Abstract
Nitrification inhibitors are commonly used to prevent nitrate leaching. However, the use of nitrification inhibitors is not free of side-effects. Some may be absorbed by the plant and cause phytotoxicity or even affect the food chain. Therefore, a solution that limits the absorption of nitrification inhibitors and its accumulation by the plant may mitigate health and environmental issues potentially associated with high levels of nitrification inhibitors. This solution may relay in the modulation of the plant’s metabolism through the interaction with specific fungal partners. This work tested the hypothesis that the symbiotic interaction between fungi and plant roots can reduce the destructive effects of the nitrification inhibitor Dicyandiamide (DCD) in plants by reducing the uptake of nitrification inhibitors. A greenhouse experiment was conducted, using a complete randomized block design, to test the effect of symbiotic fungi (plants inoculated with Piriformospora indica, Glomus etunicatum, and Glomus mosseae and noninoculated) on the phytotoxicity of DCD applied at four concentrations (0, 5, 50, and 100 mg kg−1 soil). Latuca sativa, cultivar Siyahoo, was selected for this experiment due to its economic value all over the world. The use of high DCD concentrations (100 mg kg−1 soil) affected the leaf chlorophyll content and plant growth in a manner that was significantly mitigated by the symbiosis of the plant with the fungal partner. These results highlight the benefits of using symbiotic fungal inoculants as plant protectors against the phytotoxic effects of DCD.
1. Introduction
Nitrate is one of the most important forms of nitrogen in agricultural soils. At any time, the soil nitrate concentration is the balance between the inputs (mainly fertilizer) and the outputs (biological transformations, biological immobilization and transformation, and leaching) where nitrifying microorganisms play a key role. In the soil, these organisms convert ammonium to nitrite and then nitrite to nitrate during the nitrification process [1]. Nitrate in soils is mainly lost through leaching, which, in addition to the loss of nitrogen, causes environmental pollution. One of the most important ways to control the amount of nitrate in the soil and reduce its loss is to use nitrification inhibitors (NIs). In fact, nitrification inhibitors are compounds that delay the bio-oxidation of ammonium to nitrite without affecting the oxidation of nitrite to nitrate [2]. This is carried out by preventing or interfering with the metabolism of bacteria that are effective in nitrite generation (such as Nitrosomonas) [3]. Thus far, several types of nitrification inhibitors have been marketed: 2-chloro-6-(trichloromethyl)-pyridine, dicyandiamide (DCD), and 3,4-dimethylpyrazole phosphate (DMPP) are examples of those widely used. Research shows that the use of nitrate inhibitors can have negative effects on other soil organisms (population of fungi and bacteria) and the plants grown in it [4]. Extensive studies have been performed on the toxicity of NIs such as DMPP toxicity on clover [5]. Zerulla et al. [6] observed that the application of 1.5 kg ha−1 of DCD in lettuce causes severe leaf margin burns. In addition, the use of triazole compounds such as 1,2,4-Triazole (TZ), when applied in doses between 0.2 and 10 mg L−1, disrupts the nitrification process and can have a negative effect on other bacteria (aerobic and methane-oxidizing heterotrophic bacteria) [7].
In addition to having a direct effect on plant growth, symbiotic fungi can also protect plants against abiotic stresses through distinct mechanisms, increasing crop productivity and quality. Among these microorganisms, arbuscular and endophytic fungi able to form symbiosis with plant roots are the best characterized. They are known to increase plant resistance to biotic and abiotic stresses through several mechanisms, including the production of various metabolites [8]. P. indica is an important plant endophytic fungus that increases plant growth under normal conditions and stress under various mechanisms [9]. This microorganism strengthens the antioxidant defense system of plants, which plays an important role in stress tolerance, and induces disease resistance in plants by increasing the level of antioxidants to combat oxidative stress [10]. There are several reports of the relevance of mycorrhizal symbioses for the degradation of several contaminants. For instance, ryegrass inoculation with Glomus sp. reduces the impact of petroleum hydrocarbons, especially pyrine, on plant growth [11], and the inoculation of alfalfa with G. mosseae or G. etunicatum removed more than 98% of phenanthrene and 88% of pyrine accumulated in the plants [12].
According to the studies, there is a possibility of accumulation and increase in nitrification inhibitors in the soil, and, over the years, its amount increases in agricultural soils. The toxicity of nitrate inhibitors to soil biota, and the possibility of their accumulation in plant tissues grown in soils to which these substances are added, needs a solution, an issue that, to our knowledge, has not been researched. Due to the economic relevance of lettuce, and due to its high consumption as a leafy salad, and the broad use of DCD, this study aimed to investigate the role of mycorrhizal and endophytic fungi (Glomus mosseae, Glomus etunicatum, and P. indica) as possible tools to reduce the phytotoxicity of DCD. In addition, high concentrations of DCD were used to better understand the effects of the adverse accumulation of nitrification inhibitors.
2. Materials and Methods
2.1. Experimental Design
The experimental design was a factorial randomized block design including one plant species (lettuce, Latuca sativa, cultivar Siyahoo), four fungi treatments (G. etunicatum, G. mosseae, P. indica, and no inoculation), and four doses of DCD (0, 5, 50, and 100 mg kg−1 soil) with four replicates per treatment.
2.2. Soil Preparation and Dicyandiamide Treatment
A combined sample of a sandy loam soil (from 0 to 25 cm layer) was collected from the surface horizon of the Mohaghegh Ardabili University Campus farm and then physicochemically characterized: 68% sand, 20% silt, 12% clay, 1.2% organic matter, 0.05% total N, 7 mg kg−1 available P, 35 mg/kg available K, 0.7 mg/kg total Cd, a pH of 7.3, and 1.3 ds m−1 EC. Then, DCD powder (Sigma-Aldrich, Burlington, MA, USA) was added to the surface of 1 kg of air-dried soil samples to provide the following concentrations: 0, 5, 50, and 100 mg kg−1 soil [13]. Soil amended with inhibitors was thoroughly mixed to achieve a homogeneous DCD soil concentration. Nitrogen as (NH4)2SO4 (Merck, Darmstadt, Germany) was applied in aqueous solution at a rate of 40 mg kg−1 soil [14]. An amount of 10 kg of soil, with the distinct DCD concentrations, was transferred into pots with a diameter of 25 cm and a depth of 40 cm.
