Abstract
Heilongjiang province has made great contributions to ensuring the food security of China. Grain production has increased year by year, followed by a large amount of straw—especially the production of corn straw. Straw returning is the best treatment method from the perspective of ecology. This study simulated modern mechanized operation conditions from the field of soil biological characteristics to explore the impact of straw decomposition on the changes in the soil microbial community. In this study, in the black soil region of Northeast China (45°45′27″~45°46′33″ N, 126°35′44″~126°55′54″ E), the orthogonal experimental design was used to experiment for two years (2019–2020), using straw length, amount, and buried depth as returning factors. The carbon source utilization intensity algorithm that was developed by our team was used to extract a single carbon source. A compound mathematical model was constructed based on path analysis and grey relation analysis. This study analyzed the interspecific symbiotic relationship of soil microbes in the process of straw returning and explored the regulatory methods and schemes with which to promote straw decomposition. The results showed that in the first year after straw returning, the cumulative decomposition rate of straw could reach 55.000%; the supplement of the carbon source was glycyl-l-glutamic acid, which was helpful for the decomposition of straw. It was found that cyclodextrin should be added within 90–120 days after straw returning to promote decomposition. In the second year of straw returning, the cumulative decomposition rate of straw can reach 73.523% and the carbon sources α-d-lactose and d-galactonic acid γ-lactone should be supplemented appropriately to promote straw decomposition. This study provides an experimental basis for corn straw returning to the black soil of the cold regions, along with the scientific and technological support for the sustainable development of agriculture and a guarantee of national food security.
1. Introduction
As the main corn-producing area in China, the cold black soil region plays an important role in stabilizing the balance of grain supply and demand along with ensuring national food security [1,2]. However, the abandonment or random burning of corn straw has increased haze [3], the frequency of fires, and the waste of resources [4]. Therefore, determining how to efficiently deal with straw has become a critical concern.
Returning straw to the field can improve the soil environment [5], increase the content of soil organic matter [6], and enhance the ability of soil to retain water and fertilizer [7]. It can also supply necessary elements in plants [8], promote crop growth and development [9,10], and help with nitrogen fixation and emission reduction in the agricultural ecosystem [11,12]. Moreover, the rice yield can be effectively maintained by partially replacing mineral fertilizer with straw returning [13,14]. Recently, many scholars have conducted extensive research on straw returning to explore the best scheme of the process. These include studies on the degree of straw crushing, the amount of straw returning [15,16], the research and development of applied materials [17,18], the selection of farming methods [19,20], and the impact of soil types in the straw returning area on the straw decomposition effect [21,22]. Despite multiple studies, the research rarely involved studies on the regulation of interspecific symbiosis and the cooperation of soil microbes in the process of straw returning.
Based on this research gap, this study simulated the operating conditions of modern agricultural machinery, designed a three-factor orthogonal experiment using the amount, length, and buried depth of straw return as the factors, and carried out a two-year straw returning experiment in the cold black soil area. Using the carbon source utilization intensity algorithm that was developed by our team [23], the study extracted a single carbon source and analyzed the impact of straw returning on the carbon source utilization intensity of soil microbes. This study used the path analysis model (PA) and grey relational analysis model (GRA) to analyze the interspecific symbiotic relationship of soil microbes in the process of straw returning and find the regulatory methods and schemes with which to promote straw decomposition. This study provided scientific and technological support for the sustainable development of agriculture and to guarantee national food security.
2. Materials and Methods
2.1. Test Materials
The test was conducted at the teaching experimental base of Northeast Agricultural University (45°45′27″~45°46′33″ N, 126°35′44″~126°55′54″ E). The test area belongs to the temperate continental monsoon climate with an average annual temperature of 3.6 °C, annual precipitation of 500–600 mm, an average annual frost-free period of 135–140 days, and an effective accumulated temperature of 2700 °C [24]. The detailed meteorological data during the test are given in the Supporting Information.
The soil was typical black soil with a pH of 6.30 ± 0.06. It was composed of 39.06 ± 0.42 g/kg of organic matter; 2.20 ± 0.08 g/kg of total nitrogen; 2.71 ± 0.08 g/kg of total phosphorus; and 183.25 × 10−3 ± 0.16 × 10−3 g/kg of available potassium.
The tested straw was corn straw with total carbon of 479.0 ± 0.23 g/kg; total nitrogen of 13.16 ± 0.09 g/kg; total phosphorus of 4.56 ± 0.06 g/kg; and total potassium of 15.36 ± 0.07 g/kg. The C: N ratio was 36.13–36.78.
The mesh bag was cut from 100-mesh polyamide fiber, and the bag was 35 cm long and 25 cm wide.
2.2. Test Design
According to the three-factor five-level quadratic orthogonal rotation test design, the straw length, amount, and buried depth were taken as the test factors. Referring to the previous research results [24,25], the maximum and minimum values of each test factor are determined, that is, the actual value when the coding value is 1.682 and −1.682. Then, the actual value under other coding levels is determined according to the equivalent conversion between the coded value and the actual value. The test design result is shown in Table 1.

Table 1.
Test design.
The straws with different weights and lengths were put into mesh bags (35 cm × 25 cm) and then soaked with water to enable the moisture content of the straw to reach 40%. The bags were buried in the soil horizontally. Each treatment was randomly arranged and repeated four times, with a total of 20 plots. Each plot was 15 m long and 1 m wide. During the straw returning period, no farming is carried out.
According to the three-factors five-levels quadratic orthogonal rotation experimental design, fifteen groups of experiments were carried out. The straw amount in each experimental plot (1 m2) is given in the Supporting Information.
Two kinds of decomposition tests were set up. The first was a one-year decomposition period while the second was a two-year decomposition period. In view of the climate impact of the cold black soil area, all the straws were buried in the spring on 6 May 2019. After 15 days of adaptation in the soil, 30 days cycles were taken for sampling in the one-year decomposition period until the end of autumn on 21 October. Thus, a total of five cycles were considered in the one-year decomposition period. The samples for the two-year decomposition period were taken on the same date of the next year (2020), as shown in Table 2.

Table 2.
Sampling period.
