review only focusses on discussing molecular breeding and functional genomics for pearl millet and other minor millets only.
7. Pan-Genomic and Telomere-to-Telomere Genome Resources of Major and Minor Millets
The available genomic resources for millets have been developed from a single germplasm line/genotype of millets, which provides genetic information on a single genotype. Pan-genome studies will enable the simultaneous generation of genome resources for different cultivars, landraces, and wild species [55]. This will allow researchers to search for novel genes and alleles that may have been inadvertently lost in domesticated crops during the historical process of crop breeding or extensive plant breeding. Pan-genomic resources are currently available for two major (sorghum and pearl millet) and two minor (foxtail millet and proso millet) millets. A pan-genome analysis of 13 genetically diverse genotypes of sorghum revealed extensive hidden genomic variations between cultivated and wild species [56]. For example, a pan-genome was generated for 13 genotypes of sorghum to explore the genetic variations between the cultivated and wild species. The developed pan-genome consists of core genes (58.8%), shell genes (37.9%), private genes (3.3%), and cloud genes (0.4%). Among these, core genes are found to be involved in RNA processing, reproductive system development, leaf development, seed development, cell differentiation, and chloroplast organization in sorghum [56]. They also identified 19,359 and 147,899 presence and absence variants, respectively, associated with sorghum domestications and grain color variation. In another study, the pan-genome of 176 genotypes of sorghum identified 18,898 variable genes associated with various stresses, 1788 drought-responsive genes, and 2.0 million SNPs [57]. Similarly, a pan-genome assembly of eleven pearl millet genotypes identified structural variations in endoplasmic reticulum-related genes associated with heat stress [58]. A total of 39,143 gene families, including 46.60–52.08% of core genes, 39.75–49.94% of dispensable genes, and 0.73–8.73% of private genes, were obtained from the developed pan-genome of pearl millet. Additionally, 744,364 structural variations associated with heat-related genes were identified, including 306,679 presence variations, 315,905 absence variations, 2177 inversions, 91,852 copy number variations, and 27,751 translocations [58]. In minor millets, a pan-genome sequence has been generated for foxtail millet using 110 genotypes (35 wild, 40 landraces, and 35 modern cultivated) [59]. The developed pan-genome of foxtail millet contains 73,528 gene families, of which 23.8%, 42.9%, 29.4%, and 3.9% are core, softcore, dispensable, and individual genes, respectively. In addition, 14,283 gene families involved in RNA capping, light response, and specific metabolic processes were identified from the pan-genome of foxtail millet [59]. It is interesting to note that these gene families are not already present in the available Yugu1 reference genome. In the same study, approximately 202,884 non-redundant structural variations (107,151 insertions, 76,915 deletions, 18,455 translocations, and 363 inversions) and 158,906 presence and absence variations associated with foxtail millet domestication and improvement were detected in the pan-genome of foxtail millet. Thirty-two proso millet high-quality pan-genomes contained 27,727 core, 8288 softcore, 24,494 dispensable, and 5533 private gene families [60]. From the developed pan-genome assemblies, 207,033 structural variations and 50,515 presence or absence variants (26,195 deletions and 24,320 insertions) related to 43 domestications and 31 agronomic traits of proso millet were identified. The developed millet pan-genome represents an important resource for millet improvement and gene discovery. Novel genes identified in the pan-genomes of millets can be reintroduced into high-yielding millets by implementing traditional plant breeding, genetic selection, and transgenic approaches. Apart from the pan-genome, the telomere-to-telomere genome has been developed for HYZ-T2T genotypes of sorghum to identify structural genes, transcription factors, and transporters involved in the biosynthetic pathways of tannins in sorghum [61]. These millet pan-genomic resources will be useful for achieving the SDGs in developing countries by accelerating the genetic gain in arid and semi-arid ecologies.
8. QTL Associated with Various Traits in Major and Minor Millets
In general, molecular markers help to identify QTL associated with several traits in plants. In millets, several QTL related to various agro-morphological, biochemical, and yield-related traits were identified. Several SSR, SNP, and Diversity Arrays Technology (DArT) markers were widely used to identify QTL in millets. Blast diseases cause severe yield constraints of millets in many millet-producing regions. Three QTL associated with leaf blast disease were identified in 305 recombinant inbred lines (RILs) of foxtail millet using more than 30K SSR markers [62]. Rust disease caused by the fungus (Puccinia substriata) is one of the most important yield constraints of pearl millet worldwide, leading to grain yield losses of up to 76% and major losses in fodder yield and quality [63]. A total of 256 DArT and 70 SSR markers were used for the identification of a novel QTL for pearl millet rust disease using 168 RILs [63]. Like rust disease, downy mildew caused by Sclerospora graminicola is the most destructive disease of pearl millet [64]. A total of five QTL associated with resistance to pearl millet downy mildew disease were identified in 187 RILs using 88 SSR markers [65]. The identification of disease resistance QTL will be useful in cultivar development and the study of the genetic control of disease resistance in millets. Compared to the other millets, the contents of iron and zinc are higher in pearl millet. More than 18 QTL associated with pearl millet grain iron and zinc content were identified using SSR and DArT markers [66,67]. After further validation of these QTL, they can be used for the development of high grain iron and zinc pearl millet through marker-assisted breeding programs. Soil nutrient deficiency is one of the abiotic constraints on millet production and yield. Compared to other major cereals, QTL associated with improving nutrients’ use and acquisition efficiencies have not been identified much in millets. Recently, more than 50 QTL associated with various agro-morphological, phosphorus contents in shoot and roots and biomass traits were identified in 100 RILs of finger millet using 101 SSR markers under low- and high-phosphate conditions [68]. The same group also identified eight QTL for root- and shoot-related traits under the same low- and high-phosphate conditions using 87 SSR markers in finger millet through association mapping [69]. Apart from this, 11 QTL related to shoot-, root-, and biochemical-related traits were identified in finger millet grown under drought stress [70]. Many QTL for various agro-morphological, yield, biomass, growth, and other related traits were detected in finger millet, pearl millet, foxtail millet, and proso millet by various researchers (Table 3). All the identified QTL set a foundation for fine mapping, the identification of candidate genes, the elaboration of molecular mechanisms, and use in millet breeding programs through marker-assisted selection. No QTL have been identified yet in millet under salinity, cold, heat, or other abiotic stress conditions. The availability of the genomic sequence of millets would accelerate the development of markers to assist genotypic classification and breeding practices. Previously, researchers used EST array molecular makers for the identification of QTL in many millets. These are low-throughput molecular markers that limit the efficiency and accuracy of QTL mapping. Also, it is very expensive and time-consuming. The genome sequence of millets will allow millet researchers to rapidly identify millions of markers (SSR, SNP, EST-SSR, DArT, etc.), which will enable the generation of large-scale genotypic data for linkage mapping analysis. Apart from this, using molecular markers developed from genome sequences to detect QTL will increase the efficiency and accuracy of QTL which can definitely be used for the millet breeding program. The genome sequence will also allow for the identification of candidate genes associated with QTL and is useful for conducting gene expression and functional characterization studies.

Table 3.
Summary of QTL associated with various traits in major and minor millets.
9. Genome-Wide Association Studies (GWASs) in Millets
QTL mapping based on linkage analysis provides high power to detect QTL for a trait of interest. It has a very low mapping resolution because of the few recombination events that it takes into consideration which would ultimately lead to long linkage blocks. High-throughput genotyping technologies such as genotyping-by-sequencing (GBS) and genome-wide association studies (GWASs) help to generate high-quality genome-wide genetic markers, which enable the detection of marker–trait associations (MTAs) or QTL for crop improvement. A total of 706,646 SNP markers were developed from 407 foxtail millet genotypes by GWASs [89], and the developed SNP markers were used to identify 87 QTL associated with various panicle-related traits in foxtail millet (Table 4). GWASs were also used to identify 81 MTAs for several agronomic, plant growth, and yield-related traits by 10,000 high-quality SNPs in 142 foxtail millet genotypes [90]. The same group used GWASs to identify 74 MTAs associated with ten nutritional elements using the same 10,000 SNPs in 93 foxtail millet accessions [91]. In pearl millet, a total of 87,748 DArT markers were developed from 281 cultivars by GWASs. Among these, 58,719 high-quality SNPs were used to identify 78 MTAs for iron, zinc, and protein content [92]. A total of 392 pearl millet cultivars were used to develop 21,633 SNPs by GWASs, and the developed SNPs helped to identify 18 QTL for flowering time, plant height, tillering, and biomass [93]. In another study, 1132 MTAs for six starch-related traits were identified in 166 genotypes of pearl millet by GWASs using 78,000 SNPs [94]. In proso millet, 13 MTAs for agronomic and seed-related traits were identified by 2412 high-quality SNPs developed through GWASs [95]. A total of 190 genotypes of finger millet were used to generate 169,365 SNPs by a combination of GBS and a GWAS, which were used to identify several MTAs for iron, zinc, calcium, magnesium, potassium, and sodium and protein contents [96]. Apart from the above-mentioned studies, several QTL and MTAs were identified for many traits using both GWAS and GBS techniques in millets (Table 4). To date, there is no information regarding GWASs and GBS for little millet, brown top millet, tef, fonio millet, barnyard millet, and kodo millet. A GWAS should be carried out to identify QTL and MTAs for various traits in millets under biotic and abiotic stress conditions (Figure 3), which will help to develop a new cultivar via breeding programs. The identified QTL and MTAs by GWASs and GBS might be used for millet breeding programs. The genome assembly enables the scanning of SNP markers developed by GWASs across the complete set of DNA or genomes. It helps to find genetic variations associated with any particular traits. Once the new genetic traits are identified, researchers can use the traits for a further millet breeding program. Such studies are particularly important to find genetic variations that contribute to common complex diseases and environmental stresses.

