Abstract
Biological nitrification inhibition (BNI) in the tropical grass Brachiaria humidicola could reduce net nitrification rates and nitrous oxide (N2O) emissions in soil. To determine the effect on gross nitrogen (N) transformation processes and N2O emissions, an incubation experiment was carried out using 15N tracing of soil samples collected following 2 years of cultivation with high-BNI Brachiaria and native non-BNI grass Eremochloa ophiuroide. Brachiaria enhanced the soil ammonium (NH4+) supply by increasing gross mineralization of recalcitrant organic N and the net release of soil-adsorbed NH4+, while reducing the NH4+ immobilization rate. Compared with Eremochloa, Brachiaria decreased soil gross nitrification by 37.5% and N2O production via autotrophic nitrification by 14.7%. In contrast, Brachiaria cultivation significantly increased soil N2O emissions from 90.42 μg N2O-N kg−1 under Eremochloa cultivation to 144.31 μg N2O-N kg−1 during the 16-day incubation (p < 0.05). This was primarily due to a 59.6% increase in N2O production during denitrification via enhanced soil organic C, notably labile organic C, which exceeded the mitigated N2O production rate during nitrification. The contribution of denitrification to emitted N2O also increased from 9.7% under Eremochloa cultivation to 47.1% in the Brachiaria soil. These findings confirmed that Brachiaria reduces soil gross nitrification and N2O production via autotrophic nitrification while efficiently stimulating denitrification, thereby increasing soil N2O emissions.
1. Introduction
Nitrous oxide (N2O) concentrations in the atmosphere have increased by more than 20% since pre-industrial times and are responsible for 6% of current global warming [1]. N2O emissions are also an important factor in stratospheric ozone depletion [2], with agricultural soil accounting for approximately 66% of global anthropogenic N2O emissions, mainly due to the excessive input of synthetic N fertilizers [3,4]. The increasing use of synthetic fertilizers is also causing increased nitrifier activity, transforming modern agricultural systems into high-nitrifying environments [5,6].
Ammonia oxidation is the rate-limiting first step of nitrification, producing N2O as a by-product [7]. Biological nitrification inhibition (BNI) is a rhizospheric process whereby specific inhibitors exudated or released from the plant’s roots suppress the activity of nitrifying bacteria [8]. This process is widely found in major crops, such as sorghum [9], rice [10], wheat [11,12] and maize [13], as well as in certain forage species [14] and trees [15]. Brachiaria humidicola, a tropical grass native to East and Southeast Africa, has a strong BNI capacity due to the release of the specific compound brachialactone in its root exudates [14,16]. Previous studies have shown that soil collected from established Brachiaria plots shows a remarkable decrease in the net nitrification rate during incubation compared with soil cultivated with non-BNI plants [16,17,18,19]. Meanwhile, Subbarao et al. [14] found that both the soil ammonia oxidation rates and cumulative N2O emissions were reduced by almost 90% after Brachiaria pasture planting compared with soybean or plant-free plots during a three-year field experiment in Colombia. However, in contrast, Vazquez et al. [20] found no apparent differences in the gross nitrification rates in the soil in which different Brachiaria genotypes with differing BNI capacities were grown.
N2O is produced by a number of simultaneous N transformation processes [21]. Denitrification produces N2O as an intermediate product during the reduction in nitrate (NO3−) to N2 and is considered a much more potent source of N2O than nitrification in grassland soil [22]. However, it remains unclear whether cultivation of exotic Brachiaria in tropical pastures results in a reduction in soil denitrification potential and N2O emissions due to the decrease in supply of NO3− substrates for denitrifiers. In this study, we therefore established an incubation experiment using a 15N tracing technique with soil samples collected from an experimental field cultivated with Brachiaria and the native grass Eremochloa ophiuroide, which has no BNI capacity. The objectives were to: (1) determine the effect of Brachiaria on soil N transformation rates in terms of gross nitrification and denitrification rates; and (2) understand how cultivation of Brachiaria affects soil N2O emissions. We hypothesized that Brachiaria cultivation would reduce nitrification by releasing biological nitrification inhibitors, thereby reducing the availability of NO3− for denitrification and together with nitrification, decreasing soil N2O emissions.
2. Materials and Methods
2.1. Field Experiment and Soil Sampling
The field experiment was established in Danzhou, Hainan Province, China (109°29′ E, 19°30′ N), in August 2015. The area has a tropical monsoon climate, with an annual mean air temperature of 23.1 °C and annual precipitation of 1823 mm. The soil was developed from granite and classified as Latosol according to the US soil taxonomy. The field experiment involved eight treatments consisting of two forage grasses and four N application rates, with three replicates each. The two forage species were the introduced exotic grass Brachiaria humidicola CIAT679, which has a high-BNI capacity [14], and the native tropical grass Eremochloa ophiuroide, which has no BNI capacity. The four N application rates were 0, 150, 300 and 450 kg N ha−1 year−1. Plot size was 4 × 3 m and all plots were arranged according to a randomized block design. N fertilizer urea was surface applied prior to irrigation. In the first growing season, 60% urea was applied in August 2015 as a basal fertilizer, with the remaining 40% applied in April 2016 as a top-dressing. In the second growing season, 40% urea was applied in August 2016 as a basal fertilizer, while 30% was applied in March and 30% in June 2017 as a top-dressing. The grasses were harvested using a lawnmower one day before each top-dressing. Phosphorus and potassium application rates were 120 kg P2O5 ha−1 year−1 (calcium superphosphate) and 120 kg K2O ha−1 year−1 (potassium sulfate), respectively, with both applied annually as a basal fertilizer in August.
In March 2017, approximately 2 years after establishment of the field experiment and 6 months after the last fertilization, surface soil (0–20 cm) was collected from 10 different positions in each Brachiaria and Eremochloa plot treated with 150 kg N ha−1 year−1. The samples were then pooled to form a composite sample for each treatment. After removal of visible roots and litter, the fresh soil was sieved through a 2 mm mesh then divided into two subsamples, one of which was stored at 4 °C for incubation and the other which was air-dried for further analysis. Soil pH was measured in a 1:2.5 soil:water sample ratio using a DMP-2 mV/pH detector (Quark Ltd., Nanjing, China). Soil organic C (SOC) and total N (TN) were determined by wet-digestion with H2SO4-K2Cr2O7 and on a CN analyzer (Vario Max CN, Elementar, Hanau, Germany), respectively, while NH4+ and NO3− were extracted using 2 M potassium chloride (KCl) at a 1:5 soil:solution ratio then analyzed using a continuous-flow autoanalyzer (Skalar, Breda, The Netherlands). Dissolved organic carbon (DOC) was extracted using deionized water at a soil:water ratio of 1:5 with shaking for 0.5 h then analyzed using a TOC analyzer (Vario TOC cube, Elementar, Hanau, Germany). Soil available K+ was extracted with ammonium acetate and analyzed using a flame photometer (FP640, INASA, China). Soil properties are presented in Table 1.

