Abstract
Secretion of siderophores by Pseudomonas aeruginosa F2 and P. fluorescens JY3 was evaluated on chrome azurol S (CAS) agar plates and their inhibitory effect was inspected against Fusarium oxysporum and Rhizoctonia solani. Production of siderophores as biocontrol agents from F2 and JY3 was accomplished in two optimized media. Afterward, cell-free supernatants of the bacterial cultures containing siderophores were used for the preparation of two bio-friendly formulations for the management of F. oxysporum and R. solani under greenhouse conditions. The investigated bacterial isolates, F2 and JY3, showed antagonistic activity in vitro against F. oxysporum and R. solani and produced siderophores in optimized media with high efficiency. Colonies of both bacterial isolates were grown exponentially with a constant specific growth rate of 0.07 h−1 and 0.27 h−1, correspondingly. Siderophores estimated in 10 µL reached their highest value of 16.95% at 47 h and 19.5% at 48 h for isolate F2 and JY3, respectively. Formulations of siderophore-generating F2 and JY3 reduced damping-off caused by F. oxysporum by 40% and 80%, while the reduction percentage of damping-off caused by R. solani reached 87.5% and 62.5%, correspondingly. Moreover, both formulations encouraged the growing of wheat plants where the fresh and dry weight of shoots and roots were increased compared to the treatment with each fungus. In conclusion, bio-friendly formulations resulting from this investigation can play an active role in managing soil-borne diseases.
1. Introduction
The yield and quality of food crops are primarily reduced by soil-borne fungi, including Fusarium oxysporum and Rhizoctonia solani. Control of these pathogens is very difficult because they can stay alive in the soil for many years [1]. The accumulation of pesticides used in the management of plant diseases, in addition to industrial pollution reaching agricultural land [2], encouraged scientists to search for environmentally friendly alternative approaches for controlling plant pathogens [3,4]. Biocontrol is a viable alternative strategy to conventional pesticide usage in the management of plant pathogens, as it offers the potential to mitigate their detrimental impacts without posing risks to human health or the environment [5,6]. Pseudomonas is a genus of bacteria, which is utilized as a biocontrol agent. Pseudomonas spp. have demonstrated antagonistic properties against various phytopathogens due to their ability to synthesize a range of compounds, such as phenazines, hydrogen cyanide, hydrolytic enzymes, siderophores, and others [7].
Production of siderophores by Pseudomonas spp. restricts the availability of iron to potential plant pathogens, thereby impeding their proliferation. Additionally, this process may confer competitive benefits that promote plant growth and development [8]. Production of siderophores by fluorescent pseudomonads is one of their primary mechanisms, which is elaborated in the biocontrol of different plant diseases. Siderophores are defined as specific chelating agents for ferric ions, which are produced by various microorganisms under low iron conditions that have low molecular weights below 1000 Da [9]. Siderophores are secreted by several species of fluorescent pseudomonads, for instance, P. aeruginosa, P. fluorescens, and P. syringae [10,11]. Various plant pathogens are suppressed by siderophore-producing biocontrol agents.
The effect of fluorescent pseudomonads on the production of siderophores is affected by many things, such as the concentration of iron [10], the type and concentration of carbon and nitrogen sources [12], levels of phosphate [13], pH and light [14], degree of aeration [15], temperature [16], and the occurrence of trace elements like magnesium [17], zinc [18], and molybdenum [15]. Various types of soils have been found to contain siderophores, as reported by Powell et al. in 1980. The utilization of microbial siderophores has been observed to augment the absorption of iron by plants. This is attributed to the plant’s ability to identify the bacterial ferric–siderophore complex, as reported previously [19,20,21]. Furthermore, the significance of microbial siderophores in facilitating iron uptake by plants in the presence of metals like nickel and cadmium has been established in research conducted by Burd et al. [22] and Dimkpa et al. [23]. The extent to which bacterial siderophore complexes can fulfill the iron demands of plants is yet to be conclusively determined. The production of siderophores provides a competitive edge to the growth of plants by facilitating the roots colonization by plant growth promoting rhizobacteria (PGPR) and preventing the establishment of other microorganisms in this ecological niche [24].
Siderophores like pyoverdine and pyochelin are produced by PGRB, which may be a mechanism for disease suppression [10]. Pathogens like F. oxysporum and Pythium ultimum, which are responsible for wilt and root rot disease in crops, need iron for growing, but the siderophores trap Fe near the roots, reducing the available supply [25]. Many scientists believe that pseudomonads capable of producing siderophores could be utilized as biocontrol agents to halt the spread of soil-borne plant diseases. Some examples of these strains are P. putida WCS strains [7], P. fluorescens CHA0 [26], and P. syringae pv. syringae strain 22 d/93 [27]. The incidence of wilt diseases in cucumber, radish, and flax, which are caused by Fusarium ssp., was effectively mitigated through the application of siderophore-producing P. putida as a PGBR [28]. The primary objective of the present study was to examine the synthesis of siderophores by fluorescent pseudomonads. Furthermore, the biocontrol efficacy of fluorescent pseudomonads that produce siderophores was evaluated in a greenhouse setting to mitigate the damping-off incidence attributed to F. oxysporum and R. solani.
