Abstract
Invasive plant species have been recognized as adversely affecting native ecosystems. Some of these plant species become problematic in disturbed environments such as urbanized, agricultural, and abandoned developed or farmed land. In some cases, they can dominate the invaded ground, preventing a transition back to the native plant community. In Tenerife (Canary Islands), the invasive plant species Ulex europaeus L. has established dense infestations in abandoned agricultural lands. Removing such invasive species in Tenerife through ecological restoration is crucial for restoring ecosystem functionality and promoting biodiversity. This study evaluates various management methods for U. europaeus in abandoned fields, assessing their impact on species richness, diversity, composition, and regeneration. The findings can inform management strategies to combat this invasive species, contributing to biodiversity conservation and ecosystem resilience. The study was conducted in two highly invaded areas, evaluating chemical (C), mechanical (M), mechanical and chemical (MC), and mechanical, chemical, and plantation treatments (MCP), along with a control, to analyze changes in species richness, diversity, and plant community. Results indicate that U. europaeus remains capable of reoccupying treated areas. The different treatments led to differences in species richness and composition. The MCP treatment yielded the best results if the planted native species grew faster than Ulex europaeus, preventing its establishment due to its shade-intolerant nature. However, continual control is required to eliminate U. europaeus regeneration from seeds that can persist for at least 30 years.
1. Introduction
Urbanization and the abandonment of agricultural land can significantly transform land use in developed countries [1]. These changes have the potential to deplete biodiversity and cultural values [2,3]. Furthermore, increases in land abandonment are expected in the near future [4].
In developed nations, the cessation of extensive farming has notably contributed to the expansion of dry grasslands and dwarf shrublands on marginal lands [5], while in other cases, it has resulted in the recovery of native forests [4]. Indeed, in certain circumstances, positive outcomes can emerge from land abandonment, such as alterations in vegetation cover that positively impact water availability and soil properties [6,7]. Thus, abandoned agricultural lands could benefit from restoration efforts. Moreover, the historical prevalence of intensive agriculture and fast environmental change pose challenges to the natural recovery of original plant communities. Consequently, restoring these abandoned fields is imperative [8,9].
Following abandonment, the dominance of specific species, including exotic ones, can influence vegetation-recovery patterns in disturbed sites [9], even when native species have sufficient propagules for natural recolonization [10,11,12], hence the need for a range of management tools to help restore ecological processes within the plant community, including native species reintroduction and regeneration to increase species richness [13,14].
Ecological restoration, particularly after the removal of exotic species, constitutes a key strategy for biodiversity conservation and ecosystem recovery. The benefits of such restoration efforts are multifaceted. Firstly, eliminating invasive species reduces the pressure they exert on native flora and fauna, allowing indigenous species to reclaim their ecological niches [15]. This process contributes to the restoration of ecosystem functionality, enhancing resilience, and promoting overall biodiversity. Secondly, ecological restoration often leads to the recovery of ecosystem services crucial for human well-being, such as water purification, pollination, and carbon sequestration [16]. These restored services not only benefit local communities but also bolster the broader ecological integrity of the landscape.
Furthermore, successful restoration initiatives provide valuable learning experiences, giving insights into effective conservation strategies and management practices [17]. By documenting and disseminating the outcomes of these efforts, the scientific community can refine and optimize restoration methodologies. In addition, ecological restoration following the removal of exotic species not only fosters biodiversity conservation but also advances our understanding of ecosystem dynamics and resilience, contributing to the sustainable management of Earth’s precious natural resources.
In the case of Tenerife, Ulex europaeus L., commonly known as gorse, has emerged as a challenging invasive species to control in the Canary Islands. This issue became particularly evident when studies documented its dispersion along the northern regions of Tenerife [18,19]. Recognized as one of the most invasive shrubs in the world, it has various effects, impacting soil acidity, increasing fire rates, and reducing native species and species richness, to name a few [20]. The probable introduction of U. europaeus in the Canary Islands dates to the 1960s—although previous records exist [21]—coinciding with the importation of trees, primarily Pinus radiata or Eucalyptus camaldulensis–Eucalyptus globulus, for reforestation purposes [22]. Currently, Ulex europaeus is naturalized only on the island of Tenerife [18,19], but efforts to control its spread have been unsuccessful, and several studies suggest that the species range is expanding [23,24,25].
Field abandonment and the wildfire of 2007, which affected over 19,000 ha, resulted in an unprecedented germination and establishment of this species in many abandoned fields in the following months. In this study, we analyze different eradication treatments to evaluate a management program for U. europaeus in these invaded areas. Four different treatments: mechanical (hereafter M), chemical (hereafter C), mechanical and chemical (hereafter MC), and mechanical, chemical, and plantation (hereafter MCP) were applied and evaluated, with an additional control group. We studied an area affected by the 2007 wildfire. We investigated species richness, diversity, species composition and the impact of the above treatments on regeneration. Based on these results, some proposals will be put forward to managers to deal with the management of U. europaeus.
2. Materials and Methods
2.1. Study Area
This study was conducted in two locations in the northern region of Tenerife, Canary Islands (Figure 1). Both are situated at an altitude of between 1071 and 1180 m, in two municipalities (Pista al Lomo Cho Rosado–Los Realejos for block 1 and Finca de El Guarda-San Juan de la Rambla for block 2), within the PNS (Protected Natural Space) Paisaje Protegido de Tigaiga, Campeches and Ruíz [26]. It is a region above the town of Icod El Alto, traditionally dedicated to agricultural activity [27]. Bioclimatically, it falls within the subhumid Mediterranean pluvial bioclimatic zone [28], with average temperatures of 15 °C and total annual precipitation around 560 mm/year [29]. The potential vegetation in the area corresponds to the Canarian humid Monteverde (Lauro novocanariensis-Perseetum indicae, [26]), although the presence of replacement fayal-brezal vegetation is common in this highly anthropized area. Additionally, in these terraced zones, there is vegetation consisting of perennial grasslands, brambles, and ferns, belonging to this plant community [29]. During the sampling periods, there were no deviations from the average values for precipitation and rainfall (www.aemet.es, accessed on 29 February 2023).
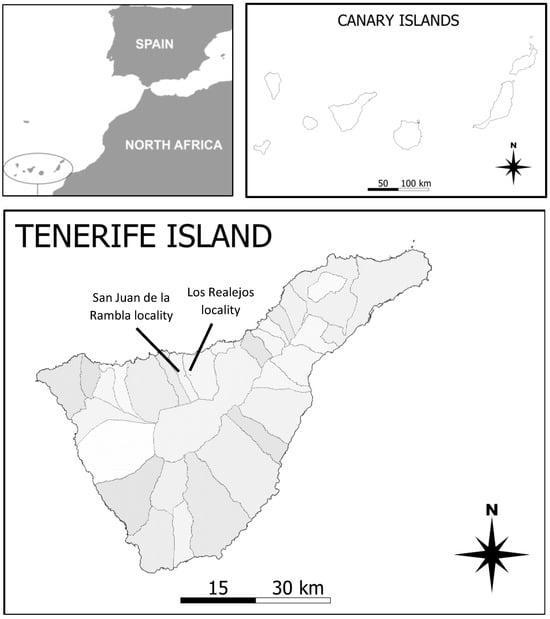
Figure 1.