Table 1.
Properties of the test soils after approximately 2 years of cultivation with Brachiaria and Eremochloa.
2.2. 15N Tracing Experiment
The soil incubation experiments consisted of two NH4NO3 treatments with three repetitions each, with labelling of either ammonium (15NH4NO3, 10.23 atom % excess) or nitrate (NH415NO3, 10.28 atom % excess) with 15N. Six sets of 250 mL incubation bottles (six bottles per set) were prepared with 30 g fresh soil (on oven-dried basis). After 24 h pre-incubation, 2 mL of 15NH4NO3 solution or NH415NO3 solution was then added at a rate of 50 mg NH4+-N kg−1 soil and 50 mg NO3−-N kg−1 soil, respectively. The bottles were sealed with cling film punctured with seven pin holes to allow gas exchange then incubated for 16 d at a water holding capacity (WHC) of 60% and a temperature of 25 °C in the dark. Water lost during incubation was compensated for by adding deionized water using a mini pipette to maintain a constant weight. Prior to incubation, a pre-experiment was conducted to confirm the optimal incubation time and gas sampling time interval for identifying the N2O flux peaks and meeting the requirement of data-input for the 15N tracing model.
Gas sampling and destructive soil sampling were carried out 2, 98, 194, 290, and 386 h after NH4NO3 application, respectively. At each sampling point, gas samples were collected using a 50 mL syringe from a specific set of bottles at 0 and 6 h after sealing with an air-tight lid. The samples were then immediately injected into two pre-evacuated gas vials with a butyl-rubber stopper for analysis of N2O concentrations and the isotopic composition of 15N2O. In advance of the first gas collection, the bottles were injected with 50 mL of fresh gas to maintain air pressure then after the second collection, the lids were replaced with the punctured cling film. At the same time as gas sampling, NH4+ and NO3− were extracted from another set of soil samples using 100 mL 2 M KCl. After extraction, the soil was rinsed repeatedly with deionized water to remove any residual inorganic N then oven-dried at 50 °C for soil organic N testing. The soil and solution samples were both stored at −20 °C until use.
N2O concentrations in the sampled gas samples were measured using a gas chromatograph (Agilent 7890, Agilent Technologies, Santa Clara, CA, USA) equipped with a 63Ni electron capture detector. For isotopic analysis, extracted NH4+ was separated by distillation with MgO, thereafter NO3− was converted to NH4+ with Devarda’s alloy in another distillation [23]. Released ammonia was absorbed in boric acid solution, and NH4+ concentration was measured using 0.02 M sulfuric acid. After acidification, the solution was dried in an oven at 50 °C and 15N enrichment of NH4+ was determined using an isotope ratio mass spectrometry (IRMS 20-22, SerCon, Crewe, UK). While 15N enrichment of N2O and organic N were measured using a MAT 253 mass spectrometer (Thermo Finnigan, Bremen, Germany).
2.3. 15N Tracing Model
A full process-based 15N tracing model (Figure 1) was used to simultaneously quantify the gross N transformation rates in each soil sample [24]. Average NH4+ and NO3− concentrations and 15N excess values (average ± standard deviations) from the two 15N-labeled treatments were included in the model. The model calculated the gross N transformation rates by simultaneously optimizing the kinetic parameters for the various N transformation processes to minimize misfit between the modeled and observed NH4+ and NO3− concentrations and respective 15N enrichments. A Markov chain Monte Carlo metropolis algorithm (MCMC-MA) was used for parameter optimization, since it is known to be efficient to simultaneously estimate a large number of parameters [25,26]. This algorithm performed a random walk in model parameter space in order to find the global minimum and was shown to be robust against local minima [24]. The optimization procedure produced a probability density function for each model parameter, from which the mean and standard deviation of three parallel sequences were then calculated [25]. To obtain the best parameter set for 15N tracing analysis that was able to simulate the observed data, various combinations of kinetic settings of individual processes were evaluated (Table 2 shows the final version of the parameter set). The most appropriate model to describe the measured N dynamics was then selected according to the Akaike information criterion for each model version [25]. The 15N tracing model was performed using MatLab (Version 7.2, The MathWorks Inc., Natick, MA, USA), which used models individually constructed in Simulink (Version 6.4, The MathWorks Inc., Natick, MA, USA).
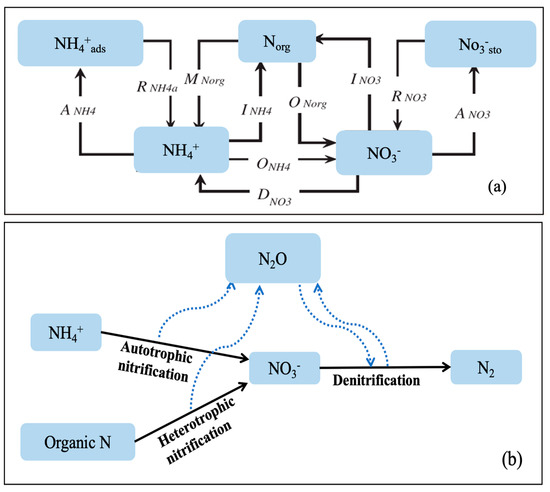
Figure 1.
The 15N tracing model used to determine gross N transformation rates (a) [24] and N2O production pathways from specific N pools (b). Norg: soil organic N (including soil labile organic N and recalcitrant organic N), NH4+: ammonium, NO3−: nitrate, NH4+ads: ammonium adsorbed to soil, NO3−sto: stored nitrate, SOM: soil organic matter. Abbreviations for the transformations are as in Table 2.

Table 2.
Descriptions and average gross N transformation rates (mean ± standard deviation, μg N g−1 soil d−1) in the Brachiaria and Eremochloa soils.
The initial pool sizes for soil NH4+-N and NO3−-N were estimated by extrapolating the first two sets of data back to the time point zero [27]. Based on the kinetic settings and the final parameters set, average gross transformation rates were then calculated over the whole incubation period and presented in units of μg N g−1 soil d−1 (Table 2).
2.4. Calculations
The N2O flux (F, μg N2O-N kg−1 h−1) was calculated as follows:
where is the density of gas under standard conditions (1.25 kg N2O-N m−3); ΔC is the variation in gas concentrations during the 6 h gas sampling period (ppbv); V is the volume of the flask (m−3); T is the incubation temperature; Δt is the incubation time (h); and W is the dry weight of the soil (kg).
Cumulative N2O emissions (E, μg N2O-N kg−1) were calculated as follows:
where F is the N2O flux (μg N2O-N kg−1 h−1); is the th measurement; and ti+1–ti represents the time interval between the two adjacent measurements.
