Abstract
Thyroid hormones (THs) exert pleiotropic effects in different mammalian organs, including gonads. Genetic and non-genetic factors, such as ageing and environmental stressors (e.g., low-iodine intake, exposure to endocrine disruptors, etc.), can alter T4/T3 synthesis by the thyroid. In any case, peripheral T3, controlled by tissue-specific enzymes (deiodinases), receptors and transporters, ensures organ homeostasis. Conflicting reports suggest that both hypothyroidism and hyperthyroidism, assessed by mean of circulating T4, T3 and Thyroid-Stimulating Hormone (TSH), could affect the functionality of the ovarian reserve determining infertility. The relationship between ovarian T3 level and functional ovarian reserve (FOR) is poorly understood despite that the modifications of local T3 metabolism and signalling have been associated with dysfunctions of several organs. Here, we will summarize the current knowledge on the role of TH signalling and its crosstalk with other pathways in controlling the physiological and premature ovarian ageing and, finally, in preserving FOR. We will consider separately the reports describing the effects of circulating and local THs on the ovarian health to elucidate their role in ovarian dysfunctions.
1. Introduction
The accelerated decline in fertility and the onset of early menopause have been associated with loss of functional ovarian reserve (FOR), resulting in premature ovarian ageing. This decline is often asymptomatic and the underlying mechanisms are still poorly understood. Genetic and environmental factors contribute to this phenomenon. Both factors influence the number of the follicles, established in early life, and the hormonal assets required for their preservation and maturation during reproductive age [1].
Although conflicting, some epidemiological studies suggest a significantly higher prevalence of hypothyroidism, both overt and subclinical, in women with a genetic cause of diminished ovarian reserve (DOR) [2].
Thyroid hormones (THs) are involved in the normal growth, development and functions of many organs, including gonads. Their circulating levels are tightly regulated by feedback mechanisms active along the hypothalamic-pituitary-thyroid (HPT)-axis. Furthermore, cells and tissues can locally customize the TH signalling by regulating the life stage-specific expression of iodothyronine deiodinases (DIOs, enzymes involved in TH metabolism), TH transporters and, lastly, TH receptors (TRs). Their local modulation represents an additional and/or an alternative mechanism to maintain the peripheral T3 quota required for physiological processes, independently from fluctuations in circulating levels of THs [3]. Therefore, the organ/tissue-specific TH signalling is the result of thyroid hormones synthesis and of their peripheral metabolism.
Our recent analyses of molecular mechanisms underlying both physiological and premature ovarian ageing revealed the impairment of several cellular functions controlled by TH signalling [4,5]. This review was conceived after these findings, when we searched PubMed using the combined terms “thyroid hormone metabolism” and “ovarian reserve” retrieving only 46 suitable articles. None article was retrieved when the terms “thyroid hormone signalling” and “ovarian reserve” were used. Both results were indicative of the poor characterization of the peripheral regulation of TH signalling in the ovary.
Here, we will summarize results regarding the role of TH signalling in ovarian development, health and disease focusing on the specific activity of circulating THs and their peripheral metabolism/signalling in regulating FOR and ovarian health.
2. HPT and Peripheral Regulation of TH Metabolism during Ageing
The synthesis and release of THs (T4 and T3) are tightly regulated by conserved mechanisms in vertebrates. Circulating T4 is controlled by a negative feedback mechanism involving the hypothalamus, the pituitary and the thyroid (HPT)-axis [6]. Specifically, the pituitary secretes the thyroid-stimulating hormone (TSH) that controls the synthesis and secretion of T4 and T3 by the thyroid. Both regulate in turn TSH release as well as the hypothalamic thyrotropin-releasing hormone (TRH) [7]. Conversely, intra-organ conversion of T4 to T3 provides negative feedback on the pituitary and on the hypothalamus inhibiting the TRH and TSH secretion, respectively (Figure 1A) [8,9,10].
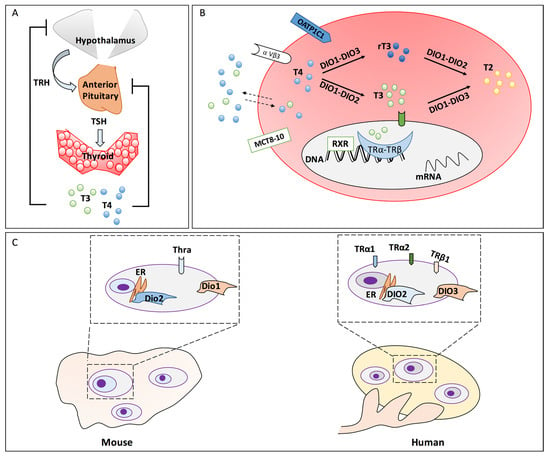
Figure 1.
HPT-axis and peripheral TH metabolism/signalling in mammals. (A) Hypothalamic-Pituitary-Thyroid (HPT)-axis and its regulatory feedback loops. (B) Mechanisms/enzymes and other proteins involved in the cell/tissue-specific TH metabolism and signalling. (C) Description of the species-specific pattern of the ensemble of proteins involved in TH metabolism and signalling in the ovary. The ovarian follicles are evidenced in the dashed rectangles. The zoom on a single follicle is reported in the upper dashed box in order to evidence the ensemble of the expressed proteins involved in peripheral TH metabolism and signalling. Abbreviations: TRH, thyrotropin releasing hormone; TSH, thyroid-stimulating hormone; T4, thyroxine; T3, triiodothyronine; T2, 3,5-diiodo-L-thyronine; rT3, reverse triiodothyronine; DIO1, DIO2, DIO3, deiodinases; MCT8–10, monocarboxylate transporter 8–10; OATP1C1, organic anion transporting polypeptide 1C1; αVβ3, integrin alpha (V) beta (3); TRα and TRβ, thyroid nuclear receptors isoform α, β; RXR, retinoic acid X receptor; ER, endoplasmic reticulum; Thra, thyroid hormone receptor alpha (Mouse); TRα1, TRα2, TRβ1 thyroid hormone receptors isoform α1, α2, β1 (Human).
The thyroid gland releases mainly the pro-hormone T4 and to a lesser extent T3, the biologically more active form of THs. Their synthesis requires the activity of a complex network of thyroid-specific enzymes. Briefly, iodine entry in thyroid follicular cells is mediated by two glycoproteins: sodium-iodide symporter (NIS) and pendrin [11,12]. The iodine oxidation is mediated by thyroid peroxidase (TPO) and, finally, it is incorporated into the thyroglobulin (TG) by a multistep process leading to the formation of T4. The prohormone T4 is converted to bioactive T3 (or to inactive rT3) in the thyroid and, mainly, in peripheral tissues by the deiodinases (Dio1, Dio2 and Dio3) [13,14,15]. Dio2 and Dio1 are T4/T3 activating enzymes and cooperate to maintain THs homeostasis, due to their differential expression in response to THs availability. Noteworthy, Dio2 regulates intracellular T3 and increases in hypothyroid subjects [16], whereas Dio1 regulates mainly circulating THs [17]. However, Dio1 can also inactivate T4 to rT3 and the sulfonated THs participating in the defence mechanism developed against iodide deficiency typical of hyperthyroidism [18]. Dio3 inactivates both T4 and T3 [19]. Moreover, both T3 and rT3 can be further metabolised to diiodothyronines (T2s), which also exhibits interesting metabolic activities (Figure 1B) [20,21,22]. Circulating THs are mostly bound to plasma proteins, such as thyroxine-binding globulin (TBG), whose level might influence TH signalling [23]. THs can be quickly liberated for entry into cells either by diffusion or by specific carrier-mediated mechanisms (e.g., OATP1C1, MCT8–10, etc.) [24,25,26].
The tissue specific TH signalling depends on the cellular content of TH receptors, which comprise the nuclear receptors (TRs), and the membrane receptors (e.g., αVβ3) [27,28,29,30]. Nuclear TRs act as transcription regulators in concert with other nuclear receptors, such as retinoic acid X receptor (RXR), for the recruitment of co-activators or repressors [31]. In mammals, four isoforms have been identified: TRα1, TRα2, TRβ1, and TRβ2 expressed in a tissue-specific manner [32,33,34]. On the other hand, integrin αVβ3 mediates THs non-genomic effects. Genomic and non-genomic pathways cooperate to determine the cellular-specific response to the TH signalling [35,36].
