Abstract
Currently, in agricultural engineering, plant growth regulators or biostimulants, immunity stimulants or bacterial vaccines are becoming standard elements in the production technology of many types of field, fruit and vegetable crops. The research was based on a three-year field experiment carried out in 2018–2021 at the Agricultural Experimental Station of northeastern Poland. The aim of the research was to determine the effect of biostimulators containing microorganisms and micro and macro elements, phosphorus and potassium and silicon on the morphological features of the leaf rosette and the increase in fresh and dry mass of the above-ground part of the rosette and the root system of three winter rape cultivars. The conducted research showed that the application of the organic preparation Ugmax significantly increased the number of rosette leaves (by an average of 13.9%), the length of the tap root (by an average of 2.3 cm), root neck diameter (by an average of 4.2%), fresh and dry weight of the above-ground part of the rosette (by an average of 6.0% and 6.6%) and fresh weight of the root system (by an average of 0.88 g) compared to the control variant. The hybrid morphotypes that were restored compared to the population cultivar Chrobry were characterized by a weaker autumn development of the leaf rosette.
1. Introduction
Many authors [1,2,3] emphasize that winter oilseed rape (Brassica napus) is one of the most important oil–protein plants in the world. It is a plant that is highly sensitive to adverse environmental conditions. Therefore, substances are sought that will reduce the impact of various types of stress negatively affecting the growth and development of plants.
Currently, in agricultural engineering, plant growth regulators or biostimulants, immunity stimulants or bacterial vaccines are becoming standard elements in the production technology of many types of field, fruit and vegetable crops [4].
Rutkowska [5] mentions that a biostimulant is a fertilizing product which, irrespective of the nutrient content, contains an active substance (substances) or microorganisms. When applied to a plant or within the rhizosphere, it stimulates natural processes that lead to increased nutrient use efficiency, tolerance to abiotic stress or improved crop quality characteristics.
Sharma et al. [6] and Kocira et al. [7] also emphasize that these substances strengthen the mechanisms of plant resistance to stress.
Ruzzi and Aroca [8] emphasize that microbiological biostimulants containing beneficial plant fungi and bacteria play an important role. Plants are not autonomous in their environment because they are associated with bacterial microorganisms and fungi which, as a result of external and internal interactions, respond to biotic and abiotic stress [9,10]. According to Du Jardin [11], the most important group in this category that stimulate plant growth are Rhizobacteria. These bacteria colonize the plant rhizosphere, improve growth, control plant pathogens, improve plant nutrient and mineral uptake and increase plant resistance to biotic and abiotic stresses. This group includes nitrogen-fixing bacteria, such as: Rhizobium, Azotobacter, Azospirillum, Pseudomonas, Bacillus [12,13].
Fageria et al. [14]; Kocoń [15]; Szewczuk and Sugier [16] emphasize that the application of foliar fertilizers containing micro and macro elements during the autumn development has a positive effect on winter plants.
It should be emphasized that preparations containing stimulants do not replace fertilization; they can only have a positive effect on the use of nutrients contained in fertilizers, mainly due to a better developed root system of plants. Despite the nutrient content, they cannot be treated as fertilizing products and used in doses higher than recommended, because they may have a toxic effect on plants [5].
Winter oilseed rape sown for harvest in 2021 amounted to over 0.9 million ha. The harvest was estimated at 3.2 million tons.
Fageria et al. [14] emphasize that winter oilseed rape has very high nutritional requirements, and both soil and foliar fertilization is important in its cultivation.
Due to the few studies on the beneficial effects of biostimulants on the morphometric features of winter oilseed rape, research was undertaken to determine the effect of biostimulators containing microorganisms and micro and macro elements, phosphorus and potassium and silicon on the morphological features of the leaf rosette and the increase in fresh and dry mass of the above-ground part of the rosette and the root system of three winter rape cultivars.