Table 4.
Identification of QTL/MTAs by genome-wide association studies (GWASs) in major and minor millets.
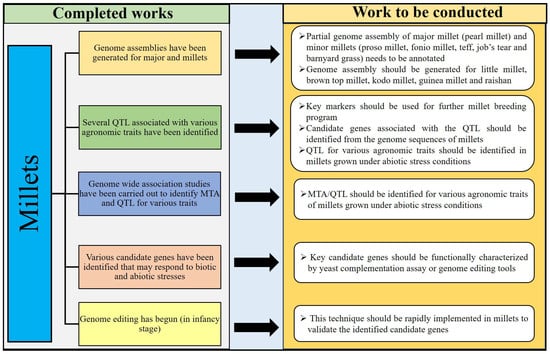
Figure 3.
Schematic diagram of resources developed and future scope of research on millets.
10. Identification and Characterization of Candidate Genes from Native Genome Sequences of Millets
In earlier studies when the genome sequences of millets were not generated, researchers used the sequences of closely related cereals to design primers and characterize the genes in millets for various biotic and abiotic stress conditions. The availability of millet genome sequences has paved the way for the identification and functional characterization of several candidate genes related to biotic, abiotic, and other stresses in many millets (Table 5). Interestingly, several candidate genes related to major environmental stresses (drought, salinity, and heat) have been identified from the annotated genome sequences of foxtail millet, which will certainly help identify the key candidate genes for further characterization studies in the future. In foxtail millet, the expression pattern of 187 basic helix–loop–helix (bHLH) genes was analyzed under several abiotic and phytohormone treatments [123] (Table 5). A total of 52 soluble-N-ethylmaleimide-sensitive-factor accessory-protein receptor (SNARE) [124], 35 DNA binding with one finger (Dof) [125], and 103 WRKY genes [126] were particularly responsive to drought stress in foxtail millet. Twelve phosphate transporter 1 (PHT1) family genes were identified specifically for foxtail millet, which help improve phosphorus uptake, translocation, and remobilization under low-phosphate stress [127]. Further, the identified key PHT1 genes were functionally characterized by yeast complementation assay [128]. The identified 29 high-affinity potassium (HAK) transporters can increase the potassium concentration in foxtail millet tissues and enhance foxtail millet growth and yield under potassium-deficiency soil [129]. Twelve natural resistance-associated macrophage proteins (NRAMPs) were developed from the foxtail millet genome support to alleviate cadmium and other heavy metals in millet tissues and grains [130]. In finger millet, 12 PHT1 and 6 zinc-regulated, iron-regulated transporter-like protein (ZIP) family transporters were detected, and their expression pattern was analyzed under an individual or combined deficiency of phosphorous and zinc conditions [131]. This study revealed the expression pattern of PHT1 and ZIP family transporters under a differential supply of phosphate and zinc. Apart from this report, 116 nucleotide-binding site–leucine-rich repeat (NBLRR) genes were identified from the native genome of finger millet, and they were analyzed in blast disease-infected finger millet [132]. Blast disease is one of the major biotic constraints on finger growth and yield. The identified NBLRR genes may contribute to alleviating the understanding of blast disease in finger millet. Eight classical drought-responsive genes were also identified from the annotated genome of finger millet, and they were analyzed in drought-tolerant and drought-sensitive genotypes [133]. More than 250 MYB genes were identified from the pearl millet genome responding to cold, high temperature, osmotic stress, drought, salinity, abscisic acid, salicylic acid, and methyl jasmonate [134,135] (Table 5). To date, only 180 NAC genes have been identified from the proso millet genome associated with drought stress [136]. There are more than 30 draft gene assemblies for proso millet, but it is regrettable that research progress is very poor compared to other millets. It is good to note that more than 1000 candidate genes have been identified and their expression pattern analyzed in various tissues of millets related to drought and salinity stress. We feel that many key genes associated with drought, salinity, and other stresses have been identified in foxtail millet. This clearly represents that foxtail millet can serve as a model millet for other minor millets such as brown top millet, fonio millet, little millet, green foxtail, tef, and other cereals. However, functional genomics research in millets is still in its infancy. In recent years, the genome editing tool CRISPR/Cas has gained popularity among plant science researchers to study the function of genes and generate stress-resistant and nutrient-rich plants. The CRISPR/Cas9 system has been widely applied in diverse plants, including millets [137]. This tool has been successfully implemented in sorghum, tef, and foxtail millet. The knockout of the SEMIDWARF-1 gene in tef through the CRISPR/Cas9 system enabled the development of lodging-resistant varieties (dwarf plants) of tef [138]. Induced site-directed mutations in the kafirin genes of sorghum by CRISPR/Cas9 improved protein digestibility and vitreous endosperm [139,140]. Herbicide-tolerant foxtail millet was developed by targeting two genes (acetolactate synthase (SiALS) and acetylcoenzyme A carboxylase (SiACC)) through CRISPR base editors (cytosine and adenosine base editors) [141]. The knockout of two carotenoid cleavage dioxygenase genes (SbCCD8a and SbCCD8b) reduced orobanchol production and parasite weed (in particular Striga) germination in sorghum [142]. Hence, a further functional characterization of key genes from already available reports through genome editing approaches will help to enhance millet growth, which will support improving food availability in 2050.

Table 5.
Details of candidate genes identified from native genome sequences of millets.
11. Will Genome Sequences of Millets Help Improve Food and Nutritional Security by 2050?
The world needs to produce more food to feed a rapidly growing world population, which is expected to reach 8.5 billion by 2030 and 9.7 billion by 2050 [182]. In addition, climate change, water scarcity, poor soil quality, and plant diseases are reducing the current availability of foods in many places worldwide. More than 50,000 plant species are available for human consumption, but less than 20 of them provide most of the world’s food supply [183]. Among them, three major cereals (rice, wheat, and maize) account for most of the calories consumed by humans every day. We cannot assume that these three major cereals will feed more than 9 million people in 2050. Also, it is difficult to cultivate these three cereals in the future’s toughest environments. Millets are one of the oldest food grains known to humans, dating back to 3500 BC. Millets are capable of growing in drought conditions, under irrigated conditions, and very low rainfall conditions and have a low water footprint [184]. Millets do not require much fertilizer or pesticides to grow, so they are a highly profitable grain for small farmers on a limited budget [3]. Millets, with their extraordinary adaptability and resilience, have proven themselves a powerful tool in the fight against global food security [185]. They are not just cereals; they are the embodiment of substances for billions of people, especially in regions where harsh climates and resources limitations pose formidable challenges to agriculture. The story of millets does not stop at feeding the world’s growing population. It extends to a profound commitment to environmental sustainability. The current annotated and draft genome sequences of major and minor millets have revolutionized the field of genomics and created significant molecular insights. Genome sequencing will help to develop millets with improved yield and nutritional values and superior resistance to various environmental stresses. Genome sequences play an important role in characterizing essential genes within a millet genotype by a variety of approaches including structural and functional analyses, linkage mapping, and gene editing. All these techniques have facilitated the genetic improvement of plants by understanding complex trait structures. The use of genome sequences in molecular breeding programs can effectively increase millet grain yield and productivity. In addition, genome sequences will help to optimize the utilization of genetic assets in different genotypes of millets. More than 30 thousand millet cultivars are globally available [31]. There is no doubt that millet genetic resources will play an important role for food security and sustainable agriculture. In a world grappling with climate change and its associated woes, the resilience of millets offers a glimmer of hope, demonstrating how a small yet mighty grain can contribute to soil health and reduce the ecological footprint of agriculture.
12. Conclusions and Future Perspectives
The availability of millet genomes facilitates the breeding and selection of millets, which are essential to support ongoing and future food security. However, researchers are trying to completely annotate the already available partial genome sequences of pearl millet, fonio millet, tef, job’s tear, proso millet, and barnyard millet (Figure 3). The complete annotation of pearl millet, fonio millet, tef, job’s tear, proso millet, and barnyard millet genome sequences will enable the rapid identification of the key genes that determine highly important traits in millets. Identifying markers and candidate genes from the available genome sequences would help millet improve against biotic and abiotic stresses. Also, this will allow for more efficient millet breeding by facilitating the selection of important traits. Genome sequences are not yet available for brown top millet, little millet, and kodo millet. Developing millet genome sequences for these three millets will support the improvement of these three millets. Genome editing is a famous tool in biotechnology to dissect the role of any candidate genes in any plants. Such novel techniques should be implemented in millets to dissect the already identified key genes related to biotic and abiotic stresses. The need for a rapid genetic improvement of millets is made more urgent by climate change, which demands new genotypes adapted to new and harsh environments. Maintaining millet genetic resources in seed banks and the conservation of the wild millet genotypes provide the genetic resources that are required for sustainable food production. More attention should be given to the collection and conservation of little millet, brown top millet, tef, fonio millet, job’s tear, kodo millet, proso millet, and barnyard millet, because most of the traditional germplasms of those millets have already disappeared from the world. Improving the nutritional contents of each millet through nutritional transporter gene manipulation may help enhance the nutritional availability in seeds of millets. In addition, several differentially expressed genes and molecular markers have already been identified for major and minor millets through transcriptomic resources. Hence, millet researchers can try to use the transcriptomic resources of millets for identifying candidate genes and developing molecular markers for those millets without complete annotated genome sequences.