N2O is thought to be derived from three N transformation process: autotrophic nitrification, heterotrophic nitrification, and denitrification. The relative contributions of each process to the N2O emissions were therefore calculated as follows [28]:
where AN, HN and DN represent autotrophic nitrification, heterotrophic nitrification and denitrification, respectively; aN2O, aa, ah and ad represent the 15N atom % excess of N2O-N, NH4+-N, organic N and NO3−-N from the paired 15NH4NO3 and NH415NO3 treatments, respectively; and fAN, fHN and fDN represent the respective fractions of N2O derived from AN, HN, and DN.
The average rate of N2O production from heterotrophic nitrification (N2Oh), autotrophic nitrification (N2Oa), and denitrification (N2Od) were then calculated as follows:
where N2OT is the total N2O production rate during the entire incubation time.
2.5. Statistical Analyses
Statistical analysis was not applied to the parameter results since the 15N tracing model contained plenty of iterations [24]. Accordingly, differences between treatments were considered significant at an alpha level of 0.05 if the 85% confidence intervals did not overlap. Differences in soil properties and N2O emissions between treatments were determined using an independent t-test. All statistical analyses were carried out using SPSS Statistics (version 26.0, IBM corp., Armonk, NY, USA) for Windows.
3. Results
3.1. Soil N Pool Sizes and 15N Enrichment
NH4+ concentrations decreased while NO3− concentrations increased during incubation of both the Brachiaria and Eremochloa soils (Figure 2). NH4+ concentrations decreased more rapidly in the Eremochloa soil, with a decrease of 95.0% during the first 8 d of incubation, with a reduction of only 49.8% in the Brachiaria soil at the end of the 16-day incubation period (Figure 2a). NO3− concentrations in the Eremochloa soil reached a maximum on day 8 after the application of NH4NO3, while a continuous increase was observed up until the end of the incubation in the Brachiaria soil (Figure 2b).
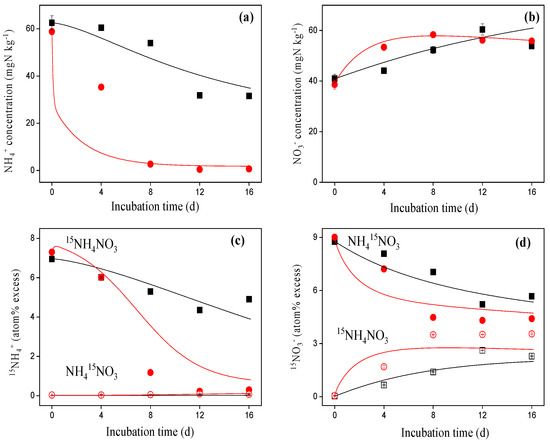
Figure 2.
Measured (points) and modeled (lines) concentrations (a,b) and 15N enrichment of NH4+ and NO3− (c,d) in the Brachiaria (squares) and Eremochloa soil (circles) treated with either 15NH4NO3 or NH415NO3. Vertical bars denote the standard deviation of the mean (n = 3).
15N enrichment of the NH4+ pool decreased, while that of the NO3− pool increased following the addition of 15NH4+, suggesting that mineralization of soil organic N and NH4+ oxidation occurred simultaneously (Figure 2c,d). Meanwhile, 15N enrichment of NO3− decreased after the application of NH415NO3, suggesting that natural or a low abundance of NO3− entered this pool. In contrast, 15N enrichment of NH4+ increased slightly after the application of NH415NO3, suggesting that the direct conversion from 15NO3− to 15NH4+ was negligible.
3.2. Gross N Transformation Rates
The 15N tracing model described the measured data in the test soil with a correlation coefficient (R2) of 0.99. The estimated gross rates of the 12 N transformation processes are shown in Table 2. The dynamic rates of labile organic N (labile organic N mineralization into NH4+ and immobilization of NH4+ into labile organic N) and adsorbed NO3− (adsorption of NO3− and release of adsorbed NO3−) were negligible in both test soils. Meanwhile, the gross mineralization rate of recalcitrant organic N in the Brachiaria soil was 2.02 μg N g−1 soil d−1, which was significantly higher than that in the Eremochloa soil. In contrast, the gross rate of mineral NH4+ immobilization in the Brachiaria soil decreased to 2.94 μg N g−1 soil d−1 from 3.57 μg N g−1 soil d−1 in the Eremochloa soil (Figure 3), and as a result, Brachiaria planting increased the mineralization–immobilization turnover of NH4+ (M/INH4+) from 44.6% in the Eremochloa soil to 68.7%. Both the adsorption rate of NH4+ and the release rate of adsorbed NH4+ were significantly lower in the Brachiaria compared to Eremochloa soil. Meanwhile, the net exchange (release minus adsorption) of mineral NH4+ between these two pools was 0.64 μg N g−1 soil d−1 in the Brachiaria soil, almost double that in the Eremochloa soil (0.33 μg N g−1 soil d−1), suggesting an increase in NH4+ supply.
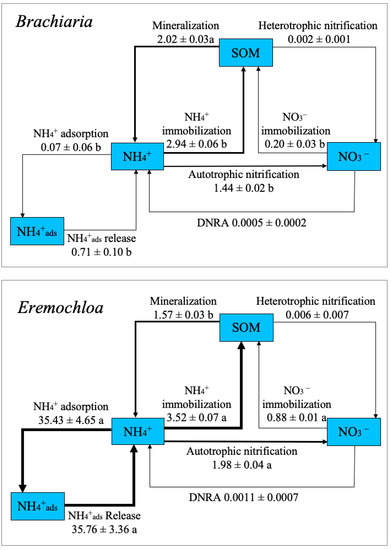
Figure 3.
Nitrogen cycles in the Brachiaria and Eremochloa soil. Values shown represent gross N transformation rates (mean ± SD, μg N g−1 d−1). The thickness of the arrows indicates the strength of each process. Different lowercase indicates denote a significant difference between treatments at p < 0.05. NH4+ads: ammonium adsorbed to soil.
Autotrophic nitrification was a dominant NO3− production process in both sets of soils, while heterotrophic nitrification was negligible. Brachiaria planting reduced the autotrophic nitrification rate to 1.44 μg N g−1 soil d−1 from 1.98 μg N g−1 soil d−1 in the Eremochloa soil. The Brachiaria soil also showed a lower nitrification capacity (ONH4+/M) than the Eremochloa soil (71.3 vs. 126.0%, respectively). Immobilization of NO3− overwhelmingly surpassed the rate of dissimilatory nitrate reduction to ammonium (DNRA) in both sets of soil, representing the primary NO3− consumption process under our experimental conditions. The NO3− immobilization rate in the Eremochloa soil was 0.88 μg N g−1 soil d−1, nearly four times greater than that in the Brachiaria soil. Meanwhile, the NO3− retention capacity and availability in the Eremochloa soil, expressed as the ratio of NO3− consumption to production, was significantly higher than in the Brachiaria soil (44.4 vs. 13.9%, respectively).