The regulatory role of peripheral TH signalling has been primarily described in metabolic processes, also becoming less efficient with ageing. Available data indicates that THs metabolism is impaired in aged organs. For instance, a reduction of Dio1 activity has been reported in thyroid and liver during ageing whereas age-related changes in TH receptors and transporters have been described in liver and kidney [37]. Circulating THs and TH signalling in peripheral organs were both reduced in a mouse model of progeria, in which the tissue-specific regulation of the activity of the deiodinases contributed to protect metabolic activity during ageing [38]. Although there is no definitive indication of the role of peripheral TH signalling in ovarian ageing, it is strongly evoked by the growing body evidence of its role in differentiation, proliferation and apoptosis in many organs, including the ovaries [39]. Indeed, in vitro and in vivo studies showed that Dio1 activity rose in the pituitary during ageing in order to maintain the local level of T3 [40,41], necessary to control the increase of circulating level of TSH [42]. Noteworthy, levels of TSH < 3.0 μIU/mL in euthyroid infertile patients have been associated with higher anti-Müllerian hormone (AMH) levels, a superior marker of FOR [43].
Taken together, the above-reported observations imply that the levels of circulating THs might not provide a sensitive and quantitative indicator of peripheral TH signalling in the ovaries, as well as in other organs, and that its deregulation could correlate with a premature loss of FOR [44,45].
3. TH and Other Pathways Involved in Preservation of FOR and Ovarian Health
Hormones, including THs, control various aspects of ageing [46]. The female reproductive system ages faster than the rest of the body: the ovaries are considered aged by the time a woman reaches the age of 45–50 years. Ovarian ageing is characterized by the progressive and silent decline of FOR, both in terms of quantity and quality of the oocytes. The menopause is the final step of this process.
Genetic and environmental factors may contribute to the premature decline of FOR resulting in the Premature Ovarian Insufficiency (POI) [5]. Mutations in the genes of the TGF-beta family, such as GDF9 and BMP15 and INHA, have been associated with POI [47,48]. Specifically, GDF9 and BMP15 are produced by the oocytes whereas INHA is secreted by granulosa cells (GCs), they are collectively involved in the physiological maintenance of FOR [49,50,51]. AMH is another GC-specific member of this family playing an important role in ovarian ageing enough to be used as a marker of FOR. Notably, studies conducted in primary mouse GCs and in a human GCs cancerous cell line (KGN cells) evidenced that GDF9 and BMP15 could directly modulate AMH expression [52].
Different environmental factors (e.g., lifestyle, diet, exposure to environmental stressors) modulate AMH expression. It has been reported that the main metabolite of methoxychlor, a chlorinated hydrocarbon pesticide, increases AMH expression in rat immature GCs as well as in vivo [53]. On the contrary, other compounds such as dibutyl phthalate did not regulate its expression in cultured rat primary GCs [54]. Accordingly, we have also reported that environmental factors, i.e., ethylene thiourea (ETU) and different diets, could alter FOR whose status was assessed investigating the expression of the aforementioned genes. Specifically, Amh mRNA was considerably reduced in mice exposed to high- dose ETU (10 mg/kg/die) along with other transcripts whose inhibition was associated with physiological ovarian ageing. In the same experimental setting, we observed the concomitant substantial decrease in circulating T4 [5]. We assume that the hypothyroidism might be involved in POI onset participating in the transcriptional regulation of these genes. Indeed, we conducted the analysis of the mouse promoter of Amh, Gdf9 and Bmp15 genes in order to verify the prediction of thyroid hormone receptor binding elements (TREs). The results, schematized in Figure 2, evidence TREs in all of them. Similar results have been obtained also with their fish and human orthologs. Considering that conserved cis-regulatory elements regulate complex gene networks tuning basic developmental processes, such as establishment and maintenance of FOR, this points out the role of TH signalling in FOR establishment and preservation [55].
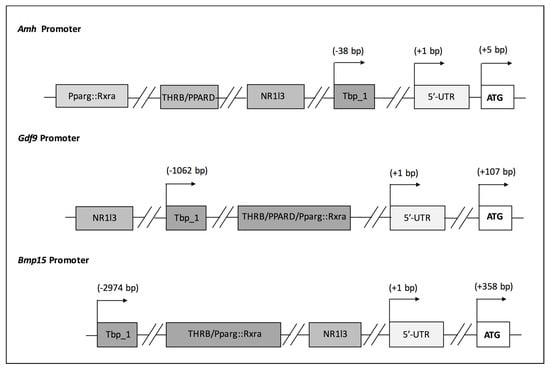
Figure 2.
Analysis of mouse Amh, Gdf9 and Bmp15 promoters. List of transcription factor binding sites that were identified by the Jaspar tool analysing the 3000 bp upstream sequence of the genes. The ENSEMBLE Transcript ID were: (Amh) ENSMUST00000036016.5; (Gdf9) ENSMUST00000018382.6; (Bmp15) ENSMUST00000024049.7.
In zebrafish, the role of TH signalling in egg production has been investigated in females exposed to propylthiouracil (PTU) for 21 days. Exposed females presented the expected reduction of T4/T3 and an increased egg production together with a reduced size of the mature oocytes [56]. Recent studies on the reproductive seasonality in birds have also revealed that normal levels of circulating THs and their peripheral signalling are crucial to the normal development/lifespan of ovarian follicles. Specifically, in laying hens it was reported that the hyperthyroid status, induced by T3 administration, caused atresia of pre-ovulatory follicles and stoppage of laying eggs as well as the impaired synthesis of hormones in ovarian follicles at various stages of development in vitro. [57].
Furthermore, the role of TH signalling in mouse ovarian ageing could be evinced by a previous gene expression profiling analysis conducted in ovaries from young- and middle-aged mice in our laboratory [4,5]. Although the TH signalling was not directly highlighted by the bioinformatic analysis, we retrieved the reduced expression of a canonical TH-responsive gene (Thrsp, also known as Spot14) in the aged ovaries [5]. Since the mitochondria are well-characterised subcellular targets of THs [58], the inhibition of the oxidative phosphorylation further corroborates the possible reduction of TH signalling in ovarian ageing. Therefore, we have investigated a potential connection between the canonical pathways, identified by IPA analysis of the transcriptomic data [4,5], and TH signalling by reviewing the literature. As evidenced in Table 1, THs modulate the first nine identified canonical pathways evidenced in our analysis. Although not surprising, this is the first piece of evidence connecting the inhibition of ovarian TH signalling to physiological ovarian ageing in mice.

Table 1.
Canonical pathways, targeted by THs, affected in physiological ovarian ageing. IPA analysis of microarray data, previously published (Cuomo et al., 2018), mapped nine top biological processes altered during physiological ovarian ageing. They are listed together with the deregulated genes that contribute to their identification. The statistical relevance, the activation status, and the regulatory role of THs of the pathways are reported as −log(p-value), z-score, and citations, respectively.
The role of the local TH metabolism and signalling in gonadal differentiation has been explored in mammals, especially in rodents and humans (Figure 1C). TH transporters (slc16a2, slc16a10 and slco1c1), the deiodinases (dio1, dio2, dio3a, dio3b) and TH receptors (thra and thrb) have been reported to modulate Zebrafish (Danio rerio) development, however, their role has not been specifically investigated in the ovaries [59].
Contrarily, their ensemble has been evaluated in rodent gonads, especially in testis. Recently reviewed data from ENCODE Consortium have evidenced Thra as the most abundant TH receptor in rodent ovary, and Mct8 (Slc16a2), Lat1 (Slc7a5) and Lat2 (Slc7a8) as the most expressed transporters. Regarding the deiodinases, the available data showed that Dio2 is more expressed than Dio1 whereas there are no data for Dio3 (Figure 1C). Since expressing the ensemble of transporters, enzymes and receptors involved in the peripheral TH signalling, rodents have been pivotal in unravelling the mechanisms regulating TH availability and activity in the development of ovarian dysfunctions [60].
In humans, the mRNA and protein levels of the ensemble of TH-transporters, receptors and deiodinases have been reported in the different cellular components of the follicles and at different their maturation stages. Precisely, TRα1, TRα2 and TRβ1 were expressed in human ovarian surface epithelium and in oocytes of primordial, primary and secondary follicles. Both receptors were faintly detected in GCs of secondary follicles whereas they were clearly detected in GCs of antral follicles (Figure 1C). Lastly, DIO2 and DIO3 transcripts were found in both mature GCs and mature (MII) oocytes [61]. Moreover, recent findings underline the TSH- and TH-signalling cooperation in ovaries in in vivo and in vitro settings [62,63,64,65,66,67].
Taken together, the data suggest that circulating THs as well as local T3 signalling may contribute to the regulation of ovarian function.