Location of the Canary Islands with respect to Africa, the Canary Island Archipelago, and the location of the two study sites on Tenerife (areas indicate municipalities).
2.2. The Species
Ulex europaeus, a highly invasive evergreen shrub, is widely recognized as one of the most problematic invasive species globally [30,31]. Introduced in the 19th century, it now spans from equatorial to temperate regions, colonizing diverse areas, such as Australia, New Zealand, South America, California, and South Africa [32]. It thrives in regions with monthly temperatures consistently above freezing, although it has also established itself in coastal regions of Europe and the Americas at higher latitudes [33,34]. Growing best in moderate to high annual rainfall (500 to 1500 mm/year), U. europaeus can endure dry spells during summer thanks to its deep root system and water-conserving features [30,35].
U. europaeus persists throughout the year; its seeds are particularly stimulated by fires, as evidenced by the 2007 wildfire on the island’s north side [35]. Its spread in non-native environments is facilitated by factors such as high reproductive fitness, prolonged seed longevity, rapid growth, and the absence of native competitors [36]. Its dispersal alters soil dynamics, hindering the growth of agricultural and native species, providing shelter for pests, and diminishing species richness, leading to economic and environmental degradation [30,37]. Also, U. europaeus depletes soil nutrients, exacerbating vegetation flammability, and complicating fire management in invaded areas [38,39]. Consequently, its invasion negatively impacts Tenerife’s native habitat and obstructs the regeneration of native plant communities in abandoned fields (personal communication of A. Naranjo-Cigala). From previous studies, its spatial distribution is increasing on the island of Tenerife, especially in the north of the island [18,22].
2.3. Sampling Design
Two blocks of plots were located with similar environmental conditions and highly invaded by U. europaeus. This area was affected by the wildfire in 2007, so this stand is likely to be 13 years old. In the selected plots, the cover by the species ranges between 80 and 100% (we selected all the plots covered homogenously by U. europaeus). Before the fire, the area was dominated by ruderal species of forbs and shrubs. In general, native species that occupied the abandoned fields were Cistus symphytifolius, Rumex maderensis, Adenocarpus foliolosus, or Sonchus canariensis, accompanied by other ruderal grasses and forbs. To evaluate the possible differences between different management treatments, a total of 15 homogeneous plots of 10 × 10 m were established in each location, separated by 10 m in all directions from the nearest plots. The treatments were applied in September–October 2018. In each plot, selected randomly, a specific eradication treatment (replicated three times) was carried out on the U. europaeus specimens. The eradication treatments tested were the following: Mechanical (M), which involved removing the specimens through mechanical cutting at ground level using specialized machinery, chainsaw and brush cutter (for an effective clear cutting). Chemical (C) treatment that entailed a non-specific and systemic herbicide (Touchdown Plus product (36% w/v Glyphosate (ammonium salt) (360 g/L)); this was applied once in both blocks on two days. The application of the product was carried out on the specimens that remained within the plots, or to their remains in the randomly mechanically treated plots. The product was applied following the instructions of the brand used, with a spray gun, and the operator carried out the treatment with the usual IPE (Individual Protection Equipment) used in this type of treatment and following the recommended doses by the manufacturer (10% of the commercial product). Mechanical and chemical (MC) treatment involved mechanically removing all the plants, and then the herbicide was applied to the remains of the target species. Additionally, a mechanical, chemical, and plantation (MCP) treatment was used. Following mechanical and chemical treatments, native species were planted. The species used were Pinus canariensis C. Sm. ex DC. (canary pine), Erica canariensis Rivas-Mart., M. Osorio and Wildpret (brezo), Morella faya (Aiton) Wilbur (faya), Teline canariensis (L.) Webb and Berthel (retamón), and Cistus symphytifolius Lam. (jara de pinar), all of them obtained from the seeds produced in the same area and grown in a nursery over the previous year (two of each species per plot). Finally, we established control plots where there was no intervention (Con) (Figure 2).

Figure 2.
Treatments applied in plots invaded by Ulex europaeus in Tenerife. (a) Control (Con), with U. europaeus individuals inside the plot (behind the field worker); (b) mechanical control (M), where the delimited plot is observed from the front of the field worker after clearing all the vegetation; (c) mechanical and chemical control (MC), in this case, systematic herbicide was applied in this plot (behind the field worker) after clearing; (d) mechanical, chemical, and planting control (MCP), after clearing and applying herbicide, planting was carried out, as shown in the figure, with the plants protected from herbivores; and (e) chemical (C), in which the herbicide was applied directly to the U. europaeus plants (aspect from the corner of the plot).
2.4. Statistical Analyses
Data on species richness, composition, and evenness for the months of July 2019 and July 2020 were analyzed. For evenness, the Smith and Wilson evenness index was used, which is considered a robust indicator of the distribution of the abundance among different species [40].
We represented the species richness variation (on average) and evenness index estimated of the three plots of each treatment and each block for July 2019 (number 1 in the figures) and July 2020 (number 2 in the figures). GLM was used with the dependent variable being species richness and using the year as the fixed factor and the block as a random factor to determine if there were differences among the treatments for both blocks (p < 0.05). The same GLM analyses were performed for evenness.
Ordination techniques can help explain community variation [41] and were used to assess temporal and spatial trends [42,43,44]. Detrended Correspondence Analysis (DCA) [45] was performed to examine how species composition changed over space and time depending on the treatment applied. The main matrix was used for this analysis. Species composition is shown for each plot in each block in the month of July for both 2019 and 2020, approximately 9 months after plot establishment and treatment application in the case of 2019. We focused on the richness of the understory, but also included the presence of woody species rooting in the plot.
Finally, the average regeneration of Ulex europaeus following the three treatments (M, MC, and MCP) was plotted and analyzed using an ANOVA test, followed by a group comparison in each block (p < 0.05). In this case, we only used the information of the second year, July 2020. Normality was previously tested with Shapiro–Wilk and homoscedasticity tested with the Levenne test (p < 0.05). CANOCO 5.1. was used for the multivariate analyses [45] and basic statistical methods according to Legendre and Legendre [46] and was performed with the SPSS v.29 statistical package [47].
3. Results
Both blocks exhibited low environmental variability, with similar altitudes, aspects, and slopes within the same bioclimatic area (Table 1). U. europaeus was dominant in both blocks (cover of 85–100%). Regarding cover, some variations were noted, with more grasses in block 2. Monitoring of the plots commenced in December 2018 and continued until July 2020, with treatments applied in September–October 2018. A total of 127 taxa were identified in this study (Appendix A). All the species planted in the MCP treatment remained alive and in good shape 18 months after planting. The number of species varied greatly depending on the season. Our analyses focused on the months of July 2019 and 2020, as this is the month when most species were present and were most easily identifiable.

Table 1.
Physical characterization of the study block (information from October 2018).
The analyses revealed differences in species richness between treatments, with the year as the fixed factor and the blocks as the random factor (F4,60 = 27.88, p < 0.001 for treatment factor; F1,60 = 5.88, p < 0.05 for the fixed factor year, and F1,60 = 0.253, p > 0.05 for the random factor block). Post-hoc Tukey tests revealed that Con and C treatments were different from the other treatments, with lower species richness, while M and MC treatments were different from MCP treatment, which presented the highest richness values (Figure 3). Regarding the planting of five species in the MCP treatment, for evenness, the same analysis revealed no differences (F4,60 = 0.525, p > 0.05 for treatment factor; F1,60 = 18.024, p > 0.05 for the fixed factor year, and F1,60 = 18.024, p > 0.05 for the random factor block; see Figure 4).