2. Materials and Methods
2.1. Source of Bacterial and Fungal Isolates
The strains utilized in this study were generously supplied by Dr. Gaber Abo-Zaid, namely P. aeruginosa F2 (MG210480), which was isolated from the rhizosphere of eggplant (El-Minoufiya governorate) and P. fluorescens JY3 (KF922490), which was isolated from the rhizosphere of cotton (El-Mansoura governorate). The soil-borne fungi, specifically F. oxysporum and R. solani, which were isolated from wheat plants, were provided by the Plant Pathology Institute at the Agricultural Research Center, Egypt. Following this, an assessment was conducted to determine their pathogenic capabilities.
2.2. Chrome Azurol S (CAS) Agar Plates for Detection of Siderophores Production
CAS agar plates were used to detect siderophore production by P. aeruginosa F2 and P. fluorescens JY3 [29]. The indicator of siderophores’ existence is the conversion of the greenish-blue color of the medium to orange. Spot inoculation of Pseudomonas isolates was achieved on the CAS agar plates, incubated for 24 h at 30 °C, and their ability to produce siderophores was demonstrated.
2.3. Antagonistic Activity of P. fluorescens JY3 and P. aeruginosa F2 (In Vitro)
The antagonistic potential of P. fluorescens JY3 and P. aeruginosa F2 against R. solani and F. oxysporum was examined per the methodology outlined by Touré et al. [30]. On potato dextrose agar plates, a line of antagonistic isolates was streaked using a loopful of a culture that was 2 days old. The plates were subsequently incubated for 48 h before inoculation with any of the tested fungi. A mycelial disc measuring 5 mm in diameter, derived from a highly active culture of the patterned fungus, was positioned in the central region of the petri plate at a standardized distance from the plate’s edge. The plate was then incubated at a temperature of 30 °C for a period ranging from 3 to 7 days. The measurement of the inhibition diameter was conducted to determine the spatial separation between the boundary of antagonistic bacterial progression and the boundary of fungal growth.
2.4. Batch Fermentation in Shake Flask
A solitary colony of P. aeruginosa F2 and P. fluorescens JY3 was introduced into Luria-Bertani (LB) medium and subjected to overnight incubation at 30 °C with agitation at 200 rpm. A batch fermentation process was conducted in a shake flask utilizing two strains. The inoculum size was maintained at a constant optical density (OD600 nm) of 0.3, achieved through inoculation from the pre-culture of LB broth of each strain. The cultivation of P. aeruginosa F2 was carried out on a medium optimized for the production of siderophores. The medium was prepared by combining 14.5 g of glucose, 20 mL of glycerol, 4 g of sodium succinate, 1 g of glutamic acid, 0.1 g of urea, 5 g of asparagine, 1 g of (NH4)2SO4, 3.5 g of K2HPO4, 1 g of MgSO4, 0.5 µM of FeCl3, 1 g of kH2PO4, and adding distilled water to reach a final volume of 1000 mL. The pH of the medium was modified to a value of 7. The process of cultivating P. fluorescens JY3 was conducted utilizing a medium that had been optimized specifically to enhance siderophore production. The medium was prepared by combining 10 mL of glycerol, 0.5 g of glutamic acid, 1 g of glucose, 3.14 g of sodium succinate, 1 g of asparagine0.1 g of urea, 0.1 g of (NH4)2SO4, 6 g of KH2PO4, 4 g of K2HPO4, 0.1 g of MgSO4, 0.62 µM of FeCl3, and sufficient dH2O to reach a final volume of 1000 mL. The resulting medium had a pH of 7 [31]. Subsequently, cultures of both strains were incubated at 25 °C while being agitated at a rate of 200 rpm for 48 h. Samples were collected periodically to determine the levels of siderophores, glycerol, glucose, and biomass. Additionally, the specific growth rate, denoted µ, was calculated from the relationship between the natural logarithm of biomass and time in the exponential phase.
2.5. Analytical Procedures
2.5.1. Siderophores Estimation
The quantification of siderophores was assessed utilizing the CAS assay technique as outlined by Schwyn and Neilands [29]. Following this, 10 µL of the culture’s supernatant was added to a 0.5 mL CAS test solution and thoroughly stirred. After completely combining, 10 µL of shuttle solution was added, and the resulting mixture was allowed to sit undisturbed at room temperature for a few minutes. The absence of the blue hue is correlated with the existence of siderophores. The measurement of absorbance was conducted at a wavelength of 630 nm, with the media serving as the blank. Siderophore levels are determined by the formula: % = [(Ar − As)/Ar] × 100. In this formula, Ar denotes the absorption of the CAS solution in combination with the media and shuttle solution, while As represents the absorption of the CAS solution in combination with the culture supernatant and shuttle solution.
2.5.2. Biomass Estimation
To determine the dry cell weight, a centrifugation process was conducted on a 10 mL sample at a force of 894× g for 10 min. The pellet underwent a process of resuspension, washing, and subsequent centrifugation. The pellets were subsequently subjected to an overnight drying process in a dry air oven operating at a temperature of 80 °C. Finally, the pellets were weighted to estimate biomass [32].
2.5.3. Glucose Estimation
An enzymatic colorimetric kit manufactured by Diamond Diagnostics in Egypt was utilized in order to illustrate the glucose levels.
2.5.4. Glycerol Estimation
The level of glycerol was determined using a technique devised by Bok and Demain [33], and the results were calculated.