2.3. Plant Growth Conditions
Lettuce seeds (Lactuca sativa cv. Siyahoo) were prepared from the Seed Bank Institute of Karaj, Iran. The seeds were sown in trays containing peat moss. When the seedlings reached the two-leaf stage, they were transferred to pots with nitrification inhibitors and inoculated using the fungi (22/18 °C day/night cycle, 60–70% relative humidity, and a photoperiod of 14 h).
2.4. Seed Inoculation with Fungi
P. indica was cultured in Petri dishes on a Hill and Käfer medium [15]. The plates were placed in a growth chamber at 29 ± 1 °C in the dark for 2 weeks. To impose the fungal treatment, one fungal plug of 10 mm in diameter was placed at a distance of 1 cm below the roots of 10-days-old lettuce seedlings.
Mycorrhizal fungi were prepared at the Department of Soil Science, University of Tabriz (Professor Nasser Aliasgharzad). The inocula of G. mosseae and G. etunicatum fungals were added to the soil of the pot, and 1 cm of soil was covered and then lettuce seedlings were placed on it. The number of spores in the inoculum was 33–35 per gram and root colonization was 78.8% [16].
2.5. SEM and Plant Growth Measurements
After 45 days of planting, the shoots of lettuce were harvested and the roots were separated from the soil substrate. Then, some young leaves were selected for imaging using scanning electron microscopy (SEM). The samples selected for imaging were dried in the shade and then imaging was performed using an electron microscope in the Department of Chemistry, Mohaghegh Ardabili University. Then, the stomata area was calculated using the obtained photos. AutoCAD software was used to measure the stomata area.
The shoots and roots were rinsed with distilled water, wiped with tissue paper, and weighted. The shoots and roots were separated, the shoot height and leaf number per plant were determined, and they were finally dried at 75 °C for 48 h in order to determine the dry weights (shoots and roots). Some root segments were placed in 50% ethanol to determine the root colonization.
2.6. Root Colonization
The Phillips and Hayman [17] method was used to color the root and determine its colonization. The roots were heated in 10% KOH solution for 5 min and then washed under water. Root samples with 1% HCl were acidified for 1 min, immersed in 20% trypan blue staining solution, and heated for 10 min. From the stained samples, 30 root segments (1 cm long) per plant were cut and observed with a light microscope (Olympus BH-2) at 20×. Root colonization was determined according to the gridline intersection method [18]. In this technique, the percentage of root colonization per plant was determined by dividing the total number of colonized root fragments (either with arbuscules, vesicles, or hyphae) by the total number of root pieces examined ×100.
2.7. Total Chlorophyll and Carotenoids Contents
Measurements of chlorophyll and carotenoids were performed using Arnon’s method [19]. Chlorophyll and carotenoids were extracted from a sample of 1 g of fresh leaves in acetone 80% (v/v). Absorption was measured at 645 and 663 nm for chlorophyll and 480 and 510 for carotenoids using a spectrophotometer (UV-600). The concentration of total chlorophyll and carotenoids was expressed as µg mL−1 fresh weight and determined using the formula:
Chlorophyll a = [15.65 (A663) − 7.340 (A645)]
Chlorophyll b = [27.05 (A645) − 11.21 (A663)]
Total chlorophyll = [20.2 (A645) + 8.02 (A663)] × V/W 1000
Carotenoids = [7.6(A480) − 1.49(A510)] × V/W 1000
2.8. Measurement of DCD in Lettuce Leaves
DCD concentration was measured using the method of Kim et al. [20]. To do this, first, a young leaf was selected from each plant (leaf sample should be the same in all plants). Then, 1 g of leaves was weighed and 40 mL of deionized water was added to it and placed on a shaker for 20 min. At the end of 20 min, the plant sample was completely crushed using a mortar and the resulting sample was passed through Whatman filter paper. Each of the 5 mL of the filtrate obtained from the two extractions of surface residues above was acidified with 0.2 mL of 0.66 M H2SO4 and allowed to stand for 30 min before centrifuging (10,000 rpm for 15 min equivalent to 11,180× g) to remove precipitated material and optimize the pH to the HPLC conditions. The concentration of DCD in the acidified supernatant was determined on a Waters 2695 HPLC using a cation-H guard column (30 × 4.6 mm internal diameter; ID) with a 0.025 M H2SO4 mobile phase at a flow rate of 0.6 mL min−1 and a 210 nm ultraviolet (UV) spectrophotometric detector.
2.9. Statistical Analysis
Two-way ANOVA was performed on all experimental data using IBM SPSS version 23 software (Chicago, IL, USA). The variance was related to the main factors (symbiotic fungi and DCD) and to the interaction between them. The differences between means were determined using Duncan’s Multiple Range Test at 0.05 and 0.01 probability levels.
3. Results and Discussion
3.1. Effect of DCD on Root Colonization by P. indica, G. etonicatum, and G. mosseae
Most arable soils are now under severe human management, and various agricultural and horticultural chemicals (such as pesticides, fungicides, and nitrification inhibitors) are applied to maintain and/or increase soil productivity [21]. Soil microbiota are commonly exposed to these chemical inputs, which have potential effects on the abundance, structure, and functionality of each community and its interactions with the communities of other microorganisms [22,23]. For instance, nitrification inhibitors, such as 3,4-dimethylpyrazole phosphate (DMPP), may interact with the nitrification community and, at the same time, reduce or suppress soil nitrogenase activity [24]. Shi et al. [25] also found that nitrification inhibitor phosphate DMPP inhibited nitrifier-induced denitrification, which is an important source of soil nitrous oxide. However, due to the nature of their physiology and their involvement in decomposition and nutrient turnover, fungi may be more responsive to soil inputs than another biota. Yet, very few studies have been performed to elucidate the impacts of agrochemical use on soil fungal communities [22,26] and on the symbiotic relationships between plants and fungi [27,28].