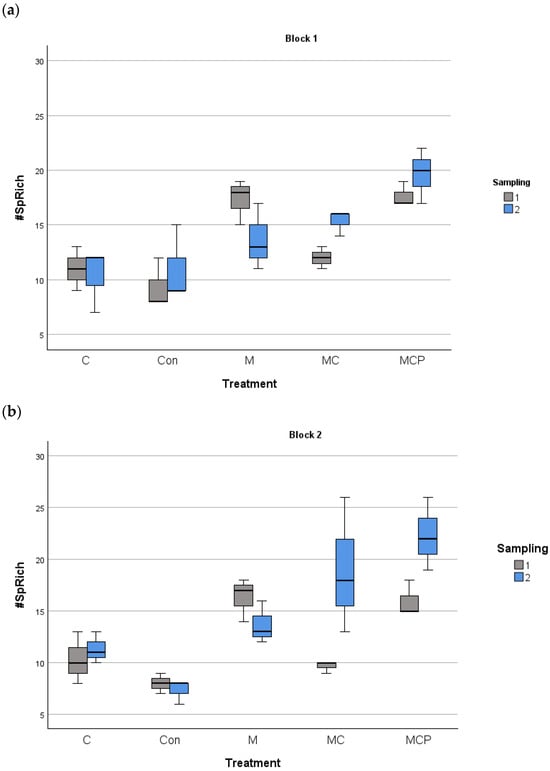
Figure 3.
Species richness in the 100 m2 plots for block 1 (a) and block 2 (b) for different treatments during the two samplings periods (July 2019 as number 1 and July 2020 as number 2). The median, the quartiles, and the range of values covered by the data are presented.
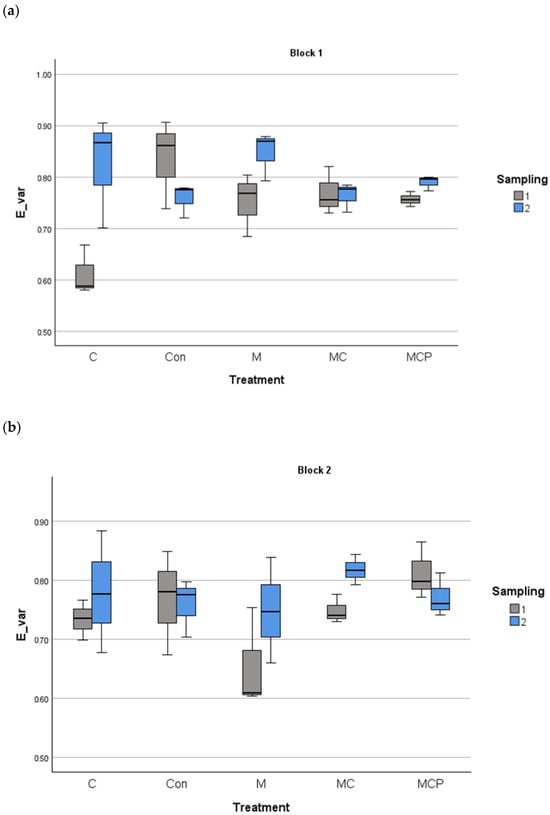
Figure 4.
Evenness index of Smith and Wilson in the 100 m2 plots for block 1 (a) and block 2 (b) for different treatments in the two sampling periods (July 2019 and July 2020). The median, the quartiles, and the range of values covered by the data are presented.
As for species composition, Detrended Correspondence Analysis (DCA) revealed that the year did not discriminate species composition in July 2019 and 2020 (Figure 5). In the case of the blocks, differences were revealed in the bidimensional space of axes I and II of the DCA. Although there is a large variability present, axis I clearly separates both groups of plots (Figure 6).
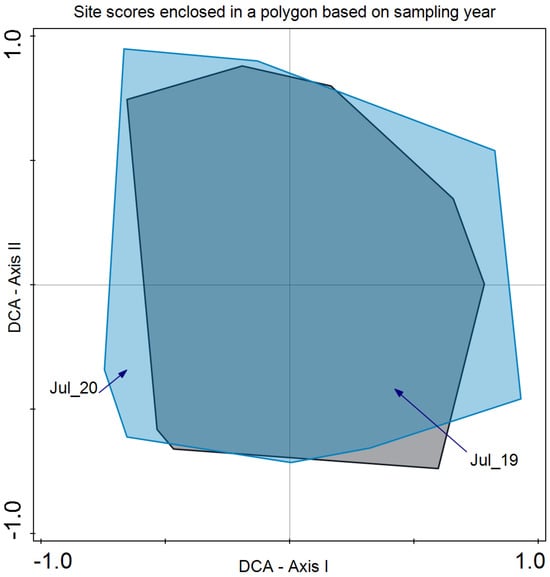
Figure 5.
Detrended Correspondence Analysis (DCA) with the site scores enclosed in a polygon for the same sampling period (July 2019 and July 2020). Eigenvalue for axis I: 0.267 and axis II: 0.185 with an explained inertia of 19.7% for both axes.
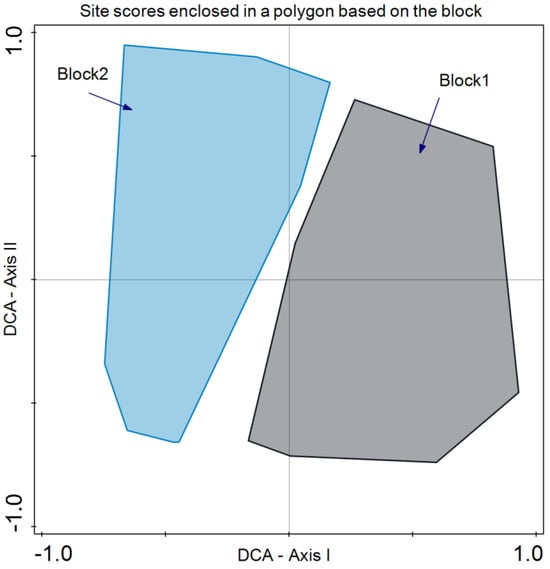
Figure 6.
Detrended Correspondence Analysis (DCA) with the site scores enclosed in a polygon for the same sampling block (blocks 1 and 2).
Finally, we enclosed the information on treatment, block, and year in different polygons (Figure 7). Although both blocks are determined by this analysis, the impact on species compositions of different treatments follows a similar pattern. In the case of control plots for both blocks, discrimination of the different treatments is apparent along axis II, with lower values for the plots. In the case of chemical treatment for block 1 (C1), dominant species include Geranium purpureum, Erica canariensis, and Oxalis pes-caprae, while for Con1, only the last two species, E. canariensis and O. pes-caprae, are representative. In the case of Con2 and C2 treatments of block 2, discrimination is low, with Adenocapus foliolosus, Vicia scandens, Agrostis castellana, and Cistus symphytifolius as characteristic species of these treatments. Along axis II, the treatment is mechanical, which in the case of block 1 (M1) has as dominant species Rumex maderensis, Briza maxima, Sonchus tenerrimus, and Andryala pinnatifida among others. Conversely, in block 2 (M2), dominant species are Tuberaria guttata, Brachypodium sylvaticum, and Orignaum vulgare.