2. Materials and Methods
2.1. Experimental Design and Research Area
The research was based on a three-year field experiment conducted in 2018–2021 at the Agricultural Experimental Station (52°03′N and 22°33′E), Siedlce, in the climatic and soil conditions of northeastern Poland. The experiment was set up in a split-plot design with three repetitions. The area of one plot for harvesting was 18 m−2.
2.2. The Research Factors
- I
- three morphotypes of winter oilseed rape:
- ▪
- population morphotype (Chrobry variety),
- ▪
- hybrid morphotype restored with a traditional type of growth (PT 271)
- ▪
- hybrid morphotype restored with a semi-dwarf growth type (PX 113)
- II
- four ways to use growth stimulants:
- ▪
- variant 1—control object—without the use of stimulators,
- ▪
- variant 2—organic preparation containing microorganisms as well as micro and macro elements (UGmax) used in the following autumn before sowing rape seeds, in doses of 0.9 dm3·ha−1. Microbiological preparation Ugmax was used, which includes yeast, lactic acid bacteria, photosynthetic bacteria, Azotobacter, Pseudomonas and Actinomycetes, and nutrients such as: potassium (3500 mg∙dm−3), nitrogen (1200 mg∙dm−3), sulphur (1000 mg∙dm−3), phosphorus (500 mg∙dm−3), sodium (200 mg∙dm−3), magnesium (100 mg∙dm−3), zinc (20 mg∙dm−3), manganese (0.3 mg∙ dm−3).
- ▪
- variant 3—biostimulator containing 13.0% P₂0₅ and 5.0% of potassium oxide (K₂O) (Rooter) applied in autumn in the 4–6 leaves phase (Biologische Bundesanstalt, Bundessortenamt und Chemical Industry—BBCH 13–15) in the doses of 1.0 dm3·ha−1
- ▪
- variant 4—biostimulator containing silicon (Optisil) used in autumn in the 4–6 leaf stage (BBCH 13–15) in doses of 0.50 dm3·ha−1.
2.3. Experimental Design
2.3.1. Soil Conditions
The experiment was carried out on soil classified according to the World Reference Base for Soil Resources [17] to the Haplic Luvisol group, sanded, belonging to the very good rye soil complex, of the IVa class. In the years of the experiment, the pH of the soil was slightly acidic and ranged from 5.68 to 5.75. The soil was characterized by a low content of the available forms of phosphorus and average bioavailability in potassium, magnesium, boron, sulphur.
2.3.2. Fertilization
After harvesting the forecrop, a set of post-harvest cultivations was made with a stubble cultivator and a string roller, and then two weeks after the first treatment, the ploughing was carried out to a depth of 20 cm along with the ring roller. In order to prepare the soil for sowing and mixing fertilizers, a combined tilling set was used. Before sowing, phosphorus and potassium fertilization was applied at a rate of 40 kg P·ha−1 and 110 kg K·ha−1 and the first rate of nitrogen at 40 kg N·ha−1. Fertilization under oilseed rape was applied in the form of Lubofos at a rate of 600 kg·ha−1, i.e., 21 kg N·ha−1, 26.4 kg P·ha−1, 92.1 kg K·ha−1, 34.8 kg S·ha−1, 1.2 kg B·ha−1. Fertilization rates were supplemented with 55.9 kg·ha−1 ammonium nitrate (19 kg N·ha−1), 29.6 kg·ha−1 triple superphosphate (13.6 kg P·ha−1) and 29 kg·ha−1 potassium salt (17.9 kg K·ha−1).
2.3.3. Sowing
Winter oilseed rape was sown in the inter-row spacing of 22.5 cm, keeping the planting density of 45 plants per m−2. Sowing was performed at the optimal date recommended for this region (from 10–15 August).
2.3.4. Chemical Protection
Chemical protection against weeds, diseases and pests was applied in accordance with the recommendations of good agricultural practice. The preparation Command 480 EC (0.25 dm3·ha−1) and Fusilade Forte 150 EG (2.0 dm3·ha−1, BBCH 13–14) were used to control weeds.