4. Circulating TH/TSH Levels and Premature Ovarian Dysfunctions
Effects of different concentrations of T3 on ovarian function have been investigated in various in vitro systems. It was reported that T3 exposure promoted (FSH)-induced pre-antral follicle growth in vitro, by activation of the Akt pathway. The last-mentioned pathway plays a crucial role as an anti-apoptotic factor for the GCs in rat [68], as also evidenced by the gene expression profiling study conducted in our laboratory (Table 1). Additionally, this observation was confirmed in a study evaluating T3 protective role in rat GCs exposed to a chemotherapeutic drug [69]. Besides, it has been shown the presence of TSH-receptor in human GCs and the increase of cAMP upon TSH stimulation [61].
HPT-axis is physiologically related to the hypothalamic-pituitary-gonads (HPG)-axis, both regulate reproductive functions [70]. As said, the zebrafish thyroid is comparable to the mammalian one in terms of genes responsible for thyroid development and/or for TSH function [71,72]. It has been shown that the hyperthyroidism in zebrafish larvae inhibited the aromatase (cyp19a1) activity, leading skewed sex ratio in favour of males [73]. Furthermore, it has been reported that adult females exposed to PTU showed the expected reduction of T4 and T3 and the increase of the steroidogenic transcripts (star, hsd3b and hsd17b) after short or long exposure. Supposedly, elevated levels of FSH and LH caused their altered expression [74]. Despite that, the role of thyroid hormones in regulating FOR in zebrafish is far from being defined.
Recently, the association of hypothyroidism with impairment of FOR has been examined in mice and rats after administration of PTU and low-iodine diet, respectively [75,76]. In both cases, the number of primordial, primary and preantral follicles was reduced whereas none significant change of atretic follicles was reported. Although none explanation was supplied, the data suggested that the numeric reduction of preantral and antral follicles was not due to their degeneration under hypothyroid condition [76,77]. Noteworthy, in a previous paper, the same authors reported an impairment of FSH and LH surge with a concomitant alteration of the antioxidant enzymes (e.g., catalase, SOD1, and NOS) in ovaries from hypothyroid rats [78]. Experimental studies conducted in Wistar rats demonstrated that hyperthyroidism increased the number of secondary and tertiary follicles whereas reduced the follicular atresia [79]. The effects of hyperthyroidism have been investigated in several reports. In prepuberal and adult rats T3 treatment altered the ovarian steroidogenesis suggested as the cause of the impaired folliculogenesis and ovulation [80]. Furthermore, T3 cooperated with FSH to promote preantral follicle development in mice by increasing Xiap and by reducing Bad mRNA levels [81]. Contrasting results have been reported regarding the effect of L-thyroxine, used to treat hypothyroidism, on ovarian health in rats. Specifically, Jiang et al. reported that L-thyroxine treatment of spontaneously hypothyroid rdw rats improved follicular development, but did not restore the pre-ovulatory surge of LH [82], whereas Zheng et al. reported a reduced number of primordial and antral follicles [83]. Other studies have been conducted in rats treated with PTU to promote prepuberal hypothyroidism. The published data evidenced that PTU reduced the proliferation of GCs in follicle-stage dependent manner [84].
Lastly, it should be accounted that maternal thyroid dysfunction in rats, both hypothyroidism and hyperthyroidism, affects the ovarian development of the offspring by reducing the follicle number at different developmental stages [80]. Despite the establishment of the OR during the foetal and the neonatal life stages and the effects of maternal hypothyroidism on the ovarian health of the offspring, the connection between local THs and ovarian dysfunctions needs further investigation.
Some of these aspects have been investigated also in humans. Although known causes of POI, include radiation, chemotherapy, X chromosome deletions and defects in genes codifying for the gonadotropin hormones or receptors, about 90% of the cases remain idiopathic [85]. THs likely play a role in POI onset and progression because of their cross-talk with other hormonal pathways (e.g., oestrogen, prolactin, IGF−1 and GnRH) impairing the folliculogenesis. The prevalence of hypothyroidism ranges between the 0.3%–4.3% in adult women and it is often associated with the presence of thyroid antibodies (e.g., AbTPO, AbTG) [86]. Although debated, the link between increased infertility/ovarian dysfunction with hypothyroidism/thyroid antibodies has been explored, evidencing the association of high levels of thyroid antibodies and several reproductive dysfunctions, including POI [87,88,89,90,91]. Michlakis and co-authors showed an increase of thyroid diseases in women affected by DOR when compared to other patients whose infertility had other origins. Therefore, the screening for TH levels and thyroid antibodies is currently recommended in women suffering from POI with unknown aetiology [92]. More recently, two conflicting studies have investigated the association between the levels of thyroid antibodies and the reduction of the FOR. The first, published in 2015, is a retrospective study involving about 5000 women. Among them, about 1/10 were affected by a diminished ovarian reserve (DOR) and about the same number had a normal ovarian reserve. Both groups did not show statistically different concentrations of fT4, TSH and AbTPO antibodies. Higher prevalence of sub-clinical hypothyroidism or hypothyroidism was observed when DOR had exclusively a genetic cause [2]. The second report, published in 2019, describes a 12-year follow-up study aimed at assessing the modulation trend of THs and AbTPO antibodies in women. FOR was determined by measuring serum AMH concentration. Its first determination, considering the age-specific AMH reference values, was used to group the patients in quartiles: Q1, grouping the women with the lowest AMH level, up to Q4 including the ones with highest AMH level. In three different follow-up visits, as at the baseline, TSH, fT4 and AbTPO antibodies were also measured. Interestingly, none statistically relevant difference in circulating THs was detected at the baseline whereas the AbTPO antibodies concentration was higher in women included in Q1. A progressive decrease of fT4 and an increased level of AbTPO antibodies were detected in all the quartiles over time [93]. Accordingly, a previous study, involving about 1000 Chinese women, reported the increase of AbTPO antibodies concomitant with idiopathic DOR [94].
Although the prevalence of hyperthyroidism is lower than hypothyroidism (1.3%), about 5.8% of hyperthyroid women are infertile [95]. This may be due to the production of anti-TSH antibodies whose increase has been associated with primary and secondary infertility.
Given the above data, we suggest that circulating THs and TSH might contribute to the establishment and maintenance of FOR.
5. Peripheral TH Metabolism/Signalling and Markers of Ovarian Reserve: Potential “Local” Crosstalk
As stated, the role of peripheral thyroid metabolism and signalling has been poorly explored in ovary both in vitro and in vivo. In vitro systems have been pivotal in suggesting the potential crosstalk between the different pathways involved in FOR preservation. They have been used to explore also the crosstalk between the gonadotropins and thyroid pathways, above all TSH, on gonadal development and vice versa. Indeed, the TH/TR complexes might exert their biological function interconnecting with other signalling pathways including AMH, GDF9, BMP15, IGF or other endocrine hormones (e.g., FSH, LH), playing a role in POI onset [96,97].
As said, these aspects have been analysed in vitro. The expression of GDF9, BMP15 and AMH during the developmental stages of the follicles is fundamental to the activation of signalling pathways directly involved in FOR preservation [52,98,99]. Despite ovarian cell lines carrying the deletion of one or more of genes of the ensemble of factors involved in cellular TH metabolism and signalling, the crosstalk of the above-reported pathways with intracellular T3 signalling has been explored in the ovaries [100,101]. Firstly, it was reported the involvement of T3 in the amplification of FSH-R signalling in the differentiation of porcine GCs, due to the increased transcription of the FSH receptor gene [102]. Subsequently, Tsang and co-authors confirmed the interaction of FSH and T3 signalling in increasing the FSH-R levels in rat pre-antral follicles via GDF9. It has also been shown that T3 and FSH co-treatment enhanced steroid biosynthesis driven by an increased expression of cytochrome P450 lanosterol 14α-demethylase (Cyp51), a mediator of T3- and FSH-induced follicular development [103]. These results indicated the potential role of TH and gonadotropin signalling crosstalk in the ovaries [104]. The crosstalk between the proteins of the TGF-beta family and THs has also been indirectly suggested in an in vitro study conducted in bovine cumulus cells stimulated with GDF9 and BMP15. After stimulation, cells showed an increase of a circular RNA hosted in TRAP80, a component of different multi-subunit complexes facilitating their function as a transcriptional factor, including TRs [105]. Although debatable, it is likely that elevated circulating TSH levels, often associated with overt or subclinical hypothyroidism, may be detrimental for FOR. For instance, the TSHR/IGF−1R cross-talk is an important mechanism for the regulation of cellular activity in thyrocytes as well as the expression of thyroid-specific genes and activation of MAPK pathways [106]. Although this crosstalk has not been explored yet in the ovary, we underscore the presence of both receptors in the tissue. Additionally, it has been reported that TSH-R expression is increased by the gonadotropin-driven cAMP cascade and inhibited by oestradiol production in cultures of rat follicles and primary GCs. Lastly, thyrostimulin, produced by the oocytes, is known to be TSH-R main activator in the ovary [107]. The crosstalk between the gonadotropins and thyroid pathways has been investigated in different animal models, including teleosts (goldfish) and mammals (rodents and humans) [104,108,109]. In goldfish, T3 inhibited the expression of LH in the pituitary [110]. Accordingly, the inhibition of circulating LH was evidenced in hyperthyroid rats [111]. Conversely, other reports have evidenced that LH mRNA was unaffected by hypothyroidism or T3 replacement in rat pituitary [112,113]. Finally, in hyperthyroid women higher levels of circulating LH have been reported, a sign of a paradoxical effect observed also in goldfish [70,114]. In the above-cited studies, the regulation of LH transcription, as well as FSH, was suggested as the mechanism by which THs modulated the steroidogenesis and the expression of the oestrogen receptors in the ovary [115]. Recently, this indication was corroborated by experiments conducted in zebrafish and mouse models carrying the homozygous deletion of the genes codifying for TH receptors and deiodinases.