Author Contributions
Conceptualization, T.M. and S.A.C.; table and image creation, T.M.; writing—original draft preparation, T.M. and T.P.A.K.; writing—review and editing, T.M., T.P.A.K. and N.M.K.; project administration, S.A.C. review, writing, and editing during revision, H.K. and M.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Rajagiri College of Social Sciences under Seed Money for Faculty Minor Research.
Acknowledgments
We would like to acknowledge the Rajagiri College of Social Sciences (RCSS) for providing internet access, software, and system facilities for writing the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gowda, N.N.; Siliveru, K.; Prasad, P.V.; Bhatt, Y.; Netravati, B.P.; Gurikar, C. Modern processing of Indian millets: A perspective on changes in nutritional properties. Foods 2022, 11, 499. [Google Scholar] [CrossRef]
- Kheya, S.A.; Talukder, S.K.; Datta, P.; Yeasmin, S.; Rashid, M.H.; Hasan, A.K.; Islam, A.K.M.M. Millets: The future crops for the tropics-Status, challenges and future prospects. Heliyon 2023, 9, e22123. [Google Scholar] [CrossRef]
- Ceasar, S.A.; Maharajan, T. The role of millets in attaining United Nation’s sustainable developmental goals. Plants People Planet 2022, 4, 345–349. [Google Scholar] [CrossRef]
- Kumar, A.; Tomer, V.; Kaur, A.; Kumar, V.; Gupta, K. Millets: A solution to agrarian and nutritional challenges. Agric. Food Secur. 2018, 7, 31. [Google Scholar] [CrossRef]
- Vinoth, A.; Ravindhran, R. Biofortification in millets: A sustainable approach for nutritional security. Front. Plant Sci. 2017, 8, 29. [Google Scholar] [CrossRef]
- Budhwar, S.; Sethi, K.; Chakraborty, M. Efficacy of germination and probiotic fermentation on underutilized cereal and millet grains. Food Prod. Process. Nutr. 2020, 2, 12. [Google Scholar] [CrossRef]
- Pramitha, L.J.; Ganesan, J.; Francis, N.; Rajasekharan, R.; Thinakaran, J. Revitalization of small millets for nutritional and food security by advanced genetics and genomics approaches. Front. Genet. 2023, 13, 1007552. [Google Scholar] [CrossRef]
- Hassan, Z.M.; Sebola, N.A.; Mabelebele, M. The nutritional use of millet grain for food and feed: A review. Agric. Food Secur. 2021, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Devi, P.B.; Vijayabharathi, R.; Sathyabama, S.; Malleshi, N.G.; Priyadarisini, V.B. Health benefits of finger millet (Eleusine coracana L.) polyphenols and dietary fiber: A review. J. Food Sci. Technol. 2014, 51, 1021–1040. [Google Scholar] [CrossRef]
- Rai, S.; Kaur, A.; Chopra, C.S. Gluten-free products for celiac susceptible people. Front. Nutr. 2018, 5, 116. [Google Scholar] [CrossRef]
- Liang, S.; Liang, K. Millet grain as a candidate antioxidant food resource: A review. Int. J. Food Prop. 2019, 22, 1652–1661. [Google Scholar] [CrossRef]
- Maharajan, T.; Ceasar, S.A.; Krishna, T.P.A.; Ignacimuthu, S. Finger millet [Eleusine coracana (L.) Gaertn]: An orphan crop with a potential to alleviate the calcium deficiency in the semi-arid tropics of Asia and Africa. Front. Sustain. Food Syst. 2021, 5, 684447. [Google Scholar] [CrossRef]
- Anitha, S.; Kane-Potaka, J.; Botha, R.; Givens, D.I.; Sulaiman, N.L.B.; Upadhyay, S.; Bhandari, R.K. Millets can have a major impact on improving iron status, hemoglobin level, and in reducing iron deficiency anemia—A systematic review and meta-analysis. Front. Nutr. 2021, 8, 725529. [Google Scholar] [CrossRef] [PubMed]
- Singhal, T.; Tara Satyavathi, C.; Singh, S.P.; Mallik, M.; Anuradha, N.; Sankar, S.M.; Singh, N. Achieving nutritional security in India through iron and zinc biofortification in pearl millet (Pennisetum glaucum (L.) R. Br.). Physiol. Mol. Biol. Plants 2022, 28, 849–869. [Google Scholar] [CrossRef] [PubMed]
- Anitha, S.; Govindaraj, M.; Kane-Potaka, J. Balanced amino acid and higher micronutrients in millets complements legumes for improved human dietary nutrition. Cereal Chem. 2020, 97, 74–84. [Google Scholar] [CrossRef]
- Saleh, A.S.; Zhang, Q.; Chen, J.; Shen, Q. Millet grains: Nutritional quality, processing, and potential health benefits. Compr. Rev. Food Sci. Food Saf. 2013, 12, 281–295. [Google Scholar] [CrossRef]
- Longvah, T.; Anantan, I.; Bhaskarachary, K.; Venkaiah, K.; Longvah, T. Indian Food Composition Tables; Longvah, T., Ed.; National Institute of Nutrition, Indian Council of Medical Research: Hyderabad, India, 2017; pp. 2–58. [Google Scholar]
- Shobana, S.; Krishnaswamy, K.; Sudha, V.; Malleshi, N.G.; Anjana, R.M.; Palaniappan, L.; Mohan, V. Finger millet (Ragi, Eleusine coracana L.): A review of its nutritional properties, processing, and plausible health benefits. Adv. Food Nutr. Res. 2013, 69, 1–39. [Google Scholar] [PubMed]
- Anitha, S.; Botha, R.; Kane-Potaka, J.; Givens, D.I.; Rajendran, A.; Tsusaka, T.W.; Bhandari, R.K. Can millet consumption help manage hyperlipidemia and obesity? a systematic review and meta-analysis. Front. Nutr. 2021, 8, 700778. [Google Scholar] [CrossRef] [PubMed]
- Sabuz, A.A.; Rana, M.R.; Ahmed, T.; Molla, M.M.; Islam, N.; Khan, H.H.; Shen, Q. Health-promoting potential of millet: A review. Separations 2023, 10, 80. [Google Scholar] [CrossRef]
- Agrawal, P.; Singh, B.R.; Gajbe, U.; Kalambe, M.A.; Bankar, M. Managing diabetes mellitus with millets: A new solution. Cureus 2023, 15, e44908. [Google Scholar] [CrossRef] [PubMed]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xian, F.; Guan, X.; Huang, K.; Yu, W.; Liu, D. Neural protective effects of millet and millet polyphenols on high-fat diet-induced oxidative stress in the brain. Plant Foods Hum. Nutr. 2020, 75, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Rani, M.; Mani, S.; Shah, P.; Singh, D.B.; Kudapa, H.; Varshney, R.K. Nutritional significance and antioxidant-mediated antiaging effects of finger millet: Molecular insights and prospects. Front. Sustain. Food Syst. 2021, 5, 684318. [Google Scholar] [CrossRef]
- Chauhan, M.; Sonawane, S.K.; Arya, S.S. Nutritional and nutraceutical properties of millets: A review. Am. J. Clin. Nutr. 2018, 1, 1–10. [Google Scholar]
- Anitha, S.; Givens, D.I.; Botha, R.; Kane-Potaka, J.; Sulaiman, N.L.B.; Tsusaka, T.W.; Bhandari, R.K. Calcium from finger millet—A systematic review and meta-analysis on calcium retention, bone resorption, and in vitro bioavailability. Sustainability 2021, 13, 8677. [Google Scholar] [CrossRef]
- Das, S.; Khound, R.; Santra, M.; Santra, D.K. Beyond bird feed: Proso millet for human health and environment. Agriculture 2019, 9, 64. [Google Scholar] [CrossRef]
- Bunkar, D.S.; Goyal, S.K.; Meena, K.K.; Kamalvanshi, V. Nutritional, functional role of kodo millet and its processing: A review. Int. J. Curr. Microbiol. Appl. Sci. 2021, 10, 1972–1985. [Google Scholar] [CrossRef]
- Singh, N.; Kumari, P.; Bassi, J.G.N. Nutrient rich Kodo millet, importance and value addition: An overview. Pharma Innov. J. 2023, 12, 3713–3719. [Google Scholar]
- Dwivedi, S.L.; Upadhyaya, H.D.; Senthilvel, S.; Hash, C.T.; Fukunaga, K.; Diao, X.; Prasad, M. Millets: Genetic and genomic resources. Genetic and Genomic Resources. In Plant Breeding Reviews; Wiley-Blackwell: Hoboken, NJ, USA, 2012; pp. 247–375. [Google Scholar]
- Vetriventhan, M.; Azevedo, V.C.; Upadhyaya, H.D.; Nirmalakumari, A.; Kane-Potaka, J.; Anitha, S.; Tonapi, V.A. Genetic and genomic resources, and breeding for accelerating improvement of small millets: Current status and future interventions. Nucleus 2020, 63, 217–239. [Google Scholar] [CrossRef]
- Bramel, P.; Giovannini, P.; Eshan Dulloo, M. Global Strategy for the Conservation and Use of Genetic Resources of Selected Millets; Paula, B., Peter, G., Eshan Dulloo, M., Eds.; Global Crop Diversity Trust: Bonn, Germany, 2022; pp. 1–88. [Google Scholar]
- Tripathi, T.; Vyas, S. From ancient grains to modern solutions: A history of millets and their significance in agriculture and food security. Int. J. Home Sci. 2023, 9, 72–78. [Google Scholar]
- Yang, X.; Wan, Z.; Perry, L.; Lu, H.; Wang, Q.; Zhao, C.; Ge, Q. Early millet use in northern China. Proc. Natl. Acad. Sci. USA 2012, 109, 3726–3730. [Google Scholar] [CrossRef] [PubMed]