2.3.5. Features of the Autumn Habit of the Plants
Immediately before the autumn vegetation was stopped, the following biometric features were determined on a randomly selected sample of 20 plants:
- ▪
- number of rosette leaves (pcs.)
- ▪
- root neck diameter (mm)
- ▪
- height of the growth cone (cm)
- ▪
- length of tap root (cm)
- ▪
- fresh weight of the above-ground part of 1 rosette (g)
- ▪
- dry weight of the above-ground part of 1 rosette (g)
- ▪
- fresh weight of the root system of 1 plant (g)
- ▪
- dry weight of the root system of 1 plant (g)
2.4. Statistical Analysis
The study results were statistically analyzed by means of the analysis of variance. The significance of the sources of variation was tested with the Fisher–Snedecor F test, and the significance of differences at the significance level α = 0.05 between the compared means was assessed using Tukey’s range test.
2.5. Water Conditions
The study calculated the value of the Sielianinov hydrothermal coefficient (K) for the summer–autumn period of plant growth, i.e., the months from August to October in the research year. The calculations of the index were made according to the formula provided by Skowera [18]:
where P—monthly sum of rainfall (mm), Ʃ t—monthly sum of average temperatures
On the basis of the values of the calculated indices, it was possible to determine the influence of temperature increases on hydrothermal conditions.
During the summer and autumn development of plants and winter dormancy, various humidity and thermal conditions prevailed (Figure 1, Figure 2 and Figure 3). In the first year of the research, during the sowing and emergence of plants, the average air temperature was higher than the multi-year average. In August, the sum of rainfall was more than twice lower than the average for the years, and during the emergence of plants, the sum of rainfall was on average 27.4 mm (Figure 1). On the basis of the calculated Sielianinov hydrothermal coefficient, which was 1.77, it was found that the season was quite humid (Table 1).
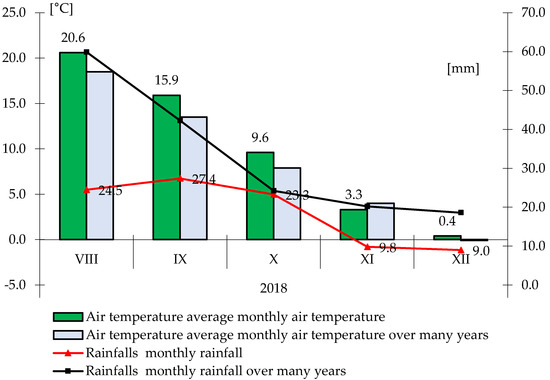
Figure 1.
Monthly rainfall totals and average air temperature during the growing season 2018.
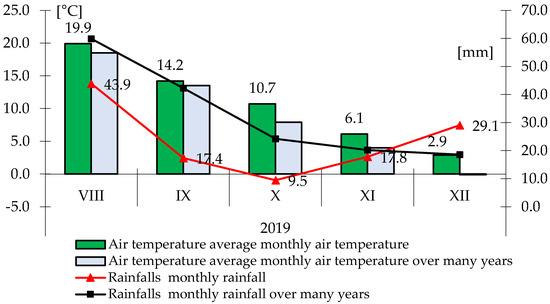
Figure 2.
Monthly rainfall totals and average air temperature during the growing season 2019.
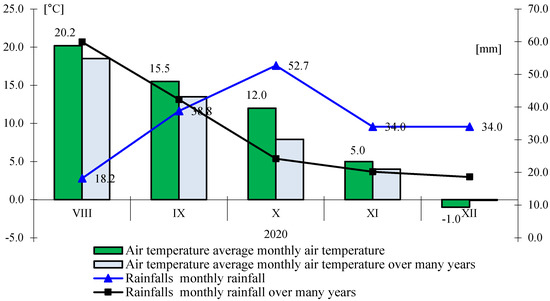
Figure 3.
Monthly rainfall totals and average air temperature during the growing season 2020.

Table 1.