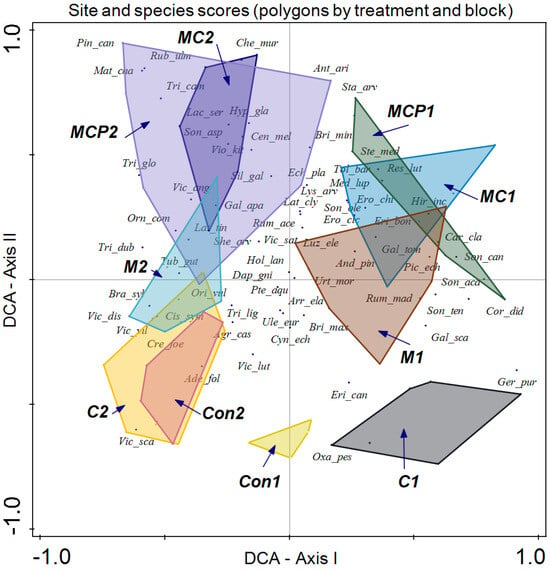
Figure 7.
Detrended Correspondence Analysis (DCA) with the site scores enclosed in a polygon for the same sampling treatment and block (treatment following the acronym indicated in the Materials and Methods section, followed by block number) and species scores (species names are indicated with the three first letters of the genus followed by the first three letters of the specific epithet).
MC and MCP treatments showed low discrimination. In block 1, both treatments (MC1 and MCP1) displayed dominant species such as Reseda luteola, Medicago lupulina, Tolpis barbata, Stellaria media, and Sonchus canariensis, while in block 2 (MC2 and MCP2), dominant species included Galium aparine, Silene gallica, Chenopoidiastrum murale, Rubus ulmifolius, Trifolium campestre, T. glomeratum, and Vicia angustifolia.
Regarding regeneration, this only occurred in the M and MC and MCP treatments. For the Con and C, the cover of the remaining Ulex europaeus individuals prevented the germination of new individuals of the species (Table 1, cover of almost 100% of U. europaeus; Figure 8). In the other treatments, we had regeneration of over 0.5 seedlings/1 m2, with a maximum of 5.2 seedlings in one of the plots. There were no differences in any of the blocks for regeneration density between treatments (F2,6 = 0.039, p > 0.05 and F2,6 = 0.041, p > 0.05 for blocks 1 and 2, respectively).
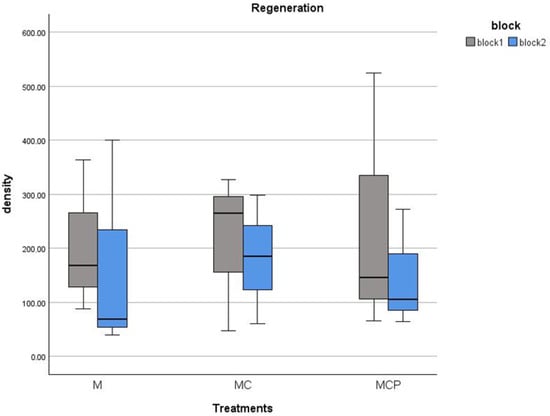
Figure 8.
Regeneration density (individuals/100 m2) for July 2020 of Ulex europaeus in the treatment plots (separately for each block).
4. Discussion
The eradication of Ulex europaeus poses significant challenges due to its highly invasive nature and robust reproductive strategies [30,31]. This species rapidly colonizes disturbed areas, forming dense thickets that outcompete native vegetation and disrupt ecosystem dynamics. Its ability to regenerate from seeds and vigorously resprout from its extensive root system contributes to its persistence and makes eradication efforts particularly challenging [37]. Moreover, U. europaeus has allelopathic properties, releasing chemicals that inhibit the growth of other plant species, further hindering restoration endeavors [48]. Despite the implementation of various control methods such as manual removal, herbicide application, and biological control agents, achieving complete eradication of U. europaeus remains elusive in many regions [20,38]. Comprehensive management strategies integrating multiple approaches are necessary to effectively address the complex challenges associated with controlling this invasive species.
In our study, the species Ulex europaeus can now be considered established and imbricated in ecological processes, as evidenced by its dispersion over the last 30 years [18,19]. Its fast dispersion can be related to a second phase of invasion characterized by the dominance of biotic relationships among the species within the community as highlighted by the invasion model of Dietz and Edwards [49].
Regarding treatments, the results for one year confirmed the persistence of the species and its high regeneration levels. Leguminous exotic species demonstrate remarkable persistence in plant communities owing to the enduring viability of their seeds. This resilience has been extensively documented in scientific literature. For instance, Baskin and Baskin [50] underline the significant role of seed longevity in the ecological success of leguminous species, emphasizing how seeds contribute to their persistence within diverse ecosystems. Similarly, Fenner [51] underscored the importance of seed persistence in shaping plant community dynamics, particularly highlighting the competitive advantage conferred by the long viability of leguminous seeds, which enhances their ability to colonize and dominate new habitats. In our study, regeneration was observed in all treatments except for the Con and C treatments due to the cover (around 100% of the plot) that prevents germination of this shade-intolerant species [33].
In the case of species richness, the treatments had a different impact on plant community. For Con and C treatment, the cover and dominance of U. europaeus had a negative impact on the number of species (these treatments presented the lowest number of species). Glyphosate is a very potent herbicide [52], whose usefulness in weed control has been recognized for over 25 years [53,54]. Nevertheless, as it is not specific, an analysis of its impact in the plant community is required before generalizing its use.
However, M eradication of U. europaeus (clear cutting the treated plots for this species) allowed solar irradiation on the surface, and increased the number of species, so there was an increase from 10 to 15 species (Figure 4a,b). In our case, there were differences in the impact of M, MC and MCP treatments from Con and C, with higher values of richness. Some studies have determined positive impact of the herbicide when used to eradicate invasive species [55] on native plant community composition.
Regarding species composition, the plots of both blocks were graphically represented, and each block was enclosed in a polygon (Figure 6). This determined that despite similar plant community and environmental conditions, both sites differed in species composition. It is important to note that even small variations in environmental conditions or natural history can result in important changes in species composition, as in this case ([56]; Figure 6). In the same analysis, we enclosed all the plot samples from July 2020 and July 2021 separately (Figure 5). In this case, no discrimination was found, thus indicating similar species composition. Finally, we enclosed in a polygon the plots sampled in both periods with the same treatment and block. The results were relevant, indicating differences between treatments in addition to variations between blocks. However, the response of species composition to treatment followed a similar pattern. First, it can be observed that both blocks are separated along axis I, which accounted for the primary source of variability. Second, treatments were discriminated along axis II (Figure 7). Con and C appeared at lower scores on axis II, with a lower number of dominant species in proximity. In the case of Con and C treatments in block 2, there were almost no differences, while in block 1, these treatments determined some differences, with hardly any dominant species in control 1. Along axis II, the next treatment was M, and finally, along axis II, again in both blocks, it was MC and MCP. Axis II is a good discriminant for the treatments in both blocks.
With respect to species scores, Ulex europaeus remains near the center of the coordinates, indicating its presence in all treatments, but with a lower dominance in MC and MCP treatments.