3.3. N2O Production Pathways and Emissions
The N2O flux peak occurred on day 4 of the incubation in the Eremochloa soil and on day 12 in the Brachiaria soil (Figure 4), although there was no apparent difference in the N2O production rates from heterotrophic nitrification between the Eremochloa and Brachiaria soil (Table 3). The average N2O production rate of autotrophic nitrification was 3.19 μg N2O-N kg−1 d−1 in the Eremochloa soil, while it was significantly lower at 2.78 μg N2O-N kg−1 d−1 in the Brachiaria soil. In contrast, the average N2O production rate during denitrification increased sharply from 0.55 μg N2O-N kg−1 d−1 in the Eremochloa soil to 4.25 μg N2O-N kg−1 d−1 in the Brachiaria soil, representing a 7.7-fold increase.
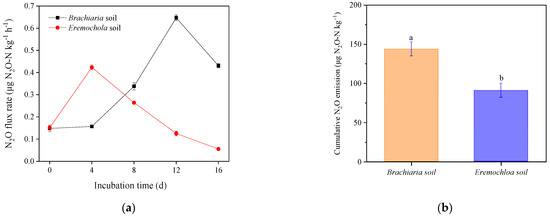
Figure 4.
Fluxes (a) and cumulative emissions (b) of N2O in the Brachiaria and Eremochloa soil during the 16-day incubation. Different lowercase indicates denote a significant difference between treatments at p < 0.05. Vertical bars denote the standard deviation of the mean (n = 3).

Table 3.
Average N2O production rates, the relative contribution of each nitrogen transformation process, and the ratio of N2O emissions from heterotrophic and autotrophic nitrification and denitrification in the Brachiaria and Eremochloa soils.
Cumulative N2O emissions during incubation were significantly higher in the Brachiaria soil (144.31 μg N2O-N kg−1) than that estimated as 90.42 μg N2O-N kg−1 in the Eremochloa soil. Meanwhile, denitrification contributed to 47.1% of the emitted N2O, exceeding the contributions of autotrophic and heterotrophic nitrification in the Brachiaria soil (Table 3). In contrast, only 9.7% of the produced N2O was derived from denitrification in the Eremochloa soil.
4. Discussion
4.1. Brachiaria Humidicola Cultivation Enhanced the Soil NH4+ Supply
This study revealed that the gross mineralization rate of soil recalcitrant organic N was significantly enhanced by 28.7% under cultivation of Brachiaria compared with Eremochloa, accelerating the renewal of soil organic N due to its slight increase. This is consistent with the findings of Teutscherová et al. [29,30] who revealed a positive priming effect of high-BNI Brachiaria on native soil organic N decomposition in Colombian pastures compared with a low-BNI genotype. It is suggested that grasses with a dense root system stimulate organic N mineralization by enhancing microbial biomass and activity through the release of large amounts of dead roots and exudates into the soil [31,32]. It is thought that the increase in organic C accelerates the formation of aggregates and reduces the effective diffusion coefficient of oxygen in the soil, in turn inducing a shift in dominant microbes from aerobes (Gram-negative bacteria) to facultative and/or anaerobic microbes (Gram-positive bacteria and fungi) [33,34,35,36]. In general, Gram-positive bacteria and fungi preferentially utilize soil recalcitrant organic matter [37,38]. It is therefore likely that the increase in soil organic C observed here was mainly due to the increase in organic C input from the high biomass of dead roots and exudates under Brachiaria cultivation, resulting in more efficient growth of Gram-positive bacteria and fungi and an increase in the turnover of recalcitrant organic C compared with Eremochloa cultivation.
In contrast, the immobilization rate of NH4+ in the Brachiaria soil decreased compared with the Eremochloa soil. This differs from the results of Vazquez et al. [20] who showed that gross NH4+ immobilization was enhanced in the soil cultivated with high-BNI Brachiaria genotypes, while the NO3− concentration and N losses remained low. It has been reported that a high C/N ratio in high-BNI plant soil reduces N availability for microbial N immobilization when no N fertilizers are added or when only limited (animal) urine deposition occurs [14,29,39,40]. In contrast, cultivation of high-BNI Brachiaria genotypes in the same field experiment results in a significant reduction in microbial NH4+ immobilization rates at 7 and 21 days after application of N fertilizers at a rate 50 kg N ha−1 [41]. These results suggest that microbial NH4+ immobilization is dependent on soil NH4+ availability.
Compared with Eremochloa, Brachiaria more efficiently reduced the gross rate of soil NH4+ adsorption than the release rate of adsorbed NH4+, causing an increase in the net release rate of adsorbed NH4+. This may have been due to two possible reasons. Firstly, roots of Brachiaria can distribute within the 20–40 cm soil layer, allowing effective absorption of non-exchangeable K+ from deeper soil layers [42], dramatically increasing available K+ in the surface soil. The higher availability of K+ also outcompetes NH4+ for soil adsorption sites, thereby reducing soil adsorption of NH4+ since both have a similar ionic radius and physical properties [43,44]. To date, however, the influence of Brachiaria planting on the soil available K+ is less studied. Further study is required to evaluate how Brachiaria cultivation affects the soil available K at the different K application rates. Secondly, the adsorption capacity of NH4+ in soil is also affected by the content of clay and organic matter [45,46]. Organic matter with plenty of polar atom groups, such as carboxyl and phenolic hydroxyl, contribute to the negative charge and is the main source of variable soil charge. It is therefore likely that NH4+ as a cation is not as efficiently adsorbed, while NH4+ from decomposed organic N is released during the mineralization of native recalcitrant organic C under cultivation of Brachiaria.
Overall, cultivation of Brachiaria therefore reduced the rates of NH4+ immobilization and adsorption and enhanced the rates of organic N mineralization and adsorbed NH4+ release, in turn increasing soil NH4+ availability and supply compared with Eremochloa cultivation. These results suggest that in the Brachiaria soil, reduced application rate of K fertilizer may increase the adsorption of NH4+ from the test soil.