2.3. Sample Collection and Index Determination
2.3.1. Determination of Soil Microbial Community
According to the sampling method of rhizosphere soil, the soil around the mesh bag should be taken to the laboratory at 4 °C. The samples were activated at 25 °C for 24 h. After that, 10 g of sample was weighed and added to 90 mL of sterilized 0.85 mol/L NaCl solution. It then oscillated at 250 r/min for 30 min and was gradually diluted to 10−3 after standing for 10 min. The bench was clean in the vertical flow, followed by the inoculation of 150 μL of soil suspension into an ECO plate, and finally cultured in a constant temperature incubator at 28 °C. The absorbance value (OD value) at 590 nm was measured by taking 24 h as a culture cycle. The measurements of seven culture cycles (168 h) were continuously taken.
In the culture cycle, the total change of 31 carbon sources in the ECO plate was given by the following:
where is the total change of 31 carbon sources; is the absorbance value of carbon source; and indicates the type of carbon source, ; . The distribution of carbon sources is given in the Supporting Information.
Therefore, the utilization intensity of carbon source by microbes was given by
where is the dimensionless data and .
2.3.2. Calculation of Straw Decomposition Rate
The straw, with the mesh bag, was placed into a sterile bag, stored at 4 °C and taken back to the laboratory. The mud and grassroots, which adhered to the mesh bag, were washed with deionized water and then dried in a constant temperature oven at 60 °C. The straw in each sampling period was accurately weighed and used to calculate the straw decomposition rate according to the Equation (3):
where is the straw decomposition rate; is the initial dry weight of the straw; and is the dry weight of the straw after days of returning.
2.4. Data Analysis
2.4.1. Path Analysis Model (PA)
PA studies the direct effect, indirect effect, and total effect by decomposing the correlation between the independent variables and dependent variables [26]. In this study, the utilization intensity of the carbon source by soil microbes is the independent variable: ; the straw decomposition rate is the dependent variable: . represents the simple correlation coefficients (spearman) of and ; represents the correlation coefficient of and ; is the direct path coefficient, which indicates the direct effect on when the other variables are fixed. can be decomposed into the following equations:
In this study, the absolute value of the path coefficient can be directly used to compare the importance of various microbial populations to straw decomposition. Among these, the direct path coefficient reflects the direct effect of this microbial population. Microbes can also affect the straw decomposition through the interaction with other microbial communities, which is expressed by the indirect path coefficient.
The indirect path of to dependent variable through other variables is , and the determination coefficient of to is calculated as follows:
where is the determination coefficient.
Secondly, the path residual effect is calculated. If the residual effect is minute (generally bounded by 0.20), it indicates that the PA included the main influencing factors; otherwise, variables need to be added to improve the model.
In this study, the residual path coefficient was less than 20.00% as the judgment standard for extracting carbon sources .
2.4.2. Grey Relational Analysis Model (GRA)
GRA is a quantitative evaluation method based on grey system theory. It reflects the similarity of the development process between sequences through displacement difference. It can make up for the defect of the mathematical statistics method having a linear relationship with the sequence, which is irrelevant. It can overcome the deficiency of relying exclusively on the model for quantification and directly find the primary and secondary factors in the process of system development [27,28]. The specific process of GRA model construction was as follows:
In this study, the straw decomposition rate, , is set as the parent sequence, and the carbon source extracted by path analysis, , is set as the sub-sequence.
Calculate the difference and take the absolute value, that is .
Calculate the maximum and minimum values for all absolute values, that is: and .
Calculate the relational coefficient according to the following formula.
where is the resolution coefficient. The smaller the value, the greater the difference between the relational coefficients and the stronger the discrimination ability. Referring to the previous research results [29], in this paper, .
Calculation of grey comprehensive correlation degree (GCD): .
3. Results and Analysis
3.1. Decomposition Rate of Straw with Different Returning Ways
As shown in Figure 1A, with the extension of straw returning time, the straw decomposition rate of each treatment group gradually increased. After 150 days of straw returning, in the T10 treatment, the cumulative decomposition rate of straw was the largest, 55.000%; in the two-year decomposition test ((A + 150) day), the cumulative decomposition rate of straw in the T10 treatment was still the largest, reaching 73.523%. In the process of straw decomposition, there was a trend of fast decomposition in the early stage and slow decomposition in the late stage. In the first two months of the one-year decomposition test, the monthly average decomposition rate was 9.310–11.000%; meanwhile, the monthly average decomposition rate of the last two months was 3.167–7.167%. The reason for this result is that, on the one hand, at the late stage of decomposition, the easily degradable organic matter in the straw gradually decreases, and the remaining part is mainly the difficult to decompose organic matter. On the other hand, it may be that the soil temperature decreases in the late stage of decomposition, resulting in the reduction in microbial activity, which is not conducive to the decomposition of straw [30]. As shown in Figure 1B,C, at the end of the test, in the two kinds of straw decomposition tests, the straw decomposition rate showed an inverted “U” shape with the increase in the coding value. High or low straw returning causes the imbalance of the soil carbon–nitrogen ratio, affects the number and activity of microbes, and leads to the reduction in straw decomposition rate [31]. Therefore, it is necessary to explore the evolution of soil microbial communities in the process of straw decomposition and find methods and schemes with which to promote straw decomposition.
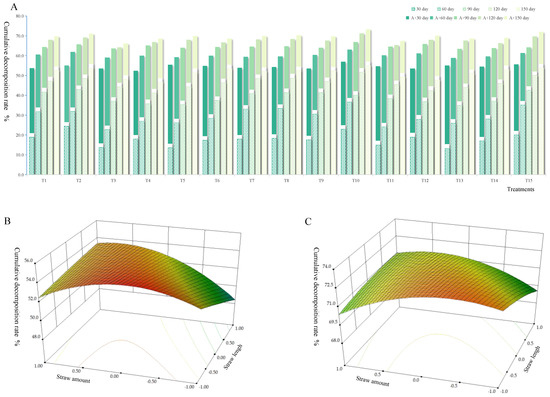
Figure 1.
Cumulative decomposition rate of straw with different returning ways. (A) Including all straw returning periods; (B) after 150 days of straw returning, the change of straw decomposition rate caused by the interaction of straw amount and straw length; (C) after (A + 150) days of straw returning, the change of straw decomposition rate caused by the interaction of straw amount and straw length. Notes: The changes of straw decomposition rates caused by the interaction of straw amount and buried depth (Figure S1), and the interaction of straw length and buried depth (Figure S2) are given in Supporting Information.