Sielianinov hydrothermal coefficient during the summer-autumn development.
In 2019, during sowing, the total rainfall was more than 17 mm lower than the multi-year average, and in September and October, more than twice as low as the multi-year average. The average air temperature was slightly higher than the average for the years 1996–2010 (Figure 2). In the second year of the study, the autumn period was optimal (K = 1.43) (Table 1).
In the last period of the autumn development, the total rainfall during sowing was three times lower than the multi-year average, while in September, it was close to the multi-year period, and while in October, it was twice as high. The average air temperature in this period was higher than the average from 1996–2010 (Figure 3). On the basis of the calculated Sielianinov coefficient, it was found that the autumn was very wet (K = 2.59) (Table 1).
The climatic conditions prevailing in the period of summer and autumn vegetation and winter dormancy significantly influenced the morphometric features of the leaf rosette.
The highest values of the examined features were found in the last, most humid and warmest year of the research, where the total rainfall was 177.7 mm, while the average air temperature was 10.3 °C.
3. Results and Discussion
3.1. Largest Number of Rosette Leaves
On the basis of the conducted research, it was found that the largest number of rosette leaves, on average 9.9 pcs., was possessed by the population cultivar Chrobry, and the lowest, significantly, on average, 8.5 pcs., by the PX113 semi-dwarf hybrid (Table 2). Gugała et al. [19] reached similar conclusions, who also received the highest number of rosette leaves in the Monolit population morphotype. Wielebski and Wójtowicz [20] showed the highest value of this trait in the Starter population cultivar, and significantly lower in the restored hybrids, Poznaniak and PR45D03. Different research results were presented by Velička et al. [21] finding, on average, 15.3% more leaves in the rosette in the Kronos restored hybrid compared to the population form, whereas Sikorska et al. [22] did not show a significant effect of the morphotype on the value of this trait.

Table 2.
Morphological features of winter rape cultivars depending on the factors of the experiment.
The conducted research proved that the greatest significant increase in the number of leaves in the rosette by 1.2 pcs. on average compared to the control variant was recorded after the application of the microbiological preparation Ugmax. However, after the use of biostimulators containing P₂0₅ and K₂0 (variant 3) and silicon (variant 4), the average values of this characteristic were, respectively, 9.4 pcs. and 9.0 pcs. Sikorska et al. [22], after using an amino-acid-containing biostimulant, showed that the number of leaves in the rosette was the same as in the control. The authors obtained a largest significant increase in the value of this feature of 1.9 pcs. In comparison to the control variant after the application of foliar fertilizer containing sulphur and boron in combination with a biostimulant, Gugała et al. [19] found a significant increase in the number of leaves in the rosette only after the use of the Asahi SL biostimulant, containing the active substance in the form of sodium o-nitrophenol, sodium para-nitrophenol and sodium 5-nitroguaiacol. However, the remaining biopreparations containing titanium and silicon, according to the authors, equally increased the number of rosette leaves in relation to the control variant. Jankowski et al. [23], after foliar application of macro and micronutrients in the BBCH 14 and 16 phase, noted an average 5% higher number of rosette leaves.
This research showed a diversified response of varieties to the applied biostimulants. The population cultivar had the same number of leaves in the rosette after the application of the organic preparation Ugmax and the stimulator containing phosphors and potassium. The silicon-containing biostimulator did not significantly increase the number of leaves in rosettes in this cultivar. A similar tendency was noted in the long-stemmed hybrid, with statistically insignificant differences in the case of this morphotype also for subjects 3 and 4. The use of biostimulators significantly increases the number of leaves in the semi-dwarf hybrid, while the values of the studied traits were the same on subjects 2, 3 and 4.
3.2. Growth Cone
The authors’ own research showed that in the population variety, the growth cone was placed the highest, while in the case of the restored hybrids, no significant differences in the value of this feature were found (Table 2). Other authors [19,22] have shown that the population morphotype did not differ significantly in the height of the growth cone from a restored hybrid with a traditional type of growth. Wielebski and Wójtowicz [20] obtained a higher growth cone by an average of 1.3 cm in the population cultivar compared to a restored hybrid with a traditional type of growth.