5. Conclusions
Although the treatments present significant effects on species richness and composition, the presence of the target species, U. europaeus, was still significant, although it was relatively reduced following the MC and MCP treatments. Each treatment has different effects, and they can be easily distinguished by species composition. Under natural conditions, Ulex europaeus seeds can remain dormant for extended periods, ranging from months to several years. The hard seed coat of gorse seeds is highly resistant to moisture and mechanical damage, allowing them to remain in the soil seed bank for extended periods until conditions become suitable for germination [57].
Based on these results, intensive and continuous work will be necessary not just to eradicate, but to control the dispersion of this species. Although not revealed by our results, treatment with plantation offers great potential in the long term, as it can provide shade, thus preventing germination. As for the present, early detection of any new species is an essential part of management. We can assume that the most potentially successful treatment for now is mechanical eradication, implemented alongside a plantation program. We have been analyzing the short-term response of the plant community, but further monitoring will be necessary to identify effective tools for controlling this species, which is considered one of the major plant pests on the planet.
Author Contributions
Conceptualization, J.R.A. and C.G.-M.; methodology, J.R.A.; validation, J.R.A.; formal analysis, J.R.A. and C.G.-M.; investigation, J.R.A., M.A.P.-M. and Z.N.-P.; resources, J.R.A. and M.G.; data curation, J.R.A., M.A.P.-M. and C.G.-M.; writing—original draft preparation, J.R.A. and C.G.-M.; writing—review and editing, J.R.A. and C.G.-M.; visualization, J.R.A. and M.G.; supervision, J.R.A.; project administration, J.R.A.; funding acquisition, J.R.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Fundación CajaCanarias and Fundación La Caixa, grant number 2017REC16. We also thank the “Cabildo Insular de Tenerife” and GESPLAN S.L. for the support of the project.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A

Table A1.
Species scientific name, family, endemic character, origin, Raunkiaer biotypes, and life forms found in this study.
Table A1.
Species scientific name, family, endemic character, origin, Raunkiaer biotypes, and life forms found in this study.
| Taxa | Family | Endemism | Origin 1 | Raunkiaer Biotypes | Life Form |
|---|---|---|---|---|---|
| Adenocarpus foliolosus (Aiton) DC. | Fabaceae | Yes | SN | Nanophanerophyte | Shrub |
| Agrostis castellana Boiss. & Reut. | Poaceae | No | PN | Hemicryptophyte | Perennial herb |
| Aira caryophyllea L. | Poaceae | No | PN | Therophyte | Herb |
| Andryala pinnatifida Aiton | Asteraceae | No | SN | Therophyte | Herb |
| Anogramma leptophylla (L.) Link | Hemionitidaceae | No | SN | Therophyte | Herb |
| Anthoxanthum aristatum Boiss. | Poaceae | No | PN | Therophyte | Herb |
| Apiaceae sp. | Apiaceae | ||||
| Arrhenatherum elatius (L.) P. Beauv. ex J. Presl & C. Presl subsp. bulbosum (Willd.) Schübl. & Mart. | Poaceae | No | PN | Hemicryptophyte | Perennial herb |
| Brachypodium sylvaticum (Huds.) P. Beauv. | Poaceae | No | PN | Therophyte | Herb |
| Briza maxima L. | Poaceae | No | PN | Therophyte | Herb |
| Briza minor L. | Poaceae | No | PN | Therophyte | Herb |
| Bromus diandrus Roth | Poaceae | No | PN | Therophyte | Herb |
| Bromus hordeaceus L. | Poaceae | No | PN | Therophyte | Herb |
| Calendula arvensis (Vaill.) L. | Asteraceae | No | SIN | Therophyte | Herb |
| Carduus clavulatus Link | Asteraceae | Yes | SN | Therophyte | Herb |
| Carduus tenuiflorus Curtis | Asteraceae | No | PN | Hemicryptophyte | Perennial herb |
| Centaurea melitensis L. | Asteraceae | No | PN | Therophyte | Herb |
| Centranthus calcitrapae (L.) Dufr. | Valerianaceae | No | PN | Therophyte | Herb |
| Cerastium glomeratum Thuill. | Caryophyllaceae | No | PN | Therophyte | Herb |
| Chenopodiastrum murale (L.) S. Fuentes, Uotila & Borsch | Chenopodiaceae | No | PI | Therophyte | Herb |
| Cistus symphytifolius Lam. | Cistaceae | Yes | SN | Nanophanerophyte | Shrub |
| Lepidium didymum L. | Brassicaceae | No | PI | Hemicryptophyte | Perennial herb |
| Cotula australis (Spreng.) & Hook. f. | Asteraceae | No | SIN | Therophyte | Herb |
| Crepis foetida L. | Asteraceae | No | PN | Hemicryptophyte | Perennial herb |
| Cynosurus echinatus L. | Poaceae | No | PN | Therophyte | Herb |
| Daphne gnidium L. | Thymelaeaceae | No | SN | Chamaephyte | Shrub |
| Echium plantagineum L. | Boraginaceae | No | PN | Hemicryptophyte | Perennial herb |
| Erica canariensis Rivas-Mart., M. Osorio & Wildpret | Ericaceae | No | SN | Microphanerophyte | Large shrub—tree |
| Erigeron bonariensis L. | Asteraceae | No | SIN | Therophyte | Herb |
| Erodium botrys (Cav.) Bertol. | Geraniaceae | No | PN | Therophyte | Herb |
| Erodium chium (L.) Willd. | Geraniaceae | No | PN | Therophyte | Herb |
| Erodium cicutarium (L.) L’Hér. in Aiton | Geraniaceae | No | PN | Therophyte | Herb |
| Euphorbia peplus L. | Euphorbiaceae | No | PN | Therophyte | Herb |
| Filago pyramidata L. | Asteraceae | No | PN | Therophyte | Herb |
| Fumaria cf. bastardii Boreau | Fumariaceae | No | PN | Therophyte | Herb |
| Fumaria montana J.A. Schmidt | Fumariaceae | No | SN | Therophyte | Herb |
| Fumaria sp. | Fumariaceae | Therophyte | Herb | ||
| Galactites tomentosus Moench | Asteraceae | No | PN | Therophyte | Herb |
| Galium aparine L. | Rubiaceae | No | PN | Therophyte | Herb |
| Galium parisiense L. | Rubiaceae | No | PN | Therophyte | Herb |
| Galium scabrum L. | Rubiaceae | No | PN | Chamaephyte | Small shrub |
| Geranium grex molle L. | Geraniaceae | Therophyte | Herb | ||
| Geranium molle L. | Geraniaceae | No | PN | Therophyte | Herb |
| Geranium purpureum Vill. | Geraniaceae | No | PN | Therophyte | Herb |