A dio2 mutant was established in zebrafish, which showed a dramatic decrease of T3 level in the gonads leading to a male-biased development. It was reported that the permanent dio2 deficiency determined severe fertility issues associated with a defect in egg laying [116]. The fertility and the ovarian phenotype were further investigated in wild type and mutant animals. The latter showed an increased expression of dio1 and thra transcripts in the ovaries. In addition, dio2 mutant presented an increase in the primary oocytes and to a lesser extent of the vitellogenic ones along with the inhibition of the ovulation. This phenotype was considered the result of the suppression of the steroidogenesis. Indeed, the ovarian reduction of T3 signalling resulted in the inhibition of ovarian oestrogen levels and concomitant down-regulation of hsd11b2 and upregulation on esr2b transcripts. This effect could be rescued by T3 supplementation [117].
In a rat model of iodine deficiency (LID), induced by a low-iodine diet, it was shown a slight reduction of Dio1 and about 2-fold increase of Dio2 activity, indicating a local compensatory mechanism [118]. Although not further characterised, similar effects were shown in zebrafish dio2 KO. It has also been reported that the reproduction was severely hampered in DIO3KO mouse. However, the effects on ovarian health have not been directly analysed [119]. On the contrary, none major effect on female fertility was described in DIO2KO and DIO1KO mice [120]. Several mouse models have been developed carrying a homozygous deletion in the TRs genes, even though never ovary-targeted. Nevertheless, some of these animal models showed fertility problems that were related to altered ovulation, as in the TRα2 knockout females [121].
Overall, the data evidence the importance of TH signalling in the preservation of FOR, as a result of its crosstalk with other signalling pathways strictly involved in ovarian health.
6. Conclusions
Taken together, the data evidence that abnormal levels of THs, especially during puberty and fertile age, might result in ovarian dysfunction throughout the entire life. Different mechanisms may contribute, ranging from the altered circulating THs levels and/or their peripheral metabolism/signalling to their crosstalk with signalling pathways pivotal for the preservation of FOR. Well documented studies indicate that thyroid dysfunctions, especially in early-life stages, may determine subfertility or infertility, menstrual/oestrous irregularity, anovulation.
Here, we have reported results from retrospective studies of women with thyroid dysfunction as well as in vivo and in vitro studies conducted in animals and/or ovarian cell cultures models of hypothyroidism or hyperthyroidism. Although still debatable, the data suggest that sub-clinical and/or overt hypothyroidism reduces the number of growing follicles and increases follicular atresia. Moreover, the results of studies investigating the relationship between the hyperthyroidism and the ovarian health are conflicting. In fact, an increase of T3 among patients suffering from the polycystic ovary syndrome (PCOS) has been associated with enhanced activity of FSH, which preserves FOR [122]. These effects could be direct or an indirect consequence of the crosstalk with other signalling pathways playing either a positive or negative role in the preservation of FOR (Figure 3).
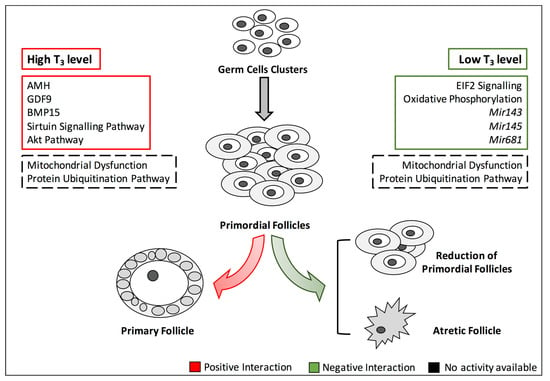
Figure 3.
Signalling pathways playing a positive or negative role in FOR homeostasis. The figure depicts the main signalling pathways involved in FOR maintenance and how they are impaired by altered TH signalling. Green and red boxes indicate negative and positive interactions playing a role in FOR homeostasis, respectively. Black dashed boxes define “unknown” interaction type. The reported molecular functions are publicly available in Cuomo et al., 2018. Abbreviation: T3, triiodothyronine; AMH, anti-Müllerian hormone; GDF9, growth differentiation factor 9; BMP15, bone morphogenetic protein 15.
This review aims at shedding the light on the peripheral TH signalling involvement in the maintenance of FOR. Very little data have been published in this regard and the retrieved publications poorly addressed the molecular mechanisms underlying the role of local thyroid hormone metabolism/signalling in the ovaries. We suppose that this depends on the models adopted in the reviewed studies, in which TH signalling impairment results from the exposure to environmental stressors, i.e., low-iodine intake, endocrine disruptors, or by the generation of whole-body knockouts.
We believe that more effort is needed to develop adequate models to characterise the role of TH signalling in the ovary such as GCs, mouse and/or zebrafish carrying an ovarian specific deletion of the genes codifying the proteins regulating TH metabolism and signalling. They are essential in understanding the effects of THs impairment in the establishment and preservation of FOR. Their development will be pivotal in dissecting the molecular mechanisms of thyroid hormone action in regulating the FOR under physiological and disease-related conditions.
Author Contributions
Conceptualisation, C.A. and M.D.F.; software, D.C.; investigation, M.C. and D.C.; writing—original draft preparation, C.A., M.C., D.C., A.G. and M.M.; writing—review and editing, C.A. and M.D.F.; visualization, C.A., M.C., and D.C.; supervision, C.A. and M.D.F.; project administration, C.A.; funding acquisition, C.A. and M.D.F.; M.C. and D.C. have contributed equally to this work.; C.A. and M.D.F. have equally contributed to this work and meant both as corresponding. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by The Italian Workers’ Compensation Authority (grant. n. 12010), Sensor Regione Campania (grant n. 23), Goodwater Regione Campania (POR Campania FESR 2014/2020 O.S. 1.1 Az. 1.1.3 E 1.1.4–CUP B63D18000150007) and POR FESR 2014–2020-Projects (RARE PLATNET, SATIN and COEPICA) Regione Campania.
Acknowledgments
We thank IRGS (Biogem) for support.
Conflicts of Interest
Authors do not have competing financial interests in relation to the work.