It can be seen from Table S2 that the minimum value of the determination coefficient appeared in the 60 days of straw returning, which was 0.967, indicating that the relative contribution of the six variables which entered the PA to the straw decomposition rate had reached 96.7%, and the remaining path coefficient was 0.182, which met the judgment standard. The results showed that the PA was suitable for analyzing the relationship between straw decomposition and the soil microbial community in the cold black soil areas.
In the 30-day straw returning test group, the carbon sources l-arginine and N-acetyl-d-glucosamine had high direct path coefficients in the positive axis direction, which were 0.846 and 0.837, respectively. However, their total effect values, which were 0.315 and 0.101, respectively, were not high due to the counteraction of their negative indirect path effect. This was lower than that of the carbon source glycyl-l-glutamic acid, which had a total effect value of 0.371. In the 60-day group, the carbon source l-phenylalanine had the largest positive direct path effect of 0.437, with a total effect of 0.343. Although the carbon source d-malic acid had the largest positive indirect path effect of 0.741, due to the offset of the negative direct path effect of −0.581, its total effect was lower than that of the carbon source l-phenylalanine, which was 0.161. After 90 days of straw returning, the carbon source α-ketobutyric acid had the highest direct path coefficient of −1.239 and indirect path coefficient of 1.253, but the total effect value was only 0.014 due to the opposite effect between them. Simultaneously, the total effect of the carbon source N-acetyl-d-glucosamine was the largest in the negative direction with a total effect value of 0.512. The carbon source glycyl-l-glutamic acid had the largest total effect value of 0.379 in the positive direction. As shown in Figure 2, the carbon source α-ketobutyric acid played a major role through the indirect effect of the carbon source glycyl-L-glutamic acid with a value of 0.824.
Figure 2.
Indirect path effect between carbon sources. (A, year; Z0, water; Z1, β−methyl−d−glucoside; Z2, d−galactonic acid γ−lactone; Z3, l−arginine; Z4, pyruvic acid methyl ester; Z5, d−xylose; Z6, d−galacturonic acid; Z7, l−asparagine; Z8, tween 40; Z9, I−erythritol; Z10, 2−hydroxy benzoic acid; Z11, l−phenylalanine; Z12, tween 80; Z13, d−mannitol; Z14, 4−hydroxy benzoic acid; Z15, l−serine; Z16, α−cyclodextrin; Z17, N−acetyl−d−glucosamine; Z18, γ−hydroxybutyric acid; Z19, l−threonine; Z20, glycogen; Z21, d−glucosaminic acid; Z22, itaconic acid; Z23, glycyl−l−glutamic acid; Z24, d−cellobiose; Z25, glucose−1−phosphate; Z26, α−ketobutyric acid; Z27, phenylethyl−amine; Z28, α−d−lactose; Z29, d,l−α−glycerol phosphate; Z30, d−malic acid; Z31, putrescine.)
After 120 days of straw returning, the carbon source l-asparagine ranked first with a positive direct effect of 0.549 and the carbon source α-cyclodextrin ranked second with a value of 0.536. However, the former counteracted the negative indirect effect of the carbon source d-glucosaminic acid, so its total effect value was lower than the latter. Simultaneously, as shown in Figure 2, the indirect effects between the carbon sources l-asparagine and α-cyclodextrin were negative and occupied large components, which were −0.107 and −0.110, respectively. In the last stage of the one-year straw returning test, the direct path effect of the carbon source glycyl-l-glutamic acid was the largest, which was 0.577. The indirect path effect of carbon source 4-hydroxy benzoic acid was the largest, which was 0.666, but the total effect value was negative at −0.469, due to the counteraction of the negative direct effect of −1.135. Although the indirect path effect of the carbon source d-xylose was also offset by the negative direct effect, its total effect value was the largest positive at 0.246.
As shown in Table 3, for the two-year straw returning test group, in the treatment of A + 30, the carbon source α-d-lactose had the maximum direct path effect of 0.506, the minimum indirect path effect of 0.052, and the maximum total path effect value of 0.558. Although the carbon source β-methyl-d-glucoside had a direct path effect of 0.500, its total effect value was only 0.062 due to the counteraction of its indirect path effect with a value of −0.439. In the treatments of A + 60 and A + 90, the total effect values of the carbon source d-galactonic acid γ-lactone were 0.714 and 0.648, respectively. These values ranked first in each group with a much higher total effect value than that of other carbon sources. This depended on them having the largest direct path effect. In the treatments of A + 150, however, the absolute values of the direct and the indirect path effect coefficients of each carbon source were large; due to the offset between positive and negative effects, only the carbon source tween 40 had a small positive total effect with a value of 0.059.

Table 3.
The results of path analysis and grey correlation analysis.
3.2. Relational Analysis between Microbes and Straw Decomposition
In the 30-day straw returning group, the carbon source glycyl-l-glutamic acid with the largest total path effect value ranked second, and the carbon source glycogen had the same correlation degree of 0.710. Meanwhile, the carbon source l-arginine had the largest correlation degree of 0.758. In the 60-day straw returning group, the correlation degree of each carbon source entering the PA model was lower than 0.700 and the differences between the carbon sources were small. In the 90-day straw returning group, the correlation degree of the carbon source glycyl-l-glutamic acid was the largest, with a value of 0.751, indicating that it was closely related to the straw decomposition. Simultaneously, the correlation degree of the d-malic acid was the second largest, with a value of 0.739. This was similar to the total effect that was obtained by PA in the positive axis direction. A similar phenomenon occurred in the 120-day straw returning group where the correlation degree of carbon source α-cyclodextrin was the largest, with a value of 0.782. Meanwhile, the carbon sources N-acetyl-d-glucosamine and d-glucosaminic acid, with the maximum negative total effect in PA, had the minimum correlation degree with values of 0.651 and 0.690, respectively (Table 3). After 150 days of straw returning, the correlation degree of the carbon sources d-xylose and l-serine ranked first at 0.726, followed by the value of the correlation degree of the carbon source glycyl-l-glutamic acid at 0.724.
In the two-year straw returning test groups, in the treatments of A + 30, A + 60, and A + 90, the carbon sources with the largest positive direct effect had the largest correlation degrees, which were 0.760, 0.805, and 0.818, respectively. The correlation degree of the carbon source α-cyclodextrin with the largest positive direct effect of 0.696 was second only to the first carbon source γ-hydroxybutyric acid with a value of 0.702 in the treatment of A + 120. However, in the treatment of A + 150, d-malic acid, the carbon source with the largest positive indirect effect, had the highest correlation degree with a straw decomposition at a value of 0.738, while phenylethylamine, the carbon source with the second positive indirect effect, also had the second correlation degree of 0.735.