| Geranium rotundifolium L. | Geraniaceae | No | PN | Therophyte | Herb |
| Geranium sp. | Geraniaceae | ||||
| Hirschfeldia incana (L.) Lagr.-Foss. | Brassicaceae | No | PN | Hemicryptophyte | Perennial herb |
| Holcus lanatus L. | Poaceae | No | PN | Hemicryptophyte | Perennial herb |
| Hypochaeris glabra L. | Asteraceae | No | PN | Therophyte | Herb |
| Juncus sp. | Juncaceae | ||||
| Lactuca serriola L. | Asteraceae | No | PN | Hemicryptophyte | Perennial herb |
| Lathyrus clymenum L. | Fabaceae | No | PI | Therophyte | Herb |
| Lathyrus sp. | Fabaceae | ||||
| Lathyrus sphaericus Retz. | Fabaceae | No | PN | Therophyte | Herb |
| Lathyrus tingitanus L. | Fabaceae | No | PI | Therophyte | Herb |
| Leontodon sp. | Asteraceae | ||||
| Luzula elegans Lowe | Juncaceae | No | SN | Therophyte | Herb |
| Lysimachia arvensis (L.) U. Manns & Anderb. | Primulaceae | No | PN | Therophyte | Herb |
| Lysimachia linum-stellatum L. | Primulaceae | No | PN | Therophyte | Herb |
| Matricaria chamomilla L. | Asteraceae | No | SIN | Therophyte | Herb |
| Medicago lupulina L. | Fabaceae | No | PI | Hemicryptophyte | Perennial herb |
| Melilotus sp. | Fabaceae | ||||
| Myosotis ramosissima Rochel | Boraginaceae | No | PN | Therophyte | Herb |
| Neotinea maculata (Desf.) Stearn | Orchidaceae | No | PN | Geophyte | Bulbous perennial herb |
| Origanum vulgare L. | Lamiaceae | No | PN | Chamaephyte | Shrub |
| Ornithopus compressus L. | Fabaceae | No | PN | Therophyte | Herb |
| Ornithopus pinnatus (Mill.) Druce | Fabaceae | No | PI | Therophyte | Herb |
| Oxalis pes-caprae L. | Oxalidaceae | No | SII | Geophyte | Bulbous perennial herb |
| Pallenis spinosa (L.) Cass. | Asteraceae | No | PN | Hemicryptophyte | Perennial herb |
| Papaver pinnatifidum Moris | Papaveraceae | No | PN | Therophyte | Herb |
| Papaver somniferum L. subsp. setigerum (DC.) Arcang. | Papaveraceae | No | PN | Therophyte | Herb |
| Parietaria debilis G. Forst. | Urticaceae | No | PN | Therophyte | Herb |
| Helminthotheca echioides (L.) Holub | Asteraceae | No | PN | Therophyte | Herb |
| Pinus canariensis C. Sm. ex DC. in Buch | Pinaceae | Yes | SN | Macrophanerophyte | Tree |
| Plantago lagopus L. | Plantaginaceae | No | PN | Therophyte | Herb |
| Poaceae sp. | Poaceae | ||||
| Polypogon viridis (Gouan) Breistr. | Poaceae | No | PN | Hemicryptophyte | Perennial herb |
| Prunus sp. | Rosaceae | ||||
| Pteridium aquilinum (L.) Kuhn in Von der Decken | Dennstaedtiaceae | No | PN | Geophyte | Rhizomatous perennial herb |
| Reseda luteola L. | Resedaceae | No | PN | Hemicryptophyte | Perennial herb |
| Rubus ulmifolius Schott | Rosaceae | No | PN | Nanophanerophyte | Liana |
| Rumex acetosella L. | Polygonaceae | No | PI | Hemicryptophyte | Perennial herb |
| Rumex bucephalophorus L. | Polygonaceae | No | SN | Therophyte | Herb |
| Rumex maderensis Lowe | Polygonaceae | No | SN | Chamaephyte | Small shrub |
| Rumex sp. | Polygonaceae | ||||
| Sagina apetala Ard. | Caryophyllaceae | No | PN | Therophyte | Herb |
| Senecio teneriffae Sch. Bip. ex Bolle | Asteraceae | No | SN | Therophyte | Herb |
| Sherardia arvensis L. | Rubiaceae | No | PN | Therophyte | Herb |
| Silene gallica L. | Caryophyllaceae | No | PN | Therophyte | Herb |
| Silene vulgaris (Moench) Garcke | Caryophyllaceae | No | PN | Therophyte | Herb |
| Sonchus acaulis Dum. Cours. | Asteraceae | Yes | SN | Hemicryptophyte | Perennial herb |
| Sonchus asper (L.) A. W. Hill | Asteraceae | No | PN | Therophyte | Herb |
| Sonchus canariensis (Sch. Bip.) Boulos | Asteraceae | Yes | SN | Nanophanerophyte | Shrub |
| Sonchus congestus Willd. | Asteraceae | Yes | SN | Nanophanerophyte | Shrub |
| Sonchus oleraceus L. | Asteraceae | No | PN | Therophyte | Herb |
| Sonchus sp. | Asteraceae | ||||
| Sonchus tenerrimus L. | Asteraceae | No | PN | Therophyte | Herb |
| Spergula arvensis L. | Caryophyllaceae | No | PN | Therophyte | Herb |
| Stachys arvensis (L.) L. | Lamiaceae | No | PN | Therophyte | Herb |
| Stellaria media (L.) Vill. | Caryophyllaceae | No | PI | Therophyte | Herb |
| Tolpis barbata (L.) Gaertn. | Asteraceae | No | PN | Therophyte | Herb |
| Trifolium campestre Schreb. in Sturm | Fabaceae | No | PN | Therophyte | Herb |
| Trifolium dubium Sibth. | Fabaceae | No | PN | Therophyte | Herb |
| Trifolium glomeratum L. | Fabaceae | No | PN | Therophyte | Herb |
| Trifolium ligusticum Balbis ex Loisel. | Fabaceae | No | PN | Therophyte | Herb |
| Trifolium sp. | Fabaceae | ||||
| Trifolium striatum L. | Fabaceae | No | PN | Therophyte | Herb |
| Trifolium subterraneum L. | Fabaceae | No | PN | Therophyte | Herb |
| Tuberaria guttata (L.) Fourr. | Cistaceae | No | PN | Therophyte | Herb |
| Ulex europaeus L. | Fabaceae | No | SII | Nanophanerophyte | Shrub |
| Urospermum picroides (L.) Scop. ex F. W. Schmidt | Asteraceae | No | PN | Therophyte | Herb |
| Urtica morifolia Poir. in Lam. | Urticaceae | No | SN | Nanophanerophyte | Liana |
| Vicia sativa L. subsp. nigra (L.) Ehrh. | Fabaceae | No | PN | Therophyte | Herb |
| Vicia disperma DC. | Fabaceae | No | PN | Therophyte | Herb |
| Vicia lutea L. | Fabaceae | No | PN | Therophyte | Herb |
| Vicia sativa L. | Fabaceae | No | PN | Therophyte | Herb |
| Vicia sativa L. subsp. sativa | Fabaceae | No | PN | Therophyte | Herb |
| Vicia scandens R. P. Murray | Fabaceae | Yes | SN | Therophyte | Herb |
| Vicia sp. | Fabaceae | Therophyte | Herb | ||
| Vicia villosa Roth | Fabaceae | No | PI | Therophyte | Herb |
| Viola kitaibeliana Schult. in Roem. & Schult. | Violaceae | No | SN | Therophyte | Herb |
| Vulpia bromoides (L.) S. F. Gray | Poaceae | No | PN | Therophyte | Herb |
| Vulpia myuros (L.) C. C. Gmel. | Poaceae | No | PN | Therophyte | Herb |
| Wahlenbergia lobelioides (L. f.) Link | Campanulaceae | No | SN | Therophyte | Herb |
1 Origin: PI: probable introduced; SII: securely introduced invasive; SIN: securely introduced non-invasive; PN: probable native; SN: secure native.