Our results clearly show that soil DCD concentration had a significant (p < 0.01) effect on the colonization of lettuce root by P. indica, G. etonicatom, and G. mosseae (Table 1). No significant differences were observed for the root colonization by each fungi at the same DCD concentration, and all the fungi presented the same root colonization pattern: a decrease in the colonization rate from 0 to 5, no big differences from 5 to 10, followed by a sharp decrease in the colonization rate from 50 to 100 mg kg−1 DCD, which, according to the impact that endophytic (P. indica) and mycorrhizal (G. etunicatum and G.mosseae) fungi have on soil and plants, can anticipate changes at the level of soil and plant functionality such as those found by Yu et al. [29] and Marschner [30].

Table 1.
Effect of distinct DCD concentrations on root colonization by Piriformospora indica (P.i), Glomus etunicatum (G.e), and Glomus mosseae (G.m), shoot dry weight, shoot height, leaf number, root fresh weight, and root length of lettuce plants.
The mechanisms through which nitrification inhibitors may reduce root colonization are debatable, but some studies have pointed to direct and undirect effects related to the increase in soil NH4+ concentration associated with nitrification inhibition. It has been observed that high soil NH4+ concentration inhibits spore germination of arbuscular mycorrhizal fungi [31], while Marschner [30] found that the increase in soil NH4+ concentration caused mitochondrial swelling and cracking of plant root cells, which resulted in reduced root colonization by mycorrhizal fungi. The hypotheses of the inhibitory effect of DCD on fungal root colonization being mediated by the toxic effect of ammonium on plant development may be supported by the observed effect of the soil concentration of DCD on plant root development (Table 1). Figure 1 also shows root colonization by symbiotic fungi.

Figure 1.
Images of root colonization by symbiotic fungi in the roots of lettuce.
3.2. Root Fresh Weight and Root Length
Our results showed that root fresh weight per plant was not significantly affected by the fungal colonization when DCD was not present in the soil. However, in the presence of DCD, the inhibition of root fresh weight was more evident in plants that were not colonized by fungi, especially at high DCD concentrations (Table 1). DCD affected root length in a similar way as root fresh weight. In general, plants inoculated with symbiotic fungi had more developed root systems than noninoculated plants, and fungal colonization protected roots from DCD inhibition. In fact, if we think that the symbiotic fungi under study are good ammonium scavengers [32], it can be argued that they may protect plant roots from ammonium toxicity. If the inhibitory effect of DCD on root colonization by symbiotic fungi is mediated by the ammonium toxicity at the root level, then the effect of DCD on fungal root colonization should differ from plant to plant according to the plant tolerance to ammonium nutrition [33].
As DCD may be absorbed by plant roots [34,35,36], one could argue about a direct effect of DCD on plant metabolism. However, this has to be a direct effect of the DCD itself as plants are not able to metabolize it [35] and the DCD taken up by plant roots accumulates in the roots or travels to the upper organs and accumulates there [34]. This brings the possibility of DCD affecting shoot metabolic activities, including photosynthesis, and by decreasing the carbon available to exchange with the fungi [35], affecting the root colonization by the symbiotic fungi (and the entire plant microbiota).
3.3. The Effect of DCD on Shoot Development
Without DCD, the symbiosis with P. indica stimulated, and those with G. etunicatum and G. mosseae did not significantly affect, shoot development of lettuce plants (p < 0.05). However, in the presence of DCD, the symbiosis with P. indica or with G. etunicatum was more effective in preventing the inhibitory effect of DCD than that with G. mosseae. Without the symbiotic partner, increasing the soil DCD concentration from 0 to 100 mg/kg decreased the shoot fresh and dry weights but increased the shoot fresh weight to dry weight ratio by about 50% (from 19.5 to 30.7), implying a much higher level of water retention in the leaf. Combining this observation with the fact that no stomata closure was observed in response to DCD treatment, it can be inferred that the water continuum between soil-plant and atmosphere was not disrupted by DCD. Thus, DCD did not cause water stress to the lettuce plants, which is consistent with the findings of Zerulla et al. [6].
Symbiotic fungi (and other biofertilizers) may promote shoot development under stress conditions [37,38] by ameliorating plant nutrition or stress. The effect of symbiotic fungi in improving shoot development in the presence of DCD was particularly evident for Piriformospora indica (Figure 2 and Figure 3). Symbiotic fungi such as P. indica and G. etunicatum may increase the production of photosynthetic pigments or reduce their degradation by helping the plant to overcome the stress conditions, in this case, driven by high DCD conditions [39,40]. Growth inhibition and reduction in leaf chlorophyll (Figure 4 and Figure 5) by DCD have been observed for plants grown under diverse conditions [41].
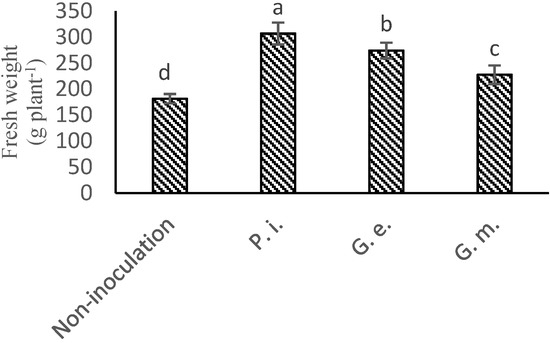
Figure 2.