The types of biostimulants used did not significantly affect the value of this feature. According to Sikorska et al. [22], after the application of the Aminoplant biostimulant, the height of the growth cone elevation was the same as in the control object, and on the sites where foliar fertilizer containing sulphur and boron was applied, the height of the growth cone elevation decreased by an average of 0.06 mm compared to the control object.
3.3. Length of the Tap Root
The population cultivar Chrobry (21.41 cm on average) had the greatest length of the tap root, and the PX113 semi-dwarf hybrid (18.98 cm) was the smallest. Similarly, Gugała et al. [19] showed that the population cultivar Monolit was characterized by a longer tap root, 17.3 cm on average, compared to the heterotic ones, PR 44D06 and PT 205. Sikorska et al. [22] reached different conclusions, finding that the largest length of the tap root was distinguished by a restored hybrid with a traditional type of growth, one significantly smaller by a semi-dwarf hybrid, and the smallest by the population variety.
The highest value of this feature was obtained after the application of the Ugmax preparation, which was, on average, higher by 2.26 cm compared to the control variant. It should be emphasized that after applying the preparation containing silicon, the length of the tap root was the same as in the control object. Aisha et al. [24] as well as Albayrak and Çarnas [25], after using humic acids, noted a higher value of this feature, while Sikorska et al. [22] concluded that the amino acid biostimulant increased the length of the tap root by only 0.19 mm. Other authors [23], after foliar application of macro and micronutrients in the BBCH 14 and 16 phase, noted an average 3% longer tap root length.
3.4. Diameter of the Root Neck
The morphotype significantly influenced the diameter of the root neck. The highest value of this feature of, on average, 8.53 mm was recorded in the population variety, significantly lower in the restored hybrid, and the lowest, on average, 7.75 mm, in the semi-dwarf hybrid (Table 2). This is confirmed by the research results of Sikorska et al. [22]. The authors noted the highest value of this trait in the population cultivar Monolit, significantly lower in the restored hybrid PT248, and the lowest in the semi-dwarf hybrid PX115. According to Veliček et al. [21] restored hybrids are distinguished by a larger diameter of the root neck compared to the traditional cultivar. Different research results were presented by Wielebski and Wójtowicz [20], who noted statistically insignificant differences between the examined morphotypes.
The largest diameter of the root neck of, on average, 8.25 mm, was obtained after the application of the organic preparation Ugmax. After the use of a silicon-containing stimulant, no significant differences were found between this object and the control object. Sikorska et al. [22], after using a biostimulant containing amino acids, noted an average increase of 0.12 mm in the value of this feature, which was a statistically insignificant difference compared to the control variant. Wenda-Piesik et al. [26], after the application of a growth activator based on amino acids of plant origin and extracts from marine brown algae, obtained a reduction in the diameter of the root neck of plants by an average of 0.1 cm compared to the control object. Jankowski et al. [23], after foliar application of macro and microelements in the BBCH 14 and 16 phase, noted an average 9% larger root neck diameter.
This research showed the interaction of the cultivars with the types of growth stimulants used. In the population cultivar and the long-stemmed hybrid, after the use of a silicon-containing stimulator, the same value of the root neck diameter was demonstrated as in the control object. In hybrids restored with PT271 and PX113, statistically insignificant differences were also found in the objects where the organic preparation UGmax and the biostimulator containing phosphorus and potassium were used.
3.5. Fresh and Dry Mass of the Above-Ground Part of the Rosette and the Root System
Fresh and dry mass of the above-ground part of the rosette and the root system was the highest in the population variety and the lowest in the restored hybrid with a semi-dwarf type of growth (Table 2). Many authors have come to similar conclusions [20,27,28,29]. On the other hand, different research results were obtained by Jankowski and Budzyński [30] as well as Wielebski [31], who showed that it was the heterotic forms that produced a rosette with a larger fresh and dry mass of one plant and the root system.