2.6. Pseudomonas Isolates Producing Siderophores as Biocontrol Agents
2.6.1. Formulation Experiment
Bio-friendly formulations were generated utilizing the cell-free supernatant of P. aeruginosa F2 and P. fluorescens JY3, both of which are capable of producing siderophores. The utilization of talc powder (TP) was employed as a means of carrying the substance. A quantity of 10 g of carboxymethylcellulose (CMC) was introduced into 400 milliliters of cell-free supernatant to utilize CMC as an adhesive agent. The homogenization of the mixture was achieved by utilizing a vortex mixer. To achieve a pH of 7.0, 15 g of calcium carbonate was incorporated into 1 kg of sterilized TP and thoroughly blended. One kilogram of TP was mixed with 400 mL of cell-free supernatant containing an additive. The formulations underwent shade drying to reduce the humidity level to below 20%. Subsequently, they were placed into polythene bags that had been sterilized with UV light, sealed, and stowed at a temperature of 4 °C until further use, as described in reference [4].
2.6.2. Preparation of Fungal Inoculum
The cultivation of F. oxysporum and R. solani inoculum was carried out using a medium composed of sorghum, coarse sand, and water in a volumetric proportion of 2:1:2. Upon the completion of the assembly of the components, they were subsequently packaged and subjected to a sterilization process lasting two hours, rendering them suitable for utilization. To inoculate the sterile media, agar discs were collected from the outermost part of a culture that were five days old for each of the fungi being investigated. According to earlier reports, fungal inoculum was obtained for soil infestation after an incubation period of two weeks at a temperature of 30 °C [34].
2.6.3. Soil Infestation
The study entailed the introduction of F. oxysporum and R. solani inoculum onto the soil surface of separate pots at a concentration of 2% w/w. A layer of sterilized soil was applied to cover the fungal inoculum. The pots that were impacted by pathogens were irrigated and subsequently left undisturbed for 7 days before being planted.
2.6.4. Greenhouse Experiment
Pots measuring 18 cm in diameter were filled with sterilized soil and subsequently exposed to infestation, following the previously outlined procedure. A total of nine treatments were implemented subsequently. The present study investigated the impact of different formulations on the enhancement of F. oxysporum and R. solani, alongside a control group comprising unaffected organisms. The formulations that were examined in this study consisted of the supernatant formulations of P. aeruginosa F2 and P. fluorescens JY3. Additionally, combinations of these formulations with F. oxysporum or R. solani were also tested. Each pot was planted with a total of five wheat seeds, and three replicates (pots) were employed for each treatment. The wheat seeds were subjected to seed drench formulations at a rate of 10 g per kilogram of seeds. The utilization of formulations was conducted at a frequency of 3 kg per feddan on two separate instances, specifically at 15 and 30 days following the initiation of seed planting, utilizing the technique of soil drenching. The evaluation of disease incidence was determined by recording the proportion of damping-off, occurring both before and after seedling emergence, at 7 and 21 days following the initiation of planting. This was cited in the reference [4]. The CoStat software was employed to perform data analysis. Analysis of Variance (ANOVA) was used as a statistical method for analyzing the information gathered. The statistical significance of changes between treatments was determined using the Least Significant changes (LSD) method at the p ≤ 0.05 level.
3. Results
3.1. Detection of Siderophore Production on CAS Agar Plates
The ability of P. aeruginosa JY3 and P. fluorescens F2 isolates to produce siderophores was confirmed through the utilization of CAS agar plates. A region displaying a color gradient from yellow to orange was detected near the colonies of F2 and JY3 that were not exposed to contamination, suggesting the presence of siderophore production (Figure 1A).
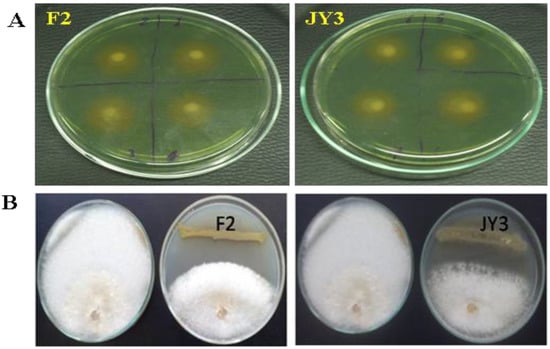
Figure 1.
(A) Displays the qualitative evaluation of siderophore synthesis on chrome azurol S (CAS) agar plates. The assessment was conducted using spot cultures of P. aeruginosa F2 and P. fluorescens JY3, with four spots for each isolate; (B) illustrates the antagonistic effect of P. aeruginosa F2 and P. fluorescens JY3 against F. oxysporum.
3.2. Antagonistic Activity of P. fluorescens JY3 and P. aeruginosa F2
The results indicate that Pseudomonas spp., F2, and JY3 exhibited great activity in suppressing the mycelial growth of F. oxysporum and R. solani (as depicted in Figure 1B). Reduction of the fungal growth expressed as an inhibition zone or diameter. F2 and JY3 isolates achieved inhibition diameter against F. oxysporum equal to 1.7 cm and 1.23 cm, respectively. On the other hand, the inhibition diameter in the case of R. solani reached 2.23 cm and 1.07 cm, respectively.