The mean effect of P. indica, G. etunicatum, and G. mosseae in lettuce plant fresh weight. (The means refer to four repetitions ± SD. Different letters for each parameter indicate significant differences assessed (p < 0.05).)

Figure 3.
The main effect of dicyanodiamide in lettuce plant fresh weight. (The means refer to four repetitions ± SD. Different letters for each parameter indicate significant differences assessed (p < 0.05).)
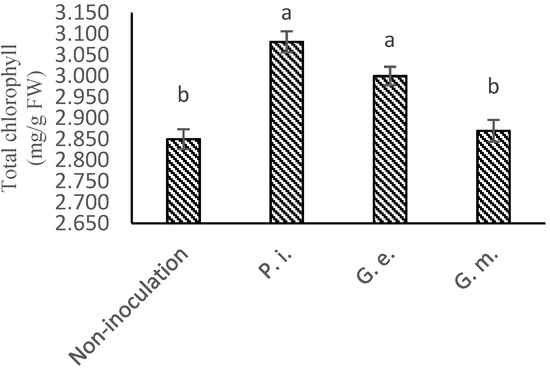
Figure 4.
The mean effect of P. indica, G. etunicatum, and G. mosseae in leaves of lettuce on total chlorophyll content. (The means refer to four repetitions ± SD. Different letters for each parameter indicate significant differences assessed (p < 0.05).)

Figure 5.
The main effect of dicyanodiamide in leaves of lettuce on total chlorophyll content. (The means refer to four repetitions ± SD. Different letters for each parameter indicate significant differences assessed (p < 0.05).)
Concentrations of 50 and 100 mg kg−1 DCD increased leaf carotenoid concentration by 34% and 60%, respectively, compared to control plants (Figure 6). Carotenoids are defense compounds with the role of antioxidants that neutralize the harmful effects of reactive oxygen species formed in excess under stress conditions [42]. Altogether, the decrease in leaf chlorophyll concentration and the increase in leaf carotenoid concentration may indicate that DCD is a cause of leaf oxidative stress.
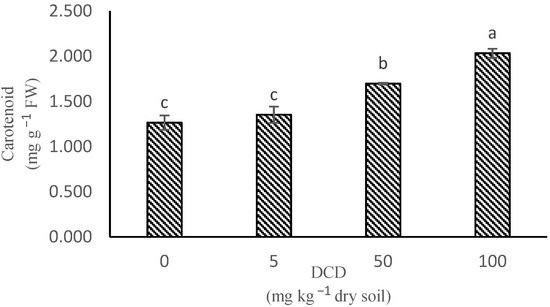
Figure 6.
The main effect of dicyanodiamide in leaves of lettuce on carotenoid content. (The means refer to four repetitions ± SD. Different letters for each parameter indicate significant differences assessed (p < 0.05).)
3.4. Dicyandiamide in the Leaves
Leaf analyses showed that DCD can accumulate in lettuce leaves, especially when present at high concentrations in the root medium, and those symbiotic fungi may decrease this accumulation (Figure 7). P. indica was more efficient in restraining leaf DCD concentrations than G. etunicatum or G. mosseae, showing that at least some fungi may be relevant to reduce the phytotoxicity of nitrification inhibitors. However, the mechanism through which the fungi act is not clear yet [43]. Fungi may help the plant to overcome the stress caused by high DCD concentrations, but they may also accelerate DCD degradation in the soil or inside the plant [2,44,45]. Two independent experiments performed by Binet et al. [46] and Aranda et al. [47] showed that mycorrhiza significantly reduced the concentration of anthracene in plants by 20–40% compared to the control. The work by Zhou et al. [48] highlighted the capacity of mycorrhizal fungi to protect Medicago sativa plants against the toxic effects of polycyclic aromatic hydrocarbon. It is possible that pollutant compounds such as DCD adsorb to the fungal cell walls, which would decrease the free concentration of the compounds to be absorbed by the plant [43]. Figure 8A–M show the result of HPLC, which is given below to complete the topic and increase understanding.
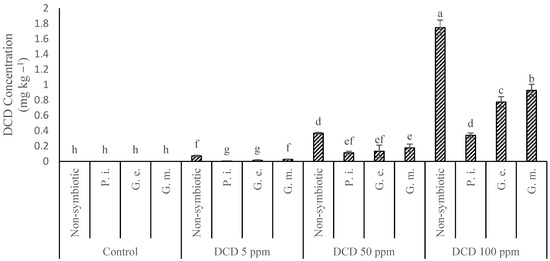
Figure 7.
Effect of Piriformospora indica, Glomus mosseae, and Glomus etunicatum on the dicyandiamide content under different concentrations of dicyandiamide nitrification inhibitor in lettuce. (The means refer to four repetitions ± SD. Different letters for each parameter indicate significant differences assessed (p < 0.05).)
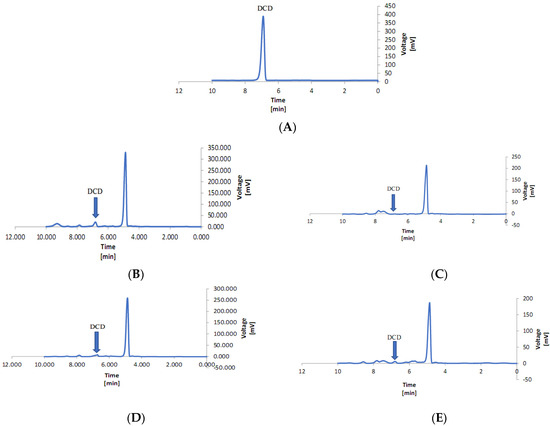

Figure 8.