4.2. Effect of Brachiaria humidicola Cultivation on Soil N2O Emissions
As expected, cultivation of Brachiaria significantly reduced autotrophic nitrification and related N2O production, compared with Eremochloa. This is consistent with the findings of Subbarao et al. [14] who reported that Brachiaria soil reduced the ammonia oxidation rate by 90% during a 3-year field experiment compared with soybean and plant-free soil. Subbarao et al. [47] revealed that the release of brachialactone exudate by Brachiaria blocked the activities of both ammonia monooxygenase and hydroxylamino oxidoreductase, thereby reducing ammonia oxidation in pure culture with the ammonia oxidizer Nitrosomonas europaea. In line with this, a reduction in the abundance of ammonia oxidizers was observed in a previous field study of Brachiaria cultivation [18,40]. It was also revealed that BNI compounds were able to persist for a long time and tended to accumulate over time, remaining effective even after Brachiaria pasture was subsequently replaced with maize [48,49,50]. However, Vazquez et al. [20] and Teutscherová et al. [41] found no comparable differences in gross nitrification rates between Brachiaria genotypes with differing BNI capacities in soil with a high organic C content in the tropical savanna in Colombia. Moreover, they attributed the reduced inhibition of potential net nitrification rates to strong microbial immobilization of NH4+, and a subsequent reduction in soil NH4+ availability for ammonia oxidizers.
In the present study, the reduction in N2O production in the Brachiaria soil via autotrophic nitrification was at the lower end of the range of 16.8–90.0% reported by previous studies [51]. This suggests that less NH4+ was converted into N2O during autotrophic nitrification in test acidic soil. In general, competition for available NH4+ exists between autotrophic nitrification and microbial immobilization or adsorption of NH4+ [25,52]. As discussed above, reduced rates of NH4+ immobilization and NH4+ adsorption together with higher mineralization rates of organic N provided more NH4+ substrates for ammonia oxidizers in the Brachiaria compared to Eremochloa soil. This suggests that the suppression effect of high-BNI Brachiaria on N2O emissions may also depend on soil NH4+ availability, with greater suppression in soil with relatively low available NH4+ [20,29,53,54].
In contrast to autotrophic nitrification, Brachiaria did not alter the N2O production rate via heterotrophic nitrification. In general, heterotrophic nitrification is carried out by a large variety of bacteria and fungi [55], with heterotrophic nitrifiers using both organic and inorganic N as a substrate, and possibly producing more N2O than autotrophic nitrifiers [56,57]. In the present study, we supposed that heterotrophic nitrifiers would use organic N as a unique substrate since the model only can select one substrate pool for running. Thus, it is very likely that the gross rate of heterotrophic nitrification in the Brachiaria and Eremochloa soil was underestimated. In general, fungal nitrification in acidic soil is not inhibited by popular synthetic nitrification inhibitors such as acetylene (C2H2), dicyandiamide (DCD) and nitrapyrin [58]. Therefore, our results suggest that the 2-year cultivation with Brachiaria had no effect on N2O production via heterotrophic nitrification.
As unexpected, cumulative N2O emissions were significantly higher in the Brachiaria soil compared to the Eremochloa soil as measured in the field (4.30 and 1.54 kg N2O-N ha−1 under Brachiaria and Eremochloa cultivation, respectively), although N2O emissions via autotrophic nitrification decreased. This is in contrast with previous results in which Brachiaria establishment was found to reduce soil N2O emissions by 20–90% compared with plants without BNI capacity [14,47]. In the present study, the N2O production rate in the Brachiaria soil during denitrification increased 6.7-fold, while the contribution ratio of denitrification to emitted N2O dramatically increased compared with Eremochloa. These results clearly suggest that the enhanced N2O emissions in the Brachiaria soil were primarily due to an increased denitrification potential. There were two suggested possibilities. Firstly, compared with Eremochloa, Brachiaria cultivation sharply reduced the NO3− immobilization rate, which outnumbered the rate of DNRA as the primary consumption process of NO3−. This suggests that cultivation of Brachiaria increased soil NO3− availability by reducing the ratio of total NO3− consumption through microbial immobilization of NO3− and dissimilatory NO3− reduction to NH4+ (INO3 + DNO3) to total NO3− production (ONH4 + ONrec), thereby providing more NO3− for denitrification. It was previously suggested that higher NO3− concentrations in the soil tend to result in a higher N2O/N2 ratio during denitrification, thereby favoring N2O emissions [59]. Secondly, in this study, Brachiaria cultivation significantly enhanced soil organic C, notably dissolved organic C, due to the increase in plant biomass and especially biomass of roots and exudates. Increases in soil organic C were also found to promote the formation of anaerobic microsites for denitrification by stimulating aggregation and soil respiration [60,61]. Meanwhile, an increase in organic C was also found to reduce the minimum soil moisture threshold for the occurrence of denitrification [62,63]. For example, Wan et al. [64] found that the addition of starch to sandy loam soil treated with nitrate-based fertilizers stimulated N2O production through denitrification. The results of this study therefore suggest that although cultivation of Brachiaria suppressed autotrophic nitrification, it significantly increased the soil denitrification potential and subsequent N2O production by increasing soil organic C, notably labile organic C, through an increase in plant biomass, thereby stimulating soil N2O emissions.
5. Conclusions
This study examined the effect of the exotic tropical grass Brachiaria, which has a high-BNI capacity, on soil N transformation processes and N2O emissions. Cultivation of Brachiaria significantly decreased the gross rate of autotrophic nitrification and N2O production during nitrification. In contrast, Brachiaria also increased the gross mineralization rate of soil recalcitrant organic N and reduced microbial NH4+ immobilization and NH4+ adsorption, thereby increasing the NH4+ supply for nitrification compared with native Eremochloa. Brachiaria planting caused a significant increase in soil N2O emissions, primarily due to an increased denitrification potential as a result of reductions in NO3− immobilization and an increase in soil labile organic C. Further studies are now required to determine the effects of K fertilizers on the adsorption and availability of NH4+. The effect of synthetic nitrification inhibitors together with the biological nitrification inhibitors released from Brachiaria on the mitigation of N2O emissions in tropical pastures also requires further clarification.