3.3. Batch Fermentation in a Shake Flask
The results depicted in Figure 2 indicate that the cellular mass of P. aeruginosa F2 underwent a gradual increase during a lag phase that persisted for approximately 5 h, before entering the logarithmic phase (also known as the exponential phase). During the logarithmic phase, the biomass exhibited a rapid increase with a consistent specific growth rate, denoted as µ, which was calculated to be 0.07 h−1 (as illustrated in Figure 3A). At this stage, the yield coefficient YX/S was determined to be 0.78 g cells per g glycerol, as illustrated in Figure 3B. The concentration of siderophores exhibited an initial value of 9.55% at 39 h, followed by a rapid increase to attain 11.31% at 43 h, and ultimately reached its peak value of 16.95% at 47 h. The maximum biomass recorded during this period was 5.4 g L−1. The concentration of glycerol exhibited a gradual decline, however, it was not entirely depleted, as evidenced by the recorded value of 9.21 g L−1 at 48 h. Conversely, the concentration of glucose exhibited a swift decline and was entirely depleted within 48 h. The data presented in Figure 4 indicates that the estimation of siderophore production for P. fluorescens JY3 reached 1.95% at 32 h and exhibited a rapid increase, reaching 9.2% after 42 h. The maximum value of siderophore production was observed to be 19.5% at 48 h. At 48 h, the biomass attained a peak level of 4.8 g per liter. The concentration of glycerol exhibited a gradual decrease and ultimately attained a value of 0.65 g L−1 after 48 h. The cell mass of P. fluorescens JY3 exhibited an exponential increase concerning a specific growth rate, µ, which was calculated to be 0.27 h−1 (as depicted in Figure 5A). The yield coefficient YX/S was determined to be 0.59 g cells per g glycerol, as illustrated in Figure 5B.
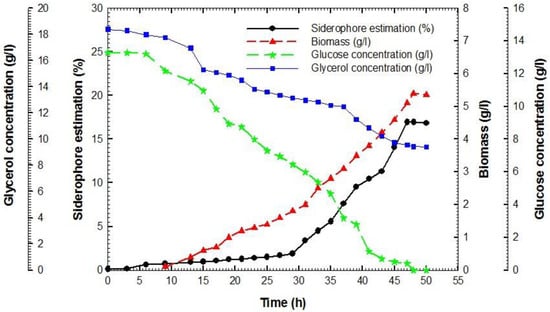
Figure 2.
The amount of siderophores, biomass, glucose, and glycerol in the broth of a batch fermentation culture of Pseudomonas aeruginosa F2 in a shaking jar as a function of time.
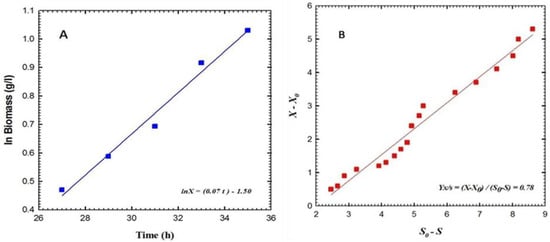
Figure 3.
(A) Shows the logarithm of the biomass (X) in g L−1 as a function of time during the exponential phase of Pseudomonas aeruginosa F2’s batch fermentation cultivation broth in a shaking flask, and (B) shows the yield coefficient on glycerol during this phase.
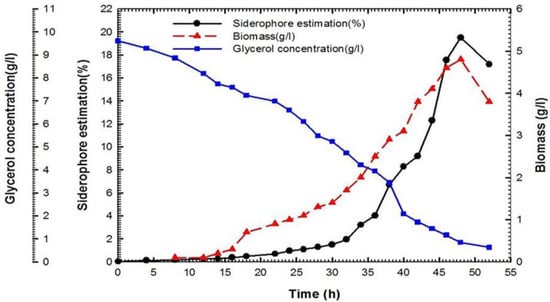
Figure 4.
The amount of siderophores, the amount of biomass, and the amount of glycerol in the cultivation broth of Pseudomonas fluorescens JY3 as a function of time in a shake jar.
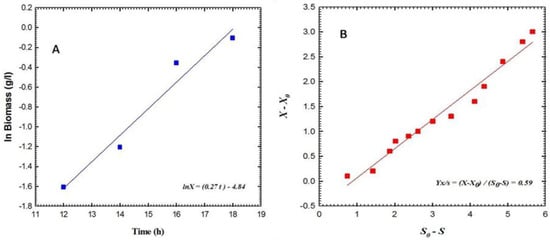
Figure 5.
(A) Shows the logarithm of the biomass (X) in g L−1 as a function of time during the exponential phase of batch fermentation culturing broth of Pseudomonas fluorescens JY3 in a shaking flask, and (B) shows the yield coefficient on glycerol during this phase.
3.4. Efficacy Evaluation of F2 and JY3 Formulations under Greenhouse Conditions
The efficacy of formulations F2 and JY3, were evaluated against plant pathogens causing damping-off. Results indicated that both formulations were operative in dropping damping-off triggered by R. solani and F. oxysporum as compared to the control group.
The prevalence of damping-off disease caused by R. solani and F. oxysporum was documented as 53.3% and 33.3%, respectively. The utilization of F2 and JY3 formulations in soil that was infested with F. oxysporum led to a noteworthy decline in the occurrence of damping-off disease, as evidenced by the reduction of values to 20% and 6.67%, respectively. Reduction percentages of 40% and 80% were observed, respectively. The results of the study suggest that using P. aeruginosa F2 in soil infested with R. solani led to a bigger drop in damping-off disease than using P. fluorescens JY3. Comparing P. aeruginosa F2 and P. fluorescens JY3, damping-off disease was found to happen 6.67% of the time with P. aeruginosa F2 and 20% of the time with P. fluorescens JY3. According to the data presented in Table 1 and Figure 6, the reduction percentages for F2 and JY3 were found to be 87.50% and 62.50%, respectively.