The types of growth stimulants used significantly increased the fresh and dry weight of the above-ground part of the rosette and the fresh weight of the root system. The highest values of these features were obtained after the application of the Ugmax preparation, which wraps microorganisms, which was applied in the autumn before sowing rape seeds at a dose of 0.9 dm3·ha−1. Similarly, Sikorska et al. [28,29] and Gawrońska et al. [32] showed an increase in the value of these features under the influence of biostimulants. The beneficial effect of humic acids on fresh and dry mass of the above-ground part of the rosette and fresh mass of the root system was demonstrated by Albayrak and Camas [25], Soheir et al. [33] and Aisha et al. [24].
The interaction found between the cultivars and the methods of using biostimulants indicates that the cultivars reacted differently to the growth bioregulators. The population cultivar had the same fresh and dry weight of the above-ground part in the control object and after the application of the preparation containing phosphorus and potassium. In the long-stem cultivar, the preparations used in the experiment significantly increased the values of the examined traits as compared to the control object, but the differences between subjects 2 and 3 as well as 3 and 4 were statistically insignificant. The same values of these properties were obtained in the semi-dwarf hybrid after the application of the preparation containing silicon as in the objects without biostimulants.
The studies showed that the fresh mass of the root system in the population morphotype and long-stemmed hybrid was the same in the control and the silicon-containing biostimulator. Population and semi-dwarf varieties had the same value of this trait in objects 2 and 3, and the hybrid semi-dwarf and long-stemmed varieties in variants 3 and 4.
4. Conclusions
The conducted research showed that the application of the organic preparation Ugmax significantly increased the number of rosette leaves (by an average of 13.9%), the length of the tap root (by an average of 2.3 cm), root neck diameter (by an average of 4.2%), fresh and dry weight of the above-ground part of the rosette (by an average of 6.0% and 6.6%) and fresh weight of the root system (by an average of 0.88 g) compared to the control variant. Regardless of the applied growth stimulator, the height of the growth cone was the same. The hybrid morphotypes that were restored, PT 271 and PX 113, compared to the population cultivar Chrobry were characterized by a weaker autumn development of the leaf rosette. Diverse climatic conditions in the periods of summer–autumn vegetation and winter dormancy in the years of the research influenced the habit of plants.
Author Contributions
Conceptualization, M.G. and K.Z.; methodology, M.G.; software, Ł.D.; formal analysis, A.S.; writing—original draft preparation, A.S.; writing—review and editing, M.G.; visualization, A.S.; supervision, I.M.; funding acquisition, M.G. All authors have read and agreed to the published version of the manuscript.
Funding
The results of the research carried out under the research theme No. 32/20/B were financed from the science grant granted by the Ministry of Science and Higher Education.