References
- Pelosi, E.; Forabosco, A.; Schlessinger, D. Genetics of the ovarian reserve. Front Genet. 2015, 6, 308. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, N.P.; Sakkas, E.; Vaiarelli, A.; Poppe, K.; Camus, M.; Tournaye, H. Thyroid autoimmunity, hypothyroidism and ovarian reserve: A cross-sectional study of 5000 women based on age-specific AMH values. Hum. Reprod. 2015, 30, 1690–1696. [Google Scholar] [CrossRef] [PubMed]
- Little, A.G. Local Regulation of Thyroid Hormone Signaling. Vitam Horm. 2018, 106, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Cuomo, D.; Ambrosino, C. Non-coding RNAs as integrators of the effects of age, genes, and environment on ovarian aging. Cell Death Dis. 2019, 10, 88. [Google Scholar] [CrossRef] [PubMed]
- Cuomo, D.; Porreca, I.; Ceccarelli, M.; Threadgill, D.W.; Barrington, W.T.; Petriella, A.; D’Angelo, F.; Cobellis, G.; De Stefano, F.; D’Agostino, M.N.; et al. Transcriptional landscape of mouse-aged ovaries reveals a unique set of non-coding RNAs associated with physiological and environmental ovarian dysfunctions. Cell Death Discov. 2018, 4, 112. [Google Scholar] [CrossRef] [PubMed]
- Shupnik, M.A.; Ridgway, E.C.; Chin, W.W. Molecular biology of thyrotropin. Endocr. Rev. 1989, 10, 459–475. [Google Scholar] [CrossRef]
- Joseph-Bravo, P.; Jaimes-Hoy, L.; Uribe, R.M.; Charli, J.L. 60 Years of Neuroendocrinology: Trh, the first hypophysiotropic releasing hormone isolated: Control of the pituitary-thyroid axis. J. Endocrinol. 2015, 226, T85–T100. [Google Scholar] [CrossRef]
- Kakucska, I.; Rand, W.; Lechan, R.M. Thyrotropin-releasing hormone gene expression in the hypothalamic paraventricular nucleus is dependent upon feedback regulation by both triiodothyronine and thyroxine. Endocrinology 1992, 130, 2845–2850. [Google Scholar] [CrossRef]
- Larsen, P.R. Thyroid-pituitary interaction: Feedback regulation of thyrotropin secretion by thyroid hormones. N. Engl. J. Med. 1982, 306, 23–32. [Google Scholar] [CrossRef]
- Fonseca, T.L.; Correa-Medina, M.; Campos, M.P.; Wittmann, G.; Werneck-de-Castro, J.P.; Arrojo e Drigo, R.; Mora-Garzon, M.; Ueta, C.B.; Caicedo, A.; Fekete, C.; et al. Coordination of hypothalamic and pituitary T3 production regulates TSH expression. J. Clin. Investig. 2013, 123, 1492–1500. [Google Scholar] [CrossRef]
- Dai, G.; Levy, O.; Carrasco, N. Cloning and characterization of the thyroid iodide transporter. Nature 1996, 379, 458–460. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.A.; Wang, R.; Kreman, T.M.; Sheffield, V.C.; Karniski, L.P. The Pendred syndrome gene encodes a chloride-iodide transport protein. Nat. Genet. 1999, 21, 440–443. [Google Scholar] [CrossRef]
- Gereben, B.; Zavacki, A.M.; Ribich, S.; Kim, B.W.; Huang, S.A.; Simonides, W.S.; Zeold, A.; Bianco, A.C. Cellular and molecular basis of deiodinase-regulated thyroid hormone signaling. Endocr. Rev. 2008, 29, 898–938. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A.C.; Salvatore, D.; Gereben, B.; Berry, M.J.; Larsen, P.R. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr. Rev. 2002, 23, 38–89. [Google Scholar] [CrossRef] [PubMed]
- Peeters, R.P.; Visser, T.J. Metabolism of Thyroid Hormone. 2000. Available online: https://www.thyroidmanager.org/chapter/metabolism-of-thyroid-hormone/ (accessed on 1 March 2020).
- Lum, S.M.; Nicoloff, J.T.; Spencer, C.A.; Kaptein, E.M. Peripheral tissue mechanism for maintenance of serum triiodothyronine values in a thyroxine-deficient state in man. J. Clin. Investig. 1984, 73, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.J.; Fiering, S.N.; Thai, B.; Wu, S.Y.; St Germain, E.; Parlow, A.F.; St Germain, D.L.; Galton, V.A. Targeted disruption of the type 1 selenodeiodinase gene (Dio1) results in marked changes in thyroid hormone economy in mice. Endocrinology 2006, 147, 580–589. [Google Scholar] [CrossRef]
- Visser, T.J. Role of sulfation in thyroid hormone metabolism. Chem. Biol. Interact. 1994, 92, 293–303. [Google Scholar] [CrossRef]
- Hernandez, A. Structure and function of the type 3 deiodinase gene. Thyroid 2005, 15, 865–874. [Google Scholar] [CrossRef]
- Moreno, M.; Giacco, A.; Di Munno, C.; Goglia, F. Direct and rapid effects of 3,5-diiodo-L-thyronine (T2). Mol. Cell Endocrinol. 2017, 458, 121–126. [Google Scholar] [CrossRef]
- Lanni, A.; Moreno, M.; Cioffi, M.; Goglia, F. Effect of 3,3’-diiodothyronine and 3,5-diiodothyronine on rat liver oxidative capacity. Mol. Cell Endocrinol. 1992, 86, 143–148. [Google Scholar] [CrossRef]
- Gnocchi, D.; Ellis, E.C.S.; Johansson, H.; Eriksson, M.; Bruscalupi, G.; Steffensen, K.R.; Parini, P. Diiodothyronines regulate metabolic homeostasis in primary human hepatocytes by modulating mTORC1 and mTORC2 activity. Mol. Cell Endocrinol. 2020, 499, 110604. [Google Scholar] [CrossRef] [PubMed]
- Pappa, T.; Ferrara, A.M.; Refetoff, S. Inherited defects of thyroxine-binding proteins. Best Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 735–747. [Google Scholar] [CrossRef] [PubMed]
- Hagenbuch, B. Cellular entry of thyroid hormones by organic anion transporting polypeptides. Best Pract. Res. Clin. Endocrinol. Metab. 2007, 21, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Friesema, E.C.; Kuiper, G.G.; Jansen, J.; Visser, T.J.; Kester, M.H. Thyroid hormone transport by the human monocarboxylate transporter 8 and its rate-limiting role in intracellular metabolism. Mol. Endocrinol. 2006, 20, 2761–2772. [Google Scholar] [CrossRef] [PubMed]
- Pizzagalli, F.; Hagenbuch, B.; Stieger, B.; Klenk, U.; Folkers, G.; Meier, P.J. Identification of a novel human organic anion transporting polypeptide as a high affinity thyroxine transporter. Mol. Endocrinol. 2002, 16, 2283–2296. [Google Scholar] [CrossRef]
- Yen, P.M. Physiological and molecular basis of thyroid hormone action. Physiol. Rev. 2001, 81, 1097–1142. [Google Scholar] [CrossRef]
- Davis, P.J.; Davis, F.B.; Mousa, S.A.; Luidens, M.K.; Lin, H.Y. Membrane receptor for thyroid hormone: Physiologic and pharmacologic implications. Annu. Rev. Pharmacol. Toxicol. 2011, 51, 99–115. [Google Scholar] [CrossRef]
- Davis, P.J.; Glinsky, G.V.; Lin, H.Y.; Leith, J.T.; Hercbergs, A.; Tang, H.Y.; Ashur-Fabian, O.; Incerpi, S.; Mousa, S.A. Corrigendum: “Cancer Cell Gene Expression Modulated from Plasma Membrane Integrin alphavbeta3 by Thyroid Hormone and Nanoparticulate Tetrac”. Front. Endocrinol. (Lausanne) 2015, 6, 98. [Google Scholar] [CrossRef]
- Bergh, J.J.; Lin, H.Y.; Lansing, L.; Mohamed, S.N.; Davis, F.B.; Mousa, S.; Davis, P.J. Integrin alphaVbeta3 contains a cell surface receptor site for thyroid hormone that is linked to activation of mitogen-activated protein kinase and induction of angiogenesis. Endocrinology 2005, 146, 2864–2871. [Google Scholar] [CrossRef]
- Zhang, X.K.; Kahl, M. Regulation of retinoid and thyroid hormone action through homodimeric and heterodimeric receptors. Trends Endocrinol. Metab. 1993, 4, 156–162. [Google Scholar] [CrossRef]