Table 1.
Effect of Pseudomonas fluorescens JY3 and Pseudomonas aeruginosa F2 supernatant mixtures on F. oxysporum and Rhizoctonia solani-caused damping-off of wheat plants.

Figure 6.
Effects of supernatant formulations of Pseudomonas fluorescens JY3 and Pseudomonas aeruginosa F2 on wheat plants as biocontrol agents for controlling Fusarium oxysporum, from the left, (A), Fusarium oxysporum; (B), Control; (C), F2 formulation; (D), JY3 formulation; (E), F2 formulation + Fusarium oxysporum, and (F), JY3 formulation + Fusarium oxysporum.
Both formulations displayed growth-enhancing characteristics on wheat plants that were cultured in soil that was infested with F. oxysporum and R. solani. This was in contrast to the remaining control treatments, in which each fungus was injected separately. When F2 and JY3 formulations were added to soil with phytopathogenic fungi, the fresh and dry biomass of both above-ground and below-ground plant structures increased significantly. The utilization of the F2 formulation on soil infested with F. oxysporum led to a noteworthy augmentation in the mass of newly harvested shoots and roots of wheat flora. The shoot and root fresh weights exhibited a respective increase of 44.50% and 49.39%, ultimately attaining values of 3.73 g and 3.3 g. The utilization of the JY3 formulation resulted in a notable augmentation in the fresh weight of both shoots and roots, exhibiting a respective rise of 45.53% and 51.87%. The shoots and roots achieved fresh weights of 3.80 g and 3.47 g, respectively. On the contrary, the solitary application of the F. oxysporum treatment yielded markedly reduced fresh weights of both shoots and roots, measuring at 2.07 g and 1.67 g, respectively. Table 2 presents the aforementioned findings. The study recorded the fresh weight of shoots in treatments that employed formulated F2 and JY3 in soil infested with R. solani. The measurements obtained were 3.53 g and 3.23 g, respectively. The observed percentage increase of 72.52% and 69.97% in fresh weight can be attributed to the current treatment, as compared to the treatment involving the sole application of the fungus, which resulted in a fresh weight of 0.97 g. The application of the aforementioned formulations yielded fresh root weights of 3.20 g and 2.87 g, respectively, representing a percentage increase of 81.25% and 79.09%, respectively, in comparison to the fungus-only treatment, which resulted in a fresh weight of 0.60 g. Table 2 displays the aforementioned results.

Table 2.
Effect of Pseudomonas fluorescens JY3 and Pseudomonas aeruginosa F2 supernatant formulations on fresh weight of wheat stems and roots.
The results of this study demonstrate that the application of F2 and JY3 formulations in soil infested with F. oxysporum and R. solani significantly enhanced the biomass of plant shoots. The experimental findings indicated that the application of the aforementioned formulations led to shoot dry weights of 2.73 g, 2.83 g, 2.50 g, and 2.33 g, accompanied by percentage increases of 62.27%, 63.60%, 86.80%, and 85.84%, respectively. On the other hand, the application of F. oxysporum and R. solani treatments yielded shoot dry weights of merely 1.03 g and 0.33 g, respectively. In contrast, the dry weight of the roots exhibited values of 2.37 g, 2.33 g, 2.03 g, and 1.83 g, indicating a percentage increase of 56.54%, 55.79%, 91.13%, and 90.16%, respectively, in comparison to treatments that employed F. oxysporum and R. solani in isolation, which registered values of 1.03 g and 0.18 g, respectively (refer to Table 3).

Table 3.
The effect of Pseudomonas fluorescens JY3 and Pseudomonas aeruginosa F2 supernatant formulations on the dry weight of wheat shoots and roots.
4. Discussion
The primary objective of the current investigation was to examine the in vitro antagonistic activity of two Pseudomonas isolates that produce siderophores against the growth of root rot fungi, namely F. oxysporum and R. solani. Isolates F2 and JY3 exhibited great activity in impeding the mycelial progress of the above-mentioned phytopathogenic fungi. The P. aeruginosa FP6 strain, which produces siderophores, exhibited antagonistic properties against R. solani, as reported in a previous study [35]. The study found that P. syringae strain BAF.1 exhibited significant antagonistic effects against F. oxysporum, resulting in a 95.24% reduction. This strain was also observed to produce siderophores of the catechol type, with a relatively low molecular weight of 488.59 Da, as reported in a previous study [36]. Based on the findings of Reddy et al. [37], it was observed that fluorescent Pseudomonas strains that produce siderophores demonstrated the capacity to inhibit the mycelial growth of Pyricularia oryzae, the pathogen responsible for rice blast, as well as R. solani AG-1 IA, which is linked to rice sheath blight disease. The study conducted by Mathiyazhagan and colleagues [38] revealed the impact of siderophores on inhibiting the proliferation and sporulation of phytopathogenic fungi. This resulted in various modifications in the morphology of the fungal mycelia due to the lack of iron.