Institutional Review Board Statement:
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bybordi, A. Effects of salinity on yield and component characters in canola (Brassica napus L.) cultivars. Not. Sci. Biol. 2010, 2, 81–83. [Google Scholar] [CrossRef][Green Version]
- Shahzadi, T.; Khan, F.A.; Zafar, F.; Ismail, A.; Amin, E.; Riaz, S. An overview of Brassica species for crop improvement. Am. Eurasian J. Agric. Environ. Sci. 2015, 15, 1568–1573. [Google Scholar] [CrossRef]
- Abdulkhaleq, D.A.; Hama, S.J.; Ahmad, R.M.; Tawfiq, S.I. Response of some Rapeseed (Brassica napus L.) varieties to Zn fertilizer Under Dryfarming Conditions. In Proceedings of the 2nd International Conference of Agricultural Sciences, Baqubah, Iraq, 17–18 August 2022; 2018; pp. 143–155. [Google Scholar]
- Kozak, M.; Wondołowska-Grabowska, A.; Serafin-Andrzejewska, M.; Gniadzik, M.; Kozak, M.K. Biostymulatory—wczoraj, dziś i jutro. W: Rolnictwo XXI wieku—problemy i wyzwania. Łuczycka, D. (red.). Idea Knowl. Future Wrocław 2016, 114–122. [Google Scholar]
- Rutkowska, A. Biostymulatory w nowoczesnej uprawie roślin. ZESZYT 2016, 48, 65–80. (In Polish) [Google Scholar] [CrossRef]
- Sharma, S.H.S.; Fleming, C.; SelBy, C.; Rao, J.R.; Trevo, R.M. Plant biostimulants: A review on the processing of macroalgae and use of extracts for crop management to reduce abiotic and biotic stresses. J. Appl. Phycol. 2014, 26, 465–490. [Google Scholar] [CrossRef]
- Kocira, A.; Świeca, M.; Kocira, S.; Złotek, U.; Jakubczyk, A. Enhancement of yield, nutritional and nutraceutical properties of two common bean cultivars following the application of seaweed extract (Ecklonia maxima). Saudi J. Biol. Sci. 2018, 25, 563–571. [Google Scholar] [CrossRef]
- Ruzzi, M.; Aroca, R. Plant growth-promoting rhizobacteria act as biostimulants in horticulture. Sci. Hortic. 2015, 196, 124–134. [Google Scholar] [CrossRef]
- Vandenkoornhuyse, P.; Quaiser, A.; Le Van Duhamel, M.; Dufresne, A. The importance of the microbiome of the plant holobiont. New Phytol. 2015, 206, 1196–1206. [Google Scholar] [CrossRef]
- Ratiu, I.A.; Al-Suod, H.; Ligor, M.; Monedeiro, F.; Buszewski, B. Effects of growth conditions and cultivability on the content of cyclitols in Medicago sativa. Int. J. Environ. Sci. Technol. 2020, 18, 33–48. [Google Scholar] [CrossRef]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Lugtenberg, B. (Ed.) Principles of Plant-Microbe Interactions: Microbes for Sustainable Agriculture; Springer: Cham, Switzerland, 2015; pp. 1–15. [Google Scholar]
- Rouphael, Y.; Colla, G. Biostimulants in Agriculture. Front. Plant Sci. 2020, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Fageria, N.K.; Barbosa Filho, M.P.; Moreira, A.; Guimarães, C.M. Foliar fertilization of crop plants. J. Plant Nutr. 2009, 32, 1044–1064. [Google Scholar] [CrossRef]
- Kocoń, A. Foliar top dressing efficiency of winter wheat and rape of chosen fertilizers in optimal fertilization and soil moisture conditions. Ann. UMCS 2009, 64, 23–28. (In Polish) [Google Scholar] [CrossRef]
- Szewczuk, C.Z.; Sugier, D. General characteristics and types of foliar fertilizers offered on the Polish market. Ann. UMCS 2009, 64, 29–36. (In Polish) [Google Scholar] [CrossRef]
- World Reference Base for Soil Resources. International Soil Classification System for Naming Soils and Creating Legends for Soil. In World Soil Resources Reports; Field Experiment; Food and Agriculture Organization: Rome, Italy, 2014; Available online: http://www.fao.org (accessed on 1 February 2022).