4. Discussion
Combined with the results of PA and GRA, the path map was drawn to analyze the interspecific symbiotic relationship of soil microbes during straw returning, and to find the methods and schemes for promoting straw decomposition.
In the one-year straw returning of the 30-day group, the carbon source l-arginine had the largest direct path effect value and correlation coefficient, but its total effect value was lower than that of the carbon source glycyl-l-glutamic acid. It can be seen from Figure 2 that the ranking of the correlation degree is affected through the indirect effect of the carbon source d-galactonic acid γ-lactone. Simultaneously, the carbon source d-galactonic acid γ-lactone had a negative maximum direct path effect and total effect. Therefore, when accelerating the decomposition of returning straw, it can be considered to reduce the input of the carbon source d-galactonic acid γ-lactone, and supplement glycyl-l-glutamic acid appropriately. As an amino acid carbon source, glycyl-l-glutamic acid plays an important role in the early stage of straw returning, which may be to balance the “carbon–nitrogen ratio” in the soil and provide suitable environmental conditions for the proliferation of microbial communities [32,33]. After 60 days of straw returning, the correlation degree difference between each carbon source entering the PA model and straw decomposition was small, indicating that the soil microbial community was in the stage of rapid reproduction and expanding population size at the time. This can be seen in Figure 3 showing that the direct demand for all kinds of carbon sources was large.
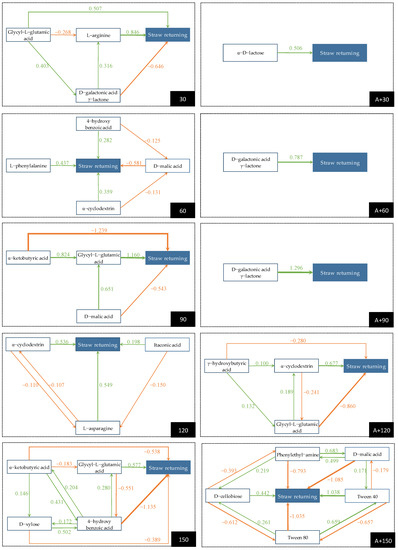
Figure 3.
Path diagram.
In the 90-day straw returning group, although the results of GRA were consistent with the total effect that was obtained by PA in the positive axis direction, the first two were carbon source glycyl-l-glutamic acid and the carbon source d-malic acid. The former mainly promoted straw decomposition through the direct effect. While the latter promoted straw decomposition through an indirect effect on the former. Simultaneously, in the indirect impact of the carbon source α-ketobutyric acid on straw decomposition (Figure 2), the carbon source glycyl-l-glutamic acid played a major role. Therefore, glycyl-l-glutamic acid was the necessary carbon source for the soil microbial community within 60–90 days of straw returning. During the decomposition of straw, cellulose and hemicellulose, formed by hexose and pentose through a single bond, were decomposed first, followed by lignin, which was linked by benzene ring compounds through the δ bond and π bond [34]. It was found that the lignin-degrading microbes had a high demand for amino acid carbon sources [35,36]. Therefore, amino acid carbon sources should be supplemented in the later stage of straw returning to accelerate the straw decomposition.
As shown in Figure 3, after 120 days of straw returning, the direct path effect of itaconic acid on straw decomposition was offset by its indirect path effect through l-asparagine. Simultaneously, the antagonistic effect between the carbon source l-asparagine and the carbon source α-cyclodextrin, and its indirect path effect through the carbon source d-glucosaminic acid made the total effect value and the correlation degree of the carbon source l-asparagine lower than that of the carbon source α-cyclodextrin. On one hand, the existence of l-asparagine may inhibit the synthesis of some substances, thus slowing down the decomposition of straw by the microbial community. l-asparagine hydrolyzes the acylamino into aspartic acid and ammonia under the action of l-asparaginase [37]. Glucosamine can be used as the starting material for the asymmetric synthesis of various amino acids [38], and it is also a special component of the lipopolysaccharide of Rhizobium leguminosarum, which is crucial for the nitrogen cycle in organisms [39]. Alternatively, the unique external hydrophilic and internal hydrophobic structures of cyclodextrin can not only increase the biological activity of microbes to accelerate the straw decomposition [40], but also increase the permeability of the cell membrane to promote microbes to absorb nutrition more effectively [41]. Simultaneously, the cylindrical three-dimensional structure with one large side and another small side is conducive to the adsorption of ammonia [42]. Moreover, given the effect of cyclodextrin on the comprehensive improvement of the physical properties of soil [27,43], it can be supplemented to the soil within 90–120 days after the straw is returned to the field.
It can be seen from the path map that the carbon source glycyl-l-glutamic acid is also a necessary carbon source for the soil microbial community within 120–150 days of straw returning. However, it can be seen from Figure 2 that the indirect path effect of the carbon source 4-hydroxy benzoic acid through the other carbon sources was the largest at this stage, and the indirect path effects of the other carbon sources through 4-hydroxy benzoic acid were also at a high level. 4-hydroxy benzoic acid has strong allelopathy on rhizosphere microbes, can inhibit the function of root mitochondria [44], promote the growth of pathogens, and lead to the occurrence of soil-borne diseases [45,46]. Studies have found that straw returning well inhibits the soil-borne pathogens, including Fusarium oxysporum [47], Rhizoctonia cerealis [48], Verticillium dahliae Kleb [49], and Plasmodiophora brassicae Woronin [50]. Therefore, it is speculated that straw returning improves the living environment of microbes [51], promotes the proliferation of microbial populations that can use the carbon source 4-hydroxybenzoic acid in the soil to form dominant species, consumes 4-hydroxybenzoic acid in the soil, and alleviates the soil-borne diseases of crops. Yang et al. [52] confirmed that the insufficient ability of microbes to metabolize 4-hydroxybenzoic acid in the soil is an important factor that causes tobacco root rot. Zhang et al. [53] found that there are microbes which can metabolize 4-hydroxybenzoic acid in the specific disease inhibiting soil of tobacco bacterial wilt.