Author Contributions
Conceptualization, W.D. and D.L.; incubation, L.X., Y.N. and D.L.; data analysis, L.X., C.M., A.J.-W. and Z.C.; writing, L.X., W.D., M.Z., L.M.; funding acquisition, W.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported from the National Natural Science Foundation of China (41730753, 41977049, 42077029), and the International Partnership Program of Chinese Academy of Sciences (151432KYSB20200001). Special appreciations to the collaboration of the German Science foundation research unit DASIM (DFG FOR 2337), and IAEA coordinated research project (D15020).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Prather, M.J.; Hsu, J.; DeLuca, N.M.; Jackman, C.H.; Oman, L.D.; Douglass, A.R.; Fleming, E.L.; Strahan, S.E.; Steenrod, S.D.; Søvde, O.A.; et al. Measuring and modeling the lifetime of nitrous oxide including its variability. J. Geophys. Res. 2015, 120, 5693–5705. [Google Scholar] [CrossRef] [PubMed]
- Ravishankara, A.R.; Daniel, J.S.; Portmann, R.W. Nitrous Oxide (N2O): The dominant ozone-depleting substance emitted in the 21st century. Science 2009, 326, 123–125. [Google Scholar] [CrossRef]
- UNEP. Drawing Down N2O to Protect Climate and the Ozone Layer; UNEP: Nairobi, Kenya, 2013; ISBN 9789280733587. [Google Scholar]
- Tian, H.; Yang, J.; Xu, R.; Lu, C.; Canadell, J.G.; Davidson, E.A.; Jackson, R.B.; Arneth, A.; Chang, J.; Ciais, P.; et al. Global soil nitrous oxide emissions since the preindustrial era estimated by an ensemble of terrestrial biosphere models: Magnitude, attribution, and uncertainty. Glob. Chang. Biol. 2019, 25, 640–659. [Google Scholar] [CrossRef] [PubMed]
- Bellamy, P.H.; Loveland, P.J.; Bradley, R.I.; Lark, R.M.; Kirk, G.J.D. Carbon losses from all soils across england and wales 1978–2003. Nature 2005, 437, 245–248. [Google Scholar] [CrossRef]
- Poudel, D.D.; Horwath, W.R.; Lanini, W.T.; Temple, S.R.; Van Bruggen, A.H.C. Comparison of soil n availability and leaching potential, crop yields and weeds in organic, low-input and conventional farming systems in northern california. Agric. Ecosyst. Environ. 2002, 90, 125–137. [Google Scholar] [CrossRef]
- Wendeborn, S. The chemistry, biology, and modulation of ammonium nitrification in soil. Angew. Chem. Int. Ed. 2019, 58, 2–23. [Google Scholar] [CrossRef] [PubMed]
- Subbarao, G.V.; Sahrawat, K.L.; Nakahara, K.; Ishikawa, T.; Kishii, M.; Rao, I.M.; Hash, C.T.; George, T.S.; Srinivasa Rao, P.; Nardi, P.; et al. Biological nitrification inhibition—A novel strategy to regulate nitrification in agricultural systems. Adv. Agron. 2012, 114, 249–302. [Google Scholar]
- Zakir, H.A.K.M.; Subbarao, G.V.; Pearse, S.J.; Gopalakrishnan, S.; Ito, O.; Ishikawa, T.; Kawano, N.; Nakahara, K.; Yoshihashi, T.; Ono, H.; et al. Detection, isolation and characterization of a root-exuded compound, methyl 3-(4-hydroxyphenyl) propionate, responsible for biological nitrification inhibition by sorghum (Sorghum bicolor). New Phytol. 2008, 180, 442–451. [Google Scholar] [CrossRef]
- Sun, L.; Lu, Y.F.; Yu, F.W.; Kronzucker, H.J.; Shi, W.M. Biological nitrification inhibition by rice root exudates and its relationship with nitrogen-use efficiency. New Phytol. 2016, 212, 646–656. [Google Scholar] [CrossRef]
- O’Sullivan, C.A.; Fillery, I.R.P.P.; Roper, M.M.; Richards, R.A.; O’Sullivan, C.A.; Fillery, I.R.P.P.; Roper, M.M.; Richards, R.A. Identification of several wheat landraces with biological nitrification inhibition capacity. Plant Soil 2016, 404, 61–74. [Google Scholar] [CrossRef]
- Subbarao, G.V.; Kishii, M.; Nakahara, K.; Ishikawa, T.; Ban, T.; Tsujimoto, H.; George, T.S.; Berry, W.L.; Hash, C.T.; Ito, O. Biological nitrification inhibition (BNI)—Is there potential for genetic interventions in the triticeae? Breed. Sci. 2009, 59, 529–545. [Google Scholar] [CrossRef]
- Otaka, J.; Subbarao, G.V.; Ono, H.; Yoshihashi, T. Biological nitrification inhibition in maize—Isolation and identification of hydrophobic inhibitors from root exudates. Biol. Fertil. Soils 2022, 58, 251–264. [Google Scholar] [CrossRef]
- Subbarao, G.V.; Nakahara, K.; Hurtado, M.P.; Ono, H.; Moreta, D.E.; Salcedo, A.F.; Yoshihashi, A.T.; Ishikawa, T.; Ishitani, M.; Ohnishi-Kameyama, M.; et al. Evidence for biological nitrification inhibition in Brachiaria pastures. Proc. Natl. Acad. Sci. USA 2009, 106, 17302–17307. [Google Scholar] [CrossRef] [PubMed]
- Castaldi, S.; Carfora, A.; Fiorentino, A.; Natale, A.; Messere, A.; Miglietta, F.; Cotrufo, M.F. Inhibition of net nitrification activity in a mediterranean woodland: Possible role of chemicals produced by arbutus unedo. Plant Soil 2009, 315, 273–283. [Google Scholar] [CrossRef]
- Subbarao, G.V.; Nakahara, K.; Ishikawa, T.; Yoshihashi, T.; Ito, O.; Ono, H.; Ohnishi-Kameyama, M.; Yoshida, M.; Kawano, N.; Berry, W.L. Free fatty acids from the pasture grass Brachiaria humidicola and one of their methyl esters as inhibitors of nitrification. Plant Soil 2008, 313, 89–99. [Google Scholar] [CrossRef]
- Karwat, H.; Egenolf, K.; Nunez, J.; Rao, I.; Rasche, F.; Arango, J.; Moreta, D.; Arevalo, A.; Cadisch, G. Low N-15 natural abundance in shoot tissue of Brachiaria humidicola is an indicator of reduced N losses due to biological nitrification inhibition (BNI). Front. Microbiol. 2018, 9, 2383. [Google Scholar] [CrossRef]
- Nunez, J.; Arevalo, A.; Karwat, H.; Egenolf, K.; Miles, J.; Chirinda, N.; Cadisch, G.; Rasche, F.; Rao, I.; Subbarao, G.V.; et al. Biological nitrification inhibition activity in a soil-grown biparental population of the forage grass, Brachiaria humidicola. Plant Soil 2018, 426, 401–411. [Google Scholar] [CrossRef]
- Egenolf, K.; Schad, P.; Arevalo, A.; Villegas, D.; Arango, J.; Karwat, H.; Cadisch, G.; Rasche, F. Inter-microbial competition for N and plant NO3− uptake rather than BNI determines soil net nitrification under intensively managed Brachiaria humidicola. Biol. Fertil. Soils 2022, 58, 307–319. [Google Scholar] [CrossRef]