In the current study, the maximum production of siderophores from isolate F2 and JY3 was achieved on optimized production media reported by Abo-Zaid et al. [31]. Glucose as a carbon source was the most effective variable in the production of siderophores from P. aeruginosa isolate F2 that was fully consumed compared to glycerol. On the other hand, glycerol was the main variable in the production of siderophores from P. fluorescens JY3. Santos et al. [39] observed the change in carbon/nitrogen ratio in the production media of siderophore had no effect. Nevertheless, Ye et al. [36] observed that different carbon/nitrogen ratios affect siderophore production significantly differently.
The present investigation revealed that F2 and JY3 formulations had high proficiency in decreasing wheat damping-off in F. oxysporum and R. solani-infected soil. Fluorescent Pseudomonas spp. have several mechanisms for managing soil-borne diseases. Secretion of siderophores by fluorescent Pseudomonas spp. is considered one of the important approaches utilized in biocontrol. It is based on the reduction of the number of ferric ions accessible for plant pathogens in the rhizosphere by chelation of these ions. The presence of low levels of iron in the rhizosphere serves as a stimulus for fluorescent pseudomonads to synthesize siderophores, which exhibit a high affinity for ferric iron. The ferric–siderophore complex resulting from this process is utilized by the producing microorganism through a very specific receptor secreted in its outer-cell membrane. On the other hand, the ferric–siderophore complex is inaccessible to other organisms [40]. This activity could potentially account for the restriction of phytopathogens within the rhizosphere, thereby reducing their capacity to establish themselves on plant roots. The P. fluorescens strains SPs9 and SPs20 were found to synthesize siderophores and exhibit significant efficacy in mitigating the incidence of F. oxysporum-induced wilt disease in tomato seedlings grown in soil [11]. Furthermore, plant systemic resistance can be accomplished by siderophores of fluorescent pseudomonads. In their study, Leeman et al. [41] documented the induction of plant systemic resistance against Fusarium wilt of radish through the production of a siderophore by P. fluorescens.
The current investigation provided evidence that the utilization of siderophore-producing F2 and JY3 formulations yielded significant enhancements in the fresh and dry weights of both shoots and roots in wheat plants cultivated in soil infested with F. oxysporum and R. solani. The results obtained by Arya et al. [11] were consistent with our findings. The previous findings may be attributed to the activation of phytohormones, specifically auxin, cytokinin, and gibberellins, through the use of both isolates. Moreover, it could be associated with the capacity of plants to absorb iron from the microbial Fe–siderophore complex that is produced. In a previous study, it was noted that the introduction of Pseudomonas strain GRP3 into Vigna radiata plants resulted in the production of siderophores, leading to an increase in iron levels as well as chlorophyll a and chlorophyll b concentrations [42].
5. Conclusions
The fluorescent Pseudomonas spp. isolates F2 and JY3 demonstrated antagonistic activity against soil-dwelling fungi. The utilization of the optimized production media led to the attainment of the highest levels of siderophore production by strains F2 and JY3, with yields of 16.95% and 19.5%, respectively, after a 48 h incubation period. The utilization of fluorescent pseudomonads that possess the ability to produce siderophores has been employed as a means of employing biocontrol agents. This approach has demonstrated significant efficacy in inhibiting the growth of F. oxysporum and R. solani, as well as reducing the percentage of damping-off caused by these pathogens. Moreover, it was observed that both formulations demonstrated a stimulatory effect on the growth of wheat plants.
Author Contributions
Conceptualization, G.A.A.-Z. and N.A.-M.S.; methodology, A.S.A.; software, G.A.A.-Z.; validation, E.E.E.-S., A.A. and S.A.-F.S.; formal analysis, G.A.A.-Z.; investigation, A.S.A.; resources, E.E.E.-S.; data curation, Y.S.; writing—original draft preparation, G.A.A.-Z.; writing—review and editing, G.A.A.-Z., A.A. and Y.S.; visualization, E.E.E.-S.; supervision, S.A.-F.S.; project administration, A.A.A.-A.; funding acquisition, A.A.A.-A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by the Researchers Supporting Project number (RSP2023R505), King Saud University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to extend their appreciation to the Researchers Supporting Project number (RSP2023R505), King Saud University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Redda, E.T.; Ma, J.; Mei, J.; Li, M.; Wu, B.; Jiang, X. Antagonistic potential of different isolates of Trichoderma against Fusarium oxysporum, Rhizoctonia solani, and Botrytis cinerea. Eur. J. Exp. Biol. 2018, 8, 12. [Google Scholar]