In the two-year straw returning test of A + 30 day treatment, seven carbon sources were mentioned to enter the PA model, but only the direct and the indirect path effect coefficients of the carbon source α-d-lactose were positive, and the correlation coefficient of α-d-lactose was the largest, indicating that α-d-lactose is the characteristic carbon source of straw decomposition at this stage. α-d-lactose, as the energy source of rod-shaped strain, can effectively improve the removal efficiency of lignin [54], and as a co-metabolic substrate, it can promote the degradation of polycyclic aromatic hydrocarbons by Pseudomonas [55]. Moreover, α-d-lactose can be used as an inducer to promote Escherichia coli to produce cyclodextrin glucosyltransferase, and then convert starch into cyclodextrin through a cyclization reaction [56,57]. Therefore, in the treatments of A + 30 and A + 60, the carbon sources α-d-lactose and α- cyclodextrin were extracted into the PA model (Table 3), while in the treatments of A + 60 and A + 90, the carbon source d-galactonic acid γ-lactone played an important role in straw decomposition, which may be related to lignin degradation. It was found that d-galactonic acid γ-lactone could be transformed into d-galactonic acid under the action of glucolactonase and enter the glycolysis process [57] to accelerate the degradation of lignocellulose [58,59].
Table 3 shows that for the treatments of A + 120 and A + 150, among the carbon sources that were extracted from the PA model, only three had a positive effect, but their total effect values were small, and the total effects between the other carbon sources and straw decomposition were negative. The results showed that the effect of the microbial community on straw decomposition was weakened at this stage. It may be possible that nutrients such as nitrogen, phosphorus, and potassium in the straw were released, and microbes no longer relied on the straw to provide the carbon source, nitrogen source, and energy. A long-term location returning test in Songnen Plain, conducted by Gong et al. [60], showed that after two years of straw returning, the degradation rates of cellulose and hemicellulose exceeded 80%, and lignin was low at 78.63%. The study by Chen et al. [61] confirmed that after half a year of straw returning, the release rate of nitrogen and phosphorus exceeded 70%, while the release rate of potassium was more than 90%.
Figure 2 shows that the indirect path effect between carbon sources increased, but the positive correlation ratio decreased, indicating that the symbiotic relationship between soil microbial species due to the straw decomposition decreased with the continuous decomposition of straw, which was consistent with the research results of Schmid et al. [62]. Simultaneously, Figure 3 shows that the complexity of the soil microbial network at this stage increased significantly. Tang et al. [63] found that the straw returning increased the network complexity to enhance the defense ability of crops against Fusarium wilt. Ma et al. [64] confirmed that straw returning can stimulate the growth of specific species clusters and inhibit the activity of pathogens by regulating the interaction between microbial populations.
5. Conclusions
In this study, straw length, amount, and buried depth were taken as the straw returning factors, and the two-year straw returning experiment was carried out by orthogonal design. The single carbon source was extracted by the carbon source utilization intensity algorithm, combined with a path analysis and grey correlation analysis to build a composite mathematical model to analyze the interspecific symbiotic relationship of soil microbes in the process of straw returning. The path map was drawn to explore the regulatory methods and schemes with which to promote straw decomposition.
It can be seen from the path map that in the black soil region of Northeast China (45°45′27″−45°46′33″ N, 126°35′44″~126°55′54″ E), in the first year of straw returning, the cumulative decomposition rate of straw can reach 55.000%. Further, supplementing the carbon source glycyl-l-glutamic acid to the soil was conducive to the decomposition of straw—especially within 90–120 days of straw returning, adding the carbon source cyclodextrin. In the second year of straw returning, the cumulative decomposition rate of straw could reach 73.523%, and carbon sources α-d-lactose and d-galactonic acid γ-lactone needed to be supplemented appropriately to promote straw decomposition. Additionally, 4-hydroxybenzoic acid degrading bacteria can be screened in the peripheral soil within 120–150 days of straw returning.
This results of this study provide an experimental basis for corn straw returning to the black soil of the cold regions, along with the scientific and technological support for the sustainable development of agriculture and a guarantee of national food security.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/agriculture12071053/s1, Figure S1: Straw decomposition rate caused by straw amount and buried delth: (A) 150 days of straw returning, (B) (A + 150) days of straw returning; Figure S2: Straw decomposition rate caused by straw length and buried delth: (A) 150 days of straw returning, (B): (A + 150) days of straw returning; Table S1: Test design (1 m2); Table S2: The determination coefficient and residual path coefficient of PA; Table S3: The meteorological conditions of the experimental site during the test. Section S1: the distribution of carbon sources in ECO plates; Section S2: the straw amount in each experimental plot (1 m2); Section S3: the changes of straw decomposition rates caused by the interaction of straw amount and buried depth; Section S4: the changes of straw decomposition rates caused by the interaction of straw length and buried depth; Section S5: the partial results of the path analysis (determination coefficient and residual path coefficient); Section S6: The meteorological conditions of the experimental site. Data S1: original data.
Author Contributions
Y.N.: writing—original draft preparation; data analysis; mathematical modeling. X.W. and Y.Y.: indices determination—soil microbial community. X.C. and Y.W.: indices determination—straw decomposition rate. D.Z. (Detang Zou): overall design; reviewing and editing. D.Z. (Dongxing Zhou): overall design; writing—reviewing and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (42107327), Heilongjiang Provincial Natural Science Foundation of China (YQ2021D002), and the Project funded by China Postdoctoral Science Foundation (2022M710650), Heilongjiang Postdoctoral Science Foundation (LBH-Z21120).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
This article does not contain any studies with human applicants or animals performed by any of the authors.