- Vazquez, E.; Teutscherova, N.; Dannenmann, M.; Töchterle, P.; Butterbach-Bahl, K.; Pulleman, M.; Arango, J. Gross nitrogen transformations in tropical pasture soils as affected by Urochloa genotypes differing in biological nitrification inhibition (BNI) capacity. Soil Biol. Biochem. 2020, 151, 108058. [Google Scholar] [CrossRef]
- Zhang, J.; Cai, Z.; Zhu, T. N2O production pathways in the subtropical acid forest soils in China. Environ. Res. 2011, 111, 643–649. [Google Scholar] [CrossRef]
- Saggar, S.; Jha, N.; Deslippe, J.; Bolan, N.S.; Luo, J.; Giltrap, D.L.; Kim, D.G.; Zaman, M.; Tillman, R.W. Denitrification and N2O: N2 Production in Temperate Grasslands: Processes, Measurements, Modelling and Mitigating Negative Impacts. Sci. Total Environ. 2013, 465, 173–195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cai, Z.; Cheng, Y.; Zhu, T. Denitrification and Total Nitrogen Gas Production from Forest Soils of Eastern China. Soil Biol. Biochem. 2009, 41, 2551–2557. [Google Scholar] [CrossRef]
- Müller, C.; Rütting, T.; Kattge, J.; Laughlin, R.J.; Stevens, R.J. Estimation of parameters in complex 15N tracing models by monte carlo sampling. Soil Biol. Biochem. 2007, 39, 715–726. [Google Scholar] [CrossRef]
- Rütting, T.; Müller, C. 15N Tracing models with a monte carlo optimization procedure provide new insights on gross n transformations in soils. Soil Biol. Biochem. 2007, 39, 2351–2361. [Google Scholar] [CrossRef]
- Knorr, W.; Kattge, J. Inversion of terrestrial ecosystem model parameter values against eddy covariance measurements by monte carlo sampling. Glob. Chang. Biol. 2005, 11, 1333–1351. [Google Scholar] [CrossRef]
- Müller, C.; Stevens, R.J.; Laughlin, R.J. A 15N tracing model to analyse n transformations in old grassland soil. Soil Biol. Biochem. 2004, 36, 619–632. [Google Scholar] [CrossRef]
- Zhu, T.; Zhang, J.; Cai, Z. The Contribution of Nitrogen Transformation Processes to Total N2O Emissions from Soils Used for Intensive Vegetable Cultivation. Plant Soil 2011, 343, 313–327. [Google Scholar] [CrossRef]
- Teutscherová, N.; Vazquez, E.; Arevalo, A.; Pulleman, M.; Rao, I.; Arango, J. Differences in arbuscular mycorrhizal colonization and P acquisition between genotypes of the tropical brachiaria grasses: Is there a relation with BNI activity? Biol. Fertil. Soils 2019, 55, 325–337. [Google Scholar] [CrossRef]
- Teutscherová, N.; Vazquez, E.; Lehndorff, E.; Pulleman, M.; Arango, J. Nitrogen acquisition by two U. humidicola genotypes differing in biological nitrification inhibition (BNI) capacity and associated microorganisms. Biol. Fertil. Soils 2022, 58, 355–364. [Google Scholar] [CrossRef]
- Lama, S.; Kuhn, T.; Lehmann, M.F.; Müller, C.; Gonzalez, O.; Eisenhauer, N.; Lange, M.; Scheu, S.; Oelmann, Y.; Wilcke, W. The biodiversity—N cycle relationship: A 15N tracer experiment with soil from plant mixtures of varying diversity to model N pool sizes and transformation rates. Biol. Fertil. Soils 2020, 56, 1047–1061. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, T.; Cai, Z.; Müller, C. Nitrogen cycling in forest soils across climate gradients in eastern China. Plant Soil 2011, 342, 419–432. [Google Scholar] [CrossRef]
- Zhang, H.; Ding, W.; Yu, H.; He, X. Carbon uptake by a microbial community during 30-day treatment with 13C-glucose of a sandy loam soil fertilized for 20 years with npk or compost as determined by a GC-C-IRMS analysis of phospholipid fatty acids. Soil Biol. Biochem. 2013, 57, 228–236. [Google Scholar] [CrossRef]
- Lin, Y.X.; Ye, G.P.; Kuzyakov, Y.; Liu, D.Y.; Fan, J.B.; Ding, W.X. Long-term manure application increases soil organic matter and aggregation, and alters microbial community structure and keystone taxa. Soil Biol. Biochem. 2019, 134, 187–196. [Google Scholar] [CrossRef]
- Zhang, H.J.; Ding, W.X.; Yu, H.Y.; He, X.H. Linking organic carbon accumulation to microbial community dynamics in a sandy loam soil: Result of 20 years compost and inorganic fertilizers repeated application experiment. Biol. Fertil. Soils 2015, 51, 137–150. [Google Scholar] [CrossRef]
- Yu, H.Y.; Ding, W.X.; Luo, J.F.; Geng, R.L.; Cai, Z.C. Long-term application of compost and mineral fertilizers on aggregation and aggregate-associated carbon in a sandy loam soil. Soil Till. Res. 2012, 124, 170–177. [Google Scholar] [CrossRef]
- Xu, G.; Chen, J.; Berninger, F.; Pumpanen, J.; Bai, J.; Yu, L.; Duan, B. Labile, recalcitrant, microbial carbon and nitrogen and the microbial community composition at two abies faxoniana forest elevations under elevated temperatures. Soil Biol. Biochem. 2015, 91, 1–13. [Google Scholar] [CrossRef]
- Fanin, N.; Kardol, P.; Farrell, M.; Nilsson, M.C.; Gundale, M.J.; Wardle, D.A. The ratio of Gram-positive to Gram-negative bacterial PLFA markers as an indicator of carbon availability in organic soils. Soil Biol. Biochem. 2019, 128, 111–114. [Google Scholar] [CrossRef]
- Horrocks, C.A.; Arango, J.; Arevalo, A.; Nuñez, J.; Cardoso, J.A.; Dungait, J.A.J. Smart forage selection could significantly improve soil health in the tropics. Sci. Total Environ. 2019, 688, 609–621. [Google Scholar] [CrossRef]
- Byrnes, R.C.; Nùñez, J.; Arenas, L.; Rao, I.; Trujillo, C.; Alvarez, C.; Arango, J.; Rasche, F.; Chirinda, N. Biological nitrification inhibition by Brachiaria grasses mitigates soil nitrous oxide emissions from bovine urine patches. Soil Biol. Biochem. 2017, 107, 156–163. [Google Scholar] [CrossRef]
- Teutscherová, N.; Vázquez, E.; Trubač, J.; Villegas, D.M.; Subbarao, G.V.; Pulleman, M.; Arango, J. Gross N transformation rates in soil system with contrasting Urochloa genotypes do not confirm the relevance of bni as previously assessed in vitro. Biol. Fertil. Soils 2022, 58, 321–331. [Google Scholar] [CrossRef]