- Kangama, C.O. Pearl millet (Pennisetum glaucum) perspectives in Africa. Int. J. Sci. Res. 2021, 2, 001–007. [Google Scholar]
- Burgarella, C.; Cubry, P.; Kane, N.A.; Varshney, R.K.; Mariac, C.; Liu, X.; Vigouroux, Y. A western Sahara centre of domestication inferred from pearl millet genomes. Nat. Ecol. Evol. 2018, 2, 1377–1380. [Google Scholar] [CrossRef] [PubMed]
- Saxena, R.; Vanga, S.K.; Wang, J.; Orsat, V.; Raghavan, V. Millets for food security in the context of climate change: A review. Sustainability 2018, 10, 2228. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, J.; Liu, K.B.; Wu, N.; Li, Y.; Zhou, K.; Li, Q. Earliest domestication of common millet (Panicum miliaceum) in East Asia extended to 10,000 years ago. Proc. Natl. Acad. Sci. USA 2009, 106, 7367–7372. [Google Scholar] [CrossRef] [PubMed]
- Animasaun, D.A.; Morakinyo, J.A.; Mustapha, O.T.; Krishnamurthy, R. Genome size and ploidy variations in pearl millet (Pennisetum glaucum) and napier grass (Pennisetum purpureum) genotypes. Acta Agron. 2019, 68, 299–305. [Google Scholar] [CrossRef]
- Bennetzen, J.L.; Schmutz, J.; Wang, H.; Percifield, R.; Hawkins, J.; Pontaroli, A.C.; Devos, K.M. Reference genome sequence of the model plant Setaria. Nat. Biotechnol. 2012, 30, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Liu, X.; Quan, Z.; Cheng, S.; Xu, X.; Pan, S.; Wang, J. Genome sequence of foxtail millet (Setaria italica) provides insights into grass evolution and biofuel potential. Nat. Biotechnol. 2012, 30, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Hittalmani, S.; Mahesh, H.B.; Shirke, M.D.; Biradar, H.; Uday, G.; Aruna, Y.R.; Mohanrao, A. Genome and transcriptome sequence of finger millet (Eleusine coracana (L.) Gaertn.) provides insights into drought tolerance and nutraceutical properties. BMC Genom. 2017, 18, 465. [Google Scholar] [CrossRef]
- Hatakeyama, M.; Aluri, S.; Balachadran, M.T.; Sivarajan, S.R.; Patrignani, A.; Grüter, S.; Shimizu, K.K. Multiple hybrid de novo genome assembly of finger millet, an orphan allotetraploid crop. DNA Res. 2018, 25, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Devos, K.M.; Qi, P.; Bahri, B.A.; Gimode, D.M.; Jenike, K.; Manthi, S.J.; Odeny, D.A. Genome analyses reveal population structure and a purple stigma color gene candidate in finger millet. Nat. Commun. 2023, 14, 3694. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Qiu, J.; Ye, C.; Jin, G.; Mao, L.; Zhang, H.; Fan, L. Echinochloa crus-galli genome analysis provides insight into its adaptation and invasiveness as a weed. Nat. Commun. 2017, 8, 1031. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.; Li, L.; Miki, D.; Li, D.; Tang, Q.; Xiao, L.; Zhang, H. The genome of broomcorn millet. Nat. Commun. 2019, 10, 436. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.; Deshpande, S.; Vetriventhan, M.; Upadhyaya, H.D.; Wallace, J.G. Genome-wide population structure analyses of three minor millets: Kodo millet, little millet, and proso millet. Plant Genome 2019, 12, 190021. [Google Scholar] [CrossRef] [PubMed]
- Paterson, A.H.; Bowers, J.E.; Bruggmann, R.; Dubchak, I.; Grimwood, J.; Gundlach, H.; Rokhsar, D.S. The Sorghum bicolor genome and the diversification of grasses. Nature 2009, 457, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Varshney, R.K.; Shi, C.; Thudi, M.; Mariac, C.; Wallace, J.; Qi, P.; Xu, X. Pearl millet genome sequence provides a resource to improve agronomic traits in arid environments. Nat. Biotechnol. 2017, 35, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Sebastin, R.; Lee, G.A.; Lee, K.J.; Shin, M.J.; Cho, G.T.; Lee, J.R.; Chung, J.W. The complete chloroplast genome sequences of little millet (Panicum sumatrense Roth ex Roem. and Schult.) (Poaceae). Mitochondrial DNA Part B 2018, 3, 719–720. [Google Scholar] [CrossRef] [PubMed]
- Cannarozzi, G.; Plaza-Wüthrich, S.; Esfeld, K.; Larti, S.; Wilson, Y.S.; Girma, D.; Tadele, Z. Genome and transcriptome sequencing identifies breeding targets in the orphan crop tef (Eragrostis tef). BMC Genom. 2014, 15, 581. [Google Scholar] [CrossRef] [PubMed]
- Abrouk, M.; Ahmed, H.I.; Cubry, P.; Šimoníková, D.; Cauet, S.; Pailles, Y.; Krattinger, S.G. Fonio millet genome unlocks African orphan crop diversity for agriculture in a changing climate. Nat. Commun. 2020, 11, 4488. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.H.; Kim, B.; Choi, B.S.; Lee, H.O.; Lee, S.J.; Oh, T.J.; Kim, C.K. Genome assembly and annotation of soft-shelled adlay (Coix lacryma-jobi Variety ma-yuen), a cereal and medicinal crop in the Poaceae family. Front. Plant Sci. 2020, 11, 523041. [Google Scholar] [CrossRef]
- Li, X.D.; Pan, H.; Lu, X.J.; Wei, X.Y.; Shi, M.; Lu, P. Complete chloroplast genome sequencing of Job’s tears (Coix l.): Genome structure, comparative analysis, and phylogenetic relationships. Mitochondrial DNA Part B 2021, 6, 1399–1405. [Google Scholar] [CrossRef] [PubMed]
- Naithani, S.; Deng, C.H.; Sahu, S.K.; Jaiswal, P. Exploring pan-genomes: An overview of resources and tools for unraveling structure, function, and evolution of crop genes and genomes. Biomolecules 2023, 13, 1403. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Luo, H.; Xu, J.; Cruickshank, A.; Zhao, X.; Teng, F.; Mace, E. Extensive variation within the pan-genome of cultivated and wild sorghum. Nat. Plants 2021, 7, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Ruperao, P.; Thirunavukkarasu, N.; Gandham, P.; Selvanayagam, S.; Govindaraj, M.; Nebie, B.; Rathore, A. Sorghum pan-genome explores the functional utility for genomic-assisted breeding to accelerate the genetic gain. Front. Plant Sci. 2021, 12, 666342. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Sun, M.; Zhang, Z.; Jin, Y.; Zhang, A.; Lin, C.; Huang, L. Pangenomic analysis identifies structural variation associated with heat tolerance in pearl millet. Nat. Genet. 2023, 55, 507–518. [Google Scholar] [CrossRef]
- He, Q.; Tang, S.; Zhi, H.; Chen, J.; Zhang, J.; Liang, H.; Diao, X. A graph-based genome and pan-genome variation of the model plant Setaria. Nat. Genet. 2023, 55, 1232–1242. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, Y.; Liu, M.; Guo, W.; Wang, Y.; He, Q.; Diao, X. Pangenome analysis reveals genomic variations associated with domestication traits in broomcorn millet. Nat. Genet. 2023, 55, 2243–2254. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Wang, Y.; Xu, J.; Jiang, F.; Li, W.; Zhang, Q.; Zhang, L. A telomere-to-telomere genome assembly of Hongyingzi, a sorghum cultivar used for Chinese Baijiu production. Crop J. 2024, 1–6. [Google Scholar] [CrossRef]
- Tian, B.; Zhang, L.; Liu, Y.; Wu, P.; Wang, W.; Zhang, Y.; Li, H. Identification of QTL for resistance to leaf blast in foxtail millet by genome re-sequencing analysis. Theor. Appl. Genet. 2021, 134, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Ambawat, S.; Senthilvel, S.; Hash, C.T.; Nepolean, T.; Rajaram, V.; Eshwar, K.; Srivastava, R.K. QTL mapping of pearl millet rust resistance using an integrated DArT-and SSR-based linkage map. Euphytica 2016, 209, 461–476. [Google Scholar] [CrossRef]
- Zoclanclounon, Y.A.B.; Kanfany, G.; Kane, A.; Fonceka, D.; Ehemba, G.L.; Ly, F. Current status of pearl millet downy mildew prevalence across agroecological zones of Senegal. Sci. World J. 2019, 2019, 1252653. [Google Scholar] [CrossRef] [PubMed]
- Chelpuri, D.; Sharma, R.; Durga, K.K.; Katiyar, P.; Mahendrakar, M.D.; Singh, R.B.; Srivastava, R.K. Mapping quantitative trait loci (QTLs) associated with resistance to major pathotype-isolates of pearl millet downy mildew pathogen. Eur. J. Plant Pathol. 2019, 154, 983–994. [Google Scholar] [CrossRef]
- Kumar, S.; Hash, C.T.; Nepolean, T.; Mahendrakar, M.D.; Satyavathi, C.T.; Singh, G.; Srivastava, R.K. Mapping grain iron and zinc content quantitative trait loci in an iniadi-derived immortal population of pearl millet. Genes 2018, 9, 248. [Google Scholar] [CrossRef] [PubMed]