Data Availability Statement
All data generated or analysed during this study are included in the online version of this article as Supplementary Material.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gu, Z.J.; Xie, Y.; Gao, Y.; Ren, X.Y.; Cheng, C.C.; Wang, S.C. Quantitative assessment of soil productivity and predicted impacts of water erosion in the black soil region of northeastern China. Sci. Total Environ. 2018, 637, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, G.; Chen, S.L.; Rasmussen, C.; Liu, B.Y. Assessing soil thickness in a black soil watershed in Northeast China using random forest and field observations. Int. Soil Water. Conse. 2021, 9, 49–57. [Google Scholar] [CrossRef]
- Shi, T.T.; Liu, Y.Q.; Zhang, L.B.; Hao, L.; Gao, Z.Q. Burning in agricultural landscapes: An emerging natural and human issue in China. Landsc. Ecol. 2014, 29, 1785–1798. [Google Scholar] [CrossRef]
- Yin, H.J.; Zhao, W.Q.; Li, T.; Cheng, X.Y.; Liu, Q. Balancing straw returning and chemical fertilizers in China: Role of straw nutrient resources. Renew. Sustain. Energy Rev. 2018, 81, 2695–2702. [Google Scholar] [CrossRef]
- Wang, M.Q.; Liu, Y.S.; Huang, Y.L.; Zhao, Y.Y.; Li, Z.X.; Han, Y.H. Research progress on effects of straw incorporation on soil micro-ecological environment. Microbiol. China 2022, 49, 807–816. [Google Scholar] [CrossRef]
- Liu, C.; Lu, M.; Cui, J.; Li, B.; Fang, C.M. Effects of straw carbon input on carbon dynamics in agricultural soils: A meta-analysis. Glob. Chang. Biol. 2014, 20, 1366–1381. [Google Scholar] [CrossRef]
- Nunes, C.; Joao, L.; Nogueirol, R.C.; Santos Menandro, L.M.; Bordonal, R.D.O.; Borges, C.D.; Cantarella, H.; Junqueira Franco, H.C. Agronomic and environmental implications of sugarcane straw removal: A major review. GCB Bioenergy 2017, 9, 1181–1195. [Google Scholar] [CrossRef]
- Sarkar, S.; Skalicky, M.; Hossain, A.; Brestic, M.; Saha, S.; Garai, S.; Ray, K.; Brahmachari, K. Management of crop residues for improving input use efficiency and agricultural sustainability. Sustainability 2020, 12, 9808. [Google Scholar] [CrossRef]
- Mabagala, F.S.; Geng, Y.H.; Cao, G.J.; Wang, L.C.; Wang, M.; Zhang, M.L. Silicon accumulation, partitioning and remobilization in spring maize (Zea mays L.) under silicon supply with straw return in Northeast China. J. Plant Nutr. 2020, 44, 1498–1514. [Google Scholar] [CrossRef]
- Cai, L.J.; Zhang, J.T.; Liu, J.Q.; Gai, Z.J.; Guo, Z.H.; Zhao, G.F. Effects of long-term no-tillage straw returning on soil organic carbon and soybean yield in cold region. Crops 2021, 6, 189–192. [Google Scholar] [CrossRef]
- Bordonal, R.D.O.; Nunes Carvalho, J.L.; Lal, R.; de Figueiredo, E.B.; de Oliveira, B.G.; Scala, N.L., Jr. Sustainability of sugarcane production in Brazil. A review. Agron. Sustain. Dev. 2018, 38, 13. [Google Scholar] [CrossRef] [Green Version]
- Yan, C.; Yan, S.S.; Jia, T.Y.; Dong, S.K.; Ma, C.M.; Gong, Z.P. Decomposition characteristics of rice straw returned to the soil in Northeast China. Nutr. Cycl. Agroecosys. 2019, 114, 211–224. [Google Scholar] [CrossRef]
- Chen, A.; Zhang, W.; Sheng, R.; Liu, Y.; Hou, H.; Liu, F.; Ma, G.; Wei, W.; Qin, H. Long-term partial replacement of mineral fertilizer with in situ crop residues ensures continued rice yields and soil fertility: A case study of a 27-year field experiment in subtropical China. Sci. Total Environ. 2021, 787, 147523. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Zeng, Y.; Wu, J.; Shi, Q.; Pan, X. Effect of crop residue retention on rice yield in China: A meta-analysis. Field Crop. Res. 2013, 154, 188–194. [Google Scholar] [CrossRef]
- Zhao, J.L.; Wang, X.G.; Zhuang, J.; Cong, Y.J.; Lu, Y.; Guo, M.Z. Fine-crush straw returning enhances dry matter accumulation rate of maize seedlings in Northeast China. Agronomy 2021, 11, 1144. [Google Scholar] [CrossRef]
- Xu, G.M.; Wang, X.C.; He, R.Y.; Ding, Q.S. Performance evaluation of rotary tillage straw returning based on composite indicators and measurement techniques. Trans. Chin. Soc. Agric. Mach. 2022, 53, 58–67. [Google Scholar] [CrossRef]
- Ma, L.J.; Kong, F.X.; Wang, Z.; Luo, Y.; Lv, X.B.; Zhou, Z.G.; Meng, Y.L. Growth and yield of cotton as affected by different straw returning modes with an equivalent carbon input. Field Crop. Res. 2019, 243, 107616. [Google Scholar] [CrossRef]
- Wang, D.; Sun, C.X.; Cui, M.; Shen, X.B.; Zhang, Y.L.; Xiao, J.H.; Liu, P.Y.; Zhang, Y.; Xie, H.T. An integrated analysis of transcriptome and metabolome provides insights into the responses of maize (Zea mays L.) roots to different straw and fertilizer conditions. Environ. Exp. Bot. 2022, 194, 104732. [Google Scholar] [CrossRef]
- Ji, B.Y.; Hu, H.; Zhao, Y.L.; Mu, X.Y.; Liu, K.; Li, C.H. Effects of deep tillage and straw returning on soil microorganism and enzyme activities. Sci. World J. 2014, 2014, 451493. [Google Scholar] [CrossRef]
- Li, Y.Z.; Song, D.P.; Dang, P.F.; Wei, L.N.; Qin, X.L.; Siddique, K.H.M. Combined ditch buried straw return technology in a ridge-furrow plastic film mulch system: Implications for crop yield and soil organic matter dynamics. Soil Tillage Res. 2020, 199, 104596. [Google Scholar] [CrossRef]