- Harvey, C.B.; Williams, G.R. Mechanism of thyroid hormone action. Thyroid 2002, 12, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, S.; Lonard, D.M.; O’Malley, B.W. Nuclear receptor coactivators: Master regulators of human health and disease. Annu. Rev. Med. 2013, 65, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.Y. Multiple mechanisms for regulation of the transcriptional activity of thyroid hormone receptors. Rev. Endocr. Metab. Disord. 2000, 1, 9–18. [Google Scholar] [CrossRef]
- Davis, P.J.; Leonard, J.L.; Davis, F.B. Mechanisms of nongenomic actions of thyroid hormone. Front. Neuroendocrinol. 2008, 29, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Davis, P.J.; Goglia, F.; Leonard, J.L. Nongenomic actions of thyroid hormone. Nat. Rev. Endocrinol. 2015, 12, 111–121. [Google Scholar] [CrossRef]
- Silvestri, E.; Lombardi, A.; de Lange, P.; Schiavo, L.; Lanni, A.; Goglia, F.; Visser, T.J.; Moreno, M. Age-related changes in renal and hepatic cellular mechanisms associated with variations in rat serum thyroid hormone levels. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E1160–E1168. [Google Scholar] [CrossRef]
- Pfundt, R.; Del Rosario, M.; Vissers, L.; Kwint, M.P.; Janssen, I.M.; de Leeuw, N.; Yntema, H.G.; Nelen, M.R.; Lugtenberg, D.; Kamsteeg, E.J.; et al. Detection of clinically relevant copy-number variants by exome sequencing in a large cohort of genetic disorders. Genet. Med. 2017, 19, 667–675. [Google Scholar] [CrossRef]
- Colicchia, M.; Campagnolo, L.; Baldini, E.; Ulisse, S.; Valensise, H.; Moretti, C. Molecular basis of thyrotropin and thyroid hormone action during implantation and early development. Hum. Reprod. Update 2014, 20, 884–904. [Google Scholar] [CrossRef]
- Maia, A.L.; Kieffer, J.D.; Harney, J.W.; Larsen, P.R. Effect of 3,5,3’-Triiodothyronine (T3) administration on dio1 gene expression and T3 metabolism in normal and type 1 deiodinase-deficient mice. Endocrinology 1995, 136, 4842–4849. [Google Scholar] [CrossRef]
- Maia, A.L.; Goemann, I.M.; Meyer, E.L.; Wajner, S.M. Deiodinases: The balance of thyroid hormone: Type 1 iodothyronine deiodinase in human physiology and disease. J. Endocrinol. 2011, 209, 283–297. [Google Scholar] [CrossRef]
- Leng, O.; Razvi, S. Hypothyroidism in the older population. Thyroid Res. 2019, 12, 2. [Google Scholar] [CrossRef] [PubMed]
- Weghofer, A.; Barad, D.H.; Darmon, S.; Kushnir, V.A.; Gleicher, N. What affects functional ovarian reserve, thyroid function or thyroid autoimmunity? Reprod. Biol. Endocrinol. 2016, 14, 26. [Google Scholar] [CrossRef] [PubMed]
- Feart, C.; Pallet, V.; Boucheron, C.; Higueret, D.; Alfos, S.; Letenneur, L.; Dartigues, J.F.; Higueret, P. Aging affects the retinoic acid and the triiodothyronine nuclear receptor mRNA expression in human peripheral blood mononuclear cells. Eur. J. Endocrinol. 2005, 152, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Veldhuis, J.D. Changes in pituitary function with ageing and implications for patient care. Nat. Rev. Endocrinol. 2013, 9, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A.C.; Dumitrescu, A.; Gereben, B.; Ribeiro, M.O.; Fonseca, T.L.; Fernandes, G.W.; Bocco, B. Paradigms of Dynamic Control of Thyroid Hormone Signaling. Endocr. Rev. 2019, 40, 1000–1047. [Google Scholar] [CrossRef]
- Kovanci, E.; Rohozinski, J.; Simpson, J.L.; Heard, M.J.; Bishop, C.E.; Carson, S.A. Growth differentiating factor-9 mutations may be associated with premature ovarian failure. Fertil. Steril. 2007, 87, 143–146. [Google Scholar] [CrossRef]
- Persani, L.; Rossetti, R.; Cacciatore, C.; Fabre, S. Genetic defects of ovarian TGF-beta-like factors and premature ovarian failure. J. Endocrinol. Investig. 2011, 34, 244–251. [Google Scholar] [CrossRef]
- Rossetti, R.; Ferrari, I.; Bonomi, M.; Persani, L. Genetics of primary ovarian insufficiency. Clin. Genet. 2016, 91, 183–198. [Google Scholar] [CrossRef]
- Huhtaniemi, I.; Hovatta, O.; La Marca, A.; Livera, G.; Monniaux, D.; Persani, L.; Heddar, A.; Jarzabek, K.; Laisk-Podar, T.; Salumets, A.; et al. Advances in the Molecular Pathophysiology, Genetics, and Treatment of Primary Ovarian Insufficiency. Trends Endocrinol. Metab. 2018, 29, 400–419. [Google Scholar] [CrossRef]
- Kumar, R.; Alwani, M.; Kosta, S.; Kaur, R.; Agarwal, S. BMP15 and GDF9 Gene Mutations in Premature Ovarian Failure. J. Reprod. Infertil. 2017, 18, 185–189. [Google Scholar]
- Roy, S.; Gandra, D.; Seger, C.; Biswas, A.; Kushnir, V.A.; Gleicher, N.; Kumar, T.R.; Sen, A. Oocyte-Derived Factors (GDF9 and BMP15) and FSH Regulate AMH Expression Via Modulation of H3K27AC in Granulosa Cells. Endocrinology 2018, 159, 3433–3445. [Google Scholar] [CrossRef] [PubMed]
- Uzumcu, M.; Kuhn, P.E.; Marano, J.E.; Armenti, A.E.; Passantino, L. Early postnatal methoxychlor exposure inhibits folliculogenesis and stimulates anti-Mullerian hormone production in the rat ovary. J. Endocrinol. 2006, 191, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Xiong, G.P.; Luo, X.M.; Huang, S.Z.; Liu, J.; Huang, X.L.; Xie, Y.Z.; Lin, W.P. Dibutyl Phthalate Inhibits the Effects of Follicle-Stimulating Hormone on Rat Granulosa Cells Through Down-Regulation of Follicle-Stimulating Hormone Receptor. Biol. Reprod. 2016, 94, 144. [Google Scholar] [CrossRef] [PubMed]
- Maeso, I.; Irimia, M.; Tena, J.J.; Casares, F.; Gomez-Skarmeta, J.L. Deep conservation of cis-regulatory elements in metazoans. Philos. Trans. R. Soc. Lond B Biol. Sci. 2013, 368, 20130020. [Google Scholar] [CrossRef] [PubMed]
- Van der Ven, L.T.; van den Brandhof, E.J.; Vos, J.H.; Power, D.M.; Wester, P.W. Effects of the antithyroid agent propylthiouracil in a partial life cycle assay with zebrafish. Environ. Sci. Technol. 2006, 40, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Sechman, A. The role of thyroid hormones in regulation of chicken ovarian steroidogenesis. Gen. Comp. Endocrinol. 2013, 190, 68–75. [Google Scholar] [CrossRef]
- Chen, Y.D.; Hoch, F.L. Mitochondrial inner membrane in hypothyroidism. Arch. Biochem. Biophys. 1976, 172, 741–744. [Google Scholar] [CrossRef][Green Version]
- Heijlen, M.; Houbrechts, A.M.; Darras, V.M. Zebrafish as a model to study peripheral thyroid hormone metabolism in vertebrate development. Gen. Comp. Endocrinol. 2013, 188, 289–296. [Google Scholar] [CrossRef]
- Duarte-Guterman, P.; Navarro-Martin, L.; Trudeau, V.L. Mechanisms of crosstalk between endocrine systems: Regulation of sex steroid hormone synthesis and action by thyroid hormones. Gen. Comp. Endocrinol. 2014, 203, 69–85. [Google Scholar] [CrossRef]
- Aghajanova, L.; Lindeberg, M.; Carlsson, I.B.; Stavreus-Evers, A.; Zhang, P.; Scott, J.E.; Hovatta, O.; Skjoldebrand-Sparre, L. Receptors for thyroid-stimulating hormone and thyroid hormones in human ovarian tissue. Reprod. Biomed. Online 2009, 18, 337–347. [Google Scholar] [CrossRef]
- Rae, M.T.; Niven, D.; Ross, A.; Forster, T.; Lathe, R.; Critchley, H.O.; Ghazal, P.; Hillier, S.G. Steroid signalling in human ovarian surface epithelial cells: The response to interleukin-1alpha determined by microarray analysis. J. Endocrinol. 2004, 183, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Rae, M.T.; Gubbay, O.; Kostogiannou, A.; Price, D.; Critchley, H.O.; Hillier, S.G. Thyroid hormone signaling in human ovarian surface epithelial cells. J. Clin. Endocrinol. Metab. 2007, 92, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Kohrle, J. Local activation and inactivation of thyroid hormones: The deiodinase family. Mol. Cell Endocrinol. 1999, 151, 103–119. [Google Scholar] [CrossRef]