Effect of Piriformospora indica, Glomus etunicatum, and Glomus mosseae on dicyandiamide content in Lettuce leaves. (A) Standard chart of the dicyandiamide, (B) 5 mg kg −1 of dicyandiamide, (C) Piriformospora indica + 5 mg kg −1 of dicyandiamide, (D) Glomus etunicatum + 5 mg kg−1, (E) Glomus mosseae + 5 mg kg −1 of dicyandiamide, (F) 50 mg kg −1 of dicyandiamide, (G) Piriformospora indica + 50 mg kg −1 of dicyandiamide, (H) Glomus etunicatum + 50 mg kg −1 of dicyandiamide, (I) Glomus mosseae + 50 mg kg −1 of dicyandiamide, (J) 100 mg kg −1 of dicyandiamide, (K) Piriformospora indica + 100 mg kg −1 of dicyandiamide, (L) Glomus etunicatum + 100 mg kg −1 of dicyandiamide, and (M) Glomus mosseae + 100 mg kg −1 of dicyandiamide.
3.5. Effect of Dicyandiamide on Stomatal Behavior
In the present study, no stomata closure was observed in response to DCD treatment (Figure 9). Researchers have shown that the presence of substances such as salt [49], heavy metals [50,51], phytohormones [52], and temperature changes and drought can affect the behavior of leaf stomata and the opening and closing of guard cells. However, the effect of nitrification inhibitors on plant leaf stomata has not been studied. In this study, the presence of high amounts of DCD caused the leaf stomata of lettuce to close. Nitric oxide (NO) affects various physiological functions, increasing plant tolerance to environmental stresses including salinity, toxic metals, and high and low temperatures by affecting the opening and closure of plant stomata [49,50,53]. Wu et al. [54] reported for the first time that the use of nitrification inhibitors prevents the production of N2O and NO greenhouse gases in the soil by acting on the bacteria that perform nitrification. Therefore, it is likely that DCD reduces NO concentration in the plant by inhibiting NO production and does not close the stomata. According to the results of previous studies and the results of the present experiment, reducing NO reduces plant growth.
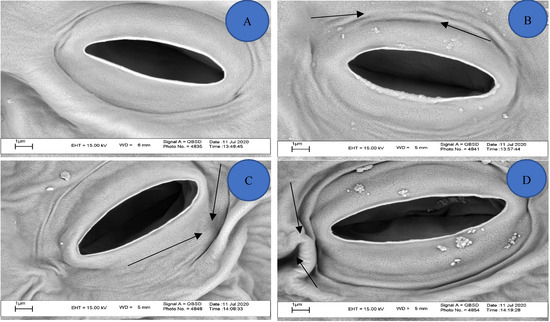
Figure 9.
Effect of DCD on lettuce leaf stomata. (A) Control, (B) concentration of 5 mg/kg DCD in soil, (C) concentration of 50 mg/kg DCD in soil, and (D) concentration of 100 mg/kg DCD in soil.
3.6. Stomata Area in the Leaves
Analysis of the pore area of the leaf stomata showed that DCD can reduce the pore area of the stomata in lettuce leaves, especially when present in high concentrations in the root environment, and symbiotic fungi may increase this stomata area (Table 2). The use of P. indica increased the pore stomata area per leaf compared to control plants. In addition, the lowest pore stomata area was presented by plants that were treated with 100 mg kg−1 DCD and without the use of symbiotic fungi (Table 2). The concentration of 5 mg kg−1 in all treatments with control plants was not significantly different, except for the interaction of 5 mg kg−1 DCD with P. indica. Vahabi et al. [55] reported that P. indica reduces stomata closure and plays an important role in increasing pore stomata size.

Table 2.
Effect of Piriformospora indica, Glomus mosseae, and Glomus etunicatum on the stomata pore area (µm2) under different concentrations of DCD in lettuce.
3.7. Effect of Dicyandiamide on Appearance of Leaves
The use of concentrations of 50 and 100 mg kg−1 DCD caused margin burning of the lower leaves (primary leaves) of lettuce (Figure 10). These symptoms appeared in 100 and 50 mg kg−1 DCD treatments 14 and 21 days after transplanting, respectively. Thus, at 50 mg kg−1 DCD, leaf margin burn was not observed in lettuce plants inoculated with P. indica and G. etunicatum. In plants inoculated with G. mosseae after 18 days of seedling cultivation, leaf burn also appeared in the presence of a DCD inhibitory concentration of 50 mg kg−1. This burn was much lesser than that of noninoculated plants with symbiotic fungi at a concentration of 50 mg kg−1 DCD. The use of DCD inhibitors at a concentration of 100 mg kg−1 caused burn marks on the leaves of all lettuce plants, but this burn in plants inoculated with P. indica, G. etunicatum, and G. mosseae was much lesser than that of noninoculated plants. In a study by Pal et al. [56], it was observed that 6.3% of DCD used in the meadow is absorbed by the plant roots within 37 days. They also stated that the plant could not metabolize the DCD inhibitor absorbed by the roots or leaves. The main reason for the harmful effects of DCD compared to other nitrification inhibitors is its use in higher concentrations, which causes side-effects on plant yield [4]. Recent research on the toxicity of DCD on plants has also shown the harmful effects of DCD on plant yield [6]. Leaf chlorosis has also been reported in plants such as clover [57] and lettuce [6]. Research has shown that DCD is absorbed by plants and that the plant cannot metabolize it. As a result, finding a way to prevent the absorption of DCD is an important issue in agriculture and the environment.

Figure 10.
Effect of dicyandiamide on appearance of leaves.
4. Conclusions
Our results showed that symbiotic fungi can, to some extent, prevent the accumulation of DCD nitrification inhibitor in the plant. In addition, the effects of DCD on the plant are very thought-provoking. Decreased root length, root colonization, and stomata area were among the significant effects of DCD at high concentrations (50 and 100 mg kg−1). However, the most important parameter that was the main purpose of this study was the concentration of DCD in the leaf and the effect that symbiotic fungi had on its reduction. According to the results, all fungi had some effect on reducing the absorption of DCD, and the greatest effect was related to the P. indica. This issue needs further research, and controlling the accumulation of nitrification inhibitors using organic methods can be the biggest challenge in agriculture.