- Zhang, H.; Liang, S.; Wang, Y.H.; Liu, S.W.; Sun, H.D. Greenhouse gas emissions of rice straw return varies with return depth and soil type in paddy systems of Northeast China. Arch. Agron. Soil Sci. 2021, 67, 1591–1602. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, H.Y.; Yang, Z.H.; Jiang, Z.Z.; Tao, Y.Z.; Liu, C.Z.; Faheem, J.M.; Li, M. Effects of straw returning modes on maize seedling growth under different soil conditions. Chin. J. Ecol. 2022, 41, 479–486. [Google Scholar] [CrossRef]
- Ning, Y.C.; Zhou, H.R.; Zhou, D.X. Study on the microbial community in earthworm and soil under cadmium stress based on contour line analysis. Environ. Sci. Pollut. Res. 2019, 26, 20989–21000. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.X.; Su, Y.; Ning, Y.C.; Rong, G.H.; Wang, G.D.; Liu, D.; Liu, L.Y. Estimation of the effects of maize straw return on soil carbon and nutrients using response surface methodology. Pedosphere 2018, 28, 411–421. [Google Scholar] [CrossRef]
- Rong, G.H.; Ning, Y.C.; Cao, X.; Su, Y.; Li, J.; Li, L.; Liu, L.Y.; Zhou, D.X. Evaluation of optimal straw incorporation characteristics based on quadratic orthogonal rotation combination design. J. Agric. Sci. 2018, 156, 367–377. [Google Scholar] [CrossRef]
- Coffman, D.L.; MacCallum, R.C. Using parcels to convert path analysis models into latent variable models. Multivar. Behav. Res. 2005, 40, 235–259. [Google Scholar] [CrossRef]
- Hu, Y.F.; Zhang, M.K. Composition characteristics of aggregates in red sandstone soil and their responses to amendments. Acta Agric. Jiangxi 2019, 31, 88–93. [Google Scholar] [CrossRef]
- Yang, Z.J.; Guo, X.T.; Sun, J.; Zhang, Y.L. Contextual and organizational factors in sustainable supply chain decision making: Grey relational analysis and interpretative structural modeling. Environ. Dev. Sustain. 2021, 23, 12056–12076. [Google Scholar] [CrossRef]
- Liu, Y.L.; Huang, X.L.; Duan, J.; Zhang, H.M. The assessment of traffic accident risk based on grey relational analysis and fuzzy comprehensive evaluation method. Nat. Hazards 2017, 88, 1409–1422. [Google Scholar] [CrossRef]
- Gao, F.; Jia, Z.K.; Lu, W.T.; Han, Q.F.; Yang, B.P.; Hou, X.Q. Effects of different straw returning treatments on soil water, maize growth and photosyntheticcharacteristics in the semi-arid area of Southern Ninaxia. Acta Ecol. Sin. 2011, 31, 777–783. [Google Scholar]
- Zhang, P.; Wei, T.; Li, Y.L.; Wang, K.; Jia, Z.K.; Han, Q.F.; Ren, X.L. Effects of straw incorporation on the stratification of the soil organic C, total N and C:N ratio in a semiarid region of China. Soil Tillage Res. 2015, 153, 28–35. [Google Scholar] [CrossRef]
- Ma, F.X.; Wang, Y.Y.; Yan, P.; Wei, F.; Sun, X.Z.; Liu, J.G. Effects of cotton straw incorporation on organic nitrogen fractions in long-term continuous cropping cotton field. Ecol. Environ. Sci. 2018, 27, 1459–1465. [Google Scholar] [CrossRef]
- Long, Z.H.; Wang, J.; Hou, Z.A. Effects of cotton straw biochar returning and N application rate on soil organic nitrogen fractions in cotton field. J. Shihezi Univ. 2019, 37, 154–161. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Zhang, A.Z.; Jiang, N. Effects of mixed fungal fermentation on degradation rate of cellulose and lignin of corn straw. Chin. J. Anim. Nutr. 2019, 31, 1385–1395. [Google Scholar] [CrossRef]
- Zeng, Z.T.; Guo, X.Y.; Xu, P.A.; Xiao, R.; Huang, D.L.; Gong, X.M.; Cheng, M.; Yi, H.; Li, T.; Zeng, G.M. Responses of microbial carbon metabolism and function diversity induced by complex fungal enzymes in lignocellulosic waste composting. Sci. Total Environ. 2018, 643, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.L.; Han, G.M.; Lan, Y.; Liu, S.N.; Gao, J.P.; Yang, X.; Meng, J.; Chen, W.F. Corn cob biochar increases soil culturable bacterial abundance without enhancing their capacities in utilizing carbon sources in Biolog Eco-plates. J. Integr. Agric. 2017, 16, 713–724. [Google Scholar] [CrossRef] [Green Version]
- Ghasemi, A.; Asad, S.; Kabiri, M.; Dabirmanesh, B. Cloning and characterization of Halomonas elongata L-asparaginase, a promising chemotherapeutic agent. Appl. Microbiol. Biotechnol. 2017, 101, 7227–7238. [Google Scholar] [CrossRef]
- Pezzotti, F.; Therisod, H.; Therisod, M. Enzymatic synthesis of D-glucosaminic acid from D-glucosamine. Carbohydr. Res. 2005, 340, 139–141. [Google Scholar] [CrossRef]
- Bhat, U.R.; Forberg, L.S.; Carlson, R.W. Structure of lipid a component of Rhizobium leguminosarum bv. phaseoli lipopolysaccharide. Unique nonphosphorylated lipid A containing 2-amino-2-deoxygluconate, galacturonate, and glucosamine. J. Biol. Chem. 1994, 269, 14402–14410. [Google Scholar] [CrossRef]
- Wu, N.; Li, X.F.; Huang, G.S.; Pan, P.; Wang, C.; Liu, X.Y.; Zeng, M. Adsorption and biodegradation functions of novel microbial embedding polyvinyl alcohol gel beads modified with cyclodextrin: A case study of benzene. Environ. Technol. 2018, 40, 1948–1958. [Google Scholar] [CrossRef]
- Tribak, M.; Ocampo, J.A.; Garcia-Romera, I. Production of xyloglucanolytic enzymes by Trichoderma viride, Paecilomyces farinosus, Wardomyces inflatus, and Pleurotus ostreatus. Mycologia 2002, 94, 404–410. [Google Scholar] [CrossRef]
- Du, L.L.; Li, G.X.; Yuan, J.; Yuan, J.B. Effect of additives on NH3 and H2S emissions during kitchen waste composting. Trans. Chin. Soc. Agric. Eng. 2015, 31, 195–200. [Google Scholar] [CrossRef]