- Zhang, S.S.; Carrillo, A.J.; Darling, D.S. Expression of multiple thyroid hormone receptor mRNAs in human oocytes, cumulus cells, and granulosa cells. Mol. Hum. Reprod. 1997, 3, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Stavreus Evers, A. Paracrine interactions of thyroid hormones and thyroid stimulation hormone in the female reproductive tract have an impact on female fertility. Front. Endocrinol. (Lausanne) 2012, 3, 50. [Google Scholar] [CrossRef]
- Cramer, D.W.; Sluss, P.M.; Powers, R.D.; McShane, P.; Ginsburgs, E.S.; Hornstein, M.D.; Vitonis, A.F.; Barbieri, R.L. Serum prolactin and TSH in an in vitro fertilization population: Is there a link between fertilization and thyroid function? J. Assist. Reprod. Genet. 2003, 20, 210–215. [Google Scholar] [CrossRef]
- Zhang, C.; Xia, G.; Tsang, B.K. Interactions of thyroid hormone and FSH in the regulation of rat granulosa cell apoptosis. Front. Biosci (Elite Ed). 2011, 3, 1401–1413. [Google Scholar] [CrossRef]
- Verga Falzacappa, C.; Timperi, E.; Bucci, B.; Amendola, D.; Piergrossi, P.; D’Amico, D.; Santaguida, M.G.; Centanni, M.; Misiti, S. T(3) preserves ovarian granulosa cells from chemotherapy-induced apoptosis. J. Endocrinol. 2012, 215, 281–289. [Google Scholar] [CrossRef]
- Doufas, A.G.; Mastorakos, G. The hypothalamic-pituitary-thyroid axis and the female reproductive system. Ann. N. Y. Acad. Sci. 2000, 900, 65–76. [Google Scholar] [CrossRef]
- Porazzi, P.; Calebiro, D.; Benato, F.; Tiso, N.; Persani, L. Thyroid gland development and function in the zebrafish model. Mol. Cell Endocrinol. 2009, 312, 14–23. [Google Scholar] [CrossRef]
- Schmidt, F.; Braunbeck, T. Alterations along the Hypothalamic-Pituitary-Thyroid Axis of the Zebrafish (Danio rerio) after Exposure to Propylthiouracil. J. Thyroid Res. 2011, 376243. [Google Scholar] [CrossRef] [PubMed]
- Mukhi, S.; Torres, L.; Patino, R. Effects of larval-juvenile treatment with perchlorate and co-treatment with thyroxine on zebrafish sex ratios. Gen. Comp. Endocrinol. 2007, 150, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhang, X.; Deng, J.; Hecker, M.; Al-Khedhairy, A.; Giesy, J.P.; Zhou, B. Effects of prochloraz or propylthiouracil on the cross-talk between the HPG, HPA, and HPT axes in zebrafish. Environ. Sci. Technol. 2010, 45, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.Y.; Ng, T.B. Effect of hypothyroidism induced by propylthiouracil and thiourea on male and female reproductive systems of neonatal mice. J. Exp. Zool. 1995, 273, 160–169. [Google Scholar] [CrossRef]
- Meng, L.; Rijntjes, E.; Swarts, H.J.; Keijer, J.; Teerds, K.J. Prolonged hypothyroidism severely reduces ovarian follicular reserve in adult rats. J. Ovarian Res. 2017, 10, 19. [Google Scholar] [CrossRef]
- Meng, L.; Jan, S.Z.; Hamer, G.; van Pelt, A.M.; van der Stelt, I.; Keijer, J.; Teerds, K.J. Preantral follicular atresia occurs mainly through autophagy, while antral follicles degenerate mostly through apoptosis. Biol. Reprod. 2018, 99, 853–863. [Google Scholar] [CrossRef]
- Meng, L.; Rijntjes, E.; Swarts, H.; Bunschoten, A.; van der Stelt, I.; Keijer, J.; Teerds, K. Dietary-Induced Chronic Hypothyroidism Negatively Affects Rat Follicular Development and Ovulation Rate and Is Associated with Oxidative Stress. Biol. Reprod. 2016, 94, 90. [Google Scholar] [CrossRef]
- Serakides, R.; Nunes, V.A.; Nascimiento, E.F.D.; Ribeiro, A.F.C.; Silva, C.M.D. Foliculogênese e esteroidogênese ovarianas em ratas adultas hipertireóideas. Arq. Bras. Endocrinol. Metab. 2001, 45, 258–264. [Google Scholar] [CrossRef]
- Fedail, J.S.; Zheng, K.; Wei, Q.; Kong, L.; Shi, F. Roles of thyroid hormones in follicular development in the ovary of neonatal and immature rats. Endocrine 2014, 46, 594–604. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, X.; Wang, Z.; Niu, W.; Zhu, B.; Xia, G. Effect of different culture systems and 3, 5, 3’-triiodothyronine/follicle-stimulating hormone on preantral follicle development in mice. PLoS ONE 2013, 8, e61947. [Google Scholar] [CrossRef]
- Jiang, J.Y.; Umezu, M.; Sato, E. Improvement of follicular development rather than gonadotrophin secretion by thyroxine treatment in infertile immature hypothyroid rdw rats. J. Reprod. Fertil. 2000, 119, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Sulieman, F.J.; Li, J.; Wei, Q.; Xu, M.; Shi, F. Nitric oxide and thyroid hormone receptor alpha 1 contribute to ovarian follicular development in immature hyper- and hypo-thyroid rats. Reprod. Biol. 2015, 15, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, G.; de Rooij, D.G.; de Jong, F.H.; van den Hurk, R. Effect of hypothyroidism on ovarian follicular development, granulosa cell proliferation and peripheral hormone levels in the prepubertal rat. Eur. J. Endocrinol. 1996, 134, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Torrealday, S.; Kodaman, P.; Pal, L. Premature Ovarian Insufficiency—An update on recent advances in understanding and management. F1000Research 2017, 6, 2069. [Google Scholar] [CrossRef] [PubMed]
- Hollowell, J.G.; Staehling, N.W.; Flanders, W.D.; Hannon, W.H.; Gunter, E.W.; Spencer, C.A.; Braverman, L.E. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J. Clin. Endocrinol. Metab. 2002, 87, 489–499. [Google Scholar] [CrossRef]
- Ayesha, V.J.; Goswami, D. Premature Ovarian Failure: An Association with Autoimmune Diseases. J. Clin. Diagn. Res. 2016, 10, QC10–QC12. [Google Scholar] [CrossRef]
- Goswami, R.; Marwaha, R.K.; Goswami, D.; Gupta, N.; Ray, D.; Tomar, N.; Singh, S. Prevalence of thyroid autoimmunity in sporadic idiopathic hypoparathyroidism in comparison to type 1 diabetes and premature ovarian failure. J. Clin. Endocrinol. Metab. 2006, 91, 4256–4259. [Google Scholar] [CrossRef]
- Novosad, J.A.; Kalantaridou, S.N.; Tong, Z.B.; Nelson, L.M. Ovarian antibodies as detected by indirect immunofluorescence are unreliable in the diagnosis of autoimmune premature ovarian failure: A controlled evaluation. BMC Womens Health 2003, 3, 2. [Google Scholar] [CrossRef]
- Gleicher, N.; Ryan, E.; Weghofer, A.; Blanco-Mejia, S.; Barad, D.H. Miscarriage rates after dehydroepiandrosterone (DHEA) supplementation in women with diminished ovarian reserve: A case control study. Reprod. Biol. Endocrinol. 2009, 7, 108. [Google Scholar] [CrossRef]
- Krassas, G.E.; Poppe, K.; Glinoer, D. Thyroid function and human reproductive health. Endocr. Rev. 2010, 31, 702–755. [Google Scholar] [CrossRef]
- Michalakis, K.G.; Mesen, T.B.; Brayboy, L.M.; Yu, B.; Richter, K.S.; Levy, M.; Widra, E.; Segars, J.H. Subclinical elevations of thyroid-stimulating hormone and assisted reproductive technology outcomes. Fertil. Steril. 2011, 95, 2634–2637. [Google Scholar] [CrossRef] [PubMed]