- Abd El-Rahim, W.M.; Mostafa, E.M.; Moawad, H. High cell density cultivation of six fungal strains efficient in azo dye bioremediation. Biotechnol. Rep. 2016, 12, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Emmert, E.A.B.; Handelsman, J. Biocontrol of plant disease: A (Gram-) positive perspective. FEMS Microbiol. Lett. 1999, 171, 1–9. [Google Scholar] [CrossRef]
- Abdel-Gayed, M.A.; Abo-Zaid, G.A.; Mohamed, M.S.; Elsayed, H.E. Fermentation, formulation and evaluation of PGPR Bacillus subtilis isolate as a bioagent for reducing occurrence of peanut soil-borne diseases. J. Integr. Agric. 2019, 18, 2080–2092. [Google Scholar]
- Lo, C.-T. General mechanisms of action of microbial biocontrol agents. Plant Pathol. Bull. 1998, 7, 155–166. [Google Scholar]
- Abo-Zaid, G.; Abdelkhalek, A.; Matar, S.; Darwish, M.; Abdel-Gayed, M. Application of Bio-Friendly Formulations of Chitinase-Producing Streptomyces cellulosae Actino 48 for Controlling Peanut Soil-Borne Diseases Caused by Sclerotium rolfsii. J. Fungi 2021, 7, 167. [Google Scholar] [CrossRef]
- Weller, D.M. Pseudomonas biocontrol agents of soilborne pathogens: Looking back over 30 years. Phytopathology 2007, 97, 250–256. [Google Scholar] [CrossRef]
- Shanmugaiah, V.; Nithya, K.; Harikrishnan, H.; Jayaprakashvel, M.; Balasubramanian, N. Biocontrol mechanisms of siderophores against bacterial plant pathogens. In Sustainable Approaches to Controlling Plant Pathogenic Bacteria; CRC Press: Boca Raton, FL, USA, 2015; pp. 167–190. [Google Scholar]
- Ngamau, C.; Matiru, V.N.; Tani, A.; Muthuri, C. Potential use of endophytic bacteria as biofertilizer for sustainable banana (Musa spp.) production. Afr. J. Hortic. Sci. 2014, 8, 1–11. [Google Scholar]
- Kloepper, J.W.; Leong, J.; Teintze, M.; Schroth, M.N. Enhanced plant growth by siderophores produced by plant growth-promoting rhizobacteria. Nature 1980, 286, 885–886. [Google Scholar] [CrossRef]
- Arya, N.; Rana, A.; Rajwar, A.; Sahgal, M.; Sharma, A.K. Biocontrol efficacy of siderophore producing indigenous Pseudomonas strains against Fusarium Wilt in Tomato. Natl. Acad. Sci. Lett. 2018, 41, 133–136. [Google Scholar] [CrossRef]
- Park, C.-S.; Paulitz, T.C.; Baker, R. Biocontrol of Fusarium wilt of cucumber resulting from interactions between Pseudomonas putida and nonpathogenic isolates of Fusarium oxysporum. Phytopathology 1988, 78, 190–194. [Google Scholar] [CrossRef]
- Barbhaiya, H.B.; Rao, K.K. Production of pyoverdine, the fluorescent pigment of Pseudomonas aeruginosa PAO1. FEMS Microbiol. Lett. 1985, 27, 233–235. [Google Scholar] [CrossRef]
- Greppin, H.; Gouda, S. Action de la lumiere sur le pigment de Pseudomonas fluorescens Migula. Arch. Sci. 1965, 18, 721–725. [Google Scholar]
- Lenhoff, H. An inverse relationship of the effects of oxygen and iron on the production of fluorescin and cytochrome c by Pseudomonas fluorescens. Nature 1963, 199, 601–602. [Google Scholar] [CrossRef] [PubMed]
- Weisbeek, P.J.; Van der Hofstad, G.; Schippers, B.; Marugg, J.D. Genetic analysis of the iron-uptake system of two plant groups promoting Pseudomonas strains. In Siderophores, and Plant Diseases; Springer: Boston, MA, USA, 1986; pp. 299–313. [Google Scholar]
- Georgia, F.R.; Poe, C.F. Study of Bacterial fluorescence in various media: I. Inorganic substances necessary for bacterial fluorescence. J. Bacteriol. 1931, 22, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarty, A.M.; Roy, S.C. Effect of trace elements on the production of pigments by a pseudomonad. Biochem. J. 1964, 93, 228. [Google Scholar] [CrossRef] [PubMed]
- Masalha, J.; Kosegarten, H.; Elmaci, Ö.; Mengel, K. The central role of microbial activity for iron acquisition in maize and sunflower. Biol. Fertil. Soils 2000, 30, 433–439. [Google Scholar] [CrossRef]
- Katiyar, V.; Goel, R. Siderophore mediated plant growth promotion at low temperature by mutant of fluorescent pseudomonad⋆. Plant Growth Regul. 2004, 42, 239–244. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; Merten, D.; Svatoš, A.; Büchel, G.; Kothe, E. Metal-induced oxidative stress impacting plant growth in contaminated soil is alleviated by microbial siderophores. Soil Biol. Biochem. 2009, 41, 154–162. [Google Scholar] [CrossRef]
- Burd, G.I.; Dixon, D.G.; Glick, B.R. A plant growth-promoting bacterium that decreases nickel toxicity in seedlings. Appl. Environ. Microbiol. 1998, 64, 3663–3668. [Google Scholar] [CrossRef]