- Yan, J.L.; Zhang, M.K.; Wang, D.Z. Different amendments: Effect on soil physical properties of newly reclaimed land in low hilly region. Chin. Agric. Sci. Bull. 2021, 37, 67–73. [Google Scholar] [CrossRef]
- Zhang, G.W.; Yang, C.Q.; Liu, R.X.; Ni, W.C. Effects of p-hydroxybenzoic acid and phloroglucinol on mitochondria function and root growth in cotton (Gossypium hirsutum L.) seedling roots. Chin. J. Appl. Ecol. 2018, 29, 231–237. [Google Scholar] [CrossRef]
- Sun, S.S.; Gong, B.; Wen, D.; Wang, X.F.; Wei, M.; Yang, F.J.; Li, Y.; Shi, Q.H. Effect of exogenous melatonin on physiological and biochemical characteristics of cucumber radicles under p-hydroxybenzoic acid. Chin. J. Appl. Ecol. 2016, 27, 897–903. [Google Scholar] [CrossRef]
- Zhao, X.S.; Qi, Y.Z.; Yan, C.M.; Zhen, W.C. Allelopathy of six organic acids on wheat sheath blight in the soil of winter wheat-summer maize double cropping straw returning system. Sci. Agric. Sin. 2020, 53, 3095–3107. [Google Scholar] [CrossRef]
- Tang, L.L.; Nie, S.R.; Li, W.H.; Fan, C.; Wang, S.Q.; Wu, F.Z.; Pan, K. Wheat straw increases the defense response and resistance of watermelon monoculture to Fusarium wilt. BMC Plant Biol. 2019, 19, 551. [Google Scholar] [CrossRef] [Green Version]
- Qi, Y.Z.; Zhen, W.C.; Li, H.Y. Allelopathy of decomposed maize straw products on three soil-born diseases of wheat and the analysis by GC-MS. J. Integr. Agric. 2015, 14, 88–97. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Li, Y.B.; Teng, L.P.; Yue, J. Allelopathy of different decomposed liquids of cotton stalk on fusarium oxysporum and verticillium dahliae. J. Agro-Environ. Sci. 2012, 31, 1696–1701. [Google Scholar]
- Han, Z.; Di, C.Q.; Rahman, M.; Gao, D.M.; Wu, F.Z.; Pan, K. Repeated application of rice straw stabilizes soil bacterial community composition and inhibits clubroot disease. Agriculture 2021, 11, 108. [Google Scholar] [CrossRef]
- Chen, L.J.; Zhou, J.H.; Chen, G.; Liu, L.; Tan, J. Research progress of influence and mechanism of field straw residue incorporation on soil-borne diseases in crops. Crop Res. 2018, 32, 535–540. [Google Scholar] [CrossRef]
- Yang, L.P.; Yao, X.Y.; Li, Q.; Jiang, Q.P. An analysis of function diversity of soil microbes in relation to the outbreak of tobacco root rot. Plant Dr. 2020, 33, 36–41. [Google Scholar] [CrossRef]
- Zhang, S.T.; Liu, X.J.; Zhou, L.H.; Deng, L.Y.; Zhao, W.A.; Liu, Y.; Ding, W. Alleviating soil acidification could increase disease suppression of bacterial wilt by recruiting potentially beneficial rhizobacteria. Microbiol. Spectr. 2022, 10, e0233321. [Google Scholar] [CrossRef]
- Raj, A.; Reddy, M.M.K.; Chandra, R.; Purohit, H.J.; Kapley, A. Biodegradation of kraft-lignin by Bacillus sp isolated from sludge of pulp and paper mill. Biodegradatioin 2007, 18, 783–792. [Google Scholar] [CrossRef]
- Liu, X.C.; Wang, J.; Guo, S.H.; Chen, C.M. Effects of co-substrates and inorganic salts on degradation of crude oil by oil degrading bacteria. Environ. Prot. Chem. Ind. 2008, 3, 218–221. [Google Scholar] [CrossRef]
- Cheng, J.; Wu, D.; Chen, S.; Wu, J.; Chen, J. Effect of complex and synthetic medium on extracellular production of α-cyclodextrin glycosyltransferase in E. coli. China Biotechnol. 2010, 30, 36–42. [Google Scholar] [CrossRef]
- Hayward, M.R.; AbuOun, M.; Woodward, M.J.; Jansen, V.A.A. Temperature and oxygen dependent metabolite utilization by salmonella enterica serovars derby and mbandaka. PLoS ONE 2015, 10, e0120450. [Google Scholar] [CrossRef] [Green Version]
- Makonde, H.M.; Mwirichia, R.; Osiemo, Z.; Boga, H.I.; Klenk, H.P. 454 Pyrosequencing-based assessment of bacterial diversity and community structure in termite guts, mounds and surrounding soils. SpringerPlus 2015, 4, 471. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.X.; Wei, J.H.; Li, J.J.; Ni, J.F. Whole-genome analysis of the dominant bacterium Dysgonomonas macrotermitis in the hindgut of Macrotermes barneyi. Acta Microbiol. Sin. 2018, 58, 995–1003. [Google Scholar] [CrossRef]
- Gong, Z.P.; Deng, N.Z.; Song, Q.L.; Li, Z.T. Decomposing characteristics of maize straw returning in Songnen Plain in long-time located experiment. Trans. Chin. Soc. Agric. Eng. 2018, 34, 139–145. [Google Scholar] [CrossRef]
- Chen, L.J.; Chen, R.; Zhou, J.H.; Yan, C.B.; Liu, L.; Li, Q.; Zhang, Y. Decomposition characteristics of three crop straws and the effects of their decomposed liquids on phytophthora nicotianae. Chin. Tob. Sci. 2021, 42, 33–39. [Google Scholar] [CrossRef]
- Schmid, C.A.O.; Schroder, P.; Armbruster, M.; Schloter, M. Organic amendments in a long-term field trial-consequences for the bulk soil bacterial community as revealed by network analysis. Microb. Ecol. 2018, 76, 226–239. [Google Scholar] [CrossRef]
- Tang, L.L.; Xia, Y.; Kou, J.M.; Wu, F.Z.; Li, W.H.; Pan, K. Control of fusarium wilt by wheat straw is associated with microbial network changes in watermelon rhizosphere. Sci. Rep. 2020, 10, 12736. [Google Scholar] [CrossRef]
- Ma, L.; Li, Y.; Wei, J.L.; Li, Z.S.; Zhou, X.L.; Zheng, F.L.; Wu, X.B.; Wang, L.; Liu, Z.H.; Tan, D.S. Effects of long-term straw returning on fungal community, enzyme activity and wheat yield in a fluvo-aquic soil. Environ. Sci. 2022. [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).