- Bahri, S.; Tehrani, F.R.; Amouzgar, A.; Rahmati, M.; Tohidi, M.; Vasheghani, M.; Azizi, F. Overtime trend of thyroid hormones and thyroid autoimmunity and ovarian reserve: A longitudinal population study with a 12-year follow up. BMC Endocr. Disord. 2019, 19, 47. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.W.; Huang, Y.L.; Tzeng, C.R.; Huang, R.L.; Chen, C.H. Idiopathic Low Ovarian Reserve Is Associated with More Frequent Positive Thyroid Peroxidase Antibodies. Thyroid 2017, 27, 1194–1200. [Google Scholar] [CrossRef] [PubMed]
- Poppe, K.; Glinoer, D.; Van Steirteghem, A.; Tournaye, H.; Devroey, P.; Schiettecatte, J.; Velkeniers, B. Thyroid dysfunction and autoimmunity in infertile women. Thyroid 2002, 12, 997–1001. [Google Scholar] [CrossRef] [PubMed]
- Belli, M.; Shimasaki, S. Molecular Aspects and Clinical Relevance of GDF9 and BMP15 in Ovarian Function. Vitam. Horm. 2018, 107, 317–348. [Google Scholar] [CrossRef]
- Mayer, A.; Fouquet, B.; Pugeat, M.; Misrahi, M. BMP15 “knockout-like” effect in familial premature ovarian insufficiency with persistent ovarian reserve. Clin. Genet. 2017, 92, 208–212. [Google Scholar] [CrossRef]
- Chu, Y.L.; Xu, Y.R.; Yang, W.X.; Sun, Y. The role of FSH and TGF-beta superfamily in follicle atresia. Aging (Albany N.Y.) 2018, 10, 305–321. [Google Scholar] [CrossRef]
- Price, C.A.; Estienne, A. The life and death of the dominant follicle. Anim. Reprod. 2018, 15 (Suppl. S1), 680–690. [Google Scholar] [CrossRef]
- Wakim, A.N.; Polizotto, S.L.; Buffo, M.J.; Marrero, M.A.; Burholt, D.R. Thyroid hormones in human follicular fluid and thyroid hormone receptors in human granulosa cells. Fertil. Steril. 1993, 59, 1187–1190. [Google Scholar] [CrossRef]
- Canipari, R.; Mangialardo, C.; Di Paolo, V.; Alfei, F.; Ucci, S.; Russi, V.; Santaguida, M.G.; Virili, C.; Segni, M.; Misiti, S.; et al. Thyroid hormones act as mitogenic and pro survival factors in rat ovarian follicles. J. Endocrinol. Investig. 2019, 42, 271–282. [Google Scholar] [CrossRef]
- Maruo, T.; Hayashi, M.; Matsuo, H.; Yamamoto, T.; Okada, H.; Mochizuki, M. The role of thyroid hormone as a biological amplifier of the actions of follicle-stimulating hormone in the functional differentiation of cultured porcine granulosa cells. Endocrinology 1987, 121, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tian, Y.; Ding, Y.; Heng, D.; Xu, K.; Liu, W.; Zhang, C. Role of CYP51 in the Regulation of T3 and FSH-Induced Steroidogenesis in Female Mice. Endocrinology 2017, 158, 3974–3987. [Google Scholar] [CrossRef]
- De Vincentis, S.; Monzani, M.L.; Brigante, G. Crosstalk between gonadotropins and thyroid axis. Minerva Ginecol. 2018, 70, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.H.; Oh, G.S.; Yoon, J.; Lee, G.G.; Lee, K.U.; Kim, S.W. Hepatic TRAP80 selectively regulates lipogenic activity of liver X receptor. J. Clin. Investig. 2018, 125, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Krieger, C.C.; Perry, J.D.; Morgan, S.J.; Kahaly, G.J.; Gershengorn, M.C. TSH/IGF-1 Receptor Cross-Talk Rapidly Activates Extracellular Signal-Regulated Kinases in Multiple Cell Types. Endocrinology 2017, 158, 3676–3683. [Google Scholar] [CrossRef]
- Sun, S.C.; Hsu, P.J.; Wu, F.J.; Li, S.H.; Lu, C.H.; Luo, C.W. Thyrostimulin, but not thyroid-stimulating hormone (TSH), acts as a paracrine regulator to activate the TSH receptor in mammalian ovary. J. Biol. Chem. 2010, 285, 3758–3765. [Google Scholar] [CrossRef]
- Silva, J.F.; Ocarino, N.M.; Serakides, R. Thyroid hormones and female reproduction. Biol. Reprod. 2018, 99, 907–921. [Google Scholar] [CrossRef]
- Habibi, H.R.; Nelson, E.R.; Allan, E.R. New insights into thyroid hormone function and modulation of reproduction in goldfish. Gen. Comp. Endocrinol. 2012, 175, 19–26. [Google Scholar] [CrossRef]
- Nelson, E.R.; Allan, E.R.; Pang, F.Y.; Habibi, H.R. Thyroid hormone and reproduction: Regulation of estrogen receptors in goldfish gonads. Mol. Reprod. Dev. 2010, 77, 784–794. [Google Scholar] [CrossRef]
- Howland, B.E.; Ibrahim, E.A. Hyperthyroidism and gonadotropin secretion in male and female rats. Experientia 1973, 29, 1398–1399. [Google Scholar] [CrossRef]
- Franklyn, J.A.; Wood, D.F.; Balfour, N.J.; Ramsden, D.B.; Docherty, K.; Chin, W.W.; Sheppard, M.C. Effect of hypothyroidism and thyroid hormone replacement in vivo on pituitary cytoplasmic concentrations of thyrotropin-beta and alpha-subunit messenger ribonucleic acids. Endocrinology 1987, 120, 2279–2288. [Google Scholar] [CrossRef] [PubMed]
- Samuels, M.H.; Wierman, M.E.; Wang, C.; Ridgway, E.C. The effect of altered thyroid status on pituitary hormone messenger ribonucleic acid concentrations in the rat. Endocrinology 1989, 124, 2277–2282. [Google Scholar] [CrossRef] [PubMed]
- Yoshiura, Y.; Sohn, Y.C.; Munakata, A.; Kobayashi, M.; Aida, K. Molecular cloning of the cDNA encoding the B subunit of thyrotropin and regulation of its gene expression by thyroid hormones in the goldfish, Carassius auratus. Fish Physiol. Biochem. 1999, 21, 201–210. [Google Scholar] [CrossRef]
- Hapon, M.B.; Simoncini, M.; Via, G.; Jahn, G.A. Effect of hypothyroidism on hormone profiles in virgin, pregnant and lactating rats, and on lactation. Reproduction 2003, 126, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Houbrechts, A.M.; Vergauwen, L.; Bagci, E.; Van Houcke, J.; Heijlen, M.; Kulemeka, B.; Hyde, D.R.; Knapen, D.; Darras, V.M. Deiodinase knockdown affects zebrafish eye development at the level of gene expression, morphology and function. Mol. Cell Endocrinol. 2016, 424, 81–93. [Google Scholar] [CrossRef]
- Houbrechts, A.M.; Van Houcke, J.; Darras, V.M. Disruption of deiodinase type 2 in zebrafish disturbs male and female reproduction. J. Endocrinol. 2019. [Google Scholar] [CrossRef]
- Lavado-Autric, R.; Calvo, R.M.; de Mena, R.M.; de Escobar, G.M.; Obregon, M.J. Deiodinase activities in thyroids and tissues of iodine-deficient female rats. Endocrinology 2012, 154, 529–536. [Google Scholar] [CrossRef]
- Hernandez, A.; Martinez, M.E.; Liao, X.H.; Van Sande, J.; Refetoff, S.; Galton, V.A.; St Germain, D.L. Type 3 deiodinase deficiency results in functional abnormalities at multiple levels of the thyroid axis. Endocrinology 2007, 148, 5680–5687. [Google Scholar] [CrossRef]
- Schneider, M.J.; Fiering, S.N.; Pallud, S.E.; Parlow, A.F.; St Germain, D.L.; Galton, V.A. Targeted disruption of the type 2 selenodeiodinase gene (DIO2) results in a phenotype of pituitary resistance to T4. Mol. Endocrinol. 2001, 15, 2137–2148. [Google Scholar] [CrossRef]
- Salto, C.; Kindblom, J.M.; Johansson, C.; Wang, Z.; Gullberg, H.; Nordstrom, K.; Mansen, A.; Ohlsson, C.; Thoren, P.; Forrest, D.; et al. Ablation of TRalpha2 and a concomitant overexpression of alpha1 yields a mixed hypo- and hyperthyroid phenotype in mice. Mol. Endocrinol. 2001, 15, 2115–2128. [Google Scholar] [CrossRef][Green Version]
- Yin, D.; Ruan, X.; Tian, X.; Du, J.; Zhao, Y.; Cui, Y.; Li, Y.; Mueck, A.O. The relationship between thyroid function and metabolic changes in Chinese women with polycystic ovary syndrome. Gynecol. Endocrinol. 2017, 33, 332–335. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).