Author Contributions
Conceptualization, R.A. and C.C.; methodology, A.P. and A.A.S.T.; validation, A.P., C.C. and R.A.; formal analysis, A.P. and R.A.; data curation, A.P. and R.A; writing—original draft preparation, A.P., C.C. and R.A.; writing—review and editing, C.C., R.A., B.E., A.P. and A.A.S.T.; supervision, R.A. and C.C.; project administration, R.A., funding acquisition, C.C., R.A. All authors have read and agreed to the published version of the manuscript.
Funding
The SOILdarity project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 952051.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
We would like to thank the University of Mohaghegh Ardabili, Ardabil, Iran, for its support during the implementation of this project, which was also financially supported by Portuguese funds through Fundação para a Ciência e a Tecnologia (project UIDB/00329/2020).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bock, E.; Wagner, M. Oxidation of Inorganic Nitrogen Compounds as an Energy Source. In The Prokaryotes; Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 457–495. [Google Scholar]
- Li, J.H.; Yu, X.Z.; Wu, S.C.; Wang, X.R.; Wang, S.H.; Tam, N.F.Y.; Wong, M.H. Responses of Bioaugmented Ryegrass to Pah Soil Contamination. Int. J. Phytoremed. 2011, 13, 441–455. [Google Scholar] [CrossRef] [PubMed]
- Ruser, R.; Schulz, R. The effect of nitrification inhibitors on the nitrous oxide (N2O) release from agricultural soils—A review. J. Plant Nutr. Soil Sci. 2015, 178, 171–188. [Google Scholar] [CrossRef]
- Yang, M.; Fang, Y.; Sun, D.; Shi, Y. Efficiency of two nitrification inhibitors (dicyandiamide and 3,4-dimethypyrazole phosphate) on soil nitrogen transformations and plant productivity: A meta-analysis. Science 2016, 6, 22075. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.M.; Lasa, B.; Aparicio-Tejo, P.M.; González-Murua, C.; Marino, D. 3,4-Dimethylpyrazole phosphate and 2-(N-3,4-dimethyl-1H-pyrazol-1-yl) succinic acid isomeric mixture nitrification inhibitors: Quantification in plant tissues and toxicity assays. Sci. Total Environ. 2018, 624, 1180–1186. [Google Scholar] [CrossRef] [PubMed]
- Zerulla, W.; Barth, T.; Dressel, J.; Erhardt, K.; Horchler von Locquenghien, K.; Pasda, G.; Rädle, M.; Wissemeier, A.H. 3,4-Dimethylpyrazole phosphate (DMPP)—A new nitrification inhibitor for agriculture and horticulture. An introduction. Biol. Fertil. Soils 2001, 34, 79–84. [Google Scholar] [CrossRef]
- Lal, K.; Kumar, L.; Kumar, A.; Kumar, A. Oxazolone–1,2,3-Triazole Hybrids: Design, Synthesis and Antimicrobial Evaluation. Curr. Top. Med. Chem. 2018, 18, 1506–1513. [Google Scholar] [CrossRef] [PubMed]
- Wani, P.A.; Omozele, A.B. Cr (VI) removal by indigenous Klebsiella species PB6 isolated from contaminated soil under the influence of various factors. Curr. Res. Bacteriol. 2018, 8, 62. [Google Scholar] [CrossRef]
- Varma, A.; Sativa, S.; Sahay, N.; Butehorn, B.; Franken, P. Piriformospora indica, a cultivable plant growth-promoting root endophyte. Appl. Environ. Microbiol. 1998, 65, 2741–2744. [Google Scholar] [CrossRef]
- Vadassery, J.; Tripathi, S.; Prasad, R.; Varma, A.; Oelmüller, R. Monodehydroascorbate reductase 2 and dehydroascorbate reductase 5 are crucial for a mutualistic interaction between Piriformospora indica and Arabidopsis. J. Plant Physiol. 2009, 166, 1263–1274. [Google Scholar] [CrossRef]
- Yu, X.Z.; Wu, S.C.; Wu, F.Y.; Wong, M.H. Enhanced dissipation of PAHs from soil using mycorrhizal ryegrass and PAH-degrading bacteria. J. Hazard. Mater. 2011, 186, 1206–1217. [Google Scholar] [CrossRef]
- Gao, Y.; Li, Q.; Ling, W.; Zhu, X. Arbuscular mycorrhizal phytoremediation of soils contaminated with phenanthrene and pyrene. J. Hazard. Mater. 2011, 185, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Maftoun, M.; Sheibany, B. Comparative phytotoxicity of several nitrification inhibitors to soybean plants. J. Agric. Food Chem. 1979, 27, 1365–1368. [Google Scholar] [CrossRef]
- Maftoun, M.; Banihashemi, Z. Effects of added sulfur, aluminium sulfate and ferrous sulfate on CO2 evolution, microbial population and nitrification in alfalfa-and straw-amended soil. Agrochimica 1981, 25, 318–326. [Google Scholar]
- Hill, T.W.; Käfer, E. Improved protocols for Aspergillus minimal medium: Trace element and minimal medium salt stock solutions. Fungal Genet. Newsl. 2001, 48, 20–21. [Google Scholar] [CrossRef]
- Hajiboland, R.; Aliasgharzadeh, N.; Laiegh, S.F.; Poschenrieder, C. Colonization with arbuscular mycorrhizal fungi improves salinity tolerance of tomato (Solanum lycopersicum L.) plants. Plant Soil 2010, 331, 313–327. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots andstaining parasitic and vesicular-arbuscular mycorrhizal fungi for rapidassessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Giovannetti, M.; Mosse, B. An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol. 1980, 84, 489–500. [Google Scholar] [CrossRef]