- Singhal, T.; Satyavathi, C.T.; Singh, S.P.; Kumar, A.; Sankar, S.M.; Bhardwaj, C.; Singh, N. Multi-environment quantitative trait loci mapping for grain iron and zinc content using bi-parental recombinant inbred line mapping population in pearl millet. Front. Sci. Plant Sci. 2021, 12, 659789. [Google Scholar] [CrossRef] [PubMed]
- Maharajan, T.; Ajeesh Krishna, T.P.; Rakkammal, K.; Ramakrishnan, M.; Ceasar, S.A.; Ramesh, M.; Ignacimuthu, S. Identification of QTL Associated with Agro-Morphological and Phosphorus Content Traits in Finger Millet under Differential Phosphorus Supply via Linkage Mapping. Agriculture 2023, 13, 262. [Google Scholar] [CrossRef]
- Ramakrishnan, M.; Ceasar, S.A.; Vinod, K.K.; Duraipandiyan, V.; Ajeesh Krishna, T.P.; Upadhyaya, H.D.; Ignacimuthu, S. Identification of putative QTLs for seedling stage phosphorus starvation response in finger millet (Eleusine coracana L. Gaertn.) by association mapping and cross species synteny analysis. PLoS ONE 2017, 12, e0183261. [Google Scholar] [CrossRef] [PubMed]
- David, R.H.A.; Ramakrishnan, M.; Maharajan, T.; BarathiKannan, K.; Babu, G.A.; Daniel, M.A.; Ignacimuthu, S. Mining QTL and genes for root traits and biochemical parameters under vegetative drought in South Indian genotypes of finger millet (Eleusine coracana (L.) Gaertn) by association mapping and in silico comparative genomics. Biocatal. Agric. Biotechnol. 2021, 32, 101935. [Google Scholar]
- Kumar, S.; Hash, C.T.; Nepolean, T.; Satyavathi, C.T.; Singh, G.; Mahendrakar, M.D.; Srivastava, R.K. Mapping QTLs controlling flowering time and important agronomic traits in pearl millet. Front. Sci. Plant Sci. 2017, 8, 1731. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Hash, C.T.; Singh, G.; Nepolean, T.; Srivastava, R.K. Mapping QTLs for important agronomic traits in an Iniadi-derived immortal population of pearl millet. Biotechnol. Notes 2021, 2, 26–32. [Google Scholar] [CrossRef]
- Vengadessan, V.; Rai, K.N.; Kannan Bapu, J.R.; Hash, C.T.; Bhattacharjee, R.; Senthilvel, S.; Nepolean, T. Construction of genetic linkage map and QTL analysis of sink-size traits in pearl millet (Pennisetum glaucum). Int. Sch. Res. Not. 2013, 2013, 471632. [Google Scholar] [CrossRef][Green Version]
- Nepolean, T.; Blummel, M.; Raj, A.B.; Rajaram, V.; Senthilvel, S.; Hash, C.T. QTLs controlling yield and stover quality traits in pearl millet. J. SAT Agric. Res. 2006, 2, 1–4. [Google Scholar]
- Gulia, S.K.; Hash, C.T.; Thakur, R.P.; Breese, W.A.; Sangwan, R.S. Mapping new QTLs for improvement of downy mildew resistance in pearl millet. In Crop Production in Stress Environments: Genetic and Management Options; Agrobios International: Jodhpur, India, 2007; pp. 373–386. [Google Scholar]
- Bidinger, F.R.; Nepolean, T.; Hash, C.T.; Yadav, R.S.; Howarth, C.J. Quantitative trait loci for grain yield in pearl millet under variable postflowering moisture conditions. Crop Sci. 2007, 47, 969–980. [Google Scholar] [CrossRef]
- Liu, T.; He, J.; Dong, K.; Wang, X.; Wang, W.; Yang, P.; Yang, T. QTL mapping of yield component traits on bin map generated from resequencing a RIL population of foxtail millet (Setaria italica). BMC Genom. 2020, 21, 141. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Zhi, H.; Tang, S.; Xing, L.; Wang, S.; Wang, H.; Diao, X. QTL mapping for foxtail millet plant height in multi-environment using an ultra-high density bin map. Theor. Appl. Genet. 2021, 134, 557–572. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, J.; Peng, J.; Du, X.; Jiang, M.; Li, Y.; Guo, E. QTL mapping for 11 agronomic traits based on a genome-wide Bin-map in a large F 2 population of foxtail millet (Setaria italica (L.) P. Beauv). Mol. Breed. 2019, 39, 18. [Google Scholar] [CrossRef]
- Guo, S.; Chai, S.; Guo, Y.; Shi, X.; Han, F.; Qu, T.; Yang, P. Mapping of major QTL and candidate gene analysis for hull colour in foxtail millet (Setaria italica (L.) P. Beauv.). BMC Genom. 2023, 24, 458. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Fan, G.; Zhang, X.; Zhao, F.; Wei, W.; Du, G.; Zhao, Z. Identification of QTLs for 14 agronomically important traits in Setaria italica based on SNPs generated from high-throughput sequencing. G3 Genes Genom. Genet. 2017, 7, 1587–1594. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; He, J.; Dong, K.; Wang, X.; Zhang, L.; Ren, R.; Zhang, Z. Genome-wide identification of quantitative trait loci for morpho-agronomic and yield-related traits in foxtail millet (Setaria italica) across multi-environments. Mol. Genet. Genom. 2022, 297, 873–888. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Z.; Du, X.; Yang, H.; Han, F.; Han, Y.; Guo, E. A high-density genetic map and QTL analysis of agronomic traits in foxtail millet [Setaria italica (L.) P. Beauv.] using RAD-seq. PLoS ONE 2017, 12, e0179717. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Hou, J.; Fu, N.; Wei, M.; Li, Y.; Yu, K.; Liu, J. Identification of QTL related to anther color and hull color by RAD sequencing in a RIL population of Setaria italica. BMC Genom. 2021, 22, 556. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, M.; Antony Ceasar, S.; Duraipandiyan, V.; Vinod, K.K.; Kalpana, K.; Al-Dhabi, N.A.; Ignacimuthu, S. Tracing QTLs for leaf blast resistance and agronomic performance of finger millet (Eleusine coracana (L.) Gaertn.) genotypes through association mapping and in silico comparative genomics analyses. PLoS ONE 2016, 11, e0159264. [Google Scholar] [CrossRef] [PubMed]
- Pendergast, T.H., IV; Qi, P.; Odeny, D.A.; Dida, M.M.; Devos, K.M. A high-density linkage map of finger millet provides QTL for blast resistance and other agronomic traits. Plant Genom. 2022, 15, e20175. [Google Scholar] [CrossRef] [PubMed]
- Kalyana Babu, B.; Agrawal, P.K.; Pandey, D.; Jaiswal, J.P.; Kumar, A. Association mapping of agro-morphological characters among the global collection of finger millet genotypes using genomic SSR markers. Mol. Biol. Rep. 2014, 41, 5287–5297. [Google Scholar] [CrossRef] [PubMed]
- Rajput, S.G.; Santra, D.K.; Schnable, J. Mapping QTLs for morpho-agronomic traits in proso millet (Panicum miliaceum L.). Mol. Breed. 2016, 36, 37. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Y.; Hou, S.; Men, Y.; Han, Y. The Integration of Genome-Wide Association Study and Homology Analysis to Explore the Genomic Regions and Candidate Genes for Panicle-Related Traits in Foxtail Millet. Int. J. Mol. Sci. 2022, 23, 14735. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, V.; Gupta, S.; Gahlaut, V.; Muthamilarasan, M.; Bandyopadhyay, T.; Ramchiary, N.; Prasad, M. Genome-wide association study of major agronomic traits in foxtail millet (Setaria italica L.) using ddRAD sequencing. Sci. Rep. 2019, 9, 5020. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, V.; Bandyopadhyay, T.; Gahlaut, V.; Gupta, S.; Dhaka, A.; Ramchiary, N.; Prasad, M. Genome-wide association study (GWAS) delineates genomic loci for ten nutritional elements in foxtail millet (Setaria italica L.). J. Cereal Sci. 2019, 85, 48–55. [Google Scholar] [CrossRef]
- Pujar, M.; Gangaprasad, S.; Govindaraj, M.; Gangurde, S.S.; Kanatti, A.; Kudapa, H. Genome-wide association study uncovers genomic regions associated with grain iron, zinc and protein content in pearl millet. Sci. Rep. 2020, 10, 19473. [Google Scholar] [CrossRef] [PubMed]
- Diack, O.; Kanfany, G.; Gueye, M.C.; Sy, O.; Fofana, A.; Tall, H.; Kane, N.A. GWAS unveils features between early-and late-flowering pearl millets. BMC Genom. 2020, 21, 777. [Google Scholar] [CrossRef] [PubMed]
- Yadav, C.B.; Srivastava, R.K.; Beynon, S.; Englyst, K.; Gangashetty, P.I.; Yadav, R.S. Genetic variability and genome-wide marker association studies for starch traits contributing to low glycaemic index in pearl millet. Food Energy Sec. 2022, 11, e341. [Google Scholar] [CrossRef]
- Boukail, S.; Macharia, M.; Miculan, M.; Masoni, A.; Calamai, A.; Palchetti, E.; Dell’Acqua, M. Genome wide association study of agronomic and seed traits in a world collection of proso millet (Panicum miliaceum L.). BMC Plant Biol. 2021, 21, 330. [Google Scholar] [CrossRef] [PubMed]