References
- Geeson, N.A.; Brandt, C.J.; Thornes, J.B. Mediterranean Desertification: A Mosaic of Processes and Responses; Wiley: Chichester, UK, 2002; 459p. [Google Scholar]
- Rackham, O. Holocene history of Mediterranean island landscapes. In Mediterranean Island Landscapes: Natural and Cultural Approaches; Vogiatzakis, I., Pungetti, G., Mannion, A.M., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 36–60. [Google Scholar]
- Palmer, C.; Colledge, S.; Bevan, S.; Conolly, J. Vegetation recolonisation of abandoned agricultural terraces on Antikythera, Greece. Environ. Archaeol. 2010, 15, 64–80. [Google Scholar] [CrossRef]
- Rounsevell, M.D.A.; Reginster, I.; Araujo, M.B.; Carter, T.R.; Dendoncker, N.; Ewert, F.; House, J.I.; Kankaanpää, S.; Leemans, R.; Metzger, M.J.; et al. A coherent set of future land use change scenarios for Europe. Agric. Ecosyst. Environt. 2006, 114, 57–68. [Google Scholar] [CrossRef]
- Hernández, M. Paisajes Agrarios y Medio Ambiente en Alicante: Evolución e Impactos Ambientales de Los Paisajes Agrarios Alicantinos, 1950–1995; Universidad de Alicante: Alicante, Spain, 1997; 290p. [Google Scholar]
- Ruecker, G.; Schad, P.; Alcubilla, M.M.; Ferrer, C. Natural regeneration of degraded soils and site changes on abandoned agricultural terraces in Mediterranean Spain. Land Degrad. Dev. 1998, 9, 179–188. [Google Scholar] [CrossRef]
- Lasanta, T.; García-Ruiz, J.M.; Pérez Rontomé, M.C.; Sancho, C. Runoff and sediment yield in a semi-arid environment: The effect of land management after farmland abandonment. Catena 2000, 38, 265–278. [Google Scholar] [CrossRef]
- Aide, T.M.; Grau, H.R. Globalization, migration, and Latin American ecosystems. Science 2004, 305, 1915–1916. [Google Scholar] [CrossRef]
- Cramer, V.A.; Hobbs, R.J.; Standish, R.J. What’s new about old fields? Land abandonment and ecosystem assembly. Trends Ecol. Evol. 2008, 23, 104–112. [Google Scholar] [CrossRef]
- Meiners, S.J.; Pickett, S.T.A.; Cadenasso, M.L. Exotic plant invasions over 40 years of old field succession: Community patterns and associations. Ecography 2002, 25, 215–223. [Google Scholar] [CrossRef]
- Corbin, J.D.; D’Antonio, C.M. Competition between native perennial and exotic annual grasses: Implications for an historical invasion. Ecology 2004, 85, 1273–1283. [Google Scholar] [CrossRef]
- Kulmatiski, A. Exotic plants establish persistent communities. Plant Ecol. 2006, 187, 261–275. [Google Scholar] [CrossRef]
- Arévalo, J.R.; Fernández-Lugo, S.; Mellado, M.; de la Concepción, T. Experimental management control of Opuntia dillenii Haw. and Agave americana L. in the Teno Rural Park, Canary Islands. Plant Spec. Biol. 2015, 30, 137–146. [Google Scholar] [CrossRef]
- Mellado, M.; Encina-Domínguez, J.A.; García, J.E.; Estrada-Castillón, E.A.; Arévalo, J.R. Vegetation response to removal of plant functional groups and grass seeding in a microphyllous desert shrubland: A 4-year field experimente. Agriculture 2021, 11, 322. [Google Scholar] [CrossRef]
- Simberloff, D.; Martin, J.L.; Genovesi, P.; Maris, V.; Wardle, D.A.; Aronson, J.; Courchamp, F.; Galil, B.; García-Berthou, E.; Pascal, M.; et al. Impacts of biological invasions: What’s what and the way forward. Trends Ecol. Evol. 2013, 28, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.G.; Schmitz, O.J. Rapid recovery of damaged ecosystems. PLoS ONE 2009, 4, e5653. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, R.J.; Harris, J.A.; Pärtel, M. Anthropogenic ecosystem disruption and the pathways of recovery. Ecol. Lett. 2006, 9, 773–782. [Google Scholar]
- García-Gallo, A.; Wildpret, W.; del Arco, M.J.; Pérez de Paz, P.L. Sobre la presencia de Ulex europaeus L. en la isla de Tenerife. Bol. Soc. Brot. 1989, 62, 221–225. [Google Scholar]
- García-Gallo, A.; Wildpret, W.; Martín, V. Especies vegetales consideradas invasoras de hábitats, en la Historia Natural de Canarias. Lazaroa 2008, 29, 49–67. [Google Scholar]
- Roberts, J.; Florentine, S. Biology, distribution, and control of the invasive species Ulex europaeus (Gorse): A global synthesis of current and future management challenges and research gaps. Weed Res. 2021, 61, 272–281. [Google Scholar] [CrossRef]
- von Buch, L. Physicalische Beschreibung der Canarischen Inseln; Gedruckt in der Druckerei der Koeninglichen Academie der Wuseebschaften: Berlin, Germany, 1825; 429p. [Google Scholar]
- Sanz, M.; Dana, E.D.; Sobrino, E. Atlas de las Plantas Alóctonas Invasoras en España; Ministerio del Medio Ambiente: Madrid, Spain, 2004; 384p.
- Arévalo, J.R.; Delgado, J.D.; Otto, R.; Naranjo-Cigala, A.; Salas, M.; Fernández-Palacios, J.M. Distribution of alien vs. native plant species in roadside communities along an altitudinal gradient in Tenerife and Gran Canaria (Canary Islands). Perspect. PlantEcol. Evol. Syst. 2005, 7, 185–202. [Google Scholar] [CrossRef]
- Pauchard, A.; Kueffer, C.; Dietz, H.; Daehler, C.C.; Alexander, J.; Edwards, P.; Arévalo, J.R.; Cavieres, L.A.; Guisan, A.; Haider, S.; et al. Ain’t not mountain high enough: Plant invasions reaching high elevations? Front. Ecol. Environ. 2009, 9, 479–486. [Google Scholar] [CrossRef]
- Iseli, E.; Chisholm, C.; Lenoir, J.; Haider, S.; Seipel, T.; Barros, A.; Hargreaves, A.L.; Kardol, P.; Lembrechts, J.J.; McDougall, K.; et al. Rapid upwards spread of non-native plants in mountains across continents. Nat. Ecol. Evol. 2023, 7, 405–413. [Google Scholar] [CrossRef]
- GRAFCAN. Sistema de Información Territorial de Canarias. 2024. Available online: https://visor.grafcan.es/visorweb/ (accessed on 2 May 2024).