- Skowera, B. Changes of hydrothermal conditions in the Polish area (1971–2010). Fragm. Agron. 2014, 31, 74–87. (In Polish) [Google Scholar]
- Gugała, M.; Sikorska, A.; Zarzecka, K.; Kapela, K.; Mystkowska, M. The effect of sowing method and biostimulators on autumn development and overwintering of winter rape. Acta Sci. Pol. Agric. 2017, 16, 111–120. [Google Scholar] [CrossRef]
- Wielebski, F.; Wójtowicz, M. Effect of date and density of sowing and weather conditions on growth in the autumn and winter survival of winter oilseed rape morfotypes with traditional and semidraft type of growth. Fragm. Agron. 2018, 35, 133–145. (In Polish) [Google Scholar] [CrossRef]
- Velička, R.; Pupalienė, R.; Butkevičienė, L.M.; Kriaučiūnienė, Z. Peculiarities of overwintering of hybrid and conventional cultivars of winter rapeseed depending on the sowing date. Acta Sci. Pol. Agric. 2012, 11, 53–66. [Google Scholar]
- Sikorska, A.; Gugała, M.; Zarzecka, K. The Effect of Foliar Nutrition with Sulphur and Boron, Amino Acids on Morphological Characteristics of Rosette and Wintering Winter Rape (Brassica napus L.). J. Ecol. Eng. 2019, 20, 190–197. [Google Scholar] [CrossRef]
- Jankowski, K.J.; Sokólski, M.; Szatkowski, A. The Effect of Autumn Foliar Fertilization on the Yield and Quality of Winter Oilseed Rape Seeds. Agronomy 2019, 9, 849. [Google Scholar] [CrossRef]
- Aisha, H.; Shafeek, M.R.A.; Mahmoud, R.; El-Desuki, M.A. Effect of Various Levels of Organic Fertilizer and Humic Acid on the Growth and Roots Quality of Turnip Plants (Brassica rapa). Curr. Sci. Int. 2014, 3, 7–14. [Google Scholar]
- Albayrak, S.; Çarnas, N. Effects of different levels and application times of humic acid on root and leaf yield components of forage turnip. J. Agron. 2005, 4, 130–133. [Google Scholar] [CrossRef]
- Wenda-Piesik, A.; Hoppe, S. Evaluation of hybrid and population cultivars on standard and high-input technology in winter oilseed rape. Acta Agric. Scand. Sect. B Soil Plant Sci. 2018, 68, 678–689. [Google Scholar] [CrossRef]
- Kotecki, A.; Malarz, W.; Kozak, M.; Pogorzelec, A. The effect of plants’ location in a canopy on the growth and yield of rape hybrids and population cultivars. Part I. Plant morphology and seed yields. Zeszyty Naukowe Uniwersytetu Przyrodniczego we Wroclawiu. Rolnictwo 2007, 90, 7–39. [Google Scholar]
- Sikorska, A.; Gugała, M.; Zarzecka, K.; Kapela, K.; Mystkowska, I. The impact of agrotechnical factors on fresh and dry matter of oilseed rape (Brassica napus L.). J. Ecol. Eng. 2017, 18, 174–179. [Google Scholar] [CrossRef]
- Sikorska, A.; Gugała, M.; Zarzecka, K. The effect of the types of foliar feeding on fresh and dry winter rape mass (Brassica napus L.). Appl. Ecol. Environ. Res. 2019, 17, 7203–7211. [Google Scholar] [CrossRef]
- Jankowski, K.J.; Budzyński, W. Response of different breeding forms of winter oilseed rape to date and density of sowing. I. Growth in the autumn and winter survival of plants. Rośliny Oleiste Oilseed Crop. 2007, 28, 177–194. (In Polish) [Google Scholar]
- Wielebski, F. Response of different types of winter oilseed rape varieties to various plant density in the field I. Seed yield and its components. Rośliny Oleiste Oilseed Crop. 2007, 28, 209–226. (In Polish) [Google Scholar]
- Gawrońska, H.; Przybysz, A.; Szalacha, E.; Słowiński, A. Physiological and molecular mode of action of Asahi SL biostimulator under optimal and stress conditions. In Biostimulators in Modern Agriculture, General Aspects; Gawronska, H., Ed.; Wieś Jutra: Warsaw, Poland, 2008; pp. 54–76. [Google Scholar]
- El-Sherbeny, S.E.; Hendawy, S.F.; Youssef, A.A.; Naguib, N.Y.; Hussein, M.S. Response of Turnip (Brassica rapa) Plants to Minerals or Organic Fertilizers Treatments. J. Appl. Sci. Res. 2012, 8, 628–634. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).