- Puranik, S.; Sahu, P.P.; Beynon, S.; Srivastava, R.K.; Sehgal, D.; Ojulong, H.; Yadav, R. Genome-wide association mapping and comparative genomics identifies genomic regions governing grain nutritional traits in finger millet (Eleusine coracana L. Gaertn.). Plants People Planet 2020, 2, 649–662. [Google Scholar] [CrossRef]
- Adeyanju, A.; Little, C.; Yu, J.; Tesso, T. Genome-wide association study on resistance to stalk rot diseases in grain sorghum. G3 Genes Genomes Genet. 2015, 5, 1165–1175. [Google Scholar] [CrossRef] [PubMed]
- Boyles, R.E.; Cooper, E.A.; Myers, M.T.; Brenton, Z.; Rauh, B.L.; Morris, G.P.; Kresovich, S. Genome-wide association studies of grain yield components in diverse sorghum germplasm. Plant Genome 2016, 9, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Mantilla Perez, M.B.; Hu, J.; Salas Fernandez, M.G. Genome-wide association study for nine plant architecture traits in Sorghum. Plant Genome 2016, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Chopra, R.; Burow, G.; Burke, J.J.; Gladman, N.; Xin, Z. Genome-wide association analysis of seedling traits in diverse Sorghum germplasm under thermal stress. BMC Plant Biol. 2017, 17, 12. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chopra, R.; Hayes, C.; Morris, G.; Marla, S.; Burke, J.; Burow, G. Genome-wide association study of developing leaves’ heat tolerance during vegetative growth stages in a sorghum association panel. Plant Genome 2017, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Parra-Londono, S.; Fiedler, K.; Kavka, M.; Samans, B.; Wieckhorst, S.; Zacharias, A.; Uptmoor, R. Genetic dissection of early-season cold tolerance in sorghum: Genome-wide association studies for seedling emergence and survival under field and controlled environment conditions. Theor. Appl. Genet. 2018, 131, 581–595. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tang, W.; Zhang, Y.W.; Chen, K.N.; Wang, C.; Liu, Y.; Wang, L. Genome-wide association studies for five forage quality-related traits in sorghum (Sorghum bicolor L.). Front. Plant Sci. 2018, 9, 1146. [Google Scholar] [CrossRef] [PubMed]
- Cuevas, H.E.; Fermin-Pérez, R.A.; Prom, L.K.; Cooper, E.A.; Bean, S.; Rooney, W.L. Genome-wide association mapping of grain mold resistance in the US sorghum association panel. Plant Genome 2019, 12, 180070. [Google Scholar] [CrossRef] [PubMed]
- Girma, G.; Nida, H.; Seyoum, A.; Mekonen, M.; Nega, A.; Lule, D.; Mengiste, T. A large-scale genome-wide association analyses of Ethiopian sorghum landrace collection reveal loci associated with important traits. Front. Plant Sci. 2019, 10, 691. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.R.; Wang, C.Y.; Wang, P.; Zhu, Z.X.; Ning, X.U.; Shi, G.S.; Huang, R.D. Genome-wide association study for starch content and constitution in sorghum (Sorghum bicolor (L.) Moench). J. Integr. Agric. 2019, 18, 2446–2456. [Google Scholar] [CrossRef]
- Tao, Y.; Zhao, X.; Wang, X.; Hathorn, A.; Hunt, C.; Cruickshank, A.W.; Jordan, D.R. Large-scale GWAS in sorghum reveals common genetic control of grain size among cereals. Plant Biotechnol. J. 2020, 18, 1093–1105. [Google Scholar] [CrossRef] [PubMed]
- Cruet-Burgos, C.; Cox, S.; Ioerger, B.P.; Perumal, R.; Hu, Z.; Herald, T.J.; Rhodes, D.H. Advancing provitamin A biofortification in sorghum: Genome-wide association studies of grain carotenoids in global germplasm. Plant Genome 2020, 13, e20013. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Upadhyaya, H.D.; Zheng, J.; Liu, Y.; Singh, S.K.; Gowda, C.L.L.; Li, J. Genome-Wide association mapping identifies novel panicle morphology loci and candidate genes in sorghum. Front. Plant Sci. 2021, 12, 743838. [Google Scholar] [CrossRef] [PubMed]
- Maina, F.; Harou, A.; Hamidou, F.; Morris, G.P. Genome-wide association studies identify putative pleiotropic locus mediating drought tolerance in sorghum. Plant Dir. 2022, 6, e413. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, Y.; Gao, L.; Yang, X.; Zhang, X.; Xie, S.; Shen, Y. Identification of candidate forage yield genes in sorghum (Sorghum bicolor L.) using integrated genome-wide association studies and RNA-seq. Front. Plant Sci. 2022, 12, 788433. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Yang, B.; Kim, W.J.; Kim, J.; Kwon, S.J.; Kim, J.H.; Ryu, J. Genome-Wide Association Study (GWAS) of the Agronomic Traits and Phenolic Content in Sorghum (Sorghum bicolor L.) Genotypes. Agronomy 2023, 13, 1449. [Google Scholar] [CrossRef]
- Xin, Y.; Gao, L.; Hu, W.; Gao, Q.; Yang, B.; Zhou, J.; Xu, C. Genome-wide association study based on plant height and drought-tolerance indices reveals two candidate drought-tolerance genes in sweet sorghum. Sustainability 2022, 14, 14339. [Google Scholar] [CrossRef]
- Ahn, E.; Botkin, J.; Ellur, V.; Lee, Y.; Poudel, K.; Prom, L.K.; Magill, C. Genome-wide association study of seed morphology traits in senegalese sorghum cultivars. Plants 2023, 12, 2344. [Google Scholar] [CrossRef] [PubMed]
- Yadav, C.B.; Srivastava, R.K.; Gangashetty, P.I.; Yadav, R.; Mur, L.A.; Yadav, R.S. Metabolite Diversity and Metabolic Genome-Wide Marker Association Studies (Mgwas) for Health Benefiting Nutritional Traits in Pearl Millet Grains. Cells 2021, 10, 3076. [Google Scholar] [CrossRef] [PubMed]
- Yadav, C.B.; Tokas, J.; Yadav, D.; Winters, A.; Singh, R.B.; Yadav, R.; Yadav, R.S. Identifying anti-oxidant biosynthesis genes in pearl millet [Pennisetum glaucum (L.) R. Br.] using genome—Wide association analysis. Front. Sci. Plant. Sci. 2021, 12, 599649. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Huang, X.; Zhi, H.; Zhao, Y.; Zhao, Q.; Li, W.; Chai, Y.; Yang, L.; Liu, K.; Lu, H.; et al. A haplotype map of genomic variations and genome-wide association studies of agronomic traits in foxtail millet (Setaria italica). Nat. Genet. 2013, 45, 957–961. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, V.; Bandyopadhyay, T.; Singh, R.K.; Gahlaut, V.; Muthamilarasan, M.; Prasad, M. Multi-environment GWAS identifies genomic regions underlying grain nutrient traits in foxtail millet (Setaria italica). Plant Cell Rep. 2024, 43, 6. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, X.; Sun, S.; Jin, C.; Su, J.; Wei, J.; Luo, X.; Wen, J.; Wei, T.; Sahu, S.K.; et al. GWAS, MWAS and mGWAS provide insights into precision agriculture based on genotype-dependent microbial effects in foxtail millet. Nat. Commun. 2022, 13, 5913. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.; Chen, Y.; Liu, Z.; Sun, M.; Han, F.; Wang, T.; Chen, L. Genome-wide association mapping for agronomic and quality traits in foxtail millet. 2019; preprint. [Google Scholar] [CrossRef]
- Sharma, D.; Tiwari, A.; Sood, S.; Jamra, G.; Singh, N.K.; Meher, P.K.; Kumar, A. Genome wide association mapping of agro-morphological traits among a diverse collection of finger millet (Eleusine coracana L.) genotypes using SNP markers. PLoS ONE 2018, 13, e0199444. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.; Sharma, D.; Sood, S.; Jaiswal, J.P.; Pachauri, S.P.; Ramteke, P.W.; Kumar, A. Genome-wide association mapping for seed protein content in finger millet (Eleusine coracana) global collection through genotyping by sequencing. J. Cereal Sci. 2020, 91, 102888. [Google Scholar] [CrossRef]
- Fan, Y.; Lai, D.; Yang, H.; Xue, G.; He, A.; Chen, L.; Cheng, J. Genome-wide identification and expression analysis of the bHLH transcription factor family and its response to abiotic stress in foxtail millet (Setaria italica L.). BMC Genom. 2021, 22, 778. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hao, D.; Wang, X.; Zhang, H.; Yang, P.; Zhang, L.; Zhang, B. Genome-wide identification and expression analysis of the SNARE genes in Foxtail millet (Setaria italica) reveals its roles in drought stress. Plant Growth Reg. 2021, 95, 355–369. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, B.; Zheng, G.; Zhang, A.; Li, R. Genome-wide characterization of the SiDof gene family in foxtail millet (Setaria italica). Biosystems 2017, 151, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Shu, H.; Zhang, A.Y.; Liu, B.L.; Xing, G.F.; Xue, J.A.; Li, R.Z. Foxtail millet WRKY genes and drought stress. J. Agric. Sci. 2017, 155, 777–790. [Google Scholar] [CrossRef]