- Dimkpa, C.; Svatoš, A.; Merten, D.; Büchel, G.; Kothe, E. Hydroxamate siderophores produced by Streptomyces acidiscabies E13 bind nickel and promote growth in cowpea (Vigna unguiculata L.) under nickel stress. Can. J. Microbiol. 2008, 54, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Haas, D.; Défago, G. Biological control of soil-borne pathogens by fluorescent pseudomonads. Nat. Rev. Microbiol. 2005, 3, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Sahu, G.K.; Sindhu, S.S. Disease control and plant growth promotion of green gram by siderophore producing Pseudomonas sp. Res. J. Microbiol. 2011, 6, 735. [Google Scholar]
- Couillerot, O.; Prigent-Combaret, C.; Caballero-Mellado, J.; Moënne-Loccoz, Y. Pseudomonas fluorescens and closely-related fluorescent pseudomonads as biocontrol agents of soil-borne phytopathogens. Lett. Appl. Microbiol. 2009, 48, 505–512. [Google Scholar] [CrossRef]
- Wensing, A.; Braun, S.D.; Büttner, P.; Expert, D.; Völksch, B.; Ullrich, M.S.; Weingart, H. Impact of siderophore production by Pseudomonas syringae pv. syringae 22d/93 on epiphytic fitness and biocontrol activity against Pseudomonas syringae pv. glycinea 1a/96. Appl. Environ. Microbiol. 2010, 76, 2704–2711. [Google Scholar] [CrossRef]
- Wang, H.; Liu, R.; You, M.P.; Barbetti, M.J.; Chen, Y. Pathogen biocontrol using plant growth-promoting bacteria (PGPR): Role of bacterial diversity. Microorganisms 2021, 9, 1988. [Google Scholar] [CrossRef] [PubMed]
- Schwyn, B.; Neilands, J.B. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 1987, 160, 47–56. [Google Scholar] [CrossRef]
- Touré, Y.; Ongena, M.; Jacques, P.; Guiro, A.; Thonart, P. Role of lipopeptides produced by Bacillus subtilis GA1 in the reduction of grey mould disease caused by Botrytis cinerea on apple. J. Appl. Microbiol. 2004, 96, 1151–1160. [Google Scholar] [CrossRef]
- Abo-Zaid, G.A.; Abdullah, A.S.; Soliman, N.A.-M.; El-Sharouny, E.E.; Sabry, S.A.-F. Optimization of siderophores production from fluorescent pseudomonads using statistical experimental designs. Biosci. Res. 2018, 15, 3040–3051. [Google Scholar]
- Van Dam-Mieras, M.C.E.; Jeu, W.H.; Vries, J.; Currell, B.R.; James, J.W.; Leach, C.K.; Patmore, R.A. Techniques Used in Bioproduct Analysis; Butterworth-Heinemann: Oxford, UK, 1992. [Google Scholar]
- Bok, S.H.; Demain, A.L. An improved colorimetric assay for polyols. Anal. Biochem. 1977, 81, 18–20. [Google Scholar] [CrossRef]
- Hussien, Z.N.; Mahmoud, E.Y.; Metwaly, A.H.; Sobhy, H.M. Effect of some antagonistic bacteria in reducing of peanut damping-off, root and pod rot incidence caused by Rhizoctonia solani. J. Plant Prot. Pathol. 2012, 3, 1173–1187. [Google Scholar] [CrossRef]
- Sasirekha, B.; Srividya, S. Siderophore production by Pseudomonas aeruginosa FP6, a biocontrol strain for Rhizoctonia solani and Colletotrichum gloeosporioides causing diseases in chilli. Agric. Nat. Resour. 2016, 50, 250–256. [Google Scholar] [CrossRef]
- Yu, S.; Teng, C.; Liang, J.; Song, T.; Dong, L.; Bai, X.; Jin, Y.; Qu, J. Characterization of siderophore produced by Pseudomonas syringae BAF. 1 and its inhibitory effects on spore germination and mycelium morphology of Fusarium oxysporum. J. Microbiol. 2017, 55, 877–884. [Google Scholar] [CrossRef]
- Reddy, B.P.; R, J.; Reddy, M.S.; Kumar, K. Siderophore isolation and its biocontrol action against rice fungal pathogens. Biochem. Mol. Biol. 2019, 1, 1–6. [Google Scholar]
- Mathiyazhagan, S.; Kavitha, K.; Nakkeeran, S.; Chandrasekar, G.; Manian, K.; Renukadevi, P.; Krishnamoorthy, A.S.; Fernando, W.G.D. PGPR mediated management of stem blight of Phyllanthus amarus (Schum and Thonn) caused by Corynespora cassiicola (Berk and Curt) Wei. Arch. Phytopathol. Plant Prot. 2004, 37, 183–199. [Google Scholar] [CrossRef]
- Santos, S.; Neto, I.F.F.; Machado, M.D.; Soares, H.M.V.M.; Soares, E.V. Siderophore production by Bacillus megaterium: Effect of growth phase and cultural conditions. Appl. Biochem. Biotechnol. 2014, 172, 549–560. [Google Scholar] [CrossRef]
- Buyer, J.S.; Leong, J. Iron transport-mediated antagonism between plant growth-promoting and plant-deleterious Pseudomonas strains. J. Biol. Chem. 1986, 261, 791–794. [Google Scholar] [CrossRef]
- Leeman, M.; Den Ouden, F.M.; Van Pelt, J.A.; Dirkx, F.P.M.; Steijl, H.; Bakker, P.; Schippers, B. Iron availability affects induction of systemic resistance to Fusarium wilt of radish by Pseudomonas fluorescens. Phytopathology 1996, 86, 149–155. [Google Scholar] [CrossRef]
- Sharma, A.; Johri, B.N.; Sharma, A.K.; Glick, B.R. Plant growth-promoting bacterium Pseudomonas sp. strain GRP3 influences iron acquisition in mung bean (Vigna radiata L. Wilzeck). Soil Biol. Biochem. 2003, 35, 887–894. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).