- Arnon, A. Method of extraction of chlorophyll in the plants. Agron. J. 1967, 23, 112–121. [Google Scholar]
- Kim, D.G.; Giltrap, D.; Saggar, S.; Palmada, T.; Berben, P.; Drysdale, D. Fate of the nitrification inhibitor dicyandiamide (DCD) sprayed on a grazed pasture: Effect of rate and time of application. Soil Res. 2012, 50, 337–347. [Google Scholar] [CrossRef]
- Yadav, I.C.; Devi, N.L.; Syed, J.H.; Cheng, Z.; Li, J.; Zhang, G.; Jones, K.C. Current status of persistent organic pesticides residues in air, water, and soil, and their possible effect on neighboring countries: A comprehensive review of India. Sci. Total Environ. 2015, 511, 123–137. [Google Scholar] [CrossRef]
- Coppola, L.; Comitini, F.; Casucci, C.; Milanovic, V.; Monaci, E.; Marinozzi, M.; Taccari, M.; Ciani, M.; Vischetti, C. Fungicides degradation in an organic biomixture: Impact on microbial diversity. New Biotechnol. 2011, 29, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Maienza, A.; Bååth, E.; Stazi, S.R.; Benedetti, A.; Grego, S.; Dell’Abate, M.T. Microbial dynamics after adding bovine manure effluent together with a nitrification inhibitor (3,4 DMPP) in a microcosm experiment. Biol. Fertil. Soils 2014, 50, 869–877. [Google Scholar] [CrossRef]
- Florio, A.; Maienza, A.; Dell’Abate, M.T.; Stazi, S.R.; Benedetti, A. Changes in the activity and abundance of the soil microbial community in response to the nitrification inhibitor 3,4-dimethylpyrazole phosphate (DMPP). J. Soils Sediments 2016, 16, 2687–2697. [Google Scholar] [CrossRef]
- Xiuzhen, S.; Hang-Wei, H.; Zhu-Barker, X.; Hayden, H.; Wang, J.; Suter, H.; Chen, D.; Ji-Zheng, H. Nitrifier-induced denitrification is an important source of soil nitrous oxide and can be inhibited by a nitrification inhibitor 3,4-dimethylpyrazole phosphate. Environ. Microbiol. 2017, 19, 4851–4865. [Google Scholar]
- Sigler, W.V.; Turco, R.F. The impact of chlorothalonil application on soil bacterial and fungal populations as assessed by denaturing gradient gel electrophoresis. Appl. Soil Ecol. 2002, 21, 107–118. [Google Scholar] [CrossRef]
- Evelin, H.; Kapoor, R. Arbuscular mycorrhizal symbiosis modules antioxidant response in salt stressed Trigonella foenum-graecum plants. Mycorrhiza 2014, 24, 197–208. [Google Scholar] [CrossRef]
- Sharma, P.; Kharkwal, A.C.; Abdin, M.Z.; Varma, A. Piriformospora indica-mediated salinity tolerance in Aloe vera plantlets. Symbiosis 2016, 72, 103–115. [Google Scholar] [CrossRef]
- Yu, Q.; Ma, J.; Zou, P.; Lin, H.; Sun, W.; Yin, J.; Fu, J. Effects of combined application of organic and inorganic fertilizers plus nitrification inhibitor DMPP on nitrogen runoff loss in vegetable soils. Environ. Sci. Pollut. Res. 2015, 22, 472–481. [Google Scholar] [CrossRef]
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants; Marschner, P., Ed.; Academic Press: New York, NY, USA, 2011. [Google Scholar]
- García, I.V.; Mendoza, R.E. Relationships among soil properties, plant nutrition and arbuscular mycorrhizal fungi–plant symbioses in a temperate grassland along hydrologic, saline and sodic gradients. FEMS Microbiol. Ecol. 2008, 63, 359–371. [Google Scholar] [CrossRef]
- Ray, P.; Abraham, P.E.; Guo, Y.; Giannone, R.J.; Engle, N.L.; Yang, Z.K.; Craven, K.D. Scavenging organic nitrogen and remodelling lipid metabolism are key survival strategies adopted by the endophytic fungi, Serendipita vermifera and Serendipita bescii to alleviate nitrogen and phosphorous starvation in vitro. Environ. Microbiol. Rep. 2019, 11, 548–557. [Google Scholar] [CrossRef]
- Teutscherova, N.; Vazquez, E.; Arango, J.; Arevalo, A.; Benito, M.; Pulleman, M. Native arbuscular mycorrhizal fungi increase the abundance of ammonia-oxidizing bacteria, but suppress nitrous oxide emissions shortly after urea application. Geoderma 2018, 338, 493–501. [Google Scholar] [CrossRef]
- Marsden, K.A.; Scowen, M.; Hill, P.W.; Jones, D.L.; Chadwick, D.R. Plant acquisition and metabolism of the synthetic nitrification inhibitor dicyandiamide and naturally- occurring guanidine from agricultural soils. Plant Soil 2015, 395, 201–214. [Google Scholar] [CrossRef]
- Pal, P.; McMillan, A.M.S.; Saggar, S. Pathways of dicyandiamide uptake in pasture plants: A laboratory study. Biol. Fertil. Soils 2016, 52, 539–546. [Google Scholar] [CrossRef]
- Vilsmeier, K. Fate of ammonium-N in pot studies as affected by DCD addition. Fert. Res. 1991, 29, 187–189. [Google Scholar] [CrossRef]
- Padash, A.; Shahabivand, S.; Behtash, F.; Aghaee, A. A practicable method for zinc enrichment in lettuce leaves by the endophyte fungus Piriformospora indica under increasing zinc supply. Sci. Hortic. 2016, 213, 367–372. [Google Scholar] [CrossRef]