- Ceasar, S.A.; Hodge, A.; Baker, A.; Baldwin, S.A. Phosphate concentration and arbuscular mycorrhizal colonisation influence the growth, yield and expression of twelve PHT1 family phosphate transporters in foxtail millet (Setaria italica). PLoS ONE 2014, 9, e108459. [Google Scholar] [CrossRef] [PubMed]
- Ceasar, S.A.; Baker, A.; Ignacimuthu, S. Functional characterization of the PHT1 family transporters of foxtail millet with development of a novel Agrobacterium-mediated transformation procedure. Sci. Rep. 2017, 7, 14064. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xiao, W.; Yu, W.; Yao, L.; Li, L.; Wei, J.; Li, R. Foxtail millet SiHAK1 excites extreme high-affinity K+ uptake to maintain K+ homeostasis under low K+ or salt stress. Plant Cell Rep. 2018, 37, 1533–1546. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, J.; Liang, Y.; Wang, X.; Li, K.; Chen, L.; Hou, S. Natural Resistance-Associated Macrophage Protein (Nramp) Family in Foxtail Millet (Setaria italica): Characterization, Expression Analysis and Relationship with Metal Content under Cd Stress. Agronomy 2023, 13, 2000. [Google Scholar] [CrossRef]
- Maharajan, T.; Krishna, T.P.A.; Shilpha, J.; Ceasar, S.A. Effects of Individual or Combined Deficiency of Phosphorous and Zinc on Phenotypic, Nutrient Uptake, and Molecular Responses of Finger Millet (Eleusine coracana): A Nutri-Rich Cereal Crop. Plants 2023, 12, 3378. [Google Scholar] [CrossRef] [PubMed]
- Balamurugan, A.; Mallikarjuna, M.G.; Bansal, S.; Nayaka, S.C.; Rajashekara, H.; Chellapilla, T.S.; Prakash, G. Genome-wide identification and characterization of NBLRR genes in Finger millet (Eleusine coracana L.) and their expression in response to Magnaporthe grisea infection. BMC Plant Biol. 2024, 24, 75. [Google Scholar] [CrossRef]
- Rakkammal, K.; Maharajan, T.; Shriram, R.N.; Ram, P.J.; Ceasar, S.A.; Ramesh, M. Physiological, biochemical and molecular responses of finger millet (Eleusine coracana) genotypes exposed to short-term drought stress induced by PEG-6000. S. Afr. J. Bot. 2023, 155, 45–59. [Google Scholar] [CrossRef]
- Lin, M.; Dong, Z.; Zhou, H.; Wu, G.; Xu, L.; Ying, S.; Chen, M. Genome-Wide Identification and Transcriptional Analysis of the MYB Gene Family in Pearl Millet (Pennisetum glaucum). Int. J. Mol. Sci. 2023, 24, 2484. [Google Scholar] [CrossRef] [PubMed]
- Chanwala, J.; Khadanga, B.; Jha, D.K.; Sandeep, I.S.; Dey, N. MYB transcription factor family in pearl millet: Genome-wide identification, evolutionary progression and expression analysis under abiotic stress and phytohormone treatments. Plants 2023, 12, 355. [Google Scholar] [CrossRef] [PubMed]
- Shan, Z.; Jiang, Y.; Li, H.; Guo, J.; Dong, M.; Zhang, J.; Liu, G. Genome-wide analysis of the NAC transcription factor family in broomcorn millet (Panicum miliaceum L.) and expression analysis under drought stress. BMC Genom. 2020, 21, 96. [Google Scholar] [CrossRef] [PubMed]
- Ceasar, A. Genome-editing in millets: Current knowledge and future perspectives. Mol. Biol. Rep. 2022, 49, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Beyene, G.; Chauhan, R.D.; Villmer, J.; Husic, N.; Wang, N.; Gebre, E.; MacKenzie, D.J. CRISPR/Cas9-mediated tetra-allelic mutation of the ‘Green Revolution’SEMIDWARF-1 (SD-1) gene confers lodging resistance in tef (Eragrostis tef). Plant Biotechnol. J. 2022, 20, 1716–1729. [Google Scholar] [CrossRef] [PubMed]
- Elkonin, L.A.; Gerashchenkov, G.A.; Borisenko, N.V.; Kenzhegulov, O.A.; Sarsenova, S.K.; Rozhnova, N.A.; Panin, V.M. Development of sorghum mutants with improved in vitro protein digestibility by CRISPR/Cas9 editing of kafirin genes. Crop J. 2023, 11, 1411–1418. [Google Scholar] [CrossRef]
- Li, X.; Liu, W.; Wang, G.; Sun, S.S.M.; Yuan, L.; Wang, J. Improving digestibility of sorghum proteins by CRISPR/Cas9-based genome editing. Food Energy Secur. 2024, 13, e506. [Google Scholar] [CrossRef]
- Liang, Z.; Wu, Y.; Ma, L.; Guo, Y.; Ran, Y. Efficient genome editing in Setaria italica using CRISPR/Cas9 and base editors. Front. Plant Sci. 2022, 12, 815946. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Yang, Y.; Futrell, S.; Kelly, E.A.; Lorts, C.M.; Nebie, B.; Schachtman, D.P. CRISPR/Cas9-mediated mutagenesis of carotenoid cleavage dioxygenase (CCD) genes in sorghum alters strigolactone biosynthesis and plant biotic Interactions. Phytobio. J. 2023, 7, 339–351. [Google Scholar] [CrossRef]
- Chanwala, J.; Satpati, S.; Dixit, A.; Parida, A.; Giri, M.K.; Dey, N. Genome-wide identification and expression analysis of WRKY transcription factors in pearl millet (Pennisetum glaucum) under dehydration and salinity stress. BMC Genom. 2020, 21, 231. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Shinde, H.; Dudhate, A.; Tsugama, D.; Gupta, S.K.; Liu, S.; Takano, T. Genome-wide investigation of SQUAMOSA promoter binding protein-like transcription factor family in pearl millet (Pennisetum glaucum (L) R. Br.). Plant Gene 2021, 27, 100313. [Google Scholar] [CrossRef]
- Dudhate, A.; Shinde, H.; Yu, P.; Tsugama, D.; Gupta, S.K.; Liu, S.; Takano, T. Comprehensive analysis of NAC transcription factor family uncovers drought and salinity stress response in pearl millet (Pennisetum glaucum). BMC Genom. 2021, 22, 70. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Cui, Y.; Zhao, Z.; Li, S.; Liang, D.; Wang, C.; Liu, Z. Genome-wide identification and characterization of the BES/BZR gene family in wheat and foxtail millet. BMC Genom. 2021, 22, 682. [Google Scholar] [CrossRef] [PubMed]
- Lai, D.; Yan, J.; He, A.; Xue, G.; Yang, H.; Feng, L.; Cheng, J. Genome-wide identification, phylogenetic and expression pattern analysis of MADS-box family genes in foxtail millet (Setaria italica). Sci. Rep. 2022, 12, 4979. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Fan, K.; Guo, X.; Liu, J.; Zhang, K.; Lu, P. Genome-wide identification, molecular evolution and expression analysis of the non-specific lipid transfer protein (nsLTP) family in Setaria italica. BMC Plant Biol. 2022, 22, 547. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Song, H.; Zhang, B.; Lu, Q.; Liu, Z.; Zhang, S.; Peng, R. Genome-wide identification, characterization, and expression analysis of superoxide dismutase (SOD) genes in foxtail millet (Setaria italica L.). 3 Biotech 2018, 8, 486. [Google Scholar] [CrossRef] [PubMed]
- Lai, D.; Yao, X.; Yan, J.; Gao, A.; Yang, H.; Xiang, D.; Cheng, J. Genome wide identification, phylogenetic and expression pattern analysis of GATA family genes in foxtail millet (Setaria italica). BMC Genom. 2022, 23, 549. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Dai, S.; Qin, N.; Zhu, C.; Qin, J.; Li, J. Genome-wide identification and expression analysis of the SAUR gene family in foxtail millet (Setaria italica L.). BMC Plant Biol. 2023, 23, 31. [Google Scholar] [CrossRef] [PubMed]
- Lai, D.; Fan, Y.; Xue, G.; He, A.; Yang, H.; He, C.; Cheng, J. Genome-wide identification and characterization of the SPL gene family and its expression in the various developmental stages and stress conditions in foxtail millet (Setaria italica). BMC Genom. 2022, 23, 389. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Fu, H.; Zhao, J.; Wang, J.; Dong, S.; Yuan, X.; Chen, M. Genome-Wide Identification and Expression Profiling of Glutathione S-Transferase Gene Family in Foxtail Millet (Setaria italica L.). Plants 2023, 12, 1138. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Chen, J.; Chen, S.; Zhang, H.; Li, L. Genome-Wide Identification and Characterization of Clavata3/Embryo Surrounding Region (CLE) Gene Family in Foxtail Millet (Setaria italica L.). Genes 2023, 14, 2046. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Liu, Y.W.; Zhou, J.M.; Zhao, S.P.; Zhang, X.H.; Min, D.H. Genome-wide analysis of the lectin receptor-like kinase family in foxtail millet (Setaria italica L.). Plant Cell Tissue Organ Cult. 2016, 127, 335–346. [Google Scholar] [CrossRef]