- Lorenzo, M.J. Los Cochineros de Icod el Alto; Comisión de Cultura de Ayuntamiento de Los Realejos: Los Realejos, Spain, 1983; 143p. [Google Scholar]
- Patiño, J.; Collart, F.; Vanderpoorten, A.; Martin-Esquivel, J.L.; Naranjo-Cigala, A.; Mirolo, S.; Karger, D.N. Spatial resolution impacts projected plant responses to climate change on topographically complex islands. Divers. Distrib. 2023, 29, 1245–1262. [Google Scholar] [CrossRef]
- Del Arco Aguilar, M.; Rodríguez-Delgado, O. Vegetation of the Canary Islands. Plant and Vegetation; Werger, M.J.A., Ed.; Springer: Utrecht, The Netherlands, 2016; Volume 16, 429p. [Google Scholar]
- Altamirano, A.; Cely, J.P.; Etter, A.; Miranda, A.; Fuentes-Ramírez, A.; Acevedo, P.; Salas, C.; Vargas, R. The invasive species Ulex europaeus (Fabaceae) shows high dynamism in a fragmented landscape of south-central Chile. Environ. Monit. Assess. 2016, 188, 495–510. [Google Scholar] [CrossRef] [PubMed]
- Broadfield, N.; McHenry, M.T. A world of gorse: Persistence of Ulex europaeus in managed landscapes. Plants 2019, 8, 523. [Google Scholar] [CrossRef] [PubMed]
- POWO. Plants of the World Online; Facilitated by the Royal Botanic Gardens, Kew. Published on the Internet. 2023. Available online: http://www.plantsoftheworldonline.org/ (accessed on 14 March 2024).
- Clements, D.R.; Peterson, D.J.; Prasad, R. The biology of Canadian weeds. 112. Ulex europaeus L. Can. J. Plant Sci. 2001, 81, 325–337. [Google Scholar] [CrossRef]
- Ángel-Vallejo, M.C.; Aguirre-Acosta, N.; Rodríguez-Rey, G.T.; García-Marín, E.J.; Álvarez-Mejía, L.M.; Feuillet-Hurtado, C. Distribution models in invasive plants with climatic niche expansion: A case study of Ulex europaeus L. in Colombian Andes. Biol. Invasions 2024, 26, 1919–1930. [Google Scholar] [CrossRef]
- Christina, M.; Gire, C.; Bakker, M.R.; Leckie, A.; Xue, J.; Clinton, P.W.; Negrin-Perez, Z.; Sierra, J.R.A.; Domec, J.-C.; Gonzalez, M. Native and invasive seedling drought-resistance under elevated temperature in common gorse populations. J. Plant Ecol. 2023, 16, rtac097. [Google Scholar] [CrossRef]
- Kariyawasam, C.S.; Ratnayake, S.S. Reproductive biology of gorse, Ulex europaeus (Fabaceae) in the Mount Lofty Ranges of South Australia and Sri Lanka. Int. J. Plant Reprod. Biol. 2019, 11, 145–152. [Google Scholar]
- Udo, N.; Tarayre, M.; Atlan, A. Evolution of germination strategy in the invasive species Ulex Europaeus. J. Plant Ecol. 2017, 10, 375–385. [Google Scholar]
- Bateman, J.B.; Vitousek, P.M. Soil fertility response to Ulex europaeus invasion and restoration efforts. Biol. Invasions 2018, 20, 2777–2791. [Google Scholar] [CrossRef]
- Atlan, A.; Limbada, F. World Distribution of Gorse Ulex europaeus in Introduced Areas. Geonetwork, OSURIS. 2019. Available online: https://www.osuris.fr/geonetwork/srv/fre/catalog.search#/search (accessed on 25 September 2024).
- Smith, B.; Wilson, J.B. A consumer’s guide to evenness indices. Oikos 1996, 76, 70–82. [Google Scholar] [CrossRef]
- Gauch, H.G., Jr. Multivariate Analysis in Community Ecology; Cambridge University Press: Cambridge, UK, 1982; 312p. [Google Scholar]
- Franklin, S.B.; Robertson, P.A.; Fralish, J.S.; Kettler, S.M. Overstory vegetation and successional trends of land between the Lakes, USA. J. Veg. Sci. 1993, 4, 509–520. [Google Scholar] [CrossRef]
- Arévalo, J.R.; Fernández-Palacios, J.M.; Palmer, M. Tree regeneration and predicted future dynamics in a laurel forest (Tenerife, Canary Islands). J. Veg. Sci. 1999, 10, 861–868. [Google Scholar] [CrossRef]
- Hill, M.O.; Gauch, H.J., Jr. Detrended Correspondence Analysis: An improved ordination technique. Vegetatio 1980, 42, 47–58. [Google Scholar] [CrossRef]
- ter Braak, C.J.F.; Šmilauer, P. CANOCO Reference Manual and CanoDraw for Windows Users Guide: Software for Canonical Community Ordination; Version 5.1; Microcomputer Power: Ithaca, NY, USA, 2018. [Google Scholar]
- Legendre, P.; Legendre, L. Numerical Ecology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 1998; 853p. [Google Scholar]
- Pallant, J. SPSS Survival Manual: A Step-by-Step Guide to Data Analysis Using IBM SPSS; Open University Press: London, UK, 2021; 378p. [Google Scholar]
- Richardson, D.M.; Pyšek, P. Plant invasions: Merging the concepts of species invasiveness and community invasibility. Prog. Phys. Geogr. 2006, 30, 409–431. [Google Scholar] [CrossRef]
- Dietz, H.; Edwards, P.J. Recognition of changing processes during plant invasions may help reconcile conflicting evidence of the causes. Ecology 2006, 87, 1359–1367. [Google Scholar] [CrossRef]
- Baskin, J.M.; Baskin, C.C. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination, 2nd ed.; Academic Press: Cambridge, MA, USA, 2014; 1586p. [Google Scholar]
- Fenner, M. The effects of the parent environment on seed germinability. Seed Sci. Res. 1991, 1, 75–84. [Google Scholar] [CrossRef]
- Baird, D.D.; Upchurch, R.P.; Homesley, W.B.; Franz, J.E. Introduction of a new broad spectrum postemergence herbicide class with utility for herbaceous perennial weed control. Proc. North Cent. Weed Control. Conf. 1971, 26, 64–68. [Google Scholar]
- Diatloff, G. New uses of glyphosate. In Proceedings of the 12th Australian Weeds Conference Proceedings, Hobart, Austria, 12–16 September 1999; pp. 299–301. [Google Scholar]
- Mokotjomela, T.M.; Nelufule, T.; Scott, Z.; Vukeya, L.R.; Xivuri, T.; Matsokane, K.; Mweli, N.; Magqabi, F.L.; Thulisile, J. The invasion threat of the emerging alien cactus Cylindropuntia pallida (Rosa), F.M. Knuth in South Africa and the potential for control using herbicides. Environ. Monit. Assess. 2024, 196, 673. [Google Scholar] [CrossRef]
- Arévalo, J.R.; Encina-Domínguez, J.A.; Juanes-Márquez, S.; Álvarez-Vázquez, P.; Nuñez-Colima, J.A.; Mellado, M. Restoration of rangelands invaded by Amelichloa clandestina (Hack.) Arriaga & Barkworth after 12 years of agriculture abandonment (Coahuila, Mexico). Agriculture 2021, 11, 886. [Google Scholar] [CrossRef]
- Rosenzweig, M.L. Species Diversity in Space and Time; Cambridge University Press: New York, NY, USA, 1995; 436p. [Google Scholar]
- Stace, C.A. New Flora of the British Isles; Cambridge University Press: New York, NY, USA, 2010; 1165p. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).