- Garcia, R.A.; Crusciol, C.A.C.; Calonego, J.C.; Rosolem, C.A. Potassium cycling in a corn-brachiaria cropping system. Eur. J. Agron. 2008, 28, 579–585. [Google Scholar] [CrossRef]
- Zhao, W.; Li, Y.; Zhao, Q.; Ning, Z.; Zhou, C.; Wang, H.; Lu, L.; Yang, P.; Zhang, K.; Wang, F.; et al. Adsorption and desorption characteristics of ammonium in eight loams irrigated with reclaimed wastewater from intensive hogpen. Environ. Earth Sci. 2013, 69, 41–49. [Google Scholar] [CrossRef]
- Rich, C.I.; Black, W.R. Pottasium exchange as affected by cation size, pH, and mineral structure. Soil Sci. 1964, 97, 384–390. [Google Scholar] [CrossRef]
- Zhu, T.; Meng, T.; Zhang, J.; Yin, Y.; Cai, Z.; Yang, W.; Zhong, W. Nitrogen mineralization, immobilization turnover, heterotrophic nitrification, and microbial groups in acid forest soils of subtropical China. Biol. Fertil. Soils 2013, 49, 323–331. [Google Scholar] [CrossRef]
- Xie, Y.; Yang, L.; Zhu, T.; Yang, H.; Zhang, J.; Yang, J.; Cao, J.; Bai, B.; Jiang, Z.; Liang, Y. Rapid recovery of nitrogen retention capacity in a subtropical acidic soil following afforestation. Soil Biol. Biochem. 2018, 120, 171–180. [Google Scholar] [CrossRef]
- Subbarao, G.V.; Ito, O.; Sahrawat, K.L.; Berry, W.L.; Nakahara, K.; Ishikawa, T.; Watanabe, T.; Suenaga, K.; Rondon, M.; Rao, I.M. scope and strategies for regulation of nitrification in agricultural systems—Challenges and opportunities. CRC. Crit. Rev. Plant Sci. 2006, 25, 303–335. [Google Scholar] [CrossRef]
- Subbarao, G.V.; Ishikawa, T.; Ito, O.; Nakahara, K.; Wang, H.Y.; Berry, W.L. A bioluminescence assay to detect nitrification inhibitors released from plant roots: A case study with Brachiaria humidicola. Plant Soil 2006, 288, 101–112. [Google Scholar] [CrossRef]
- Subbarao, G.V.V.; Sahrawat, K.L.; Nakahara, K.; Rao, I.M.; Ishitani, M.; Hash, C.T.; Kishii, M.; Bonnett, D.G.; Berry, W.L.; Lata, J.C. A paradigm shift towards low-nitrifying production systems: The role of biological nitrification inhibition (BNI). Ann. Bot. 2013, 112, 297–316. [Google Scholar] [CrossRef]
- Karwat, H.; Moreta, D.; Arango, J.; Nunez, J.; Rao, I.; Rincon, A.; Rasche, F.; Cadisch, G. Residual effect of BNI by Brachiaria Humidicola pasture on nitrogen recovery and grain yield of subsequent maize. Plant Soil 2017, 420, 389–406. [Google Scholar] [CrossRef]
- Wang, X.; Bai, J.; Xie, T.; Wang, W.; Zhang, G.; Yin, S.; Wang, D. Effects of biological nitrification inhibitors on nitrogen use efficiency and greenhouse gas emissions in agricultural soils: A review. Ecotoxicol. Environ. Saf. 2021, 220, 112338. [Google Scholar] [CrossRef]
- Portier, E.; Silver, W.L.; Yang, W.H. Invasive perennial forb effects on gross soil nitrogen cycling and nitrous oxide fluxes depend on phenology. Ecology 2019, 100, e02716. [Google Scholar] [CrossRef] [PubMed]
- Nardi, P.; Müller, C.; Pietramellara, G.; Subbarao, G.V.; Nannipieri, P. Recommendations about soil biological nitrification inhibition (BNI) studies. Biol. Fertil. Soils 2022, 58, 613–615. [Google Scholar] [CrossRef]
- Nardi, P.; Laanbroek, H.J.; Nicol, G.W.; Renella, G.; Cardinale, M.; Pietramellara, G.; Weckwerth, W.; Trinchera, A.; Ghatak, A.; Nannipieri, P. Biological nitrification inhibition in the rhizosphere: Determining interactions and impact on microbially mediated processes and potential applications. FEMS Microbiol. Rev. 2020, 44, 874–908. [Google Scholar] [CrossRef]
- Braker, G.; Conrad, R. Diversity, structure, and size of N2O-producing microbial communities in soils-what matters for their functioning? Adv. Appl. Microbiol. 2011, 75, 33–70. [Google Scholar]
- Zhang, J.; Müller, C.; Cai, Z. Heterotrophic nitrification of organic N and its contribution to nitrous oxide emissions in soils. Soil Biol. Biochem. 2015, 84, 199–209. [Google Scholar] [CrossRef]
- Anderson, I.C.; Poth, M.; Homstead, J.; Burdige, D. A Comparison of NO and N2O production by the autotrophic nitrifier nitrosomonas europaea and the heterotrophic nitrifier Alcaligenes faecalis. Appl. Environ. Microbiol. 1993, 59, 3525–3533. [Google Scholar] [CrossRef]
- De Boer, W.; Kowalchuk, G.A. Nitrification in acid soils: Micro-organisms and mechanisms. Soil Biol. Biochem. 2001, 33, 853–866. [Google Scholar] [CrossRef]
- Luo, J.; Tillman, R.W.; Ball, P.R. Factors regulating denitrification in a soil under pasture. Soil Biol. Biochem. 1999, 31, 913–927. [Google Scholar] [CrossRef]
- Garcia-Montiel, D.C.; Melilo, J.M.; Steudler, P.A.; Cerri, C.C.; Piccolo, M.C. Carbon limitations to nitrous oxide emissions in a humid tropical forest of the Brazilian Amazon. Biol. Fertil. Soils 2003, 38, 267–272. [Google Scholar] [CrossRef]
- Bollmann, A.; Conrad, R. Influence of O2 Availability on NO and N2O release by nitrification and denitrification in soils. Glob. Chang. Biol. 2004, 4, 387–396. [Google Scholar] [CrossRef]
- Groenigen, J.W.; Kasper, G.J.; Velthof, G.L.; Dasselaar van den Pol-van, A.; Kuikman, P.J. Nitrous oxide emissions from silage maize fields under different mineral nitrogen fertilizer and slurry application. Plant Soil 2004, 263, 101–111. [Google Scholar] [CrossRef]
- Chantigny, M.H.; Pelster, D.E.; Perron, M.H.; Rochette, P.; Angers, D.A.; Parent, L.E.; Massé, D.; Ziadi, N. Nitrous oxide emissions from clayey soils amended with paper sludges and biosolids of separated pig slurry. J. Environ. Qual. 2013, 42, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.J.; Ju, X.T.; Ingwersen, J.; Schwarz, U.; Stange, C.F.; Zhang, F.S.; Streck, T. Gross nitrogen transformations and related nitrous oxide emissions in an intensively used calcareous soil. Soil Sci. Soc. Am. J. 2009, 73, 102–112. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).