- Sharaf-Eldin, M.A.; Elkholy, S.; Fernández, J.A.; Junge, H.; Cheetham, R.D.; Guardiola, J.L.; Weathers, P.J. The effect of Bacillus subtilis FZB24® on flowers quantity and quality of saffron (Crocus sativus L.). Planta Medica 2007, 73, P_607. [Google Scholar] [CrossRef]
- Tanha, S.R.; Ghasemnezhad, A.; Babaeizad, V. A study on the effect of endophyte fungus, Piriformospora indica, on the yield and phytochemical changes of globe artichoke (Cynara scolymus L.) leaves under water stress. Int. J. Adv. Biol. Biomed. Res. 2014, 2, 1907–1921. [Google Scholar]
- Rathod, D.P.; Brestic, M.; Shao, H.B. Chlorophyll a fluorescence determines the drought resistance capabilities in two varieties of mycorrhized and non-mycorrhized Glycine max Linn. Afr. J. Microbiol. Res. 2011, 5, 4197–4206. [Google Scholar] [CrossRef]
- Reeves, D.W.; Touchton, J.T. Relative Phytotoxicity of Dicyandiamide and Availability of its Nitrogen to Cotton, Corn, and Grain Sorghum. Soil Sci. Soc. Am. J. 1986, 50, 1353–1357. [Google Scholar] [CrossRef]
- Lawlor, D.W.; Cornic, G. Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant Cell Environ. 2002, 25, 275–294. [Google Scholar] [CrossRef]
- Lenoir, I.; Lounes-Hadj Sahraoui, A.; Laruelle, F.; Dalpe, Y.; Fontaine, J. Arbuscular mycorrhizal wheat inoculation promotes alkane and polycyclic aromatic hydrocarbon biodegradation: Microcosm experiment on aged-contaminated soil. Environ. Pollut. 2016, 213, 549–560. [Google Scholar]
- Sainz, M.J.; Gonzalez, P.B.; Vilarino, A. Effects of hexachlorocyclohexane on rhizosphere fungal propagules and root colonization by arbuscular mycorrhizal fungi in Plantago lanceolata. Eur. J. Soil Sci. 2006, 57, 83–90. [Google Scholar] [CrossRef]
- Liu, J.; Liu, J.; Liu, J.; Cui, M.; Huang, Y.; Tian, Y.; Chen, A.; Xu, G. The Potassium Transporter SlHAK10 Is Involved in Mycorrhizal Potassium Uptake. Plant Physiol. 2019, 180, 465–479. [Google Scholar] [CrossRef] [PubMed]
- Binet, P.; Portal, J.M.; Leyval, C. Application of GC–MS to the study of anthracene disappearance in the rhizosphere of ryegrass. Org. Geochem. 2001, 32, 217–222. [Google Scholar] [CrossRef]
- Aranda, E.; Scervino, J.M.; Godoy, P.; Reina, R.; Ocampo, J.A.; Wittich, R.M.; García-Romera, I. Role of arbuscular mycorrhizal fungus Rhizophagus custos in the dissipation of PAHs under root-organ culture conditions. Environ. Pollut. 2019, 181, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhou, J.; Xiang, X. Impact of four plant species and arbuscular mycorrhizal (AM) fungi on polycyclic aromatic hydrocarbon (PAH) dissipation in spiked soil. Polish J. Environ. Stud. 2013, 22, 1239–1245. [Google Scholar]
- Fatma, M.; Masood, A.; Per, T.S.; Khan, N.A. Nitric oxide alleviates salt stress inhibited photosynthetic performance by interacting with sulfur assimilation in mustard. Front. Plant Sci. 2016, 7, 521. [Google Scholar] [CrossRef]
- Per, T.S.; Masood, A.; Khan, N.A. Nitric oxide improves S-assimilation and GSH production to prevent inhibitory effects of cadmium stress on photosynthesis in mustard (Brassica juncea L.). Nitric Oxide 2017, 68, 111–124. [Google Scholar] [CrossRef]
- Sehar, Z.; Masood, A.; Khan, N.A. Nitric oxide reverses glucose-mediated photosynthetic repression in wheat (Triticum aestivum L.) under salt stress. Environ. Exp. Bot. 2019, 16, 277–289. [Google Scholar] [CrossRef]
- Fatma, M.; Khan, M.I.R.; Masood, A.; Khan, N.A. Coordinate changes in assimilatory sulfate reduction are correlated to salt tolerance: Involvement of phytohormones. Annu. Rev. Res. Biol. 2013, 3, 267–295. [Google Scholar]
- Asgher, M.; Per, T.S.; Masood, A.; Fatma, M.; Freschi, L.; Corpas, F.J.; Khan, N.A. Nitric oxide signaling and its crosstalk with other plant growth regulators in plant responses to abiotic stress. Environ. Sci. Pollut. Res. Int. 2017, 24, 2273–2285. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Cárdenas, L.M.; Calvet, S.; Brüggemann, N.; Loick, N.; Liu, S.; Bol, R. The effect of nitrification inhibitor on N2O, NO and N2 emissions under different soil moisture levels in a permanent grassland soil. Soil Biol. Biochem. 2017, 113, 153–160. [Google Scholar] [CrossRef]
- Vahabi, K.; Dorcheh, S.K.; Monajembashi, S.; Westermann, M.; Reichelt, M.; Falkenberg, D.; Hemmerich, P.; Sherameti, I.; Oelmüller, R. Stress promotes Arabidopsis-Piriformospora indica interaction. Plant Signal. Behav. 2016, 11, 1136763. [Google Scholar] [CrossRef] [PubMed]
- Pal, P.; McMillan, A.; Saggar, S. Routes of Dicyandiamide Uptake in Pasture Plants: A Preliminary Laboratory Study. Int. Grassl. Congr. Proc. 2020, 28, 1–9. [Google Scholar]
- Macadam, X.M.B.; Del Prado, A.; Merino, P.; Estavillo, J.M.; Pinto, M.; González-Murua, C. Dicyandiamide and 3,4-dimethyl pyrazole phosphate decrease N2O emissions from grassland but dicyandiamide produces deleterious effects in clover. J. Plant Physiol. 2003, 160, 1517–1523. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).