- Li, Y.; Yu, S.; Zhang, Q.; Wang, Z.; Liu, M.; Zhang, A.; Li, C. Genome-wide identification and characterization of the CCT gene family in foxtail millet (Setaria italica) response to diurnal rhythm and abiotic stress. Genes 2022, 13, 1829. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Wei, S.; Guo, Y.; Wu, Y. Genome wide identification of MPK and MKK gene families and their responses to phytohormone treatment and abiotic stress in foxtail millet. Plant Growth Regul. 2023, 99, 85–99. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, C.; Kang, X.; Pei, Z.Q.; Bai, X.; Wang, J.; Zhang, T.G. Identification and expression analysis of MAPK cascade gene family in foxtail millet (Setaria italica). Plant Signal. Behav. 2023, 18, 2246228. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.F.; Zhao, W.Y.; Fu, J.D.; Liu, Y.W.; Chen, M.; Zhou, Y.B.; Xi, Y.J. Genome-wide analysis of CDPK family in foxtail millet and determination of SiCDPK24 functions in drought stress. Front. Sci. Plant. Sci. 2018, 9, 651. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, L.; Li, W.; Zhang, X.; Zhang, Y.; Dong, S.; Yuan, X. Genome-Wide Identification and Expression Profiling of Cytochrome P450 Monooxygenase Superfamily in Foxtail Millet. Int. J. Mol. Sci. 2023, 24, 11053. [Google Scholar] [CrossRef] [PubMed]
- Lata, C.; Mishra, A.K.; Muthamilarasan, M.; Bonthala, V.S.; Khan, Y.; Prasad, M. Genome-wide investigation and expression profiling of AP2/ERF transcription factor superfamily in foxtail millet (Setaria italica L.). PLoS ONE 2014, 9, e113092. [Google Scholar] [CrossRef]
- Li, W.; Chen, M.; Wang, E.; Hu, L.; Hawkesford, M.J.; Zhong, L.; Ma, Y. Genome-wide analysis of autophagy-associated genes in foxtail millet (Setaria italica L.) and characterization of the function of SiATG8a in conferring tolerance to nitrogen starvation in rice. BMC Genom. 2016, 17, 797. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.J.; He, G.H.; Zheng, W.J.; Lu, P.P.; Chen, M.; Gong, Y.M.; Xu, Z.S. Foxtail millet NF-Y families: Genome-wide survey and evolution analyses identified two functional genes important in abiotic stresses. Front. Sci. Plant. Sci. 2015, 6, 1142. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cheng, J.; Han, H.; Sun, R.; Li, Y.; Zhang, Y.; Li, X. Genome-wide identification of the HKT transcription factor family and their response to salt stress in foxtail millet (Setaria italica). Plant Growth Regul. 2023, 99, 113–123. [Google Scholar] [CrossRef]
- Muthamilarasan, M.; Khandelwal, R.; Yadav, C.B.; Bonthala, V.S.; Khan, Y.; Prasad, M. Identification and molecular characterization of MYB transcription factor superfamily in C4 model plant foxtail millet (Setaria italica L.). PLoS ONE 2014, 9, e109920. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wei, Y.; Wang, Y.; Tan, B.; Chen, S.; Li, H. Genome-Wide Identification of LBD Genes in Foxtail Millet (Setaria italica) and Functional Characterization of SiLBD21. Int. J. Mol. Sci. 2023, 24, 7110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yu, Y.; Song, T.; Zhang, M.; Li, N.; Yu, M.; Zhang, X. Genome-wide identification of foxtail millet’s TRX family and a functional analysis of SiNRX1 in response to drought and salt stresses in transgenic Arabidopsis. Front. Sci. Plant. Sci. 2022, 13, 946037. [Google Scholar] [CrossRef] [PubMed]
- Xing, G.; Jin, M.; Qu, R.; Zhang, J.; Han, Y.; Han, Y.; Zhao, X. Genome-wide investigation of histone acetyltransferase gene family and its responses to biotic and abiotic stress in foxtail millet (Setaria italica [L.] P. Beauv). BMC Plant Biol. 2022, 22, 292. [Google Scholar] [CrossRef] [PubMed]
- Chai, W.; Si, W.; Ji, W.; Qin, Q.; Zhao, M.; Jiang, H. Genome-wide investigation and expression profiling of HD-zip transcription factors in foxtail millet (Setaria italica L.). BioMed Res. Int. 2018, 2018, 8457614. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Varshney, V.; Tak, N.; Jha, S. Genome-wide identification and expression analysis of glycogen synthase kinase encoding genes in foxtail millet (Setaria italica L.) under salinity, dehydration, and oxidative stress. Plant Stress 2023, 8, 100165. [Google Scholar] [CrossRef]
- Cheng, J.; Tan, H.; Shan, M.; Duan, M.; Ye, L.; Yang, Y.; Wang, X. Genome-wide identification and characterization of the NPF genes provide new insight into low nitrogen tolerance in Setaria. Front. Sci. Plant. Sci. 2022, 13, 1043832. [Google Scholar] [CrossRef] [PubMed]
- Huangfu, Y.; Pan, J.; Li, Z.; Wang, Q.; Mastouri, F.; Li, Y.; Liu, W. Genome-wide identification of PTI1 family in Setaria italica and salinity-responsive functional analysis of SiPTI1–5. BMC Plant Biol. 2021, 21, 319. [Google Scholar] [CrossRef]
- Gong, L.; Li, B.; Zhu, T.; Xue, B. Genome-wide identification and expression profiling analysis of DIR gene family in Setaria italica. Front. Sci. Plant. Sci. 2023, 14, 1243806. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Guo, Y.; Wang, H.; Wang, X.; Lv, M.; Yang, P.; Zhang, L. Identification and characterization of Shaker K+ Channel Gene Family in Foxtail Millet (Setaria italica) and their role in stress response. Front. Sci. Plant. Sci. 2022, 13, 907635. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chai, Y.; Liu, J.; Zheng, J.; Zhao, Z.; Amo, A.; Hu, Y.G. Amino acid transporter (AAT) gene family in foxtail millet (Setaria italica L.): Widespread family expansion, functional differentiation, roles in quality formation and response to abiotic stresses. BMC Genom. 2021, 22, 519. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhao, Y.; Zhang, J.; Li, X.; Ma, F.; Duan, M.; Li, H. The responses of the lipoxygenase gene family to salt and drought stress in foxtail millet (Setaria italica). Life 2021, 11, 1169. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, J.; Li, M.; Li, Y.; Zhao, Z.; Li, C.; Yue, J. Genome-wide characterization of remorin genes in terms of their evolution and expression in response to hormone signals and abiotic stresses in foxtail millet (Setaria italica). Diversity 2022, 14, 711. [Google Scholar] [CrossRef]
- Nadeem, F.; Ahmad, Z.; Wang, R.; Han, J.; Shen, Q.; Chang, F.; Li, X. Foxtail millet [Setaria italica (L.) Beauv.] grown under low nitrogen shows a smaller root system, enhanced biomass accumulation, and nitrate transporter expression. Front. Sci. Plant. Sci. 2018, 9, 205. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Tang, S.; Zhi, H.; Xing, L.; Zhang, H.; Tang, C.; Diao, X. The boron transporter SiBOR1 functions in cell wall integrity, cellular homeostasis, and panicle development in foxtail millet. Crop J. 2022, 10, 342–353. [Google Scholar] [CrossRef]
- Alagarasan, G.; Dubey, M.; Aswathy, K.S.; Chandel, G. Genome wide identification of orthologous ZIP genes associated with zinc and iron translocation in Setaria italica. Front. Sci. Plant. Sci. 2017, 8, 775. [Google Scholar] [CrossRef] [PubMed]
- Blume, R.; Yemets, A.; Korkhovyi, V.; Radchuk, V.; Rakhmetov, D.; Blume, Y. Genome-wide identification and analysis of the cytokinin oxidase/dehydrogenase (ckx) gene family in finger millet (Eleusine coracana). Front. Genet. 2022, 13, 963789. [Google Scholar] [CrossRef] [PubMed]
- Gu, D.; Andreev, K.; Dupre, M.E. Major trends in population growth around the world. China CDC Wkly. 2021, 3, 604. [Google Scholar] [CrossRef] [PubMed]
- Hemathilake, D.M.K.S.; Gunathilake, D.M.C.C. Agricultural productivity and food supply to meet increased demands. In Future Foods Global Trends, Opportunities, and Sustainability Challenges; Academic Press: Cambridge, MA, USA, 2022; pp. 539–553. [Google Scholar]
- Antony Ceasar, S.; Maharajan, T.; Ajeesh Krishna, T.P.; Ramakrishnan, M.; Victor Roch, G.; Satish, L.; Ignacimuthu, S. Finger millet [Eleusine coracana (L.) Gaertn.] improvement: Current status and future interventions of whole genome sequence. Front. Sci. Plant Sci. 2018, 9, 1054. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.M.; Arora, S.; Mirza, N.; Pande, A.; Lata, C.; Puranik, S.; Kumar, A. Finger millet: A “certain” crop for an “uncertain” future and a solution to food insecurity and hidden hunger under stressful environments. Front. Plant Sci. 2017, 8, 643. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).